The Copper Chaperone Protein Gene GmATX1 Promotes Seed Vigor and Seedling Tolerance under Heavy Metal and High Temperature and Humidity Stresses in Transgenic Arabidopsis
Abstract
:1. Introduction
2. Results
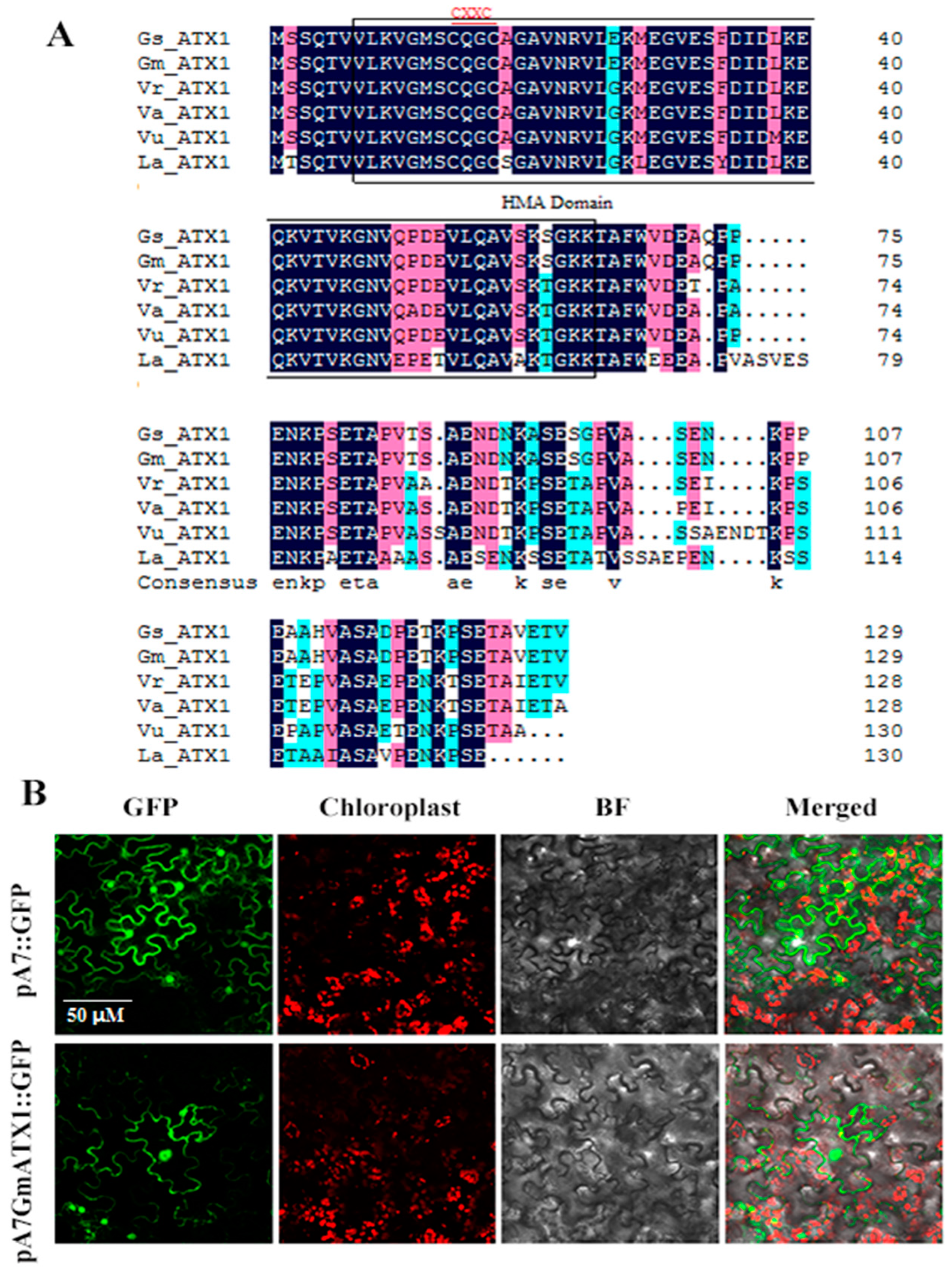
2.1. Sequence Characterization and Subcellular Location of GmATX1
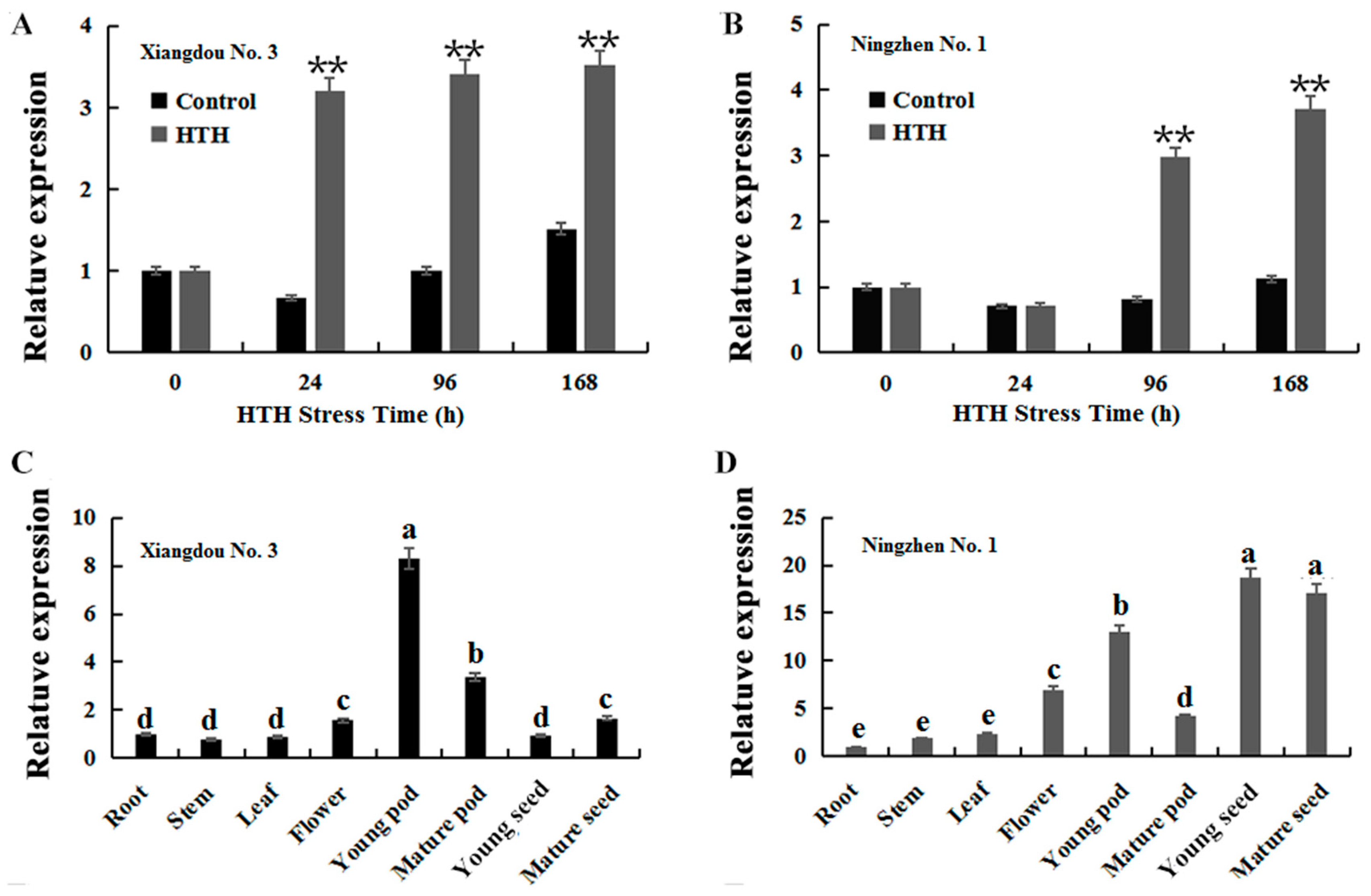
2.2. GmATX1 Expression in Various Tissues and under HTH Stress in Soybean
2.3. GmATX1 Enhancement of Seedling Tolerance, Antioxidase Activity, and ROS Scavenging Ability in Soybean under HT and HTH Stresses
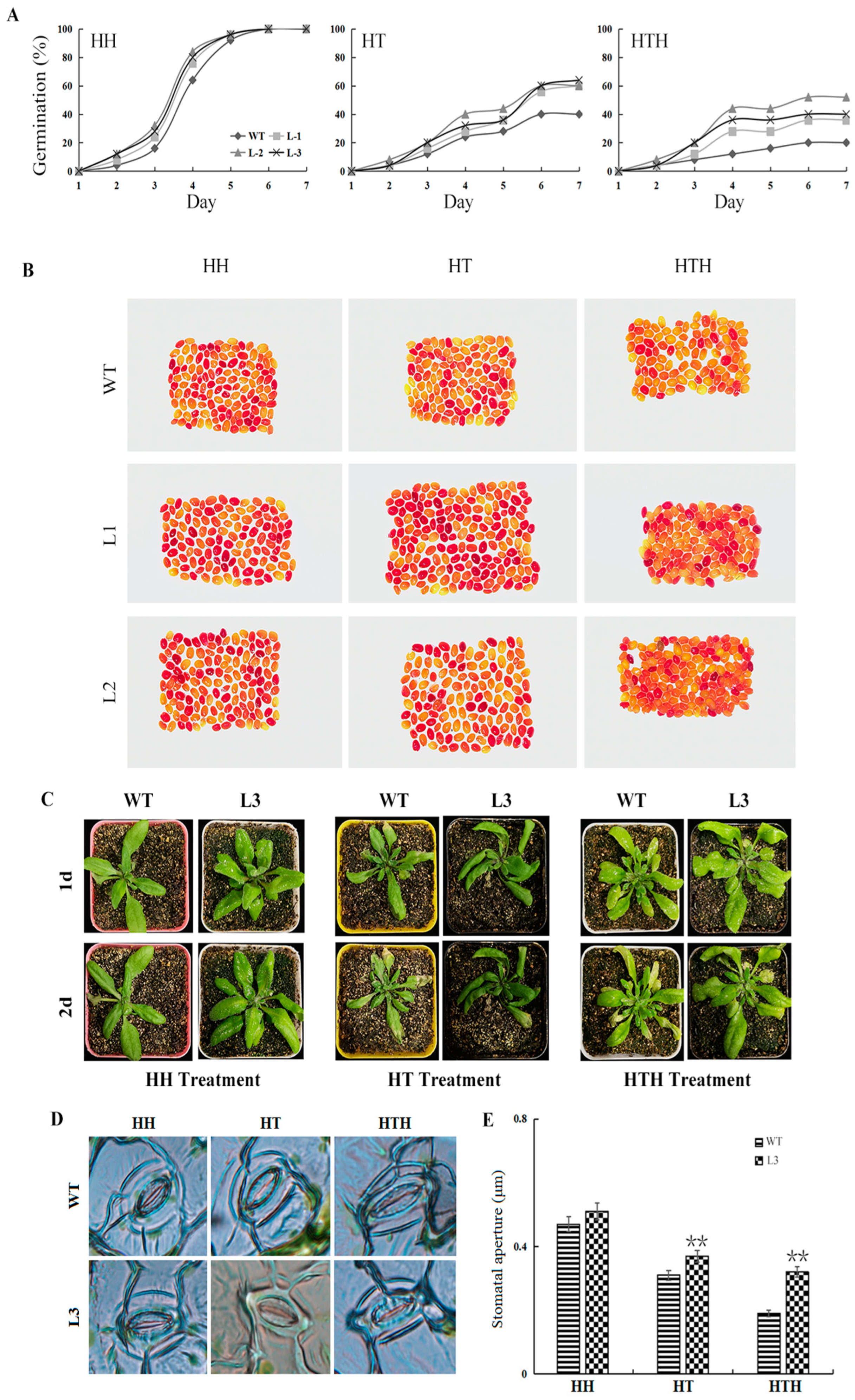
2.4. GmATX1 Overexpression in Arabidopsis Promoting Seed Vitality, Seedling Tolerance, Antioxidase Activity, and ROS Scavenging Ability under HT and HTH Stresses
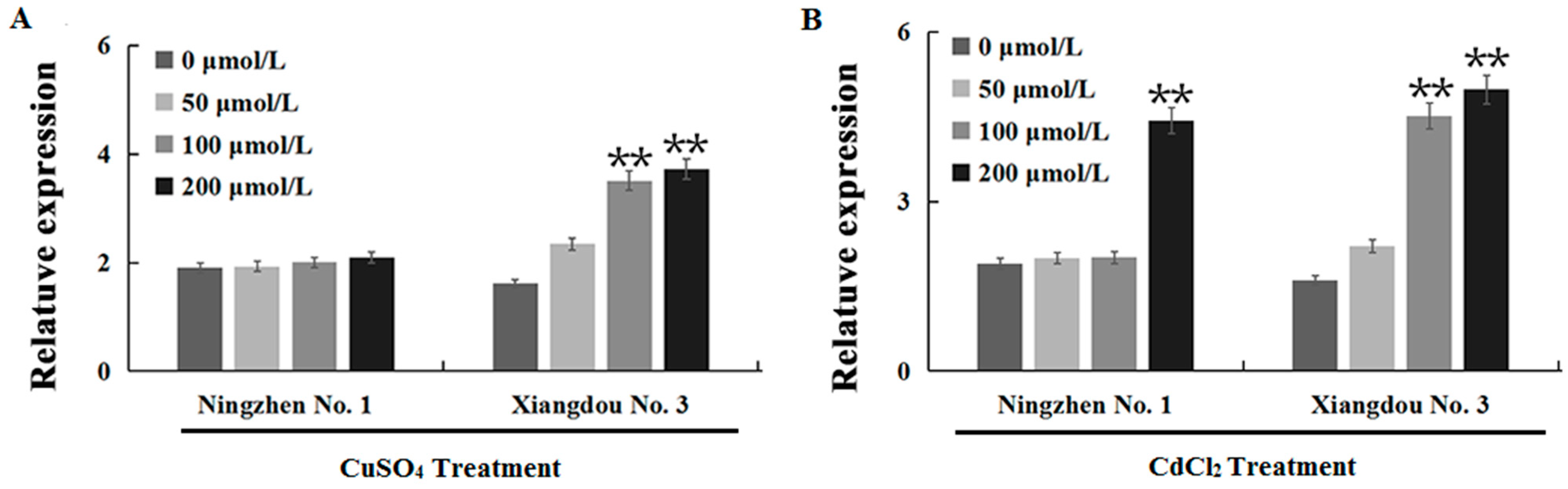
2.5. GmATX1 Enhances Seedling Tolerance and Antioxidase Activity in Arabidopsis under Heavy Metal Stress
3. Discussion
4. Materials and Methods
4.1. Isolation of GmATX1 and Subcellular Localization
4.2. Generation of the GmATX1-Silent Soybean Line
4.3. Generation of GmATX1-Overexpressed Arabidopsis
4.4. Stress Experiments
4.5. Total RNA Extraction and qRT-PCR Analysis
4.6. Germination and TTC Assay
4.7. Phenotype and Stomatal Morphology Analysis of GmATX1-Overexpressed Arabidopsis
4.8. Antioxidase Activity and Lipid Peroxidation Assay
4.9. Hydrogen Peroxide (H2O2) Staining and ROS Release Assay
4.10. Statistics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DAB | Staining, 3,3′-diaminobenzidine staining |
| ATX1 | Copper chaperone protein |
| BF | Brightfield |
| Cvs | Cultivars |
| GFP | Green fluorescent protein |
| HTH | High temperature and humidity |
| HT | High temperature |
| HH | High humidity |
| ORF | Open reading frame |
| PCR | Polymerase chain reaction |
| CAT | Catalase |
| SOD | Superoxide |
| POD | Peroxidase |
| MDA | Malondialdehyde |
| H2O2 | Hydrogen peroxide |
| PM | Plasma membrane |
| qRT-PCR | Quantitative real-time PCR |
| TTC | 2,3,5-Triphenyltetrazolium chloride |
| RH | Relative humidity |
| ROS | Reactive oxygen species |
| WT | Wild type |
References
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [Green Version]
- Zarkadas, C.G.; Gagnon, C.; Gleddie, S.; Khanizadeh, S.; Cober, E.R.; Guillemette, R.J.D. Assessment of the protein quality of fourteen soybean [Glycine max (L.) Merr.] cultivars using aminoacid analysis and two-dimensional electrophoresis. Food Res. Int. 2007, 40, 129–146. [Google Scholar] [CrossRef]
- Shu, Y.J.; Tao, Y.; Wang, S.; Huang, L.Y.; Yu, X.W.; Wang, Z.K.; Chen, M.; Gu, W.H.; Ma, H. GmSBH1, a homeobox transcription factor gene, related to growth and development and involves in response to high temperature and humidity stress in soybean. Plant Cell Rep. 2015, 34, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.X.; Song, W.J.; Xu, L.; Jin, Z.L.; Subrahmaniyan, K.; Zhou, W.J. Sowing seasons and drying methods during post-harvest influence the seed vigour of soybean (Glycine max (L.) Merr.). Acta Physiol. Plant. 2006, 28, 273–280. [Google Scholar] [CrossRef]
- Ren, C.; Bilyeu, K.D.; Beuselinck, P.R. Composition, vigor, and proteome of mature soybean seeds developed under high temperature. Crop Sci. 2009, 49, 1010–1022. [Google Scholar] [CrossRef]
- Egli, D.B.; TeKrony, D.M.; Heitholt, J.J.; Rupe, J. Air temperature during seed filling and soybean seed germination and vigor. Crop Sci. 2005, 45, 1329–1335. [Google Scholar] [CrossRef]
- Wang, L.Q.; Ma, H.; Song, L.R.; Shu, Y.J.; Gu, W.H. Comparative proteomics analysis reveals the mechanism of pre-harvest seed deterioration of soybean under high temperature and humidity stress. J. Proteome. 2012, 75, 2109–2127. [Google Scholar] [CrossRef]
- Zhuang, P.; Zou, B.; Li, N.Y.; Li, Z.A. Heavy metal contamination in soils and food crops around Dabaoshan mine in Guangdong, China: Implication for human health. Environ. Geochem. Health 2009, 31, 707–715. [Google Scholar] [CrossRef]
- Burkhead, J.L.; Reynolds, K.A.; Abdel-Ghany, S.E.; Cohu, C.M.; Pilon, M. Copper homeostasis. New Phytol. 2009, 182, 799–816. [Google Scholar] [CrossRef]
- Sommer, F.; Kropat, J.; Malasarn, D.; Grossoehme, N.E.; Chen, X.; Giedroc, D.P.; Merchant, S.S. The CRR1 nutritional copper sensor in Chlamydomonas contains two distinct metal-responsive domains. Plant Cell 2010, 22, 4098–4113. [Google Scholar] [CrossRef] [Green Version]
- Mendel, R.R.; Kruse, T. Cell biology of molybdenum in plants and humans. Biochim. Biophys. Acta 2012, 1823, 1568–1579. [Google Scholar] [CrossRef] [Green Version]
- Ravet, K.; Pilon, M. Copper and iron homeostasis in plants: The challenges of oxidative stress. Antioxid. Redox Signal. 2013, 19, 919–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, X.; Mu, Q.; Wang, X.; Li, X.; Zhu, X.; Shangguan, L.; Fang, J. Transporters, chaperones, and P-type ATPases controlling grapevine copper homeostasis. Funct. Integr. Genom. 2015, 15, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Y.; Deng, F.; Yamaji, N.; Pinson, S.R.; Fujii-kashino, M.; Danku, J.; Douglas, A.; Guerinot, M.L.; Salt, D.E.; Ma, J.F. A heavy metal P-type ATPase OsHMA4 prevents copper accumulation in rice grain. Nat. Commun. 2016, 7, 12138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilon, M.; Abdel-ghany, S.E.; Cohu, C.M.; Gogolin, K.A.; Ye, H. Copper cofactor delivery in plant cells. Curr. Opin. Plant Biol. 2006, 9, 256–263. [Google Scholar] [CrossRef]
- Huffman, D.L.; O’Halloran, T.V. Function, structure, and mechanism of intracellular copper trafficking proteins. Annu. Rev. Biochem. 2001, 70, 677–701. [Google Scholar] [CrossRef] [PubMed]
- Puig, S.; Mira, H.; Dorcey, E.; Sancenon, V.; Andres-Colas, N.; Garcia-Molina, A.; Burkhead, J.L.; Gogolin, K.A.; Abdel-Ghany, S.E.; Thiele, D.J.; et al. Higher plants possess two different types of ATX1-like copper chaperones. Biochem. Biophys. Res. Commun. 2007, 354, 385–390. [Google Scholar] [CrossRef]
- Shin, L.J.; Yeh, K.C. Overexpression of Arabidopsis ATX1 retards plant growth under severe copper deficiency. Plant Signal. Behav. 2012, 7, 1082–1083. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, G.K.; Rakwal, R.; Jwa, N.S.; Agrawal, V.P. Characterization of a novel rice gene OsATX and modulation of its expression by components of the stress signalling pathways. Physiol. Plant 2002, 116, 87–95. [Google Scholar] [CrossRef]
- Wei, J.P.; Liu, X.L.; Li, L.Z.; Zhao, H.H.; Liu, S.S.; Yu, X.W.; Shen, Y.Z.; Zhou, Y.L.; Zhu, Y.J.; Shu, Y.J.; et al. Quantitative proteomics analysis reveals effects of the high temperature and high humidity stress on seed vigor in soybean. BMC Plant Biol. 2020, 20, 127. [Google Scholar] [CrossRef]
- Turchetto-Zolet, A.C.; Pinheiro, F.; Salgueiro, F.; Palma-Silva, C. Phylogeographical patterns shed light on evolutionary process in South America. Mol. Ecol. 2013, 22, 1193–1213. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Wei, X.Z.; Yu, P.L.; Deng, X.; Xu, W.X.; Ma, M.; Zhang, H.Y. Expression of cadR enhances its specific activity for Cd detoxification and accumulation in Arabidopsis. Plant Cell Physiol. 2016, 57, 1720–1731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, L.J.; Lo, J.C.; Yeh, K.C. Copper chaperone antioxidant protein1 is essential for copper homeostasis. Plant Physiol. 2012, 159, 1099–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Lee, J.S.; Bae, E.K.; Choi, Y.I.; Noh, E.W. Differential expression of a poplar copper chaperone gene in response to various abiotic stresses. Tree Physiol. 2005, 25, 395–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saed-Moucheshi, A.; Shekoofa, A.; Pessarakli, M. Reactive oxygen species (ROS) generation and detoxifying in plants. J. Plant Nutr. 2014, 37, 1573–1585. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [Green Version]
- Dvorak, P.; Krasylenko, Y.; Zeiner, A.; Samaj, J.; Takac, T. Signaling toward reactive oxygen species-scavenging enzymes in plants. Front. Plant Sci. 2021, 11, 618835. [Google Scholar] [CrossRef]
- Sfaxi-boushbih, A.; Chaoui, A.; Ferjani, E.E. Cadmium impairs mineral and carbohydrate mobilization during the germination of bean seeds. Ecotoxicol. Environ. Saf. 2010, 73, 1123–1129. [Google Scholar] [CrossRef]
- Zhao, F.J.; Ma, Y.B.; Zhu, Y.G.; Tang, Z.; Mcgrath, S.P. Soil contamination in China: Current status and mitigation strategies. Environ. Sci. Technol. 2015, 49, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Kulikova, A.L.; Kuznetsova, N.A.; Kholodova, V.P. Effect of copper excess in environment on soybean root viability and morphology. Russ. J. Plant Physl. 2011, 58, 836–843. [Google Scholar] [CrossRef]
- Sun, T.J.; Ma, L.; Sun, L.Y.; Wang, M.X.; Sun, X.Z.; Zhang, J.; Wang, D.M. A TRV-VIGS-based approach for high throughput gene function verification in soybean (Glycine max (L.)). J. Agric. Biotechnol. 2020, 28, 2080–2090. [Google Scholar]
- Chen, H.H.; Chu, P.; Zhou, Y.L.; Ding, Y.; Li, Y.; Liu, J.; Jiang, L.W.; Huang, S.Z. Ectopic expression of NnPER1, a Nelumbo nucifera 1-cysteine peroxiredoxin antioxidant, enhances seed longevity and stress tolerance in Arabidopsis. Plant J. 2016, 88, 608–619. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for a grobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, Y.; Chen, M.; Shu, Y.J.; Zhu, Y.J.; Wang, S.; Huang, L.Y.; Yu, X.W.; Wang, Z.K.; Qian, P.P.; Gu, W.H.; et al. Identification and functional characterization of a novel BEL1-LIKE homeobox transcription factor GmBLH4 in soybean. Plant Cell Tiss. Org. 2018, 128, 607–618. [Google Scholar] [CrossRef]
- Song, L.R.; Liu, Z.Q.; Tong, J.H.; Xiao, L.T.; Ma, H.; Zhang, H.Q. Comparative proteomics analysis reveals the mechanism of fertility alternation of thermosensitive genic male sterile rice lines under low temperature inducement. Proteomics 2015, 15, 1884–1905. [Google Scholar] [CrossRef]
- Chu, P.; Chen, H.H.; Zhou, Y.L.; Li, Y.; Ding, Y.; Jiang, L.W.; Tsang, E.W.; Wu, K.Q.; Huang, S.Z. Proteomic and functional analyses of Nelumbo nucifera annexins involved in seed thermotolerance and germination vigor. Planta 2012, 235, 1271–1288. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.L.; Sun, W.N. Arabidopsis seed-specific vacuolar aquaporins are involved in maintaining seed longevity under the control of abscisic acid insensitive 3. J. Exp. Bot. 2015, 66, 4781–4794. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.S.; Liu, Y.M.; Jia, Y.H.; Wei, J.P.; Wang, S.; Liu, X.L.; Zhou, Y.L.; Zhu, Y.J.; Gu, W.H.; Ma, H. Gm1-MMP is involved in growth and development of leaf and seed, and enhances tolerance to high temperature and humidity stress in transgenic Arabidopsis. Plant Sci. 2017, 259, 48–61. [Google Scholar] [CrossRef]



| Treatments | Arabidopsis Lines | |||
|---|---|---|---|---|
| WT | L1 | L2 | L3 | |
| HH | 100 | 100 | 100 | 100 |
| HT | 40 b | 60 a | 60 a | 64 a |
| HTH | 20 c | 36 b | 52 a | 40 b |
| Treatments | Arabidopsis Lines | ||
|---|---|---|---|
| WT | L1 | L2 | |
| HH | 76.38 b | 93.64 a | 94.55 a |
| HT | 74.74 b | 87.23 a | 89.36 a |
| HTH | 36.84 b | 50.53 a | 44.21 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Wei, J.; Wang, S.; Zhang, X.; Mu, K.; Liu, S.; Ma, H. The Copper Chaperone Protein Gene GmATX1 Promotes Seed Vigor and Seedling Tolerance under Heavy Metal and High Temperature and Humidity Stresses in Transgenic Arabidopsis. Plants 2022, 11, 1325. https://doi.org/10.3390/plants11101325
Shen Y, Wei J, Wang S, Zhang X, Mu K, Liu S, Ma H. The Copper Chaperone Protein Gene GmATX1 Promotes Seed Vigor and Seedling Tolerance under Heavy Metal and High Temperature and Humidity Stresses in Transgenic Arabidopsis. Plants. 2022; 11(10):1325. https://doi.org/10.3390/plants11101325
Chicago/Turabian StyleShen, Yingzi, Jiaping Wei, Shuang Wang, Xi Zhang, Kebing Mu, Sushuang Liu, and Hao Ma. 2022. "The Copper Chaperone Protein Gene GmATX1 Promotes Seed Vigor and Seedling Tolerance under Heavy Metal and High Temperature and Humidity Stresses in Transgenic Arabidopsis" Plants 11, no. 10: 1325. https://doi.org/10.3390/plants11101325
APA StyleShen, Y., Wei, J., Wang, S., Zhang, X., Mu, K., Liu, S., & Ma, H. (2022). The Copper Chaperone Protein Gene GmATX1 Promotes Seed Vigor and Seedling Tolerance under Heavy Metal and High Temperature and Humidity Stresses in Transgenic Arabidopsis. Plants, 11(10), 1325. https://doi.org/10.3390/plants11101325






