Associations between Integument Color and Physical and Physiological Quality in Pterodon pubescens Seeds
Abstract
:1. Introduction
2. Results
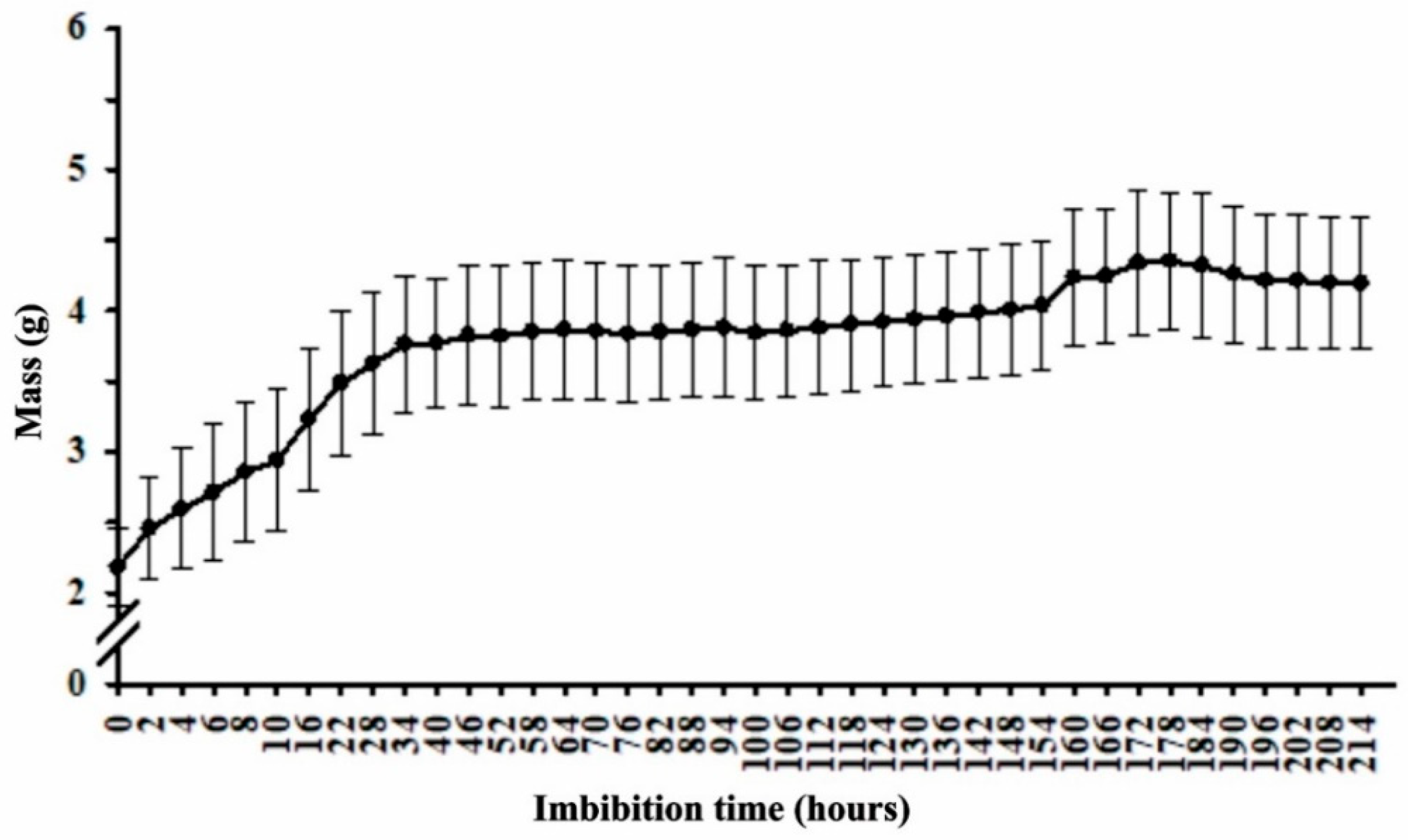
2.1. Imbibition Curve
2.2. Colorimetric Assessments
2.3. Germination Test
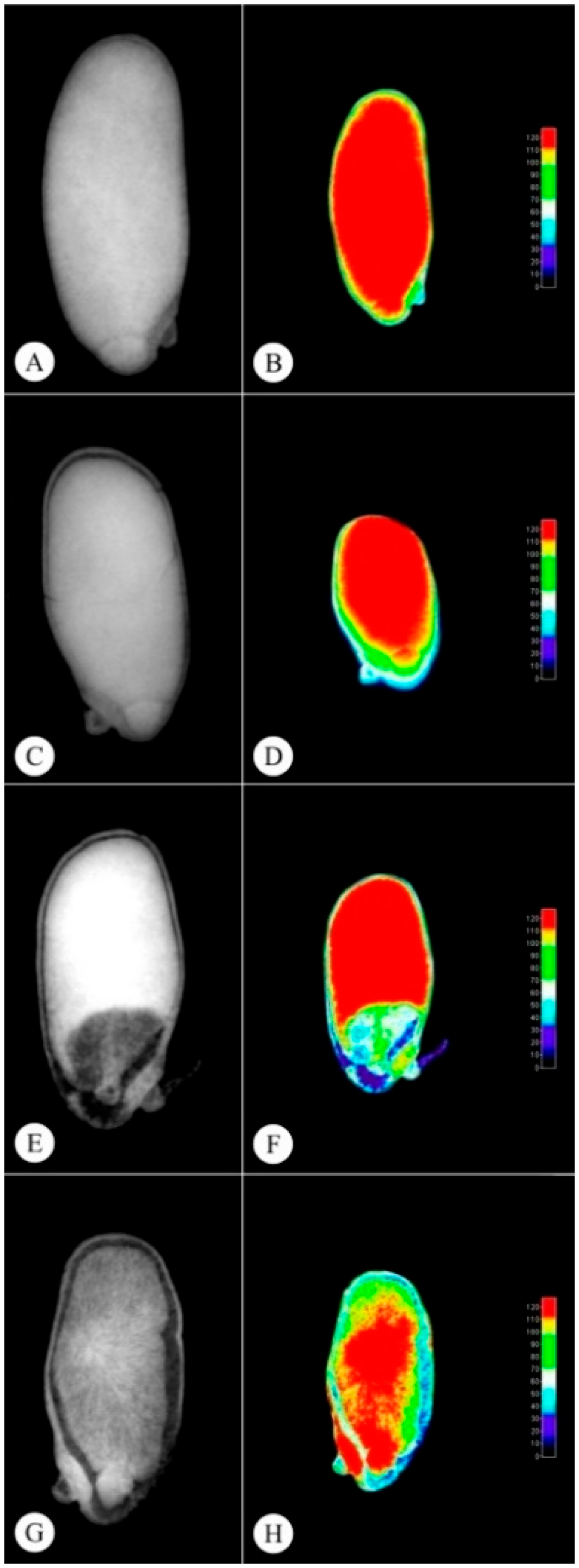
2.4. Radiographic Imaging
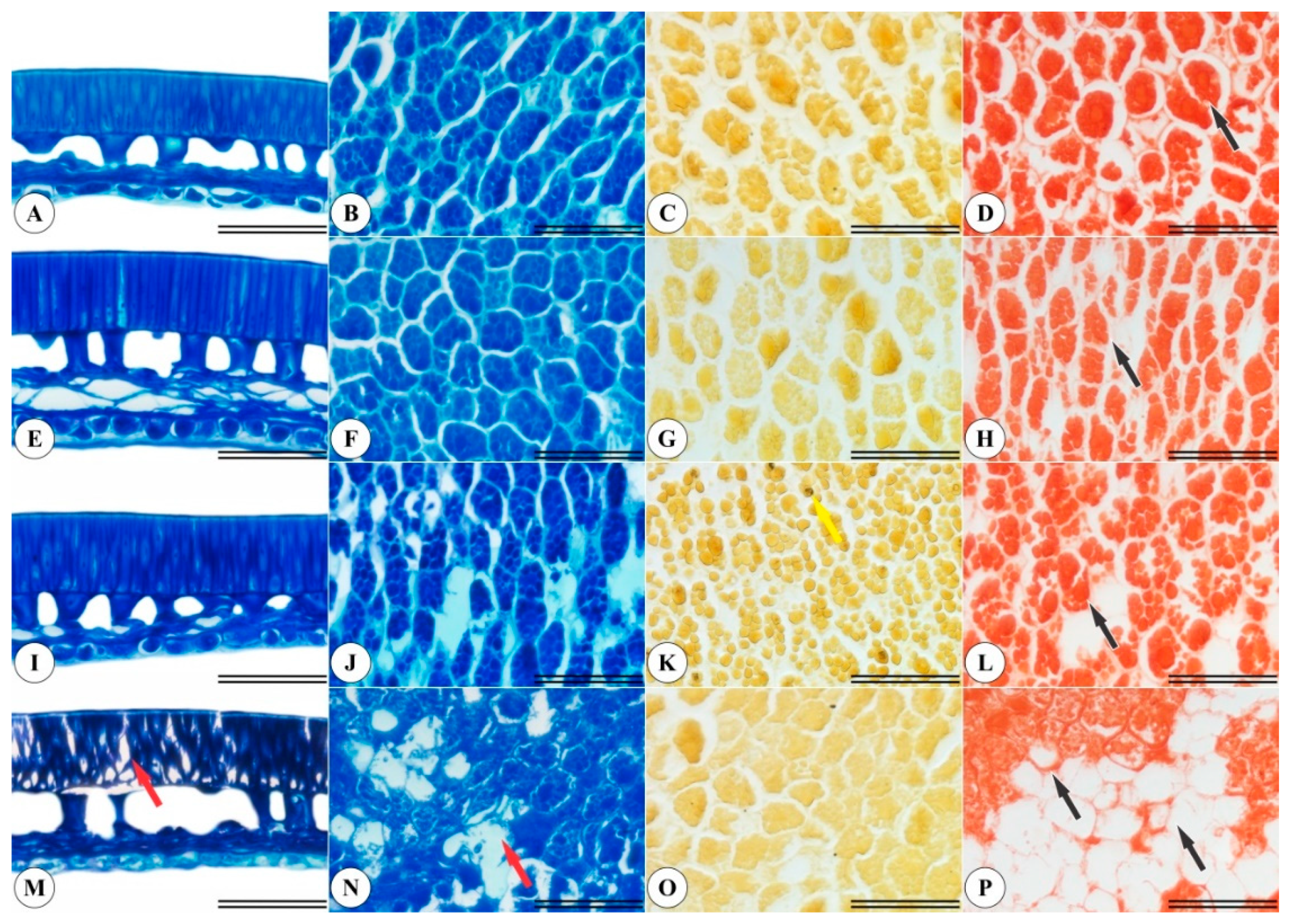
2.5. Anatomical and Histochemical Seed Characterizations
2.6. Biochemical Analyses
3. Discussion
4. Material and methods
4.1. Sampling
4.2. Imbibition Curve Test
4.3. Colorimetric Assessments
4.4. Radiographic Images
4.5. Physiological Assays
4.6. Morphoanatomical Seed Characterization
4.7. Determination of Antioxidant System Enzymatic Activity
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lorenzi, H.; Mato, F.J.A. Medicinal Plants in Brazil Native and Exotic; Plantarum Institute: Nova Odessa, Brazil, 2002; p. 428. [Google Scholar]
- Carvalho, C.S.; Cardoso, D.B.O.S.; Lima, H.C. Pterodon in Flora do Brasil 2020. Botanical Garden of Rio de Janeiro. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB29843 (accessed on 5 April 2022).
- Arriaga, A.M.C.; Castro, M.A.; Silveira, E.R.; Braz-Filho, R. Further diterpenoids isolated from Pterodon polygalaeflorus. J. Braz. Chem. Soc. 2000, 11, 187–190. [Google Scholar] [CrossRef] [Green Version]
- Agra, M.F.; Silva, K.N.; Basílio, I.J.L.D.; França, P.F.; Barbosa-Filho, J.M. Survey of medicinal plants used in the region Northeast of Brazil. Rev. Bras. Farm. 2008, 18, 472–508. [Google Scholar] [CrossRef]
- Hoscheid, J.; Outuki, P.M.; Kleinubing, S.A.; Silva, M.F.; Bruschi, M.L.; Cardoso, M.L.C. Development and characterization of Pterodon pubescens oil nanoemulsions as a possible delivery system for the treatment of rheumatoid arthritis, Colloids and Surfaces. Physicochem. Eng. Asp. 2015, 484, 19–27. [Google Scholar] [CrossRef]
- Basting, R.T.; Spindola, H.M.; Sousa, I.M.O.; Queiroz, N.C.A.; Trigo, J.R.T.; Carvalho, J.E.; Foglio, M.A. Pterodon pubescens and Cordia verbenacea association promotes a synergistic response in antinociceptive model and improves the anti-inflammatory results in animal models. Biomed. Pharmacother. 2019, 112, 108693. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, M.Y.M.; Zamora, L.O.; Araújo, R.S.; Fernandes, C.P.; Ricotta, T.Q.N.; Oliveira, L.G.; Queiroz-Junior, C.M.; Fernandes, A.P.; Conceição, E.C.; Ferreira, L.A.M.; et al. Efficacy of nanoemulsion with Pterodon emarginatus Vogel oleoresin for topical treatment of cutaneous leishmaniasis. Biomed. Pharmacother. 2021, 134, 111109. [Google Scholar] [CrossRef]
- Woods, A.J.; Martín-García, J.; Bulman, L.; Vasconcelos, M.W.; Boberg, J.; La Porta, N.; Peredo, H.; Vergara, G.; Ahumada, R.; Brown, A.; et al. Dothistroma needle blight, weather and possible climatic triggers for the disease’s recent emergence. For. Pathol. 2016, 46, 443–452. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Smets, P.; Chourmouzis, C.; Aitken, S.N.; Kolotelo, D. Conservation status of native tree species in British Columbia. Glob. Ecol. Conserv. 2020, 24, e01362. [Google Scholar] [CrossRef]
- Guidoni-Martins, K.G.; Maracahipes, L.; Melo, A.S.; Cianciaruso, M.V. Annual fires reduce local species richness but do not homogenize the composition of savanna woody species. Flora 2021, 281, 151868. [Google Scholar] [CrossRef]
- Zemouri, Z.; Djabeur, A.; Frimehdi, N.; Khelil, O.; Kaid-Harche, M. The seed diversity of Carob (Ceratonia siliqua L.) and the relationship between seeds color and coat dormancy. Sci. Hortic. 2020, 274, 109679. [Google Scholar] [CrossRef]
- Xia, Y.; Xu, Y.; Li, J.; Zhang, C.; Fan, S. Recent advances in emerging techniques for non-destructive detection of seed viability: A review. Artif. Intell. Agric 2019, 1, 35–47. [Google Scholar] [CrossRef]
- Medeiros, A.D.; Pinheiro, D.T.; Xavier, W.A.; Silva, L.J.; Dias, D.C.F.S. Quality classification of Jatropha curcas seeds using radiographic images and machine learning. Ind. Crops Prod. 2020, 146, 112162. [Google Scholar] [CrossRef]
- Oliveira, G.E. Physiological Quality and Expression of Amylase Enzymes in Seeds of Maize Lines. Dissertation, Federal University of Lavras, Lavras, Brazil, 2012. [Google Scholar]
- Chen, Y.; Fanourakis, D.; Tsaniklidis, G.; Aliniaeifard, S.; Yang, Q.; Li, T. Low UVA intensity during cultivation improves the lettuce shelf-life, an effect that is not sustained at higher intensity. Postharvest Biol. Technol. 2020, 172, 111376. [Google Scholar] [CrossRef]
- Degl’Innocenti, E.; Guidi, L.; Pardossi, A.; Tognoni, F. Biochemical study of leaf browning in minimally processed leaves of lettuce (Lactuca sativa L. var. Acephala). J. Agric. Food Chem. 2005, 53, 9980–9984. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013; p. 392. [Google Scholar]
- Marcos-Filho, J. Physiology of Seeds of Cultivated Plants; ABRATES: Londrina, Brazil, 2015; p. 659. [Google Scholar]
- Leão-Araújo, E.F.; Souza, E.R.B.; Peixoto, N.; Santos, W.V.; Costa, L.L.; Gomes-Júnior, F. Seed and fruit size affect soaking and physiological seed quality in Campomanesia adamantium? J. Seed Sci. 2020, 42, e202042035. [Google Scholar] [CrossRef]
- Jian-bo, C.; Li-min, H.; Charles, N.C.; Li-hong, Q.; Chun-yu, Z.; Yantun, S.; Rong, H. Ultrastructural studies of seed coat and cotyledon during rapeseed maturation. J. Integr. Agric. 2021, 20, 1239–1249. [Google Scholar] [CrossRef]
- Rios, P.A.F.; Araújo Neto, J.C.; Ferreira, V.M.; Neves, M.I.R.S. Seed Morphometry and Germination of Aechmea costantinii (Mez) L. B. Sm. (bromeliaceae). Caatinga Mag. 2016, 29, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Botelho, F.M.; Granella, S.J.; Botelho, S.C.C.; Garcia, T.R.B. Influence of drying temperature on the physical properties of soybeans. Eng. Agric. 2013, 23, 212–219. [Google Scholar]
- Atis, I.; Atak, M.; Can, E.; Mavi, K. Seed coat color effects on seed quality and salt tolerance of red clover (Trifolium pratense). Int. J. Agric. Biol. 2011, 13, 363–368. [Google Scholar]
- Velijevic, N.; Štrbanović, R.; Poštić, D.; Stanisavljevic, R.; Djukanovic, L. Effects of seed coat colour on the seed quality and initial seedling growth of red clover cultivars (Trifolium pratense). J. Process. Energy Agric. 2017, 21, 174–177. [Google Scholar] [CrossRef] [Green Version]
- Medeiros, A.D.; Bernardes, R.C.; Silva, L.J.S.; Freitas, B.A.L.; Dias, D.C.F.S.; Silva, C.B.S. Deep learning-based approach using X-ray images for classifying Crambe abyssinica seed quality. Ind. Crops Prod. 2021, 164, 113378. [Google Scholar] [CrossRef]
- Oliveira, D.M.T.; Paiva, E.A.S. Anatomy and Ontogeny of Pterodon emarginatus (fabaceae: Faboideae) Seed. Braz. J. Biol. 2005, 65, 483–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zienkiewicz, A.; Zienkiewicz, K.; Rejón, J.D.; de Dios Alché, J.; Castro, A.J.; Rodríguez-García, M.I. Olive seed protein bodies store degrading enzymes involved in mobilization of oil bodies. J. Exp. Bot 2014, 65, 103–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzottini-dos-Santos, H.C.; Ribeiro, L.M.; Oliveira, D.M.T. Roles of the haustorium and endosperm during the development of seedlings of Acrocomia aculeata (Arecaceae): Dynamics of reserve mobilization and accumulation. Protoplasma 2017, 254, 1563–1578. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2022, 7, 405–410. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Scandalios, J.G. Oxidative stress: Molecular perception and transduction of signals triggering antioxidant gene defenses. Braz. J. Med. Biol. Res. 2005, 38, 995–1014. [Google Scholar] [CrossRef]
- Bailly, C. Active oxygen species and antioxidants in seed biology. Seed Sci. Res. 2004, 14, 93–107. [Google Scholar] [CrossRef]
- Ramalheiro, J.P.S.C. Contribution to the Biochemical Characterization of the Ripening State of Olives of Different Varieties. Dissertation, Technical University of Lisbon, Lisbon, Portugal, 2009; p. 51. [Google Scholar]
- Mei, Y.; Song, S. Response to temperature stress of reactive oxygen species scavengig enzymes in the cross-tolerance of barley seed germination. J. Zhejiang Univ. Sci. B 2010, 11, 965–972. [Google Scholar] [CrossRef]
- Freitas, A.A.; Francelin, M.F.; Hirata, G.F.; Clemente, E.; Schmidt, F.L. Activities of peroxidase (POD) and polyphenoloxidase (PPO) enzymes in benitaka and rubi grapes and in their juices and jellies. Food Sci. Technol 2008, 28, 172–177. [Google Scholar] [CrossRef] [Green Version]
- Clemente, E.; Pastore, G.M. Peroxidase and polyphenoloxidase, the importance for food technology. Ciênc. Tecnol. Aliment 1988, 32, 167–171. [Google Scholar]
- Amorim, H.V.; Josephson, R.V. Water-soluble protein and non-protein components of brasilian green coffes beans. J. Food Sci. 1975, 40, 1179–1185. [Google Scholar] [CrossRef]
- Deuner, C.; Maia, M.S.; Deuner, S.; Almeida, A.S.; Meneghello, G.E. Viability and antioxidant activity of kidney bean genotypes seeds submitted to saline stress. Braz. Seed Mag. 2011, 33, 711–720. [Google Scholar]
- Regras Para Análise de Sementes. Ministério da Agricultura e Reforma Agrária; Coordenação de Laboratório Vegetal: Brasília, Brazil, 2009; ISBN 978-85-99851-70-8. [Google Scholar]
- Martinazzo, A.P.; Corrêa, P.C.; Melo, E.C.; Carneiro, A.P.S. Colorimetric evaluation of dried leaves of Cymbopogon citratus (D.C.) Stapf during storage in different packages. Braz. J. Agroind. Prod. 2008, 10, 131–140. [Google Scholar]
- Silva, R.C.; Castrillon, S.K.I.; Fernandes, J.R.C.; Morais, F.F.; Morini, A.A.E.T.; Nunes, J.R.S. Overcoming dormancy in seeds of Pterodon pubescens (Benth.) Benth. (Leguminosae, Papilionoideae). Res. Soc. Dev. 2021, 10, e3221019144. [Google Scholar] [CrossRef]
- Maguire, J.D. Speed of germination in selection and evaluation of seedling emergence and vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Karnovsky, M.J. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron-microscopy. J. Cell Biol. 1965, 27, 137–138A. [Google Scholar]
- O’Brien, T.P.; Feder, N.; Mccully, M.E. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 1964, 59, 368–373. [Google Scholar] [CrossRef]
- Jensen, W.A. Botanical Histochemistry: Principles and Practice; WH Freeman: San Francisco, CA, USA, 1962; p. 408. [Google Scholar]
- O’Brien, T.P.; Mccully, M.E. The Study of Plant Structure Principles and Selected Methods; Termarcarphi Pty Ltd.: Melbourne, Australia, 1981. [Google Scholar]
- Bernfeld, P. Amylase α and β. Methods Enzymol. 1955, 1, 149–151. [Google Scholar] [CrossRef]
- Tárrago, J.F.; Nicolás, G. Starch degradation in the cotyledons of germinating lentils. Plant Physiol. 1976, 58, 618–621. [Google Scholar] [CrossRef] [Green Version]
- Kishorekumar, A.; Jaleel, C.A.; Manivannan, P.; Sankar, B.; Sridharan, R.; Panneerselvam, R. Comparative effects of different triazole compaunds on growth, photosynthetic pigments and carbohydrate metabolism of Solenostemon rotundifolius. Colloids Surf. 2007, 60, 207–212. [Google Scholar] [CrossRef]
- Del Longo, O.T.; González, C.A.; Pastori, G.M.; Trippi, V.S. Antioxidant defences under hyperoxygenic and hyperosmotic conditions in leaves of two lines of maize with differential sensitivity to drought. Plant Cell Physiol. 1993, 34, 1023–1028. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Analyt. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium Deficiency and High Light Intensity Enhance Activities of Superoxide Dismutase, Ascorbate Peroxidase, and Glutathione Reductase in Bean Leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [Green Version]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Kar, M.; Mishra, D. Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol. 1976, 57, 315–319. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.N. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Cakmak, I.; Horst, W.J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol. Plant 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Ferreira, D.F. SISVAR: A computer analysis system to fixed effects split plot type designs. Braz. J. Biom. 2020, 37, 529–535. [Google Scholar] [CrossRef] [Green Version]

| Seed Class | L | a * | b * | Cr | °h |
|---|---|---|---|---|---|
| Yellow | 36.19 ± 0.305 a | 8.05 ± 0.125 a | 22.88 ± 0.517 a | 24.26 ± 0.511 a | 1.53 ± 0.001 a |
| Light brown | 32.24 ± 0.462 b | 9.00 ± 0.254 a | 20.08 ± 0.248 a | 22.01 ± 0.218 a | 1.47 ± 0.001 a |
| Dark brown | 23.89 ± 0.435 c | 8.64 ± 0.669 a | 12.44 ± 1.824 b | 15.18 ± 1.820 b | 1.32 ± 0.040 b |
| Black | 20.58 ± 0.181 d | 1.44 ± 0.275 b | 1.63 ± 0.193 c | 2.17 ± 0.327 c | 0.39 ± 0.041 c |
| One-Way ANOVA | |||||
| F (t-test) | 395.13 * | 85.19 * | 97.86 * | 105.80 * | 315.27 * |
| P | <0.0000 | <0.0000 | <0.0000 | <0.0000 | <0.0000 |
| CV (%) | 2.23 | 9.92 | 11.67 | 10.52 | 4.43 |
| Seed Class | G% 5 DAS | G% 13 DAS | PN% | PA% | GSI |
|---|---|---|---|---|---|
| Yellow | 45 ± 5.701 a | 87 ± 4.062 a | 64 ± 6.403 a | 23 ± 6.042 a | 3.61 ± 0.166 a |
| Light brown | 31 ± 4.848 a | 77 ± 6.245 a | 52 ± 6.042 a | 25 ± 5.244 a | 3.42 ± 0.394 a |
| Dark brown | 8 ± 2.550 b | 34 ± 6.782 b | 16 ± 4.301 b | 18 ± 4.062 ab | 1.43 ± 0.253 b |
| Black | 1 ± 1.000 b | 1 ± 1.000 c | 1 ± 1.000 b | 0 ± 0.000 b | 0.04 ± 0.040 c |
| One-Way ANOVA | |||||
| F (t-test) | 26.14 * | 61.85 * | 36.22 * | 6.44 * | 46.85 * |
| P | 0.0000 | 0.0000 | 0.0000 | 0.0046 | 0.0000 |
| CV (%) | 41.93 | 22.75 | 33.12 | 60.80 | 26.20 |
| Seed Class | Area (mm2) | Perimeter (mm2) | Relative Density (Gray.Pixel−1) | Gray Levels (Gray.Pixel−1) | Filling (%) |
|---|---|---|---|---|---|
| Yellow | 20.605 ± 0.4164 a | 19.079 ± 0.2325 a | 127.911 ± 1.4009 a | 138.20 ± 1.6553 a | 98.35 ± 0.0441 a |
| Light brown | 19.713 ± 0.3781 a | 18.616 ± 0.2562 a | 127.049 ± 2.6518 a | 138.60 ± 2.3152 a | 98.10 ± 0.2027 a |
| Dark brown | 17.642 ± 0.9103 a | 17.285 ± 0.4826 a | 121.843 ± 2.0572 a | 132.00 ± 2.0976 a | 96.20 ± 0.8651 a |
| Black | 19.550 ± 2.0304 a | 18.207 ± 1.0513 a | 88.969 ± 9.0533 b | 90.20 ± 9.9870 b | 88.21 ± 2.2419 b |
| One-Way ANOVA | |||||
| F (t-test) | 1.18 ns | 1.60 ns | 14.40 * | 19.23 * | 15.63 * |
| P | 0.3485 | 0.2295 | 0.0001 | 0.0000 | 0.0000 |
| CV (%) | 13.24 | 7.38 | 9.37 | 9.49 | 2.83 |
| Seed Class | α-Amylase | β-Amylase | SOD | CAT |
|---|---|---|---|---|
| Yellow | 9.41 ± 1.330 ab | 12.51 ± 0.284 a | 1.59 ± 0.096 b | 2.91 ± 0.249 b |
| Light brown | 9.98 ± 1.633 a | 10.62 ± 2.031 a | 1.56 ± 0.024 b | 2.78 ± 0.233 b |
| Dark brown | 7.80 ± 0.602 ab | 9.89 ± 1.116 ab | 1.98 ± 0.098 b | 3.31 ± 0.411 b |
| Black | 4.79 ± 0.764 b | 5.00 ± 0.727 b | 7.68 ± 0.959 a | 13.11 ± 1.356 a |
| One-Way ANOVA | ||||
| F (t-test) | 4.03 * | 6.86 * | 38.16 * | 48.29 * |
| P | 0.0339 | 0.0060 | 0.0000 | 0.0000 |
| CV (%) | 29.00 | 25.72 | 30.23 | 26.34 |
| Seed Class | APX | POX | PPO | PROT | MDA |
|---|---|---|---|---|---|
| Yellow | 66.05 ± 13.837 a | 0.09 ± 0.017 b | 9.22 ± 0.358 a | 0.22 ± 0.016 a | 49.46 ± 2.032 b |
| Light brown | 70.97 ± 8.884 a | 0.12 ± 0.029 b | 6.29 ± 0.700 b | 0.18 ± 0.029 a | 50.81 ± 1.050 b |
| Dark brown | 27.40 ± 4.630 b | 0.09 ± 0.005 b | 4.12 ± 0.349 b | 0.17 ± 0.002 a | 47.03 ± 4.024 b |
| Black | 0.00 ± 0.000 b | 0.48 ± 0.041 a | 5.21 ± 0.688 b | 0.06 ± 0.011 b | 75.85 ± 0.652 a |
| One-Way ANOVA | |||||
| F (t-test) | 15.49 * | 51.04 * | 15.84 * | 15.15 * | 33.21 * |
| P | 0.0002 | 0.0000 | 0.0002 | 0.0002 | 0.0000 |
| CV (%) | 41.56 | 27.47 | 17.74 | 22.72 | 8.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira Medeiros, R.; de Fátima Sales, J.; Juliane Telles Nascimento, K.; Rúbio Neto, A.; Zuchi, J.; Resende, O.; Almeida Rodrigues, D.; Almeida Rodrigues, A. Associations between Integument Color and Physical and Physiological Quality in Pterodon pubescens Seeds. Plants 2022, 11, 1302. https://doi.org/10.3390/plants11101302
Vieira Medeiros R, de Fátima Sales J, Juliane Telles Nascimento K, Rúbio Neto A, Zuchi J, Resende O, Almeida Rodrigues D, Almeida Rodrigues A. Associations between Integument Color and Physical and Physiological Quality in Pterodon pubescens Seeds. Plants. 2022; 11(10):1302. https://doi.org/10.3390/plants11101302
Chicago/Turabian StyleVieira Medeiros, Renato, Juliana de Fátima Sales, Kelly Juliane Telles Nascimento, Aurélio Rúbio Neto, Jacson Zuchi, Osvaldo Resende, Douglas Almeida Rodrigues, and Arthur Almeida Rodrigues. 2022. "Associations between Integument Color and Physical and Physiological Quality in Pterodon pubescens Seeds" Plants 11, no. 10: 1302. https://doi.org/10.3390/plants11101302
APA StyleVieira Medeiros, R., de Fátima Sales, J., Juliane Telles Nascimento, K., Rúbio Neto, A., Zuchi, J., Resende, O., Almeida Rodrigues, D., & Almeida Rodrigues, A. (2022). Associations between Integument Color and Physical and Physiological Quality in Pterodon pubescens Seeds. Plants, 11(10), 1302. https://doi.org/10.3390/plants11101302






