Burial Environment Drives Seed Mortality of Kochia (Bassia scoparia), Wild Oat (Avena fatua), and Volunteer Canola (Brassica napus) Irrespective of Crop Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Treatment Structure
2.2. Experimental Logistics
2.3. Data Collection
2.4. Statistical Analysis
3. Results
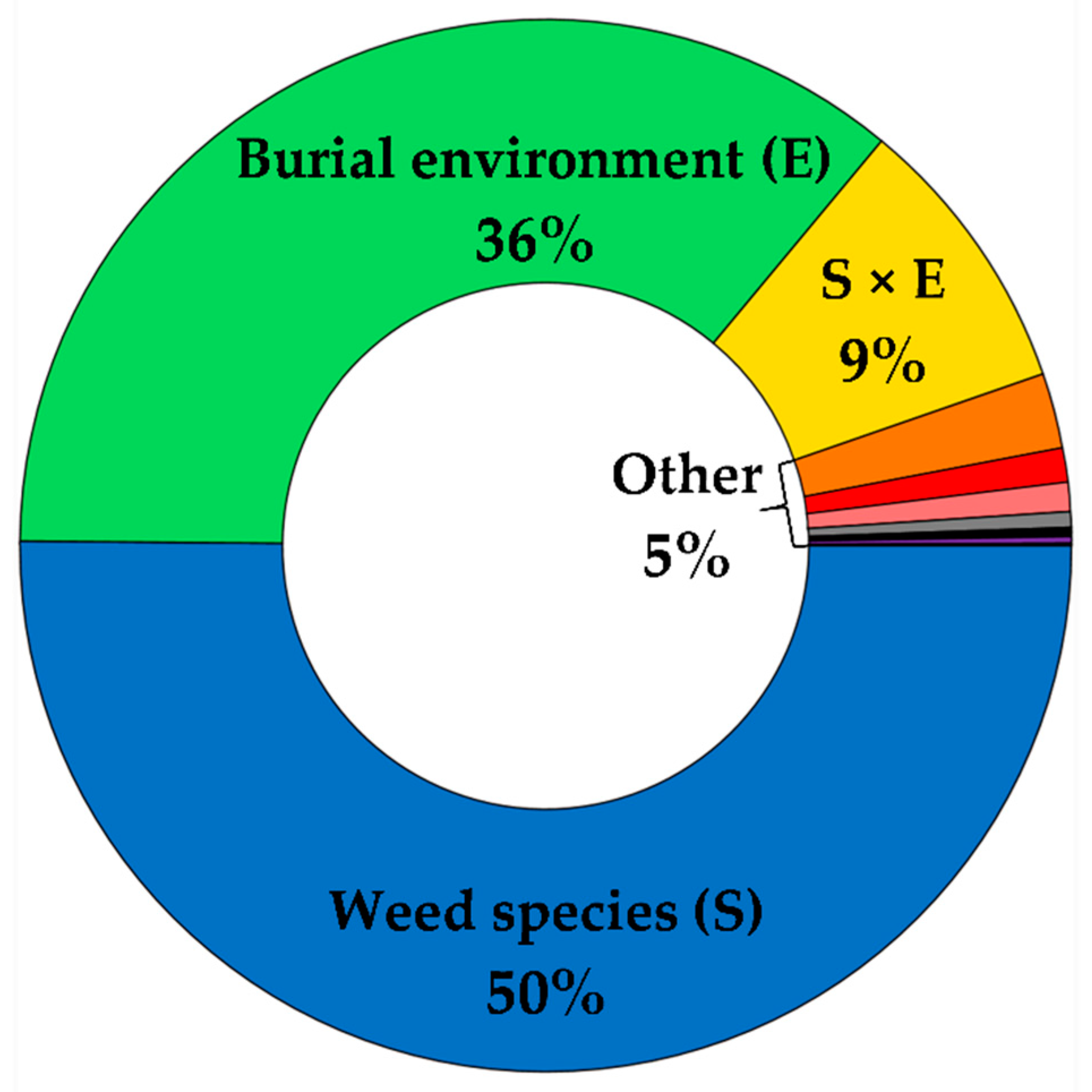
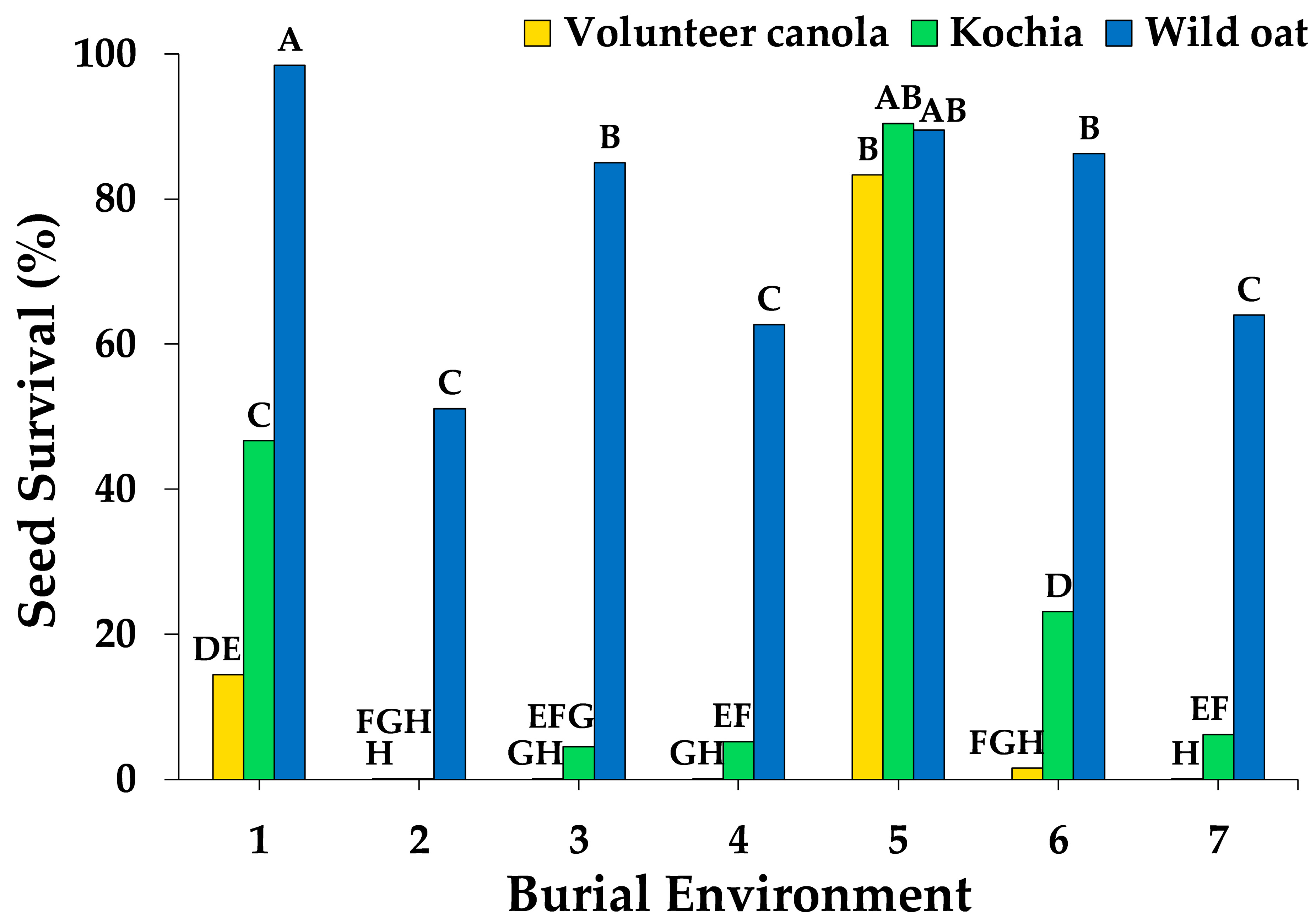
3.1. Weed Seed Survival
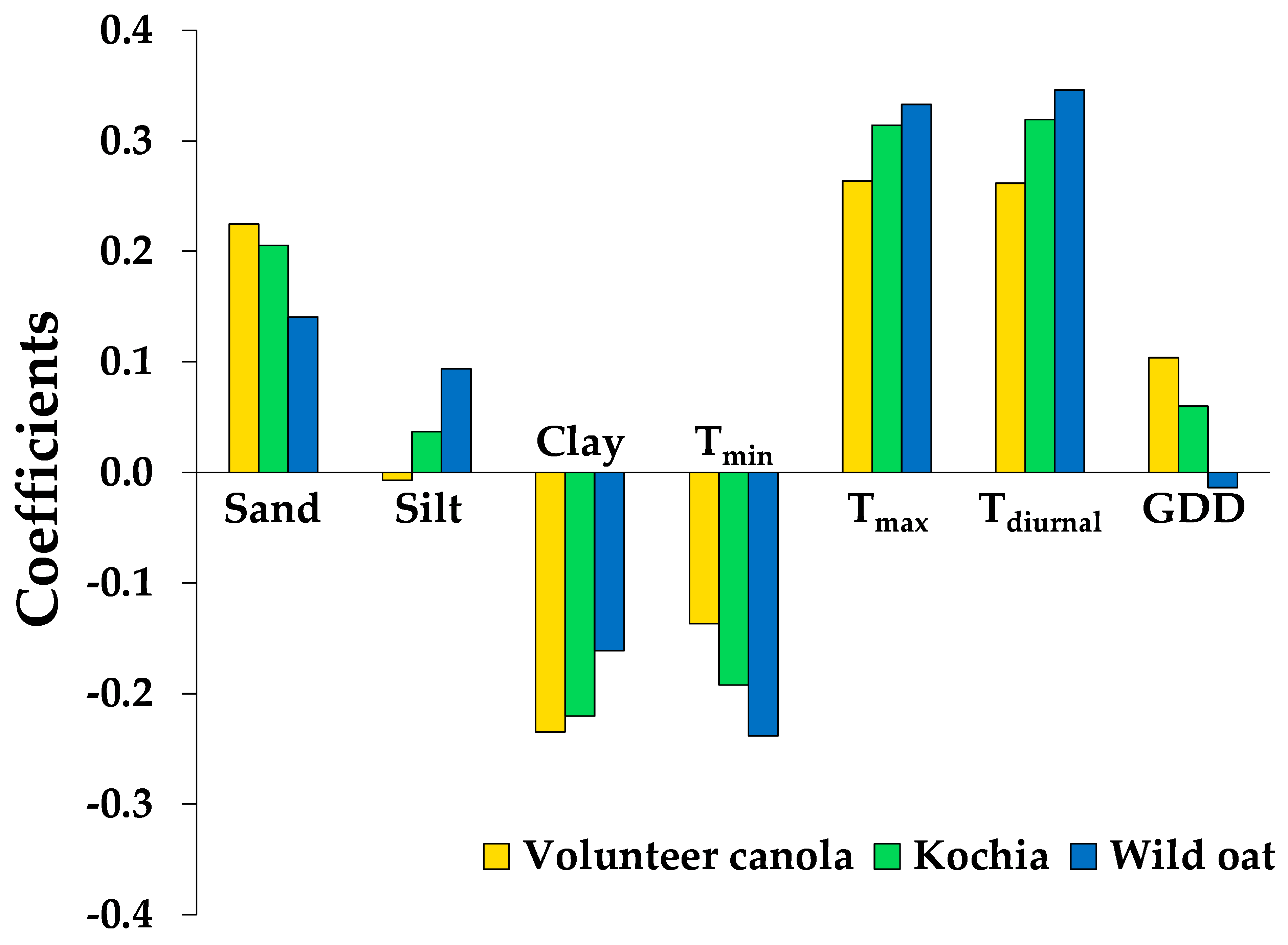
3.2. Contribution of Soil Edaphic Factors and Seed Microsite Characteristics
4. Discussion
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beckie, H.J.; Shirriff, S.W.; Leeson, J.Y.; Hall, L.M.; Harker, K.N.; Dokken-Bouchard, F.; Brenzil, C.A. Herbicide-resistant weeds in the Canadian prairies: 2012 to 2017. Weed Technol. 2020, 34, 461–474. [Google Scholar] [CrossRef]
- Leeson, J.Y.; Hall, L.M.; Neeser, C.; Tidemann, B.; Harker, K.N. Alberta survey of annual crops in 2017. In Weed Survey Series Publ. 19-1; Agriculture and Agri-Food Canada: Saskatoon, SK, Canada, 2019; p. 275. [Google Scholar]
- Beckie, H.J.; Weiss, R.M.; Leeson, J.Y.; Olfert, O.O. Range expansion of kochia (Kochia scoparia) in North America under a changing climate. In Climate Change and the Canadian Agricultural Environment. Topics in Canadian Weed Science; Ivany, J.A., Blackshaw, R.E., Eds.; Canadian Weed Science Society–Société canadienne de malherbologie: Pinawa, MB, Canada, 2012; Volume 8, pp. 31–44. [Google Scholar]
- Geddes, C.M.; Davis, A.S. The critical period for weed seed control: A proposed framework to limit weed seed return. Weed Res. 2021, 61, 282–287. [Google Scholar] [CrossRef]
- Mierau, A.; Kurtenbach, M.E.; Johnson, E.N.; Gulden, R.H.; Weber, J.D.; May, W.E.; Willenborg, C.J. Herbicide programs for control of glyphosate-resistant canola (Brassica napus) in glyphosate-resistant soybean. Weed Technol. 2020, 34, 540–546. [Google Scholar] [CrossRef]
- Torbiak, A.T.; Blackshaw, R.E.; Brandt, R.N.; Hall, L.M.; Hamman, B.; Geddes, C.M. Herbicide mixtures control glyphosate-resistant kochia (Bassia scoparia) in chemical fallow, but their longevity warrants careful stewardship. Can. J. Plant Sci. 2021, 101, 188–198. [Google Scholar] [CrossRef]
- Torbiak, A.T.; Blackshaw, R.E.; Brandt, R.N.; Hamman, B.; Geddes, C.M. Herbicide strategies for managing glyphosate-resistant and -susceptible kochia (Bassia scoparia) in spring wheat. Can. J. Plant Sci. 2021, 101, 607–621. [Google Scholar] [CrossRef]
- Heap, I.M. The International Herbicide Resistant Weed Database. Available online: http://www.weedscience.org (accessed on 3 September 2021).
- Beckie, H.J.; Hall, L.M.; Shirriff, S.W.; Martin, E.; Leeson, J.Y. Triple-resistant kochia [Kochia scoparia (L.) Schrad.] in Alberta. Can. J. Plant Sci. 2019, 99, 281–285. [Google Scholar] [CrossRef]
- Geddes, C.M.; Ostendorf, T.E.; Owen, M.L.; Leeson, J.Y.; Sharpe, S.M.; Shirriff, S.W.; Beckie, H.J. Fluroxypyr-resistant kochia [Bassia scoparia (L.) A.J. Scott] confirmed in Alberta. Can. J. Plant Sci. 2021, in press. [Google Scholar] [CrossRef]
- Geddes, C.M.; Owen, N.L.; Ostendorf, T.E.; Leeson, J.Y.; Sharpe, S.M.; Shirriff, S.W.; Beckie, H.J. Herbicide diagnostics reveal multiple patterns of synthetic auxin resistance in kochia (Bassia scoparia). Weed Technol. 2021, in press. [Google Scholar] [CrossRef]
- Beckie, H.J.; Harker, K.N.; Hall, L.M.; Warwick, S.I.; Légère, A.; Sikkema, P.H.; Clayton, G.W.; Thomas, A.G.; Leeson, J.Y.; Séguin-Swartz, G.; et al. A decade of herbicide-resistant crops in Canada. Can. J. Plant Sci. 2006, 86, 1243–1264. [Google Scholar] [CrossRef]
- Geddes, C.M. Life Cycle Management of Volunteer Canola (Brassica napus L.) in Western Canada. Ph.D. Thesis, University of Manitoba, Winnipeg, MB, Canada, 2017. [Google Scholar]
- Geddes, C.M.; Gulden, R.H. Early autumn soil disturbance decreases persistence of volunteer summer-annual oilseed rape (Brassica napus). Weed Res. 2017, 57, 182–192. [Google Scholar] [CrossRef]
- Geddes, C.M.; Gulden, R.H. Candidate tools for integrated weed management in soybean at the northern frontier of production. Weed Sci. 2018, 66, 662–672. [Google Scholar] [CrossRef]
- Liebman, M.; Gallandt, E.R. Many little hammers: Ecological management of crop-weed interactions. In Ecology in Agriculture; Jackson, L.E., Ed.; Academic Press: San Diego, CA, USA, 1997; pp. 291–343. [Google Scholar]
- Cousens, R.; Mortimer, M. Dynamics of Weed Populations; Cambridge University Press: Cambridge, UK, 1995; p. 332. [Google Scholar]
- Davis, A.S. When does it make sense to target the weed seed bank? Weed Sci. 2006, 54, 558–565. [Google Scholar] [CrossRef]
- Beckie, H.J.; Blackshaw, R.E.; Leeson, J.Y.; Stahlman, P.W.; Gaines, T.A.; Johnson, E.N. Seedbank persistence, germination and early growth of glyphosate-resistant Kochia scoparia. Weed Res. 2018, 58, 177–187. [Google Scholar] [CrossRef]
- Dille, J.A.; Stahlman, P.E.; Fu, J.; Geier, P.W.; Riffel, J.D.; Currie, R.S.; Wilson, R.G.; Sbatella, G.M.; Westra, P.; Kniss, A.R.; et al. Kochia (Kochia scoparia) emergence profiles and seed persistence across the central great plains. Weed Sci. 2017, 65, 614–625. [Google Scholar] [CrossRef]
- Gulden, R.H.; Shirtliffe, S.J.; Thomas, A.G. Harvest losses of canola (Brassia napus) cause large seedbank inputs. Weed Sci. 2003, 51, 83–86. [Google Scholar] [CrossRef]
- Gulden, R.H.; Shirtliffe, S.J.; Thomas, A.G. Secondary seed dormancy prolongs persistence of volunteer canola in western Canada. Weed Sci. 2003, 51, 904–913. [Google Scholar] [CrossRef]
- Gulden, R.H.; Thomas, A.G.; Shirtliffe, S.J. Relative contribution of genotype, seed size and environment to secondary seed dormancy potential in Canadian spring oilseed rape (Brassica napus). Weed Res. 2004, 44, 97–106. [Google Scholar] [CrossRef]
- Gulden, R.H.; Thomas, A.G.; Shirtliffe, S.J. Secondary dormancy, temperature, and burial depth regulate seedbank dynamics in canola. Weed Sci. 2004, 52, 382–388. [Google Scholar] [CrossRef]
- Van Acker, R.C. Weed biology serves practical weed management. Weed Res. 2009, 49, 1–5. [Google Scholar] [CrossRef]
- Osipitan, O.A.; Dille, J.A.; Bagavathiannan, M.V.; Knezevic, S.Z. Modelling population dynamics of kochia (Bassia scoparia) in response to diverse weed control options. Weed Sci. 2018, 67, 57–67. [Google Scholar] [CrossRef]
- Tidemann, B.D.; Hall, L.M.; Harker, K.N.; Alexander, B.C.S. Identifying critical control points in the wild oat (Avena fatua) life cycle and the potential effects of harvest weed-seed control. Weed Sci. 2016, 64, 463–473. [Google Scholar] [CrossRef]
- Harper, J.L. Population Biology of Plants, 4th ed.; Academic Press: New York, NY, USA, 1977; p. 306. [Google Scholar]
- Harper, J.L.; Williams, J.T.; Sager, G.R. The behavior of seeds in the soil. I. The heterogeneity of soil surfaces and its role in determining the establishment of plants from seed. J. Ecol. 1965, 53, 273–286. [Google Scholar] [CrossRef]
- Weston, L.A.; Duke, S.O. Weed and crop allelopathy. Crit. Rev. Plant Sci. 2003, 22, 367–389. [Google Scholar] [CrossRef]
- Geddes, C.M.; Cavalieri, A.; Daayf, F.; Gulden, R.H. The allelopathic potential of hairy vetch (Vicia villosa Roth.) mulch. Am. J. Plant Sci. 2015, 6, 2651–2663. [Google Scholar] [CrossRef][Green Version]
- Davis, A.S.; Cardina, J.; Forcella, F.; Johnson, G.A.; Kegode, G.; Lindquist, J.L.; Luschei, E.E.C.; Renner, K.A.; Sprague, C.L.; Williams, M.M., II. Environmental factors affecting seed persistence of annual weeds across the U.S. corn belt. Weed Sci. 2005, 53, 860–868. [Google Scholar] [CrossRef]
- Schwinghamer, T.D.; Van Acker, R.C. Emergence timing and persistence of kochia (Kochia scoparia). Weed Sci. 2008, 56, 37–41. [Google Scholar] [CrossRef]
- Littell, R.C.; Milken, G.A.; Stroup, W.W.; Wolfinger, R.R.; Schabenberger, O. SAS for Mixed Models, 2nd ed.; SAS Institute Inc.: Cary, NC, USA, 2006; p. 834. [Google Scholar]
- SAS Institute Inc. Chapter 74: The PLS procedure. In SAS/STAT® 13.1 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2013; pp. 6248–6298. [Google Scholar]
- Sawatsky, M.L.; Clyde, M.; Meek, F. Partial least squares regression in the social sciences. Quant. Methods Psychol. 2015, 11, 52–62. [Google Scholar] [CrossRef]
- Wold, H. Soft modeling by latent variables: The nonlinear iterative partial least squares approach. In Perspectives in Probability and Statistics, Papers in Honour of M. S. Bartlett; Gani, J., Ed.; Academic Press: London, UK, 1975; pp. 520–540. [Google Scholar]
- Pakeman, R.J.; Small, J.L.; Torvell, L. Edaphic factors influence the longevity of seeds in the soil. Plant Ecol. 2012, 213, 57–65. [Google Scholar] [CrossRef]
- Schutte, B.J.; Davis, A.S.; Renner, K.A.; Cardina, C. Maternal and burial environment effects on seed mortality of velvetleaf (Abutilon theophrasti) and giant foxtail (Setaria faberi). Weed Sci. 2008, 56, 834–840. [Google Scholar] [CrossRef]
- Gruber, S.; Weber, E.Q.; Claupein, W. Which soils are comfortable for oilseed rape seeds (Brassica napus) to survive? Plant Soil Environ. 2014, 60, 280–284. [Google Scholar] [CrossRef]
- Long, R.L.; Steadman, K.J.; Panetta, F.D.; Adkins, S.E. Soil type does not affect seed ageing when soil water potential and temperature are controlled. Plant Soil 2009, 320, 131–140. [Google Scholar] [CrossRef]
- Narwal, S.; Sindel, B.M.; Jessop, R.S. Dormancy and longevity of annual ryegrass (Lolium rigidum) as affected by soil type, depth, rainfall, and duration of burial. Plant Soil 2008, 310, 225–234. [Google Scholar] [CrossRef]
- Mickelson, J.A.; Grey, W.E. Effect of soil water content on wild oat (Avena fatua) seed mortality and seedling emergence. Weed Sci. 2006, 54, 255–262. [Google Scholar] [CrossRef]
- Chee-Sanford, J.C.; Williams, M.M., II; Davis, A.S.; Sims, G.K. Do microorganisms influence seed-bank dynamics? Weed Sci. 2006, 54, 575–587. [Google Scholar] [CrossRef]
- Davis, A.S.; Anderson, K.I.; Hallett, S.G.; Renner, K.A. Weed seed mortality in soils with contrasting agricultural management histories. Weed Sci. 2006, 54, 281–297. [Google Scholar] [CrossRef]
- Davis, A.S. Nitrogen fertilizer and crop residue effects on seed mortality and germination of eight annual weed species. Weed Sci. 2007, 55, 123–128. [Google Scholar] [CrossRef]
- Gruber, S.; Bühler, A.; Möhring, J.; Claupein, W. Sleepers in the soil–Vertical distribution by tillage and long-term survival of oilseed rape seeds compared with plastic pellets. Eur. J. Agron. 2010, 33, 81–88. [Google Scholar] [CrossRef]
- Miller, S.D.; Nalewaja, J.D. Influence of burial depth on wild oats (Avena fatua) seed longevity. Weed Technol. 1990, 4, 514–517. [Google Scholar] [CrossRef]
- Zorner, P.S.; Zimdahl, R.L.; Schweizer, E.E. Effect of depth and duration of seed burial on kochia (Kochia scoparia). Weed Sci. 1984, 32, 602–607. [Google Scholar] [CrossRef]
- Zorner, P.S.; Zimdahl, R.L.; Schweizer, E.E. Sources of viable seed loss in buried dormant and non-dormant populations of wild oat (Avena fatua L.) seed in Colorado. Weed Res. 1984, 24, 143–150. [Google Scholar] [CrossRef]
| Soil Collection Location | Experiment Location | ||||||
|---|---|---|---|---|---|---|---|
| Burial Environ. | Latitude | Longitude | Soil Texture | Previous Vegetation | City, Province | Growth Environ. b | Direct Sunlight |
| ° N | ° W | h day−1 | |||||
| 1 | 49.70 | −112.69 | CL | Wheat (Triticum aestivum L.) | Lethbridge, AB | Outdoors | 7 |
| 2 | 49.69 | −112.76 | CL | Wheat (Triticum aestivum L.) | Lethbridge, AB | Indoors | 3 |
| 3 | 49.69 | −112.76 | CL | Canola (Brassica napus L.) | Lethbridge, AB | Outdoors | 4 |
| 4 | 49.33 | −123.05 | SL | Buttercup (Ranunculus spp.) | Vancouver, BC | Outdoors | 8 |
| 5 | 50.74 | −119.24 | SL | Alfalfa (Medicago sativa L.) | Salmon Arm, BC | Outdoors | 7 |
| 6 | 54.17 | −113.00 | CL | Wheat (Triticum aestivum L.) | Redwater, AB | Outdoors | 8 |
| 7 | 49.70 | −112.70 | L | Soybean [Glycine max (L.) Merr.] | Lethbridge, AB | Outdoors | 7 |
| Burial Environ. | Sand | Silt | Clay | OM | NO3-N | P | K | SO4-S | pH | Soluble Salts | Tmin | Tmax | Tavg | Tdiurnal | GDD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | kg ha−1 | dS m−1 | °C | ||||||||||||
| 1 | 36 | 34 | 30 | 3.7 | 7 | 38 | 1310 | 2 | 7.4 | 0.4 | 11.3 | 29.0 | 19.0 | 17.8 | 1263 |
| 2 | 37 | 29 | 34 | 3.8 | 16 | 124 | 1128 | 6 | 7.6 | 0.4 | 17.9 | 23.7 | 20.4 | 5.8 | 1483 |
| 3 | 34 | 35 | 31 | 3.7 | 20 | 124 | 1501 | 6 | 7.6 | 0.5 | 11.3 | 31.7 | 19.7 | 20.4 | 1053 |
| 4 | 62 | 30 | 8 | 10.9 | 7 | 7 | 166 | 23 | 5.6 | 0.2 | 17.5 | 25.1 | 20.7 | 7.6 | 1471 |
| 5 | 62 | 30 | 8 | 2.8 | 33 | 157 | 355 | 14 | 6.5 | 0.2 | 15.1 | 30.1 | 20.3 | 15.0 | 1476 |
| 6 | 41 | 31 | 28 | 7.6 | 34 | 160 | 590 | 54 | 7.3 | 0.6 | 12.5 | 31.9 | 19.9 | 19.4 | 1394 |
| 7 | 40 | 34 | 26 | 3.3 | 12 | 15 | 858 | 4 | 7.5 | 0.3 | 13.7 | 24.3 | 18.5 | 10.5 | 1268 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Her Majesty the Queen in Right of Canada. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geddes, C.M. Burial Environment Drives Seed Mortality of Kochia (Bassia scoparia), Wild Oat (Avena fatua), and Volunteer Canola (Brassica napus) Irrespective of Crop Species. Plants 2021, 10, 1961. https://doi.org/10.3390/plants10091961
Geddes CM. Burial Environment Drives Seed Mortality of Kochia (Bassia scoparia), Wild Oat (Avena fatua), and Volunteer Canola (Brassica napus) Irrespective of Crop Species. Plants. 2021; 10(9):1961. https://doi.org/10.3390/plants10091961
Chicago/Turabian StyleGeddes, Charles M. 2021. "Burial Environment Drives Seed Mortality of Kochia (Bassia scoparia), Wild Oat (Avena fatua), and Volunteer Canola (Brassica napus) Irrespective of Crop Species" Plants 10, no. 9: 1961. https://doi.org/10.3390/plants10091961
APA StyleGeddes, C. M. (2021). Burial Environment Drives Seed Mortality of Kochia (Bassia scoparia), Wild Oat (Avena fatua), and Volunteer Canola (Brassica napus) Irrespective of Crop Species. Plants, 10(9), 1961. https://doi.org/10.3390/plants10091961






