Alleviation of Lead Stress on Sage Plant by 5-Aminolevulinic Acid (ALA)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Germination of Salvia officinalis Seeds
2.3. Treatment of Salvia officinalis Seedlings with Pb(NO3)2
2.4. Preparation of Leaf Extract of Salvia officinalis
2.5. Determination of Total Soluble Protein (TSP) Content
2.6. Determination of MDA Content
2.7. Determination of Hydrogen Peroxide Content
2.8. Determination of Antioxidant Enzyme Activity
2.8.1. Preparation of Enzymes Extract
2.8.2. Assay of Enzymes
Assay of Ascorbate Peroxidase (APX, EC: 1.11.1.11)
Assay of Glutathione Peroxidase (GPX, EC: 1.11.1.9)
Assay of Superoxide Dismutase (SOD, EC: 1.15.1.1)
Assay of Glutathione Reductase (GR, EC: 1.6.4.2)
2.9. Statistical Analysis
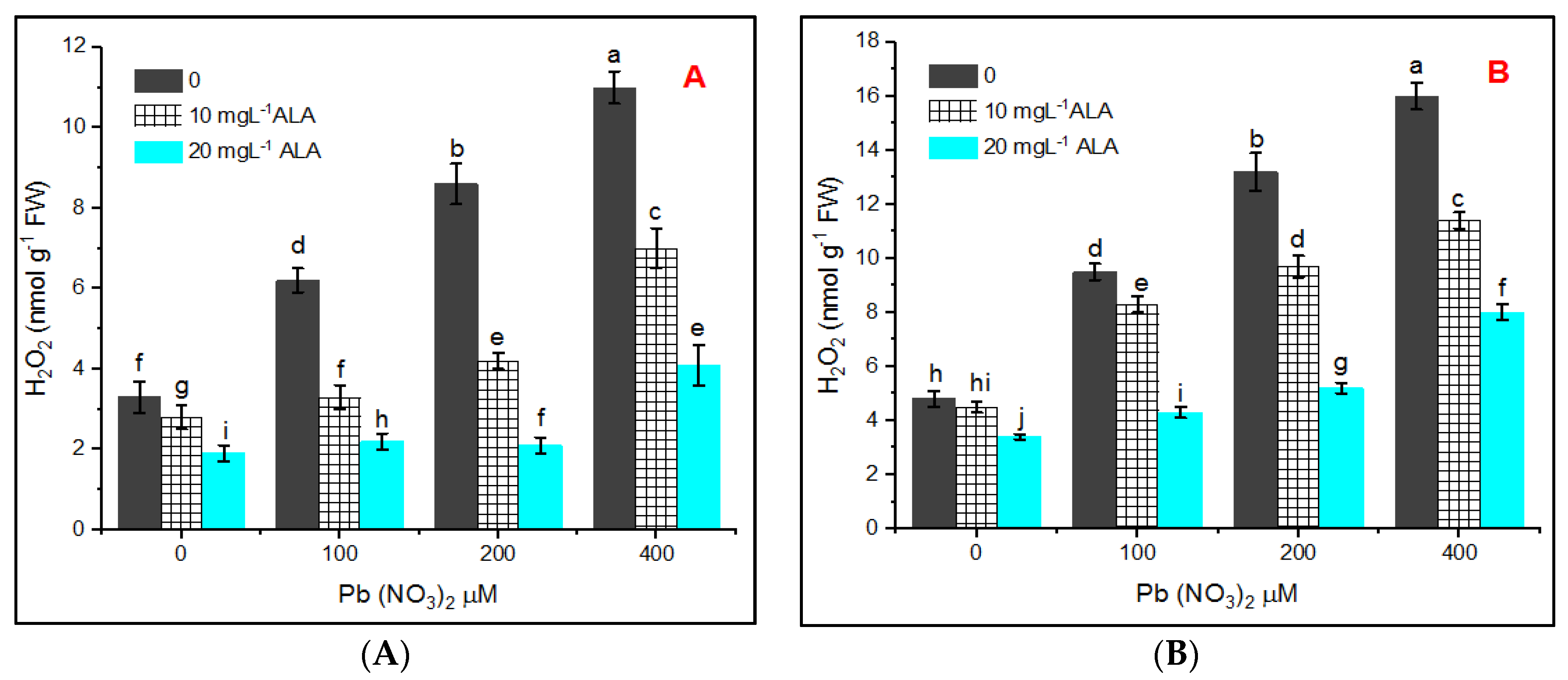
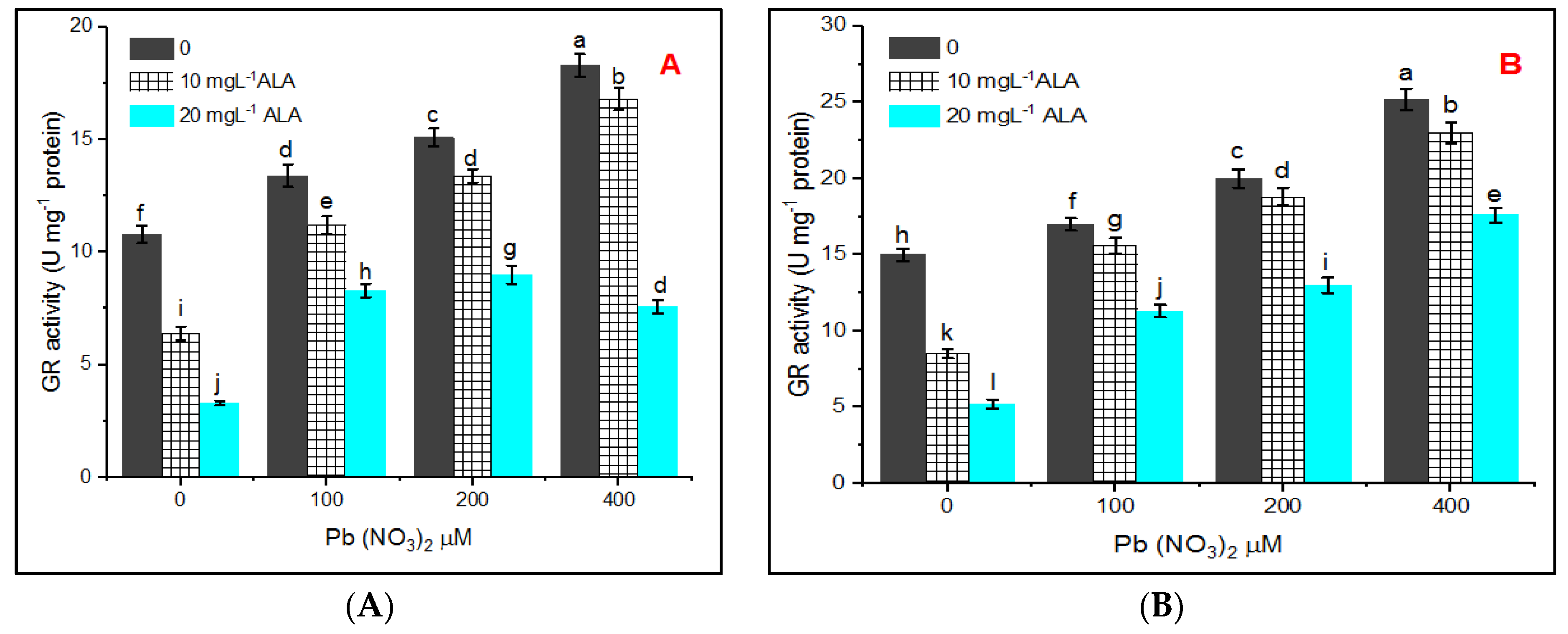
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Sai, B.; Ali, S.; Farooq, M.A.; Iqbal, N.; Abbas, F.; Ahmad, M. Alleviation of lead toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes suppressed lead uptake and oxidative stress in cotton. J. Biorem. Biodeg. 2013, 4, 187. [Google Scholar] [CrossRef]
- Demarco, C.F.; Afonso, T.F.; Pieniz, S.; Quadro, M.S.; Camargo, F.A.; Andreazza, R. In situ phytoremediation characterization of heavy metals promoted by Hydrocotyle ranunculoides at Santa Bárbara stream, an anthropogenic polluted site in southern of Brazil. Environ. Sci. Pollut. Res. 2018, 25, 28312–28321. [Google Scholar] [CrossRef] [PubMed]
- Fazekašová, D.; Fazekaš, J. Soil quality and heavy metal pollution assessment of iron ore mines in Nizna Slana (Slovakia). Sustainability 2020, 12, 2549. [Google Scholar] [CrossRef] [Green Version]
- Bączek-Kwinta, R.; Antonkiewicz, J.; Łopata-Stasiak, A.; Kępka, W. Smoke compounds aggravate stress inflicted on Brassica seedlings by unfavourable soil conditions. Photosynthetica 2019, 57, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Giannakoula, A.; Therios, I.; Chatzissavvidis, C. Effect of lead and copper on photosynthetic apparatus in citrus (Citrus aurantium L.) plants. The role of antioxidants in oxidative damage as a response to heavy metal stress. Plants 2021, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Piechalak, A.; Tomaszewska, B.; Barałkiewicz, D. Enhancing phytoremediative ability of Pisum sativum by EDTA application. Phytochemistry 2003, 64, 1239–1251. [Google Scholar] [CrossRef]
- Navabpour, S.; Yamchi, A.; Bagherikia, S.; Kafi, H. Lead-induced oxidative stress and role of antioxidant defense in wheat (Triticum aestivum L.). Physiol. Molec. Biol. Plants 2020, 26, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Dey, U.; Mondal, N.K. Ultrastructural deformation of plant cell under heavy metal stress in Gram seedlings. Cogent Environ. Sci. 2016, 2, 1196472. [Google Scholar] [CrossRef]
- Islam, E.; Liu, D.; Li, T.; Yang, X.; Jin, X.; Mahmood, Q.; Tian, S.; Li, J. Effect of Pb toxicity on leaf growth, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. J. Hazard. Mater. 2008, 154, 914–926. [Google Scholar] [CrossRef]
- Shahid, M.; Khalid, S.; Abbas, G.; Shahid, N.; Nadeem, M.; Sabir, M.; Aslam, M.; Dumat, C. Heavy metal stress and crop productivity. In Crop Production and Global Environmental Issues; Hakeem, K., Ed.; Springer Int.: Cham, Switzerland, 2015. [Google Scholar]
- Tao, L.; Guo, M.; Ren, J. Effects of cadmium on seed germination, coleoptile growth, and root elongation of six pulses. Pol. J. Environ. Stud. 2015, 24, 295–299. [Google Scholar]
- Mishra, K.K.; Rai, U.N.; Prakash, O. Bio concentration and phytotoxicity of Cd in Eichhornia crassipes. Environ. Monit. Assess 2007, 130, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, L.; Li, J.; Chen, M.; An, R. Comparative study on the bioaccumulation of lead, cadmium and nickel and their toxic effects on the growth and enzyme defense strategies of a heavy metal accumulator, Hydrilla verticillata (L.f.) Royle. Environ. Sci. Pollut. Res. 2020, 27, 9853–9865. [Google Scholar] [CrossRef]
- Otsuka, S.; Matsumoto, K.; Nakajima, M.; Tanaka, T.; Ogura, S.I. Oxygen availability for porphyrin biosynthesis enzymes determines the production of protoporphyrin IX (PpIX) during hypoxia. PLoS ONE 2015, 10, e0146026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habiba, U.; Ali, S.; Rizwan, M.; Hussain, M.; Hussain, A.; Alam, P.; Alqarawi, A.; Hashem, A.; AbdAllah, E. The ameliorative role of 5-aminolevulinic acid (ALA) under Cr stress in two maize cultivars showing differential sensitivity to Cr stress tolerance. J. Plant Growth Regul. 2018, 38, 788–798. [Google Scholar] [CrossRef]
- Gill, R.A.; Ali, B.; Islam, F.; Farooq, M.A.; Gill, M.B.; Mwamba, T.M.; Zhou, W. Physiological and molecular analyses of black and yellow seeded Brassica napus regulated by 5-aminolivulinic acid under chromium stress. Plant Physiol. Biochem. 2015, 94, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Herman, S.; Marco, G.; Cecilia, B.; Alfonso, V.; Luis, M.; Cristin, V.; Sebastin, P.; Sebastin, A. Effect of water availability on growth, water use efficiency and omega 3 (ALA) content in two phenotypes of chia (Salvia hispanica L.) established in the arid Mediterranean zone of Chile. Agric. Water Manag. 2016, 173, 67–75. [Google Scholar] [CrossRef]
- Naeem, M.S.; Rasheed, M.; Liu, D.; Jin, Z.L.; Ming, D.F.; Yoneyama, K.; Takeuchi, Y.; Zhou, W.J. 5-Aminolevulinic acid ameliorates salinity-induced metabolic, water-related and biochemical changes in Brassica napus L. Acta Physiol Plant 2011, 33, 517–528. [Google Scholar] [CrossRef]
- Tian, T.; Ali, B.; Qin, Y.; Malik, Z.; Rafaqat, A.; Ali, S.; Zhou, W. Alleviation of lead toxicity by 5-aminolevulinic acid is related to elevated growth, photosynthesis, and suppressed ultra-structural damages in oilseed rape. BioMed Res. Intern. 2014, 2014, 1–11. [Google Scholar]
- Zhang, W.F.; Zhang, F.; Raziuddin, R.; Gong, H.J.; Yang, Z.M.; Lu, L.; Ye, Q.F.; Zhou, W.J. Effects of 5-aminolevulinic acid on oilseed rape seedling growth under herbicide toxicity stress. J. Plant Growth Regul. 2008, 27, 159–169. [Google Scholar] [CrossRef]
- Bisset, N.G.; Wichtl, M. Herbal Drugs and Phytopharmaceuticals: A Handbook for Practice on a Scientific Basis with Reference to German Commission E Monographs, 2nd ed.; CRC Press: Boca Raton, FI, USA, 2001; pp. 440–443. [Google Scholar]
- Miura, K.; Kikuzaki, H.; Nakatani, N. Apianane terpenoids from Salvia officinalis. Phytochemistry 2001, 58, 1171–1175. [Google Scholar] [CrossRef]
- Kintzios, S.E. Medicinal and aromatic plants—Industrial profiles. In Sage, the Genus Salvia; Harwood Academic Publishers: Amsterdam, The Netherlands, 2000; Volume 14. [Google Scholar]
- Garcia, C.S.C.; Menti, C.; Lambert, A.P.F. Pharmacological perspectives from Brazilian Salvia officinalis (Lamiaceae): Antioxidant, and antitumor in mammalian cells. An. Acad. Bras. Ciências 2016, 88, 281–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cegiełka, A.; Hać-Szymańczuk, E.; Piwowarek, K.; Dasiewicz, K.; Słowiński, M.; Wrońska, K. The use of bioactive properties of sage preparations to improve the storage stability of low-pressure mechanically separated meat from chickens. Poult. Sci. 2019, 98, 5045–5053. [Google Scholar] [CrossRef]
- Ben Taarit, M.; Msaada, K.; Hosni, K.; Hammami, M.; Kchouk, M.E.; Marzouk, B. Plant growth, essential oil yield and composition of sage (Salvia officinalis L.) fruits cultivated under salt stress conditions. Ind. Crop. Prod. 2009, 30, 333–337. [Google Scholar] [CrossRef]
- El-Shora, H.M.; El-Farrash, A.H.; Kamal, H.; Abdelrazek, A.L. Enhancement of antioxidant defense system by UV- Radiation in Fenugreek as a medical plant. Int. J. Adv. Res. 2015, 3, 529–535. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for the growing plants without soil. Calif. Agric. Exp. Stn. 1950, 347, 1–32. [Google Scholar]
- Li, Y. Effect of salt stress on seed germination and seedling growth of three salinity plants. Pakistan J Biolog. Sci. 2008, 11, 1268–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, N.M. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–190. [Google Scholar] [CrossRef]
- Alexieve, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Abd El-Gawad, A.M.; El-Shora, H.M. Assessment of allelopathic potential of Hyoscyamus muticus L. on antioxidant system and nucleic acids of purslane. Fresen. Environ. Bull 2017, 36, 2147–2155. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.B.; Hafeman, D.G.; Hoekstra, W.G. Selenium: Biochemical role as a component of glutathione peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Klapheck, S.; Zimmer, I.; Cosse, H. Scavenging of hydrogen peroxide in the endosperm of Ricinus communis by ascorbate peroxidase. Plant Cell Physiol. 1990, 31, 1005–1013. [Google Scholar]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Maestri, E.; Marmiroli, M.; Visioli, G.; Marmiroli, N. Metal tolerance and hyper accumulation: Costs and trade-offs between traits and environment. Environ. Exp. Bot. 2010, 68, 1–13. [Google Scholar] [CrossRef]
- Moustakas, M.; Lanaras, T.; Symeonidis, L.; Karataglis, S. Grow and some photosynthetic characteristics on field grown Avena Sativa under copper and lead stress. Photosynthetica 1994, 30, 389–396. [Google Scholar]
- Akram, N.A.; Ashraf, M.; Al-Qurainy, F. Aminolevulinic acid-induced regulation in some key physiological attributes and activities of antioxidant enzymes in sunflower (Helianthus annuus L.) under saline regimes. Sci. Hortic. 2012, 142, 143–148. [Google Scholar] [CrossRef]
- Ali, B.; Wang, B.; Ali, S.; Ghani, M.A.; Hayat, M.T.; Yang, C.; Xu, L.; Zhou, W.J. 5-Aminolevulinic acid ameliorates the growth, photosynthetic gas exchange capacity and ultrastructural changes under cadmium stress in Brassica napus L. J. Plant Growth Regul. 2013, 32, 604–614. [Google Scholar] [CrossRef]
- Tamás, M.J.; Sharma, S.K.; Ibstedt, S.; Jacobson, T.; Christen, P. Heavy metals and metalloids as a cause for protein misfolding and aggregation. Biomolecules 2014, 4, 252–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Shora, H.M.; Habib, H.M.; Kamel, H.A.; Mostafa, I.Y. Pretreatment with low-doses of gamma irradiation enhances Vicia faba plant tolerance to lead stress. Biosci. Res. 2019, 16, 1528–1537. [Google Scholar]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, M.; Choudhuri, M. Effect of lead and Cadmium on the biochemical changes in the leaves of terrestrial (Vigna) and aquatic (hydrilla) plants under solution culture. Indian J. Plant Physiol. 1997, 32, 99–103. [Google Scholar]
- Gupta, D.K.; Nicoloso, F.T.; Schetinger, M.R.; Rossato, L.V.; Pereira, L.B.; Castro, G.Y. Antioxidant defense mechanism in hydroponically grown Zea mays seedlings under moderate lead stress. J. Hazard. Mater. 2009, 172, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Vassavi, A.; Sudha Madhavi, K.; Ush, R. Effect of lead toxicity on the growth and antioxidant enzymes in Helianthus annuus L. seedlings. J. Pharm. Res. 2012, 5, 2395–2401. [Google Scholar]
- Ali, B.; Gill, R.A.; Yang, S.; Gill, M.B.; Farooq, M.A.; Liu, D.; Daud, M.K.; Ali, S.; Zhou, W. Regulation of cadmium-induced proteomic and metabolic changes by 5-aminolevulinic acid in leaves of Brassica napus L. PLoS ONE 2015, 10, 328. [Google Scholar] [CrossRef]
- Chen, Y.X.; He, Y.F.; Luo, Y.M.; Yu, Y.L.; Lin, Q.; Wang, M.H. Physiological mechanism of plant root exposed to cadmium. Chemoshere 2003, 50, 789–793. [Google Scholar] [CrossRef]
- Muradoglu, F.; Gundogd, U.; Ercisli, S.; Encu, T.; Balta, F.; Jafar, H.Z.; Zia-Ul-Haq, M. Cadmium toxicity affect chlorophyll a and b content antioxidant enzyme activities and mineral nutrient accumulation in strawberry. Biol. Res. 2015, 48, 11. [Google Scholar] [CrossRef] [Green Version]
- Hu, R.; Sunc, K.; Suc, X.; Pana, Y.; Zhanga, Y.; Wanga, X. Physiological responses and tolerance mechanisms to Pb in two xerophils; Salsola passerina Bunge and Chenopodium album L. J. Hazard. Mater. 2012, 205, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Prasad, M.N.; Sytar, O. Lead toxicity, defense strategies and associated indicative biomarkers in Talinum triangulare grown hydroponically. Chemosphere 2012, 89, 1056–1165. [Google Scholar] [CrossRef]
- El-Shora, H.M.; El-Gawad, A.M.A. Response of Cicer arietinum to allelopathic effect of Portulaca oleracea root extract. Phyton 2015, 55, 215–232. [Google Scholar]
- Sayari, B.; Swati, B. Oxidative stress parameters and antioxidant status in middle aged AMD elderly subjects: An age-related comparative study. Int. J. Bioassays 2014, 3, 3131–3136. [Google Scholar]
- Srivastava, M.M.; Singh, N.; Singh, S. Antioxidant responses of hyper accumulator and sensitive fern species to arsenic. J. Exp. Bot. 2005, 56, 1335–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Shora, H.M.; Abd El-Gawad, A.M. Evaluation of Allelopathic Effect of White Lupin (Lupinus termis L.) Leaf Extract on the Biochemical Dynamics of Common Purslane (Portulaca oleracea L.). Egypt. J. Botany 2015, 54, 317–332. [Google Scholar]
- Shahid, M.; Pinelli, E.; Pourrut, B.; Dumat, C. Effect of organic ligands on lead-induced oxidative damage and enhanced antioxidant defense in the leaves of Vicia faba plants. J. Geochem. Explor. 2014, 144, 282–289. [Google Scholar] [CrossRef]
- Rasheed, R.; Yasmeen, H.; Hussain, I.; Iqbal, M.; Ashraf, M.A.; Parveen, A. Exogenously applied 5-aminolevulinic acid modulates growth, secondary metabolism and oxidative defense in sunflower under water deficit stress. Physiol. Molec. Biol. Plants 2020, 26, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Iannone, M.F.; Rosales, E.P.; Zawoznik, M.S.; Groppa, M.D.; Benavides, M.P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Miao, M.M.; Wang, C.L. Effects of ALA on photosynthesis, antioxidant enzyme activity, and gene expression, and regulation of proline accumulation in tomato seedlings under NaCl stress. J. Plant Growth Regul. 2015, 34, 637–650. [Google Scholar] [CrossRef]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef] [Green Version]
- El-Shora, H.M.; El-Gawad, A.; Ahmed, M. Evaluation of allelopathic potential of Rumex dentatus root extract and allelochemicals on Cicer arietinum. J. Stress Physiol. Biochem. 2014, 10, 167–180. [Google Scholar]
- Malecka, A.; Piechalak, A.; Mensinger, A.; Hanć, A.; Baralkiewicz, D.; Tomaszewska, B. Antioxidative defense system in Pisum sativum roots exposed to heavy metals (Pb, Cu, Cd, Zn). Pol. J. Environ. Studies 2012, 21, 1721–1730. [Google Scholar]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- El-Shora, H.M.; Abd El-Gawad, A.M. Environmental toxicity of arsenic on Lupine (Lupinus termis L.) as C3 Crop Plant and possible alleviation. Int. J. Agric. Crop. Sci. 2014, 7, 687. [Google Scholar]
- Ali, B.; Xu, X.; Gill, R.A.; Yang, S.; Ali, S.; Tahir, M.; Zhou, W. Promotive role of 5-aminolevulinic acid on mineral nutrients and antioxidative defense system under lead toxicity in Brassica napus. Ind. Crop. Prod. 2014, 52, 617–626. [Google Scholar] [CrossRef]
- Wu, Y.; Jin, X.; Liao, W.; Hu, L.; Dawuda, M.M.; Zhao, X.; Yu, J. 5-Aminolevulinic acid (ALA) alleviated salinity stress in cucumber seedlings by enhancing chlorophyll synthesis pathway. Front. Plant Sci. 2018, 9, 635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anwar, A.; Yan, Y.; Liu, Y.; Li, Y.; Yu, X. 5-aminolevulinic acid improves nutrient uptake and endogenous hormone accumulation, enhancing low-temperature stress tolerance in cucumbers. Int. J. Mol. Sci. 2018, 19, 3379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.Q.; Wang, Y.; Lv, T.T.; Xiao, Y.H. Role of 5-aminolevulinic acid on growth, photosynthetic parameters and antioxidant enzyme activity in NaCl-stressed Isatis indigotica Fort. Russ. J. Plant Physiol. 2017, 64, 198–206. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Shora, H.M.; Massoud, G.F.; El-Sherbeny, G.A.; Alrdahe, S.S.; Darwish, D.B. Alleviation of Lead Stress on Sage Plant by 5-Aminolevulinic Acid (ALA). Plants 2021, 10, 1969. https://doi.org/10.3390/plants10091969
El-Shora HM, Massoud GF, El-Sherbeny GA, Alrdahe SS, Darwish DB. Alleviation of Lead Stress on Sage Plant by 5-Aminolevulinic Acid (ALA). Plants. 2021; 10(9):1969. https://doi.org/10.3390/plants10091969
Chicago/Turabian StyleEl-Shora, Hamed M., Gehan F. Massoud, Ghada A. El-Sherbeny, Salma Saleh Alrdahe, and Doaa B. Darwish. 2021. "Alleviation of Lead Stress on Sage Plant by 5-Aminolevulinic Acid (ALA)" Plants 10, no. 9: 1969. https://doi.org/10.3390/plants10091969





