Abstract
Drought and salinity are the major environmental abiotic stresses that negatively impact crop development and yield. To improve yields under abiotic stress conditions, drought- and salinity-tolerant crops are key to support world crop production and mitigate the demand of the growing world population. Nevertheless, plant responses to abiotic stresses are highly complex and controlled by networks of genetic and ecological factors that are the main targets of crop breeding programs. Several genomics strategies are employed to improve crop productivity under abiotic stress conditions, but traditional techniques are not sufficient to prevent stress-related losses in productivity. Within the last decade, modern genomics studies have advanced our capabilities of improving crop genetics, especially those traits relevant to abiotic stress management. This review provided updated and comprehensive knowledge concerning all possible combinations of advanced genomics tools and the gene regulatory network of reactive oxygen species homeostasis for the appropriate planning of future breeding programs, which will assist sustainable crop production under salinity and drought conditions.
1. Introduction
Global crop productivity is restricted due to abiotic stresses such as drought, salinity, flooding, nutrient deficiency, and environmental toxicity. Among these abiotic stresses, salinity and drought are the most severe constraints for sustainable agriculture on a global scale. Nearly 7% of terrestrial land is affected by salinity [1], while drought is widespread and increasingly common in recent years due to climate change. Altogether, salinity- and drought-affected lands cover approximately 10.5 and 60 million km2, respectively [2]. Furthermore, climatic changes worsen the frequency and intensity of water shortages in subtropical areas of Asia and Africa. As stated by the UN climatic report [http://www.solcomhouse.com/drought.htm; accessed date on 12 July 2021], rising temperatures are melting the Himalayan glaciers that feed Asia’s largest rivers (Indus, Ganges, Brahmaputra, Yangtze, Mekong, Salween, and Yellow), and those glaciers may disappear by 2035. Additionally, long-term trends indicate that the progressive proliferation of salinity has caused the dilapidation of arable land [3]. For instance, in California, over the last century, 4.5 out of 8.6 million hectares of wetted agricultural land have become salt-affected [4]. Currently, it has become a pertinent problem for crop production [5], mostly in arid and semiarid areas.
Based on numerous estimations, the world population will increase to over 9.7 billion by 2050, which will continue to exacerbate current global food insecurity issues. It is estimated that, over the past 50 years, improved crop productivity has brought about an increase in world food production by up to 20% per capita and decreased the proportion of food-insecure people existing in developing countries from 57% to 27% of the world population [6]. As a result, crops will need to cope with abiotic stresses such as drought and salinity and double productivity to further diminish food insecurity and support the growing human population in more ecologically sustainable ways.
Both drought and salinity stresses induce cellular dehydration, which causes osmotic stress, removal of water from the cytoplasm into the apoplast, and eventually evaporation into the atmosphere [2]. Moreover, early responses to salt stress and drought are comparable in plants. For example, plant cells prevent water loss by increasing the ionic constituents and decreasing the osmotic potential in stressed cells. Due to the similar mechanisms of the stress response in plants, it appears that drought and salinity tolerance mechanisms might be functionally interchangeable [7]. It is well known that stress response mechanisms involve several particular physiological and biochemical pathways that allow plants to adapt to unfavorable conditions. A number of abiotic stress factors, such as salinity, drought, high temperatures, and osmotic stresses, lead to the overproduction of reactive oxygen species (ROS), which cause serious cellular damage and hamper photosynthesis. To protect or repair these injuries, plant cells use an intricate defense system, including a number of antioxidative stress-related defense genes that, in turn, prompt changes in the biochemical plant machinery [8]. ROS production and antioxidant regulation all occur in a synergistic, additive, or antagonistic way and are associated with the control of oxidative stress.
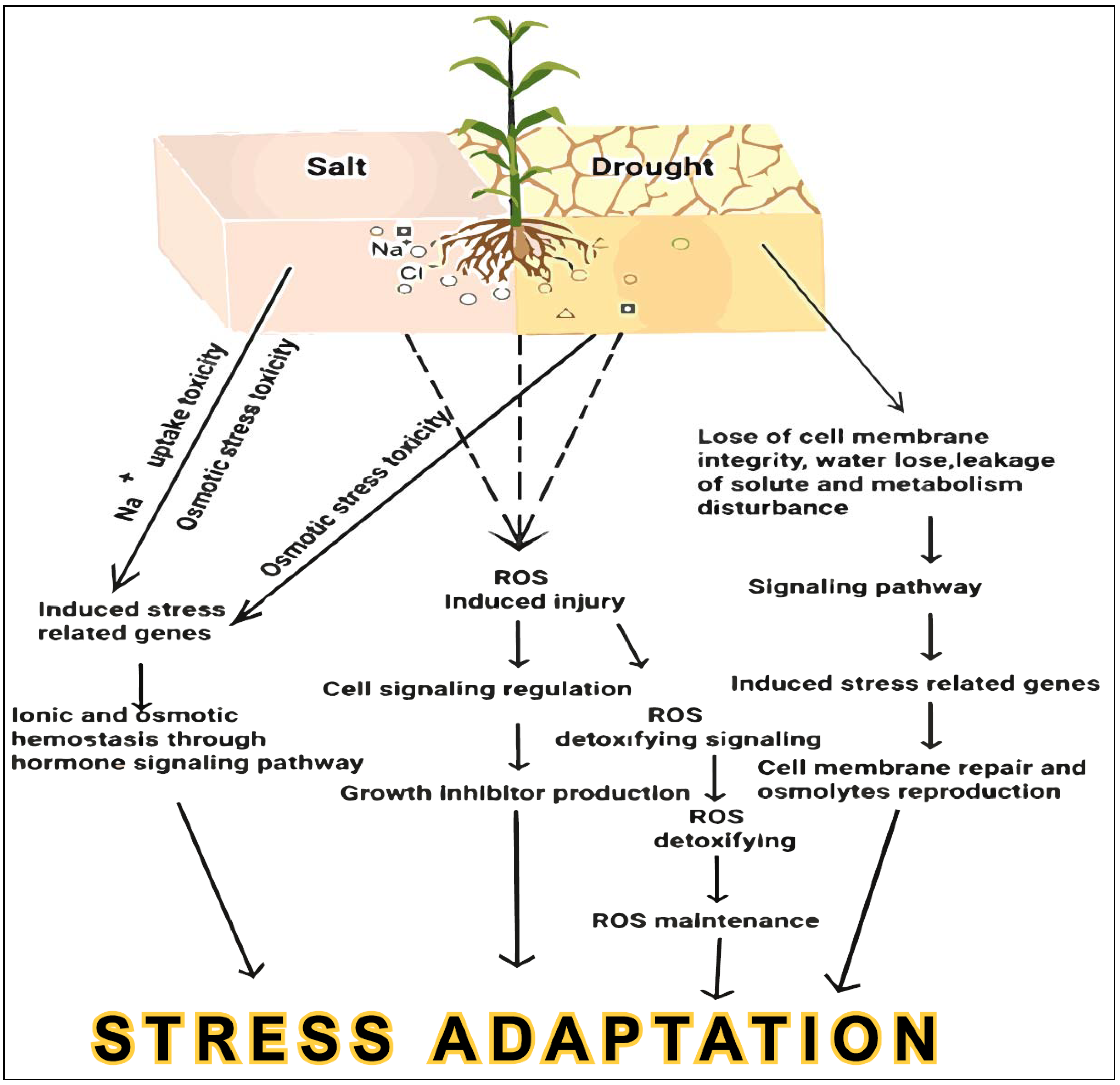
Nevertheless, plant stress response mechanisms are controlled by convoluted networks that are determined by environmental and genetic factors that are often difficult to untangle, thereby impeding traditional breeding approaches [9]. Considering that the conventional breeding strategies for crop improvement are largely aimed at improving yield to meet the demands of an ever-growing world population, breeders have to implement innovative approaches in agriculture to combine high-yield and abiotic stress-tolerant traits in crops [10]. Recent scientific advances and the abovementioned challenges in agriculture have directed the development of high-throughput techniques to pursue and take advantage of plant genome research for the improvement of stress-tolerant crops. Thus, these genomics approaches focus on the entire genome, involving genic and intergenic positions, to attain new insights into the functional and molecular responses of plants, which will sequentially offer specific techniques for crop plant improvement. Recently, many scientists have revealed promising outcomes toward understanding the molecular mechanisms of abiotic stress tolerance in prospective crops using progressive molecular biology practices [10,11,12,13,14,15,16,17,18,19,20,21]. The mechanisms involved in crop salt and drought stress responses are discussed in Figure 1. In this review, we described in detail how to mine the functional genes involved in drought and salt response in plants, using methods such as traditional QTL, transcriptomic analysis, and GWAS. Then, we explored approaches such as epigenetic regulators, gain-of-function, RNAi, TALENs, ZFNs, CRISPR, base editing, and primer editing for functional verification with an example of target genes generated by the aforementioned approaches. Finally, we summarized the methods for generating salt and drought-tolerant crops. The review aimed to offer comprehensive knowledge for improving salt- and drought-tolerant crops using modern genomics strategies regarding ROS regulatory networks. The overview of earlier studies on the advancement of genomics approaches will help in the investigation of upcoming research instructions for improving salt- and drought-tolerant crops.
Figure 1.
The mechanisms involved in crop salt and drought stress responses.
2. Mining Approaches for Salt and Drought Stress Response Genes
To improve drought and salinity stress tolerance in crops, we first require comprehensive knowledge concerning the complex mechanisms of plants that respond to stresses. Detecting the genes/markers/QTL regions associated with drought and salinity stress responses is the first crucial step toward reaching the required understanding for breeding drought and salinity stress-tolerant crop varieties. For the discovery of a gene, various strategies are available in both model and nonmodel crops; here, some of the most advanced are discussed briefly.
2.1. Quantitative Trait Loci (QTL) Analysis
A quantitative trait locus (QTL) is a gene or a region of DNA that is associated with the variation of a quantitative/phenotypic trait that must be polymorphic to affect the biological population. QTL mapping has been a powerful tool for dissecting genetic variants underlying quantitative traits in numerous biological studies and breeding programs. There are two primary concerns when using QTL mapping. One is the power for QTL identification under a controlled false-positive rate, and the other is the accuracy of QTL localization [22]. QTL mapping has been applied as a technique for identifying genomic regions significantly correlated with grain output and various genetically intricate characteristics in cereal crops. This technique is particularly powerful when genetic variation is studied concerning numerous complex traits, where it is possible to identify and differentiate genomic regions that contribute to different characteristics of interest. The data relevant to QTL mapping can be conducive to enhancing the genetic potential of crops via marker-assisted breeding [23]. Currently, scientists can link the molecular mechanisms of genes found in QTLs to demonstrate the genetic and physiological basis of traits such as grain yield. A nice example of this cooperation of QTL mapping, trait scoring, and breeding can be found in using green coloration as a metric of drought resistance in sorghum. The genetic dissection of molecular QTLs associated with green coloration during drought lends convenience to demonstrate the basic mechanisms of physiology and investigate the molecular causes of drought tolerance in sorghum and different grasses [24]. Reducing the genomic sizes of QTLs facilitates enhanced targeting of pertinent genomic regions. Improving the fine mapping of QTLs improves the efficiency with which breeders can understand the significance and mechanisms of QTLs relevant to their traits of interest [24]. Enhanced QTL mapping is particularly relevant when deconvolving complex genomic regions. For example, hypostasis of alleles within QTLs, QTL-QTL genetic interactions, context-dependent activities of QTLs, and the QTL marker position itself impact the articulation of a complex trait such as the yield of grain under drought stress [25]. Interestingly, fine mapping of QTLs revealed that an individual main QTL controlling membrane potential vastly improved marker-assisted selection for salinity-stressed barley [26]. Thus, fine QTL mapping is required for marker-aided QTL pyramiding to improve drought tolerance [27]. Identification of QTLs for abiotic stress tolerance suggests augmentations that can be used for further genomics studies toward the detection of noble genes of salt and drought tolerance to develop a new variety [28]. Several examples of QTLs (quantitative trait loci) for improving crop plant production under salinity and drought stresses are discussed in Table 1.

Table 1.
Known QTLs (quantitative trait loci) for improving crop plant production under salinity and drought stresses.
2.2. Forward Genetics and the Candidate Gene Strategy
Crop plants with stress tolerance have been generated by the transference of genes/loci from definite donor parents, either through a forwarding genetics method that includes the determination of a gene function linked with a phenotype or the identification of novel stress-tolerant donor lines created by the use of mutagenesis. In contrast, reverse genetic breeding approaches could offer an understanding of gene functions and structure/sequence information to predict traits for adapting stress-tolerant cultivars using transgenic and advanced breeding tools. In genomic studies, researchers have implemented these approaches for the genetic improvement of various model and nonmodel species toward salt and drought stress tolerance [34,35,36,37,38,39,40,41].
2.3. Transcriptomics Analysis
Transcriptome analysis refers to the study of the transcriptome of the entire set of RNA transcripts that are generated by a genome, under given times and circumstances or in a specific cell. Transcriptomic analysis techniques play a crucial role in the identification of candidate gene functions and pathways that respond to specific environments [42]. In the last decade, universal transcriptome analysis approaches have been particularly advantageous for functional genomic studies that offer comprehensive molecular mechanisms of certain phenomena. Primarily, a global transcriptome study was initiated with suppression subtractive hybridization (SSH) and cDNA-AFLP and acquired a quantum dive to RNA-seq with the progression of NGS platforms [10]. The information content of an organism is held in its genome and articulated through transcription. The basic purposes of transcriptomics are to record the transcription of all species, including mRNAs, noncoding RNAs, and small RNAs; to determine the transcriptional configuration of genes in terms of their start sites, 5′ and 3′ ends, splice variants, and other posttranscriptional modifications; and to calculate the varying expression patterns of every transcript throughout development and under diverse conditions [40,41]. Currently, transcriptome profiling has progressed into nearly all organisms and represents how information attained from sequence data can be converted into a wide knowledge of gene functionality [42]. Plant stress-response mechanisms frequently employ the use of transcription factors (TFs). A TF is a protein that targets, typically, multiple genes that comprise a regulon and influences their expression patterns. Thus, TFs are a powerful tool for the genetic regulation of many downstream genes and processes, including abiotic stress responses [43]. In the case of salt and drought stress, transcripts related to the upregulation of vital biochemical pathways required for cellular osmotic balance, abscisic acid, and cellular water uptake are controlled by TFs [44]. The role and example of various transcription families through transcriptome analysis relevant to salt and drought stress tolerance are discussed in Table 2.

Table 2.
Identification of different TF families through transcriptome analysis relevant to salt and drought stress tolerance.
2.4. Association Mapping
In genetics studies, association mapping (also well known as linkage disequilibrium mapping) refers to the regular genome-wide distribution of several genes together with other measurable loci (markers) in predicting marker-trait relatives [52] applied in various crops, including rice, barley, maize, sorghum, and wheat, to identify the significant genes or markers that confer a given trait [53]. Numerous genes have been indicated as being connected with abiotic resistance by applying association mapping [54]. It has also been applied to inquire about the limitations within focused parameters and molecular markers in different crops [55]. Association mapping is extremely efficient in experimental varieties with complex or unknown genotypes or those that have a large regeneration time. Association mapping of drought-related varieties in barley was applied to terminate a conventional biparental system of QTL mapping [56]. Furthermore, association mapping has been used to progress the development of QTL maps [57]. A detailed discussion on the association mapping for the sustainability of crop production under salinity and drought stresses is available in Table 3.

Table 3.
Association mapping for improving crop production under salinity and drought stresses.
2.5. Genome-Wide Association
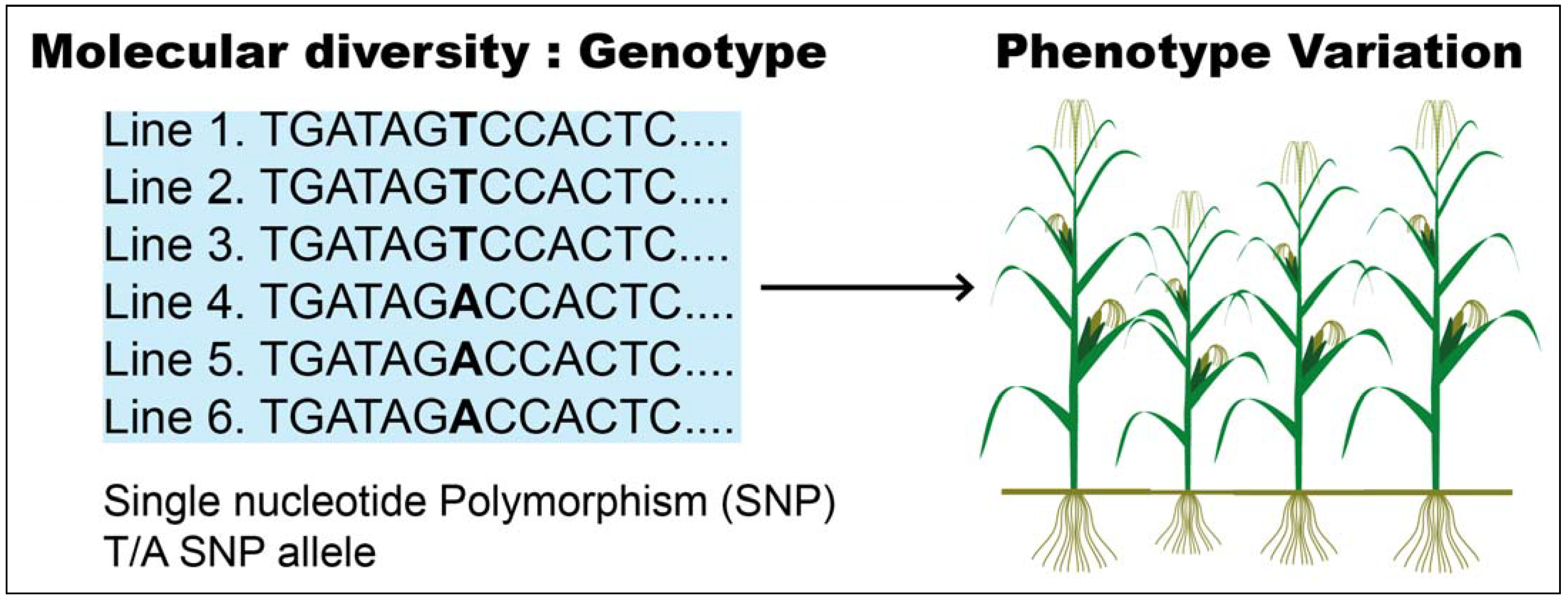
GWAS (genome-wide association study) is a potent presumption-free method used to identify and dissect the genetic regions associated with a certain trait. Typically, GWAS is performed by scoring the phenotypes and sequencing many individuals to link genotype to phenotype, thereby linking genetic variants to a given trait (Figure 2) [61].
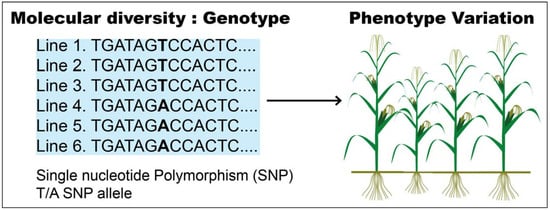
Figure 2.
Connection of genotype to phenotype variation.
GWAS applies large markers and several populations of non-cross-executed lines to provide larger mapping exploration than traditional QTL mapping based on a cross-evolved segregating population, leading to the detection of unknown or unexpected genes. It has been applied to separate complicated genetic parameters in leading crops such as rice and wheat under salt and drought stress. Additionally, GWAS has been effectively conducted to designate QTLs for particular characteristics in wheat (e.g., grain yield, morphology relevant to leaf rust disease, and end-usage quality), thereby applying various systems of molecular markers to bolster breeding resources [62,63]. GWAS has detected more than 2000 loci for simple human diseases to date [64]. Therefore, compared with QTL mapping, GWAS delivers an in-depth, cost-efficient mode of gene investigation and detection of molecular markers.
GWAS focused on the flowering period of saline-treated rice identified 11 loci bearing 22 important SNPs linked to stress responses. The potential genetic determinant of germination was identified on chromosome one, close to the saline conditional QTL regulating Na+ and K+ levels. Approximately 1200 candidate genes linking development to sodium and potassium ion allowances were detected [65]. Thus, GWAS offered an informed list of candidates for saline tolerance-connected gene cloning and uncovered responsive genetic elements relevant to salt stress [66]. GWAS is also important to perceive the genetic architecture of complex characteristics to improve drought tolerance [67]. Recently, “No-Genome-Required-GWAS” approaches have provided easy and efficient identification of genetic variants underlying phenotypic variation in plants [68]. Details on genome-wide association mapping for identifying QTLs under salinity and drought stresses are discussed in Table 4.

Table 4.
Genome-wide association mapping for identifying QTLs under salinity and drought stresses.
2.6. Next-Generation Sequencing
Sequencing technologies include several techniques that generally consist of template preparation, sequencing and imaging, and data analysis [79]. Next-generation sequencing (NGS) integrates technologies that inexpensively and efficiently produce millions of short DNA sequence reads mainly in the range of 25 to 700 bp in length [80]. These technologies have made it possible for scientists to investigate crops at the genomic and transcriptomic levels to assist diversity analysis and marker-assisted breeding [80]. The relevance of NGS appears to be endless, permitting quick presses forward in numerous fields associated with the biological sciences. NGS has also afforded a wealth of knowledge for biology studies via end-to-end whole-genome sequencing of a broad diversity of organisms [81]. Whole-genome sequence studies have focused particularly on detailed information on genomics criteria, including regulatory sequences, coding and noncoding genes, GC content, and repetitive elements, which would be utilized in functional characterization, such as microarray or tiling arrays. Additionally, NGS can be used to address many remaining biological questions by means of resequencing targeted areas of concern or whole genomes (as is being performed for the human genome [82]), de novo assemblies of bacterial and lower eukaryotic genomes, cataloging the transcriptomes of cells, tissues, and organisms (RNA sequencing), genome-wide profiling of epigenetic markers and chromatin structure using additional seq-based methods (ChIP-seq, methyl-seq, and DNase-seq), and species classification and/or gene discovery by metagenomics studies [50].
3. Functional Genomics Approaches
After identifying a QTL/allele/gene, the next sensible step is to characterize the gene before incorporation into a cultivar by studying several physiological, molecular, and biochemical pathways of genes. Thus, functional genomics approaches were extensively implemented to determine the gene functions and the connections between genes in a regulatory network that would be utilized to produce improved crop varieties. Consequently, there have been multiple tools developed for the characterization of gene function; some of the most exploited are described briefly.
3.1. Epigenetic Regulators
In wider definitions, the term ‘epigenetics’ frequently refers to a type of overall nongenetic (unrelated to DNA sequence per se) heredity at various levels. That is, epigenetics illustrates a number of dissimilar methods of genetic regulation whose temporal and heritable constituents have not in all cases been decided [83]. For example, methylation of DNA generally interferes with gene expression by way of gene silencing [84]. The reduction of methylation in resistance-associated genes activates chromatin and the expression of genes, which offers long-term or enduring resistance under stress conditions [85]. Epigenetics sustains the identity of stress memory in plants, which helps pre-exposed plants fight comparable stress throughout subsequent exposures. Histone modifications, DNA methylation and demethylation, and ATP-dependent chromatin remodeling are some of the epigenetic changes performed by plants during drought stress [86]. Epigenetic responses to drought stress have been studied in numerous plants, particularly the stress memory and gene activation marker H3K4me3, which has been used to carry out genome-wide ChIPseq analyses in Arabidopsis [87]. Furthermore, the HAT genes in rice (OsHAC703, OsHAG703, OsHAF701, and OsHAM70) [88] and the HvMYST and HvELP3 genes in barley were also shown to be involved in epigenetic regulation in drought responses [89]. DNA methylation and histone modifications may have a similar result on stress-inducible genes, as salinity stress influences the expression of a range of transcripts in soybean [48]. Work in rice underlined that hypomethylation in reaction to salt stress may be associated with changes in the expression of DNA demethylases [90]. The transcriptional adaptor ADA2b (a modulator of histone acetyltransferase activity) is responsible for hypersensitivity to salt stress in Arabidopsis thaliana [91].
3.2. Gain-of-Function Lines
Gain-of-function methods have been extensively used for the study of gene function in plants and are considered among the most useful tools for gain-of-function phenotypes. Gain-of-function lines are generated through the arbitrary activation of endogenous genes by transcriptional enhancers and the regular expression of individual transgenes by transformation [9]. This method employs the phenotype of gain-of-function lines that overexpress a selected gene family and can be executed without meddling from other gene family members that allow the categorization of functionally unwanted genes [10]. Alternatively, the overexpression of a mutant gene can be expressed due to the presence of higher levels of nonfunctional protein causing a superseding negative interface with the wild-type protein. To overcome this event, a mutant type could be used to compare the wild-type protein allies, resulting in a mutant phenotype. Conversely, heterologous expression of a gene in the yeast-hybrid system is an alternative way to characterize genes. In the first gain-of-function approach, a strong promoter or enhancer element is arbitrarily inserted into the plant genome with the help of T-DNA [11], which stimulates a gene near the site of the harbor. Other gain-of-function approaches involve cDNA overexpression and open reading frame (ORF) overexpression, whereas full-length cDNAs or ORFs have been cloned into a strong promoter downstream. Under the switch of the CaMV35S promoter, various abiotic stress response genes have been characterized by the use of ectopic overexpression of cDNAs [11,12,13,14].
3.3. Gene Silencing and RNA Interference Techniques for Salinity and Drought Stress
Suppression of a gene is referred to as gene silencing in plants and fungi and interference RNA (RNAi) in animals and is generally thought of as a controlling mechanism of gene expression mostly in eukaryotic cells [92]. RNA interference (RNAi) has been considered one of the most crucial discoveries in molecular genetics during the last several years for posttranscriptional gene silencing (PTGS) cosuppression [93]. RNA silencing hints at a nucleotide sequence-specific procedure that prompts mRNA degradation or translation termination at the posttranscriptional level in plants arbitrated by small RNAs (sRNAs), which are divided into two classes: microRNAs (miRNAs) and small interfering RNAs (siRNAs). However, RNAi was properly adapted into antisense-stranded RNA as an operative technique to constrain gene expression [94]. Silencing a gene through transgenic expression of sRNAs has been extensively implemented for abiotic stress-related gene function functional efforts. Currently, the virus-induced gene silencing (VIGS) technique for posttranscriptional gene silencing is extensively used for rapid and efficient gene function studies related to salt and drought stresses [95,96,97,98]. It can also be used for both forward and reverse genetic studies. Target gene silencing techniques for improving crops under salinity and drought stresses are discussed in Table 5.

Table 5.
Target gene-silencing techniques for improving crop variety under salinity and drought stresses.
3.4. Genome Engineering (TALENs, ZFNs, CRISPR/Cas)
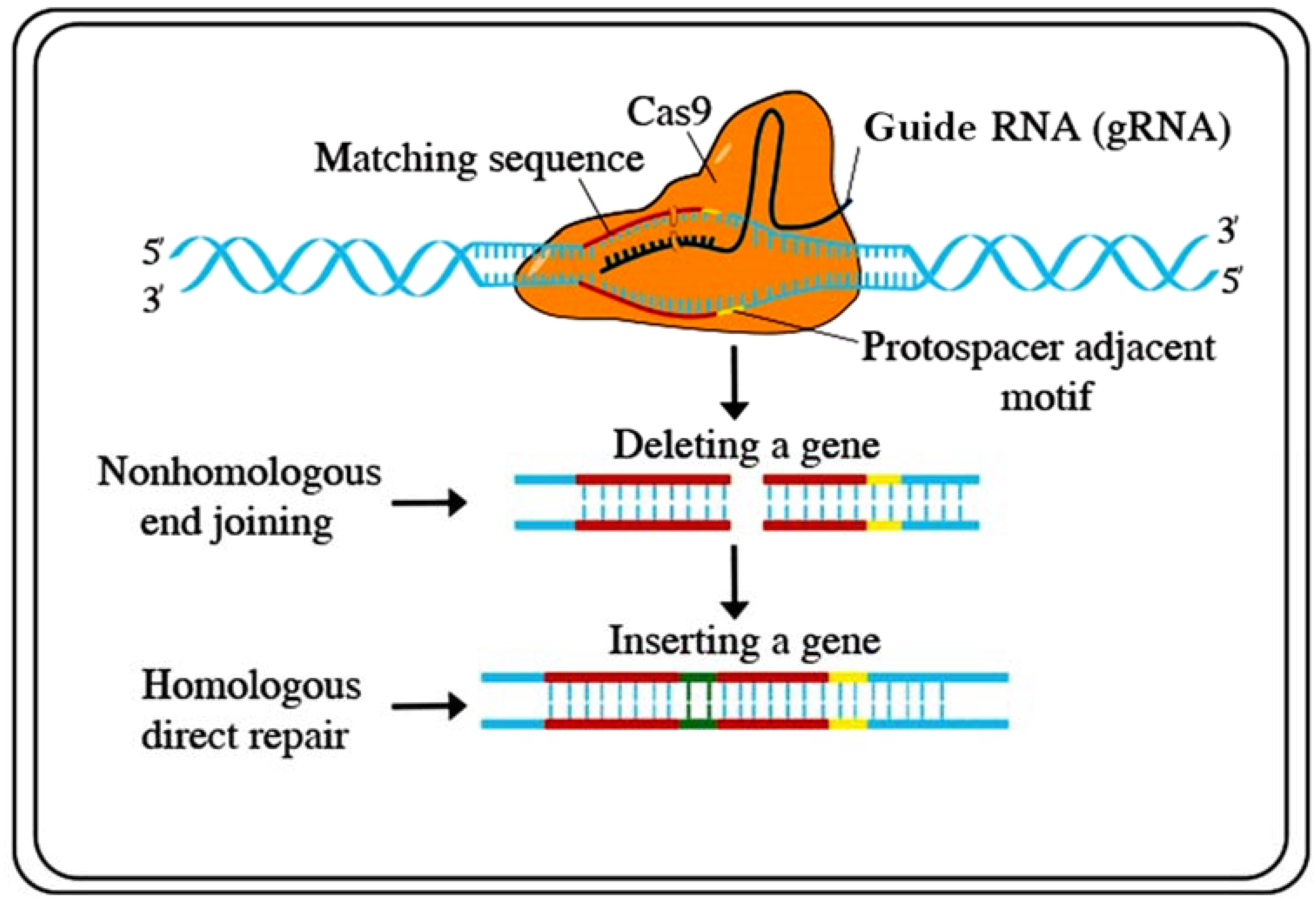
Recently, several functional genomics-based strategies have been developed for genetic engineering. To improve crops for sustainable food production, targeted genome engineering has become a substitute for conventional plant breeding and transgenic (GMO) strategies, including transcription regulators, epigenetic modifiers, DNA integrators, TAL effector nucleases (TALENs), zinc-finger nucleases (ZFNs), clustered regularly interspaced short palindromic repeats (CRISPR)/Cas (CRISPR-associated proteins), and base editors and prime editors. Until recently, the existing methods have been considered to be unwieldy. Both TALENs and ZFNs could be used to mutagenize genomes at exact loci. However, the problem is that these systems need two altered DNA-binding proteins flanking a sequence of interest, each with a C-end FokI nuclease unit [106]. For plant research, these techniques have not been extensively implemented. Recently, a technique based on the bacterial clustered regularly interspaced short palindromic repeats (CRISPR)/Cas (CRISPR-associated proteins) type-2 prokaryotic adaptive invulnerable system has been developed as an alternate process for genome engineering [106]. The CRISPR/Cas (clustered regularly interspaced short palindromic repeats/CRISPR-associated proteins) system was first identified in bacteria and archaea and can cleave exogenous DNA substrates [107]. CRISPR/Cas has since been modified to be used as a gene-editing technology. However, CRISPR/Cas9 has largely overtaken the other aforementioned gene editing practices. Investigators express similar stories: a few years ago, they started working on projects using both TALENs and CRISPR/Cas9 side-by-side but rapidly established CRISPR systems [108]. Graphical presentations of the CRISPR/Cas9 techniques are available in Figure 3.
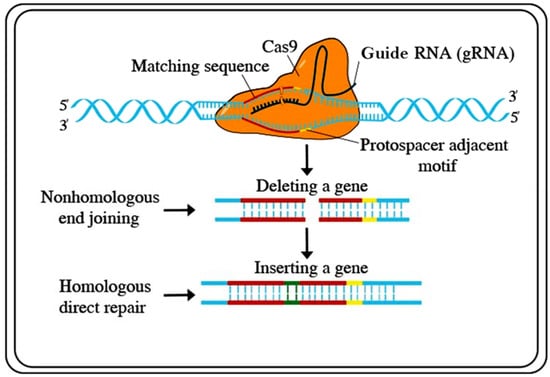
Figure 3.
CRISPR/Cas9 is a powerful tool for genome editing of Cas9 to a guide RNA that directs the complex to a place on the DNA double helix and contains the code for the addition of a new DNA sequence at the double-stranded break. Source: adapted and modified from [www.stockadobe.com; accessed date on 12 July 2021].
The beginning of CRISPR has made it conceivable to rewrite host DNA by introducing some major amendments. These modifications include gene replacement, deletions, inversion, knockouts, and translocations [109]. Using CRISPR/Cas9 tools, several genes, such as OsERF922, OsPDS, OsERF922, ERFs, OsHAK1, Badh2, OsRR22, and TMS5, were knocked out, and a predictable phenotype was attained [110,111,112,113,114,115,116]. More promising are the potential forecasts of this technique for producing plants with specifically tailored purposes, i.e., biofuel production, synthetic biology, disease resistance, phytoremediation, etc. [117]. This technique also offers a new method for abiotic stress breeding programs [113]. Several examples of CRISPR/Cas9 technology-mediated improvements to plant tolerance to abiotic stress are discussed in Table 6.

Table 6.
CRISPR/Cas9 technology-mediated improvements to plant tolerance to abiotic stress.
3.5. CRISPR-Mediated Base Editing and Prime Genome Editor
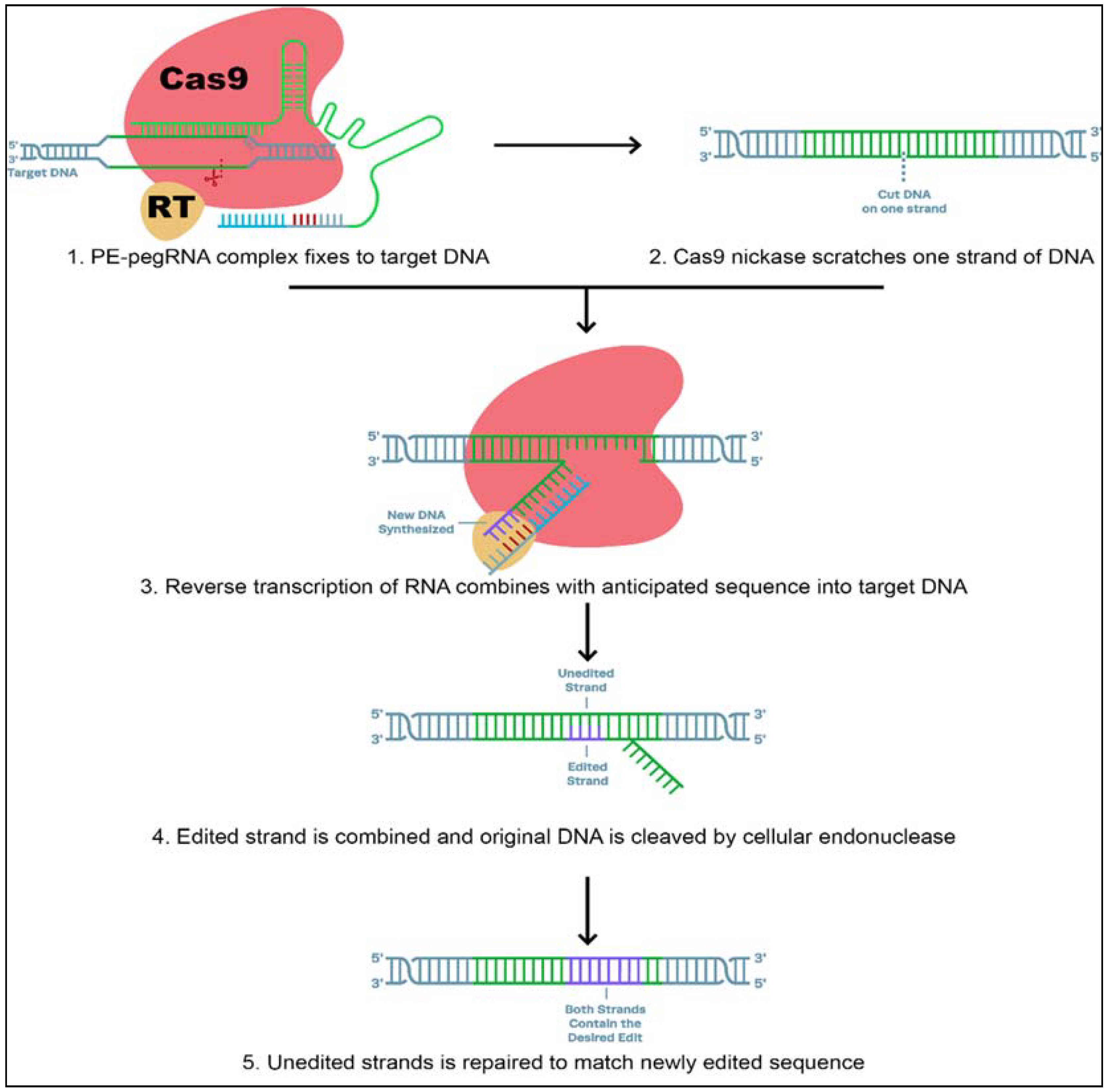
It is well known that CRISPR is a powerful genome-editing technique. CRISPR can change genes and edit DNA sequences by producing double-strand breaks in double-helical DNA, leaving the cell to repair the breakage (Figure 4).
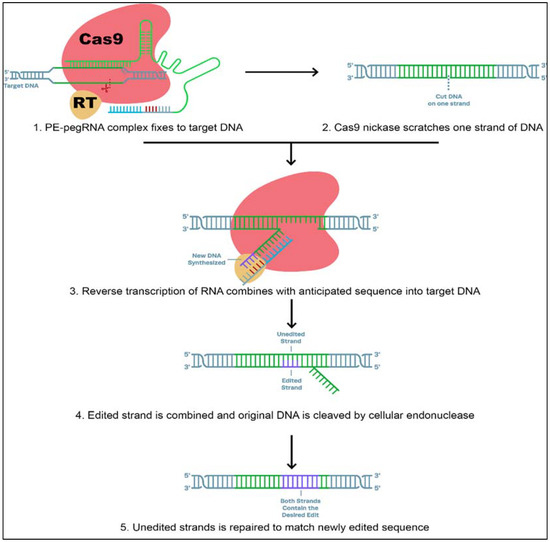
Figure 4.
A new tool (prime editor) of DNA manipulation that couples two enzymes, Cas9 (brown) and reverse transcriptase (yellow), to a guide RNA (red) that directs the complex to an exact place on the DNA double helix and contains the code for the addition of a new DNA sequence at the double-stranded break. [Figure modified from: https://www.synthego.com/guide/crispr-methods/prime-editing; accessed date on 12 July 2021].
The control mechanisms over the repair process are the main limitations in basic research and plant sciences. However, several groups recently reported the “base editing” system, a new approach for site-directed mutagenesis of genomic DNA. Base editing tools are highly efficient, reduce the rate of off-target effects, and do not require DNA double-strand cleavage or donor template repair. These methods make use of a Cas9 nickase fused to various deaminases. Specific C-to-T or A-to-G transitions in genomic DNA are catalyzed by these fusion proteins. The base editor and Target-AID (target-activation-induced cytidine deaminase) systems are two representative architectures of cytidine base [127,128]. Therefore, engineering of single-plasmid CRISPR-mediated base editing tools for S. meliloti that included adenosine base editors (ABEs), cytidine base editors (CBEs), and glycosylase base editors (GBEs) is capable of achieving both base transitions (A-to-G, C-to-T) and transversions (C-to-G) [129]. Base editing has become a widely applicable tool for gene disruption in a variety of bacteria [17,22,28,130]. Nevertheless, the new invention “prime editor” makes the successful addition or deletion of exact sequences within the genome possible with minimum off-target effects [129].
The creators claim that their tools can precisely target approximately 89% of recognized pathogenic human genetic variants. Prime editing may have fewer bystander mutations than base editing, especially when multiple Cs or As are present in the editing activity window [131]. It is also less constrained by the availability of protospacer adjacent motif (PAM) than other methods such as homology directed repair (HDR), non-homologous end joining (NHEJ), or base editing, because the PAM-to-edit distance can be greater than 30 bp on average [26]. Nevertheless, there is a large suite of base editors that have been developed with improved efficiency, product purity, and DNA specificity, as well as broad applicability [25]. Although prime editing has the potential to replace base editors, the technology is still in its early stages and is typically less efficient than current generation base-editing systems with superior on and off-target DNA editing profiles [20]. Consequently, a suitable editing strategy for specific applications must be chosen based on various criteria for gene-editing, such as the desired edit, the availability of PAMs, the efficiency of editing, and off-target/bystander mutations.
4. The Development of Salt- and Drought-Tolerant Crops with High Yielding Capacity
The generation of crop varieties with a high level of tolerance to salinity and drought is vital for creating full yield potential and sustainable production. Generally, there are two methods to integrate enhanced traits such as drought and salinity stresses in plants: genetic engineering and breeding programs.
4.1. Genetics Engineering
The advent of modern genetic engineering strategies offers the generation of plants with rising abiotic stress tolerance. Under abiotic stress conditions, several genes of crop plants in different pathways lead to upregulation of expression. Stress-responsive genes and their controlling genes can be transferred and expressed in different species using an Agrobacterium-mediated transformation system involving molecular, biochemical, and physiological changes that direct an increase in plant growth, development, and yield under stress environments [16]. Currently, the use of stress-inducible promoters for the expression of stress response genes has confirmed a time-specific and optimal level of expression. Salinity and drought are major environmental stresses that adversely affect the growth and development of crops; thus, a number of genes encoding proteins involved in the biosynthesis of stress defensive elements, including glycine betaine, mannitol, and heat shock proteins, have been used for abiotic stress tolerance, as well as several transcription factors, such as MAPK, bZIP, AP2/EREBP, WRKY, and DREB1 [17,18]. However, overexpressed transgenes can function as positive regulators of tolerance to a single stress or multiple stresses, such as salinity, drought or both. Therefore, the newly developed transgenic plant might have to be tolerant to single or multiple stresses, have high yields, and be devoid of harmful pleiotropic traits. Posttranslational modifications, orthologous gene expression of effectors from wild relatives or halophytes, gene expression by regulating miRNA activity, osmoprotectants, gene pyramiding, engineering of transcription factors, chaperones, late embryogenesis, metabolic pathways, abundant proteins, epigenetics, and even chaperones have been implemented to produce a new generation of transgenic plants [130]. Successful salinity- and drought-tolerant transgenic crops were produced and approved for cultivation as food and feed [23,27,28,29].
4.2. Gene Introgression
Introgressiomics is designated as an extensive systematic improvement of plant genomes and populations through bearing introgressions of genomic fragments from wild crop relatives relative to the genetic background of established crops to develop new cultivars with promising traits [24]. Through introgression, greater genomic plasticity can be attained in a crop using exotic genetic material that was previously nonexistent within the genome [104]. For crop improvement, genetic engineering strategies are relatively faster than traditional breeding programs, as well as cloning of genes responsible for imperative traits and introgression into plants [104]. To develop salt- and drought-tolerant varieties, a particular breeding program can be established through an understanding of the physiological and genetic mechanisms of these stresses. MAS improves the speed and efficacy of breeding because genetic markers are unaffected by the environment, are efficient to use in early generations [105], and can be useful for the introgression of target genes. Successful stories of introgression in various crops for many traits, including both abiotic and biotic stress tolerance/resistance, have been implicated from wild relatives in cultivation without affecting yield and quality [24,106,107].
4.3. Marker-Assisted Breeding and Transference of Genes
Marker-assisted breeding is a process that permits breeders to track traits over generations of breeding using genetic markers associated with a given trait. In marker-assisted breeding, DNA markers associated with desirable traits are used to identify and choose plants containing the genetic locus that confers the desirable trait. DNA markers have a high probability of increasing the capability and accuracy of traditional plant breeding via marker-assisted selection (MAS). MAS allows for quicker and more efficient selection of desired crops, as cultivators can reliably test for the presence of a genetic marker associated with a trait rather than waiting to assess the trait itself. The most efficient and extensively applied method for MAS is marker-assisted backcrossing [132]. Marker-assisted breeding is in contrast to the direct addition of a gene or multiple genes to enhance a trait, such as genetic modification. Using genetic markers in breeding depends on the phenological acclimatization of the acceptor genotype, and the introduction of a new marker or allele may be necessary to increase the yield. With the advent of molecular markers and MAS technology, numerous studies have capitalized on such technology to identify genes or QTLs affecting sequence tagging in different plant species during different developmental stages, to identify genes or QTLs that were introduced into different plant varieties, and to gain an overall deeper and more efficient understanding of QTLs that contribute to complex traits [133]. Marker-assisted breeding for improving crop quality under salinity and drought stresses is discussed in Table 7.

Table 7.
Marker-assisted breeding results for improving crop quality under salinity and drought stresses.
Current advances in genomics and genome sequencing in rice have made it feasible to locate and precisely map a certain number of genes via linkage to DNA markers. MAS can be applied to control the presence or absence of genes and has also been applied to assess the contributions of such genes conferring traits that have been introduced into extensively developed varieties [26]. Coupling genomic resources with the utility of MAS, breeders can now gain unprecedented insight into the genetic regulation of complex traits. MAS is a large advantage for developing new crop varieties because crops with ineligible gene aggregations can be dispelled from the selection process. This offers breeders the opportunity to focus on a reduced number of candidate lines for breeding targets in successive generations [131]. It has been shown that association mapping along with population formation and screening of cotton germplasm can improve QTL assignment and MAS [131]. Combining MAS and GS (genomic selection) with adequate genetic variety, databases, analytical instruments, and well-established climate and soil data is a powerful way to produce modern varieties with high drought resistance that can be readily inaugurated into appropriate agricultural programs [27]. These methods could produce a high number of lines of a crop appropriate for propagating crops in a range of drought and salinity stress ecosystems. Furthermore, incorporating these data can lead to the creation of varieties that can be further optimized to control largely heritable principal secondary characteristics. MAS delivers precise, rapid, and profitable progress toward the development of crop varieties that can be applied to abiotic stress tolerance [26]. A graphical presentation of the development of a new crop variety by marker-assisted selection is available in Figure 5.
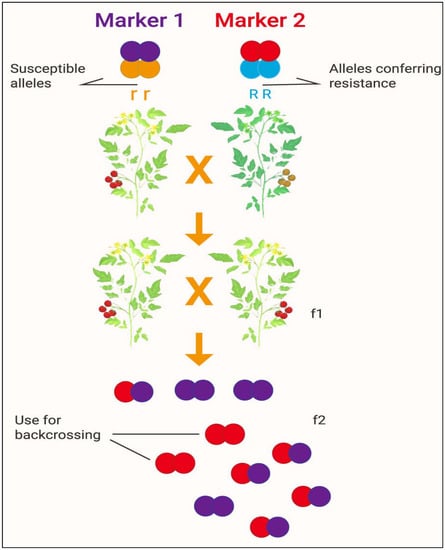
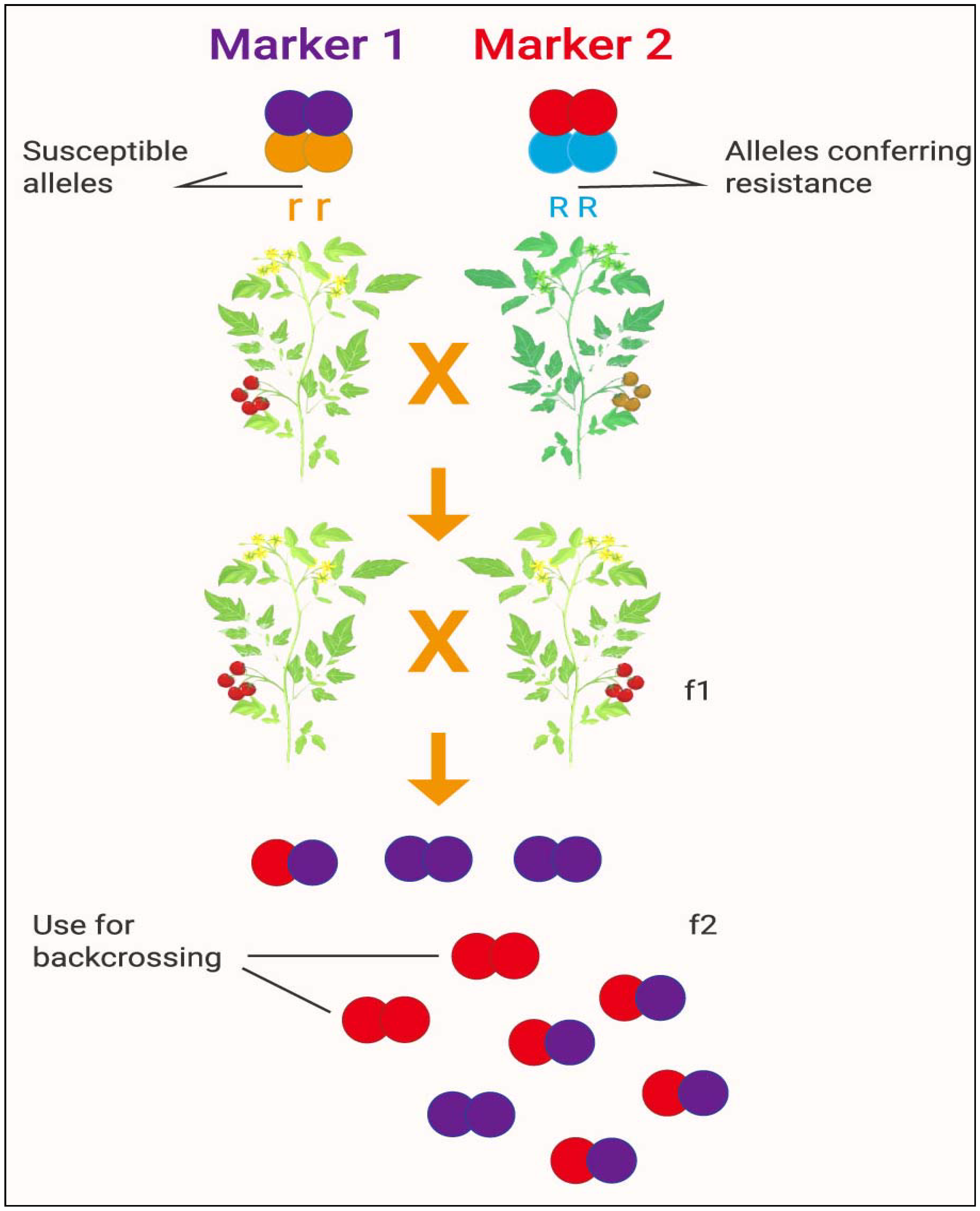
Figure 5.
Development of a new crop variety by marker-assisted selection. Source: modified from: [http://b4fa.org/bioscience-in-brief/plantbreeding/how-do-you-develop-a-new-crop-variety-by-marker-assisted-selection-mas/; accessed date on 12 July 2021]. Note: Marker 1 and Marker 2 confer susceptible and resistance alleles, respectively; f1 and f2 indicate the first and second filial generations of offspring, respectively.
5. Involvement of Genes in the Regulation of ROS in Abiotic Stress Tolerance
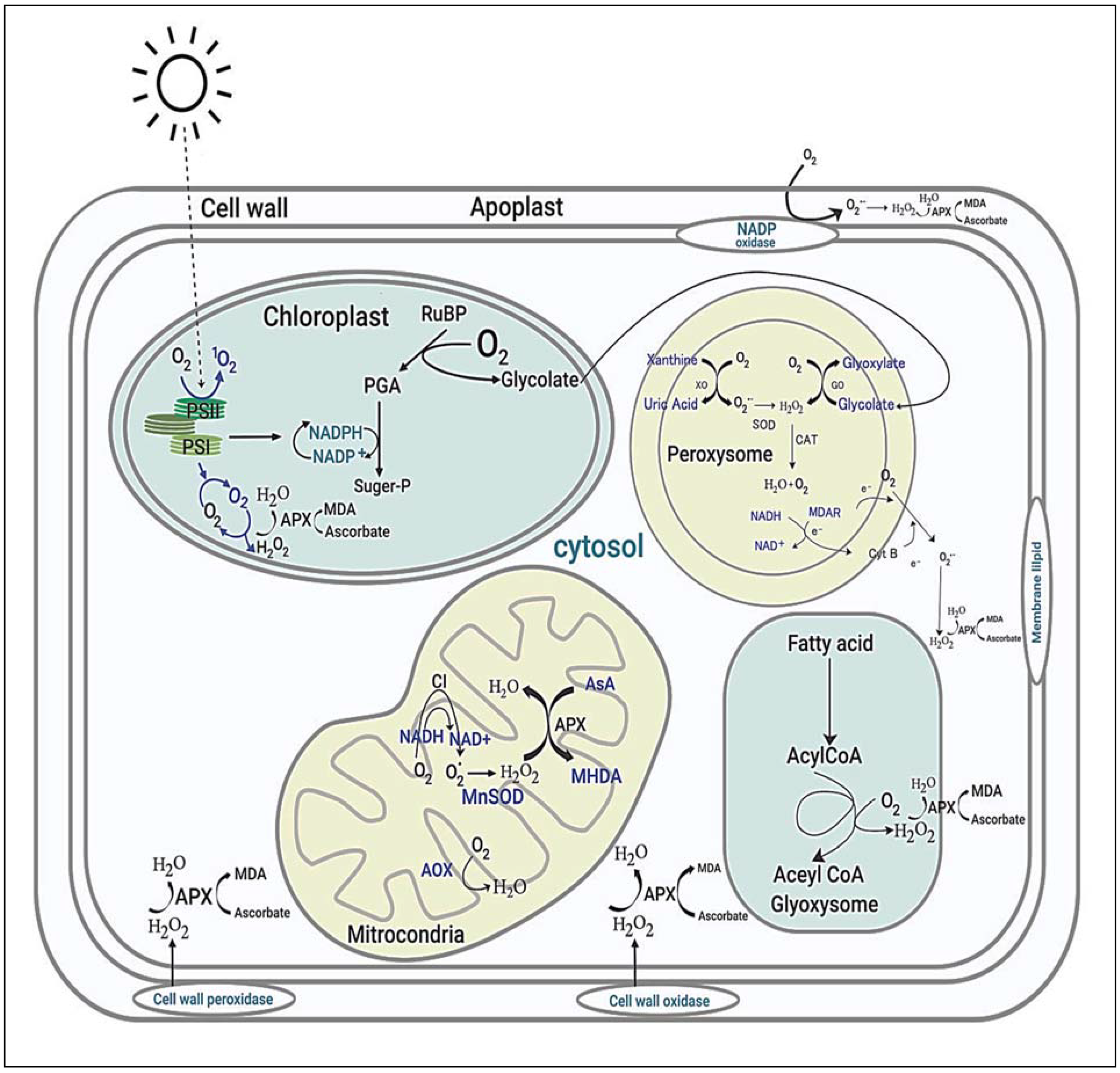
Reactive oxygen species (ROS) are assumed to play roles in many noteworthy signaling reactions in plant metabolism. Under drought and salinity environments, interrupting photosynthesis and increasing photorespiration intermittently alter the regular homeostasis of cells and influence the production of ROS in mitochondria, chloroplasts, and peroxisomes (Figure 6) [142,143].
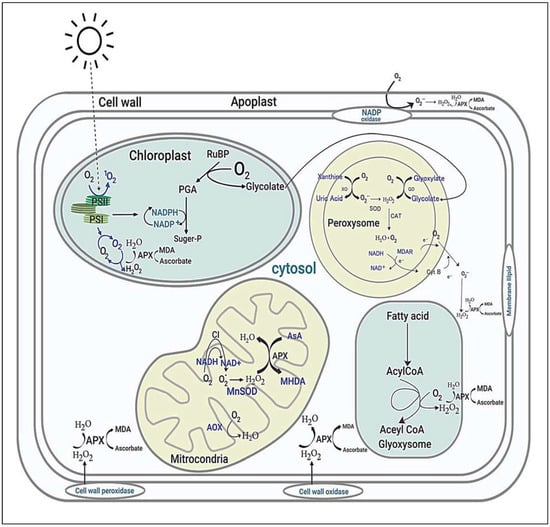
Figure 6.
Sites and typical regulation of ROS in plant cells. PSII; Photosystem II, PSI; Photosystem I, MDA; Malondialdehyde, APX; Ascorbate Peroxidase, PGA; 3-Phosphoglyceric acid, H2O2; Hydrogen peroxide, SOD; Superoxide dismutases, CAT; Catalase, MDAR; Monodehydroascorbate reductase, NADH; Nicotinamide adenine dinucleotide, Alternative oxidase.
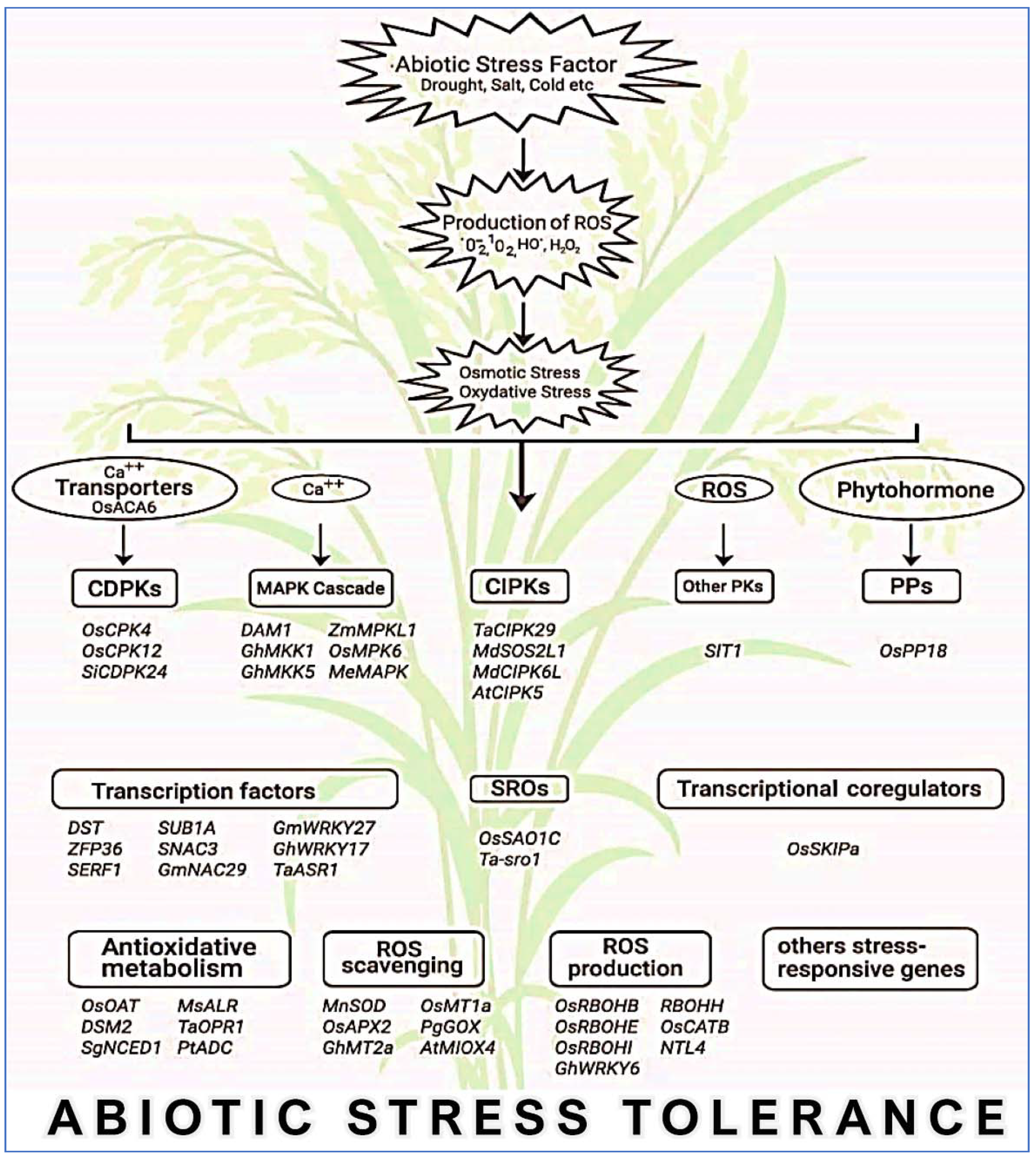
In addition to organelles, the plasma membrane together with the apoplast is the main site for ROS production in response to endogenous signals and exogenous environmental stimuli [144]. Overproduction of ROS in plant cells is extremely reactive and noxious to proteins, lipids, and nucleic acids, which finally results in cellular damage and death initiated by stressful environments [142]. ROS-scavenging enzymatic antioxidants (SOD, APX, CAT, GPX, MDHAR, DHAR, GR, GST, and PRX) and nonenzymatic antioxidants (GSH, AsA, carotenoids, tocopherols, and flavonoids) are located in different sites of plant cells, and they directly or indirectly play a key role in ROS homeostasis via different unique pathways to avoid oxidative damage. In addition, soluble sugars as well as disaccharides, raffinose family oligosaccharides, and fructans play a dual role in ROS maintenance [145]. Consequently, crop plants have executed several interrelated signaling pathways to operate different groups of genes (Figure 7), which are induced under stress conditions to generate different classes of proteins, for example, protein kinases, enzymes, transcription factors, molecular chaperones, and other efficient proteins, subsequent to various physiological and metabolic reactions to improve tolerance to multiple environmental stresses.
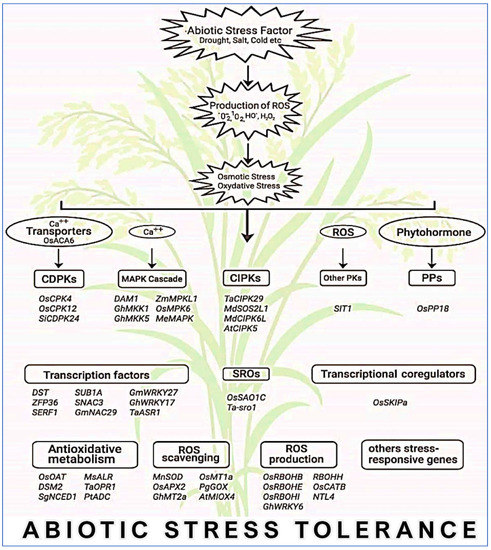
Figure 7.
A general view of major genes that are intricate in abiotic stress resistance through ROS maintenance in crops. MAPK, mitogen-activated protein kinase; CDPK, calcium-dependent protein kinase; CIPK, calcineurin B-like protein-interacting protein kinase; PK, protein kinase; PP, protein phosphatase; SRO, similar to RCD.
It is well known that antioxidants stimulate gene expression linked with responses to various environmental signals to exploit protection through the regulation of cellular ROS levels and redox state [146]. The characteristics and roles of selected genes and their processes under salinity and drought stresses are discussed in detail in Table 8 and Table 9.

Table 8.
Characterized genes involved in abiotic stress tolerance through ROS regulation in crops.

Table 9.
A summary of identified genes and their processes under salinity and drought stresses.
6. Conclusions
The adverse effects of climatic change and an increasing population pose a momentous challenge to crop production and food security, particularly in developing countries. Thus, it is a prerequisite to understand plant response mechanisms to abiotic stresses, namely, salinity and drought, at the molecular level to improve crop productivity. To overcome these circumstances, conventional breeding systems are no longer appropriate avenues to bolster crop production. In this review, we mainly discussed advanced molecular genomics tools focusing on plant genes in response to abiotic stress mechanisms to update our knowledge on the rapid development of high-yielding crop varieties under salt and drought stresses. Moreover, we summarized the recent studies of plant genes and differentiated them according to their molecular functions in response to salt and drought and reported recent advances in these stress-response mechanisms. Finally, the integration of any two or all three genomics approaches would be used to generate salinity- and drought-tolerant crops.
Author Contributions
Conceptualization, M.B. (Masum Billah); writing—original draft, M.B. (Masum Billah), S.A. and T.G.M.; review and editing, M.S.U., S.A.B., A.B.M.K., T.G.M., M.B. (Marian Brestic), M.Z., X.Y., M.S., S.M. and A.H.; funding, M.B. (Marian Brestic), M.Z., X.Y., M.S. and A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the ‘Slovak University of Agriculture’, Nitra, Tr. A. Hlinku 2,949 01 Nitra, Slovak Republic under the projects ‘APVV-1465 and EPPN2020-OPVaI-VA-ITMS313011T813′.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Most of the recorded data are available in the tables and figures of the manuscript.
Acknowledgments
The authors extend their appreciation to the editor and anonymous reviewers for their valuable comments, which have allowed for considerable improvement of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rozema, J.; Flowers, T. Crops for a Salinized World. Science 2008, 322, 1478–1480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, G.; Long, W.; Zou, X.; Li, F.; Nishio, T. Recent progress in drought and salt tolerance studies in Brassica crops. Breed. Sci. 2014, 64, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Athar, H.R.; Ashraf, M. Strategies for Crop Improvement against Salinity and Drought Stress: An Overview. In Salinity and Water Stress; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–16. [Google Scholar]
- Catlin, P.; Hoffman, G.; Mead, R.; Johnson, R. Long-term response of mature plum trees to salinity. Irrig. Sci. 1993, 13, 171–176. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- FAO. Unlocking the Water Potential of Agriculture. 2003. Available online: https://agris.fao.org/agris-search/search.do?recordID=XF2006444621 (accessed on 7 April 2006).
- EL Sabagh, A.; Islam, M.S.; Skalicky, M.; Raza, M.A.; Singh, K.; Hossain, M.A.; Hossain, A.; Mahboob, W.; Iqbal, M.A.; Ratnasekera, D.; et al. Adaptation and Management Strategies of Wheat (Triticum aestivum L.) Against Salinity Stress to Increase Yield and Quality. Front. Agron. 2021, 3, 43. [Google Scholar] [CrossRef]
- Kumari, V.V.; Roy, A.; Vijayan, R.; Banerjee, P.; Verma, V.; Nalia, A.; Pramanik, M.; Mukherjee, B.; Ghosh, A.; Reja, H.; et al. Drought and Heat Stress in Cool-Season Food Legumes in Sub-Tropical Regions: Consequences, Adaptation, and Mitigation Strategies. Plants 2021, 10, 1038. [Google Scholar] [CrossRef]
- Sinclair, T.R. Challenges in breeding for yield increase for drought. Trends Plant Sci. 2011, 16, 289–293. [Google Scholar] [CrossRef]
- Abbai, R.; Subramaniyam, S.; Mathiyalagan, R.; Yang, D.C. Functional genomic approaches in plant research. In Plant Bioinformatics; Springer: Berlin/Heidelberg, Germany, 2017; pp. 215–239. [Google Scholar]
- Kartseva, T.; Dobrikova, A.; Kocheva, K.; Alexandrov, V.; Georgiev, G.; Brestič, M.; Misheva, S. Optimal Nitrogen Supply Ameliorates the Performance of Wheat Seedlings under Osmotic Stress in Genotype-Specific Manner. Plants 2021, 10, 493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Tong, J.; He, X.; Xu, Z.; Xu, L.; Wei, P.; Huang, Y.; Ebrestic, M.; Ema, H.; Eshao, H.-B. A Novel Soybean Intrinsic Protein Gene, GmTIP2;3, Involved in Responding to Osmotic Stress. Front. Plant Sci. 2016, 6, 1237. [Google Scholar] [CrossRef]
- dos Reis, S.P.; Marques, D.N.; Barros, N.L.F.; Costa, C.d.N.M.; de Souza, C.R.B. Genetically engineered food crops to abiotic stress tolerance. In Genetically Engineered Foods; Elsevier: Amsterdam, The Netherlands, 2018; pp. 247–279. [Google Scholar]
- Lawlor, D.W. Genetic engineering to improve plant performance under drought: Physiological evaluation of achievements, limitations, and possibilities. J. Exp. Bot. 2013, 64, 83–108. [Google Scholar] [CrossRef] [PubMed]
- Rasel, M.; Tahjib-Ul-Arif, M.; Hossain, M.A.; Hassan, L.; Farzana, S.; Brestic, M. Screening of Salt-Tolerant Rice Landraces by Seedling Stage Phenotyping and Dissecting Biochemical Determinants of Tolerance Mechanism multidimensional roles in salt-stressed plants. J. Plant Growth Regul. 2020, 1–16. [Google Scholar] [CrossRef]
- Plaut, Z.; Edelstein, M.; Ben-Hur, M. Overcoming Salinity Barriers to Crop Production Using Traditional Methods. Crit. Rev. Plant Sci. 2013, 32, 250–291. [Google Scholar] [CrossRef]
- Gepts, P. The contribution of genetic and genomic approaches to plant domestication studies. Curr. Opin. Plant Biol. 2014, 18, 51–59. [Google Scholar] [CrossRef]
- Shi, J.; Gao, H.; Wang, H.; Lafitte, H.R.; Archibald, R.L.; Yang, M.; Hakimi, S.M.; Mo, H.; Habben, J.E. ARGOS8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant Biotechnol. J. 2016, 15, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Ibrahimova, U.; Zivcak, M.; Gasparovic, K.; Rastogi, A.; Allakhverdiev, S.I.; Yang, X.; Brestic, M. Electron and proton transport in wheat exposed to salt stress: Is the increase of the thylakoid membrane proton conductivity responsible for decreasing the photosynthetic activity in sensitive genotypes? Photosynth. Res. 2021, 1–17. [Google Scholar] [CrossRef]
- Singh, B.; Salaria, N.; Thakur, K.; Kukreja, S.; Gautam, S.; Goutam, U. Functional genomic approaches to improve crop plant heat stress tolerance. F1000Research 2019, 8, 1721. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Shao, H.; Shao, C.; Chen, P.; Zhao, S.; Brestic, M.; Chen, X. Physiological adaptive mechanisms of plants grown in saline soil and implications for sustainable saline agriculture in coastal zone. Acta Physiol. Plant. 2013, 35, 2867–2878. [Google Scholar] [CrossRef]
- Cheng, R.; Doerge, R.W.; Borevitz, J. Novel Resampling Improves Statistical Power for Multiple-Trait QTL Mapping. G3 Genes Genomes Genet. 2017, 7, 813–822. [Google Scholar] [CrossRef]
- Aktar, S.; Hossain, N.; Azam, M.G.; Billah, M.; Biswas, P.L.; Latif, M.A.; Rohman, M.; Bagum, S.A.; Uddin, M.S. Phenotyping of Hybrid Maize (Zea mays L.) at Seedling Stage under Drought Condition. Am. J. Plant Sci. 2018, 9, 2154–2169. [Google Scholar] [CrossRef][Green Version]
- Sanchez, A.; Subudhi, P.; Rosenow, D.; Nguyen, H. Mapping QTLs associated with drought resistance in sorghum (Sorghum bicolor L. Moench). Plant Mol. Biol. 2002, 48, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, N.; Singh, A.; Dixit, S.; Cruz, M.T.S.; Maturan, P.C.; Jain, R.K.; Kumar, A. Identification and mapping of stable QTL with main and epistasis effect on rice grain yield under upland drought stress. BMC Genet. 2014, 15, 63. [Google Scholar] [CrossRef]
- Prohens, J.; Gramazio, P.; Plazas, M.; Dempewolf, H.; Kilian, B.; Diez, M.J.; Fita, A.; Herraiz, F.J.; Rodríguez-Burruezo, A.; Soler, S.; et al. Introgressiomics: A new approach for using crop wild relatives in breeding for adaptation to climate change. Euphytica 2017, 213, 158. [Google Scholar] [CrossRef]
- Swamy, B.P.M.; Shamsudin, N.A.A.; Rahman, S.N.A.; Mauleon, R.; Ratnam, W.; Cruz, M.T.S.; Kumar, A. Association Mapping of Yield and Yield-related Traits Under Reproductive Stage Drought Stress in Rice (Oryza sativa L.). Rice 2017, 10, 21. [Google Scholar] [CrossRef]
- Abdelraheem, A.; Thyssen, G.N.; Fang, D.D.; Jenkins, J.N.; Mccarty, J.C.; Wedegaertner, T.; Zhang, J. GWAS reveals consistent QTL for drought and salt tolerance in a MAGIC population of 550 lines derived from intermating of 11 Upland cotton (Gossypium hirsutum) parents. Mol. Genet. Genom. 2020, 296, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, H.; Shao, B.; Chen, W.; Guo, Z.; Gong, H.; Sang, X.; Wang, J.; Ye, W.W. SSR-based association mapping of salt tolerance in cotton (Gossypium hirsutum L.). Genet. Mol. Res. 2016, 15, gmr.15027370. [Google Scholar] [CrossRef]
- Bennett, D.; Reynolds, M.P.; Mullan, D.; Izanloo, A.; Kuchel, H.; Langridge, P.; Schnurbusch, T. Detection of two major grain yield QTL in bread wheat (Triticum aestivum L.) under heat, drought and high yield potential environments. Theor. Appl. Genet. 2012, 125, 1473–1485. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fan, Y.; Mak, M.; Babla, M.; Holford, P.; Wang, F.; Chen, G.; Scott, G.; Wang, G.; Shabala, S.; et al. QTLs for stomatal and photosynthetic traits related to salinity tolerance in barley. BMC Genom. 2017, 18, 1–13. [Google Scholar] [CrossRef]
- Fan, Y.; Shabala, S.; Ma, Y.; Xu, R.; Zhou, M. Using QTL mapping to investigate the relationships between abiotic stress tolerance (drought and salinity) and agronomic and physiological traits. BMC Genom. 2015, 16, 43. [Google Scholar] [CrossRef] [PubMed]
- Courtois, B.; McLaren, G.; Sinha, P.; Prasad, K.; Yadav, R.; Shen, L. Mapping QTLs associated with drought avoidance in upland rice. Mol. Breed. 2000, 6, 55–66. [Google Scholar] [CrossRef]
- Bahuguna, R.N.; Gupta, P.; Bagri, J.; Singh, D.; Dewi, A.K.; Tao, L.; Islam, M.; Sarsu, F.; Singla-Pareek, S.L.; Pareek, A. Forward and reverse genetics approaches for combined stress tolerance in rice. Indian J. Plant Physiol. 2018, 23, 630–646. [Google Scholar] [CrossRef]
- Gupta, B.K.; Sahoo, K.K.; Ghosh, A.; Tripathi, A.K.; Anwar, K.; Das, P.; Singh, A.K.; Pareek, A.; Sopory, S.K.; Singla-Pareek, S.L. Manipulation of glyoxalase pathway confers tolerance to multiple stresses in rice. Plant Cell Environ. 2017, 41, 1186–1200. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Prashat, R.; Sharma, P.C.; Singla-Pareek, S.L.; Pareek, A. Physiological characterization of gamma-ray induced mutant population of rice to facilitate biomass and yield improvement under salinity stress. Indian J. Plant Physiol. 2016, 21, 545–555. [Google Scholar] [CrossRef]
- Martinez-Atienza, J.; Jiang, X.; Garciadeblas, B.; Mendoza, I.; Zhu, J.-K.; Pardo, J.M.; Quintero, F.J. Conservation of the Salt Overly Sensitive Pathway in Rice. Plant Physiol. 2006, 143, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Mickelbart, M.V.; Hasegawa, P.M.; Bailey-Serres, J. Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat. Rev. Genet. 2015, 16, 237–251. [Google Scholar] [CrossRef]
- Nutan, K.K.; Kumar, G.; Singla-Pareek, S.L.; Pareek, A. A Salt Overly Sensitive Pathway Member from Brassica juncea BjSOS3 Can Functionally Complement ΔAtsos3 in Arabidopsis. Curr. Genom. 2017, 19, 60–69. [Google Scholar] [CrossRef]
- Olías, R.; Eljakaoui, Z.; Pardo, J.M.; Belver, A. The Na+/H+ exchanger SOS1 controls extrusion and distribution of Na+ in tomato plants under salinity conditions. Plant Signal. Behav. 2009, 4, 973–976. [Google Scholar] [CrossRef]
- Wu, S.-J.; Ding, L.; Zhu, J.-K. SOS1, a genetic locus essential for salt tolerance and potassium acquisition. Plant Cell 1996, 8, 617–627. [Google Scholar] [CrossRef]
- Blumenberg, M. Introductory Chapter: Transcriptome Analysis; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Gahlaut, V.; Jaiswal, V.; Kumar, A.; Gupta, P.K. Transcription factors involved in drought tolerance and their possible role in developing drought tolerant cultivars with emphasis on wheat (Triticum aestivum L.). Theor. Appl. Genet. 2016, 129, 2019–2042. [Google Scholar] [CrossRef]
- Bowman, M.J.; Park, W.; Bauer, P.; Udall, J.A.; Page, J.T.; Raney, J.; Scheffler, B.; Jones, D.C.; Campbell, B.T. RNA-Seq Transcriptome Profiling of Upland Cotton (Gossypium hirsutum L.) Root Tissue under Water-Deficit Stress. PLoS ONE 2013, 8, e82634. [Google Scholar] [CrossRef]
- Rong, W.; Qi, L.; Wang, A.; Ye, X.; Du, L.; Liang, H.; Xin, Z.; Zhang, Z. The ERF transcription factor Ta ERF 3 promotes tolerance to salt and drought stresses in wheat. Plant Biotechnol. J. 2014, 12, 468–479. [Google Scholar] [CrossRef]
- Gao, S.-Q.; Chen, M.; Xu, Z.-S.; Zhao, C.-P.; Li, L.; Xu, H.-J.; Tang, Y.-M.; Zhao, X.; Ma, Y.-Z. The soybean GmbZIP1 transcription factor enhances multiple abiotic stress tolerances in transgenic plants. Plant Mol. Biol. 2011, 75, 537–553. [Google Scholar] [CrossRef]
- Shin, D.; Moon, S.-J.; Han, S.; Kim, B.-G.; Park, S.R.; Lee, S.-K.; Yoon, H.-J.; Lee, H.E.; Kwon, H.-B.; Baek, D.; et al. Expression of StMYB1R-1, a Novel Potato Single MYB-Like Domain Transcription Factor, Increases Drought Tolerance. Plant Physiol. 2010, 155, 421–432. [Google Scholar] [CrossRef]
- Song, Y.; Ji, D.; Li, S.; Wang, P.; Li, Q.; Xiang, F. The Dynamic Changes of DNA Methylation and Histone Modifications of Salt Responsive Transcription Factor Genes in Soybean. PLoS ONE 2012, 7, e41274. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.-J.; Wei, W.; Song, Q.-X.; Chen, H.-W.; Zhang, Y.-Q.; Wang, F.; Zou, H.-F.; Lei, G.; Tian, A.; Zhang, W.-K.; et al. Soybean NAC transcription factors promote abiotic stress tolerance and lateral root formation in transgenic plants. Plant J. 2011, 68, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zeng, J.; Li, Y.; Rong, X.; Sun, J.; Sun, T.; Li, M.; Wang, L.; Feng, Y.; Chai, R.; et al. Expression of TaWRKY44, a wheat WRKY gene, in transgenic tobacco confers multiple abiotic stress tolerances. Front. Plant Sci. 2015, 6, 615. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Fujita, Y.; Maruyama, K.; Mogami, J.; Todaka, D.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Four A rabidopsis AREB/ABF transcription factors function predominantly in gene expression downstream of SnRK2 kinases in abscisic acid signalling in response to osmotic stress. Plant Cell Environ. 2014, 38, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, A.K. Marker-Assisted Plant Breeding: Principles and Practices; Springer: Berlin/Heidelberg, Germany, 2015; p. 514. [Google Scholar] [CrossRef]
- Agrama, H.A.; Eizenga, G.C.; Yan, W. Association mapping of yield and its components in rice cultivars. Mol. Breed. 2007, 19, 341–356. [Google Scholar] [CrossRef]
- Edae, E.A.; Byrne, P.F.; Manmathan, H.; Haley, S.D.; Reynolds, M.P. Association Mapping and Nucleotide Sequence Variation in Five Drought Tolerance Candidate Genes in Spring Wheat. Plant Genome 2013, 6, 547–562. [Google Scholar] [CrossRef]
- Saeed, M.; Wangzhen, G.; Tianzhen, Z. Association mapping for salinity tolerance in cotton (Gossypium hirsutum L.) germplasm from US and diverse regions of China. Aust. J. Crop Sci. 2014, 8, 338–346. [Google Scholar]
- Purcărea, C.; Cachiţă-Cosma, D. Studies regarding the effects of salicylic acid on maize (Zea mays L.) seedling under salt stress. Studia Univ. Ser. Tiintele Vietii 2010, 20, 63–68. [Google Scholar]
- Wójcik-Jagła, M.; Fiust, A.; Kościelniak, J.; Rapacz, M. Association mapping of drought tolerance-related traits in barley to complement a traditional biparental QTL mapping study. Theor. Appl. Genet. 2017, 131, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Song, S.-Y.; Chen, Y.; Chen, J.; Dai, X.-Y.; Zhang, W.-H. Physiological mechanisms underlying OsNAC5-dependent tolerance of rice plants to abiotic stress. Planta 2011, 234, 331–345. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, C.; Ren, Q.; Chang, X.; Liu, G.; Jing, R. Association mapping of dynamic developmental plant height in common wheat. Planta 2011, 234, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, D.; Skot, L.; Singh, R.; Srivastava, R.K.; Das, S.P.; Taunk, J.; Sharma, P.C.; Pal, R.; Raj, B.; Hash, C.T. Exploring Potential of Pearl Millet Germplasm Association Panel for Association Mapping of Drought Tolerance Traits. PLoS ONE 2015, 10, e0122165. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, S.; Dreisigacker, S.; Lopes, M.; Chavez, P.; Reynolds, M.P. Genome-wide association study for grain yield and related traits in an elite spring wheat population grown in temperate irrigated environments. Theor. Appl. Genet. 2014, 128, 353–363. [Google Scholar] [CrossRef]
- Crossa, J.; Burgueño, J.; Dreisigacker, S.; Vargas, M.; Herrera-Foessel, S.A.; Lillemo, M.; Singh, R.P.; Trethowan, R.; Warburton, M.; Franco, J. Association Analysis of Historical Bread Wheat Germplasm Using Additive Genetic Covariance of Relatives and Population Structure. Genetics 2007, 177, 1889–1913. [Google Scholar] [CrossRef]
- Rasheed, A.; Xia, X.; Ogbonnaya, F.; Mahmood, T.; Zhang, Z.; Mujeeb-Kazi, A.; He, Z. Genome-wide association for grain morphology in synthetic hexaploid wheats using digital imaging analysis. BMC Plant Biol. 2014, 14, 128. [Google Scholar] [CrossRef]
- Rolvien, T.; Kornak, U.; Linke, S.J.; Amling, M.; Oheim, R. Whole-Exome Sequencing Identifies Novel Compound Heterozygous ZNF469 Mutations in Two Siblings with Mild Brittle Cornea Syndrome. Calcif. Tissue Int. 2020, 107, 294–299. [Google Scholar] [CrossRef]
- Lekklar, C.; Pongpanich, M.; Suriya-Arunroj, D.; Chinpongpanich, A.; Tsai, H.; Comai, L.; Chadchawan, S.; Buaboocha, T. Genome-wide association study for salinity tolerance at the flowering stage in a panel of rice accessions from Thailand. BMC Genom. 2019, 20, 76. [Google Scholar] [CrossRef]
- Shi, Y.; Gao, L.; Wu, Z.; Zhang, X.; Wang, M.; Zhang, C.; Zhang, F.; Zhou, Y.; Li, Z. Genome-wide association study of salt tolerance at the seed germination stage in rice. BMC Plant Biol. 2017, 17, 92. [Google Scholar] [CrossRef]
- Hasan, M.; Skalicky, M.; Jahan, M.; Hossain, N.; Anwar, Z.; Nie, Z.; Alabdallah, N.; Brestic, M.; Hejnak, V.; Fang, X.-W. Spermine: Its Emerging Role in Regulating Drought Stress Responses in Plants. Cells 2021, 10, 261. [Google Scholar] [CrossRef]
- Voichek, Y.; Weigel, D. Identifying genetic variants underlying phenotypic variation in plants without complete genomes. Biol. Res. 2020, 52, 534–540. [Google Scholar] [CrossRef]
- Ogbonnaya, F.C.; Rasheed, A.; Okechukwu, E.C.; Jighly, A.; Makdis, F.; Wuletaw, T.; Hagras, A.; Uguru, M.I.; Agbo, C.U. Genome-wide association study for agronomic and physiological traits in spring wheat evaluated in a range of heat prone environments. Theor. Appl. Genet. 2017, 130, 1819–1835. [Google Scholar] [CrossRef] [PubMed]
- Hoang, G.T.; Van Dinh, L.; Nguyen, T.T.; Ta, N.K.; Gathignol, F.; Mai, C.D.; Jouannic, S.; Tran, K.D.; Khuat, T.H.; Do, V.N.; et al. Genome-wide Association Study of a Panel of Vietnamese Rice Landraces Reveals New QTLs for Tolerance to Water Deficit During the Vegetative Phase. Rice 2019, 12, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Cui, P.; Wang, Z.; Zhang, S.; Ali, S.; Xiong, L. Genome-wide analysis of alternative splicing of pre-mRNA under salt stress in Arabidopsis. BMC Genom. 2014, 15, 431. [Google Scholar] [CrossRef] [PubMed]
- Hübner, S.; Korol, A.B.; Schmid, K.J. RNA-Seq analysis identifies genes associated with differential reproductive success under drought-stress in accessions of wild barley Hordeum spontaneum. BMC Plant Biol. 2015, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pucholt, P.; Sjödin, P.; Weih, M.; Rönnberg-Wästljung, A.C.; Berlin, S. Genome-wide transcriptional and physiological responses to drought stress in leaves and roots of two willow genotypes. BMC Plant Biol. 2015, 15, 1–16. [Google Scholar] [CrossRef]
- Pham, A.-T.; Maurer, A.; Pillen, K.; Brien, C.; Dowling, K.; Berger, B.; Eglinton, J.K.; March, T.J. Genome-wide association of barley plant growth under drought stress using a nested association mapping population. BMC Plant Biol. 2019, 19, 134. [Google Scholar] [CrossRef]
- Ibrahimova, U.; Kumari, P.; Yadav, S.; Rastogi, A.; Antala, M.; Suleymanova, Z.; Zivcak, M.; Arif, T.U.; Hussain, S.; Abdelhamid, M.; et al. Progress in understanding salt stress response in plants using biotechnological tools. J. Biotechnol. 2021, 329, 180–191. [Google Scholar] [CrossRef]
- Yu, L.-X.; Liu, X.; Boge, W.; Liu, X.-P. Genome-Wide Association Study Identifies Loci for Salt Tolerance during Germination in Autotetraploid Alfalfa (Medicago sativa L.) Using Genotyping-by-Sequencing. Front. Plant Sci. 2016, 7, 956. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, X.; Pan, Y.; Zhu, L.; Fu, B.; Li, Z. DNA methylation changes detected by methylation-sensitive amplified polymorphism in two contrasting rice genotypes under salt stress. J. Genet. Genom. 2011, 38, 419–424. [Google Scholar] [CrossRef]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Alam, M.A.; Abu Syed, M.; Hossain, J.; Sarkar, S.; Saha, S.; Bhadra, P.; et al. Consequences and Mitigation Strategies of Abiotic Stresses in Wheat (Triticum aestivum L.) under the Changing Climate. Agronomy 2021, 11, 241. [Google Scholar] [CrossRef]
- Metzker, M.L. Sequencing technologies—the next generation. Nat. Rev. Genet. 2010, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Unamba, C.I.N.; Nag, A.; Sharma, R.K. Next Generation Sequencing Technologies: The Doorway to the Unexplored Genomics of Non-Model Plants. Front. Plant Sci. 2015, 6, 1074. [Google Scholar] [CrossRef] [PubMed]
- Grada, A.; Weinbrecht, K. Next-Generation Sequencing: Methodology and Application. J. Investig. Dermatol. 2013, 133, 1–4. [Google Scholar] [CrossRef]
- Koboldt, D.C.; Steinberg, K.M.; Larson, D.; Wilson, R.K.; Mardis, E.R. The Next-Generation Sequencing Revolution and Its Impact on Genomics. Cell 2013, 155, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; Hümpel, A. Introduction to epigenetics. In Epigenetics; Springer: New York, NY, USA, 2017; pp. 11–29. [Google Scholar]
- Chan, S.W.-L.; Henderson, I.; Jacobsen, S.E. Gardening the genome: DNA methylation in Arabidopsis thaliana. Nat. Rev. Genet. 2005, 6, 351–360. [Google Scholar] [CrossRef]
- Singroha, G.; Sharma, P. Epigenetic Modifications in Plants under Abiotic Stress. In Epigenetics; IntechOpen: London, UK, 2019. [Google Scholar]
- Banerjee, A.; Roychoudhury, A. Epigenetic regulation during salinity and drought stress in plants: Histone modifications and DNA methylation. Plant Gene 2017, 11, 199–204. [Google Scholar] [CrossRef]
- Ding, Y.; Fromm, M.E.; Avramova, Z. Multiple exposures to drought ‘train’ transcriptional responses in Arabidopsis. Nat. Commun. 2012, 3, 740. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Liu, X.; Thorn, G.; Duan, J.; Tian, L. Expression analysis of histone acetyltransferases in rice under drought stress. Biochem. Biophys. Res. Commun. 2013, 443, 400–405. [Google Scholar] [CrossRef]
- Kim, J.-M.; Sasaki, T.; Ueda, M.; Sako, K.; Seki, M. Chromatin changes in response to drought, salinity, heat, and cold stresses in plants. Front. Plant Sci. 2015, 6, 114. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.J.; Azevedo, V.S.; Maroco, J.; Oliveira, M.M.; Santos, A.P. Salt Tolerant and Sensitive Rice Varieties Display Differential Methylome Flexibility under Salt Stress. PLoS ONE 2015, 10, e0124060. [Google Scholar] [CrossRef] [PubMed]
- Kaldis, A.; Tsementzi, D.; Tanriverdi, O.; Vlachonasios, K.E. Arabidopsis thaliana transcriptional co-activators ADA2b and SGF29a are implicated in salt stress responses. Planta 2010, 233, 749–762. [Google Scholar] [CrossRef]
- Duan, C.-G.; Wang, C.-H.; Guo, H.-S. Application of RNA silencing to plant disease resistance. Silence 2012, 3, 5. [Google Scholar] [CrossRef]
- Ricaño-Rodríguez, J.; Adame-García, J.; Patlas-Martínez, C.I.; Hipólito-Romero, E.; Ramos-Prado, J.M. Plant gene co-suppression; basis of the molecular machinery of interfering RNA. Plant Omics 2016, 9, 261–269. [Google Scholar] [CrossRef]
- Hunter, C.P. Gene silencing: Shrinking the black box of RNAi. Curr. Biol. 2000, 10, R137–R140. [Google Scholar] [CrossRef]
- Bekele, D.; Tesfaye, K.; Fikre, A. Applications of Virus Induced Gene Silencing (VIGS) in Plant Functional Genomics Studies. J. Plant Biochem. Physiol. 2019, 7, 1–7. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, L.; Fan, X.; Xu, P.; Xu, Z.; Zhang, X.; Meng, S.; Shen, X.J. Transcription Factor GarWRKY5 Is Involved in Salt Stress Response in Diploid Cotton Species (Gossypium aridum L.). Int. J. Mol. Sci. 2019, 20, 5244. [Google Scholar] [CrossRef] [PubMed]
- Kirungu, J.N.; Magwanga, R.O.; Pu, L.; Cai, X.; Xu, Y.; Hou, Y.; Zhou, Y.; Cai, Y.; Hao, F.; Zhou, Z.; et al. Knockdown of Gh_A05G1554 (GhDHN_03) and Gh_D05G1729 (GhDHN_04) Dehydrin genes, Reveals their potential role in enhancing osmotic and salt tolerance in cotton. Genomics 2019, 112, 1902–1915. [Google Scholar] [CrossRef]
- Wang, X.; Ren, Y.; Li, J.; Wang, Z.; Xin, Z.; Lin, T. Knock-down the expression of TaH2B-7D using virus-induced gene silencing reduces wheat drought tolerance. Biol. Res. 2019, 52, 14. [Google Scholar] [CrossRef]
- Oh, D.-H.; Leidi, E.; Zhang, Q.; Hwang, S.-M.; Li, Y.; Quintero, F.J.; Jiang, X.; D’Urzo, M.P.; Lee, S.Y.; Zhao, Y. Loss of halophytism by interference with SOS1 expression. Plant Physiol. 2009, 151, 210–222. [Google Scholar] [CrossRef]
- Zhai, Y.; Wang, H.; Liang, M.; Lu, M. Both silencing-and over-expression of pepper CaATG8c gene compromise plant tolerance to heat and salt stress. Environ. Exp. Bot. 2017, 141, 10–18. [Google Scholar] [CrossRef]
- Bai, C.; Wang, P.; Fan, Q.; Fu, W.-D.; Wang, L.; Zhang, Z.-N.; Song, Z.; Zhang, G.-L.; Wu, J.-H. Analysis of the Role of the Drought-Induced Gene DRI15 and Salinity-Induced Gene SI1 in Alternanthera philoxeroides Plasticity Using a Virus-Based Gene Silencing Tool. Front. Plant Sci. 2017, 8, 1579. [Google Scholar] [CrossRef]
- Li, C.; Yan, J.-M.; Li, Y.-Z.; Zhang, Z.-C.; Wang, Q.-L.; Liang, Y. Silencing the SpMPK1, SpMPK2, and SpMPK3 genes in tomato reduces abscisic acid—mediated drought tolerance. Int. J. Mol. Sci. 2013, 14, 21983–21996. [Google Scholar] [CrossRef]
- Manmathan, H.; Shaner, D.; Snelling, J.; Tisserat, N.; Lapitan, N. Virus-induced gene silencing of Arabidopsis thaliana gene homologues in wheat identifies genes conferring improved drought tolerance. J. Exp. Bot. 2013, 64, 1381–1392. [Google Scholar] [CrossRef]
- Kirungu, J.N.; Magwanga, R.O.; Lu, P.; Cai, X.; Zhou, Z.; Wang, X.; Peng, R.; Wang, K.; Liu, F. Functional characterization of Gh_A08G1120 (GH3.5) gene reveal their significant role in enhancing drought and salt stress tolerance in cotton. BMC Genet. 2019, 20, 62. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, L.; Zhou, K.; Zhang, Y.; Han, X.; Ding, Y.; Ge, X.; Wang, P.; Li, F.; Ma, Z. GhWRKY6 acts as a negative regulator in both transgenic Arabidopsis and cotton during drought and salt stress. Front. Genet. 2019, 10, 392. [Google Scholar] [CrossRef] [PubMed]
- Belhaj, K.; Chaparro-Garcia, A.; Kamoun, S.; Nekrasov, V. Plant genome editing made easy: Targeted mutagenesis in model and crop plants using the CRISPR/Cas system. Plant Methods 2013, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, S.; Xu, J.; Sui, C.; Wei, J. Application of CRISPR/Cas9 in plant biology. Acta Pharm. Sin. B 2017, 7, 292–302. [Google Scholar] [CrossRef]
- Jia, H.; Zhang, Y.; Orbović, V.; Xu, J.; White, F.F.; Jones, J.B.; Wang, N. Genome editing of the disease susceptibility gene CsLOB1 in citrus confers resistance to citrus canker. Plant Biotechnol. J. 2016, 15, 817–823. [Google Scholar] [CrossRef]
- Fernandez i Marti, A.; Dodd, R.S. Using CRISPR as a gene editing tool for validating adaptive gene function in tree landscape genomics. Front. Ecol. Evol. Dev. 2018, 6, 76. [Google Scholar] [CrossRef]
- Debbarma, J.; Sarki, Y.N.; Saikia, B.; Boruah, H.P.D.; Singha, D.L.; Chikkaputtaiah, C. Ethylene response factor (ERF) family proteins in abiotic stresses and CRISPR–Cas9 genome editing of ERFs for multiple abiotic stress tolerance in crop plants: A review. Mol. Biotechnol. 2019, 61, 153–172. [Google Scholar] [CrossRef]
- Nieves-Cordones, M.; Mohamed, S.; Tanoi, K.; Kobayashi, N.I.; Takagi, K.; Vernet, A.; Guiderdoni, E.; Périn, C.; Sentenac, H.; Véry, A.A. Production of low-Cs+ rice plants by inactivation of the K+ transporter Os HAK 1 with the CRISPR-Cas system. Plant J. 2017, 92, 43–56. [Google Scholar] [CrossRef]
- Shao, G.; Xie, L.; Jiao, G.; Wei, X.; Sheng, Z.; Tang, S.; Hu, P. CRISPR/CAS9-mediated editing of the fragrant gene Badh2 in rice. Chin. J. Rice Sci. 2017, 31, 216–222. [Google Scholar]
- Wang, F.; Wang, C.; Liu, P.; Lei, C.; Hao, W.; Gao, Y.; Liu, Y.-G.; Zhao, K. Enhanced Rice Blast Resistance by CRISPR/Cas9-Targeted Mutagenesis of the ERF Transcription Factor Gene OsERF922. PLoS ONE 2016, 11, e0154027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Liu, Y.; Wang, F.; Li, T.; Chen, Z.; Kong, D.; Bi, J.; Zhang, F.; Luo, X.; Wang, J.; et al. Enhanced rice salinity tolerance via CRISPR/Cas9-targeted mutagenesis of the OsRR22 gene. Mol. Breed. 2019, 39, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.; Wei, P.; Zhang, B.; Gou, F.; Feng, Z.; Mao, Y.; Yang, L.; Zhang, H.; Xu, N. The CRISPR/C as9 system produces specific and homozygous targeted gene editing in rice in one generation. Plant Biotechnol. J. 2014, 12, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Song, N.; Sun, S.; Yang, W.; Zhao, H.; Song, W.; Lai, J. Efficiency and Inheritance of Targeted Mutagenesis in Maize Using CRISPR-Cas9. J. Genet. Genom. 2016, 43, 25–36. [Google Scholar] [CrossRef]
- Hunter, C.T. CRISPR/Cas9 Targeted Mutagenesis for Functional Genetics in Maize. Plants 2021, 10, 723. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Alptekin, B.; Budak, H. CRISPR/Cas9 genome editing in wheat. Funct. Integr. Genom. 2017, 18, 31–41. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, J.; Zhang, X.; Yang, Y.; Zhou, D.; Yu, Q.; Que, Y.; Xu, L.; Guo, J. A Novel Non-specific Lipid Transfer Protein Gene from Sugarcane (NsLTPs), Obviously Responded to Abiotic Stresses and Signaling Molecules of SA and MeJA. Sugar Tech. 2016, 19, 17–25. [Google Scholar] [CrossRef]
- Miao, H.; Sun, P.; Liu, Q.; Liu, J.; Xu, B.; Jin, Z. The AGPase Family Proteins in Banana: Genome-Wide Identification, Phylogeny, and Expression Analyses Reveal Their Involvement in the Development, Ripening, and Abiotic/Biotic Stress Responses. Int. J. Mol. Sci. 2017, 18, 1581. [Google Scholar] [CrossRef]
- Ou, W.; Mao, X.; Huang, C.; Tie, W.; Yan, Y.; Ding, Z.; Wu, C.; Xia, Z.; Wang, W.; Zhou, S.; et al. Genome-Wide Identification and Expression Analysis of the KUP Family under Abiotic Stress in Cassava (Manihot esculenta Crantz). Front. Physiol. 2018, 9, 17. [Google Scholar] [CrossRef]
- Ye, J.; Yang, H.; Shi, H.; Wei, Y.; Tie, W.; Ding, Z.; Yan, Y.; Luo, Y.; Xia, Z.; Wang, W.; et al. The MAPKKK gene family in cassava: Genome-wide identification and expression analysis against drought stress. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Zhao, P.; Wang, L.; Zhang, Y.; Wang, X.; Xiao, H.; Yu, J.; Xiao, G. The PIN gene family in cotton (Gossypium hirsutum): Genome-wide identification and gene expression analyses during root development and abiotic stress responses. BMC Genom. 2017, 18, 507. [Google Scholar] [CrossRef] [PubMed]
- Dass, A.; Abdin, M.Z.; Reddy, V.S.; Leelavathi, S. Isolation and characterization of the dehydration stress-inducible GhRDL1 promoter from the cultivated upland cotton (Gossypium hirsutum). J. Plant Biochem. Biotechnol. 2016, 26, 113–119. [Google Scholar] [CrossRef]
- Arroyo-Herrera, A.; Yáñez, F.; Castano, E.; Santamaria, J.; Pereira-Santana, A.; Espadas-Alcocer, J.; Teyer, L.F.S.; Espadas-Gil, F.; Alcaraz, L.D.; López-Gómez, R.; et al. A novel Dreb2-type gene from Carica papaya confers tolerance under abiotic stress. Plant Cell Tissue Organ Cult. 2015, 125, 119–133. [Google Scholar] [CrossRef]
- Kumar, V.V.S.; Verma, R.K.; Yadav, S.K.; Yadav, P.; Watts, A.; Rao, M.V.; Chinnusamy, V. CRISPR-Cas9 mediated genome editing of drought and salt tolerance (OsDST) gene in indica mega rice cultivar MTU1010. Physiol. Mol. Biol. Plants 2020, 26, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, C.; Zhao, R.; Wang, L.; Chen, L.; Yu, W.; Zhang, S.; Sheng, J.; Shen, L. CRISPR/Cas9-Mediated SlNPR1 mutagenesis reduces tomato plant drought tolerance. BMC Plant Biol. 2019, 19, 38. [Google Scholar] [CrossRef]
- Liao, S.; Qin, X.; Luo, L.; Han, Y.; Wang, X.; Usman, B.; Nawaz, G.; Zhao, N.; Liu, Y.; Li, R. CRISPR/Cas9-Induced Mutagenesis of Semi-Rolled Leaf1,2 Confers Curled Leaf Phenotype and Drought Tolerance by Influencing Protein Expression Patterns and ROS Scavenging in Rice (Oryza sativa L.). Agronomy 2019, 9, 728. [Google Scholar] [CrossRef]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.; Levy, J.M.; Chen, P.; Wilson, C.; Newby, G.A.; Raguram, A.; et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 2019, 576, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Wiens, C.P.; Shabala, L.; Zhang, J.; Pottosin, I.; Bose, J.; Zhu, M.; Fuglsang, A.T.; Velarde, A.; Massart, A.; Hill, C. Cell-type specific H+-ATPase activity enables root K+ retention and mediates acclimatation to salinity. Plant Physiol. 2016, 172, 2445–2458. [Google Scholar]
- Gill, M.B.; Zeng, F.; Shabala, L.; Zhang, G.; Fan, Y.; Shabala, S.; Zhou, M. Cell-Based Phenotyping Reveals QTL for Membrane Potential Maintenance Associated with Hypoxia and Salinity Stress Tolerance in Barley. Front. Plant Sci. 2017, 8, 1941. [Google Scholar] [CrossRef] [PubMed]
- Monneveux, P.; Reynolds, M.P.; Aguilar, J.G.; Singh, R.P.; Weber, W.E. Effects of the 7DL.7Ag translocation from Lophopyrum elongatum on wheat yield and related morphophysiological traits under different environments. Plant Breed. 2003, 122, 379–384. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Crop breeding for salt tolerance in the era of molecular markers and marker-assisted selection. Plant Breed. 2012, 132, 10–20. [Google Scholar] [CrossRef]
- thi Lang, N.; Buu, B.; Ismail, A. Molecular mapping and marker-assisted selection for salt tolerance in rice (Oryza sativa L.). OmonRice 2008, 16, 50–56. [Google Scholar]
- Prince, S.J.; Beena, R.; Gomez, S.M.; Senthivel, S.; Babu, R.C. Mapping Consistent Rice (Oryza sativa L.) Yield QTLs under Drought Stress in Target Rainfed Environments. Rice 2015, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Zheng, T.; Wang, X.; Wang, Y.; Chen, K.; Wang, S.; Xu, J.; Li, Z. QTL mapping and candidate gene analysis of peduncle vascular bundle related traits in rice by genome-wide association study. Rice 2018, 11, 13. [Google Scholar] [CrossRef]
- Shamsudin, N.A.A.; Swamy, B.P.M.; Ratnam, W.; Cruz, M.T.S.; Raman, A.; Kumar, A. Marker assisted pyramiding of drought yield QTLs into a popular Malaysian rice cultivar, MR219. BMC Genet. 2016, 17, 1–14. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Talabi, A.O.; Fakorede, M.A.B.; Fasanmade, Y.; Gedil, M.; Magorokosho, C.; Asiedu, R. Yield gains and associated changes in an early yellow bi-parental maize population following genomic selection for Striga resistance and drought tolerance. BMC Plant Biol. 2019, 19, 1–17. [Google Scholar] [CrossRef]
- Wang, J.; Li, R.; Mao, X.; Jing, R. Functional Analysis and Marker Development of TaCRT-D Gene in Common Wheat (Triticum aestivum L.). Front. Plant Sci. 2017, 8, 1557. [Google Scholar] [CrossRef]
- Bimpong, I.K.; Manneh, B.; Sock, M.; Diaw, F.; Amoah, N.K.A.; Ismail, A.M.; Gregorio, G.; Singh, R.K.; Wopereis, M. Improving salt tolerance of lowland rice cultivar ‘Rassi’ through marker-aided backcross breeding in West Africa. Plant Sci. 2016, 242, 288–299. [Google Scholar] [CrossRef]
- Gu, J.; Yin, X.; Zhang, C.; Wang, H.; Struik, P.C. Linking ecophysiological modelling with quantitative genetics to support marker-assisted crop design for improved yields of rice (Oryza sativa) under drought stress. Ann. Bot. 2014, 114, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Van Breusegem, F. Reactive oxygen species in plant development. Development 2018, 145, dev164376. [Google Scholar] [CrossRef]
- You, J.; Chan, Z. ROS Regulation During Abiotic Stress Responses in Crop Plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef]
- Keunen, E.; Peshev, D.; Vangronsveld, J.; Ende, W.V.D.; Cuypers, A. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox Homeostasis and Antioxidant Signaling: A Metabolic Interface between Stress Perception and Physiological Responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef]
- Lu, W.; Chu, X.; Li, Y.; Wang, C.; Guo, X. Cotton GhMKK1 Induces the Tolerance of Salt and Drought Stress, and Mediates Defence Responses to Pathogen Infection in Transgenic Nicotiana benthamiana. PLoS ONE 2013, 8, e68503. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Li, X.; Hicks, L.; Xiong, L. A Raf-Like MAPKKK Gene DSM1 Mediates Drought Resistance through Reactive Oxygen Species Scavenging in Rice. Plant Physiol. 2009, 152, 876–890. [Google Scholar] [CrossRef]
- Du, H.; Wang, N.; Cui, F.; Li, X.; Xiao, J.; Xiong, L. Characterization of the β-Carotene Hydroxylase Gene DSM2 Conferring Drought and Oxidative Stress Resistance by Increasing Xanthophylls and Abscisic Acid Synthesis in Rice. Plant Physiol. 2010, 154, 1304–1318. [Google Scholar] [CrossRef]
- Nadarajah, K.K. ROS Homeostasis in Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- Dongdong, L.; Ming, Z.; Lili, H.; Xiaobo, C.; Yang, G.; Xingqi, G.; Han, L. GhMAPKKK49, a novel cotton (Gossypium hirsutum L.) MAPKKK gene, is involved in diverse stress responses. Acta Physiol. Plant. 2015, 38, 13. [Google Scholar] [CrossRef]
- Gao, M.; Liu, J.; Bi, D.; Zhang, Z.; Cheng, F.; Chen, S.; Zhang, Y. MEKK1, MKK1/MKK2 and MPK4 function together in a mitogen-activated protein kinase cascade to regulate innate immunity in plants. Cell Res. 2008, 18, 1190–1198. [Google Scholar] [CrossRef]
- Bundó, M.; Coca, M. Enhancing blast disease resistance by overexpression of the calcium-dependent protein kinaseOsCPK4in rice. Plant Biotechnol. J. 2015, 14, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Hayashi, N.; Kobayashi, M.; Aoki, N.; Miyao, A.; Mitsuhara, I.; Ichikawa, H.; Komatsu, S.; Hirochika, H.; Kikuchi, S.; et al. A rice calcium-dependent protein kinase OsCPK12 oppositely modulates salt-stress tolerance and blast disease resistance. Plant J. 2011, 69, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.-F.; Zhao, W.-Y.; Fu, J.-D.; Liu, Y.-W.; Chen, M.; Zhou, Y.-B.; Ma, Y.-Z.; Xu, Z.-S.; Xi, Y.-J. Genome-Wide Analysis of CDPK Family in Foxtail Millet and Determination of SiCDPK24 Functions in Drought Stress. Front. Plant Sci. 2018, 9, 651. [Google Scholar] [CrossRef]
- Deng, X.; Hu, W.; Wei, S.; Zhou, S.; Zhang, F.; Han, J.; Chen, L.; Li, Y.; Feng, J.; Fang, B.; et al. TaCIPK29, a CBL-Interacting Protein Kinase Gene from Wheat, Confers Salt Stress Tolerance in Transgenic Tobacco. PLoS ONE 2013, 8, e69881. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-K.; Li, L.-L.; Cao, Z.-H.; Zhao, Q.; Li, M.; Zhang, L.-Y.; Hao, Y.-J. Molecular cloning and functional characterization of a novel apple MdCIPK6L gene reveals its involvement in multiple abiotic stress tolerance in transgenic plants. Plant Mol. Biol. 2012, 79, 123–135. [Google Scholar] [CrossRef]
- Hu, D.-G.; Ma, Q.-J.; Sun, C.-H.; Sun, M.-H.; You, C.-X.; Hao, Y.-J. Overexpression of MdSOS2L1, a CIPK protein kinase, increases the antioxidant metabolites to enhance salt tolerance in apple and tomato. Physiol. Plant. 2015, 156, 201–214. [Google Scholar] [CrossRef]
- Singh, N.K.; Shukla, P.; Kirti, P.B. A CBL-interacting protein kinase AdCIPK5 confers salt and osmotic stress tolerance in transgenic tobacco. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Li, C.-H.; Wang, G.; Zhao, J.-L.; Zhang, L.-Q.; Ai, L.-F.; Han, Y.-F.; Sun, D.-Y.; Zhang, S.-W.; Sun, Y. The receptor-like kinase SIT1 mediates salt sensitivity by activating MAPK3/6 and regulating ethylene homeostasis in rice. Plant Cell 2014, 26, 2538–2553. [Google Scholar] [CrossRef]
- Ueno, Y.; Yoshida, R.; Kishi-Kaboshi, M.; Matsushita, A.; Jiang, C.-J.; Goto, S.; Takahashi, A.; Hirochika, H.; Takatsuji, H. Abiotic Stresses Antagonize the Rice Defence Pathway through the Tyrosine-Dephosphorylation of OsMPK6. PLoS Pathog. 2015, 11, e1005231. [Google Scholar] [CrossRef]
- Zhu, D.; Chang, Y.; Pei, T.; Zhang, X.; Liu, L.; Li, Y.; Zhuang, J.; Yang, H.; Qin, F.; Song, C.; et al. MAPK-like protein 1 positively regulates maize seedling drought sensitivity by suppressing ABA biosynthesis. Plant J. 2019, 102, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, L.; Ding, Z.; Tie, W.; Ding, X.; Zeng, C.; Wei, Y.; Zhao, H.; Peng, M.; Hu, W. Genome-Wide Identification and Expression Analysis of the Mitogen-Activated Protein Kinase Gene Family in Cassava. Front. Plant Sci. 2016, 7, 1294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Pan, J.; Kong, X.; Zhou, Y.; Liu, Y.; Sun, L.; Li, D. ZmMKK3, a novel maize group B mitogen-activated protein kinase kinase gene, mediates osmotic stress and ABA signal responses. J. Plant Physiol. 2012, 169, 1501–1510. [Google Scholar] [CrossRef]
- You, J.; Zong, W.; Hu, H.; Li, X.; Xiao, J.; Xiong, L. A SNAC1-regulated protein phosphatase gene OsPP18 modulates drought and oxidative stress tolerance through ABA-independent reactive oxygen species scavenging in rice. Plant Physiol. 2014, 166, 2100–2114. [Google Scholar] [CrossRef] [PubMed]
- Deveshwar, P.; Prusty, A.; Sharma, S.; Tyagi, A.K. Phytohormone-Mediated Molecular Mechanisms Involving Multiple Genes and QTL Govern Grain Number in Rice. Front. Genet. 2020, 11, 586462. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-Y.; Chao, D.-Y.; Gao, J.-P.; Zhu, M.-Z.; Shi, M.; Lin, H.-X. A previously unknown zinc finger protein, DST, regulates drought and salt tolerance in rice via stomatal aperture control. Genes Dev. Chang. 2009, 23, 1805–1817. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Wen, F.; Yao, D.; Wang, L.; Guo, J.; Ni, L.; Zhang, A.; Tan, M.; Jiang, M. A novel rice C2H2-type zinc finger protein, ZFP36, is a key player involved in abscisic acid-induced antioxidant defence and oxidative stress tolerance in rice. J. Exp. Bot. 2014, 65, 5795–5809. [Google Scholar] [CrossRef]
- Jan, A.; Maruyama, K.; Todaka, D.; Kidokoro, S.; Abo, M.; Yoshimura, E.; Shinozaki, K.; Nakashima, K.; Yamaguchi-Shinozaki, K. OsTZF1, a CCCH-Tandem Zinc Finger Protein, Confers Delayed Senescence and Stress Tolerance in Rice by Regulating Stress-Related Genes. Plant Physiol. 2013, 161, 1202–1216. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Liu, C.; Zhang, Y.; Meng, X.; Zhou, X.; Chu, C.; Wang, X. OsWRKY30 is activated by MAP kinases to confer drought tolerance in rice. Plant Mol. Biol. 2012, 80, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, K.; Mahadeva, A.; Sreevathsa, R.; Ramachandra Swamy, N.; Swamy, N. In Vitro Screening and Identification of Putative Sunflower (Helianthus annus L.) Transformants Expressing ECNAC1 Gene by Salt Stress Method. Trends Biosci. 2013, 6, 108–111. [Google Scholar]
- Lee, S.; Seo, P.J.; Lee, H.-J.; Park, C.-M. A NAC transcription factor NTL4 promotes reactive oxygen species production during drought-induced leaf senescence in Arabidopsis. Plant J. 2012, 70, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Babitha, K.; Vemanna, R.S.; Nataraja, K.N.; Udayakumar, M. Overexpression of EcbHLH57 transcription factor from Eleusine coracana L. in tobacco confers tolerance to salt, oxidative and drought stress. PLoS ONE 2015, 10, e0137098. [Google Scholar] [CrossRef]
- Zhangwu, H.; Liu, W.; Wan, L.; Li, F.; Dai, L.; Li, D.; Zhang, Z.; Huang, R. Functional analyses of ethylene response factor JERF3 with the aim of improving tolerance to drought and osmotic stress in transgenic rice. Transgenic Res. 2010, 19, 809–818. [Google Scholar] [CrossRef]
- McKersie, B.D.; Bowley, S.R.; Harjanto, E.; Leprince, O. Water-Deficit Tolerance and Field Performance of Transgenic Alfalfa Overexpressing Superoxide Dismutase. Plant Physiol. 1996, 111, 1177–1181. [Google Scholar] [CrossRef]
- Zhang, Q.; Cui, M.; Xin, X.; Ming, X.; Jing, L.; WU, J.-X. Overexpression of a cytosolic ascorbate peroxidase gene, OsAPX2, increases salt tolerance in transgenic alfalfa. J. Integr. Agric. 2014, 13, 2500–2507. [Google Scholar] [CrossRef][Green Version]
- Islam, T.; Manna, M.; Reddy, M.K. Glutathione Peroxidase of Pennisetum glaucum (PgGPx) Is a Functional Cd2+ Dependent Peroxiredoxin that Enhances Tolerance against Salinity and Drought Stress. PLoS ONE 2015, 10, e0143344. [Google Scholar] [CrossRef]
- Oberschall, A.; Deák, M.; Török, K.; Sass, L.; Vass, I.; Kovács, I.; Fehér, A.; Dudits, D.; Horváth, G.V. A novel aldose/aldehyde reductase protects transgenic plants against lipid peroxidation under chemical and drought stresses. Plant J. 2000, 24, 437–446. [Google Scholar] [CrossRef]
- Nepal, N.; Yactayo-Chang, J.P.; Medina-Jiménez, K.; Acosta-Gamboa, L.M.; González-Romero, M.E.; Arteaga-Vázquez, M.A.; Lorence, A. Mechanisms underlying the enhanced biomass and abiotic stress tolerance phenotype of an Arabidopsis MIOX over-expresser. Plant Direct 2019, 3, e00165. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, K.; Niu, X.; Wang, Q.; Wan, Y.; Yang, F.; Li, G.; Wang, Y.; Wang, R. Genome-wide Identification of PP2C Genes and Their Expression Profiling in Response to Drought and Cold Stresses in Medicago truncatula. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Z.; Liu, Y.; Kong, D.; Li, T.; Yu, S.; Mei, H.; Xu, X.; Liu, H.; Chen, L.; et al. A novel gene OsAHL1 improves both drought avoidance and drought tolerance in rice. Sci. Rep. 2016, 6, 30264. [Google Scholar] [CrossRef]
- Wen, F.; Qin, T.; Wang, Y.; Dong, W.; Zhang, A.; Tan, M.; Jiang, M. OsHK3 is a crucial regulator of abscisic acid signaling involved in antioxidant defense in rice. J. Integr. Plant Biol. 2014, 57, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Chen, M.; Li, S. Isolation and Identification of Ipomoea cairica (L.) Sweet Gene IcSRO1 Encoding a SIMILAR TO RCD-ONE Protein, Which Improves Salt and Drought Tolerance in Transgenic Arabidopsis. Int. J. Mol. Sci. 2020, 21, 1017. [Google Scholar] [CrossRef]
- Ye, N.; Zhu, G.; Liu, Y.; Li, Y.; Zhang, J. ABA Controls H2O2 Accumulation Through the Induction of OsCATB in Rice Leaves Under Water Stress. Plant Cell Physiol. 2011, 52, 689–698. [Google Scholar] [CrossRef]
- Yamauchi, T.; Yoshioka, M.; Fukazawa, A.; Mori, H.; Nishizawa, N.K.; Tsutsumi, N.; Yoshioka, H.; Nakazono, M. An NADPH Oxidase RBOH Functions in Rice Roots during Lysigenous Aerenchyma Formation under Oxygen-Deficient Conditions. Plant Cell 2017, 29, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, J.; Zhu, M.; Jia, H.; Liu, D.; Hao, L.; Guo, X. A cotton Raf-like MAP3K gene, GhMAP3K40, mediates reduced tolerance to biotic and abiotic stress in Nicotiana benthamiana by negatively regulating growth and development. Plant Sci. 2015, 240, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.-J.; Choi, H.-K.; Park, M.Y.; Choi, S.-W.; Vo, K.T.X.; Jeon, J.-S.; Kim, S.Y. OsMAPKKK63 is involved in salt stress response and seed dormancy control. Plant Signal. Behav. 2019, 14, e1578633. [Google Scholar] [CrossRef]
- Wang, G.; Liang, Y.-H.; Zhang, J.-Y.; Cheng, Z.-M. Cloning, molecular and functional characterization by overexpression in Arabidopsis of MAPKK genes from grapevine (Vitis vinifera). BMC Plant Biol. 2020, 20, 194. [Google Scholar] [CrossRef]
- Jagodzik, P.; Tajdel-Zielinska, M.; Cieśla, A.; Marczak, M.; Ludwikow, A. Mitogen-Activated Protein Kinase Cascades in Plant Hormone Signaling. Front. Plant Sci. 2018, 9, 1387. [Google Scholar] [CrossRef] [PubMed]
- Farooq, S.; Azam, F. Co-Existence of Salt and Drought Tolerance in Triticeae. Hereditas 2004, 135, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Baranwal, V.K.; Khurana, P. Genome-wide analysis of bZIP transcription factors in wheat and functional characterization of a TabZIP under abiotic stress. Sci. Rep. 2019, 9, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-S.; Liu, J.-H.; Chen, X.-J. Overexpression of PtrABF gene, a bZIP transcription factor isolated from Poncirus trifoliata, enhances dehydration and drought tolerance in tobacco via scavenging ROS and modulating expression of stress-responsive genes. BMC Plant Biol. 2010, 10, 230. [Google Scholar] [CrossRef]
- Joo, J.; Lee, Y.H.; Song, S.I. Overexpression of the rice basic leucine zipper transcription factor OsbZIP12 confers drought tolerance to rice and makes seedlings hypersensitive to ABA. Plant Biotechnol. Rep. 2014, 8, 431–441. [Google Scholar] [CrossRef]
- Kang, J.-Y.; Choi, H.-I.; Im, M.-Y.; Kim, S.Y. Arabidopsis Basic Leucine Zipper Proteins That Mediate Stress-Responsive Abscisic Acid Signaling. Plant Cell 2002, 14, 343–357. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, H.; Wang, X.; Kang, J.; Lv, B.; Dong, Q.; Li, C.; Chen, H.; Wu, Q. Tartary Buckwheat Transcription Factor FtbZIP5, Regulated by FtSnRK2.6, Can Improve Salt/Drought Resistance in Transgenic Arabidopsis. Int. J. Mol. Sci. 2020, 21, 1123. [Google Scholar] [CrossRef]
- Liu, C.; Mao, B.; Ou, S.; Wang, W.; Liu, L.; Wu, Y.; Chu, C.; Wang, X. OsbZIP71, a bZIP transcription factor, confers salinity and drought tolerance in rice. Plant Mol. Biol. 2013, 84, 19–36. [Google Scholar] [CrossRef]
- Lu, G.; Gao, C.; Zheng, X.; Han, B. Identification of OsbZIP72 as a positive regulator of ABA response and drought tolerance in rice. Planta 2008, 229, 605–615. [Google Scholar] [CrossRef]
- Ma, H.; Liu, C.; Li, Z.; Ran, Q.; Xie, G.; Wang, B.; Fang, S.; Chu, J.; Zhang, J. ZmbZIP4 Contributes to Stress Resistance in Maize by Regulating ABA Synthesis and Root Development. Plant Physiol. 2018, 178, 753–770. [Google Scholar] [CrossRef]
- Tang, N.; Zhang, H.; Li, X.; Xiao, J.; Xiong, L. Constitutive activation of transcription factor OsbZIP46 improves drought tolerance in rice. Plant Physiol. 2012, 158, 1755–1768. [Google Scholar] [CrossRef]
- Xiang, Y.; Tang, N.; Du, H.; Ye, H.; Xiong, L. Characterization of OsbZIP23 as a key player of the basic leucine zipper transcription factor family for conferring abscisic acid sensitivity and salinity and drought tolerance in rice. Plant Physiol. 2008, 148, 1938–1952. [Google Scholar] [CrossRef]
- Babitha, K.; Ramu, S.; Pruthvi, V.; Mahesh, P.; Nataraja, K.N.; Udayakumar, M. Co-expression of at bHLH17 and at WRKY 28 confers resistance to abiotic stress in Arabidopsis. PLoS ONE 2013, 22, 327–341. [Google Scholar]
- Dombrecht, B.; Xue, G.P.; Sprague, S.J.; Kirkegaard, J.A.; Ross, J.J.; Reid, J.B.; Fitt, G.P.; Sewelam, N.; Schenk, P.M.; Manners, J.M.; et al. MYC2 Differentially Modulates Diverse Jasmonate-Dependent Functions inArabidopsis. Plant Cell 2007, 19, 2225–2245. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, C.; Han, X.; Tang, S.; Liu, S.; Xia, X.; Yin, W. A novel bHLH transcription factor PebHLH35 from Populus euphratica confers drought tolerance through regulating stomatal development, photosynthesis and growth in Arabidopsis. Biochem. Biophys. Res. Commun. 2014, 450, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, P.; Vishal, B.; Khoo, K.; Rajappa, S.; Loh, C.-S.; Kumar, P.P. Expression of AoNHX1 increases salt tolerance of rice and Arabidopsis, and bHLH transcription factors regulate AtNHX1 and AtNHX6 in Arabidopsis. Plant Cell Rep. 2019, 38, 1299–1315. [Google Scholar] [CrossRef] [PubMed]
- Le Hir, R.; Castelain, M.; Chakraborti, D.; Moritz, T.; Dinant, S.; Bellini, C. At bHLH68 transcription factor contributes to the regulation of ABA homeostasis and drought stress tolerance in Arabidopsis thaliana. Physiol. Plant. 2017, 160, 312–327. [Google Scholar] [CrossRef]
- Seo, J.-S.; Joo, J.; Kim, M.-J.; Kim, Y.-K.; Nahm, B.H.; Song, S.I.; Cheong, J.-J.; Lee, J.S.; Kim, J.-K.; Choi, Y.D. OsbHLH148, a basic helix-loop-helix protein, interacts with OsJAZ proteins in a jasmonate signaling pathway leading to drought tolerance in rice. Plant J. 2011, 65, 907–921. [Google Scholar] [CrossRef]
- Yao, P.; Sun, Z.; Li, C.; Zhao, X.; Li, M.; Deng, R.; Huang, Y.; Zhao, H.; Chen, H.; Wu, Q. Overexpression of Fagopyrum ta-taricum FtbHLH2 enhances tolerance to cold stress in transgenic Arabidopsis. Front. Plant Sci. 2018, 125, 85–94. [Google Scholar]
- Yao, P.-F.; Li, C.-L.; Zhao, X.-R.; Li, M.-F.; Zhao, H.-X.; Guo, J.-Y.; Cai, Y.; Chen, H.; Wu, Q. Overexpression of a Tartary Buckwheat Gene, FtbHLH3, Enhances Drought/Oxidative Stress Tolerance in Transgenic Arabidopsis. Front. Plant Sci. 2017, 8, 625. [Google Scholar] [CrossRef] [PubMed]
- Shoucheng, C.; Chai, S.; McIntyre, C.; Xue, G.-P. Overexpression of a predominantly root-expressed NAC transcription factor in wheat roots enhances root length, biomass and drought tolerance. Plant Cell Rep. 2017, 37, 225–237. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kim, Y.S.; Baek, K.H.; Jung, H.; Ha, S.-H.; Choi, Y.D.; Kim, M.; Reuzeau, C.; Kim, J.-K. Root-Specific Expression of OsNAC10 Improves Drought Tolerance and Grain Yield in Rice under Field Drought Conditions. Plant Physiol. 2010, 153, 185–197. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kim, Y.S.; Redillas, M.C.F.R.; Jang, G.; Jung, H.; Bang, S.W.; Choi, Y.D.; Ha, S.-H.; Reuzeau, C.; Kim, J.-K. OsNAC5overexpression enlarges root diameter in rice plants leading to enhanced drought tolerance and increased grain yield in the field. Plant Biotechnol. J. 2012, 11, 101–114. [Google Scholar] [CrossRef]
- Lee, D.K.; Chung, P.J.; Jeong, J.S.; Jang, G.; Bang, S.W.; Jung, H.; Kim, Y.S.; Ha, S.H.; Choi, Y.D.; Kim, J.K. The rice Os NAC 6 transcription factor orchestrates multiple molecular mechanisms involving root structural adaptions and nicotianamine biosynthesis for drought tolerance. Plant Biotechnol. J. 2017, 15, 754–764. [Google Scholar] [CrossRef]
- Ohnishi, T.; Sugahara, S.; Yamada, T.; Kikuchi, K.; Yoshiba, Y.; Hirano, H.-Y.; Tsutsumi, N. OsNAC6, a member of the NAC gene family, is induced by various stresses in rice. Genes Genet. Syst. 2005, 80, 135–139. [Google Scholar] [CrossRef]
- Redillas, M.C.; Jeong, J.S.; Kim, Y.S.; Jung, H.; Bang, S.W.; Choi, Y.D.; Ha, S.H.; Reuzeau, C.; Kim, J.K. The overexpression of OsNAC9 alters the root architecture of rice plants enhancing drought resistance and grain yield under field conditions. Plant Biotechnol. J. 2012, 10, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Shahnejat-Bushehri, S.; Mueller-Roeber, B.; Balazadeh, S. Arabidopsis NAC transcription factor JUNGBRUNNEN1 affects thermomemory-associated genes and enhances heat stress tolerance in primed and unprimed conditions. Plant Signal. Behav. 2012, 7, 1518–1521. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.-S.; Nakashima, K.; Sakuma, Y.; Simpson, S.D.; Fujita, Y.; Maruyama, K.; Fujita, M.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Isolation and Functional Analysis of Arabidopsis Stress-Inducible NAC Transcription Factors That Bind to a Drought-Responsive cis-Element in the early responsive to dehydration stress 1 Promoter. Plant Cell 2004, 16, 2481–2498. [Google Scholar] [CrossRef]
- Xu, Z.; Gong, Z.; Wang, C.; Xue, F.; Zhang, H.; Ji, W. Wheat NAC transcription factor TaNAC29 is involved in response to salt stress. Plant Physiol. Biochem. 2015, 96, 356–363. [Google Scholar] [CrossRef]
- Yang, X.; Kim, M.Y.; Ha, J.; Lee, S.-H. Overexpression of the Soybean NAC Gene GmNAC109 Increases Lateral Root Formation and Abiotic Stress Tolerance in Transgenic Arabidopsis Plants. Front. Plant Sci. 2019, 10, 1036. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, F.; Wang, X.; Liu, S.; Saeed, U.H.; Hou, X.; Zhang, Y.; Luo, D.; Meng, Y.; Zhang, W.; et al. Molecular and Functional Characterization of CaNAC035, an NAC Transcription Factor From Pepper (Capsicum annuum L.). Front. Plant Sci. 2020, 11, 14. [Google Scholar] [CrossRef]
- Dubouzet, J.G.; Sakuma, Y.; Ito, Y.; Kasuga, M.; Dubouzet, E.G.; Miura, S.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. OsDREBgenes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J. 2003, 33, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, E.-J.; Cheng, M.-C.; Lin, T.-P. Functional characterization of an abiotic stress-inducible transcription factor AtERF53 in Arabidopsis thaliana. Plant Mol. Biol. 2013, 82, 223–237. [Google Scholar] [CrossRef]
- Jisha, V.; Dampanaboina, L.; Vadassery, J.; Mithöfer, A.; Kappara, S.; Ramanan, R. Overexpression of an AP2/ERF Type Transcription Factor OsEREBP1 Confers Biotic and Abiotic Stress Tolerance in Rice. PLoS ONE 2015, 10, e0127831. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, X.; Zhang, M.; Wang, X.; Zhao, Y.; Yin, Z.; Zhang, Z.; Wang, Y.; Xiong, H.; Zhang, H.; et al. OsERF71 confers drought tolerance via modulating ABA signaling and proline biosynthesis. Plant Sci. 2018, 270, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Seymour, G.B.; Lu, C.; Hu, Z.; Chen, X.; Chen, G. An ethylene response factor (ERF5) promoting adaptation to drought and salt tolerance in tomato. Plant Cell Rep. 2011, 31, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, C.; Doherty, C.J.; Gilmour, S.J.; Kim, Y.; Thomashow, M.F. Regulation of the Arabidopsis CBF regulon by a complex low-temperature regulatory network. Plant J. 2015, 82, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Kakimoto, M.; Sakuma, Y.; Maruyama, K.; Osakabe, Y.; Tran, L.S.P.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Regu-lation and functional analysis of ZmDREB2A in response to drought and heat stresses in Zea mays L. Plant J. 2007, 50, 54–69. [Google Scholar] [CrossRef]
- Yao, Y.; He, R.J.; Xie, Q.L.; Zhao, X.H.; Deng, X.M.; He, J.B.; Song, L.; He, J.; Marchant, A.; Chen, X.Y. Ethylene Response Factor 74 (ERF74) plays an essential role in controlling a respiratory burst oxidase homolog D (RbohD)-dependent mechanism in response to different stresses in Arabidopsis. New Phytol. 2017, 213, 1667–1681. [Google Scholar] [CrossRef]
- Zhang, B.; Su, L.; Hu, B.; Li, L.J. Expression of AhDREB1, an AP2/ERF transcription factor gene from peanut, is af-fected by histone acetylation and increases abscisic acid sensitivity and tolerance to osmotic stress in Arabidopsis. Int. J. Mol. Sci. 2018, 19, 1441. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, M.; Li, L.; Xu, Z.; Chen, X.; Guo, J.; Ma, Y. Overexpression of the soybean GmERF3 gene, an AP2/ERF type transcription factor for increased tolerances to salt, drought, and diseases in transgenic tobacco. J. Exp. Bot. 2009, 60, 3781–3796. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, Z.; Xie, S.; Si, T.; Li, Y.; Zhu, J.-K. Mutational Evidence for the Critical Role of CBF Transcription Factors in Cold Acclimation in Arabidopsis. Plant Physiol. 2016, 171, 2744–2759. [Google Scholar] [CrossRef]
- Cui, M.H.; Yoo, K.S.; Hyoung, S.; Nguyen, H.T.K.; Kim, Y.Y.; Kim, H.J.; Ok, S.H.; Yoo, S.D.; Shin, J.S. An Arabidopsis R2R3-MYB transcription factor, AtMYB20, negatively regulates type 2C serine/threonine protein phosphatases to enhance salt tolerance. FEBS Lett. 2013, 587, 1773–1778. [Google Scholar] [CrossRef]
- Jung, C.; Seo, J.S.; Han, S.W.; Koo, Y.J.; Kim, C.H.; Song, S.I.; Nahm, B.H.; Choi, Y.D.; Cheong, J.-J. Overexpression of AtMYB44 Enhances Stomatal Closure to Confer Abiotic Stress Tolerance in Transgenic Arabidopsis. Plant Physiol. 2007, 146, 323–324. [Google Scholar] [CrossRef]
- Lee, S.B.; Kim, H.; Kim, R.J.; Suh, M.C. Overexpression of Arabidopsis MYB96 confers drought resistance in Camelina sativa via cuticular wax accumulation. Plant Cell Rep. 2014, 33, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Zou, H.-F.; Wang, H.-W.; Zhang, W.-K.; Ma, B.; Zhang, J.-S.; Chen, S.-Y. Soybean GmMYB76, GmMYB92, and GmMYB177 genes confer stress tolerance in transgenic Arabidopsis plants. Cell Res. 2008, 18, 1047–1060. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Qi, T.; Huang, H.; Ren, Q.; Wu, D.; Chang, C.; Peng, W.; Liu, Y.; Peng, J.; Xie, D. The Jasmonate-ZIM domain proteins interact with the R2R3-MYB transcription factors MYB21 and MYB24 to affect Jasmonate-regulated stamen development in Arabidopsis. Plant Cell 2011, 23, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Bao, X.; Zhi, Y.; Wu, Q.; Guo, Y.; Yin, X.; Zeng, L.; Li, J.; Zhang, J.; He, W.; et al. Overexpression of a MYB Family Gene, OsMYB6, Increases Drought and Salinity Stress Tolerance in Transgenic Rice. Front. Plant Sci. 2019, 10, 168. [Google Scholar] [CrossRef]
- Vannini, C.; Locatelli, F.; Bracale, M.; Magnani, E.; Marsoni, M.; Osnato, M.; Mattana, M.; Baldoni, E.; Coraggio, I. Overex-pression of the rice Osmyb4 gene increases chilling and freezing tolerance of Arabidopsis thaliana plants. Plant J. 2004, 37, 115–127. [Google Scholar] [CrossRef]
- Xiong, H.; Li, J.; Liu, P.; Duan, J.; Zhao, Y.; Guo, X.; Li, Y.; Zhang, H.; Ali, J.; Li, Z. Overexpression of OsMYB48-1, a Novel MYB-Related Transcription Factor, Enhances Drought and Salinity Tolerance in Rice. PLoS ONE 2014, 9, e92913. [Google Scholar] [CrossRef]
- Zhu, N.; Cheng, S.; Liu, X.; Du, H.; Dai, M.; Zhou, D.-X.; Yang, W.; Zhao, Y. The R2R3-type MYB gene OsMYB91 has a function in coordinating plant growth and salt stress tolerance in rice. Plant Sci. 2015, 236, 146–156. [Google Scholar] [CrossRef] [PubMed]
- He, G.-H.; Xu, J.-Y.; Wang, Y.-X.; Liu, J.-M.; Li, P.-S.; Chen, M.; Ma, Y.-Z.; Xu, Z.-S. Drought-responsive WRKY transcription factor genes TaWRKY1 and TaWRKY33 from wheat confer drought and/or heat resistance in Arabidopsis. BMC Plant Biol. 2016, 16, 1–16. [Google Scholar] [CrossRef]
- Li, H.; Gao, Y.; Xu, H.; Dai, Y.; Deng, D.; Chen, J. ZmWRKY33, a WRKY maize transcription factor conferring enhanced salt stress tolerances in Arabidopsis. Plant Growth Regul. 2013, 70, 207–216. [Google Scholar] [CrossRef]
- Mzid, R.; Zorrig, W.; ben Ayed, R.; Ben Hamed, K.; Ayadi, M.; Damak, Y.; Lauvergeat, V.; Hanana, M. The grapevine VvWRKY2 gene enhances salt and osmotic stress tolerance in transgenic Nicotiana tabacum. 3 Biotech 2018, 8, 277. [Google Scholar] [CrossRef]
- Qiu, Y.; Yu, D. Over-expression of the stress-induced OsWRKY45 enhances disease resistance and drought tolerance in Arabidopsis. Environ. Exp. Exp. Bot. 2009, 65, 35–47. [Google Scholar] [CrossRef]
- Villano, C.; Esposito, S.; D’Amelia, V.; Garramone, R.; Alioto, D.; Zoina, A.; Aversano, R.; Carputo, D. WRKY genes family study reveals tissue-specific and stress-responsive TFs in wild potato species. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Shiroto, Y.; Kishitani, S.; Ito, Y.; Toriyama, K. Enhanced heat and drought tolerance in transgenic rice seedlings overexpressing OsWRKY11 under the control of HSP101 promoter. Plant Cell Rep. 2009, 28, 21–30. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Lin, K.-H.; Sei, S.-C.; Su, Y.-H.; Chiang, C.-M. Overexpression of the Arabidopsis and winter squash superoxide dismutase genes enhances chilling tolerance via ABA-sensitive transcriptional regulation in transgenic Arabidopsis. Plant Signal. Behav. 2019, 14, 1685728. [Google Scholar] [CrossRef]
- Zhou, C.; Zhu, C.; Fu, H.; Li, X.; Chen, L.; Lin, Y.; Lai, Z.; Guo, Y. Genome-wide investigation of superoxide dismutase (SOD) gene family and their regulatory miRNAs reveal the involvement in abiotic stress and hormone response in tea plant (Camellia sinensis). PLoS ONE 2019, 14, e0223609. [Google Scholar] [CrossRef]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef]
- Pandey, S.; Fartyal, D.; Agarwal, A.; Shukla, T.; James, D.; Kaul, T.; Negi, Y.K.; Arora, S.; Reddy, M.K. Abiotic stress toler-ance in plants: Myriad roles of ascorbate peroxidase. Front. Plant Sci. 2017, 8, 581. [Google Scholar] [CrossRef]
- Liu, J.-X.; Feng, K.; Duan, A.-Q.; Li, H.; Yang, Q.-Q.; Xu, Z.-S.; Xiong, A.-S. Isolation, purification and characterization of an ascorbate peroxidase from celery and overexpression of the AgAPX1 gene enhanced ascorbate content and drought tolerance in Arabidopsis. BMC Plant Biol. 2019, 19, 1–13. [Google Scholar] [CrossRef]
- Liu, Z.; Bao, H.; Cai, J.; Han, J.; Zhou, L. A Novel Thylakoid Ascorbate Peroxidase from Jatrophacurcas Enhances Salt Tolerance in Transgenic Tobacco. Int. J. Mol. Sci. 2013, 15, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cai, J.; Yang, F.; Zhou, B.; Zhou, L. Ascorbate peroxidase from Jatropha curcas enhances salt tolerance in transgenic Arabidopsis. Genet. Mol. Res. 2015, 14, 4879–4889. [Google Scholar] [CrossRef] [PubMed]
- Filiz, E.; Ozyigit, I.I.; Saracoglu, I.A.; Uras, M.E.; Sen, U.; Yalcin, B. Abiotic stress-induced regulation of antioxidant genes in different Arabidopsis ecotypes: Microarray data evaluation. Biotechnol. Biotechnol. Equip. 2019, 33, 128–143. [Google Scholar] [CrossRef]
- Nie, Q.; Gao, G.-L.; Fan, Q.-J.; Qiao, G.; Wen, X.-P.; Liu, T.; Peng, Z.-J.; Cai, Y.-Q. Isolation and characterization of a catalase gene “HuCAT3” from pitaya (Hylocereus undatus) and its expression under abiotic stress. Gene 2015, 563, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, S.; Yang, Z.; Yang, Y.; Jiang, L.; Hu, L. CsCAT3, a catalase gene from Cucumis sativus, confers resistance to a variety of stresses to Escherichia coli. Biotechnol. Biotechnol. Equip. 2017, 31, 886–896. [Google Scholar] [CrossRef]
- Milla, M.A.R.; Maurer, A.; Huete, A.R.; Gustafson, J.P. Glutathione peroxidase genes in Arabidopsis are ubiquitous and reg-ulated by abiotic stresses through diverse signaling pathways. Plant J. 2003, 36, 602–615. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.; Wang, J.; Yang, W.; Yang, Y. Identification and Characterization of the Glutathione Peroxidase (GPX) Gene Family in Watermelon and Its Expression under Various Abiotic Stresses. Agronomy 2018, 8, 206. [Google Scholar] [CrossRef]
- Diao, Y.; Xu, H.; Li, G.; Yu, A.; Yu, X.; Hu, W.; Zheng, X.; Li, S.; Wang, Y.; Hu, Z. Cloning a glutathione peroxidase gene from Nelumbo nucifera and enhanced salt tolerance by overexpressing in rice. Mol. Biol. Rep. 2014, 41, 4919–4927. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fang, G.; Yang, J.; Li, Y. A Thioredoxin-Dependent Glutathione Peroxidase (OsGPX5) Is Required for Rice Normal Development and Salt Stress Tolerance. Plant Mol. Biol. Rep. 2017, 35, 333–342. [Google Scholar] [CrossRef]
- Sultana, S.; Khew, C.Y.; Morshed, M.; Namasivayam, P.; Napis, S.; Ho, C.-L. Overexpression of monodehydroascorbate reductase from a mangrove plant (AeMDHAR) confers salt tolerance on rice. J. Plant Physiol. 2011, 169, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Eltayeb, A.E.; Kawano, N.; Badawi, G.H.; Kaminaka, H.; Sanekata, T.; Shibahara, T.; Inanaga, S.; Tanaka, K. Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta 2006, 225, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.H.; Wang, M.-H. Expression Pattern of Two Dehydroascorbate Reductase Genes from Tomato (Solanum lycopersicum L.) in Response to Stress. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 668–676. [Google Scholar] [CrossRef]
- Hao, Z.; Wang, X.; Zong, Y.; Wen, S.; Cheng, Y.; Li, H. Enzymatic activity and functional analysis under multiple abiotic stress conditions of a dehydroascorbate reductase gene derived from Liriodendron Chinense. Environ. Exp. Bot. 2019, 167, 103850. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Peng, Y.; Wang, X.; Peng, D.; Li, Y.; McPhee, D.J.; Zhang, X.; Ma, X.; Huang, L.; et al. Clones of FeSOD, MDHAR, DHAR Genes from White Clover and Gene Expression Analysis of ROS-Scavenging Enzymes during Abiotic Stress and Hormone Treatments. Molecules 2015, 20, 20939–20954. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).