Study on the Effects of Salt Tolerance Type, Soil Salinity and Soil Characteristics on the Element Composition of Chenopodiaceae Halophytes
Abstract
:1. Introduction
2. Results
2.1. Patterns of Element Concentrations in the Leaf, Stem and Root
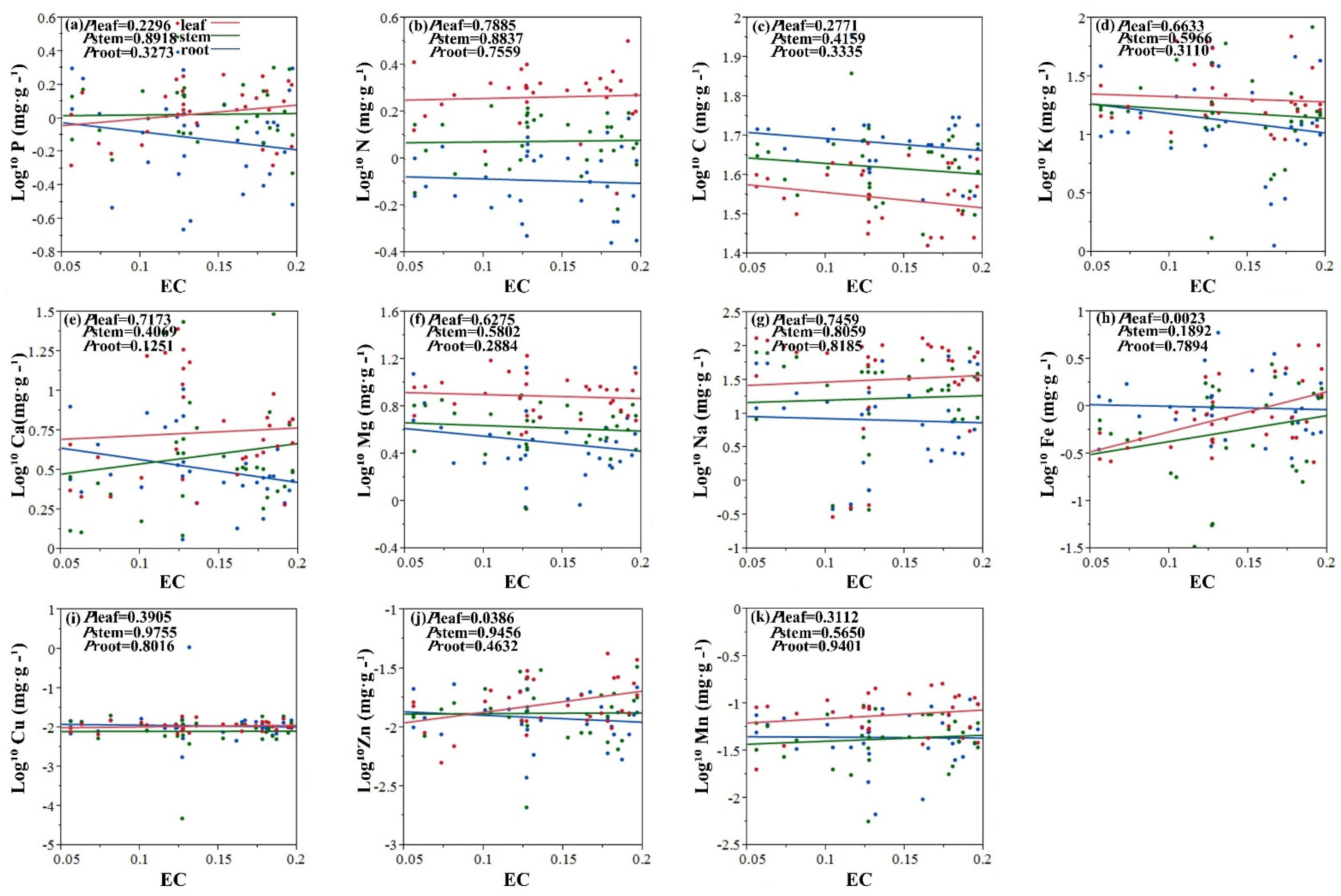
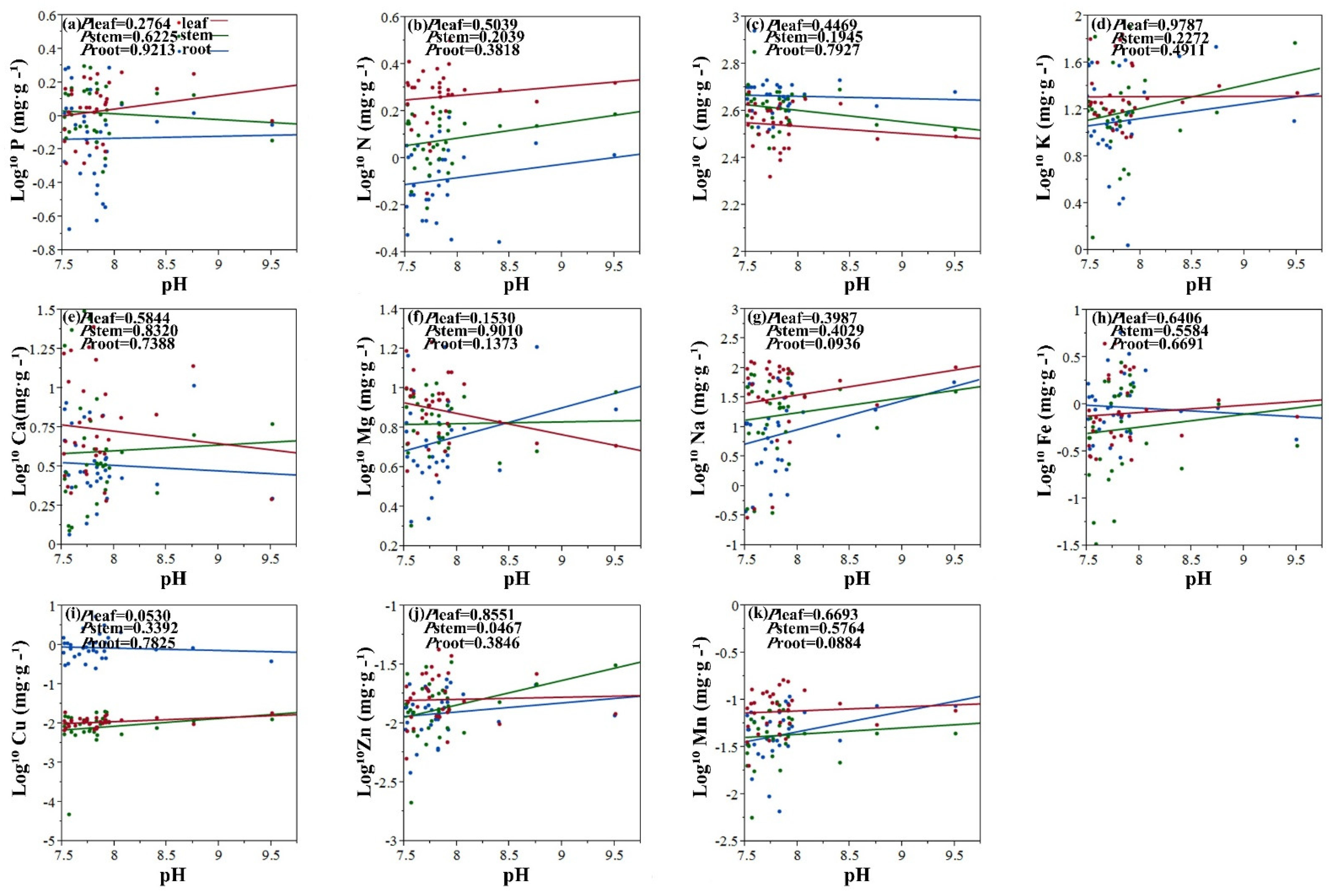
2.2. Relationships between Element Concentrations of Halophytes and Soil Salinity
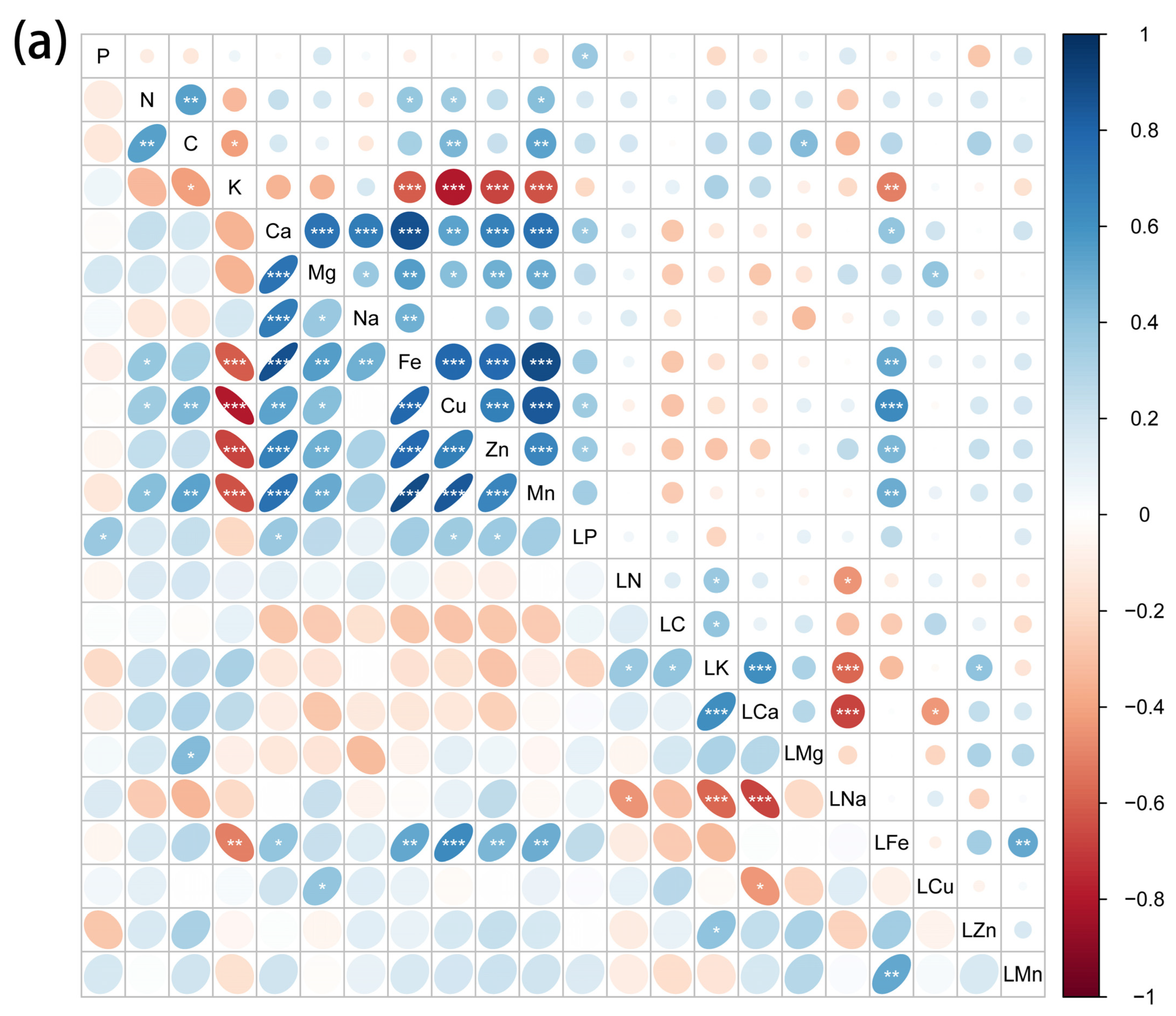
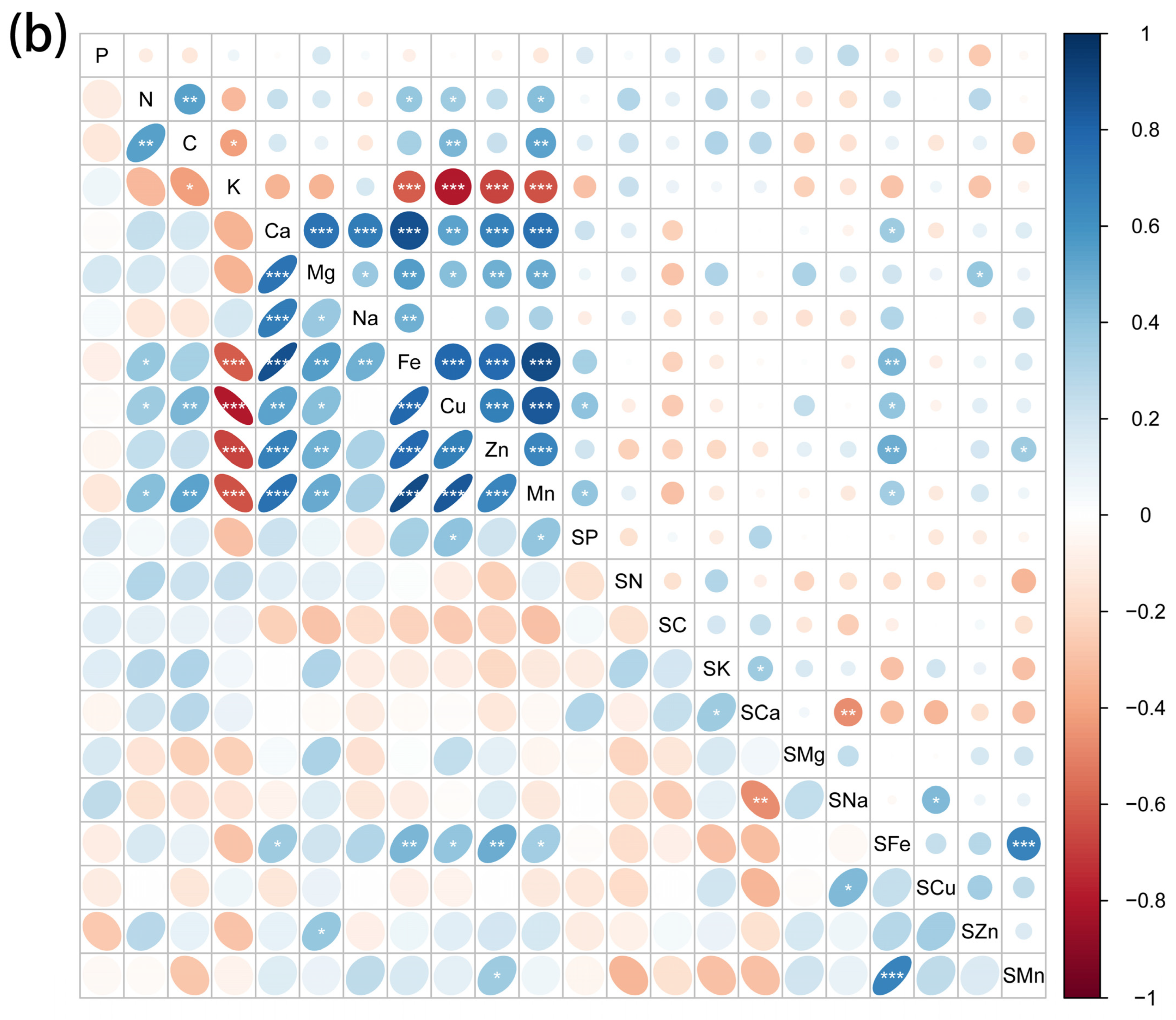
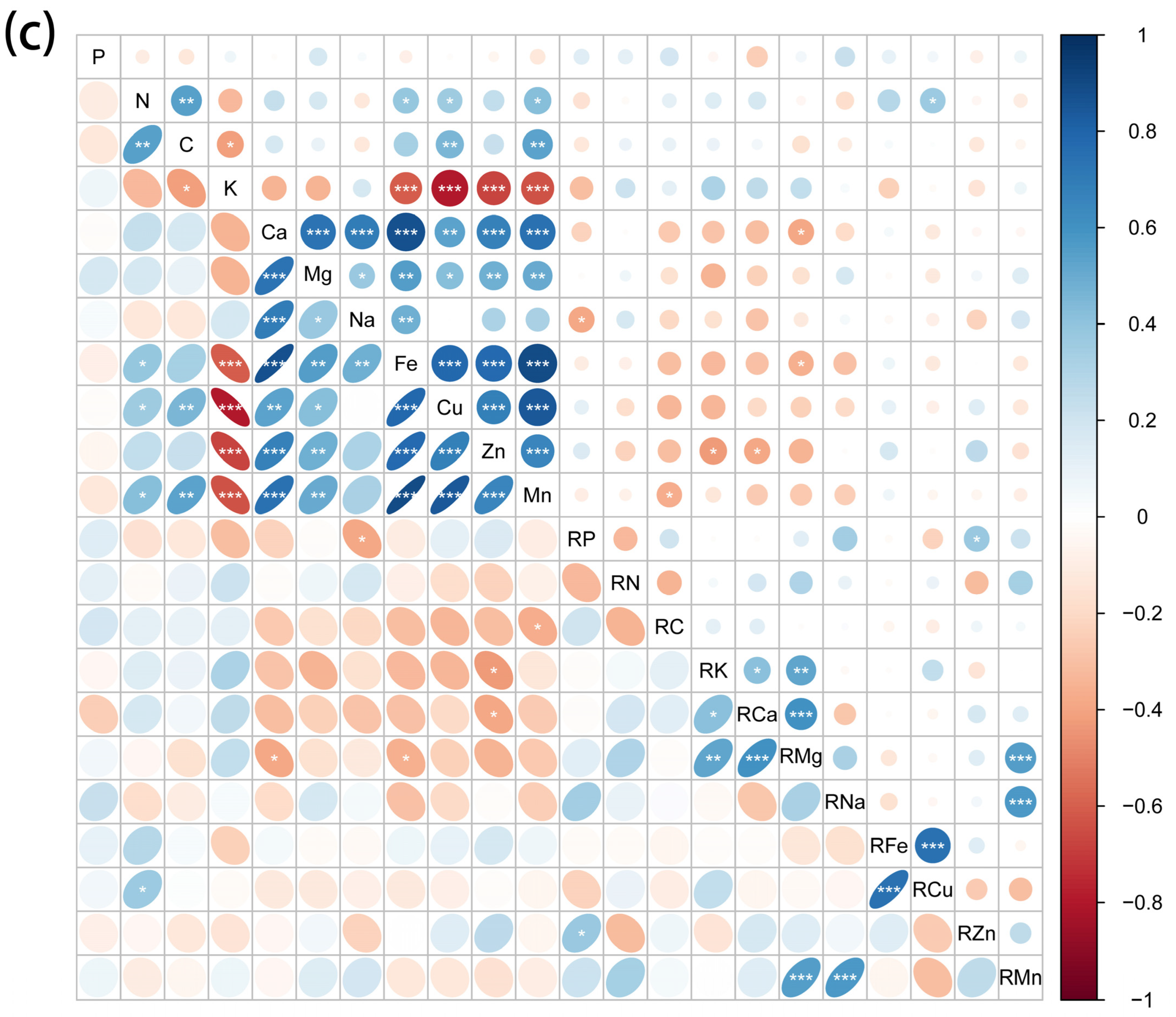
2.3. Correlations between Element Concentrations of Halophytes and Soil Mineral Elements
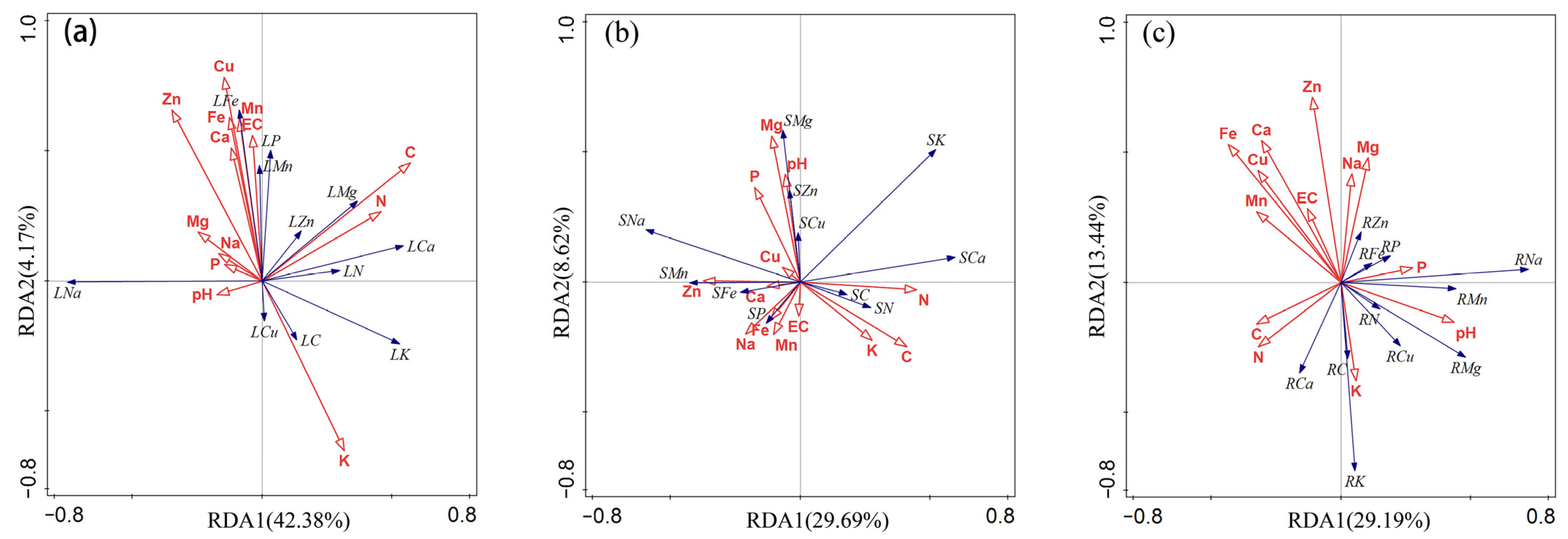
2.4. Relationship between the Elemental Composition of Plants, Soil Mineral Elements, and Soil Salinity
2.5. Element Composition in the Leaf, Stem and Root and Environmental Control
3. Discussion
3.1. The Effect of Salt Tolerance Type on Element Concentrations in Different Plant Parts
3.2. The Effects of Soil Salinity on Element Concentrations in Different Plant Parts
3.3. Element Composition in the Leaf, Stem and Root and Environmental Control
4. Materials and Methods
4.1. Study Area and Species
4.2. Soil and Plant Sampling and Chemical Analysis
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lobell, D.B.; Lesch, S.M.; Corwin, D.L.; Ulmer, M.G.; Anderson, K.A.; Potts, D.J.; Doolittle, J.A.; Matos, M.R.; Baltes, M.J. Regional-scale Assessment of Soil Salinity in the Red River Valley Using Multi-year MODIS EVI and NDVI. J. Environ. Qual. 2010, 39, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.X.; Shi, Z.Q.; Tian, Y.J.; Zhou, Q.; Cai, J.; Dai, T.B.; Cao, W.X.; Pu, H.C.; Jiang, D. Salt stress increases content and size of glutenin macropolymers in wheat grain. Food Chem. 2016, 197, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Rozema, J.; Flowers, T. Crops for a Salinized World. Science 2008, 322, 1478–1480. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Singh, A. Soil salinization and waterlogging: A threat to environment and agricultural sustainability. Ecol. Indic. 2015, 57, 128–130. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Liu, H.; Li, S.; Wang, Y.; Liu, Y. Ecological stoichiometry-based study of the influence of soil saline-alkali stress on nutrient homeostasis in L. chinensis. Ecotoxicol. Environ. Saf. 2018, 165, 243–249. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Q.; Wang, H.; Nie, S.; Liang, Z. Effects of soil salinity on the content, composition, and ion binding capacity of glomalin-related soil protein (GRSP). Sci. Total Environ. 2017, 581–582, 657–665. [Google Scholar] [CrossRef]
- Rodrigues, P.M.S.; Goncalves, C.E.; Schaefer, R.; Silva, J.D.; Ferreira, W.G.; Dos Santos, R.M.; Neri, A.V. The influence of soil on vegetation structure and plant diversity in different tropical savannic and forest habitats. J. Plant Ecol. 2018, 11, 226–236. [Google Scholar] [CrossRef]
- Sardans, J.; Rivas-Ubach, A.; Peñuelas, J. The C: N: P stoichiometry of organisms and ecosystems in a changing world: A review and perspectives. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 33–47. [Google Scholar] [CrossRef]
- Sun, X.; Gao, Y.; Wang, D.; Chen, J.; Zhang, F.; Zhou, J.; Yan, X.; Li, Y. Stoichiometric variation of halophytes in response to changes in soil salinity. Plant Biol. 2017, 19, 360–367. [Google Scholar] [CrossRef]
- Benko, Z.R. Survival strategies of annual desert plants (Adaptations of desert organisms). Community Ecol. 2003, 4, 115–117. [Google Scholar]
- Bromham, L. Macroevolutionary patterns of salt tolerance in angiosperms. Ann. Bot. 2014, 115, 333–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Zhao, K.F.; Song, J.; Feng, G.; Zhao, M.; Liu, J.P. Species, types, distribution, and economic potential of halophytes in China. Plant Soil 2011, 342, 495–509. [Google Scholar] [CrossRef]
- Rufo, L.; Iglesias-Lopez, M.T.; de la Fuente, V. The endemic halophyte Sarcocornia carinata Fuente, Rufo & Sanchez-Mata (Chenopodiaceae) in relation to environmental variables: Elemental composition and biominerals. Plant Soil 2021, 460, 189–209. [Google Scholar]
- Barakat, N.A.M.; Cazzato, E.; Nedjimi, B.; Kabiel, H.F.; Laudadio, V.; Tufarelli, V. Ecophysiological and species-specific responses to seasonal variations in halophytic species of the chenopodiaceae in a Mediterranean salt marsh. Afr. J. Ecol. 2013, 52, 163–172. [Google Scholar] [CrossRef]
- White, P.J.; Bowen, H.C.; Broadley, M.; El-Serehy, H.A.; Neugebauer, K.; Taylor, A.; Thompson, J.A.; Wright, G. Evolutionary origins of abnormally large shoot sodium accumulation in nonsaline environments within the Caryophyllales. New Phytol. 2016, 214, 284–293. [Google Scholar] [CrossRef]
- Mori, S.; Akiya, M.; Yamamura, K.; Murano, H.; Arao, T.; Kawasaki, A.; Higuchi, K.; Maeda, Y.; Yoshiba, M.; Tadano, T. Physiological Role of Sodium in the Growth of the Halophyte Suaeda salsa (L.) Pall. under High-Sodium Conditions. Crop Sci. 2010, 50, 2492–2498. [Google Scholar] [CrossRef]
- Matinzadeh, Z.; Breckle, S.-W.; Mirmassoumi, M.; Akhani, H. Ionic relationships in some halophytic Iranian Chenopodiaceae and their rhizospheres. Plant Soil 2013, 372, 523–539. [Google Scholar] [CrossRef]
- Kudo, N.; Sugino, T.; Oka, M.; Fujiyama, H. Sodium tolerance of plants in relation to ionic balance and the absorption ability of microelements. Soil Sci. Plant Nutr. 2010, 56, 225–233. [Google Scholar] [CrossRef]
- Pérez-López, U.; Miranda-Apodaca, J.; Mena-Petite, A.; Muñoz-Rueda, A. Responses of nutrient dynamics in barley seedlings to the interaction of salinity and carbon dioxide enrichment. Environ. Exp. Bot. 2014, 99, 86–99. [Google Scholar] [CrossRef]
- Ramoliya, P.J.; Patel, H.M.; Pandey, A.N. Effect of salinisation of soil on growth and macro- and micro-nutrient accumulation in seedlings of Acacia catechu (Mimosaceae). Ann. Appl. Biol. 2004, 144, 321–332. [Google Scholar] [CrossRef]
- Ball, M.; Pidsley, S.M. Growth Responses to Salinity in Relation to Distribution of Two Mangrove Species, Sonneratia alba and S. lanceolata, in Northern Australia. Funct. Ecol. 1995, 9, 77. [Google Scholar] [CrossRef]
- Loupassaki, M.H.; Chartzoulakis, K.S.; Digalaki, N.B.; Androulakis, I.I. Effects of salt stress on concentration of nitrogen, phosphorus, potassium, calcium, magnesium, and sodium in leaves, shoots, and roots of six olive cultivars. J. Plant Nutr. 2002, 25, 2457–2482. [Google Scholar] [CrossRef]
- Patel, A.D.; Jadeja, H.; Pandey, A.N. Effect of Salinization of Soil on Growth, Water status and nutrient accumulation in seedlings of Acacia auriculiformis (Fabaceae). J. Plant Nutr. 2010, 33, 914–932. [Google Scholar] [CrossRef]
- Miatto, R.C.; Batalha, M.A. Leaf chemistry of woody species in the Brazilian cerrado and seasonal forest: Response to soil and taxonomy and effects on decomposition rates. Plant Ecol. 2016, 217, 1467–1479. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Liu, Y.-Q.; Duan, H.-R.; Yin, X.-X.; Cui, Y.-N.; Chai, W.-W.; Song, X.; Flowers, T.J.; Wang, S.-M. SsHKT1;1 is coordinated with SsSOS1 and SsNHX1 to regulate Na+ homeostasis in Suaeda salsa under saline conditions. Plant Soil 2020, 449, 117–131. [Google Scholar] [CrossRef]
- Zhang, K.; Su, Y.; Yang, R. Biomass and nutrient allocation strategies in a desert ecosystem in the Hexi Corridor, northwest China. J. Plant Res. 2017, 130, 699–708. [Google Scholar] [CrossRef]
- Matinzadeh, Z.; Akhani, H.; Abedi, M.; Palacio, S. The elemental composition of halophytes correlates with key morphological adaptations and taxonomic groups. Plant Physiol. Biochem. 2019, 141, 259–278. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Song, X.; Tian, F.; Zhang, K.; Zhang, Z.; Chen, N.; Li, X. Divergent variations in concentrations of chemical elements among shrub organs in a temperate desert. Sci. Rep. 2016, 6, 20124. [Google Scholar] [CrossRef] [Green Version]
- Eva, V.Z.; Zhang, Y.X.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar]
- Flowers, T.J.; Troke, P.F.; Yeo, A.R. The mechanism of salt tolerance in halophytes. Annu. Rev. Plant Biol. 1977, 28, 89–121. [Google Scholar] [CrossRef]
- Donovan, L.A.; Richards, J.H.; Schaber, E.J. Nutrient relations of the halophytic shrub, Sarcobatus vermiculatus, along a soil salinity gradient. Plant Soil 1997, 190, 105–117. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J.; Colmer, T.D. Plant salt tolerance: Adaptations in halophytes. Ann. Bot. 2015, 115, 327–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, S.T.; Sima, N.A.K.K.; Mirzaei, H.H. Effects of sodium chloride on physiological aspects of Salicornia persica growth. J. Plant Nutr. 2013, 36, 401–414. [Google Scholar] [CrossRef]
- Maathuis, F.J.M. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef]
- Barbosa, J.P.R.A.D.; Rambal, S.; Soares, A.M.; Mouillot, F.; Nogueira, J.M.P.; Martins, G.A. Plant physiological ecology and the global changes. Cienc. Agrotecnol. 2012, 36, 253–269. [Google Scholar] [CrossRef] [Green Version]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef] [Green Version]
- Azeem, A.; Wu, Y.; Xing, D.; Javed, Q.; Ullah, I. Photosynthetic response of two okra cultivars under salt stress and re-watering. J. Plant Interact. 2017, 12, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Fujimaki, S.; Maruyama, T.; Suzui, N.; Kawachi, N.; Miwa, E.; Higuchi, K. Base to tip and long-distance transport of sodium in the root of common reed [Phragmites australis (Cav.) Trin. ex Steud.] at steady state under constant high-salt conditions. Plant Cell Physiol. 2015, 56, 943–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corwin, D.L.; Yemoto, K. Measurement of Soil Salinity: Electrical Conductivity and Total Dissolved Solids. Soil Sci. Soc. Am. J. 2019, 83, 1–2. [Google Scholar] [CrossRef]
- Elser, J.J.; Fagan, W.F.; Kerkhoff, A.J.; Swenson, N.G.; Enquist, B. Biological stoichiometry of plant production: Metabolism, scaling and ecological response to global change. N. Phytol. 2010, 186, 593–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouached, H.; Rhee, S.Y. System-level understanding of plant mineral nutrition in the big data era. Curr. Opin. Syst. Biol. 2017, 4, 71–77. [Google Scholar] [CrossRef]
- Yu, Q.; Elser, J.J.; He, N.; Wu, H.; Chen, Q.; Zhang, G.; Han, X. Stoichiometric homeostasis of vascular plants in the Inner Mongolia grassland. Oecologia 2011, 166, 1–10. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Guo, D.; Liang, S.; Wang, Y. Ecological stoichiometry homeostasis of Leymus chinensis in degraded grassland in western Jilin Province, NE China. Ecol. Eng. 2016, 90, 387–391. [Google Scholar] [CrossRef]
- Schreeg, L.A.; Santiago, L.S.; Wright, S.J.; Turner, B.L. Stem, root, and older leaf N:P ratios are more responsive indicators of soil nutrient availability than new foliage. Ecology 2014, 95, 2062–2068. [Google Scholar] [CrossRef] [Green Version]
- Krüger, H.R.; Peinemann, N. Coastal plain halophytes and their relation to soil ionic composition. Vegetatio 1996, 122, 143–150. [Google Scholar] [CrossRef]
- Agriculture, U. Diagnosis and improvement of saline and alkali soils. Soil Sci. 1954, 78, 154–155. [Google Scholar]

| Elements (mg·g−1) | Salt-Dilution Halophyte | Recretohalophyte | ||||
|---|---|---|---|---|---|---|
| Leaf | Stem | Root | Leaf | Stem | Root | |
| P | 1.07 ± 0.07 a | 1.07 ± 0.08 a | 0.89 ± 0.13 a | 1.26 ± 0.14 a | 1.19 ± 0.11 a | 0.91 ± 0.1 a |
| N | 18.87 ± 1.2 a | 11.69 ± 0.65 b | 7.99 ± 0.62 c | 19.08 ± 1.04 a | 13.63 ± 0.75 b | 9.81 ± 0.69 c |
| C | 344.01 ± 16.27 c | 418.66 ± 15.49 b | 485.57 ± 13.88 a | 371.94 ± 14.92 b | 436.67 ± 29.84 ab | 501.75 ± 40.58 a |
| K | 21.4 ± 3.65 a | 18.19 ± 4.38 a | 15.88 ± 3.22 a | 29.12 ± 4.82 a | 26.1 ± 4.83 a | 23.62 ± 4.47 a |
| Ca | 5.83 ± 1.25 a | 4.59 ± 1.47 a | 2.64 ± 0.17 a | 9.35 ± 1.61 a | 8.79 ± 2.58 a | 5.73 ± 0.72 a |
| Mg | 7.41 ± 0.5 a | 4.55 ± 0.4 b | 3.1 ± 0.36 c | 9.75 ± 1.13 a | 4.81 ± 0.55 b | 5.95 ± 1.36 b |
| Na | 72.26 ± 8.47 a | 40.57 ± 5.94 b | 24.08 ± 5.85 b | 38.64 ± 10.23 a | 18.27 ± 5.33 b | 10.81 ± 2.96 b |
| Fe | 1.15 ± 0.25 a | 0.98 ± 0.18 a | 1.33 ± 0.3 a | 1.12 ± 0.32 a | 0.73 ± 0.18 a | 1.25 ± 0.24 a |
| Cu | 0.01 ± 0 a | 0.01 ± 0 a | 0.07 ± 0.06 a | 0.01 ± 0 a | 0.01 ± 0 a | 0.01 ± 0 a |
| Zn | 0.02 ± 0 a | 0.02 ± 0 a | 0.01 ± 0 b | 0.02 ± 0 a | 0.02 ± 0 a | 0.01 ± 0 a |
| Mn | 0.09 ± 0.01 a | 0.06 ± 0.01 b | 0.05 ± 0.01 a | 0.07 ± 0.01 a | 0.03 ± 0 b | 0.06 ± 0.01 a |
| Na/K | 5.02 ± 0.8 a | 4.41 ± 1.51 a | 2.16 ± 0.49 a | 2.29 ± 0.71 a | 1.11 ± 0.36 ab | 0.52 ± 0.13 b |
| Elements (mg·g−1) | Salt Tolerance Type | Part | Salt Tolerance Type * Part | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| P | 1.43 | 0.23 | 3.531 | 0.033 | 0.294 | 0.746 |
| N | 3.126 | 0.080 | 61.601 | 0.000 | 0.555 | 0.576 |
| C | 1.380 | 0.243 | 19.752 | 0.000 | 0.043 | 0.958 |
| K | 4.903 | 0.029 | 0.822 | 0.443 | 0.000 | 1.000 |
| Ca | 9.688 | 0.002 | 3.097 | 0.050 | 0.077 | 0.926 |
| Mg | 10.149 | 0.002 | 21.717 | 0.000 | 1.935 | 0.150 |
| Na | 14.881 | 0.000 | 14.079 | 0.000 | 0.970 | 0.383 |
| Fe | 0.287 | 0.593 | 1.332 | 0.269 | 0.093 | 0.911 |
| Cu | 0.751 | 0.389 | 0.607 | 0.547 | 0.599 | 0.552 |
| Zn | 0.468 | 0.496 | 2.890 | 0.061 | 0.181 | 0.835 |
| Mn | 5.200 | 0.025 | 12.252 | 0.000 | 2.747 | 0.070 |
| Na/K | 10.329 | 0.002 | 2.872 | 0.062 | 0.378 | 0.686 |
| Leaf Element | Total Effects (r2, %) | |||
|---|---|---|---|---|
| Full | Salt Tolerance Type | Soil Mineral Element | Soil Salinity | |
| P | 68.5 | 5.5 | 52.7 | 10.3 |
| N | 29.4 | 0.5 | 20.6 | 8.3 |
| C | 26.2 | 4.3 | 19.5 | 2.4 |
| K | 41.9 | 5.2 | 33.9 | 2.8 |
| Ca | 45.5 | 9.1 | 35.9 | 0.5 |
| Mg | 56.8 | 13.5 | 40.7 | 2.6 |
| Na | 54 | 17.1 | 32.9 | 4 |
| Fe | 53.3 | 0.01 | 52.2 | 1.1 |
| Cu | 50.7 | 12.5 | 30.9 | 7.3 |
| Zn | 58.9 | 1.4 | 55.1 | 2.4 |
| Mn | 47.9 | 9.7 | 32.4 | 5.8 |
| Sites | Location | Geodesic Coordinates | Altitude (m) |
|---|---|---|---|
| 1 | Xin Barag Youqi | 48°49′12.7″ N, 116°51′40.83″ E | 547 |
| 2 | Xin Barag Youqi | 48°27′50.29″ N, 117°15′21.43″ E | 593 |
| 3 | Xin Barag Youqi | 48°20′55.06″ N, 117°52′11.22″ E | 574 |
| 4 | Xin Barag Zuoqi | 48°15′9.14″ N, 118°25′15.04″ E | 689 |
| 5 | Xin Barag Zuoqi | 48°16′43.55″ N, 118°3′44.56″ E | 585 |
| 6 | Xin Barag Zuoqi | 48°48′40.02″ N, 118°50′6.88″ E | 685 |
| Salt Tolerance Type | Species | Sites | Organ of Salt Concentration | Soil Texture | EC | pH | C | N | P |
|---|---|---|---|---|---|---|---|---|---|
| Salt-dilution halophyte | Suaeda salsa (L.) Pall. | 1, 3, 4 | Vacuole | Calcareous chernozem | 0.104 | 7.71 | 11.60 | 0.29 | 0.65 |
| Suaeda glauca (Bunge) Bunge | 1, 2, 3, 6 | 0.147 | 8.3 | 14.38 | 0.37 | 0.32 | |||
| Kalidium foliatum (Pall.) Moq. | 1 | 0.167 | 7.84 | 12.91 | 0.43 | 0.41 | |||
| Salsola collina Pall. | 1, 3 | 0.169 | 7.71 | 9.48 | 0.22 | 0.26 | |||
| Kochia scoparia (L.) Schrad. var. sieversiana (Pall.) Ulbr. ex Asch. & Graebn. | 1, 3 | 0.142 | 7.84 | 15.52 | 0.54 | 0.40 | |||
| Recretohalophyte | Atriplex patens (Litv.) Iljin | 1, 2, 4, 5 | Salt gland | Calcareous chernozem | 0.130 | 7.73 | 18.62 | 0.48 | 0.25 |
| Atriplex sibirica L. | 1, 2, 4, 5 | 0.140 | 8.01 | 11.44 | 0.40 | 0.41 | |||
| Chenopodium glaucum L. | 1, 2, 5, 6 | 1.25 | 7.74 | 17.97 | 0.58 | 0.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Su, Y.; Zheng, J.; Zhang, Z.; Liang, Z.; Tang, Z. Study on the Effects of Salt Tolerance Type, Soil Salinity and Soil Characteristics on the Element Composition of Chenopodiaceae Halophytes. Plants 2022, 11, 1288. https://doi.org/10.3390/plants11101288
Song X, Su Y, Zheng J, Zhang Z, Liang Z, Tang Z. Study on the Effects of Salt Tolerance Type, Soil Salinity and Soil Characteristics on the Element Composition of Chenopodiaceae Halophytes. Plants. 2022; 11(10):1288. https://doi.org/10.3390/plants11101288
Chicago/Turabian StyleSong, Xiaoqian, Yuhang Su, Jingwen Zheng, Zhonghua Zhang, Zhengwei Liang, and Zhonghua Tang. 2022. "Study on the Effects of Salt Tolerance Type, Soil Salinity and Soil Characteristics on the Element Composition of Chenopodiaceae Halophytes" Plants 11, no. 10: 1288. https://doi.org/10.3390/plants11101288
APA StyleSong, X., Su, Y., Zheng, J., Zhang, Z., Liang, Z., & Tang, Z. (2022). Study on the Effects of Salt Tolerance Type, Soil Salinity and Soil Characteristics on the Element Composition of Chenopodiaceae Halophytes. Plants, 11(10), 1288. https://doi.org/10.3390/plants11101288







