Comparison between the Biological Active Compounds in Plants with Adaptogenic Properties (Rhaponticum carthamoides, Lepidium meyenii, Eleutherococcus senticosus and Panax ginseng)
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Rhaponticum carthamoides
3.1.1. Phytochemical Composition of Rhaponticum carthamoides Roots
3.1.2. Phytochemical Composition of Rhaponticum carthamoides Leaves
3.1.3. Phytochemical Composition of Rhaponticum carthamoides Seeds
3.2. Lepidium meyenii
3.2.1. Phytochemicals Isolated from Maca Root
3.2.2. Bioactive Compounds Detected in Lepidium meyenii Tuber
3.2.3. Bioactive Compounds Isolated from Lepidium meyenii Hypocotyls
3.2.4. Nutritional Ingredients Isolated from Maca
3.3. Eleutherococcus senticosus
3.3.1. Phytochemical Compounds Isolated from Eleutherococcus senticosus Roots
3.3.2. Phytochemicals Isolated from Eleutherococcus senticosus Stem and Leaves
3.4. Panax ginseng
3.4.1. Phytochemicals Isolated from Panax ginseng Roots
3.4.2. Phytochemicals Isolated from Panax ginseng Leaves and Flower Buds
4. Comparison between Rhaponticum carthamoides, Lepidium meyenii, Eleutherococcus senticosus and Panax ginseng and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wagner, H.; Nörr, H.; Winterhoff, H. Plant Adaptogens. Phytomedicine 1994, 1, 63–76. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G.; Wagner, H. Plant Adaptogens III. Earlier and More Recent Aspects and Concepts on Their Mode of Action. Phytomedicine 1999, 6, 287–300. [Google Scholar] [CrossRef]
- Panossian, A.G.; Efferth, T.; Shikov, A.N.; Pozharitskaya, O.N.; Kuchta, K.; Mukherjee, P.K.; Banerjee, S.; Heinrich, M.; Wu, W.; Guo, D.A.; et al. Evolution of the Adaptogenic Concept from Traditional Use to Medical Systems: Pharmacology of Stress- and Aging-Related Diseases. Med. Res. Rev. 2021, 41, 630–703. [Google Scholar] [CrossRef]
- Brekhman, I.I.; Dardymov, I.V. New Substances of Plant Origin Which Increase Nonspecific Resistance. Annu. Rev. Pharmacol. 1969, 9, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.G. Adaptogens: Tonic Herbs for Fatigue and Stress. Altern. Complement. Ther. 2003, 9, 327–331. [Google Scholar] [CrossRef]
- Rai, D.; Bhatia, G.; Palit, G.; Pal, R.; Singh, S.; Singh, H.K. Adaptogenic Effect of Bacopa Monniera (Brahmi). Pharmacol. Biochem. Behav. 2003, 75, 823–830. [Google Scholar] [CrossRef]
- Shivakumar, H.; Javed, T.; Prakash, T.; Rao, R.N.; Swamy, B.H.M.J.; Goud, A.V. Adaptogenic Activity of Ethanolic Extract of Tribulus terrestris L. J. Nat. Remedies 2006, 6, 87–95. [Google Scholar] [CrossRef]
- Ley, B.M. Maca!: Adaptogen and Hormonal Regulator; BL Publications: Memphis, TN, USA, 2003; ISBN 9781890766252. [Google Scholar]
- Gupta, G.; Rana, A.C. Withania Somnifera (Ashwagandha): A Review. Pharmacogn. Rev. 2007, 1, 129–136. [Google Scholar]
- Iwu, M.M.; Diop, A.D.; Meserole, L.; Okunji, C.O. Chapter 17—Garcinia Kola: A New Look at an Old Adaptogenic Agent. In Advances in Phytomedicine: Ethnomedicine and Drug Discovery; Iwu, M.M., Wootton, J.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; Volume 1, pp. 191–199. [Google Scholar]
- Kamal, M.; Arif, M.; Jawaid, T. Adaptogenic Medicinal Plants Utilized for Strengthening the Power of Resistance during Chemotherapy—A Review. Orient. Pharm. Exp. Med. 2017, 17, 1–18. [Google Scholar] [CrossRef]
- Davydov, M.; Krikorian, A.D. Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. (Araliaceae) as an Adaptogen: A Closer Look. J. Ethnopharmacol. 2000, 72, 345–393. [Google Scholar] [CrossRef]
- Kokoska, L.; Janovska, D. Chemistry and Pharmacology of Rhaponticum carthamoides: A Review. Phytochemistry 2009, 70, 842–855. [Google Scholar] [CrossRef]
- Gan, J.; Feng, Y.; He, Z.; Li, X.; Zhang, H. Correlations between Antioxidant Activity and Alkaloids and Phenols of Maca (Lepidium meyenii). J. Food Qual. 2017, 2017, e3185945. [Google Scholar] [CrossRef] [Green Version]
- Shergis, J.L.; Zhang, A.L.; Zhou, W.; Xue, C.C. Panax ginseng in Randomised Controlled Trials: A Systematic Review. Phytother. Res. 2013, 27, 949–965. [Google Scholar] [CrossRef]
- Coleman, C.I.; Hebert, J.H.; Reddy, P. The Effects of Panax ginseng on Quality of Life. J. Clin. Pharm. Ther. 2003, 28, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Biskup, E.; Lojkowska, E. Evaluation of Biological Activities of Rhaponticum carthamoides Extracts. JMPR 2009, 3, 1092–1098. [Google Scholar] [CrossRef]
- Peres, N.D.S.L.; Bortoluzzi, L.C.P.; Marques, L.L.M.; Formigoni, M.; Barros Fuchs, R.H.; Aparecida Droval, A.; Cardoso, F.A.R. Medicinal Effects of Peruvian Maca (Lepidium meyenii): A Review. Food Funct. 2020, 11, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Rebecca, H.; Kadioglu, O.; Georg, W.; Efferth, T. Understanding Adaptogens: New Evidence on Their Possible Effectiveness in Stress-Induced and Ageing-Associated Disorders from a DNA Microarray Study of Neuroglia Cells. Planta Med. 2013, 79, PM8. [Google Scholar] [CrossRef]
- Pawar, V.S.; Shivakumar, H. A Current Status of Adaptogens: Natural Remedy to Stress. Asian Pac. J. Trop. Dis. 2012, 2, S480–S490. [Google Scholar] [CrossRef]
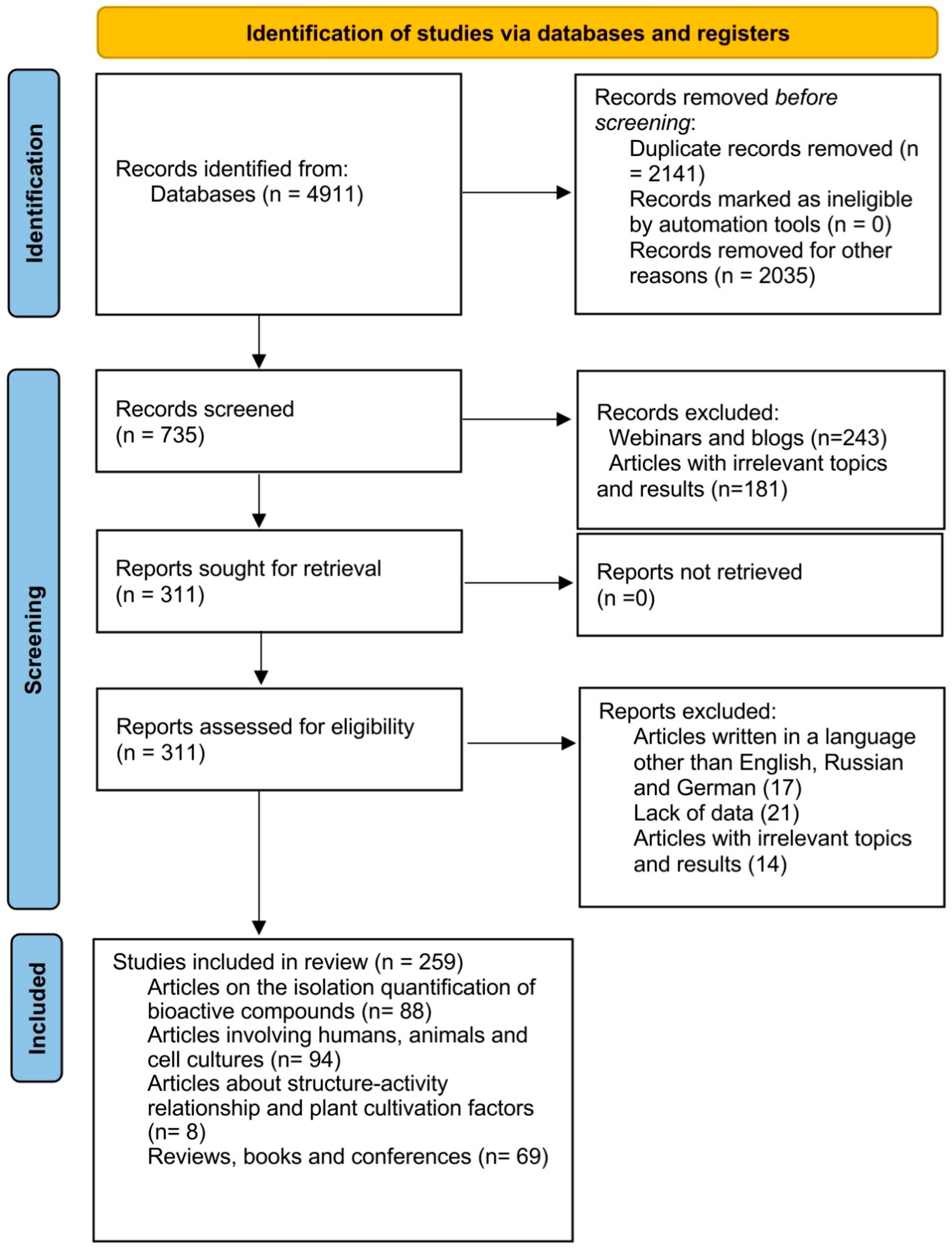
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Dini, A.; Migliuolo, G.; Rastrelli, L.; Saturnino, P.; Schettino, O. Chemical Composition of Lepidium meyenii. Food Chem. 1994, 49, 347–349. [Google Scholar] [CrossRef]
- Lee, S.M.; Bae, B.S.; Park, H.W.; Ahn, N.G.; Cho, B.G.; Cho, Y.L.; Kwak, Y.S. Characterization of Korean Red Ginseng (Panax ginseng Meyer): History, Preparation Method, and Chemical Composition. J. Ginseng Res. 2015, 39, 384–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koleckar, V.; Brojerova, E.; Rehakova, Z.; Kubikova, K.; Cervenka, F.; Kuca, K.; Jun, D.; Hronek, M.; Opletalova, V.; Opletal, L. In Vitro Antiplatelet Activity of Flavonoids from Leuzea carthamoides. Drug Chem. Toxicol. 2008, 31, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Koleckar, V.; Opletal, L.; Macakova, K.; Jahodar, L.; Jun, D.; Kunes, J.; Kuca, K. New Antioxidant Flavonoid Isolated from Leuzea carthamoides. J. Enzyme Inhib. Med. Chem. 2010, 25, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, M.; Skiba, A.; Weglarz, Z.; El-Ansari, M.A. Two Flavonol 5-O-Glycosides from the Roots of Leuzea carthamoides. Fitoterapia 2001, 72, 940–942. [Google Scholar] [CrossRef]
- Skiba, A.; Weglarz, Z. Phenolic Acids of Rhaponticum carthamoides. Acta Hortic. 2003, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Wagner, H.; Heur, Y.H.; Obermeier, A.; Tittel, G.; Bladt, S. Die DC-and HPLC-analyse der Eleutherococcus Droge. Planta Med. 1982, 44, 193–198. [Google Scholar] [CrossRef]
- Kurkin, V.A.; Zapesochnaya, G.G.; Bandyshev, V.V. Phenolic Compounds of Eleutherococcus senticosus. Chem. Nat. Compd. 1991, 27, 755–756. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Li, J.; Duan, Z.; Zhu, S.; Fan, L. The Composition Analysis of Maca (Lepidium meyenii Walp.) from Xinjiang and Its Antifatigue Activity. J. Food Qual. 2017, 2017, e2904951. [Google Scholar] [CrossRef] [Green Version]
- Timofeev, N.P. Leuzea carthamoides DC.: Application Prospects as Pharmpreparations and Biologically Active Components. In Functional Foods for Chronic Diseases: The Modern Day Cure without the Side Effects of Traditional Treatments; Martirosyan, D.M., Ed.; D & A Inc.: Richardson, TX, USA, 2006; pp. 105–120. ISBN 9780976753520. [Google Scholar]
- Petkov, V.; Roussinov, K.; Todorov, S.; Lazarova, M.; Yonkov, D.; Draganova, S. Pharmacological Investigations on Rhaponticum carthamoides. Planta Med. 1984, 50, 205–209. [Google Scholar] [CrossRef]
- Ewa, S.; Picot, L.; Saluk-Bijak, J.; Szemraj, J.; Kicel, A.; Olszewska, M.; Sitarek, P.; Bijak, M. An Efficient Plant Regeneration from Rhaponticum carthamoides Transformed Roots, Enhanced Caffeoylquinic Acid Derivatives Production in PRi-Transformed Plants and Their Biological Activity. Ind. Crop. Prod. 2019, 129, 327–338. [Google Scholar] [CrossRef]
- State Pharmacopoeia of the Russian Federation/Ministry of Health of the Russian Federation, 14th ed.; The Ministry of Health of the Russian Federation: Moscow, Russia, 2018; Volume 4, pp. 6360–6368.
- Todorova, V.; Ivanov, K.; Delattre, C.; Nalbantova, V.; Karcheva-Bahchevanska, D.; Ivanova, S. Plant Adaptogens-History and Future Perspectives. Nutrients 2021, 13, 2861. [Google Scholar] [CrossRef]
- Isenmann, E.; Ambrosio, G.; Joseph, J.F.; Mazzarino, M.; de la Torre, X.; Zimmer, P.; Kazlauskas, R.; Goebel, C.; Botrè, F.; Diel, P.; et al. Ecdysteroids as Non-Conventional Anabolic Agent: Performance Enhancement by Ecdysterone Supplementation in Humans. Arch. Toxicol. 2019, 93, 1807–1816. [Google Scholar] [CrossRef]
- Wilborn, C.D.; Taylor, L.W.; Campbell, B.I.; Kerksick, C.; Rasmussen, C.J.; Greenwood, M.; Kreider, R.B. Effects of Methoxyisoflavone, Ecdysterone, and Sulfo-Polysaccharide Supplementation on Training Adaptations in Resistance-Trained Males. J. Int. Soc. Sports Nutr. 2006, 3, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Ryan, E.D.; Gerstner, G.R.; Mota, J.A.; Trexler, E.T.; Giuliani, H.K.; Blue, M.N.M.; Hirsch, K.R.; Smith-Ryan, A.E. The Acute Effects of a Multi-Ingredient Herbal Supplement on Performance Fatigability: A Double-Blind, Randomized, and Placebo-Controlled Trial. J. Diet. Suppl. 2021, 18, 507–516. [Google Scholar] [CrossRef]
- Głazowska, J.; Kamiński, M.M.; Kamiński, M. Chromatographic Separation, Determination and Identification of Ecdysteroids: Focus on Maral Root (Rhaponticum carthamoides, Leuzea carthamoides). J. Sep. Sci. 2018, 41, 4304–4314. [Google Scholar] [CrossRef]
- Skała, E.; Rijo, P.; Garcia, C.; Sitarek, P.; Kalemba, D.; Toma, M.; Szemraj, J.; Pytel, D.; Wysokińska, H.; Śliwiński, T. The Essential Oils of Rhaponticum carthamoides Hairy Roots and Roots of Soil-Grown Plants: Chemical Composition and Antimicrobial, Anti-Inflammatory, and Antioxidant Activities. Oxid. Med. Cell. Longev. 2016, 2016, 8505384. [Google Scholar] [CrossRef]
- Dinan, L. Phytoecdysteroids: Biological Aspects. Phytochemistry 2001, 57, 325–339. [Google Scholar] [CrossRef]
- Das, N.; Mishra, S.K.; Bishayee, A.; Ali, E.S.; Bishayee, A. The Phytochemical, Biological, and Medicinal Attributes of Phytoecdysteroids: An Updated Review. Acta Pharm. Sin. B 2021, 11, 1740–1766. [Google Scholar] [CrossRef] [PubMed]
- Bathori, M.; Toth, N.; Hunyadi, A.; Marki, A.; Zador, E. Phytoecdysteroids and Anabolic-Androgenic Steroids—Structure and Effects on Humans. CMC 2008, 15, 75–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarkowská, D.; Strnad, M. Plant Ecdysteroids: Plant Sterols with Intriguing Distributions, Biological Effects and Relations to Plant Hormones. Planta 2016, 244, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Szendrei, K.; Varga, E.; Hajdú, Z.; Herke, I.; Lafont, R.; Girault, J.P. Ajugasterone C and 5-Deoxykaladasterone, an Ecdysteroid Artifact, from Leuzea carthamoides. J. Nat. Prod. 1988, 51, 993–995. [Google Scholar] [CrossRef]
- Bathori, M.; Pongracz, Z. Phytoecdysteroids—From Isolation to Their Effects on Humans. Curr. Med. Chem. 2005, 12, 153–172. [Google Scholar] [CrossRef] [PubMed]
- Timofeev, N.P.; Chukhchin, D.G. Accumulation and Composition of Extractive Substances from Above-Ground and Underground Bodies of Levzei safloroid. In Proceedings of the New Achievements in Chemistry and Chemical Technology of Vegetable Raw Materials: Materials of the IV All-Russian Conference. April 21–23, 2009; Bazarnova, N.G., Markin, V.I., Eds.; Altajskogo Gos. Univ.: Burnau, Russia, 2009; pp. 145–147. ISBN 978-5-7904-0903-5. [Google Scholar]
- Píš, J.; Buděšínsky, M.; Vokáč, K.; Laudová, V.; Harmatha, J. Ecdysteroids from the Roots of Leuzea carthamoides. Phytochemistry 1994, 37, 707–711. [Google Scholar] [CrossRef]
- Vokáč, K.; Budesinsky, M.; Harmatha, J. Minor Ecdysteroid Components of Leuzea carthamoides. Collect. Czechoslov. Chem. Commun. 2002, 67, 124–139. [Google Scholar] [CrossRef]
- Buděšínský, M.; Vokáč, K.; Harmatha, J.; Cvačka, J. Additional Minor Ecdysteroid Components of Leuzea carthamoides. Steroids 2008, 73, 502–514. [Google Scholar] [CrossRef]
- Girault, J.P.; Lafont, R.; Varga, E.; Hajdu, Z.; Herke, I.; Szendrei, K. Ecdysteroids from Leuzea carthamoides. Phytochemistry 1988, 27, 737–741. [Google Scholar] [CrossRef]
- Mamatkhanov, A.U.; Shamsutdinov, M.-R.I.; Shakirov, T.T. Isolation of Ecdysterone from the Roots of Rhaponticum carthamoides. Chem. Nat. Compd. 1980, 16, 381–382. [Google Scholar] [CrossRef]
- Timofeev, N.P.; Volodin, V.V.; Frolov, Y.M. Distribution of 20-hydroxyecdysone in the structure of the biomass of the aboveground part of Rhaponticum carthamoides (Willd.) Iljin. 7. Russ. Acad. Sci. 1998, 34, 63–69. [Google Scholar]
- Ramazanov, N.S.; Makshimov, E.S.; Saatov, Z.; Mamatkhanov, A.U.; Abdullaev, N.D. Phytoecdysteroids of Plants of the Genus Rhaponticum I. Carthamosterone a FromRh. Carthamoides. Chem. Nat. Compd. 1997, 33, 301–302. [Google Scholar] [CrossRef]
- Sovová, H.; Opletal, L.; Sajfrtová, M.; Bártlová, M. Supercritical Fluid Extraction of Cynaropicrin and 20-Hydroxyecdysone from Leuzea carthamoides DC. J. Sep. Sci. 2008, 31, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Sadykov, Z.T.; Ramazanov, N.S.; Saatov, Z. Phytoecdysteroids of Plants of the GenusRhaponticum Polypodin B 22-O-Benzoate from Rhaponticum carthamoides. Chem. Nat. Compd. 1997, 33, 665–666. [Google Scholar] [CrossRef]
- Ferro, N.; Tacoronte, J.E.; Reinard, T.; Bultinck, P.; Montero, L.A. Structure–Activity Analysis on Ecdysteroids: A Structural and Quantum Chemical Approach Based on Two Biological Systems. J. Mol. Struct. Theochem. 2006, 758, 263–274. [Google Scholar] [CrossRef]
- Dinan, L. Ecdysteroid Structure-Activity Relationships. In Studies in Natural Products Chemistry: Bioactive Natural Products (Part J); Attaur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 29, pp. 3–71. [Google Scholar]
- Syrov, V.N.; Saatov, Z.; Sagdullaev, S.S.; Mamatkhanov, A.U. Study of the Structure—Anabolic Activity Relationship for Phytoecdysteroids Extracted from Some Plants of Central Asia. Pharm. Chem. J. 2001, 35, 667–671. [Google Scholar] [CrossRef]
- Cahlíková, L.; Macáková, K.; Chlebek, J.; Hošt’álková, A.; Kulhánková, A.; Opletal, L. Ecdysterone and Its Activity on Some Degenerative Diseases. Nat. Prod. Commun. 2011, 6, 1934578X1100600527. [Google Scholar] [CrossRef] [Green Version]
- Lafont, R.; Dinan, L. Practical Uses for Ecdysteroids in Mammals Including Humans: And Update. J. Insect Sci. 2003, 3. [Google Scholar] [CrossRef]
- Dinan, L.; Dioh, W.; Veillet, S.; Lafont, R. 20-Hydroxyecdysone, from Plant Extracts to Clinical Use: Therapeutic Potential for the Treatment of Neuromuscular, Cardio-Metabolic and Respiratory Diseases. Biomedicines 2021, 9, 492. [Google Scholar] [CrossRef]
- Bakrim, A.; Maria, A.; Sayah, F.; Lafont, R.; Takvorian, N. Ecdysteroids in Spinach (Spinacia oleracea L.): Biosynthesis, Transport and Regulation of Levels. Plant Physiol. Biochem. 2008, 46, 844–854. [Google Scholar] [CrossRef]
- Imai, S.; Toyosato, T.; Sakai, M.; Sato, Y.; Fujioka, S.; Murata, E.; Goto, M. Isolation of Cyasterone and Ecdysterone from Plant Materials. Chem. Pharm. Bull. 1969, 17, 340–342. [Google Scholar] [CrossRef] [Green Version]
- The World Anti-Doping Agency—WADA. The 2020 Monitoring Program. Available online: https://www.wada-ama.org/sites/default/files/resources/files/wada_2020_english_monitoring_program_pdf (accessed on 30 November 2021).
- Tóth, N.; Szabó, A.; Kacsala, P.; Héger, J.; Zádor, E. 20-Hydroxyecdysone Increases Fiber Size in a Muscle-Specific Fashion in Rat. Phytomedicine 2008, 15, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Syrov, V.N. Comparative Experimental Investigation of the Anabolic Activity of Phytoecdysteroids and Steranabols. Pharm. Chem. J. 2000, 34, 193–197. [Google Scholar] [CrossRef]
- Seidlova-Wuttke, D.; Ehrhardt, C.; Wuttke, W. Metabolic Effects of 20-OH-ecdysone in Ovariectomized Rats. J. Steroid Biochem. Mol. Biol. 2010, 119, 121–126. [Google Scholar] [CrossRef]
- Buniam, J.; Chukijrungroat, N.; Rattanavichit, Y.; Surapongchai, J.; Weerachayaphorn, J.; Bupha-Intr, T.; Saengsirisuwan, V. 20-Hydroxyecdysone Ameliorates Metabolic and Cardiovascular Dysfunction in High-fat-high-fructose-fed Ovariectomized Rats. BMC Complement. Med. Ther. 2020, 20, 140. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, X.; Xu, T.; Qin, S. β-Ecdysterone Suppresses Interleukin-1β-Induced Apoptosis and Inflammation in Rat Chondrocytes via Inhibition of NF-ΚB Signaling Pathway. Drug Dev. Res. 2014, 75, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Krasutsky, A.G.; Cheremisinov, V.N. The use of Levzey’s extract to increase the efficiency of the training process in fitness clubs students. In Proceedings of the Actual Problems of Biochemistry and Bioenergy of Sport of the XXI Century, Moscow, Russia, 10–26 April 2017; pp. 382–388. [Google Scholar]
- Vanyuk, A.I. Evaluation of the Effectivnness of Rehabilitation Measures Among Female Volleyball Players 18-22 Years Old in the Competitive Period of the Annual Training Cycle. Slobozhanskiy Sci. Sports Visnik. 2012, 5, 95–98. [Google Scholar]
- Timofeev, N.P.; Koksharov, A.V. Study of Leuzea from Leaves: Results of 15 Years of Trials in Athletics. New Unconv. Plants Prospect. Use 2016, 12, 502–505. [Google Scholar]
- Krasutsky, A.G.; Cheremisinov, V.N. Research of the Influence of Adaptogens on Increasing the Efficacy of the Training Process in Fitness Clubs. In Proceedings of the Current Problems of Biochemistry and Bioenergy Sport of the XXI Century, Moscow, Russia, 10–12 April 2018; pp. 267–282. [Google Scholar]
- Chen, Q.; Xia, Y.; Qiu, Z. Effect of Ecdysterone on Glucose Metabolism in Vitro. Life Sci. 2006, 78, 1108–1113. [Google Scholar] [CrossRef]
- Romaniuk-Drapała, A.; Lisiak, N.; Totoń, E.; Matysiak, A.; Nawrot, J.; Nowak, G.; Kaczmarek, M.; Rybczyńska, M.; Rubiś, B. Proapoptotic and Proautophagic Activity of 20-Hydroxyecdysone in Breast Cancer Cells in Vitro. Chem. Biol. Interact. 2021, 342. [Google Scholar] [CrossRef]
- Shuvalov, O.; Fedorova, O.; Tananykina, E.; Gnennaya, Y.; Daks, A.; Petukhov, A.; Barlev, N.A. An Arthropod Hormone, Ecdysterone, Inhibits the Growth of Breast Cancer Cells via Different Mechanisms. Front. Pharmacol. 2020, 11, 561537. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Niu, C.; Zhang, X.; Dong, M. β-Ecdysterone Protects SH-SY5Y Cells against β-Amyloid-Induced Apoptosis via c-Jun N-Terminal Kinase- and Akt-Associated Complementary Pathways. Lab. Investig. 2018, 98, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.H.; Yue, Z.S.; Li, G.S.; Zeng, L.R.; Xin, D.W.; Hu, Z.Q.; Xu, C.D. Effect of Β-ecdysterone on Glucocorticoid-induced Apoptosis and Autophagy in Osteoblasts. Mol. Med. Rep. 2018, 17, 158–164. [Google Scholar] [CrossRef] [Green Version]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietta, P.G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Faizieva, S.K.; Khushbaktova, Z.A.; Syrov, V.N.; Yuldashev, M.P.; Batirov, É.K.; Sagdullaev, S.S. The Total Flavonoids from Thermopsis Alterniflora, Th. Dolichocarpa, Vexibia Alopecuroides, and Rhaponticum carthamoides and Their Hypolipidemic Activity. Chem. Nat. Compd. 1999, 35, 155–158. [Google Scholar] [CrossRef]
- Glevitzky, I.; Dumitrel, G.A.; Glevitzky, M.; Pasca, B.; Otrisal, P.; Bungau, S.; Cioca, G.; Pantis, C.; Popa, M. Statistical Analysis of the Relationship Between Antioxidant Activity and the Structure of Flavonoid Compounds. Rev. Chim. 2019, 70, 3103–3107. [Google Scholar] [CrossRef]
- Havlik, J.; Budesinsky, M.; Kloucek, P.; Kokoska, L.; Valterova, I.; Vasickova, S.; Zeleny, V. Norsesquiterpene Hydrocarbon, Chemical Composition and Antimicrobial Activity of Rhaponticum carthamoides Root Essential Oil. Phytochemistry 2009, 70, 414–418. [Google Scholar] [CrossRef]
- Geszprych, A.; Weglarz, Z. Composition of Essential Oil from Underground and Aboveground Organs of Rhaponticum carthamoides [Willd.] Iljin. Herba Pol. 2002, 4, 188–192. [Google Scholar]
- Bastaev, U.A.; Abubakirov, N.K. Phytoecdysteroids of Rhaponticum carthamoides. Chem. Nat. Compd. 1987, 23, 565–568. [Google Scholar] [CrossRef]
- Timofeev, N.P. Variability of Contnet Ecdysterone in Leaves Rhaponticum carthamoides During Season of Vegetation. In Renewable Wood and Plant Resources: Chemistry, Technology, Pharmacology, Medicine; Saint-Petersburg State Forest Technical Academy: St. Petersburg, Russia, 2011; pp. 228–229. [Google Scholar]
- Timofeev, N.P.; Lapin, A.A.; Zelenkov, V.N. Quality Assessment of Rhaponticum carthamoides (Willd.) Iljin as Medicinal Raw Material by the Bromic Antioxidant Capacity Estimation. In Functional Foods for Chronic Diseases; D & A Inc.: St. Anthony, ID, USA, 2006; pp. 164–172. [Google Scholar]
- Krasnov, E.A.; Saratikov, A.S.; Yakunina, G.D. Inokosterone and Ecdysterone from Rhaponticum carthamoides. Chem. Nat. Compd. 1976, 12, 494–495. [Google Scholar] [CrossRef]
- Borovikova, E.B.; Shangaraeva, G.S.; Baltaev, U.A. Rhapisterone D 20-Acetate from the Seeds of Leuzea carthamoides. Chem. Nat. Compd. 1999, 35, 184–185. [Google Scholar] [CrossRef]
- Baltaev, U.A. Phytoecdysteroids of Rhaponticum carthamoides. II. Rhapisterone B. Chem. Nat. Compd. 1991, 27, 712–713. [Google Scholar] [CrossRef]
- Baltaev, U.A. Phytoecdysteroids of Rhaponticum carthamoides III. Rhapisterone C. Chem. Nat. Compd. 1992, 28, 198–200. [Google Scholar] [CrossRef]
- Baltaev, U.A. Rapisterone D, a Phytoecdysteroid from Rhaponticum carthamoides. Phytochemistry 1995, 38, 799–800. [Google Scholar] [CrossRef]
- Huang, M.F.; Li, N.; Ni, H.; Jia, X.G.; Li, X. Studies on Chemical Constituents from Rhaponticum carthamoides (Willd.) Iljin. Chin. Pharm. J. 2009, 17, 1287–1290. [Google Scholar]
- Wang, Y.; Wang, Y.; McNeil, B.; Harvey, L.M. Maca: An Andean Crop with Multi-Pharmacological Functions. Food Res. Int. 2007, 40, 783–792. [Google Scholar] [CrossRef]
- Kasprzak, D.; Jodlowska-Jedrych, B.; Borowska, K.; Wojtowicz, A. Lepidium meyenii (Maca)—Multidirectional Health Effects—Review. Curr. Issues Pharm. Med. Sci. 2018, 31, 107–112. [Google Scholar] [CrossRef]
- Muhammad, I.; Zhao, J.; Dunbar, D.C.; Khan, I.A. Constituents of Lepidium meyenii ‘Maca’. Phytochemistry 2002, 59, 105–110. [Google Scholar] [CrossRef]
- León, J. The “Maca” (Lepidium meyenii), a Little Known Food Plant of Peru. Econ. Bot. 1964, 18, 122–127. [Google Scholar] [CrossRef]
- Gonzales, C.; Rubio, J.; Gasco, M.; Nieto, J.; Yucra, S.; Gonzales, G.F. Effect of Short-Term and Long-Term Treatments with Three Ecotypes of Lepidium meyenii (MACA) on Spermatogenesis in Rats. J. Ethnopharmacol. 2006, 103, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Ganzera, M.; Zhao, J.; Muhammad, I.; Khan, I.A. Chemical Profiling and Standardization of Lepidium meyenii (Maca) by Reversed Phase High Performance Liquid Chromatography. Chem. Pharm. Bull. 2002, 50, 988–991. [Google Scholar] [CrossRef] [Green Version]
- Cicero, A.F.G.; Bandieri, E.; Arletti, R. Lepidium meyenii Walp. Improves Sexual Behaviour in Male Rats Independently from Its Action on Spontaneous Locomotor Activity. J. Ethnopharmacol. 2001, 75, 225–229. [Google Scholar] [CrossRef]
- Piacente, S.; Carbone, V.; Plaza, A.; Zampelli, A.; Pizza, C. Investigation of the Tuber Constituents of Maca (Lepidium meyenii Walp.). J. Agric. Food Chem. 2002, 50, 5621–5625. [Google Scholar] [CrossRef]
- Zhao, J.; Muhammad, I.; Dunbar, D.C.; Mustafa, J.; Khan, I.A. New Alkamides from Maca (Lepidium meyenii). J. Agric. Food Chem. 2005, 53, 690–693. [Google Scholar] [CrossRef]
- Melnikovova, I.; Havlik, J.; Cusimamani, E.F.; Milella, L. Macamides and fatty acids content comparison in maca cultivated plant under field conditions and greenhouse. Bol. Latinoam. Caribe Plantas Med. Aromat. 2012, 11, 420–427. [Google Scholar]
- Shalaby, E.; Azzam, G.M. Antioxidants in Foods and Its Applications; BoD—Books on Demand: Norderstedt, Germany, 2018; ISBN 9781789233780. [Google Scholar]
- Del Valle Mendoza, J.; Pumarola, T.; Gonzales, L.A.; del Valle, L.J. Antiviral Activity of Maca (Lepidium meyenii) against Human Influenza Virus. Asian Pac. J. Trop. Med. 2014, 7, S415–S420. [Google Scholar] [CrossRef] [Green Version]
- Chung, F.; Rubio, J.; Gonzales, C.; Gasco, M.; Gonzales, G.F. Dose–Response Effects of Lepidium meyenii (Maca) Aqueous Extract on Testicular Function and Weight of Different Organs in Adult Rats. J. Ethnopharmacol. 2005, 98, 143–147. [Google Scholar] [CrossRef]
- Lee, Y.; Jung, S.K.; Chang, Y.H. Rheological Properties of a Neutral Polysaccharide Extracted from Maca (Lepidium meyenii Walp.) Roots with Prebiotic and Anti-Inflammatory Activities. Int. J. Biol. Macromol. 2020, 152, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Lentz, A.; Gravitt, K.; Carson, C.C.; Marson, L. Acute and Chronic Dosing of Lepidium meyenii (Maca) on Male Rat Sexual Behavior. J. Sex. Med. 2007, 4, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, G.F. Ethnobiology and Ethnopharmacology of Lepidium meyenii (Maca), a Plant from the Peruvian Highlands. Evid.-Based Complement. Altern. Med. 2011, 2012, e193496. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhu, F. Chemical Composition and Health Effects of Maca (Lepidium meyenii). Food Chem. 2019, 288, 422–443. [Google Scholar] [CrossRef]
- Ronald, J.; Davis, S.J. Measuring Hypocotyl Length in Emphasis Type Arabidopsis. Plant Circadian Netw. 2022, 99–106. [Google Scholar] [CrossRef]
- Chen, R.; Wei, J.; Gao, Y. A Review of the Study of Active Components and Their Pharmacology Value in Lepidium meyenii (Maca). Phytother. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Esparza, E.; Hadzich, A.; Kofer, W.; Mithöfer, A.; Cosio, E.G. Bioactive Maca (Lepidium meyenii) Alkamides Are a Result of Traditional Andean Postharvest Drying Practices. Phytochemistry 2015, 116, 138–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCollom, M.M.; Villinski, J.R.; McPhail, K.L.; Craker, L.E.; Gafner, S. Analysis of Macamides in Samples of Maca (Lepidium meyenii) by HPLC-UV-MS/MS. Phytochem. Anal. 2005, 16, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.X.; Li, K.K.; Pubu, D.; Jiang, S.P.; Chen, B.; Chen, L.R.; Yang, Z.; Ma, C.; Gong, X.J. Optimization of Ultrasound-Assisted Extraction, HPLC and UHPLC-ESI-Q-TOF-MS/MS Analysis of Main Macamides and Macaenes from Maca (Cultivars of Lepidium meyenii Walp). Molecules 2017, 22, 2196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, L.; Wei, J.; Gao, Y.; Chen, R. Antioxidant and Antitumoral Activities of Isolated Macamide and Macaene Fractions from Lepidium meyenii (Maca). Talanta 2021, 221, 121635. [Google Scholar] [CrossRef]
- Ostlund, R.E. Phytosterols in Human Nutrition. Annu. Rev. Nutr. 2002, 22, 533–549. [Google Scholar] [CrossRef]
- Lagarda, M.J.; García-Llatas, G.; Farré, R. Analysis of Phytosterols in Foods. J. Pharm. Biomed. Anal. 2006, 41, 1486–1496. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Xu, F.; Zheng, M.; Xi, X.; Cui, X.; Han, C. Maca Polysaccharides: A Review of Compositions, Isolation, Therapeutics and Prospects. Int. J. Biol. Macromol. 2018, 111, 894–902. [Google Scholar] [CrossRef]
- Sinha, V.R.; Kumria, R. Polysaccharides in Colon-Specific Drug Delivery. Int. J. Pharm. 2001, 224, 19–38. [Google Scholar] [CrossRef]
- Nosáľová, G.; Prisenžňáková, L.; Paulovičová, E.; Capek, P.; Matulová, M.; Navarini, L.; Liverani, F.S. Antitussive and immunomodulating activities of instant coffee arabinogalactan-protein. Int. J. Biol. Macromol. 2011, 49, 493–497. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, R.-Q.; Chen, Y.-J.; Liao, L.-M.; Sun, Y.-Q.; Ma, Z.-H.; Yang, Q.-F.; Li, P.; Ye, Y.-Q.; Hu, Q.-F. Three New Pyrrole Alkaloids from the Roots of Lepidium meyenii. Phytochem. Lett. 2018, 23, 137–140. [Google Scholar] [CrossRef]
- Kostova, I.; Bhatia, S.; Grigorov, P.; Balkansky, S.; Parmar, V.S.; Prasad, A.K.; Saso, L. Coumarins as Antioxidants. Curr. Med. Chem. 2011, 18, 3929–3951. [Google Scholar] [CrossRef]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple Coumarins and Analogues in Medicinal Chemistry: Occurrence, Synthesis and Biological Activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef]
- Yábar, E.; Pedreschi, R.; Chirinos, R.; Campos, D. Glucosinolate Content and Myrosinase Activity Evolution in Three Maca (Lepidium meyenii Walp.) Ecotypes during Preharvest, Harvest and Postharvest Drying. Food Chem. 2011, 127, 1576–1583. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, G.; Lai, F.; Wu, H. Structural Characterization and Immunomodulatory Activity of a Novel Polysaccharide from Lepidium meyenii. J. Agric. Food Chem. 2016, 64, 1921–1931. [Google Scholar] [CrossRef] [PubMed]
- Natella, F.; Maldini, M.; Leoni, G.; Scaccini, C. Glucosinolates Redox Activities: Can They Act as Antioxidants? Food Chem. 2014, 149, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Mithen, R. Glucosinolates—Biochemistry, Genetics and Biological Activity. Plant Growth Regul. 2001, 34, 91–103. [Google Scholar] [CrossRef]
- Manici, L.M.; Lazzeri, L.; Palmieri, S. In Vitro Fungitoxic Activity of Some Glucosinolates and Their Enzyme-Derived Products toward Plant Pathogenic Fungi. J. Agric. Food Chem. 1997, 45, 2768–2773. [Google Scholar] [CrossRef]
- Rubio, J.; Dang, H.; Gong, M.; Liu, X.; Chen, S.-L.; Gonzales, G.F. Aqueous and Hydroalcoholic Extracts of Black Maca (Lepidium meyenii) Improve Scopolamine-Induced Memory Impairment in Mice. Food Chem. Toxicol. 2007, 45, 1882–1890. [Google Scholar] [CrossRef]
- Pino-Figueroa, A.; Nguyen, D.; Maher, T.J. Neuroprotective Effects of Lepidium meyenii (Maca): Lepidium meyenii Neuroprotective Effects. Ann. N. Y. Acad. Sci. 2010, 1199, 77–85. [Google Scholar] [CrossRef]
- Rodríguez-Huamán, Á.; Casimiro-Gonzales, S.; Chávez-Pérez, J.A.; Gonzales-Arimborgo, C.; Cisneros-Fernández, R.; Aguilar-Mendoza, L.Á.; Gonzales, G.F. Antioxidant and Neuroprotector Effect of Lepidium meyenii (Maca) Methanol Leaf Extract against 6-Hydroxy Dopamine (6-OHDA)-Induced Toxicity in PC12 Cells. Toxicol. Mech. Methods 2017, 27, 279–285. [Google Scholar] [CrossRef]
- Ohta, Y.; Kawate, N.; Inaba, T.; Morii, H.; Takahashi, K.; Tamada, H. Feeding Hydroalcoholic Extract Powder of Lepidium meyenii (Maca) Enhances Testicular Gene Expression of 3β-Hydroxysteroid Dehydrogenase in Rats. Andrologia 2017, 49. [Google Scholar] [CrossRef]
- Vecera, R.; Orolin, J.; Skottová, N.; Kazdová, L.; Oliyarnik, O.; Ulrichová, J.; Simánek, V. The Influence of Maca (Lepidium meyenii) on Antioxidant Status, Lipid and Glucose Metabolism in Rat. Plant Foods Hum. Nutr. 2007, 62, 59–63. [Google Scholar] [CrossRef]
- Qiu, C.; Zhu, T.; Lan, L.; Zeng, Q.; Du, Z. Analysis of Maceaene and Macamide Contents of Petroleum Ether Extract of Black, Yellow, and Purple Lepidium meyenii (Maca) and Their Antioxidant Effect on Diabetes Mellitus Rat Model. Braz. Arch. Biol. Technol. 2016, 59, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yucra, S.; Gasco, M.; Rubio, J.; Nieto, J.; Gonzales, G.F. Effect of Different Fractions from Hydroalcoholic Extract of Black Maca (Lepidium meyenii) on Testicular Function in Adult Male Rats. Fertil. Steril. 2008, 89, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Meissner, H.O.; Kapczynski, W.; Mscisz, A.; Lutomski, J. Use of Gelatinized Maca (Lepidium Peruvianum) in Early Postmenopausal Women. Int. J. Biomed. Sci. 2005, 1, 33–45. [Google Scholar] [PubMed]
- Dording, C.M.; Fisher, L.; Papakostas, G.; Farabaugh, A.; Sonawalla, S.; Fava, M.; Mischoulon, D. A Double-Blind, Randomized, Pilot Dose-Finding Study of Maca Root (L. Meyenii) for the Management of SSRI-Induced Sexual Dysfunction. CNS Neurosci. Ther. 2008, 14, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Stojanovska, L.; Law, C.; Lai, B.; Chung, T.; Nelson, K.; Day, S.; Apostolopoulos, V.; Haines, C. Maca Reduces Blood Pressure and Depression, in a Pilot Study in Postmenopausal Women. Climacteric 2015, 18, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Gonzales-Arimborgo, C.; Yupanqui, I.; Montero, E.; Alarcón-Yaquetto, D.E.; Zevallos-Concha, A.; Caballero, L.; Gasco, M.; Zhao, J.; Khan, I.A.; Gonzales, G.F. Acceptability, Safety, and Efficacy of Oral Administration of Extracts of Black or Red Maca (Lepidium meyenii) in Adult Human Subjects: A Randomized, Double-Blind, Placebo-Controlled Study. Pharmaceuticals 2016, 9, 49. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Jin, W.; Lv, X.; Dai, P.; Ao, Y.; Wu, M.; Deng, W.; Yu, L. Effects of Macamides on Endurance Capacity and Anti-Fatigue Property in Prolonged Swimming Mice. Pharm. Biol. 2016, 54, 827–834. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, W.C.; Wu, Z.Y.; Fu, C.X.; Hui, A.L.; Gao, H.; Chen, P.P.; Du, B.; Zhang, H.W. Two Macamide Extracts Relieve Physical Fatigue by Attenuating Muscle Damage in Mice: Anti-Fatigue Activity of Two Macamide Extracts. J. Sci. Food Agric. 2019, 99, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Zha, R.; Ge, E.; Guo, L.; Gao, Q.; Lin, Q.; Zhou, W.; Jin, X.; Xie, W.; Yin, H.; Liu, T. A Newly Identified Polyunsaturated Macamide Alleviates Dextran Sulfate Sodium-Induced Colitis in Mice. Fitoterapia 2021, 152, 104916. [Google Scholar] [CrossRef] [PubMed]
- Gugnani, K.S.; Vu, N.; Rondón-Ortiz, A.N.; Böhlke, M.; Maher, T.J.; Pino-Figueroa, A.J. Neuroprotective Activity of Macamides on Manganese-Induced Mitochondrial Disruption in U-87 MG Glioblastoma Cells. Toxicol. Appl. Pharmacol. 2018, 340, 67–76. [Google Scholar] [CrossRef]
- Clément, C.; Diaz Grados, D.A.; Avula, B.; Khan, I.A.; Mayer, A.C.; Ponce Aguirre, D.D.; Manrique, I.; Kreuzer, M. Influence of Colour Type and Previous Cultivation on Secondary Metabolites in Hypocotyls and Leaves of Maca (Lepidium meyenii Walpers). J. Sci. Food Agric. 2010, 90, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, J.; Fan, L. The Nutritional Composition of Maca in Hypocotyls (Lepidium meyenii Walp.) Cultivated in Different Regions of China. J. Food Qual. 2017, 2017, e3749627. [Google Scholar] [CrossRef] [Green Version]
- Bai, N.; He, K.; Roller, M.; Lai, C.S.; Bai, L.; Pan, M.H. Flavonolignans and Other Constituents from Lepidium meyenii with Activities in Anti-Inflammation and Human Cancer Cell Lines. J. Agric. Food Chem. 2015, 63, 2458–2463. [Google Scholar] [CrossRef]
- Dini, I.; Tenore, G.C.; Dini, A. Glucosinolates from Maca (Lepidium meyenii). Biochem. Syst. Ecol. 2002, 30, 1087–1090. [Google Scholar] [CrossRef]
- Li, G.; Ammermann, U.; Quirós, C.F. Glucosinolate Contents in Maca (Lepidium Peruvianum Chacón) Seeds, Sprouts, Mature Plants and Several Derived Commercial Products. Econ. Bot. 2001, 55, 255–262. [Google Scholar] [CrossRef]
- Cui, B.; Zheng, B.L.; He, K.; Zheng, Q.Y. Imidazole Alkaloids from Lepidium meyenii. J. Nat. Prod. 2003, 66, 1101–1103. [Google Scholar] [CrossRef]
- Zha, S.; Zhao, Q.; Chen, J.; Wang, L.; Zhang, G.; Zhang, H.; Zhao, B. Extraction, Purification and Antioxidant Activities of the Polysaccharides from Maca (Lepidium meyenii). Carbohydr. Polym. 2014, 111, 584–587. [Google Scholar] [CrossRef]
- Loyer, J. Communicating Superfoods: A Case Study of Maca Packaging In Food and Communication: Proceedings of the Oxford Symposium on Food and Cookery 2015; McWilliams, M., Ed.; Prospect Books: London, UK, 2016; pp. 236–246. ISBN 978-1-909-248-49-6. [Google Scholar]
- Rondán-Sanabria, G.G.; Finardi-Filho, F. Physical–Chemical and Functional Properties of Maca Root Starch (Lepidium meyenii Walpers). Food Chem. 2009, 114, 492–498. [Google Scholar] [CrossRef]
- Zhang, L.; Li, G.; Wang, S.; Yao, W.; Zhu, F. Physicochemical Properties of Maca Starch. Food Chem. 2017, 218, 56–63. [Google Scholar] [CrossRef]
- Lee, Y.K.; Chang, Y.H. Physicochemical and Antioxidant Properties of Methanol Extract from Maca (Lepidium meyenii Walp.) Leaves and Roots. Food Sci. Technol. 2019, 39, 278–286. [Google Scholar] [CrossRef] [Green Version]
- Caba, Z.T. The Concept of Superfoods in Diet. In The Role of Alternative and Innovative Food Ingredients and Products in Consumer Wellness; Elsevier: Amsterdam, The Netherlands, 2019; pp. 73–101. [Google Scholar] [CrossRef]
- Wolfe, D. Superfoods: The Food and Medicine of the Future; North Atlantic Books: Berkeley, CA, USA, 2009; ISBN 9781556437762. [Google Scholar]
- Driessche, J.J.; van den Plat, J.; Mensink, R.P. Effects of Superfoods on Risk Factors of Metabolic Syndrome: A Systematic Review of Human Intervention Trials. Food Funct. 2018, 9, 1944–1966. [Google Scholar] [CrossRef]
- Yan-Lin, S.; Lin-De, L.; Soon-Kwan, H. Eleutherococcus senticosus as a Crude Medicine: Review of Biological and Pharmacological Effects. JMPR 2011, 5, 5946–5952. [Google Scholar] [CrossRef]
- Baczek, K.; Węglarz, Z.; Przybył, J. Accumulation of Biologically Active Compounds in the Rhizomes and Roots of Eleuthero (Eleutherococcus senticosus/Maxim. Et Rupr./Maxim.). Adv. Environ. Biol. 2011, 5, 325–328. [Google Scholar]
- European Directorate for the Quality of Medicines & Health Care. Eleutherococci radix. In European Pharmacopoeia, Monograph 01/2008:1419: Corrected 7.0; European Directorate for the Quality of Medicines & Health Care: Strasburg, France, 2016. [Google Scholar]
- Huang, L.; Zhao, H.; Huang, B.; Zheng, C.; Peng, W.; Qin, L. Acanthopanax senticosus: Review of Botany, Chemistry and Pharmacology. Pharm. Int. J. Pharm. Sci. 2011, 66, 83–97. [Google Scholar] [CrossRef]
- Bleakney, T.L. Deconstructing an Adaptogen: Eleutherococcus senticosus. Holist. Nurs. Pract. 2008, 22, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Bokelmann, J.M. 43—Eleuthero/Siberian Ginseng (Eleutherococcus senticosus/Acanthopanax senticosus): Root. In Medicinal Herbs in Primary Care; Bokelmann, J.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 327–333. ISBN 9780323846769. [Google Scholar]
- Dowling, E.A.; Redondo, D.R.; Branch, J.D.; Jones, S.; McNabb, G.; Williams, M.H. Effect of Eleutherococcus senticosus on Submaximal and Maximal Exercise Performance. Med. Sci. Sports Exerc. 1996, 28, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Eschbach, L.C.; Webster, M.J.; Boyd, J.C.; McArthur, P.D.; Evetovich, T.K. The Effect of Siberian Ginseng (Eleutherococcus senticosus) on Substrate Utilization and Performance during Prolonged Cycling. Int. J. Sport Nutr. Exerc. Metab. 2000, 10, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Derosa, G.; Brillante, R.; Bernardi, R.; Nascetti, S.; Gaddi, A. Effects of Siberian Ginseng (Eleutherococcus senticosus Maxim.) on Elderly Quality of Life: A Randomized Clinical Trial. Arch. Gerontol. Geriatr. 2004, 38, 69–73. [Google Scholar] [CrossRef]
- Shen, M.L.; Zhai, S.K.; Chen, H.L.; Luo, Y.D.; Tu, G.R.; Ou, D.W. Immunomopharmacological Effects of Polysaccharides from Acanthopanax senticosus on Experimental Animals. Int. J. Immunopharmacol. 1991, 13, 549–554. [Google Scholar] [CrossRef]
- Augustin, J.M.; Kuzina, V.; Andersen, S.B.; Bak, S. Molecular Activities, Biosynthesis and Evolution of Triterpenoid Saponins. Phytochemistry 2011, 72, 435–457. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M. Effects of Various Eleutherococcus senticosus Cortex on Swimming Time, Natural Killer Activity and Corticosterone Level in Forced Swimming Stressed Mice. J. Ethnopharmacol. 2004, 95, 447–453. [Google Scholar] [CrossRef]
- Ahn, J.; Um, M.Y.; Lee, H.; Jung, C.H.; Heo, S.H.; Ha, T.Y. Eleutheroside E, An Active Component of Eleutherococcus senticosus, Ameliorates Insulin Resistance in Type 2 Diabetic Db/Db Mice. Evid.-Based Complement. Altern. Med. 2013, 2013, e934183. [Google Scholar] [CrossRef] [Green Version]
- Soine, T.O. Naturally Occurring Coumarins and Related Physiological Activities. J. Pharm. Sci. 1964, 53, 231–264. [Google Scholar] [CrossRef]
- Fang, J.N.; Proksch, A.; Wagner, H. Immunologically Active Polysaccharides of Acanthopanax senticosus. Phytochemistry 1985, 24, 2619–2622. [Google Scholar] [CrossRef]
- Załuski, D.; Olech, M.; Galanty, A.; Verpoorte, R.; Kuźniewski, R.; Nowak, R.; Bogucka-Kocka, A. Phytochemical Content and Pharma-Nutrition Study on Eleutherococcus senticosus Fruits Intractum. Oxid. Med. Cell. Longev. 2016, 2016, e9270691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asano, K.; Takahashi, T.; Miyashita, M.; Matsuzaka, A.; Muramatsu, S.; Kuboyama, M.; Kugo, H.; Imai, J. Effect of Eleutheroccocus Senticosus Extract on Human Physical Working Capacity. Planta Med. 1986, 52, 175–177. [Google Scholar] [CrossRef]
- Kuo, J.; Chen, K.W.C.; Cheng, I.S.; Tsai, P.H.; Lu, Y.J.; Lee, N.Y. The Effect of Eight Weeks of Supplementation with Eleutherococcus senticosus on Endurance Capacity and Metabolism in Human. Chin. J. Physiol. 2010, 53, 105–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamauchi, Y.; Ge, Y.W.; Yoshimatsu, K.; Komatsu, K.; Kuboyama, T.; Yang, X.; Tohda, C. Memory Enhancement by Oral Administration of Extract of Eleutherococcus senticosus Leaves and Active Compounds Transferred in the Brain. Nutrients 2019, 11, 1142. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Park, J.; Yoon, J.; Kim, M.; Choi, H.; Kim, H. Neuroprotective Effects of Eleutherococcus senticosus Bark on Transient Global Cerebral Ischemia in Rats. J. Ethnopharmacol. 2012, 139, 6–11. [Google Scholar] [CrossRef]
- Ahmed, S.; Moni, D.A.; Sonawane, K.D.; Paek, K.Y.; Shohael, A.M. A Comprehensive in Silico Exploration of Pharmacological Properties, Bioactivities and COX-2 Inhibitory Potential of Eleutheroside B from Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. J. Biomol. Struct. Dyn. 2021, 39, 6553–6566. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Luo, J.; Meng, C.; Jiang, N.; Cao, J.; Zhao, J. Syringin Exerts Neuroprotective Effects in a Rat Model of Cerebral Ischemia through the FOXO3a/NF-ΚB Pathway. Int. Immunopharmacol. 2021, 90, 107268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gu, H.; Zhao, Q.J.Y.; Li, H.; Shen, S.; Liu, X.; Wang, G.; Shi, Q. Syringin Protects against Colitis by Ameliorating Inflammation. Arch. Biochem. Biophys. 2020, 680, 108242. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Z.; Guo, Q.; Dong, Y.; Zhao, Q.; Ma, X. Syringin Prevents Bone Loss in Ovariectomized Mice via TRAF6 Mediated Inhibition of NF-ΚB and Stimulation of PI3K/AKT. Phytomedicine 2018, 42, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.Z.; Wei, L.; Zhao, H.F.; Huang, B.-K.; Rahman, K.; Qin, L.-P. The Effect of Eleutheroside E on Behavioral Alterations in Murine Sleep Deprivation Stress Model. Eur. J. Pharmacol. 2011, 658, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, X. Eleutheroside E Decreases Oxidative Stress and NF-ΚB Activation and Reprograms the Metabolic Response against Hypoxia-Reoxygenation Injury in H9c2 Cells. Int. Immunopharmacol. 2020, 84, 106513. [Google Scholar] [CrossRef]
- Ovodov, Y.S.; Frolova, G.M.; Nefedova, M.Y.; Elyakov, G.B. The Glycosides of Eleutherococcus senticosus II. The Structure of Eleutherosides A, B1, C, and D. Chem. Nat. Compd. 1967, 3, 53–54. [Google Scholar] [CrossRef]
- Kang, J.S.; Linh, P.T.; Cai, X.F.; Kim, H.S.; Lee, J.J.; Kim, Y.H. Quantitative Determination of Eleutheroside B and E From Acanthopanax Species by High Performance Liquid Chromatography. Arch. Pharm. Res. 2001, 24, 407–411. [Google Scholar] [CrossRef]
- Apers, S.; Naessens, T.; Van Miert, S.; Pieters, L.; Vlietinck, A. Quality Control of Roots of Eleutherococcus senticosus by HPLC. Phytochem. Anal. 2005, 16, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Yat, P.N.; Arnason, J.T.; Awang, D.V.C. An Improved Extraction Procedure for the Rapid, Quantitative High-Performance Liquid Chromatographic Estimation of the Main Eleutherosides (b and e) in Eleutherococcus senticosus (Eleuthero). Phytochem. Anal. 1998, 9, 291–295. [Google Scholar] [CrossRef]
- Li, X.C.; Barnes, D.L.; Khan, I.A. A New Lignan Glycoside from Eleutherococcus senticosus. Planta Med. 2001, 67, 776–778. [Google Scholar] [CrossRef] [PubMed]
- Hikino, H.; Takahashi, M.; Otake, K.; Konno, C. Isolation and Hypoglycemic Activity of Eleutherans A, B, C, D, E, F, and G: Glycans of Eleutherococcus senticosus Roots. J. Nat. Prod. 1986, 49, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Chang, S.Y.; Yook, C.S.; Nohara, T. New 3,4-Seco-Lupane-Type Triterpene Glycosides from Acanthopanax senticosus Forma Inermis. J. Nat. Prod. 2000, 63, 1630–1633. [Google Scholar] [CrossRef]
- Lee, S.; Shin, D.S.; Oh, K.B.; Shin, K.H. Antibacterial Compounds from the Leaves of Acanthopanax senticosus. Arch. Pharm. Res. 2003, 26, 40–42. [Google Scholar] [CrossRef]
- Suprunov, N.I.; Dzizenko, S.N. (−)-Sesamin from Eleutherococcus senticosus. Chem. Nat. Compd. 1971, 7, 502. [Google Scholar] [CrossRef]
- Choi, K. Botanical Characteristics, Pharmacological Effects and Medicinal Components of Korean Panax ginseng C A Meyer. Acta Pharmacol. Sin. 2008, 29, 1109–1118. [Google Scholar] [CrossRef] [Green Version]
- Zhuo, D. Preventive Geriatrics: An Overview from Traditional Chinese Medicine. Am. J. Chin. Med. 1982, 10, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Coon, J.T.; Ernst, E. Panax ginseng: A Systematic Review of Adverse Effects and Drug Interactions. Drug Saf. 2002, 25, 323–344. [Google Scholar] [CrossRef]
- Nocerino, E.; Amato, M.; Izzo, A.A. The Aphrodisiac and Adaptogenic Properties of Ginseng. Fitoterapia 2000, 71, S1–S5. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines & Health Care. Ginseng radix. In European Pharmacopoeia, Monograph 07/2019:1523; European Directorate for the Quality of Medicines & Health Care: Strasburg, France, 2019. [Google Scholar]
- Mancuso, C.; Santangelo, R. Panax ginseng and Panax Quinquefolius: From Pharmacology to Toxicology. Food Chem. Toxicol. 2017, 107, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Rauf, A. Adaptogenic Herb Ginseng (Panax) as Medical Food: Status Quo and Future Prospects. Biomed. Pharmacother. 2017, 85, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, D.S.; Pantuso, T. Panax ginseng. AFP 2003, 68, 1539–1542. [Google Scholar]
- Kim, S.H.; Park, K.S. Effects of Panax ginseng Extract on Lipid Metabolism in Humans. Pharmacol. Res. 2003, 48, 511–513. [Google Scholar] [CrossRef]
- Engels, H.-J.; Said, J.M.; Wirth, J.C. Failure of Chronic Ginseng Supplementation to Affect Work Performance and Energy Metabolism in Healthy Adult Females. Nutr. Res. 1996, 16, 1295–1305. [Google Scholar] [CrossRef]
- Zarabi, L.; Arazi, H.; Izadi, M. The Effects of Panax ginseng Supplementation on Growth Hormone, Cortisol and Lactate Response to High-Intensity Resistance Exercise. Biomed. Hum. Kinet. 2018, 10, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.G.; Cho, J.H.; Yoo, S.R.; Lee, J.S.; Han, J.M.; Lee, N.H.; Son, C.G. Antifatigue Effects of Panax ginseng CA Meyer: A Randomised, Double-Blind, Placebo-Controlled Trial. PLoS ONE 2013, 8, E61271. [Google Scholar]
- Christensen, L.P. Chapter 1 Ginsenosides: Chemistry, Biosynthesis, Analysis, and Potential Health Effects. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2008; Volume 55, pp. 1–99. ISBN 10434526. [Google Scholar]
- Leung, K.W.; Wong, A.S.T. Pharmacology of Ginsenosides: A Literature Review. Chin. Med. 2010, 5, 20. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Ko, M.J.; Chung, M.S. Subcritical Water Extraction of Bioactive Components from Red Ginseng (Panax ginseng C.A. Meyer). J. Supercrit. Fluids 2018, 133, 177–183. [Google Scholar] [CrossRef]
- Park, J.D.; Rhee, D.K.; Lee, Y.H. Biological Activities and Chemistry of Saponins from Panax ginseng C. A. Meyer. Phytochem. Rev. 2005, 4, 159–175. [Google Scholar] [CrossRef] [Green Version]
- Rausch, W.D.; Liu, S.; Gille, G.; Radad, K. Neuroprotective Effects of Ginsenosides. Acta Neurobiol. Exp. 2006, 66, 369–375. [Google Scholar]
- Smith, I.; Williamson, E.M.; Putnam, S.; Farrimond, J.; Whalley, B.J. Effects and Mechanisms of Ginseng and Ginsenosides on Cognition. Nutr. Rev. 2014, 72, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Qin, J.; Wang, W.; Wang, M.H.; Wang, H.; Zhang, R. Ginsenosides as Anticancer Agents: In Vitro and in Vivo Activities, Structure-Activity Relationships, and Molecular Mechanisms of Action. Front. Pharmacol. 2012, 3, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, L.W.; Wang, C.Z.; Yuan, C.S. Ginsenosides from American Ginseng: Chemical and Pharmacological Diversity. Phytochemistry 2011, 72, 689–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Qiao, X.; Li, K.; Fan, J.; Bo, T.; Guo, D.; Ye, M. Identification and Differentiation of Panax ginseng, Panax Quinquefolium, and Panax Notoginseng by Monitoring Multiple Diagnostic Chemical Markers. Acta Pharm. Sin. B 2016, 6, 568–575. [Google Scholar] [CrossRef] [Green Version]
- Du, Z.; Li, J.; Zhang, X.; Pei, J.; Huang, L. An Integrated LC-MS-Based Strategy for the Quality Assessment and Discrimination of Three Panax Species. Molecules 2018, 23, 2988. [Google Scholar] [CrossRef] [Green Version]
- Reay, J.L.; Scholey, A.B.; Kennedy, D.O. Panax ginseng (G115) Improves Aspects of Working Memory Performance and Subjective Ratings of Calmness in Healthy Young Adults. Hum. Psychopharmacol. Clin. Exp. 2010, 25, 462–471. [Google Scholar] [CrossRef]
- Bhattacharjee, I.; Bandyopadhyay, A. Effects of Acute Supplementation of Panax ginseng on Endurance Performance in Healthy Adult Males of Kolkata, India. Int. J. Clin. Exp. Physiol. 2020, 7, 63–68. [Google Scholar] [CrossRef]
- Etemadifar, M.; Sayahi, F.; Abtahi, S.H.; Shemshaki, H.; Dorooshi, G.-A.; Goodarzi, M.; Akbari, M.; Fereidan-Esfahani, M. Ginseng in the Treatment of Fatigue in Multiple Sclerosis: A Randomized, Placebo-Controlled, Double-Blind Pilot Study. Int. J. Neurosci. 2013, 123, 480–486. [Google Scholar] [CrossRef]
- Lee, S.A.; Kang, S.G.; Lee, H.J.; Jung, K.Y.; Kim, L. Effect of Korean Red Ginseng on Sleep: A Randomized, Placebo-Controlled Trial. Sleep Med. Psychophysiol. 2010, 17, 85–90. [Google Scholar]
- Attele, A.S.; Zhou, Y.-P.; Xie, J.T.; Wu, J.A.; Zhang, L.; Dey, L.; Pugh, W.; Rue, P.A.; Polonsky, K.S.; Yuan, C.-S. Antidiabetic Effects of Panax ginseng Berry Extract and the Identification of an Effective Component. Diabetes 2002, 51, 1851–1858. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Germolec, D.R.; Luster, M.I. Panax ginseng as a Potential Immunomodulator: Studies in Mice. Immunopharmacol. Immunotoxicol. 1990, 12, 257–276. [Google Scholar] [CrossRef] [PubMed]
- Ban, J.Y.; Kang, S.W.; Lee, J.S.; Chung, J.-H.; Ko, Y.G.; Choi, H.S. Korean Red Ginseng Protects against Neuronal Damage Induced by Transient Focal Ischemia in Rats. Exp. Ther. Med. 2012, 3, 693–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, K.H.; Cho, J.Y.; Kim, B.; Bae, B.-S.; Kim, J.-H. Red Ginseng (Panax ginseng) Decreases Isoproterenol-Induced Cardiac Injury via Antioxidant Properties in Porcine. J. Med. Food 2014, 17, 111–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.S.; Kim, Y.H.; Noh, J.R.; Cho, E.S.; Park, J.H.; Son, H.Y. Protective Effect of Korean Red Ginseng against Aflatoxin B1-Induced Hepatotoxicity in Rat. J. Ginseng Res. 2011, 35, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Abdelfattah-Hassan, A.; Shalaby, S.I.; Khater, S.I.; El-Shetry, E.S.; Abd El Fadil, H.; Elsayed, S.A. Panax ginseng Is Superior to Vitamin E as a Hepatoprotector against Cyclophosphamide-Induced Liver Damage. Complement. Ther. Med. 2019, 46, 95–102. [Google Scholar] [CrossRef]
- Han, Y.; Wang, T.; Li, C.; Wang, Z.; Zhao, Y.; He, J.; Fu, L.; Han, B. Ginsenoside Rg3 Exerts a Neuroprotective Effect in Rotenone-Induced Parkinson’s Disease Mice via Its Anti-Oxidative Properties. Eur. J. Pharmacol. 2021, 909, 174413. [Google Scholar] [CrossRef]
- Sun, J.; Wang, R.; Chao, T.; Peng, J.; Wang, C.; Chen, K. Ginsenoside Re Inhibits Myocardial Fibrosis by Regulating MiR-489/Myd88/NF-ΚB Pathway. J. Ginseng Res. 2021, S1226845321001688. [Google Scholar] [CrossRef]
- Gu, D.; Yi, H.; Jiang, K.; Fakhar, S.H.; Shi, J.; He, Y.; Liu, B.; Guo, Y.; Fan, X.; Li, S. Transcriptome Analysis Reveals the Efficacy of Ginsenoside-Rg1 in the Treatment of Nonalcoholic Fatty Liver Disease. Life Sci. 2021, 267, 118986. [Google Scholar] [CrossRef]
- Cui, J.; Shan, R.; Cao, Y.; Zhou, Y.; Liu, C.; Fan, Y. Protective Effects of Ginsenoside Rg2 against Memory Impairment and Neuronal Death Induced by Aβ25-35 in Rats. J. Ethnopharmacol. 2021, 266, 113466. [Google Scholar] [CrossRef]
- Mu, Q.; Zuo, J.; Zhao, D.; Zhou, X.; Hua, J.; Bai, Y.; Mo, F.; Fang, X.; Fu, M.; Gao, S. Ginsenoside Rg3 Reduces Body Weight by Regulating Fat Content and Browning in Obese Mice. J. Tradit. Chin. Med. Sci. 2021, 8, 65–71. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, Y.; Sun, N.; Zhou, L.; Zhang, D.; Chen, H.; Miao, W.; Gao, W.; Zhang, C.; Liu, C.; et al. Ginsenosides Rc, as a Novel SIRT6 Activator, Protects Mice against High Fat Diet Induced NAFLD. J. Ginseng Res. 2020, S1226845320301160. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, H.; Qiao, O.; Li, Z.; Pecoraro, L.; Zhang, X.; Han, X.; Wang, W.; Zhang, X.; Man, S.; et al. Nanoparticle Conjugation of Ginsenoside Rb3 Inhibits Myocardial Fibrosis by Regulating PPARα Pathway. Biomed. Pharmacother. 2021, 139, 111630. [Google Scholar] [CrossRef] [PubMed]
- Jihee, H.; Gwon, D.; Jang, C. Ginsenoside Rg1 Suppresses Cancer Cell Proliferation through Perturbing Mitotic Progression. J. Ginseng Res. 2021. [Google Scholar] [CrossRef]
- Hu, C.; Yang, L.; Wang, Y.; Zhou, S.; Luo, J.; Gu, Y. Ginsenoside Rh2 Reduces M6A RNA Methylation in Cancer via the KIF26B-SRF Positive Feedback Loop. J. Ginseng Res. 2021, 45, 734–743. [Google Scholar] [CrossRef]
- Ke, C.; Peng, Y.; Yuan, Z.; Cai, J. Ginsenoside Rb1 Protected PC12 Cells from Aβ25-35-Induced Cytotoxicity via PPARγ Activation and Cholesterol Reduction. Eur. J. Pharmacol. 2021, 893, 173835. [Google Scholar] [CrossRef]
- Lu, M.L.; Wang, J.; Sun, Y.; Li, C.; Sun, T.R.; Hou, X.W.; Wang, H.-X. Ginsenoside Rg1 Attenuates Mechanical Stress-Induced Cardiac Injury via Calcium Sensing Receptor-Related Pathway. J. Ginseng Res. 2021, 45, 683–694. [Google Scholar] [CrossRef]
- Besso, H.; Kasai, R.; Saruwatari, Y.; Fuwa, T.; Tanaka, O. Ginsenoside-Ra1 and Ginsenoside-Ra2, New Dammarane-Saponins of Ginseng Roots. Chem. Pharm. Bull. 1982, 30, 2380–2385. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-S.; Chang, Y.-J.; Zedk, U.; Zhao, P.; Liu, Y.-Q.; Yang, C.-R. Dammarane Saponins from Panax ginseng. Phytochemistry 1995, 40, 1493–1497. [Google Scholar] [CrossRef]
- Ma, X.Q.; Liang, X.-M.; Xu, Q.; Zhang, X.-Z.; Xiao, H.-B. Identification of Ginsenosides in Roots of Panax ginseng by HPLC-APCI/MS. Phytochem. Anal. 2005, 16, 181–187. [Google Scholar] [CrossRef]
- Fuzzati, N.; Gabetta, B.; Jayakar, K.; Pace, R.; Peterlongo, F. Liquid Chromatography–Electrospray Mass Spectrometric Identification of Ginsenosides in Panax ginseng Roots. J. Chromatogr. A 1999, 854, 69–79. [Google Scholar] [CrossRef]
- Samukawa, K.I.; Yamashita, H.; Matsuda, H.; Kubo, M. Simultaneous Analysis of Saponins in Ginseng Radix by High Performance Liquid Chromatography. Chem. Pharm. Bull. 1995, 43, 137–141. [Google Scholar] [CrossRef]
- Sun, B.S.; Gu, L.J.; Fang, Z.M.; Wang, C.; Wang, Z.; Lee, M.-R.; Li, Z.; Li, J.J.; Sung, C.-K. Simultaneous Quantification of 19 Ginsenosides in Black Ginseng Developed from Panax ginseng by HPLC–ELSD. J. Pharm. Biomed. Anal. 2009, 50, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Sanada, S.; Kondo, N.; Shoji, J.; Tanaka, O.; Shibata, S. Studies on the Saponins of Ginseng. I. Studies on the saponins of ginseng. I. Structure of ginseng-R0, Rb1, Rb2, RC and Rd. Chem. Pharm. Bull. 1974, 22, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Yahara, S.; Tanaka, O.; Komori, T. Saponins of the Leaves of Panax ginseng C. A. Meyer. Chem. Pharm. Bull. 1976, 24, 2204–2208. [Google Scholar] [CrossRef] [Green Version]
- Sanada, S.; Kondo, N.; Shoji, J.; Tanaka, O.; Shibata, S. Studies on the Saponins of Ginseng. II. Structures of Ginsenoside-Re, -Rf and -Rg2. Chem. Pharm. Bull. 1974, 22, 2407–2412. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Lu, Z.; Tan, G.T.; Qiu, S.; Farnsworth, N.R.; Pezzuto, J.M.; Fong, H.H.S. Polyacetyleneginsenoside-Ro, a Novel Triterpene Saponin from Panax ginseng. Tetrahedron Lett. 2002, 43, 973–977. [Google Scholar] [CrossRef]
- Dou, D.Q.; Chen, Y.J.; Liang, L.H.; Pang, F.G.; Shimizu, N.; Takeda, T. Six New Dammarane-Type Triterpene Saponins from the Leaves of Panax ginseng. Chem. Pharm. Bull. 2001, 49, 442–446. [Google Scholar] [CrossRef] [Green Version]
- Park, I.H.; Kim, N.Y.; Han, S.B.; Kim, J.M.; Kwon, S.W.; Kim, H.J.; Park, M.K.; Park, J.H. Three New Dammarane Glycosides from Heat Processed Ginseng. Arch. Pharm. Res. 2002, 25, 428. [Google Scholar] [CrossRef]
- Park, I.H.; Han, S.B.; Kim, J.M.; Piao, L.; Kwon, S.W.; Kim, N.Y.; Kang, T.L.; Park, M.K.; Park, J.H. Four New Acetylated Ginsenosides from Processed Ginseng (Sun Ginseng). Arch. Pharm. Res. 2002, 25, 837. [Google Scholar] [CrossRef]
- Qiu, F.; Ma, Z.-Z.; Xu, S.-X.; Yao, X.-S.; Che, C.-T.; Chen, Y.-J. A Pair of 24-Hydroperoxyl Epimeric Dammarane Saponins from Flower-Buds of Panax ginseng. J. Asian Nat. Prod. Res. 2001, 3, 235–240. [Google Scholar] [CrossRef]
- Uvarova, N.I.; Makhan’kova, V.V.; Malinovskaya, G.V.; Samoshina, N.F.; Atopkina, L.N.; Likhatskaya, G.N.; Kim, N.Y.; Anisimov, M.M.; Elyakov, G.B. Triterpene Glycosides from Wild and Cultivated Ginseng Occurring in Maritime Territory: Chemical Characterization, Comparative Quantitative Analysis, and Biological Activity Study. Pharm. Chem. J. 2000, 34, 122–129. [Google Scholar] [CrossRef]
- Lee, D.Y.; Cha, B.J.; Lee, Y.S.; Kim, G.S.; Noh, H.J.; Kim, S.Y.; Kang, H.C.; Kim, J.H.; Baek, N.I. The Potential of Minor Ginsenosides Isolated from the Leaves of Panax ginseng as Inhibitors of Melanogenesis. Int. J. Mol. Sci. 2015, 16, 1677–1690. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.; Song, S.B.; Tung, N.H.; Kim, K.E.; Kim, Y.H. Inhibition of TNF-α-Mediated NF-ΚB Transcriptional Activity by Dammarane-Type Ginsenosides from Steamed Flower Buds of Panax ginseng in HepG2 and SK-Hep1 Cells. Biomol. Ther. 2014, 22, 55–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.K.; Xu, F.; Li, S.S.; Cao, G.Y.; Gong, X.-J. Cytotoxic Epimeric Ginsenosides from the Flower Buds of Panax ginseng. Steroids 2019, 143, 1–5. [Google Scholar] [CrossRef]
- Samuel, A.D.; Tit, D.M.; Melinte, C.E.; Iovan, C.; Purza, L.; Gitea, M.; Bungau, S. Enzymological and Physicochemical Evaluation of the Effects of Soil Management Practices. Rev. Chim. 2017, 68, 2243–2247. [Google Scholar] [CrossRef]
- Bungau, S.; Behl, T.; Aleya, L.; Bourgeade, P.; Aloui-Sossé, B.; Purza, A.L.; Abid, A.; Samuel, A.D. Expatiating the Impact of Anthropogenic Aspects and Climatic Factors on Long-Term Soil Monitoring and Management. Environ. Sci. Pollut. Res. 2021, 28, 30528–30550. [Google Scholar] [CrossRef] [PubMed]
- Geszprych, A.; Weglarz, Z. Accumulation of Biologically Active Compounds in the Seeds of Rhaponticum carthamoides (Willd.) Iljin Cultivated in Poland. Folia Hortic. 2002, 14, 195–199. [Google Scholar]
- Kizelsztein, P.; Govorko, D.; Komarnytsky, S.; Evans, A.; Wang, Z.; Cefalu, W.T.; Raskin, I. 20-Hydroxyecdysone Decreases Weight and Hyperglycemia in a Diet-Induced Obesity Mice Model. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E433–E439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marschall, M.J.M.; Ringseis, R.; Gessner, D.K.; Grundmann, S.M.; Most, E.; Wen, G.; Maheshwari, G.; Zorn, H.; Eder, K. Effect of Ecdysterone on the Hepatic Transcriptome and Lipid Metabolism in Lean and Obese Zucker Rats. Int. J. Mol. Sci. 2021, 22, 5241. [Google Scholar] [CrossRef] [PubMed]




| Biological Active Compound | Plant Part | References |
|---|---|---|
| Phytosteroids | ||
| 20-Hydroxyecdysone | Roots Leaves Seeds | [47,48,49,50,51,52,55,86] [53,55,87,88] [53,54,56] |
| 20-Hydroxyecdysone 2-acetate | Roots | [50] |
| 20-Hydroxyecdysone 3-acetate | Roots | [50] |
| 20-Hydroxyecdysone 2,3-monoacetonide | Roots | [48,49,86] |
| 20-Hydroxyecdysone 20,22-monoacetonide | Roots | [48,49,86] |
| 20-Hydroxyecdysone 2,3;20,22-diacetonide | Roots | [48,49] |
| 2-Deoxyecdysterone | Roots | [86] |
| 3-epi-20-Hydroxyecdysone | Roots | [49] |
| 5-α-20-Hydroxyecdysone | Roots | [49] |
| 22-Oxo-20-Hydroxyecdysone | Roots | [49] |
| Leuzeasterone | Roots | [49] |
| Polypodine B | Roots Seeds | [48,49,51,86] [54] |
| Polypodin B-22-O-benzoate | Seeds | [56] |
| Polypodine B-20,22-acetonide | Roots | [48] |
| Inokosterone | Roots | [50,89] |
| Inokosterone 20,22-acetonide | Roots | [50] |
| Integristerone A | Roots | [49,50,86] |
| Integristeone A 20,22-acetonide | Roots | [50] |
| Integristerone B | Roots | [49] |
| 14-epi-Ponasterone A 22-glucoside | Roots | [50] |
| 15-Hydroxyponasterone A | Roots | [50] |
| Makisterone | Roots | [51] |
| Makisterone A | Roots Seeds | [48,49] [90] |
| Makisterone C | Roots | [49,50] |
| 24-epi-Makisterone A | Roots | [50] |
| 24(28)-Dehydromakisterone A | Roots Seeds | [50,51,86] [54] |
| 26-Hydroxymakisterone C | Roots | [50] |
| 1-Hydroxymakisterone C | Roots | [50] |
| (24Z)-29-Hydroxy-24(28)-dehydromakisterone C | Roots | [49,50] |
| 22-Deoxy-28-hydroxymakisterone C | Roots | [50] |
| Isovitexirone | Roots | [48,49] |
| Rhapisterone | Roots | [86] |
| Rhapisterone B | Seeds | [91] |
| Rhapisterone C | Seeds | [92] |
| Rhapisterone D | Seeds | [93] |
| Rhapisterone D 20-acetate | Seeds | [90] |
| Kaladasterone | Roots | [45] |
| 5-Deoxykaladasterone | Roots | [45,51] |
| Munisterone A | Roots | [45] |
| Taxisterone | Roots | [49] |
| Rubrosterone | Roots | [49] |
| Dihydrorubrosterone | Roots | [49] |
| Carthamosterone | Roots | [49,50,51] |
| Carthamosterone A | Seeds | [54] |
| Ajugasterone C | Roots | [45,48,49,50,51] |
| Amarasterone A | Roots | [50] |
| 24(28)-Dehydroamarasterone B | Roots | [50] |
| Turkesteron | Roots | [50] |
| Poststerone | Roots | [49] |
| Eriodictyol-7-β-glucopyranoside | Leaves | [24] |
| Flavonoids | ||
| Quercetin 5-O-galactoside | Roots | [26] |
| Isorhamnetin 5-O-rhamnoside | Roots | [26] |
| Patuletin 3′-β-xylofuranoside | Leaves | [25] |
| 6-Hydroxykaempferol-7-O-(6″-O-acetyl-β-D-glucopyranoside) | Leaves | [24] |
| Phenolic acids | ||
| Protocatechuic acid Benzoic acid o-Hydroxyphenylacetic acid p-Hydroxyphenylacetic acid m-Hydroxybenzoic acid p-Hydroxybenzoic acid Salicylic acid Gentisic acid Elagic acid Chlorogenic acid Vanillic acid o-Coumaric acid p-Coumaric acid Synapic acid Caffeic acid Ferulic acid Gallic acid Syringic acid | Roots | [27] |
| Essential oil-components | ||
| Geraniol | Roots and leaves | [85] |
| α-Pinene | Roots | [40,84] |
| β-Pinene | Roots | [84] |
| Limonene | Roots | [40,84] |
| β-Caryophyllene | Roots and leaves | [84,85] |
| 13-Norcypera-1(5),11(12)-diene | Roots | [40] |
| Cyperene | Roots | [40,84] |
| 2,5,8-Trimethyl-1-naphthol | Roots | [40] |
| Cadalene | Roots | [40] |
| Cyclosativene | Roots | [40,84] |
| β-Elemene | Roots | [40,84] |
| Biological Active Compound | Plant Part | References |
|---|---|---|
| Sterols | ||
| Brassicasteryl acetate | Tuber | [22] |
| Ergosteryl acetate | Tuber | [22] |
| Campesteryl acetate | Tuber | [22] |
| Δ22-Ergostadienyl acetate | Tuber | [22] |
| Sitosteryl acetate | Tuber | [22] |
| Campesterol | Hypocotyls and Leaves | [146] |
| β-Sytosterol | Hypocotyls and Leaves | [146] |
| Glucosinolates | ||
| Glucosinolate | Root | [30] |
| Benzyl Glucosinolate (Glucotropaeolin) | Hypocotyls Root/Tuber Fresh hypocotyls; Fresh leaf; Seed; Sprout; Dry hypocotyls | [114,126,147] [102,148,149] [150] |
| Desulfoglucotropaeolin | Root | [148] |
| m-Methoxybenzylglucosinolate | Tuber | [102,149] |
| 5-Methylsulfinylpentyt glucosinolate (glucoalyssin) | Fresh hypocotyls; Fresh leaf; Seed; Sprout; Dry hypocotyls | [150] |
| p-Hydroxybenzyl glucosinolate/4-Hydroxybenzyl glucosinolate (glucosinalbin) | Fresh hypocotyls; Fresh leaf; Seed; Sprout; Dry hypocotyls | [150] |
| p-Hydroxybenzyl glucosinolate/4-Hydroxybenzyl glucosinolate (glucosinalbin) | Hypocotyls | [126] |
| m-Hydroxybenzyl-glucosinolate | Fresh hypocotyls; Fresh leaf; Seed | [150] |
| Pent-4-enyl glucosinolate (glucobrassicanapin) | Fresh hypocotyls; Fresh leaf | [150] |
| Indolyl 3-methyl glucosinolate (glucobrassicin) | Fresh hypocotyls; Fresh leaf; Dry hypocotyls | [150] |
| p-Methoxybenzylglucosinolate | Fresh hypocotyls; Fresh leaf; Sprout; Dry hypocotyls | [150] |
| 4-Methoxyindolyl-3-methyl glucosinolate (4-methoxyglucobrassicin) | Fresh hypocotyls; Fresh leaf; Seed | [150] |
| 4-Methoxyindolyl-3-methyl glucosinolate (4-methoxyglucobrassicin) | Hypocotyls | [126] |
| 4-Hydroxy-3-indolylmethyl glucosinolate (4-Hydroxyglucobrassicin) | Hypocotyls | [126] |
| 3-Methoxybenzyl glucosinolate (Glucolimnanthin) | Hypocotyls | [126] |
| 5-Methylsulfinylpentyl glucosinolate (Glucoalyssin) | Hypocotyls | [126] |
| Alkaloids | ||
| Total Alkaloids | Root Hypocotyls | [30] [147] |
| Imidazole alkaloids | ||
| Lepidiline A (1,3-dibenzyl-4,5-dimethylimidazolium chloride) | Root | [151] |
| Lepidiline B (1,3-dibenzyl-2,4,5-trimethylimidazolium chloride) | Root | [148,151] |
| Pyrrole alkaloids | ||
| Macapyrrolins A | Root | [123] |
| Macapyrrolins B | Root | [123] |
| Macapyrrolins C | Root | [123] |
| Macamides | ||
| Macamides (benzylalkamides) | Root/Tuber | [30,97] |
| Hypocotyls | [114] | |
| Total macamides | Hypocotyls | [115] |
| Hypocotyls and Leaves | [146] | |
| N-benzylhexadecanamide | Hypocotyls | [104,115,147] |
| N-benzyl-(9Z)-octadecanamide | Hypocotyls | [104,115] |
| Methoxy-N-benzyl-(9Z,12Z)-octadecadienamide | Hypocotyls | [104] |
| N-benzyloctadecanamide | Hypocotyls | [104,115] |
| N-Benzylhexadecanamide | Hypocotyls Tuber | [115] [97] |
| N-benzyl-(9Z,12Z)-octadecadienamide | Hypocotyls | [104,115] |
| N-benzyl-(9Z,12Z,15Z)-octadecatrienamide | Hypocotyls | [104,115] |
| Methoxy-N-benzyl-(9Z,12Z,15Z)-octadecatrienamide | Hypocotyls | [104] |
| N-benzyl-5-oxo-6E,8E-octadecadienamide | Tuber | [97] |
| Makamide 1 (N-benzyl palmitamide) | Hypocotyls and Leaves | [146] |
| Makamide 2 (N-benzyl-5-oxo-6E, 8E-octadecadienamide) | Hypocotyls and Leaves | [146] |
| Macaridine (benzylated derivative of 1,2-dihydro-N-hydroxypyridine) | Tuber | [97] |
| Makaenes | ||
| Makaene (5-oxo-6E,8E-octadecadienoic acid) | Tuber | [97] |
| Makaene (5-oxo-6E, 8E-octadecadienoic acid) | Hypocotyls and Leaves | [146] |
| Flavolignans | ||
| Tricin 4′-O [threo-β-guaiacyl-(7″-O-methyl)-glyceryl] ether | Root | [148] |
| Tricin 4′-O-(erythro-β-guaiacyl-glyceryl) ether | Root | [148] |
| Others | ||
| Alkamides | Tuber | [103] |
| Total Phenols | Hypocotyls and Leaves | [146] |
| Benzylamine | Hypocotyls | [114] |
| Tricin | Root | [148] |
| Pinoresinol | Root | [148] |
| 4-Hydroxycinnamic acid | Root | [148] |
| Guanosine | Root | [148] |
| 3-Hydroxybenzylisothiocyanate | Root | [148] |
| 5-(Hydroxymethyl)-2-furfural | Root | [148] |
| Vanillic acid 4-O-β-D-glucoside | Root | [148] |
| Malic acid | Tuber | [102] |
| Malic acid benzoate | Root | [148] |
| Benzoyl derivative of malic acid | Tuber | [102] |
| Uridine acid | Tuber | [102] |
| Benzoyl derivates of uridine acid | Tuber | [102] |
| (1R,3S)-1-Methyltetrahydro-β-carboline-3-carboxylic acid | Tuber | [102] |
| Benzylisothiocyanate | Tuber Hypocotyls | [102] [114] |
| Polysaccharide MC-1 | Root | [127,152] |
| Nutritional Ingredient | Plant Part | References |
|---|---|---|
| Proteins | Root/Tuber Hypocotyls | [22,30] [147] |
| Oil | Root | [30] |
| Lipids | Tuber | [22] |
| Hydrolyzable carbohydrates | Tuber | [22] |
| Whole fibre | Tuber | [22] |
| Total dietary fibre | Hypocotyls | [147] |
| Amino acids | Root/Tuber | [22,30] |
| Aspartic acid | Root/Tuber | [22,30] |
| Hypocotyls | [147] | |
| Glutamic acid | Root/Tuber | [22,30] |
| Hypocotyls | [147] | |
| Serine | Root/Tuber | [22,30] |
| Hypocotyls | [147] | |
| Glycine | Root/Tuber | [22,30] |
| Hypocotyls | [147] | |
| Cysteine | Root/Tuber | [22,30] |
| Hypocotyls | [147] | |
| Alanine | Root/Tuber | [22,30] |
| Hypocotyls | [147] | |
| Arginine | Root/Tuber | [22,30] |
| Hypocotyls | [147] | |
| Tyrosine | Root/Tuber | [22,30] |
| Hypocotyls | [147] | |
| Hydroxy-Proline | Tuber | [22] |
| Proline | Root/Tuber | [22,30] |
| Hypocotyls | [147] | |
| Histidine | Root/Tuber | [22,30] |
| Hypocotyls | [147] | |
| Threonine | Root/Tuber | [22,30] |
| Hypocotyls | [147] | |
| Phenylalanine | Root/Tuber Hypocotyls | [22,30] [147] |
| D-phenylalanine | Root | [148] |
| Valine | Root/Tuber Hypocotyls | [22,30] [147] |
| Methionine | Root/Tuber Hypocotyls | [22,30] [147] |
| Isoleucine | Root/Tuber Hypocotyls | [22,30] [147] |
| Leucine | Root/Tuber Hypocotyls | [22,30] [147] |
| Lysine | Root/Tuber Hypocotyls | [22,30] [147] |
| Tryptophan | Tuber | [22] |
| Sarcosine | Tuber | [22] |
| Fatty acids | Root/Tuber | [22,114] |
| C12: 0 dodecanoic (lauric) | Tuber | [22] |
| C13:0 tridecanoic | Tuber | [22] |
| C13:1 7-tridecenoic | Tuber | [22] |
| C14:0 tetradecanoic (myristic) | Tuber | [22] |
| C15:0 pentadecanoic | Tuber | [22] |
| C15:1 7-pentadecenoic | Tuber | [22] |
| Cl6:0 esadecanoic (palmitic) | Tuber | [22] |
| C16:1 9-esadecenoic (palmitoleic) | Tuber | [22] |
| C17:0 heptadecanoic | Tuber | [22] |
| C17: l 9-heptadecenoic | Tuber | [22] |
| C18:0 octadecanoic (stearic) | Tuber | [22] |
| C18:1 9-octadecenoic (oleic) | Tuber | [22] |
| C18: 2 9, 12-octadecadienoic (linoleic) | Root/Tuber Hypocotyls | [22,114] [104] |
| C19:1 11-nonadecenoic | Tuber | [22] |
| Cl9:0 nonadecanoic | Tuber | [22] |
| C20: l 15-eicosenoic | Tuber | [22] |
| C20:0 eicosanoic (arachidic) | Tuber | [22] |
| C22:0 docosanoic (behenic) | Tuber | [22] |
| C24:0 tetracosanoic (lignoceric) | Tuber | [22] |
| C24:1 15-tetracosenoic (nervonic) | Tuber | [22] |
| Linolenic acid | Hypocotyls Root | [104] [114] |
| Minerals | Root/Tuber | [22,30] |
| Hypocotyls | [147] | |
| Fe | Root/Tuber Hypocotyls | [22,30] [147] |
| Mn | Root/Tuber Hypocotyls | [22,30] [147] |
| Cu | Root/Tuber Hypocotyls | [22,30] [147] |
| Na | Root/Tuber Hypocotyls | [22,30] [147] |
| K | Root/Tuber Hypocotyls | [22,30] [147] |
| Ca | Root/Tuber Hypocotyls | [22,30] [147] |
| Mg | Root Hypocotyls | [30] [147] |
| Zn | Root/Tuber Hypocotyls | [22,30] [147] |
| Biological Active Compound | Plant Part | References |
|---|---|---|
| Saponins and their glycosides | ||
| Eleutheroside A | Roots | [186] |
| Eleutheroside B (syringine) | Stem Roots | [187] [28,29,187,188,189] |
| Eleutheroside B1 (isofraxidine glucoside) | Roots | [28,29,186] |
| Isofraxidine—aglykone of Eleutheroside B1 | Roots | [28,29] |
| Eleutheroside C | Roots | [186] |
| Eleutheroside D (syringaresinol diglucoside) | Roots | [29] |
| Eleutheroside E ((-)syringaresinoldiglucoside) | Stem Roots | [187] [28,187,188] |
| Eleutheroside E (syringaresinol di-O-β-D-glucoside; liriodendrin) | Roots | [189] |
| Eleutheroside E2 | Roots | [190] |
| Syringaresinol (aglykone of Eleutherosde E) | Roots | [28,29] |
| Eleutherans A, B, C, D, E, F, G | Roots | [191] |
| Phenolic acids | ||
| Chlorogenic acid | Roots | [28,29] |
| p-Hydroxybenzoic acid | Roots | [29] |
| Vanillic acid | Roots | [29] |
| Syringic acid | Roots | [29] |
| p-Coumaric acid | Roots | [29] |
| Caffeic acid | Roots | [29] |
| Ethyl ester of caffeic acid | Roots | [28] |
| Ferulic acid | Roots | [29] |
| Triterpene glycosides | ||
| Inermoside | Leaves | [192] |
| 1-Deoxychiisanoside | Leaves | [192] |
| 24-Hydroxychiisanoside | Leaves | [192] |
| 11-Deoxyisochiisanoside | Leaves | [192] |
| Others | ||
| Chiisanoside | Leaves | [193] |
| Chiisanogenin | Leaves | [193] |
| Hyperin | Leaves | [193] |
| Isomaltol 3-O-alpha-D-glucopyranoside | Roots | [190] |
| (-) Sesamine | Roots | [28,194] |
| Sytoterole | Roots | [28] |
| Coniferine | Roots | [29] |
| Coniferylaldehyde | Roots | [28] |
| Coniferyl alcohol | Roots | [29] |
| Cumarine | Roots | [28] |
| Oleanolic acid | Roots | [28] |
| Polysaccharides | Roots | [174] |
| Biological Active Compound | Plant Part | References |
|---|---|---|
| Saponins and their glycosides | ||
| Ginsenoside Ra1 (20(S)-protopanaxadiol 3-O-β-D-glucopyranosyl(1–2)-β-D-glucopyranoside-20-O-β-D-xylopyranosyl(1–4)-α-L-arabinosyl(1–6)-β-D-glucopyranoside) | Roots | [238,239] |
| Ginsenoside Ra2 | Roots | [238,239] |
| Ginsenoside Ra3 | Roots | [240,241] |
| Ginsenoside Rb1 | Roots | [239,240,241,242,243,244] |
| Ginsenoside Rb2 | Roots | [239,240,241,242,243,244] |
| Ginsenoside Rb3 | Roots | [240,241,242] |
| Malonyl-Rb | Roots | [241] |
| Malonyl-Rb1 | Roots | [240,241] |
| Ginsenodide Rc | Roots | [239,240,241,242,243,244] |
| Ginsenoside Rd | Leaves Roots | [245] [239,240,241,242,243,244] |
| Malonyl-Rd | Roots | [240] |
| Ginsenoside Re | Roots Leaves | [239,240,241,242,243,246] [245] |
| Ginsenoside Rf | Roots | [239,240,241,242,243,246,247] |
| 20-Glc-Rf | Roots | [240] |
| Ginsenoside Rg1 | Roots Leaves | [239,240,241,242,243,247] [245] |
| Ginsenoside Rg2 | Roots | [239,241,246,247] |
| 20(S)-Ginsenoside-Rg2 | Roots | [240,242] |
| 20(R)-Ginsenoside-Rg2 | Roots | [242] |
| Ginsenoside Rg3 | Roots | [239] |
| 20(S)-Ginsenoside-Rg3 | Roots | [243] |
| 20(R)-Ginsenoside-Rg3 | Roots | [242,243] |
| Rg3/isomer | Roots | [240] |
| Ginsenoside Rg5 | Roots | [243] |
| Ginsenoside Rg6 | Roots | [243] |
| Ginsenoside Rg7 (3-O-β-D-glucopyranosyl 3β,12β,20(S),24(R)-tetrahydroxy-dammar-25-ene 20-O-β-D-glucopyranoside) | Leaves | [248] |
| Ginsenoside Rh | Roots | [239,242] |
| Ginsenoside 20(S)-Rh1 | Roots | [240,242] |
| Ginsenoside Rh4 | Roots | [240,243] |
| Ginsenoside Rh5 (3β,6α,12β,24xtetrahydroxy-dammar-20(22),25-diene 6-O-β-D-glucopyranoside) | Leaves | [248] |
| Ginsnoside Rh6 (3β,6α,12β,20(S)-tetrahydroxy-25-hydroperoxy-dammar-23-ene 20-O-β-D-glucopyranoside) | Leaves | [248] |
| Ginsenoside Rh7 (3β,7β,12β,20(S)-tetrahydroxy-dammar-5,24-diene 20-O-β-D-glucopyranoside) | Leaves | [248] |
| Ginsenoside Rh8 (3β,6α,20(S)-trihydroxy-dammar-24-ene-12-one 20-O-β-D-glucopyranoside) | Leaves | [248] |
| Ginsenoside Rh9 (3β,6α,20(S)-trihydroxy-12b,23-epoxy-dammar-24-ene 20-O-β-D-glucopyranoside) | Leaves | [248] |
| Ginsenoside Rk1 | Roots | [243,249] |
| Ginsenoside Rk2 | Roots | [249] |
| Ginsenoside Rk3 | Roots | [243,249] |
| Ginsenoside Ro | Roots | [239,241,242,244] |
| Ginsenoside Ro isomer | Roots | [240] |
| Polyacetyleneginsenoside-Ro | Roots | [247] |
| Ginsenoside-Ro methyl ester | Roots | [247] |
| Ginsenoside Rs1 | Roots | [242] |
| 20(S)-Ginsenoside Rs3 | Roots | [243] |
| 20(R)-Ginsenoside Rs3 | Roots | [243] |
| Ginsenoside Rs4 (3β,12β-dihydroxydammar-20(22),24-diene-3-O-β-D-glucopyranosyl(1→2)-P-D-6″-O-acetylglucopyranoside) | Roots | [243,250] |
| Ginsenoside Rs5 (3β,12β-dihydroxydammar-20(21), 24-diene-3-O-β-D-glucopyranosyl(1→2)-β-D-6″-O-acetylglucopyranoside) | Roots | [243,250] |
| Ginsenoside Rs6 (3β, 6α,12p-trihydro-xydammar-20(22),24-diene-6-O-β-D-6′-O-acetylglucopyranoside) | Roots | [250] |
| Ginsenoside Rs7 (3β,6α, 12β-trihydroxydam-mar-20(21),24-diene-6-O-β-D-6′-O-acetylglucopyranoside) | Roots | [250] |
| Ginsenoside F1 (20-O-β-glucopyranosyl-20(S)-protopanaxatriol) | Leaves | [245] |
| Ginsenoside F2 (3, 20-di-O-β-glucopyranosyl-20(S)-protopanaxadiol) | Leaves | [245] |
| Ginsenoside F3 (20-O-(α-arabinopyranosyl-(1→6)-β-glucopyranosyl)-20(S)-protopanaxatriol) | Leaves | [245] |
| Ginsenoside I | Flower buds | [251] |
| Ginsenoside II | Flower buds | [251] |
| Ginsenoside F4 | Roots | [243] |
| Malonyl-Ra1/Ra2 | Roots | [241] |
| Malonyl-Rb2/Rb3/Rc | Roots | [241] |
| Malonyl-Rd Notoginsenoside R2/F3 | Roots | [241] |
| Malonyl-Rd isomer | Roots | [241] |
| Ra1/Ra2/isomer | Roots | [240,241] |
| Gingerglycolipid B | Roots | [247] |
| Quinginsenoside R1 | Roots | [242] |
| Koryoginsenoside-R1 (6-O-[trans butenoyl-(1→6)-β-D-glucopyranosyl]-20-O-β-D-glucopyranosyl dammar-24-en-3β, 6α,12β,20(S)-tetrol) | Roots | [239] |
| Koryoginsenoside-R2 3-O-[β-D-glucopyranosyl-(1→2)-β-D-glucopyranosyl]-20-O-[β-D-glucopyranosyl-(1→6)-β-D-glucopyranosyl] dammar-22-en-3β, 12β, 20(S), -25-tetrol | Roots | [239] |
| Notoginsenoside R1 | Roots | [239] |
| Notoginsenoside R2 | Roots | [240] |
| Notoginseng R2 | Roots | [252] |
| Malonyl-Rg1 | Roots | [240] |
| Malonyl-Rc/Rb2/Rb3 | Roots | [240] |
| Rg6/F4 | Roots | [240] |
| Rg5/Rk1 | Roots | [240] |
| Bioactive Compounds | Rhaponticum carthamoides | Lepidium meyenii | Eleutherococcus senticosus | Panax ginseng |
|---|---|---|---|---|
| Phytosteroids | [24,47,48,49,50,51,52,53,54,55,56,86,87] | - | - | - |
| Glucosinolates | - | [30,102,114,126,147,148,149,150] | - | - |
| Alkaloids | - | [30,123,147,148,151] | - | - |
| Macamides and makaaenes | - | [30,97,104,114,115,146,147,148] | - | - |
| Eleutherosides | - | [127,152] | [28,29,186,187,188,190,191,192] | - |
| Ginsenosides | - | - | - | [238,239,240,241,242,243,244,245,246,247,248,249,250,251,252] |
| Effects/Activity | Rhaponthicum carthamoides | Lepidium meyenii | Eleutherococcus senticosus | Panax ginseng |
|---|---|---|---|---|
| Weight loss management | + | - | - | - |
| Lipid profile management | + | + | + | + |
| Nootropic activity | + | + | + | + |
| Diabetes management | + | + | + | + |
| Ergogenic activity | More data are needed. In process of monitoring | - | - | - |
| Hormones regulation | + | + | - | - |
| Antiviral activity | More data are needed | More data are needed | More data are needed | More data are needed |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorova, V.; Ivanov, K.; Ivanova, S. Comparison between the Biological Active Compounds in Plants with Adaptogenic Properties (Rhaponticum carthamoides, Lepidium meyenii, Eleutherococcus senticosus and Panax ginseng). Plants 2022, 11, 64. https://doi.org/10.3390/plants11010064
Todorova V, Ivanov K, Ivanova S. Comparison between the Biological Active Compounds in Plants with Adaptogenic Properties (Rhaponticum carthamoides, Lepidium meyenii, Eleutherococcus senticosus and Panax ginseng). Plants. 2022; 11(1):64. https://doi.org/10.3390/plants11010064
Chicago/Turabian StyleTodorova, Velislava, Kalin Ivanov, and Stanislava Ivanova. 2022. "Comparison between the Biological Active Compounds in Plants with Adaptogenic Properties (Rhaponticum carthamoides, Lepidium meyenii, Eleutherococcus senticosus and Panax ginseng)" Plants 11, no. 1: 64. https://doi.org/10.3390/plants11010064
APA StyleTodorova, V., Ivanov, K., & Ivanova, S. (2022). Comparison between the Biological Active Compounds in Plants with Adaptogenic Properties (Rhaponticum carthamoides, Lepidium meyenii, Eleutherococcus senticosus and Panax ginseng). Plants, 11(1), 64. https://doi.org/10.3390/plants11010064








