Melatonin and Carbohydrate Metabolism in Plant Cells
Abstract
:1. Introduction
2. Biosynthesis of Melatonin in Plants
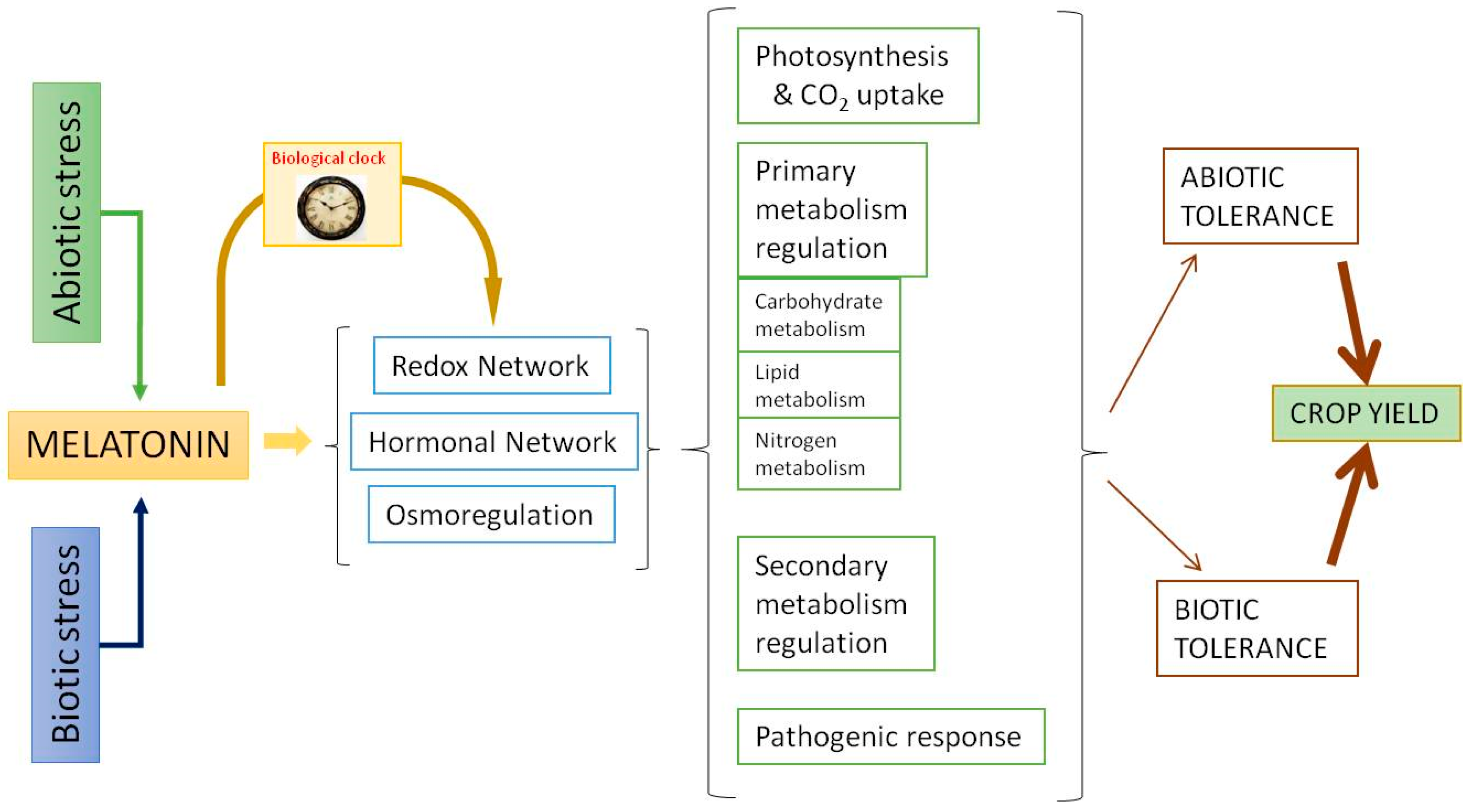
3. Roles of Melatonin in Plants
4. Effect of Melatonin in Simple Carbohydrates, Starch, and Polyalcohols
5. Regulatory Action of Melatonin on Carbohydrate Metabolism
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| accD | acetyl-coenzyme A carboxylase-carboxyl transferase |
| ACLA,B | cytrate synthase |
| ADH1,2 | alcohol dehydrogenase |
| ADP-G | ADP-glucose |
| ALDH3H,3F | aldehyde dehydrogenase |
| ALDEP | aldose epimerase |
| ALDO | aldoketoreductase |
| ATPF1A | ATP synthase alpha subunit |
| COX2 | cytochrome c oxidase |
| CWI | cell wall invertase |
| D1 (PSII) | integral part of the reaction center of photosystem II |
| F | fructose |
| F6P | fructose-6-phosphate |
| FRK2 | fructokinase |
| FUM2 | fumarate hydratase |
| G | glucose |
| G1P | glucose-1-phosphate |
| G6P | glucose-6-phosphate |
| GAPC1 | cytosolic glyceraldehyde-3-phosphate dehydrogenase |
| GAPCP2 | chloroplastic glyceraldehyde-3-phosphate dehydrogenase |
| HxK | hexokinase |
| IDH | isocitrate dehydrogenase |
| INVINH | invertase inhibitor |
| MDH | malate dehydrogenase |
| NAPs | senescence-induced genes |
| NINV | neutral invertase |
| PaO | pheophorbide a oxygenase |
| PEP | phosphoenolpyruvate |
| PEPC | phosphoenolpyruvate carboxylase |
| PEPCK | phosphoenolpyruvate carboxykinase |
| PetF1 | ferredoxin |
| PDC | pyruvate decarboxylase |
| PDH | pyruvate dehydrogenase |
| PFK | phosphofructo kinase |
| PGK | phosphoglycerate kinase |
| PGM | phosphogluco mutase |
| PHO | plastidial a-glucan phosphorylase |
| PK | cytosolic |
| PKP2 | plastidial pyruvate kinase |
| PNSL2 | photosynthetic NADPH subunit of lumenal location |
| PSI | Psa-A,F,G,H,K,O, Photosystem I subunits |
| PSII | Psb-E,O,P,Q,Y,Z,28, Photosystem II subunits |
| PSY | phytoene synthase |
| RbcS | Rubisco small subunit |
| (RCCR1, SGR, NYC1,3) | chlorophyll degradation-related genes |
| SAG12 | senescence-related gene |
| SDH | succinate dehydrogenase |
| SPS1/2/3 | sucrose phosphate synthase irreversible |
| SUC | sucrose |
| SUC6P | sucrose-6-phosphate |
| SUS | sucrose synthase reversible |
| TIM | triose isomerase |
| UDP-G | UDP-glucose |
| vAINV | vacuolar acid invertase |
| VINV | vacuolar invertase |
References
- Bryant, J.; Burrell, M.M.; Kruger, N.N. Plant Carbohydrate Biochemistry; Garland Sci./Taylor & Francis: New York, NY, USA, 1999. [Google Scholar]
- Ernst, B.; Hart, G.W.; Sinay, P. Carbohydrates in Chemistry and Biology; John Wiley & Sons: Weinheim, Germany, 2000. [Google Scholar]
- Sharkey, T.D. Pentose Phosphate Pathway Reactions in Photosynthesizing Cells. Cells 2021, 10, 1547. [Google Scholar] [CrossRef]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of sugars under abiotic stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Versluys, M.; Tarkowski, L.P.; Van den Ende, W. Fructans As DAMPs or MAMPs: Evolutionary Prospects, Cross-Tolerance, and Multistress Resistance Potential. Front. Plant Sci. 2017, 7, 2061. [Google Scholar] [CrossRef] [Green Version]
- Hattori, A.; Migitaka, H.; Iigo, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar] [PubMed]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by HPLC-MS. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Kolar, J.; Machackova, I.; Illnerova, H.; Prinsen, E.; van Dongen, W.; van Onckelen, H. Melatonin in higher plant determined by radioimmunoassay and liquid chromatography-mass spectrometry. Biol. Rhythm Res. 1995, 26, 406–409. [Google Scholar]
- Majidinia, M.; Reiter, R.J.; Shakouri, S.K.; Yousefi, B. The role of melatonin, a multitasking molecule, in retarding the processes of ageing. Age Res. Rev. 2018, 47, 198–213. [Google Scholar] [CrossRef]
- Socaciu, A.I.; Ionut, R.; Socaciu, M.A.; Ungur, A.P.; Bârsan, M.; Chiorean, A.; Socaciu, C.; Râjnoveanu, A.G. Melatonin, an ubiquitous metabolic regulator: Functions, mechanisms and effects on circadian disruption and degenerative diseases. Rev. Endocr. Metabol. Dis. 2020, 21, 465–478. [Google Scholar] [CrossRef]
- Shukla, M.; Govitrapong, P.; Boontem, P.; Reiter, R.J.; Satayavivad, J. Mechanisms of Melatonin in Alleviating Alzheimer’s Disease. Curr. Neuropharmacol. 2017, 15, 1010–1031. [Google Scholar] [CrossRef] [Green Version]
- Cardinali, D.; Brown, G.; Pandi-Perumal, S.R. Can Melatonin Be a Potential “Silver Bullet” in Treating COVID-19 Patients? Diseases 2020, 8, 44. [Google Scholar] [CrossRef]
- Cardinali, D. Melatonin and healthy aging. Vitam. Horm. 2021, 115, 67–88. [Google Scholar]
- Talib, W.H.; Alsayed, A.R.; Abuawad, A.; Daoud, S.; Mahmod, A.I. Melatonin in Cancer Treatment: Current Knowledge and Future Opportunities. Molecules 2021, 26, 2506. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Rodriguez, C.; Martin, V.; Rosales-Corral, S.; Zuccari, D.A.P.d.C.; Chuffa, L.G.d.A. Part-time cancers and role of melatonin in determining their metabolic phenotype. Life Sci. 2021, 278, 119597. [Google Scholar] [CrossRef]
- Delpino, F.M.; Figueiredo, L.M.; Nunes, B.P. Effects of melatonin supplementation on diabetes: A systematic review and meta-analysis of randomized clinical trials. Clin. Nutr. 2021, 40, 4595–4605. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Cardinali, D.; Reiter, R.; Brown, G. Low melatonin as a contributor to SARS-CoV-2 disease. Melatonin Res. 2020, 3, 558–576. [Google Scholar] [CrossRef]
- Back, K.; Tan, D.X.; Reiter, R.J. Melatonin biosynthesis in plants: Multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J. Pineal Res. 2016, 61, 426–437. [Google Scholar] [CrossRef]
- Tan, D.X.; Hardeland, R.; Back, K.; Manchester, L.C.; Latorre-Jimenez, M.A.; Reiter, R.J. On the significance of an alternate pathway of melatonin synthesis via 5-methoxytryptamine: Comparisons across species. J. Pineal Res. 2016, 61, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Back, K. Melatonin metabolism, signaling, and possible roles in plants. Plant J. 2020, 105, 376–391. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin synthesis and function: Evolutionary history in animals and plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Reiter, R.J. An evolutionary view of melatonin synthesis and metabolism related to its biological functions in plants. J. Exp. Bot. 2020, 71, 4677–4689. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.R.; Kim, Y.J.; Lim, Y.J.; Duan, S.; Eom, S.H.; Jung, K.H. Key Genes in the Melatonin Biosynthesis Pathway with Circadian Rhythm Are Associated with Various Abiotic Stresses. Plants 2021, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Buttar, Z.A.; Wu, S.N.; Arnao, M.B.; Wang, C.; Ullah, I.; Wang, C. Melatonin suppressed the heat stress-induced damage in wheat seedlings by modulating the antioxidant machinery. Plants 2020, 9, 809. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin against environmental plant stressors: A review. Curr. Prot. Pept. Sci. 2021, 22, 1–17. [Google Scholar]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a plant biostimulant in crops and during post-harvest: A new approach is needed. J. Sci. Food Agric. 2021, 101, 5297–5304. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Balabusta, M.; Wieczorek, M.; Sliwinska, E.; Janas, K.M. Melatonin applied to cucumber (Cucumis sativus L.) seeds improves germination during chilling stress. J. Pineal Res. 2009, 46, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Gong, B.; Sun, S.; Liu, S.; Wang, X.; Wei, M.; Yang, F.; Li, Y.; Shi, Q. Promoting roles of melatonin in adventitious root development of Solanum lycopersicum L. by regulating auxin and nitric oxide signaling. Front. Plant Sci. 2016, 7, 718. [Google Scholar] [CrossRef] [Green Version]
- Korkmaz, A.; Karakas, A.; Kocacinar, F.; Cuci, Y. The effects of seed treatment with melatonin on germination and emergence performance of pepper seeds under chilling stress. J. Agric. Sci. 2017, 23, 167–176. [Google Scholar]
- Sarropoulou, V.N.; Dimassi-Theriou, K.N.; Therios, I.N.; Koukourikou-Petridou, M. Melatonin enhances root regeneration, photosynthetic pigments, biomass, total carbohydrates and proline content in the cherry rootstock PHL-C (Prunus avium x Prunus cerasus). Plant Physiol. Biochem. 2012, 61, 162–168. [Google Scholar] [CrossRef]
- Wang, P.; Sun, X.; Chang, C.; Feng, F.; Liang, D.; Cheng, L.; Ma, F. Delay in leaf senescence of Malus hupehensis by long-term melatonin application is associated with its regulation of metabolic status and protein degradation. J. Pineal Res. 2013, 55, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Li, Q.; Chu, Y.-N.; Reiter, R.J.; Yu, X.M.; Zhu, D.H.; Zhang, W.K.; Ma, B.; Lin, Q.; Zhang, J.S.; et al. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2015, 66, 695–707. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Chang, J.; Chen, H.; Wang, Z.; Gu, X.; Wei, C.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X. Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front. Plant Sci. 2017, 8, 295. [Google Scholar] [CrossRef] [Green Version]
- Galano, A.; Castañeda-Arriaga, R.; Pérez-González, A.; Tan, D.X.; Reiter, J.R. Phenolic melatonin-related compounds: Their role as chemical protectors against oxidative stress. Molecules 2016, 21, 1442. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Yue, Q.; Bian, F.; Sun, H.; Zhai, H.; Yao, Y. Melatonin enhances phenolics accumulation partially via ethylene signaling and resulted in high antioxidant capacity in grape berries. Front. Plant Sci. 2017, 8, 1426. [Google Scholar] [CrossRef] [Green Version]
- Bahcesular, B.; Yildirim, E.D.; Karaçocuk, M.; Kulak, M.; Karaman, S. Seed priming with melatonin effects on growth, essential oil compounds and antioxidant activity of basil (Ocimum basilicum L.) under salinity stress. Ind. Crops Prod. 2020, 146, 112165. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Z.; Yang, M.; Li, D.; Qi, M.; Xu, Y.; Abdelshafy, A.M.; Ban, Z.; Wang, F.; Li, L. Role of exogenous melatonin in table grapes: First evidence on contribution to the phenolics-oriented response. Food Chem. 2020, 329, 127155. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Growth activity, rooting capacity, and tropism: Three auxinic precepts fulfilled by melatonin. Acta Physiol. Plant. 2017, 39, 127. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin promotes adventitious- and lateral root regeneration in etiolated hypocotyls of Lupinus albus L. J. Pineal Res. 2007, 42, 147–152. [Google Scholar] [CrossRef]
- Murch, S.J.; Campbell, S.S.B.; Saxena, P.K. The role of serotonin and melatonin in plant morphogenesis. Regulation of auxin-induced root organogenesis in in vitro-cultured explants of Hypericum perforatum L. In Vitro Cell Dev. Biol.-Plant 2001, 37, 786–793. [Google Scholar] [CrossRef]
- Pelagio-Flores, R.; Muñoz-Parra, E.; Ortiz-Castro, R.; Lopez-Bucio, J. Melatonin regulates Arabidopsis root system architecture likely acting independently of auxin signaling. J. Pineal Res. 2012, 53, 279–288. [Google Scholar] [CrossRef]
- Park, S.; Back, K. Melatonin promotes seminal root elongation and root growth in transgenic rice after germination. J. Pineal Res. 2012, 53, 385–389. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J. Pineal Res. 2009, 46, 58–63. [Google Scholar] [CrossRef]
- Ahmad, S.; Su, W.; Kamran, M.; Ahmad, I.; Meng, X.; Wu, X.; Javed, T.; Han, Q. Foliar application of melatonin delay leaf senescence in maize by improving the antioxidant defense system and enhancing photosynthetic capacity under semi-arid regions. Protoplasma 2020, 257, 1079–1092. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Zhang, Z.W.; Chen, Y.E.; Ding, C.B.; Yuan, S.; Reiter, R.J.; Yuan, M. Melatonin: A Potential Agent in Delaying Leaf Senescence. Crit. Rev. Plant Sci. 2021, 40, 1–22. [Google Scholar] [CrossRef]
- Wang, P.; Yin, L.; Liang, D.; Li, C.; Ma, F.; Yue, Z. Delayed senescence of apple leaves by exogenous melatonin treatment: Toward regulating the ascorbate-glutathione cycle. J. Pineal Res. 2012, 53, 11–20. [Google Scholar] [CrossRef]
- Byeon, Y.; Park, S.; Kim, Y.S.; Park, D.H.; Lee, S.; Back, K. Light-regulated melatonin biosynthesis in rice during the senescence process in detached leaves. J. Pineal Res. 2012, 53, 107–111. [Google Scholar] [CrossRef]
- Wang, P.; Sun, X.; Xie, Y.; Li, M.; Chen, W.; Zhang, S.; Liang, D.; Ma, F. Melatonin regulates proteomic changes during leaf senescence in Malus hupehensis. J. Pineal Res. 2014, 57, 291–307. [Google Scholar] [CrossRef]
- Zhai, R.; Liu, J.; Liu, F.; Zhao, Y.; Liu, L.; Fang, C.; Wang, H.; Li, X.; Wang, Z.; Ma, F.; et al. Melatonin limited ethylene production, softening and reduced physiology disorder in pear (Pyrus communis L.) fruit during senescence. Postharv. Biol. Technol. 2018, 139, 38–46. [Google Scholar] [CrossRef]
- Xu, L.; Yue, Q.; Xiang, G.; Bian, F.; Yao, Y. Melatonin promotes ripening of grape berry via increasing the levels of ABA, H2O2, and particularly ethylene. Hortic. Res. 2018, 5, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.; Liu, L.; Zhang, L.; Lv, H.; He, Q.; Guo, L.; Zhang, X.; He, H.; Ren, S.; Zhang, N.; et al. Melatonin promotes carotenoid biosynthesis in an ethylene-dependent manner in tomato fruits. Plant Sci. 2020, 298, 110580. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin in flowering, fruit set and fruit ripening. Plant Reprod. 2020, 33, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.J.; Alan, A.R.; Cao, J.; Saxena, P.K. Melatonin and serotonin in flowers and fruits of Datura metel L. J. Pineal Res. 2009, 47, 277–283. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Almoneafy, A.; Mahmoud, A.; Elkelish, A.; Arnao, M.B.; Li, L.; Ai, S. Melatonin and its protective role against biotic stress impacts on plants. Biomolecules 2020, 10, 54. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A new plant hormone and/or a plant master regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 2014, 19, 789–797. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a regulatory hub of plant hormone levels and action in stress situations. Plant Biol. 2021, 23, 7–19. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin in its relationship to plant hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef] [Green Version]
- Reiter, J.R.; Tan, X.D.; Rosales-Corral, S.; Galano, A.; Zhou, J.X.; Xu, B. Mitochondria: Central organelles for melatonin’s antioxidant and anti-aging actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef] [Green Version]
- Galano, A.; Reiter, R.J. Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J. Pineal Res. 2018, 65, 12514. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin and reactive oxygen and nitrogen species: A model for the plant redox network. Melatonin Res. 2019, 2, 152–168. [Google Scholar] [CrossRef]
- Li, D.; Wei, J.; Peng, Z.; Ma, W.; Yang, Q.; Song, Z.; Sun, W.; Yang, W.; Yuan, L.; Xu, X.; et al. Daily rhythms of phytomelatonin signaling modulate diurnal stomatal closure via regulating reactive oxygen species dynamics in Arabidopsis. J. Pineal Res. 2020, 68, e12640. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Mora-Poblete, F.; Arnao, M.B.; Naz, S.; Anwar, M.; Altaf, M.M.; Shahid, S.; Shakoor, A.; et al. Phytomelatonin: An overview of the importance and mediating functions of melatonin against environmental stresses. Physiol. Plant. 2021, 172, 820–846. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Is phytomelatonin a new plant hormone? Agronomy 2020, 10, 95. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, H.M.; Alamri, S.; Al-Khaishany, Y.M.; Khan, N.M.; Al-Amri, A.; Ali, M.H.; Alaraidh, A.I.; Alsahli, A.A. Exogenous melatonin counteracts NaCl-induced damage by regulating the antioxidant system, proline and carbohydrates metabolism in tomato seedlings. Int. J. Mol. Sci. 2019, 20, 353. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Jiang, C.; Ye, T.; Tan, D.; Reiter, R.J.; Zhang, H.; Liu, R.; Chan, Z. Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L). Pers.] by exogenous melatonin. J. Exp. Bot. 2015, 66, 681–694. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, M.H.; Alamri, S.; Nasir Khan, M.; Corpas, F.J.; Al-Amri, A.A.; Alsubaie, Q.D.; Ali, H.M.; Kalaji, H.M.; Ahmad, P. Melatonin and calcium function synergistically to promote the resilience through ROS metabolism under arsenic-induced stress. J. Hazard. Mater. 2020, 398, 122882. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, R.; Sun, Y.; Liu, Z.; Jin, W.; Sun, Y. The beneficial effects of exogenous melatonin on tomato fruit properties. Sci. Hortic. 2016, 207, 14–20. [Google Scholar] [CrossRef]
- Liu, J.; Yue, R.; Si, M.; Wu, M.; Cong, L.; Zhai, R.; Yang, C.; Wang, Z.; Ma, F.; Xu, L. Effects of exogenous application of melatonin on quality and sugar metabolism in Zaosu pear fruit. J. Plant Growth Regul. 2019, 38, 1161–1169. [Google Scholar] [CrossRef]
- Sun, Q.Q.; Zhang, N.; Wang, J.; Zhang, H.J.; Li, D.B.; Shi, J.; Li, R.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin promotes ripening and improves quality of tomato fruit during postharvest life. J. Exp. Bot. 2015, 66, 657–668. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Yang, H.; Tie, W.; Yan, Y.; Ding, Z.; Liu, Y.; Wu, C.; Wang, J.; Reiter, R.J.; Tan, D.X.; et al. Natural Variation in Banana Varieties Highlights the Role of Melatonin in Postharvest Ripening and Quality. J. Agric. Food Chem. 2017, 65, 9987–9994. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Tan, D.X.; Reiter, R.J.; Shi, H. Comparative metabolomic analysis highlights the involvement of sugars and glycerol in melatonin-mediated innate immunity against bacterial pathogen in Arabidopsis. Sci. Rep. 2015, 5, 15815. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Kamran, M.; Abbasi, G.H.; Saleem, M.H.; Ahmad, S.; Parveen, A.; Malik, Z.; Afzal, S.; Ahmar, S.; Dawar, K.M.; et al. Melatonin-Induced Salinity Tolerance by Ameliorating Osmotic and Oxidative Stress in the Seedlings of Two Tomato (Solanum lycopersicum L.) Cultivars. J. Plant Growth Regul. 2020. [Google Scholar] [CrossRef]
- Fan, J.; Hu, Z.; Xie, Y.; Chan, Z.; Chen, K.; Amombo, E.; Chen, L.; Fu, J. Alleviation of cold damage to photosystem II and metabolisms by melatonin in Bermudagrass. Front. Plant Sci. 2015, 6, 925. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Su, T.; Huo, L.; Wei, H.; Jiang, Y.; Xu, L.; Ma, F. Unveiling the mechanism of melatonin impacts on maize seedling growth: Sugar metabolism as a case. J. Pineal Res. 2015, 59, 255–266. [Google Scholar] [CrossRef]
- Erdal, S. Melatonin promotes plant growth by maintaining integration and coordination between carbon and nitrogen metabolisms. Plant Cell Rep. 2019, 38, 1001–1012. [Google Scholar] [CrossRef]
- Mir, A.R.; Siddiqui, H.; Alam, P.; Hayat, S. Melatonin modulates photosynthesis, redox status, and elemental composition to promote growth of Brassica juncea: A dose-dependent effect. Protoplasma 2020, 257, 1685–1700. [Google Scholar] [CrossRef]
- Zhong, L.; Lin, L.; Yang, L.; Liao, M.; Wang, X.; Wang, J.; Lv, X.; Deng, H.; Liang, D.; Xia, H.; et al. Exogenous melatonin promotes growth and sucrose metabolism of grape seedlings. PLoS ONE 2020, 15, e0232033. [Google Scholar] [CrossRef]
- Samanta, S.; Singh, A.; Banerjee, A.; Roychoudhury, A. Exogenous supplementation of melatonin alters representative organic acids and enzymes of respiratory cycle as well as sugar metabolism during arsenic stress in two contrasting indica rice cultivars. J. Biotech. 2020, 324, 220–232. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, C.; Wang, Z.; Sun, S.; Zhan, R.; Zhao, Y.; Ma, B.; Ma, F.; Li, M. Melatonin-Mediated Sugar Accumulation and Growth Inhibition in Apple Plants Involves Down-Regulation of Fructokinase 2 Expression and Activity. Front. Plant Sci. 2019, 10, 150. [Google Scholar] [CrossRef] [Green Version]
- Kobylinska, A.; Borek, S.; Posmyk, M.M. Melatonin redirects carbohydrates metabolism during sugar starvation in plant cells. J. Pineal Res. 2018, 64, e12466. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Wang, J.; Xu, D.; Tao, S.; Chong, S.; Yan, D.; Li, Z.; Yuan, H.; Zheng, B. Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Carya cathayensis plants. Sci. Total Environ. 2020, 713, 136675. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Elkelish, A.; Dafea, M.; Khan, M.; Arnao, M.B.; Abdelhamid, M.T.; El-Ezz, A.A.; Almoneafy, A.; Mahmoud, A.; Awad, M.; et al. Role of melatonin in plant tolerance to soil stressors: Salinity, pH and heavy metals. Molecules 2020, 25, 5359. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Mahmoud, A.; Arnao, M.B.; Sheteiwy, M.; Dafea, M.; Soltan, M.; Elkelish, A.; Hasanuzzaman, M.; Ai, S. Melatonin-induced water stress tolerance in plants: Recent advances. Antioxidants 2020, 9, 809. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Role of melatonin to enhance phytoremediation capacity. Appl. Sci. 2019, 9, 5293. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.L.; Fan, Z.Q.; Kuang, J.F.; Lu, W.J.; Reiter, R.J.; Lakshmanan, P.; Su, X.G.; Zhou, J.; Chen, J.Y.; Shan, W. Melatonin delays leaf senescence of Chinese flowering cabbage by suppressing ABFs-mediated abscisic acid biosynthesis and chlorophyll degradation. J. Pineal Res. 2019, 67, e12570. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Guo, J.; Reiter, R.J.; Wei, Y.; Shi, H. Melatonin synthesis enzymes interact with ascorbate peroxidase to protect against oxidative stress in cassava. J. Exp. Bot. 2020, 71, 5645–5655. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, H.; Chen, S.; Yu, D.; Reiter, R. Phytomelatonin: An emerging regulator of plant biotic stress resistance. Trends Plant Sci. 2020, 26, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, H.J.; Zhao, B.; Sun, Q.Q.; Cao, Y.Y.; Li, R.; Wu, X.X.; Weeda, S.; Li, L.; Ren, S.; et al. The RNA-seq approach to discriminate gene expression profiles in response to melatonin on cucumber lateral root formation. J. Pineal Res. 2014, 56, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Weeda, S.; Zhang, N.; Zhao, X.; Ndip, G.; Guo, Y.D.; Buck, G.; Fu, C.; Ren, S. Arabidopsis transcriptome analysis reveals key roles of melatonin in plant defense systems. PLoS ONE 2014, 9, e93462. [Google Scholar] [CrossRef] [Green Version]
- Khanna-Chopra, R.; Semwal, V.; Lakra, N.; Pareek, A. Proline: A key regulator conferring plant tolerance to salinity and drought. In Plant Tolerance to Environmental Stress. Role of Phytoprotectants; Hasanuzzaman, M., Fujita, M., Oku, H., Tofazzal Islam, M., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 59–80. [Google Scholar]
- Ilyas, M.; Nisar, M.; Khan, N.; Hazrat, A.; Khan, A.H.; Hayat, K.; Fahad, S.; Khan, A.; Ullah, A. Drought Tolerance Strategies in Plants: A Mechanistic Approach. J. Plant Growth Regul. 2021, 40, 926–944. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Y.; Yun, Z.; Hu, M.; Liu, J.; Jiang, Y.; Zhang, Z. Melatonin enhances cold tolerance by regulating energy and proline metabolism in litchi fruit. Foods 2020, 9, 454. [Google Scholar] [CrossRef] [Green Version]
- Aghdam, M.S.; Luo, Z.; Jannatizadeh, A.; Sheikh-Assadi, M.; Sharafi, Y.; Farmani, B.; Fard, J.R.; Razavi, F. Employing exogenous melatonin applying confers chilling tolerance in tomato fruits by upregulating ZAT2/6/12 giving rise to promoting endogenous polyamines, proline, and nitric oxide accumulation by triggering arginine pathway activity. Food Chem. 2019, 275, 549–556. [Google Scholar] [CrossRef]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Lal, M.K.; Naga, K.C.; Kumar, R.; Chourasia, K.N.; Subhash, S.; Kumar, D.; Sharma, S. Emerging roles of melatonin in mitigating abiotic and biotic stresses of horticultural crops. Sci. Hortic. 2020, 272, 109592. [Google Scholar] [CrossRef]
- Debnath, B.; Islam, W.; Li, M.; Sun, Y.; Lu, X.; Mitra, S.; Hussain, M.; Liu, S.; Qiu, D. Melatonin mediates enhancement of stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 1040. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, N.; Fatma, M.; Gautam, H.; Umar, S.; Sofo, A.; D’ippolito, I.; Khan, N.A. The crosstalk of melatonin and hydrogen sulfide determines photosynthetic performance by regulation of carbohydrate metabolism in wheat under heat stress. Plants 2021, 10, 1778. [Google Scholar] [CrossRef]
- Wu, X.; Ren, J.; Huang, X.; Zheng, X.; Tian, Y.; Shi, L.; Dong, P.; Li, Z. Melatonin: Biosynthesis, content, and function in horticultural plants and potential application. Sci. Hortic. 2021, 288, 110392. [Google Scholar] [CrossRef]
- Wang, S.Y.; Shi, X.C.; Wang, R.; Wang, H.L.; Liu, F.; Laborda, P. Melatonin in fruit production and postharvest preservation: A review. Food Chem. 2020, 320, 126642. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Nawaz, G.; Cao, Q.; Xu, T. Melatonin is a potential target for improving horticultural crop resistance to abiotic stress. Sci. Hortic. 2022, 291, 110560. [Google Scholar] [CrossRef]
- Ahmad, S.; Kamran, M.; Zhou, X.; Ahmad, I.; Meng, X.; Javed, T.; Iqbal, A.; Wang, G.; Su, W.; Wu, X.; et al. Melatonin improves the seed filling rate and endogenous hormonal mechanism in grains of summer maize. Physiol. Plant. 2021, 172, 1059–1072. [Google Scholar] [CrossRef]
- Ye, J.; Yang, W.; Li, Y.; Wang, S.; Yin, L.; Deng, X. Seed pre-soaking with melatonin improves wheat yield by delaying leaf senescence and promoting root development. Agronomy 2020, 10, 84. [Google Scholar] [CrossRef] [Green Version]

 , increased level of transcript expression and
, increased level of transcript expression and  , decreased level, or transcript expression (see abbreviations in text).
, decreased level, or transcript expression (see abbreviations in text).
 , increased level of transcript expression and
, increased level of transcript expression and  , decreased level, or transcript expression (see abbreviations in text).
, decreased level, or transcript expression (see abbreviations in text).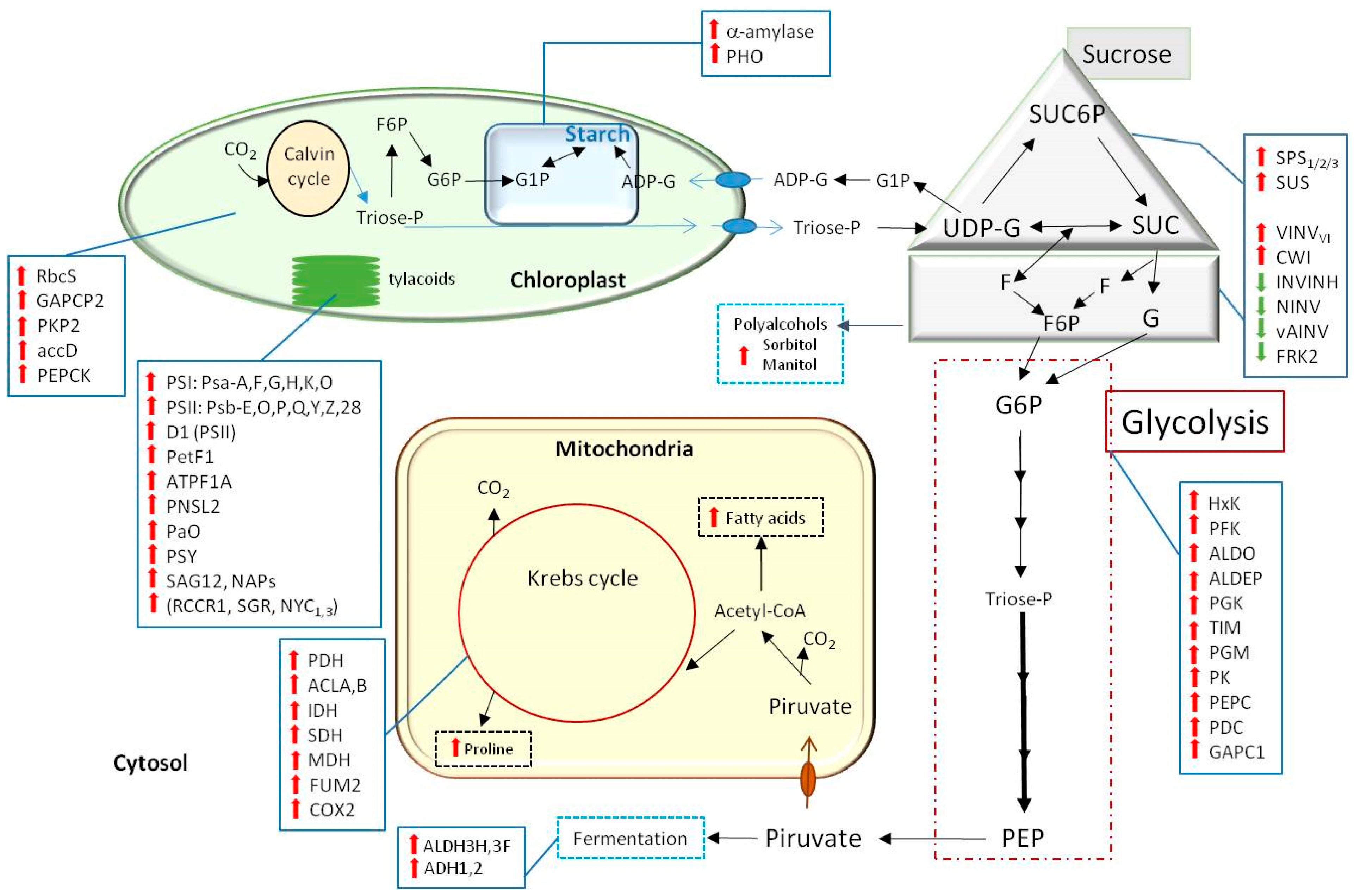
| Plant | Melatonin Treatment (µM) | Compound Level vs. Un-Treated | Response vs. Un-Treated | Reference |
|---|---|---|---|---|
| Prunus avium x Prunus cerasus (in vitro) | 0.05–10 | ↑ total carbohydrates | ↑ rooting ↑ plant biomass | [31] |
| Malus hupehensis tree | 100 | ↑ fructose, glucose, sucrose, starch ↑ sorbitol | ↑ photosynthesis ↓ senescence ↓ autophagy | [32] |
| Tomato fruits | 1–500 | ↑ soluble sugars | ↑ fruit ripening and quality | [71] |
| Tomato plants | 100 | ↑ glucose, sucrose, inositol ↓ fructose, galactose | ↑ photosynthesis ↑ plant biomass ↑ fruit number and size | [69] |
| 20–50 | ↑ soluble sugars ↑ ascorbate and GSH | ↑ photosynthesis ↑ plant growth ↑ NaCl tolerance | [66] [74] | |
| Soybean | 50 and 100 | ↑ carbohydrate metabolism, fatty acid biosynthesis, and ascorbate metabolism ↑ light reactions, Calvin cycle, carbohydrate, amino acid, fatty acid metabolism and Krebs cycle | ↑ germination, biomass ↑ photosynthesis ↑ cell division ↑ NaCl tolerance | [33] |
| Bermudagrass (Cynodon dactylon) | 4–100 | 54 metabolites, including amino acids, organic acids, sugars, and sugar alcohols ↑ photosyntesis, Calvin cycle and carbohydrate metabolism | ↑ NaCl tolerance ↑ cold tolerance ↑ drought tolerance | [67] |
| 100 | ↑ arabinose, mannose, gluco-pyranose, maltose and turanose | ↑ cold tolerance ↑ photosynthesis | [75] | |
| Maize | 10–100 | ↑ fructose, glucose, sucrose, starch and its biosynthesis genes | ↑ photosynthesis ↑ leaf and root growth | [76] |
| 10–1000 | ↑ total soluble sugars ↑ nitrogen compounds ↑ expressions of genes involved in C- and N- metabolisms | ↑ photosynthesis ↑ plant growth | [77] | |
| Banana fruits | 50–500 | ↑ total soluble sugars ↑ starch | ↑ fruit ripening and quality ↓ ethylene | [72] |
| Vicia faba | 50 | ↑ soluble sugars ↑ ascorbate and GSH | ↑ As tolerance ↑ photosynthesis ↑ plant growth | [68] |
| Brassica juncea | 10–50 | ↑ total soluble sugars ↑ reducing sugars | ↑ photosynthesis ↑ plant growth ↑ mineral nutrition | [78] |
| Grape plants | 50–200 | ↑ fructose, sucrose, starch, reducing sugars ↑ sucrose biosynthesis genes | ↑ photosynthesis ↑ plant growth ↑ mineral nutrition | [79] |
| Rice plants | 20 | ↑ fructose, sucrose, starch, reducing sugars ↑ sucrose biosynthesis genes | ↑ As tolerance ↑ Krebs cycle | [80] |
| Pear tree | 100 | ↑ total soluble sugars ↑ sucrose, starch, reducing sugars, sorbitol ↑ sucrose synthase, invertases | ↑ photosynthesis ↑ fruit size and quality | [70] |
| Malus domestica (plants) | 1000 | ↑ fructose, glucose, sucrose, sorbitol ↓ fructokinase gene | ↑ melatonin-induced sugar accumulation ↑ growth inhibition | [81] |
| Nicotiana tabacum (in vitro) | 0.2 | ↑ starch ↑ PEPCK and α-amylase genes | ↑ sugar starved ↑ gluconeogenesis | [82] |
| Chinese hickory (plants) | 100 | ↑ total soluble sugars, starch ↑ proline | ↑ drought tolerance ↑ photosynthesis, transpiration | [83] |
| Arabidopsis thaliana (Pseudomonas syringe infected) | 20 | ↑ fructose, glucose, melibose, sucrose, maltose, galatose, tagatofuranose and glycerol | ↑ bacterial innate immunity ↑ disease resistance | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnao, M.B.; Hernández-Ruiz, J.; Cano, A.; Reiter, R.J. Melatonin and Carbohydrate Metabolism in Plant Cells. Plants 2021, 10, 1917. https://doi.org/10.3390/plants10091917
Arnao MB, Hernández-Ruiz J, Cano A, Reiter RJ. Melatonin and Carbohydrate Metabolism in Plant Cells. Plants. 2021; 10(9):1917. https://doi.org/10.3390/plants10091917
Chicago/Turabian StyleArnao, Marino B., Josefa Hernández-Ruiz, Antonio Cano, and Russel J. Reiter. 2021. "Melatonin and Carbohydrate Metabolism in Plant Cells" Plants 10, no. 9: 1917. https://doi.org/10.3390/plants10091917
APA StyleArnao, M. B., Hernández-Ruiz, J., Cano, A., & Reiter, R. J. (2021). Melatonin and Carbohydrate Metabolism in Plant Cells. Plants, 10(9), 1917. https://doi.org/10.3390/plants10091917









