Abstract
Dioscorea caucasica Lipsky is a tertiary relict endemic plant naturally growing in the western part of the trans-Caucasus regions; it has adapted and successfully grows in the temperate region of the Baltic countries. Information about its phytochemical composition and bioactivities is rather scarce. This study reports the results of the identification of 41 compounds in D. caucasica leaf and tuber hydroethanolic extracts using UPLC-QTOF/MS. Organic acids were found in both extracts; hydroxycinnamates and flavonoids were the main phytochemicals in the leaves, while steroidal glycosides, fatty acids (mainly hydroxylated) and carbohydrates were found in the tubers. Leaf extracts inhibited enzymes in a dose-dependent manner and were remarkably stronger inhibitors of physiologically important enzymes, namely α-amylase (48.6% at 480 µg/mL), α-glucosidase (IC50 = 41.99 and 47.95 µg/mL with and without 0.1 M Na2CO3), acetylcholinesterase (45.85% at 100 µg/mL) and angiotensin-converting enzyme (IC50 = 829.7 µg/mL), most likely due to the presence of some quantified polyphenolic antioxidants. The mode of inhibition of α-glucosidase and acetylcholinesterase was assessed via kinetic studies based on Lineweaver–Burk inhibition plots. Leaf and tuber extracts acted as mixed-type and competitive inhibitors of α-glucosidase, respectively; the leaf extract demonstrated an uncompetitive inhibition mode of acetylcholinesterase. It is expected that this new knowledge of D. caucasica will serve for its valorization in developing new health beneficial ingredients for functional foods and nutraceuticals.
1. Introduction
The interest in health beneficial natural substances has been steadily increasing during the last few decades. This tendency has been fostered by the fast developments in the area of functional foods and nutraceuticals, which have contributed in shifting healthcare concepts towards the increasing role of preventive medicine. In addition, such a generic characteristic of nutritional and healthcare products as ‘naturalness’ has become very popular among consumers [1]. Due to the vast number of plant species, which are the main sources of bioactive phytochemicals, this tendency has stable and sustainable support, particularly considering the presence of many under-investigated plants.
Plant phytochemicals exert various bioactivities, which may provide numerous health benefits, particularly in preventing and/or delaying the development of age-dependent degenerative and chronic diseases [2]. Among the mechanisms of their bioactivities, antioxidant and antimicrobial properties, molecular signaling, inhibition of enzymes and other effects have been reported for various plant preparations and purified natural compounds [3,4,5,6,7]. Many studies have reported the inhibition of physiologically important enzymes by extracts isolated from botanicals, which correlates with various health benefits [8,9,10,11]. For instance, it is well established that the inhibition of amylase and glucosidase enzymes may reduce the postprandial glycemic index, which is linked to the development of diabetes [12], while inhibition of angiotensin-converting enzyme (ACE) may assist in maintaining normal blood pressure [13].
This study is focused on the phytochemical characterization of hydroethanolic extracts of Dioscorea caucasica Lipsky leaves and tubers and the evaluation of their effects on the activity of physiologically important enzymes. Dioscorea is a large genus of the Dioscoreaceae family, comprising around 715 species, which probably originates from the South-East Asia region [14]. The yam is a common name for some edible tuber-forming Dioscorea species. Although the largest diversity of yams is present in the humid tropical and subtropical regions of the world, a number of Dioscorea spp. grow in the temperate and mountainous regions as well. All yams are perennial, herbaceous, dioecious twining climbers producing dry capsules, although, occasionally, both male and female flowers can be found on the same plant [15]. Despite the botanical similarities, the biochemical composition of Dioscorea species may differ significantly; therefore, raw materials of different origin have been claimed to possess diverse nutritional, economic and medicinal value. Some yams, which are cultivated in the humid tropical regions, are economically important crops as a source of cheap starchy food [16], whereas in the temperate climate regions, Dioscorea plants are mainly used due to their health benefits. It should be noted that the majority of Dioscorea spp. studies have been focused on yam tubers, whereas plant leaves may also present potential as renewable resources for various bioactive compounds [17].
D. caucasica is one of the most valuable representatives of the genus. This is a rare tertiary relict endemic plant naturally growing in the Caucasus and mainly found in the western part of the trans-Caucasus regions of Georgia (Abkhazia), Krasnodar (Adler) and some others [18]. It is widely grown in the botanical gardens of Northern Europe as well [18]. Despite the limitations in natural resources, this medicinal plant has received increasing scientific and commercial attention due to the expanding information on the properties of its bioactive compounds. D. caucasica is considered as a high-value native medicinal plant in the Caucasus, whose rhizomes with roots are used for producing folk medicines and health-promoting preparations in the form of ground powder, decoctions, herbal teas or ethanol extracts. Such preparations are prescribed as anti-atherosclerotic, strengthening the function of the brain and contributing to the improvement of cognitive capabilities, as well as being beneficial to the blood circulatory/cardiovascular system as a natural medicine. It has also been reported that D. caucasica nutraceuticals may assist in normalizing blood pressure, both in hypertensive and hypotensive patients; therefore, in Russia, D. caucasica rhizomes and roots are used in the pharmaceutical industry to manufacture novogalene drugs for treating atherosclerosis [19].
However, the majority of the ethnopharmacological health benefits of D. caucasica preparations are not sufficiently supported scientifically, both in terms of their bioactivities and phytochemical composition. Several bioactive compounds isolated from D. caucasica, especially steroidal saponins dioscin and diosgenin, have shown important pharmacological activities, such as suppressing cellular viability and inducing apoptosis in ovarian cancer cells [20], protecting against coronary heart diseases, exerting anti-inflammatory effects by mitigating oxidative stress [21,22,23] and reducing neuropathic pain [10]. The studies conducted with Dioscorea species have also revealed that their constituents might reduce the expression of α-amylase and α-glucosidase [7,9] and inhibit angiotensin-converting enzyme [24] and pancreatic lipase [8]. To the best of our knowledge, only one report is available on D. caucasica phytochemicals, particularly polyphenolic constituents: Szakiel et al. (2017) [25] reported the presence of non-glycosylated triterpenoids in the lipophilic fraction of D. caucasica leaves from Poland.
Currently, as D. caucasica has been introduced and successfully adapted in the temperate region of the Baltic States, the interest in this plant, as a promising source of functional ingredients for foods and nutraceuticals, is increasing in the northern and other countries as well. The overall aim of this study was to screen the phytochemicals in hydroethanolic extracts of D. caucasica leaves and tubers and to evaluate their effects on some physiologically important enzymes, namely α-amylase, α-glucosidase, acethylcholinesterase and angiotensin-converting enzyme. It is expected that this new scientific knowledge of D. caucasica will be useful for its valorization in developing new health beneficial ingredients for functional foods and nutraceuticals.
2. Results
2.1. Phytochemical Composition of Extracts
2.1.1. Identification of Secondary Metabolites Using UPLC-QTOF/MS
The compounds detected in the extracts are listed in Table 1. Several organic acids were identified in D. caucasica leaf and tuber extracts: quinic 1 ([M − H]− ion at m/z 191.0556), malic 2 ([M − H]− ion at m/z 133.0137) and citric/isocitric 4 ([M − H]− ion at m/z 191.0191) acids were detected at tR 0.4 and 1.2 min, respectively. Shikimic acid 3 ([M − H]− ion at m/z 173.0450) with tR 0.5 min was detected only in the leaf extract, while piscidic acid 5 ([M − H]− ion at m/z 255.0505) with tR 1.8 min was found only in the tuber extract. Shikimic acid was reported in D. elephantipes, D. sylvatica and D. mexicana [26], while piscidic acid was reported in D. nipponica tuber [27]. However, this acid was not reported previously in D. caucasica tubers. The MS data of 2-deoxy-2,3-dehydro-N-acetylneuraminic acid (Neu2en5Ac) 33 ([M − H]− m/z 290.0875) at tR 0.9 min perfectly matched the fragmentation pattern of this sialic acid derivative, which has been reported in various biomaterials, including plants. The identity of pyroglutamic acid 34 ([M − H]− ion m/z 128.0347) at tR 0.9 min and glucoheptonic acid 32 ([M − H]− ion m/z 225.0610) at tR 0.3 min is also in agreement with the previously reported data [28,29]. These compounds were found only in the leaf extract.

Table 1.
Identification data of the constituents detected in D. caucasica leaf and tuber extracts by UPLC/Q-TOF-MS in negative ion mode.
It is evident that phenolic acids and flavonoids are the most important phytochemicals in the leaf extract: based on MS and other data, eight hydroxycinnamic acid derivatives and seven flavonoids were identified (Table 1). Caffeoylquinic acid (CQA) isomers have the chemical formula of C16H18O9 and the monoisotopic mass of 354.0950. Similar [M − H]− precursor ions with m/z 353.0872 were recorded for the compounds 6, 10, 12 (tR = 1.5, 1.7, 1.9 min), which indicates the presence of several CQA isomers. The first compound 6 was identified as 3-caffeoylquinic acid. The second compound 10 was assigned to 5-chlorogenic acid based on its dimer m/z 707.1823 [2M − H]−, which yielded the peaks of m/z 353.0872 [M − H]− and m/z 191.0556 [M − H]−. The spectra are in agreement with those previously reported [31,32] and, in addition, were confirmed with an authentic standard. Based on a precursor ion at m/z 297.0612 [M − H]−, the compound 9 was tentatively identified as caffeoylthreonic acid. The compounds 11, 12 produced molecular ions [M − H]− with m/z 337.0923, 353.0872 and the fragments of m/z 191.0556 [M–H–146]− and m/z 191.0556 [M–H–162]−, respectively; these spectral data enabled us to assign the peaks to coumaroylquinic and 4-caffeoylquinic acids, respectively. Compound 13 (tR = 2.0 min) yielded an ion at m/z 335.0766 [M − H]− and was identified as caffeoylshikimic acid [33], while the compound 14 (tR = 2.1 min) yielded an ion at m/z 367.1029 [M − H]− and was assigned to feruloylquinic acid.
The compounds 15–21 were identified as flavonoids, all being quercetin derivatives (Table 1). The chemical formula of quercetin (3,3′,4′,5,7-pentahydroxyl-flavone) is C15H10O7 and the monoisotopic mass is 302.04265. In general, D. caucasica leaf extract flavonoids produced spectra characteristic for [M − H (3-O-glycoside)]−. Thus, in the negative ion mode, the compounds 15, 16 with molecular ions at m/z 609.1455 and 463.0876 and retention times of 2.3, 2.4 min, correspond to quercetin 3-O-rutinoside (rutin) and quercetin 3-O-glucoside (isoquercitrin). The identity was also confirmed by the reference standards. The compound 19 (tR = 2.6 min) gave m/z of 447.0927 and, based on comparison with the spectral data available in the literature [35], it was assigned to quercetin 3-O-rhamnoside (quercitrin). The compounds 17, 18 (tR = 2.5 min) demonstrated precursor ions [M − H]− at m/z 549.0880 and m/z 505.0982, respectively. Consequently, the compounds were identified as quercetin-3-O-malonylglucoside and quercetin-3-O-acetylglucoside, respectively h [34]. The ions of the compound 20 (m/z 533.0931) and compound 21 (m/z 489.1033), corresponding to the molecular formulas C24H21O14 and C23H21O12, respectively, enabled the tentative identification of quercetin 3-O-malonylrhamnoside and quercetin 3-O-acethylrhamnoside, respectively.
Except for hexose 22 of unknown structure, other sugars were detected only in D. caucasica tubers. Sucrose 23 ([M − H]− ion m/z 341.1083) [26,30,36] and the following compounds 24 ([2M − H]− ion at m/z 683.2246) and 25 ([3M − H]− ion at m/z 1025.3408) correspond to the spectral characteristics of deprotonated dimer and trimer oligosaccharide ions [37]; they are included in Table 1 as unseparated sugars.
Long-chain fatty acids and their hydroxylated derivatives are common in various plant materials, including yam tubers of Dioscorea species [38,43,44]. Spectral data of the compound 31, [M − H]− ion m/z 279.2324 (tR = 8.8 min, fits linoleic acid (C18:2), and those of 29 [M − H]− ion m/z 295.2273 (tR = 6.9 and 8 min) correspond to linolenic acid (C18:3). Other derivatives suggest the structures of hydroxylated fatty acids: 30 [M − H]− ion m/z 271.2273 at (tR) 8.4 min and 26 [M − H]− ion m/z 329.2328 at (tR) 4.6 min correspond to hydroxyhexadecanoic and trihydroxyoctadecenoic acids, respectively. Hydroxyoctadecatrienoic acid 27 [M − H]− ion m/z 293.2117 was detected only in the leaf extract at (tR) 5.8 min. MS data do not provide sufficient information on the exact position of the hydroxy group in the fatty acid chain. Furthermore, MS data of the compound 28, [M − H]− ion m/z 358.2593 at (tR) 6.8 min, suggest the molecular formula of C19H36NO5, which fits a fatty acid ester (https://www.lipidmaps.org accessed on 12 December 2021 [39]). The latter was assigned to a carnitine derivative, hydroxy dodecanoylcarnitine. Conjugates of fatty acids with amino acids occur widely in food from animal sources, but there is limited availability in plants. The obtained mass spectra do not allow us to identify the precise site of hydroxylation.
It was not possible to elucidate the exact structures of 36, 37, 38, 39, 40; however, based on their [M − H]− m/z of 1109.5379 (tR = 3.3 min), 1093.5431(tR = 3.7 min) and 1075.5325 (tR = 4.3 min), 929.4746 (tR = 6.5 min), 913.4797 (tR = 6.6 min), which correspond to formulae of C52H85O25, C52H85O24, C52H83O23, C46H73O19 and C46H73O18, respectively, most likely belong to steroidal glycosides, which are abundant in yams [41] and some other plants [40,42]. Spectral data of the compound 41 [M − H]− ion m/z of 767.4217 (tR = 6.8 min) also indicate steroidal saponin pentandroside B [42]. Furthermore, the compound 45 [M − H]− ion m/z 723.3803 corresponding to C34H59O16 detected in tubers at 6.8 min and the compound 44 [M − H]− ion m/z 721.3646 detected in leaves at 5.6 min, and corresponding to C34H57O16, most likely belong to galactolipids. The compound 35 producing [M–H]− ion at m/z 345.1186 at (tR = 1.4 min) fit well with the relevant data of an iridoid glycoside and was tentatively identified as an aucubin. This iridoid is commonly found in different anatomical parts of plants and is responsible for the defensive function.
2.1.2. Quantitative Analysis of the Main Secondary Metabolites Using HPLC
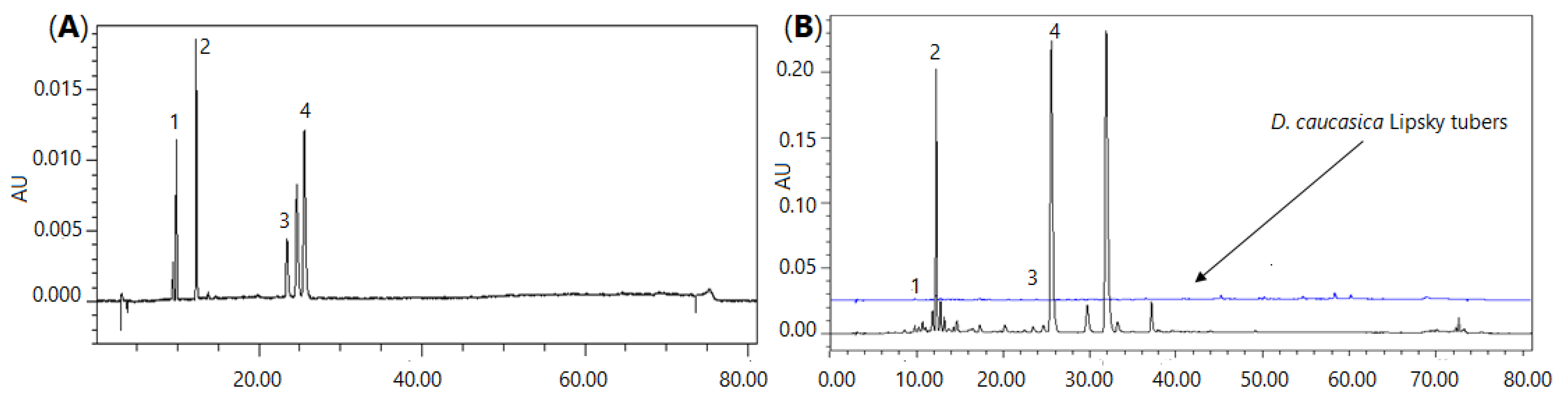
The content of neochlorogenic acid, chlorogenic acid, rutin and isoquercitrin in the D. caucasica leaf extract was 5.89, 116.6, 4.02 and 187.2 µg/mL, respectively. Thus, quercetin glycoside and isoquercitrin were the most abundant phenolic constituents recovered from the plant leaves. In general, these data agree with the UPLC-QTOF/MS analysis, which demonstrated the highest m/z intensities for the major quantified compounds. As may be judged from the peak area in the chromatographic profile (Figure 1), some other constituents may also be present in the extract at high concentrations. Based on the peak m/z intensities in the UPLC-QTOF/MS chromatograms, these compounds might be quinic acid, which forms esters with phenolic acids, and quercitrin, which eluted after isoquercitrin (Figure 1), i.e., similarly as in the UPLC-QTOF/MS chromatogram. The content of biologically active phenolic compounds in the leaves was remarkably higher than in the tubers. For instance, the content of neochlorogenic and chlorogenic acids in the tuber extract was only 0.687 and 1.869 µg/mL, respectively. Quantification of other tuber phytochemicals was beyond the scope of this study; however, based on the peak m/z intensities in the UPLC-QTOF/MS chromatograms, the major compounds in the tuber extract might be some sugars, piscidic acid, linolenic acid and two steroidal glycosides with tR 3.7 and 6.6 min (Table 1).
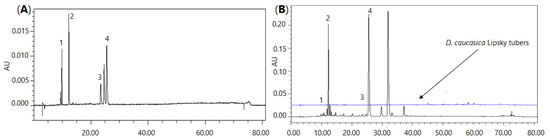
Figure 1.
Chromatographic profile of D. caucasica leaf and tuber extracts (B) and the mixture of reference phenolic compounds (A): 1—neochlorogenic acid, 2—chlorogenic acid, 3—rutin, 4—isoquercitrin.
2.2. Enzyme Inhibitory Properties of D. caucasica Extracts
2.2.1. α-Glucosidase
α-Glucosidase (otherwise known as α-1,4-glucosidase; IUMB enzyme nomenclature number EC 3.2.1.20 [45]), from baker’s yeast (Saccharomyces cerevisiae) Type I, belongs to the Glycoside Hydrolase 13 (GH_13) family [46], whose specificity is directed mainly towards the exohydrolysis of (1→4)-α-glucosidic linkages [47]. Under the conditions used (T = 37 °C; pH 6.8), α-glucosidase catalyzes the cleavage of the p-nitrophenyl-α-D-glucopyranoside (pNPG) substrate, resulting in the formation of α-D-glucose and p-nitrophenol (pNp). The amount of yellow color pNp liberated during the reaction was monitored by absorbance measurements at λ = 405 nm [48]
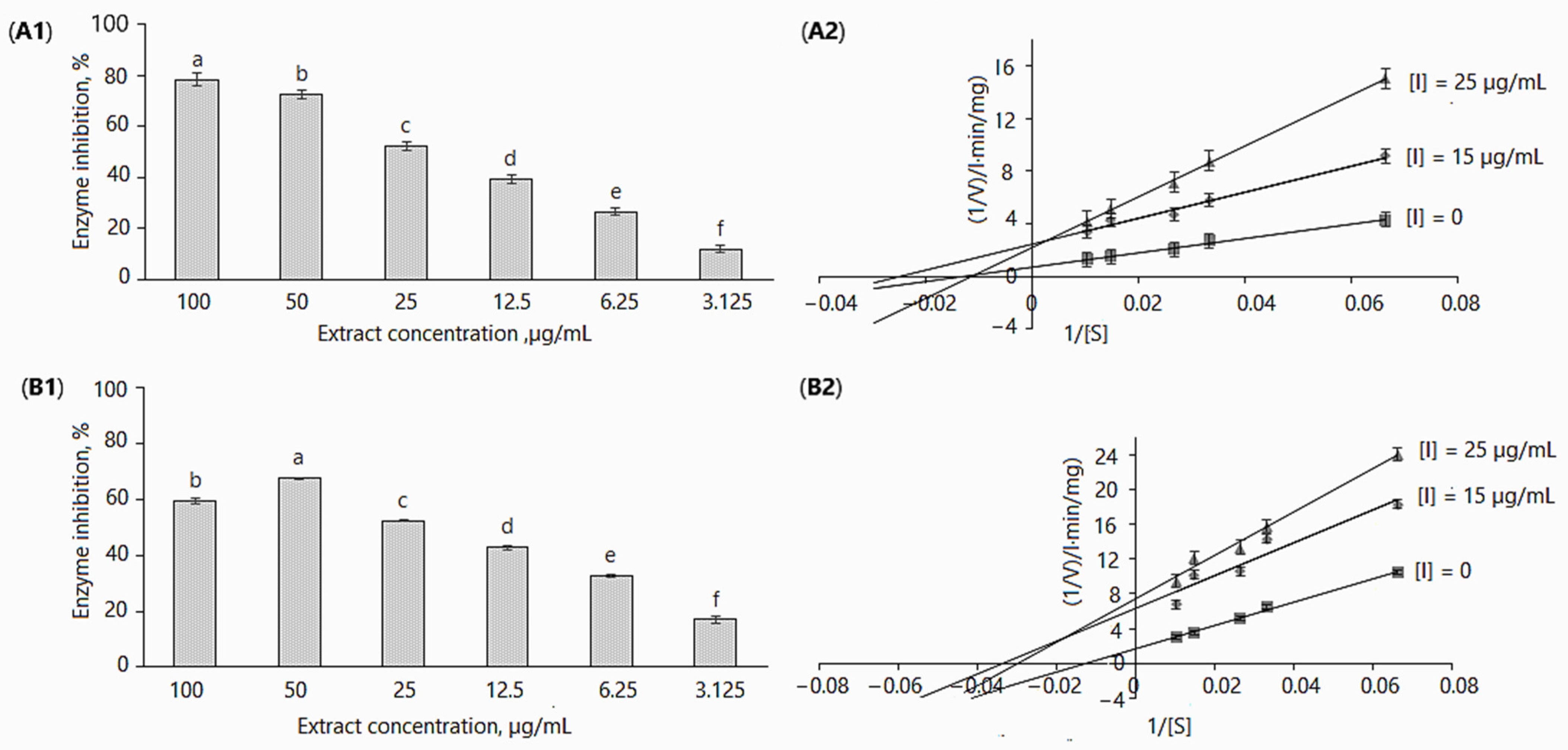
All in vitro experiments were performed at extract concentrations of 3.125, 6.25, 12.5, 25, 50 and 100 µg/mL. The extracts inhibited α-glucosidase activity in a dose-dependent manner; in the assays without adding 0.1 M Na2CO3 (Figure 2A1 and Table 2), enzyme activity as compared to control (without inhibitor) was reduced from 11.94 ± 1.22% to 78.49 ± 2.39% by increasing the concentration from 3.125 to 100 µg/mL. In case of the addition of 0.1 M Na2CO3, enzyme inhibition for the maximal and minimal applied concentrations was 59.30 ± 1.1% and 16.93 ± 1.43% (Figure 2A2 and Table 2). It may be observed that the differences in enzymatic activity with/without using 0.1 M Na2CO3 were not remarkable; however, they were statistically significant, except for the 25 µg/mL concentration (Table 2). Obviously, these differences may be related to the concentration of biologically active and other compounds present in the extracts.
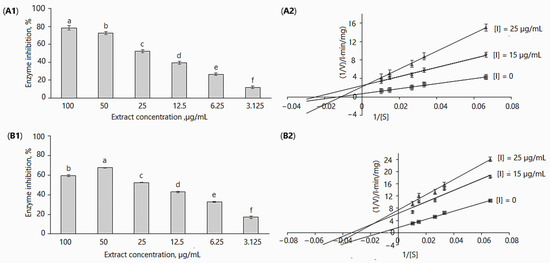
Figure 2.
Inhibition of α-glucosidase by D. caucasica 70% ethanol leaf extracts at different concentrations (A1,B1) and Lineweaver–Burk plot using 0.05–0.33 mM of pNPG (A2,B2): (A1,A2) without adding 0.1 M Na2CO3; (B1,B2) with adding 0.1 M Na2CO3. The values are the means ± standard deviation SD (n = 3). Different letters in (A1,B1) indicate that the values are significantly different, according to Fisher’s LSD test (p < 0.05) after one-way ANOVA.

Table 2.
Effect of D. caucasica 70% ethanol extract of leaves on the percentage inhibition of α-glucosidase enzymatic activity.
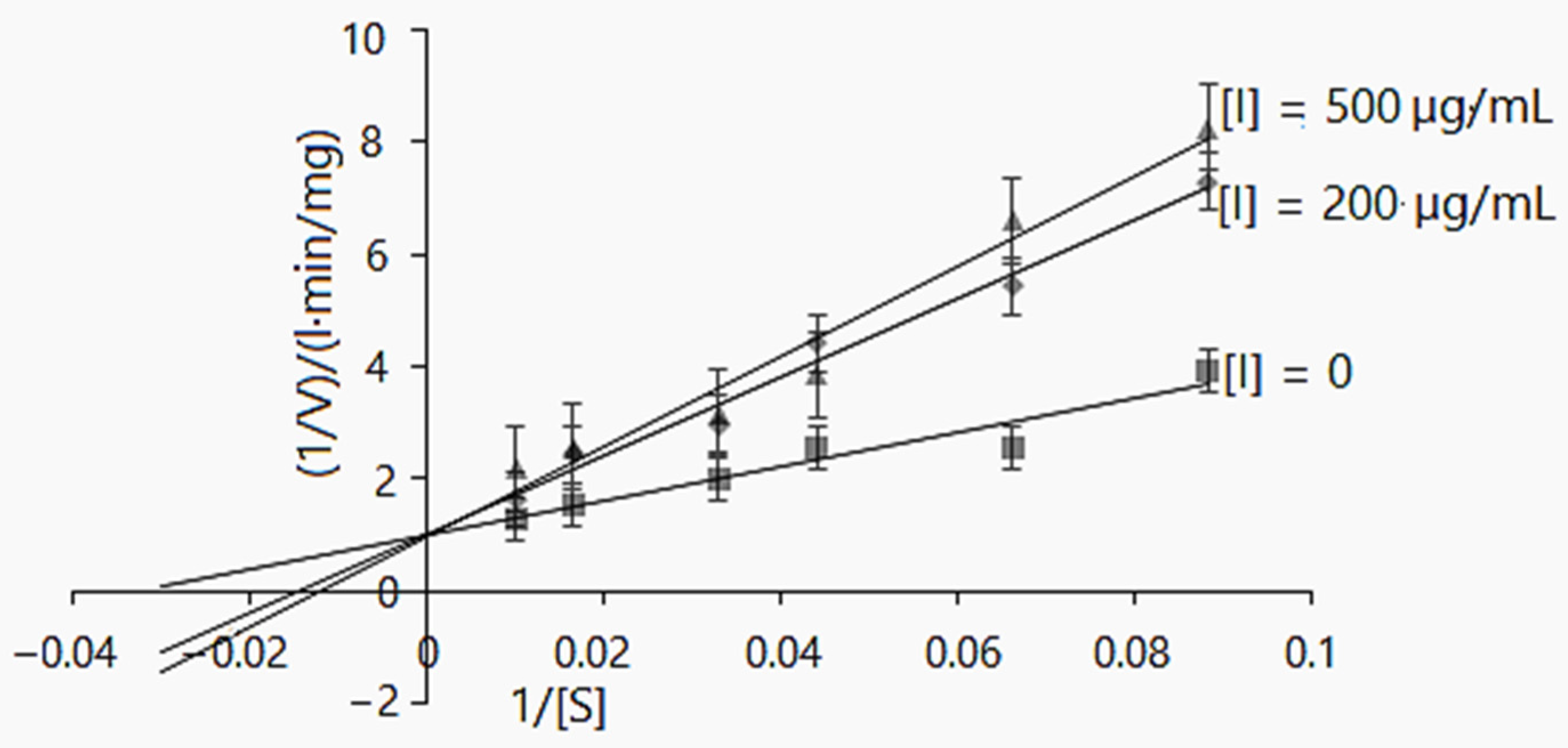
The IC50 values of D. caucasica leaf extracts with/without 0.1 M Na2CO3 at the tested concentrations were 41.99 µg/mL and 47.95 µg/mL, respectively. For comparison, acarbose at 200 µg/mL concentration reduced α-glucosidase activity by 71.17%. Most likely, the high inhibitory activity of D. caucasica leaf extracts may be attributed to the chlorogenic acids, quercitrin and isoquercitrin, although the contribution of other non-identified compounds cannot be discounted. In contrast, the tuber extracts of D. caucasica displayed rather weak inhibitory activity against α-glucosidase; for instance, at 500 µg/mL, enzyme activity was reduced by 40.79 ± 2.71% (Figure 3).
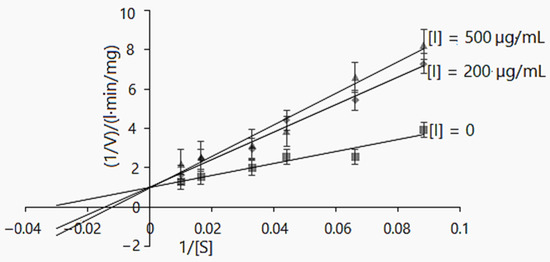
Figure 3.
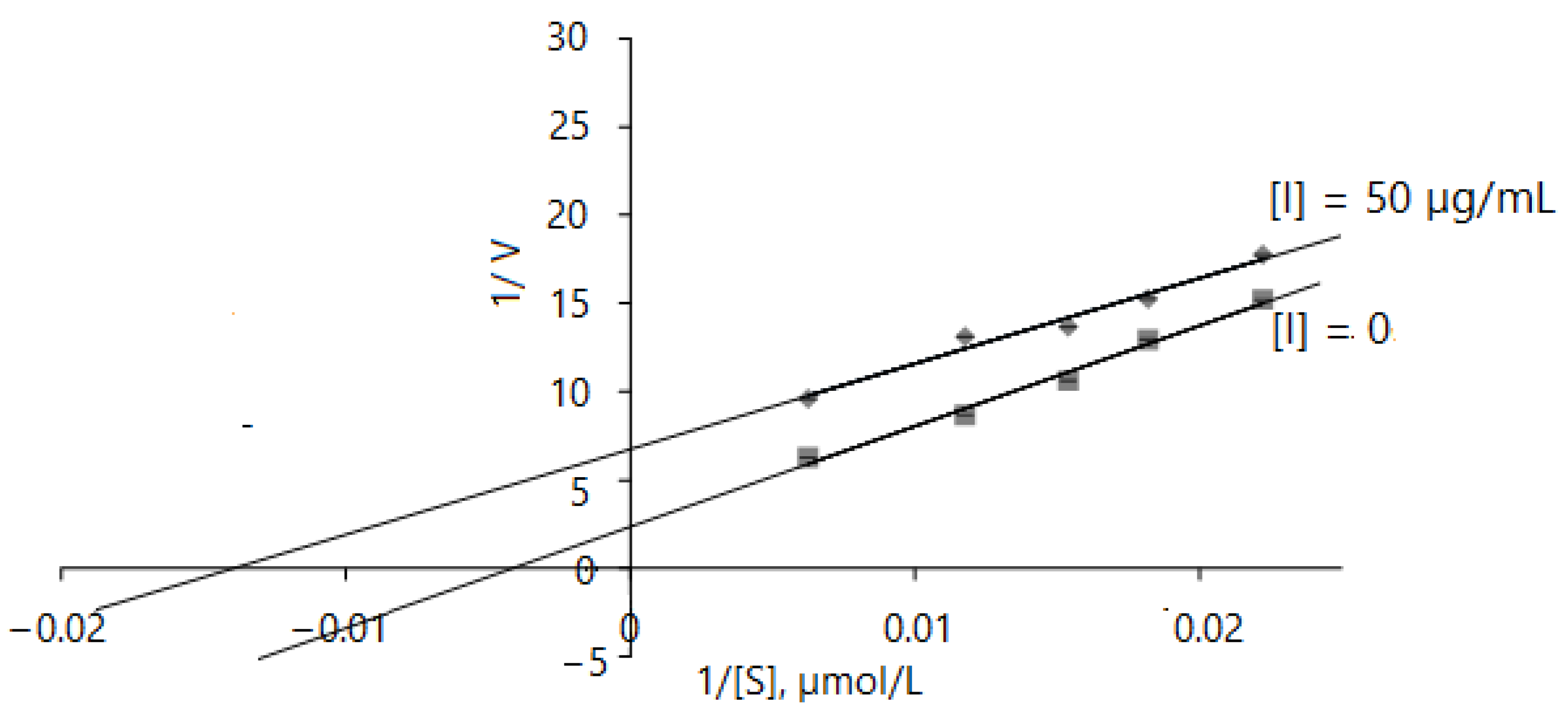
Lineweaver–Burk inhibition plot of α-glucosidase enzyme activity using D. caucasica tuber extract and different concentrations of pNPG (0, 0.004, 0.005, 0.008, 0.01, 0.02 and 0.33 mM). The signs and symbols on the graphic refers: squares: none inhibitor, rhombuses: 200 µg/mL of tubers extract concentration; triangles: 500 µg/mL of tubers extract concentration, [I]: inhibitor/extract; [S]: substrate. The values are the means ± SD (n = 3).
2.2.2. α-Glucosidase Inhibition Kinetics
A good deal of information about enzymatic reactions can be obtained by observing the changes in the reaction caused by the presence of inhibitors. Based on the inhibitory results of the yam extracts used in this study, the mode of inhibition of α-glucosidase activities was investigated via kinetic studies in the absence and the presence of the inhibitor. The Lineweaver–Burk inhibition plots [49] of 1/V versus 1/[pNPG] gave the following equations: (1) without inhibitor: y = 55.052x + 0.7009, r2 = 0.9935; 15 µg/mL leaf extract: y = 99.316x + 2.482, r2 = 0.9852; 25 µg/mL leaf extract: y = 193.4x + 2.2253, r2 = 0.9989; (2) without inhibitor: y = 30.698x + 0.9772, r2 = 0.9292; 20 µg/mL tuber extract: y = 69.91x + 1.0085, r2 = 0.9833; 50 µg/mL tuber extract: y = 80.37x + 0.967, r2 = 0.966 (Figure 2A2). Similarly, a double reciprocal plot (Lineweaver–Burk plot) was constructed when the enzymatic reaction was stopped with 0.1M Na2CO2 (Figure 2B2). The following mathematical equations were obtained: (1) without inhibitor: y = 134.64x + 1.7083, r2 = 0.9943; 15 µg/mL of leaf extract: y = 188.35x + 6.3407, r2 = 0.9072; 25 µg/mL leaf extract: y = 249.6x + 7.4252, r2 = 0.9862.
D. caucasica leaf extracts displayed a mixed-type non-competitive mode of inhibition of α-glucosidase (Figure 2A2,B2). By virtue of the Lineweaver–Burk plot in the enzymatic reaction system I (Table 3), the Vmax(app) decreased from 0.157 ± 0.03 to 0.134 ± 0.004 mg/L·min after increasing the inhibitor concentration from 15 to 25 µg/mL; Km(app) values were 29.76 ± 1.54 and 33.61 ± 0.75 mg/L, respectively. In contrast, in the enzymatic reaction system II (Table 3), the increase in extract concentration resulted in an increase in the Vmax(app) value, from 0.402 ± 0.041 to 0.449 ± 0.026 mg/L·min. The Km(app) value in this case was more than two-fold higher (86.91 mg/L) at 25 µg/mL than at 15 µg/mL (39.984 mg/L). It was established that an increased Km and unchanged Vmax show competitive inhibition, while the decreased Vmax and increased/decreased Km values (Table 3) indicate the mixed-type inhibition model. Despite significant differences in kinetic constant (Vmax(app), Km(app)) values among enzymatic reaction systems, noticeable is the hallmark of non-competitive inhibition. The double reciprocal plot showed a group of straight lines with different slopes, which intersect at the third quadrant (Figure 2B2)/second quadrant (Figure 2A2), suggesting that the extracts act as mixed-type inhibitors [50].

Table 3.
Parameters of α-glucosidase inhibition kinetics.
The catalytic efficiency (CE) of α-glucosidase in the different enzymatic reaction systems decreased as follows: in enzymatic reaction I, from 0.007 ([I] = 0) to 0.005 ([I] = 15 µg/mL) and 0.004 ([I] = 25 µg/mL); in enzymatic reaction II, from 0.018 ([I] = 0) to 0.01 ([I] = 15 µg/mL) and 0.005 ([I] = 25 µg/mL) (Table 3). The decreased rate of catalysis confirms the interaction of the inhibitor with α-glucosidase, which reduces product formation.
The kinetic reaction model was also elaborated for D. caucasica tuber extract. At the concentrations of 200 and 500 µg/mL, it competitively inhibited α-glucosidase: the Km(app) values increased from 69.34 ± 0.31 to 83.33 mg/L, whereas Vmax(app) 0.99 ± 0.22 and 1.034 ± 0.38 mg/L·min were not significantly different (Table 3). Further, the Lineweaver–Burk plot provides information about 1/Vmax and −1/Km for the α-glucosidase kinetics. As may be observed (Figure 3), the increased Km value and insignificant changes in the Vmax value in the case of increasing inhibitor concentrations suggests the mechanisms of a competitive mode of inhibition [50,51]
2.2.3. α-Amylase
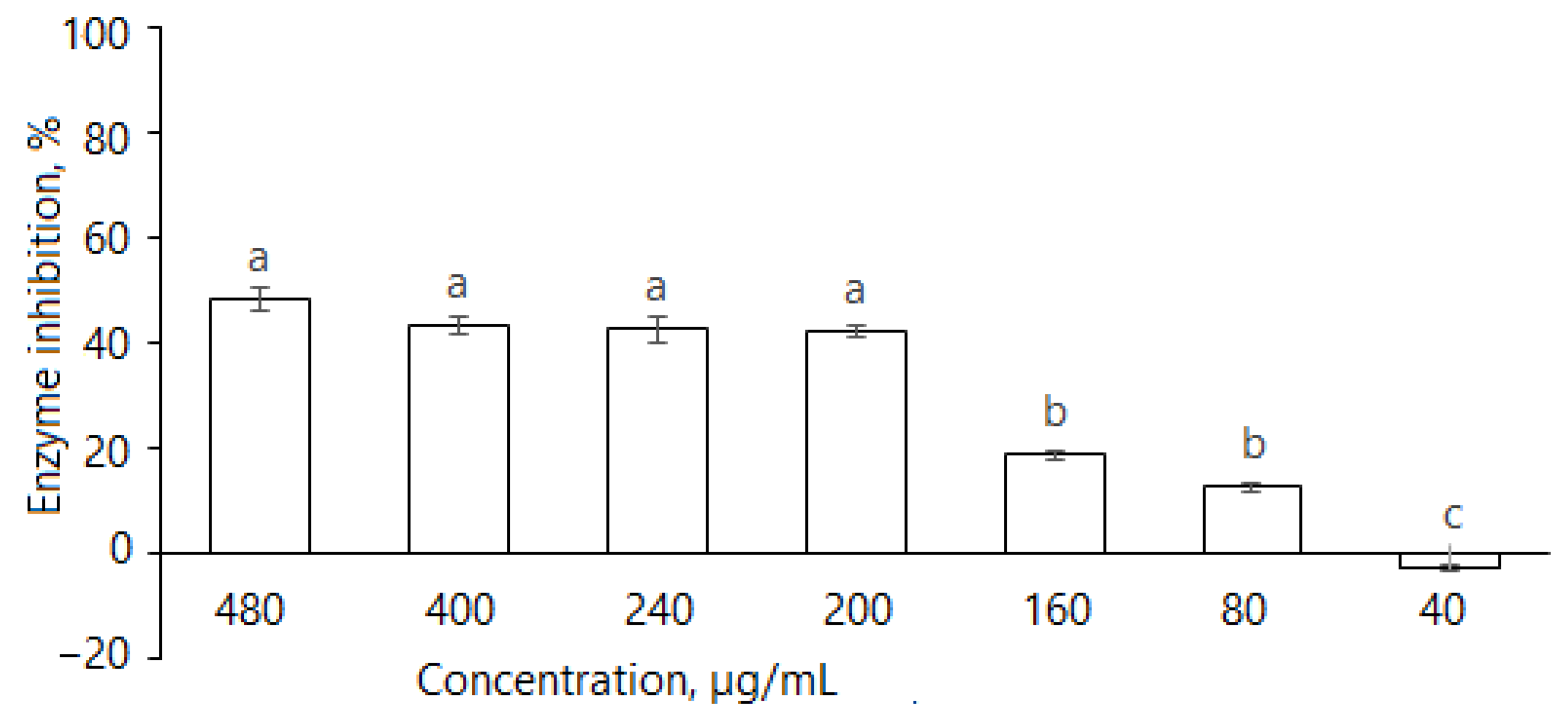
As the tuber extract possessed remarkably weaker α-glucosidase inhibitory effects, further studies were focused on the leaf extract. It is evident that D. caucasica leaf extracts inhibited the α-amylase enzyme (Figure 4); at 480, 400, 240, 200, 160 and 80 μg/mL concentrations, it reduced α-amylase activity by 48.6 ± 2.2%, 43.4 ± 1.7%, 42.6 ± 2.3%, 42.2 ± 1.3%, 19.0 ± 0.9% and 12.8 ± 0.9%, respectively. The inhibitory effect of the plant was compared with the standard drug, acarbose, which reduced enzyme activity by approximately 93.8% at the concentration of 50 µg/mL. The inhibition increased with extract concentration; however, there was no linear dependence. At the concentration of ≥200 μg/mL, the changes in percentage inhibition were not significant. Most likely, it depends on the substrate composition and the mode of inhibition, which will be demonstrated further in the studies of reaction kinetics with other two tested enzymes. From these data, we can conclude that D. caucasica leaf extract was a moderate inhibitor (40–50% inhibition at ≥200 μg/mL concentration) of α-amylase, with a remarkably lower effect than acarbose.
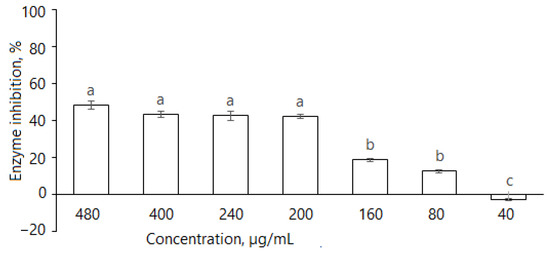
Figure 4.
Inhibition of α-amylase by D. caucasica 70% ethanol leaf extracts at different concentrations (enzyme activity in the absence of extract was considered as 100%). The values are the means ± standard error (SE) (n = 3); different letters indicate that the values are significantly different, according to Fisher’s LSD test (p < 0.05) after one-way ANOVA.
2.2.4. Acetylcholinesterase (AChE) Inhibition Assay
AChE (IUMB enzyme nomenclature number EC 3.1.1.7), also known as acetylcholine hydrolase, choline esterase I, cholinesterase, acetylthiocholinesterase and acetyl-β-methylcholinesterase, belongs to the cholinesterase (ChEs) family, which is most widely known for hydrolyzing the neurotransmitter acetylcholine (ACh) to choline (Ch) and acetic acid in the synaptic cleft. The principle of this assay method is based on the production of tiocholine (SCh), which is formed from acetylthiocholine during enzymatic hydrolysis with Ellman’s reagent (DTNB) and produces yellow-colored chromophore 5-thio-2-nitrobenzoate. The rate of appearance of the yellow derivative is measured spectrophotometrically at 412 nm [52].
The samples for the assay were prepared from the lyophilized powder and tested for their ability to inhibit AChE catalysis in the concentration range of 25–100 µg/mL. The results are listed in Table 4.

Table 4.
The effect of D. caucasica 70% ethanol leaf extracts on specific activity of AChE (in µM/min/mg protein) and percentage inhibition (in brackets).
It may be observed that the inhibition noticeably increased after increasing the concentration of the leaf extract from 25 µg/mL (inhibition = 27.55%) to 40 μg/mL (inhibition = 40.58%), while a further increase in the concentration to 80 µg/mL resulted in a significant increase in AChE inhibition that was, however, less remarkable. However, at 80 and 100 µg/mL extract concentrations, there were no significant (p > 0.05) differences in decreased enzyme activity. The inhibitory effect of D. caucasica solution on AChE was compared with the synthetic inhibitor Donepezil HCl. As expected, the positive control was a more potent AChE inhibitor than the extracts of D. caucasica: at a 16 µg/mL concentration, it reduced enzyme activity by 70.62 ± 2% and Vmax to 0.0593 µM/min/mg protein.
2.2.5. Acetylcholinesterase Inhibition Kinetics
The results obtained indicate that the AChE inhibition mechanism of D. caucasica leaf extracts may be rather complex; therefore, enzyme inhibition kinetic studies were performed by monitoring enzyme activity at varying concentrations of acethylthiocholine iodide in the range of 45–160 µmol/L. The Lineweaver–Burk inhibition plots of 1/V versus 1/[ATChI] gave the following equations: without inhibitor: y = 569.49 + 2.3524, r2 = 0.9874; with 50 µg/mL of leaf extract: y = 483.75 + 6.7466, r2 = 0.9755. D. caucasica leaf extracts displayed an uncompetitive mode of inhibition with respect to AChE (Figure 5). By virtue of the Lineweaver–Burk plot in the enzymatic reaction system with pure enzyme, the Vmax, Km values were 0.425 ± 0.11 µM/mg protein/min and 242.08 ± 15.76 µmol/L, respectively; meanwhile, in the case of using extracts at the concentration of 50 µg/mL, the V max(app), Km(app) values were remarkably lower, 0.148 ± 0.015 µM/min/mg protein and 71.56 ± 6.55 µmol/L, respectively. This profile of inhibition indicates that the inhibitor only binds to the enzyme–substrate complex [51].
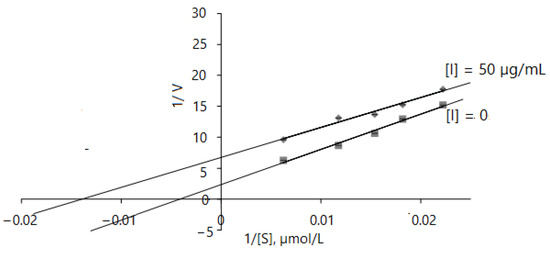
Figure 5.
Mathematical modeling of Lineweaver–Burk inhibition plot on enzyme activity using different concentrations of ATChI (0, 45, 55, 65, 85 and 160 µM). The signs and symbols on the graphic refers: squares: none inhibitor, rhombuses: 50 µg/mL of leaves extract concentration; [I]: inhibitor/extract; [S]: substrate. Values represent mean ± standard deviation of triplicate samples.
2.2.6. Angiotensin-Converting Enzyme (ACE)
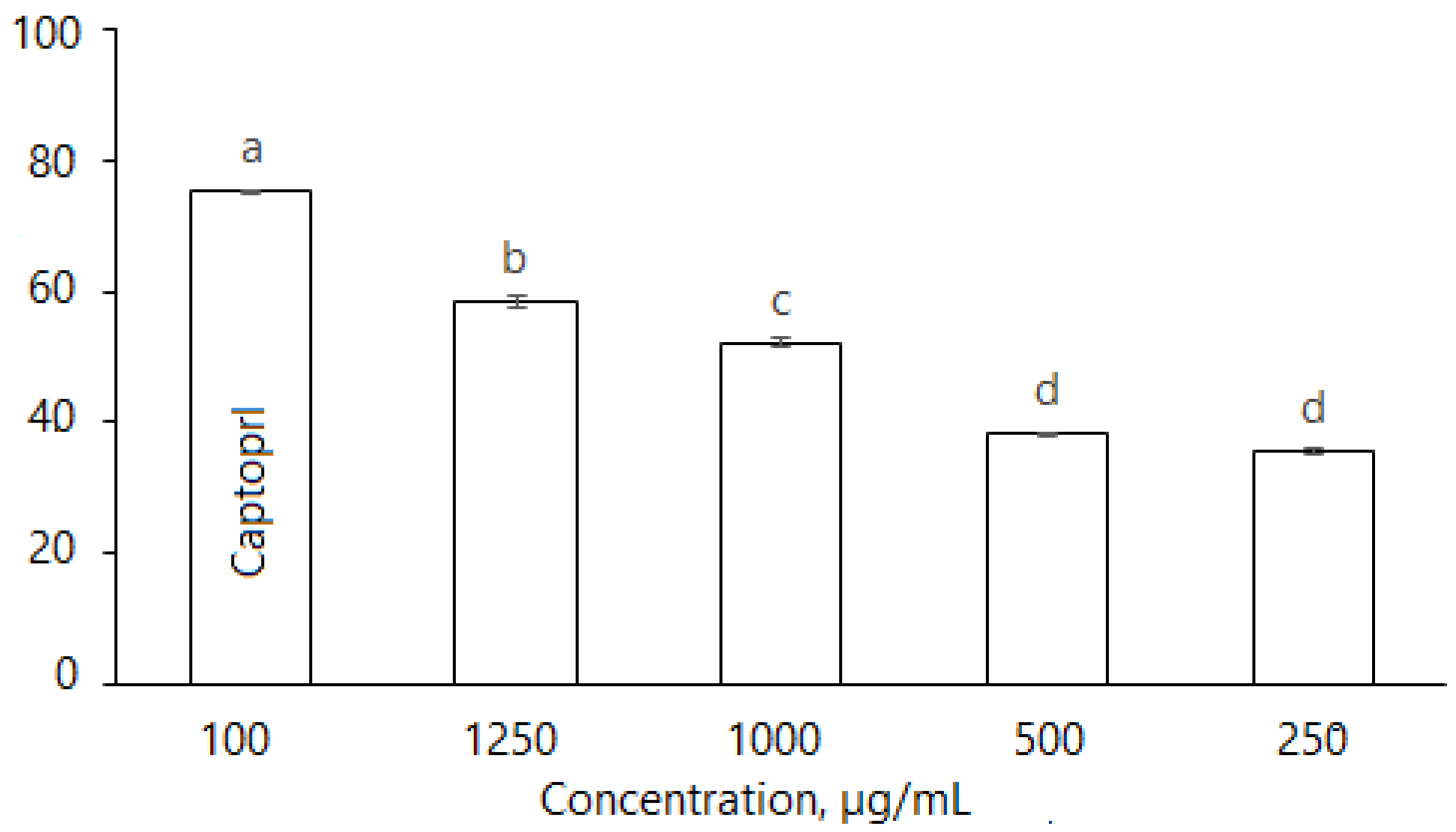
D. caucasica leaf extracts also inhibited ACE in a dose dependent manner (Figure 6): at the concentrations of 250, 500, 1000 and 1250 μg/mL, the enzyme inhibition was 35.46 ± 0.76%, 38.45 ± 0.52%, 51.12 ± 0.81% and 58.34 ± 1.01%. The inhibitory effect of plant extracts was compared with the synthetic antihypertension drug Captopril; at 100 µg/mL, the inhibition level was 74.3 ± 0.57%, while the effective concentration IC50 (ACE inhibition 50%) for the botanical extract was 829.7 µg/mL. Nevertheless, D. caucasica leaf extract may be considered a promising ingredient for the soft control of low/moderate hypertension.
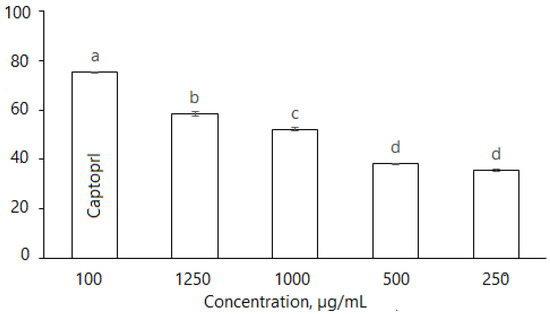
Figure 6.
Inhibition of ACE by D. caucasica 70% ethanol leaf extracts at different concentrations (Captopril—positive control; negative control—no inhibition). Enzyme inhibition was expressed as percentage versus control (100%) (Control = 0 inhibition). The values are the means ± standard error (SE) (n = 3). The values with different letters are significantly different, according to Fisher’s LSD test (p < 0.05) after one-way ANOVA.
3. Discussion
The search for and evaluation of biologically active nutrients in under-investigated plant materials is an important task, which may serve as a good platform in developing new effective ingredients for nutraceuticals and functional foods. In general, studies on the enzyme inhibitory activities of Dioscorea preparations are rather scarce. For instance, the extracts of D. alata and D. bulbifera tubers were tested as α-glucosidase and α-amylase inhibitors [20,53]. Our study substantially expands the knowledge on phytochemicals in D. caucasica leaves and tubers and their effects on physiologically important enzymes, namely α-amylase, α-glucosidase, angiotensin-converting enzyme and acetylcholinesterase. It is evident that the polyphenolic-rich extract of leaves is a remarkably stronger inhibitor of all tested enzymes than that of the tubers. Sterols (campesterol, sitosterol, stigmasterol), triterpene acids (oleanolic and ursolic acid), pentacyclic triterpenoids and their esters (α-amyrin, β-amyrin, taraxasterol, taraxerol) were reported in previously published articles on D. caucasica leaf constituents [25], while saponins such as parvifloside, protodeltonin, protodioscin, deltonin, dioscin [54] and diosgenin [54,55] were the dominant compounds in plant tubers. Some studies reported the hypoglycemic and α-glucosidase/α-amylase inhibitory activities of phytosterols [56] present in seed oils and triterpenoid saponins from botanicals [57]; however, it is highly unlikely that liposoluble (hydrophobic) leaf phytochemicals play a significant role as enzyme inhibitors. Therefore, the stronger enzyme inhibition by leaf extracts may be related to the presence of the main polyphenolic antioxidants, 3-O-glycoside flavonoid and hydroxycinnamic acid derivatives (Table 1).
Inhibiting the activity of amylolytic enzymes is important in controlling the postprandial glycemic index, which may help in managing type-2 diabetes mellitus. Chlorogenic and neochlorogenic acids and quercetin derivatives are well-known inhibitors of α-glucosidase and α-amylase [58,59]. For instance, the inhibition of α-glucosidase very strongly correlated with chlorogenic acid present in the young inflorescence tissues of selected Rosaceae plants [60]. However, the inhibitory activity depends on the structural peculiarities of the phenolic compounds. Recently, Zhang et al. [61] reported that the fraction of bound polyphenolics of red quinoa composed mainly of ferulic acid more strongly inhibited α-glucosidase than the fraction of free polyphenolics consisting mainly of hydroxybenzoic acid and its derivatives; the former fraction inhibited α-glucosidase in an uncompetitive mode, while the latter one in a non-competitive mode. This was explained by the peculiarities of the interactions between ferulic acid and enzyme amino acid sites. Flavonols such as quercetin were also reported as potent inhibitors of α-amylase and α-glucosidase in an activity-guided study of chokeberry, pomegranate and red grape extracts [62]. Based on the strong inhibition of glucose release from complex carbohydrates, herbal infusions containing phenolic acids were suggested as natural preparations for preventing type II diabetes [6]. The hypoglycemic effect of di-caffeoylquinic acid derivatives may be also explained by the modulation of α-glucosidase, while chlorogenic acid is a potent inhibitor of glucose 6-phosphate translocase [63].
Quite interesting behavior was observed by evaluating extract-induced α-glucosidase inhibition (Table 2) and reaction kinetics (Figure 2 and Table 3). It may be noted that the main findings were valid regardless of some modification of the method described in the Sigma-Aldrich protocol [48]. Our data suggest that using 0.1 M Na2CO3 solution may significantly change the manner of extract-mediated α-glucosidase inhibition. Significantly negative differences in the activity shift were observed at concentrations of up to 25 µg/mL, while, at higher concentrations, the inhibition in the reaction without 0.1 M Na2CO3 was remarkably higher. To the best of our knowledge, the peculiarities of botanical extract-induced α-glucosidase inhibition using this approach have not been reported previously. Most likely, the significant differences in inhibition between the reaction systems, which were particularly clearly pronounced in the case of increasing extract concentrations, were due to changes in the ratios of the compounds with different effects on the enzyme.
Lineweaver–Burk, Eadie–Hofstee and Hanes–Woolf plots are widely used for determining important parameters in enzyme kinetics, although they may result in some erroneous data [64]. Chon et al. [65] compared five estimation methods, including Lineweaver–Burk and Eadie–Hofstee plots, for simulation data using additive error and combined error models for the parameters of the Michaelis–Menten equation and concluded that the estimation of Vmax and Km by nonlinear methods provided the most accurate results, while in case of linear methods, the Eadie–Hofstee plot exhibited some advantages over the Lineweaver–Burk plot, although, for the simulation data incorporating additive error, estimated Vmax values were similar. In addition, it was noted that the double reciprocal Lineweaver–Burk plot overestimates rate measurements recorded at low substrate concentrations, where the experimental error is liable to be greatest [66], while the Eadie–Hofstee plot is less biased at low [S]; however, it can result in significant errors since both coordinates contain V [64]. Marasović et al. (2017) [67] suggested that the Hanes–Woolf plot is the most accurate of the three; however, its major drawback is that both ordinate and abscissa are dependent on the substrate concentration. Despite the perceived errors of the described methods, they are all presented in the scientific literature and are applied for determining kinetic parameters. We chose the most widely used Lineweaver–Burk method, which enabled us to demonstrate that the D. caucasica leaf and tuber extracts displayed mixed-type and competitive modes of inhibition of α-glucosidase, respectively.
Numerous plant origin bioactive compounds and food-derived peptides may inhibit ACE in a very wide range of IC50. Regarding yams, D. opposita autolysate and enzymatic hydrolysates of tuber mucilage were tested as ACE inhibitors [68], while the inhibitory properties of leaf extracts have not been reported. D. causacica leaf extract inhibited ACE in a dose-dependent manner (Figure 6). Polyphenolic compounds in this case may also play the most important role. For instance, wine lees of Cabernet grape variety, which contained significantly higher amounts of catechin flavanols than other evaluated varieties, effectively inhibited ACE and demonstrated similar potency to the drug Captopril in hypertensive rats [69]. In vivo studies with mice showed that a dietary supplement consisting of selected botanicals, chlorogenic acid and inulin reduced the risk factors of cardiovascular diseases by lowering hepatic angiotensinogen and angiotensin-II levels, among other factors [70]. The hypotensive effects of the phenolic-rich fraction of red wine were also demonstrated by an in vivo study with spontaneously hypertensive rats [5].
The AChE enzyme is involved in numerous noncholinergic physiological functions, such as promoting cell development and differentiation, participating in apoptosis and relating to pathogenic processes including Alzheimer’s disease and tumorigenesis [71]. Various compounds, e.g., galanhtamine, huperizine A, N-demethyl-puqietinone, yibeinoside A, quercetin, etc., which are abundant in some plant species, were reported as AChE inhibitors [4,72]. The anti-cholinesterase ability of Phyllanthus emblica fruit extract was also related to a high amount of flavonols and phenolic acids [73], which were abundant in the D. caucasica leaf extract evaluated in our study. The diethyl ether and ethyl acetate extracts of D. communis tubers tested in previous studies did not show an AChE inhibitory effect; however, some individual Dioscorea constituents (2,3,4-trimethoxy-7,8-methylenedioxyphenanthrene, 2,4-dimethoxy-7,8-methylenedioxy-3-phenanthrenol, 2,4,8-trimethoxy-3,7-phenanthrene-diol) inhibited the enzyme [3]. Phenanthrene derivates we not detected in ethanol extracts, but the possibility cannot be ruled out that some other nonidentified compound may have inhibited this enzyme. For example, Tenfen et al. (2019) [74] reported that the strong inhibition of Eugenia genus leaf extracts against AChE was associated with the presence of isoquercitrin, quercetin, catechin, epicatechin, procatecuic acid and myricitrin content. Thus, the extract from the leaves of D. caucasica inhibits the AChE enzyme in a dose-dependent manner, as with other, previously tested enzymes.
4. Materials and Methods
4.1. Reagents
α-Glucosidase (EC 3.2.1.20) from Saccharomyces cerevisiae Type I; lyophilized powder, ≥10 units/mg protein (G5003—100 UN) [75]; α-amylase (EC 3.2.1.1) from porcine pancreas (Sigma A6255, 1151 U/mg of protein) [76], lung acetone powder from rabbit (L-0756) [77], N-[3-(2-furyl)acryloyl]-L-phenylalanine-glycyl-glycine (FAPGG), p-nitrophenyl α-D-glucopyranoside (pNPG) (N1377-1G), 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB, ≥99%), acetylthiolcholine iodide (ATChI); and starch were obtained from Sigma-Aldrich (St. Louis, MO, USA). Acetylcholinesterase (EC 3.1.17) from Electrophorus electricus, Type VI-S; lyophilized powder, 200–1000 units mg/protein (C3389) [78], was from Sigma-Aldrich Chemie (Taufkirchen, Germany); 3.5-dinitrosalicilyc acid (DNS) from Acros Organics (Geel, Belgium), 4-nitrophenol (C6H5NO3) from Alfa Aesar (Kandel, Germany). Potassium phosphate dibasic (K2HPO4), potassium phosphate monobasic (KH2PO4), sodium phosphate monobasic (NaH2PO4), sodium phosphate dibasic (Na2HPO4), tris(hydroxymethyl) aminomethane hydrochloride, boric acid (H3BO3), anhydrous sodium carbonate (Na2CO3), sodium chloride (NaCl) and sodium hydrocarbonate (NaHCO3) were purchased from Eurochemicals (Vilnius, Lithuania); 1M hydrochloric acid (HCl) from Merck KGaA (Darmstadt, Germany). Agricultural origin ethanol (96.6 %) was from Stumbras (Kaunas, Lithuania). Captopril, 1-[(2S)-3-mercapto-2-methyl propionyl] (Salutas Pharma GmbH, Barleben, Germany) and donepezil hydrochloridum (Accord Healthcare Limited, London, UK) were purchased from the local pharmacy. Acarbose (C25H43NO18) was from Merck KGaA (Darmstadt, Germany); reference compounds, neochlorogenic acid, chlorogenic acid, rutin and quercitrin were from Sigma Chemical Co. (St. Louis, MO, USA). Other chemicals and reagents used in the current study were of analytical grade and obtained from Sigma-Aldrich (St. Louis, MO, USA), unless other sources are indicated. Ultrapure water was produced using a Simplicity 185 system (Millipore, MA, USA). All buffers were made using ultrapure water and pH measurements were performed at room temperature using an Agilent 3200P pH Meter (Agilent Technologies, Inc., Shanghai, China).
4.2. Preparation of D. caucasica Extracts
Dioscorea caucasica Lipsky leaves and tubers were collected from the Kaunas Botanical Garden of Vytautas Magnus University in Lithuania (55°52′14′′ N 23°54′37′′ E) [79] in June 2019. The collected materials were air-dried at room temperature and milled in a centrifugal mill, Retsch ZM200 (Haan, Germany), using a sieve with 0.5 mm diameter holes. Twenty grams of powdered sample were extracted with 200 mL 70% (v/v) ethanol in a rotary shaker (200 rpm) during 14 h at room temperature. The extract obtained was filtered through Whatman filter paper no. 1 (Whatman® International Ltd., Maidstone, UK) to obtain the first extract. This procedure was repeated on the residue using 100 mL 70% ethanol for 1 h to obtain the second filtrate. Both filtrates were combined and the solvent was removed in a rotary vacuum evaporator, the Büchi Rotavapor R-210 (Büchi Labortechnik, Flawil, Switzerland), at 40 °C. The residual water was evaporated by freeze-drying. The yield of dry extract powder was 17.86% and 15.65% from leaves and tubers, respectively. Freeze-dried extracts (100 ± 0.1 mg) were dissolved in 10 mL of 70% (v/v) ethanol in a volumetric flask to prepare 10 mg/mL extract stock solutions, which were stored at 4 °C until further use.
4.3. Identification and Quantitative Analysis of the Main Phytochemicals
4.3.1. Identification of Phytochemicals by UPLC-QTOF-MS
The extracts were separated using a Waters Acquity UPLC system [80,81] consisting of a binary solvent manager, autosampler, column heater and a photodiode-array detector (PDA) (Waters Corporation, Milford, MA, USA). A Waters Acquity BEH C18 (100 × 2.1 mm; 1.7 μm) [82] column was used for compound separation. Eluent A was 0.4% (v/v) formic acid solution in ultrapure water, and B was acetonitrile. The gradient was formed as follows: initially, the separation was started with 100% A; then, in 9 min, B was increased to 100%, and was held at 100% for 1 min. After this, the column was returned to initial conditions in 1 min and then was allowed to equilibrate for 1 min. The column was equilibrated for 2 min before each run. Flow rate was 0.4 mL/min, injection volume 1 µL and column temperature 40 °C.
The eluted compounds were analyzed on a MAXIS 4G QTOF mass spectrometer (Bruker Daltonik GmbH, Bremen, Germany) equipped with an electrospray ionization (ESI) source. The spectrometer was operated in negative ionization mode, and capillary voltage was maintained at 4000 V. Nitrogen (N2) was used as a nebulizing and drying gas at 2.5 bar pressure and 10 L/min flow rate. Drying gas temperature was maintained at 200 °C. MS were recorded in the range from 80 to 1200 m/z; spectra recording rate was 3 Hz. The compounds profiles were identified by comparing MS results with previously reported data.
4.3.2. Quantification of the Main Phytochemicals by HPLC
The extracts were prepared by dissolving 200 ± 0.1 mg of freeze-dried powdered leaves/tubers in 10 mL of methanol in an ultrasonic bath for 30 min at 20 ± 0.2 °C. Subsequently, the aliquots of 10 µL samples were injected into the column of a Waters Alliance 2695 HPLC, and the oven temperature was kept at 25 °C. The mobile phase A (0.1% trifluoroacetic acid (v/v) in ultrapure water) and mobile phase B (acetonitrile) were operated with a fast eluting gradient as follows: 0–8 min, 95% A, 5% B; 8–30 min, 90–80% A, 10–20% B; 20–30 min, 80–60% A, 20–40% B; 30–40 min, 60–40% A, 40–60% B; 40–45 min, 20–40% B; 30–40 min, 60–40% A, 40–60% B; 40–45 min, 40–30% A, 60–70% B; and 45–50 min, 30–90% A, 70–10% B. Chromatographic separation was performed in an Atlantis C18 column (250 mm × 4.6 mm i.d., 5 µm). Detector was set at 320 nm for phenolic acids (neochlorogenic and chlorogenic), 360 nm for flavonoids (rutin, isoquercitrin). Neochlorogenic acid, chlorogenic acid, rutin and isoquercitrin were eluted at 9.84, 12.28, 23.43 and 25.55 min, respectively (Figure 1). Quantitative determination was performed by using calibration curves, which were produced using reference compounds.
4.4. Preparation of Solutions for Enzyme Inhibition Assays
4.4.1. Rabbit Lung and Captopril Solutions for Angiotensin-Converting Enzyme (ACE) Inhibition Assays
Rabbit lung acetone powder [76] was prepared as described by Vermerissen et al. (2002) [83], with slight modification. Briefly, 100 ± 0.1 mg of powder was dissolved in 100 mL of cold borate buffer (80 mmol/L, pH 8.3 ± 0.05) and kept at 5 °C temperature overnight. Insoluble matter was centrifuged in a centrifuge, the MPW 260RH (Med. Instruments, Warsaw, Poland), at 6000 rpm for 30 min at 4 °C temperature. After centrifugation, the clear wine-red supernatant was carefully transferred into a clean test tube and used for experiments.
Captopril solution was prepared as described by Donath-Nagy et al. (2011) [84], with slight modifications. Two tablets containing 25 mg of Captopril were ground in a mortar and extracted with approximately 15 mL water in a 25 mL volumetric flask in the ultrasonic bath (Bandelin Sanorex, Berlin, Germany) for 10 min, and then brought to volume with water and filtered through Whatman filter paper. Solution of Captopril (1 mg/mL) was used as a positive control in the ACE inhibition assay.
4.4.2. Donepezil HCl Solution for Acetylcholinesterase (AChE) Inhibitory Activity
Donepezil hydrochloride solution was prepared from two tablets containing 5 mg of Donepezil Accord drug. The tablets were crushed and 100 ± 0.1 mg of fine powder was dissolved in 100 mL of ultrapure water in a 100 mL volumetric flask to obtain a 1 mg/mL Donepezil HCl stock solution. Working solutions of Donepezil HCl were obtained by the appropriate dilution of the stock solution and used as positive controls in AChE inhibition assays.
4.4.3. Acarbose Solution for α-Amylase and α-Glucosidase Inhibition Assays
Aqueous solution of acarbose was prepared by dissolving 100 ± 0.1 mg acarbose in a 100 mL volumetric flask to obtain a 1 mg/mL acarbose stock solution. Working solutions of acarbose were obtained by appropriate dilution of the stock solution and used as positive controls in α-amylase/α-glucosidase inhibition assays.
4.5. Determination of Enzyme Inhibition Activities
4.5.1. α-Glucosidase
The α-glucosidase inhibition was evaluated by the chromogenic method using pNPG as a substrate [48]. The α-glucosidase activity measurements were conducted at 37 ± 0.2 °C using a 1 mL mixture composed of 0.33 mM pNPG, dissolved in potassium phosphate buffer (pH 6.8) and different concentrations of inhibitors, namely 0, 3.125, 6.25, 12.5, 25, 50 and 100 µg/mL. The assay was initiated by the addition of 5 µL solution of enzyme. α-Glucosidase and product formation (pNPG + α-glucosidase → α-D-glucose + pNp was monitored after 30 min at 405 nm using the Spectronic Genesys 8 spectrophotometer (Thermo Spectronic, Rochester, NY, USA). The data were recorded in two ways: with the stop solution 0.1 M Na2CO3 and without it. The control solution was prepared using the same buffer without inhibitor. The percentage of inhibition of α-glucosidase was calculated similarly to α-amylase.
For kinetic studies, α-glucosidase activity was measured spectrophotometrically by following the absorbance at 405 nm in assay mixture containing various concentrations of substrate: 0.1mM, 0.125 mM, 0.23 mM and 0.33 mM pNPG in 0.1 M K2HPO4/ KH2PO4 phosphate buffer, pH 6.8 ± 0.05, fixed amount of α-glucosidase (5 µL) solution and the absence or presence of the leaf and tuber extracts at 15 µg/mL, 25 µg/mL and 200 and 500 µg/mL concentrations, respectively.
4.5.2. α-Amylase
In vitro α-amylase inhibition was assayed by the method by described in the Sigma-Aldrich protocol [85], with slight modifications. α-Amylase [76] was diluted (1:1000) in 0.02 mM sodium phosphate buffer (pH 6.9) containing 6.7 mM sodium chloride to produce stock solution. The reaction mixture containing 400 µL of α-amylase (0.5 IU/mL) and 400 μL of varying concentrations of extracts (40–480 µg/mL) was incubated in a test tube at 25 °C ± 0.2 °C for 30 min, followed by the addition of 400 µL of 1% (w/v) potato starch solution (0.02 mM sodium phosphate buffer, pH 6.9 ± 0.5), as a substrate, and further incubation for 3 min. The reaction was terminated by the addition of 800 µL of 3.5-dinitrosalicylic acid (DNSA) and heating in a water bath for 10 min at 85 ± 0.2 °C. Afterwards, the mixture was removed from the water bath, cooled under tap water and diluted with 3 mL of distilled water. The absorbance (A) was measured at 540 nm and the inhibition activity was calculated by the following equation: % Inhibition = (Acontrol − Asample)/Acontrol × 100. The blank sample was prepared without enzyme; control samples were those without extracts (100% enzyme activity). All tests were performed in triplicate. Acarbose was used as a positive control.
4.5.3. Acetylcholinesterase (AChE)
The AChE inhibition was estimated in vitro by Ellman’s method [52] using ATChI as a substrate. The spectrophotometric method for the estimation of AChE activity is based on the determination of yellow-colored chromophore (5-thio-2-nitrobenzoate; C7H5NO4S; λ = 412 nm) during enzymatic hydrolysis with DTNB reagent (5,5′-dithiobis-(2-nitrobenzoic acid), which reacts with the sulfhydryl groups of the protein. Briefly, AChE activity measurements were carried out at a constant temperature of 20 ± 0.5 °C using a 1 mL mixture composed of 50 mmol/L Tris/HCl (pH 8.0 ± 0.05) buffer, different concentrations (25–100 µg/mL) of inhibitor, 0.05 mmol/L DTNB and AChE at 0.55U. After pre-incubation for 10 min, the reaction was initiated by the addition of 2.5 mM ATChI. The formation of a DTNB-tios complex (C7H5NO4S) was measured at 412 nm by using a GENESYS 50 UV/Vis spectrophotometer (GENESYS Instruments Limited, Cambridge, UK). Donepezil, a standard AChE inhibitor, was used as a positive control and Tris/HCl (pH 8.0 ± 0.05) was used as a negative control. Enzyme-specific activity was calculated using nitrobenzoate (TNB) molar extinction coefficient ε =14150 M−1 cm−1 [86] and expressed as µmol thiocholine formed/min/mg protein. The enzyme percentage inhibition was calculated by comparing the enzymatic activity with/without inhibitor using the following equation: % I = (Acontrol − Asample) /Acontrol × 100 %, where Acontol is AChE activity for the negative control, and Asample is the presence of the plant extract or Donepezil.
For kinetic studies, AChE activity was measured spectrophotometrically by following the absorbance at 412 nm in assay mixture containing various concentrations of substrate, 45, 50, 65, 85 and 160 µM ATChI, in 50 mmol/L Tris/HCl, pH 8.0 ± 0.05, fixed amount of AChE (10 µL) solution and the absence or presence of the leaf extracts at 50 µg/mL concentration.
4.5.4. Angiotensin-Converting Enzyme (ACE)
ACE inhibition was estimated in vitro by measuring the release of N-[3-(2-furyl)acryloyl]-L-phenylalanine (FAP) and glycine-glycine (Gly-Gly) from the substrate FAPGG (C20H21N3O6) according to the simple, rapid and sensitive method of Vermerissen et al. (2002) [83]. Ethanol solutions of plant extract at concentrations of 250, 500, 1000 and 1250 µg/mL were used for the ACE inhibition assay. Three hundred µL of rabbit lung ACE (rabbit lung acetone powder reconstituted in 80 mM sodium borate buffer containing 300 mmol/L sodium chloride, pH 8.3 ± 0.05) and 200 μL of inhibitor solution were added into a test tube and pre-incubated for 3–5 min at room temperature. After pre-incubation, the reaction was started (t = 0 min) by adding 800 μL ACE-specific substrate solution comprising 0.8 mmol/L FAPGG dissolved in a borate buffer, to the final volume of 1300 μL. The mixture was allowed to stand at room temperature for 1 min and absorbance was recorded at 340 nm using the Biochrom Libra S4+ visible spectrophotometer (Biochrom Ltd., Cambridge, UK). Subsequently, the absorbance was monitored after 20 min at 37 ± 0.2 °C. Hydrolysis of FAPGG results in a decrease in absorbance at 340 nm. The rate of decrease in absorbance is directly proportional to ACE activity in the sample. The percentage inhibition of ACE activity by plant extracts was calculated with the following equation: Inhibition, % = [100 − (ΔA340/min inhibitor/ΔA340/min control × 100], where ΔA is the difference between the initial and final absorbance of the test sample during its incubation.
4.6. Mathematical Modeling of Enzyme Inhibition Kinetics
The application of kinetic modeling can provide important insights into how interacting components behave in biological systems. The changes in enzyme catalytic activity in a biochemical system are frequently modeled by choosing a mathematical model to evaluate the following fundamental parameters: Michaelis–Menten constant (Km), maximal velocity (Vmax) and kinetic constant (Ki). In this study, kinetic data were estimated by virtue of plotting the data on a double reciprocal graph, called a Lineaweaver–Burk plot [49], to the following equation: 1/V = Km/Vmax × 1/[S] + 1/Vmax, where V is the reaction rate, Km is the Michaelis–Menten constant, Vmax is the maximum reaction rate (mg/L·min), [S] is the pNPG concentration (mg/L). The resultant plot is a straight line, with X- and Y-axis intercepts representing −1/Km and 1/Vmax, respectively, and the slope is Km/Vmax. The inhibition type of the inhibitors was determined by analyzing the Lineaweaver–Burk plots, which were competitive, non-competitive or uncompetitive, and the inhibition constant (Ki) values for botanical extracts were estimated by fitting the equation Km(app) = Km (1 + [I]/Ki) for competitive inhibition and equation Vmax(app) = Vmax (1 + [I]/Ki) for non-competitive inhibition, where Km(app) and Km are the concentrations of a substrate required to produce 50% of its maximum velocity (Vmax) in the presence and absence of an inhibitor, which were determined in parallel; [I] is the compound concentration; Ki is the inhibition constant of the reactions. Table 5 summarizes the types of inhibition and their effects on these parameters.

Table 5.
Enzyme inhibition patterns [50].
4.7. Statistical Data Analysis
Data are expressed as mean ± standard error (SE) and/or standard deviation (SD). Reaction velocities and enzyme kinetics and IC50 values of the extracts were calculated using Microsoft Excel 2016. Statistical analysis was performed by Student’s t-test, paired-samples t-test and Fisher’s least significance difference (LSD) test (p < 0.05).
5. Conclusions
The first systematic phytochemical study of D. caucasica revealed that the plant leaves are rich in phenolic compounds, mainly caffeic acid esters and quercetin glycosides, while its tubers accumulate steroidal glycosides, as the major secondary metabolites. D. caucasica leaf extracts were remarkably stronger inhibitors of α-glucosidase, α-amylase, acetylcholinesterase and angiotensin-converting enzyme than the tuber extracts. Kinetic studies of enzyme inhibition suggest that the mode of inhibition, depending on the extract origin, may be mixed-type (leaves) and competitive (tubers). Based on the results obtained in this study and previously reported data, it may be sufficiently reasonably assumed that D. caucasica leaf polyphenolics play the most important role in the enzyme inhibition, while the tuber steroidal glycosides might be remarkably weaker enzyme inhibitors or their effects may be hampered by other, antagonistically competitive constituents. In general, the new data on D. caucasica phytochemicals and bioactivities may aid in the valorization of this plant for its wider use in the development of health beneficial ingredients for functional foods and nutraceuticals.
Author Contributions
Conceptualization, A.A. and P.R.V.; methodology, A.A., A.P. and P.R.V.; investigation, A.A. and A.P.; writing—review and editing, A.A. and P.R.V.; resources, P.R.V. and O.R.; supervision, P.R.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nodland, S. Food’s Role as an Immunomodulator. Health and Nutrition Institute–Science for Healthier Food. 2021. Available online: https://khni.kerry.com/tag/nutrion/ (accessed on 21 April 2022).
- World Health Organization. Diet, nutrition, and the prevention of chronic diseases. World Health Organ. Tech. Rep. Ser. 2003, 916, 1–149. [Google Scholar]
- Boudjada, A.; Touil, A.; Bensouici, C.; Bendif, H.; Rhouati, S. Phenanthrene and dihydrophenanthrene derivatives from Dioscorea communis with anticholinesterase, and antioxidant activities. Nat. Prod. Res. 2018, 33, 3278–3282. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.P.; Faraoni, M.B.; Castro, M.J.; Alza, N.P.; Cavallaro, V. Natural AChE Inhibitors from plants and their contribution to Alzheimer’s disease therapy. Curr. Neuropharmacol. 2013, 11, 388–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueiredo, E.A.d.; Alves, N.F.B.; Monteiro, M.M.d.O.; Cavalcanti, C.d.O.; Silva, T.M.S.d.; Silva, T.M.G.d.; Braga, V.d.A.; Oliveira, E.d.J. Antioxidant and antihypertensive effects of a chemically defined fraction of Syrah red wine on spontaneously hypertensive rats. Nutrients 2017, 9, 574. [Google Scholar] [CrossRef] [Green Version]
- Studzińska-Sroka, E.; Galanty, A.; Gościniak, A.; Wieczorek, M.; Kłaput, M.; Dudek-Makuch, M.; Cielecka-Piontek, J. Herbal infusions as a valuable functional food. Nutrients 2021, 13, 4051. [Google Scholar] [CrossRef]
- Zhao, C.; Li, X.; Miao, J.; Jing, S.; Li, X.; Huang, L.; Gao, W. The effect of different extraction techniques on property and bioactivity of polysaccharides from Dioscorea hemsleyi. Int. J. Biol. Macromol. 2017, 102, 847–856. [Google Scholar] [CrossRef]
- Kwon, C.S.; Sohn, H.Y.; Kim, S.H.; Kim, J.H.; Son, K.H.; Lee, J.S.; Lim, J.K.; Kim, J.S. Anti-obesity effect of Dioscorea nipponica Makino with lipase-inhibitory activity in rodents. Biosci. Biotechnol. Biochem. 2003, 67, 1451–1456. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; More, P.; Derle, A.; Patil, A.B.; Markad, P.; Asok, A.; Kumbhar, N.; Shaikh, M.L.; Ramanamurthy, B.; Shinde, V.S.; et al. Diosgenin from Dioscorea bulbifera: Novel hit for treatment of Type II Diabetes Mellitus with inhibitory activity against α-amylase and α-glucosidase. PLoS ONE 2014, 9, e106039. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.X.; Wang, P.F.; Song, H.G.; Sun, N. Diosgenin attenuates neuropathic pain in a rat model of chronic constriction injury. Mol. Med. Rep. 2017, 16, 1559–1564. [Google Scholar] [CrossRef]
- Guo, X.; Sha, X.; Liu, J.; Cai, S.; Wang, Y.; Ji, B. Chinese purple yam (Dioscorea alata L.) extracts inhibit diabetes-related enzymes and protect HepG2 cells against oxidative stress and insulin resistance induced by FFA. Food Sci. Technol. Res. 2015, 21, 677–683. [Google Scholar] [CrossRef] [Green Version]
- Yadav, D.; Mishra, M.; Jadaun, P.; Choi, E.; Priyadharsini, R.; Kong, I.D. Glycemic index and glycemic load in cardiovascular disease risk. Prog. Nutr. 2016, 18, 95–101. [Google Scholar]
- Goyal, A.; Cusick, A.S.; Thielemier, B. ACE Inhibitors. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430896/ (accessed on 22 April 2022).
- Christenhusz, M.J.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef] [Green Version]
- Lebot, V. Tropical Root and Tuber Crops: Cassava, Sweet Potato, Yams and Aroids, 2nd ed.; CABI: Wallingford, UK; Boston, MA, USA, 2020; pp. 218–220. [Google Scholar]
- FAOASTAT Food and Agricultural Organization. Available online: https://www.fao.org/ (accessed on 20 August 2021).
- Kim, M.J.; Son, S.Y.; Jeon, S.G.; Kim, J.G.; Lee, C.-H. Metabolite Profiling of Dioscorea (Yam) leaves to identify bioactive compounds reveals their potential as renewable resources. Plants 2021, 10, 1751. [Google Scholar] [CrossRef] [PubMed]
- GBIF. Global Biodiversity Information Facility. Available online: https://www.gbif.org/ (accessed on 20 August 2021).
- Korochinsky, A.V.; Korochinskaya, V.V.; Zilfikarov, I.N.; Daironas, J.V. Innovative technology of tablets «Diosklephyt» based on Dioscorea caucasica Lypsky. Drug Dev. Regist. 2015, 4, 74–81. [Google Scholar]
- Guo, X.; Ding, X. Dioscin suppresses the viability of ovarian cancer cells by regulating the VEGFR2 and PI3K/AKT/MAPK signaling pathways. Oncol. Lett. 2018, 15, 9537–9542. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Xu, B.; Zhao, H.; Wang, Y.; Zhang, J.; Li, C.; Wu, Q.; Cao, Y.; Li, Y.; Cao, F. Dioscin protects against coronary heart disease by reducing oxidative stress and inflammation via Sirt1/Nrf2 and p38 MAPK pathways. Mol. Med. Rep. 2018, 18, 973–980. [Google Scholar] [CrossRef] [Green Version]
- Son, I.S.; Kim, J.H.; Sohn, H.Y.; Son, K.H.; Kim, J.S.; Kwon, C.S. Antioxidative and hypolipidemic effects of diosgenin, a steroidal saponin of yam (Dioscorea spp.), on high-cholesterol fed rats. Biosci. Biotechnol. Biochem. 2007, 71, 3063–3071. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Sun, H.J.; Liu, S.M.; Jiang, X.H.; Wang, Q.Y.; Zhang, S.; Yu, D.H. Anti-inflammation effects of the total saponin fraction from Dioscorea nipponica Makino on rats with gouty arthritis by influencing MAPK signalling pathway. BMC Complement. Med. Ther. 2020, 20, 261. [Google Scholar] [CrossRef]
- Al Shukor, N.; Van Camp, J.; Gonzales, G.B.; Staljanssens, D.; Struijs, K.; Zotti, M.J.; Raes, K.; Smagghe, G. Angiotensin-converting enzyme inhibitory effects by plant phenolic compounds: A study of structure activity relationships. J. Agric. Food Chem. 2013, 61, 11832–11839. [Google Scholar] [CrossRef]
- Szakiel, A.; Grabarczyk, M.; Pączkowski, C.; Mieczkowski, A. Comparison of the profiles of non-glycosylated triterpenoids from leaves of plants of selected species of genus Dioscorea. Phytochem. Lett. 2017, 20, 350–355. [Google Scholar] [CrossRef]
- Price, E.J.; Wilkin, P.; Sarasan, V.; Fraser, P.D. Metabolite profiling of Dioscorea (yam) species reveals underutilised biodiversity and renewable sources for high-value compounds. Sci. Rep. 2016, 6, 29136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bj, H.; Zy, L.; Gs, J.; Ym, L. Studies on an aqueous soluble active constituent of Chuan-Shan-Long (Dioscorea nipponica Makino). I. Isolation and identification of p-hydroxy benzyl tartaric acid (piscidic acid) (author’s transl). Acta Pharm. Sin. 1980, 15, 764–765. [Google Scholar]
- Zeng, X.; Liu, D.; Huang, L. Metabolome Profiling of Eight Chinese Yam (Dioscorea polystachya Turcz.) Varieties Reveals Metabolite Diversity and Variety Specific Uses. Life 2021, 11, 687. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jia, Z.; Zhang, Z.; Wang, Y.; Liu, X.; Wang, L.; Lin, R. Analysis of chemical constituents of Melastoma dodecandrum Lour. by UPLC-ESI-Q-Exactive Focus-MS/MS. Molecules 2017, 22, 476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, W.; Zhang, C.; Zhao, D.; Wang, L.; Li, P.; Li, H. Discovery of hepatotoxic equivalent combinatorial markers from Dioscorea bulbifera tuber by fingerprint-toxicity relationship modeling. Sci. Rep. 2018, 8, 462. [Google Scholar] [CrossRef] [PubMed]
- Parveen, I.; Winters, A.; Threadgill, M.D.; Hauck, B.; Morris, P. Extraction, structural characterisation and evaluation of hydroxycinnamate esters of orchard grass (Dactylis glomerata) as substrates for polyphenol oxidase. Phytochem 2008, 69, 2799–2806. [Google Scholar] [CrossRef]
- Xue, M.; Shi, H.; Zhang, J.; Liu, Q.Q.; Guan, J.; Zhang, J.Y.; Ma, Q. Stability and degradation of caffeoylquinic acids under different storage conditions studied by high-performance liquid chromatography with photo diode array detection and high-performance liquid chromatography with electrospray ionization collision-induced dissociation tandem mass spectrometry. Molecules 2016, 21, 948. [Google Scholar]
- Zhou, L.; Shi, X.M.; Ren, X.M.; Zhang, J.P.; Qin, Z.H. Identification of phenolic components in the root and leaf of purple yam (Dioscorea alata) by UHPLC-DAD-ESI-MS/MS. Mod. Food Sci. Technol. 2016, 32, 310–315. [Google Scholar]
- Ammar, S.; Contreras, M.; del Mar Contreras, M.; Belguith-Hadrich, O.; Bouaziz, M.; Segura-Carretero, A. New insights into the qualitative phenolic profile of Ficus carica L. fruits and leaves from Tunisia using ultra-high-performance liquid chromatography coupled to quadrupole-time-of-flight mass spectrometry and their antioxidant activity. RSC Adv. 2015, 5, 20035–20050. [Google Scholar] [CrossRef]
- Ablajan, K.; Tuoheti, A. Fragmentation characteristics and isomeric differentiation of flavonol O -rhamnosides using negative ion electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 27, 451–460. [Google Scholar] [CrossRef]
- Lebot, V.; Malapa, R.; Molisalé, T. Development of HP-TLC method for rapid quantification of sugars, catechins, phenolic acids and saponins to assess yam (Dioscorea spp.) tuber flour quality. Plant Genet. Resour. 2019, 17, 62–72. [Google Scholar] [CrossRef]
- Wan, D.; Yang, H.; Yan, C.; Song, F.; Liu, Z.; Liu, S. Differentiation of glucose-containing disaccharides isomers by fragmentation of the deprotonated non-covalent dimers using negative electrospray ionization tandem mass spectrometry. Talanta. 2013, 115, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Abd Kadir, N.A.A. Fatty acid profile, phytochemicals, and other substances in Canarium odontophyllum fat extracted using supercritical carbon dioxide. Front. Chem. 2019, 7, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LIPID MAPS. Available online: https://www.lipidmaps.org (accessed on 12 December 2021).
- Xie, T.; Liang, Y.; Hao, H.; A, J.; Xie, L.; Gong, P.; Dai, C.; Liu, L.; Kang, A.; Zheng, X.; et al. Rapid identification of ophiopogonins and ophiopogonones in Ophiopogon japonicus extract with a practical technique of mass defect filtering based on high resolution mass spectrometry. J. Chromatogr. A 2012, 1227, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, H.Y.; Chao, L.P.; Qu, L.; Ruan, J.Y.; Liu, Y.X.; Dong, Y.Z.; Han, L.F.; Wang, T. Anti-inflammatory steroids from the rhizomes of Dioscorea septemloba Thunb. Steroids 2016, 112, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Yu, D. 3.11 Sterodal saponins. In ¹H NMR Handbook of Natural Products: Saponins; De Gruyter: Berlin, Germany; Boston, MA, USA, 2021; Volume 2, pp. 349–486. [Google Scholar]
- Osagie, A.U.; Opute, F.I. Major lipid constituents of Dioscorea rotundata tuber during growth and maturation. J. Exp. Bot. 1981, 32, 737–740. [Google Scholar] [CrossRef]
- Shajeela, P.S.; Tresina, P.S.; Mohan, V.R. Fatty acid composition of wild yam (Dioscorea spp.). Trop. Subtrop. Agroecosyst. 2013, 16, 35–38. [Google Scholar]
- IUBMB. Enzyme Nomenclature: Recommendations (1992) of the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology. EC 3.2.1.20. Available online: https://www.qmuil.ac.uk/enzyme/EC3/2/1/20.html (accessed on 20 April 2022).
- Carbohydrate-Active Enzymes Database (CAZy). Available online: www.cazy.org/GH13.html (accessed on 20 April 2022).
- ExplorEnz–The Enzyme Database. ExplorEnz: EC 3.2.1.20. Available online: Enzyme-database.org (accessed on 20 April 2022).
- Sigma-Aldrich protocol. Enzymatic Assay of α-GLUCOSIDASE (EC 3.2.1.20) p-Nitrophenyl α-D-Glucoside as Substrate. Sigma-Aldrich. [Online]. Available online: http://www.sigmaaldric.com/technical-documents/protocol/enzymatic-assay-of-a-glucosidase.html (accessed on 19 April 2022).
- Lineweaver, H.; Burk, D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
- Page, M.I.; Engel, P.C. (Eds.) Chapter 3 Enzyme kinetics. In The Chemnistry of Enzyme Action; Reprinted from New Comprehensive Biochemistry; Elsevier: Amsterdam, The Netherlands, 1984; Volume 6, pp. 73–110. [Google Scholar]
- Berg, J.M.; Stryer, T.J.L. Appendix: Vmax and Km can be determined by double-reciprocal plots. In Biochemistryi, 5th ed.; W H Freeman: New York, NY, USA, 2002. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Ghosh, S.; Ahire, M.; Patil, S.; Jabgunde, A.; Bhat Dusane, M.; Joshi, B.N.; Pardesi, K.; Jachak, S.; Dhavale, D.D.; Chopade, B.A. Antidiabetic activity of Gnidia glaucaand and Dioscorea bulbifera: Potent amylase and glucosidase inhibitors. Evid. Based Complement. Alter. Med. 2012, 2012, 929051. [Google Scholar]
- Avula, B.; Wang, Y.H.; Ali, Z.; Smillie, T.J.; Khan, I.A. Chemical fingerprint analysis and quantitative determination of steroidal compounds from Dioscorea villosa, Dioscorea species and dietary supplements using UHPLC-ELSD. Biomed. Chromatogr. 2014, 28, 281–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vendl, O.; Wawrosch, C.; Noe, C.; Molina, C.; Kahl, G.; Kopp, B. Diosgenin contents and DNA fingerprint screening of various yam (Dioscorea sp.) genotypes. Z. Nat. C 2006, 61, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Chen, L. α-Glucosidase and α-amylase inhibitors from seed oil: A review of liposoluble substance to treat diabetes. Crit. Rev. Food Sci. Nutr. 2017, 57, 3438–3448. [Google Scholar] [CrossRef] [PubMed]
- Navarro del Hierroa, J.; Herrera, T.; Fornari, T.; Reglero, G.; Martin, D. The gastrointestinal behavior of saponins and its significance for their bioavailability and bioactivities. J. Funct. Foods. 2018, 40, 484–497. [Google Scholar] [CrossRef]
- Li, Y.Q.; Zhou, F.C.; Gao, F.; Bian, J.S.; Shan, F. Comparative evaluation of quercetin, isoquercetin and rutin as inhibitors of α-glucosidase. J. Agric. Food Chem. 2009, 57, 11463–11468. [Google Scholar] [CrossRef]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of chlorogenic acid on regulating glucose and lipids metabolism: A review. Evid. Based Complement. Alter. Med. 2013, 2013, 801457. [Google Scholar] [CrossRef]
- Šola, I.; Poljuha, D.; Mikulic-Petkovsek, M.; Davosir, D.; Pinterić, M.; Bilić, J.; Veberic, R.; Hudina, M.; Rusak, G. Biopotential of underutilized Rosaceae inflorescences: LC-DAD-MS phytochemical profiles associated with antioxidant, antidiabetic, anti-inflammatory and antiproliferative activity in vitro. Plants 2022, 11, 271. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, B.; Yan, Y.; Liang, J.; Guan, X. Bound polyphenols from red quinoa prevailed over free polyphenols in reducing postprandial blood glucose rises by inhibiting α-glucosidase activity and starch digestion. Nutrients 2022, 14, 728. [Google Scholar] [CrossRef]
- Ostberg-Potthoff, J.J.; Berger, K.; Richling, E.; Winterhalter, P. Activity-guided fractionation of red fruit extracts for the identification of compounds influencing glucose metabolism. Nutrients 2019, 11, 1166. [Google Scholar] [CrossRef] [Green Version]
- Rondanelli, M.; Riva, A.; Petrangolini, G.; Allegrini, P.; Bernardinelli, L.; Fazia, T.; Peroni, G.; Gasparri, C.; Nichetti, M.; Faliva, M.A.; et al. The metabolic effects of Cynara supplementation in overweight and obese class I subjects with newly detected impaired fasting glycemia: A double-blind, placebo-controlled, randomized clinical trial. Nutrients 2020, 12, 3298. [Google Scholar] [CrossRef]
- Chempedia. Available online: http://chempedia.info/info/eadie_hofstee (accessed on 23 April 2022).
- Cho, Y.S.; Lim, H.S. Comparison of various estimation methods for the parameters of Michaelis–Menten equation based on in vitro elimination kinetic simulation data. Transl. Clin. Pharmacol. 2018, 26, 39–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owusu-Apenten, R.K. Food Enzymes. In Introduction to Food Chemistry, 1st ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 174–185. [Google Scholar]
- Marasović, M.; Marasović, T.; Miloš, M. Robust nonlinear regression in enzyme kinetic parameters estimation. J. Chem. 2017, 2017, 6560983. [Google Scholar] [CrossRef]
- Nagai, T.; Suzuki, N.; Nagashima, T. Autolysate and enzymatic hydrolysates from yam (Dioscorea opposita Thunb) tuber mucilage tororo have antioxidant and angiotensin I-converting enzyme inhibitory activities. J. Food Agric. Environ. 2007, 5, 39–43. [Google Scholar]
- López-Fernández-Sobrino, R.; Soliz-Rueda, J.R.; Margalef, M.; Arola-Arnal, A.; Suárez, M.; Bravo, F.I.; Muguerza, B. ACE Inhibitory and antihypertensive activities of wine lees and relationship among bioactivity and phenolic profile. Nutrients 2021, 13, 679. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Caldara, G.-F.; Nuzzo, D.; Baldassano, S.; Picone, P.; Rizzo, M.; Mulè, F.; Di Carlo, M. NAFLD and atherosclerosis are prevented by a natural dietary supplement containing curcumin, silymarin, guggul, chlorogenic acid and inulin in mice fed a high-fat diet. Nutrients 2017, 9, 492. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.J.; Yang, L.; Zhao, Q.; Caen, J.P.; He, H.Y.; Jin, Q.H.; Guo, L.H.; Alemany, M.; Zhang, L.Y.; Shi, Y.F. Induction of acetylcholinesterase expression during apoptosis in various cell types. Cell Death Differ. 2002, 9, 790–800. [Google Scholar] [CrossRef] [Green Version]
- Orhan, I.; Kartal, M.; Tosun, F.; Şener, B. Screening of various phenolic acids and flavonoid derivatives for their anticholinesterase potential. Z. Nat. C 2007, 62, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Cai, J.; Fang, Z.; Li, S.; Huang, Z.; Tang, Z.; Luo, Q.; Chen, H. The composition and anti-aging activities of polyphenol extract from Phyllanthus emblica L. fruit. Nutrients 2022, 14, 857. [Google Scholar] [CrossRef]
- Tenfen, A.; Vechi, G.; Cechinel-Zanchett, C.C.; Lorenzett, T.S.; Reginato-Couto, C.E.; Siebert, D.A.; Vitali, L.; Micke, G.; Klein-Júnior, L.C.; Cechinel Filho, V. Phenolic profile by HPLC-ESI-MS/MS of six Brazilian Eugenia species and their potential as cholinesterase inhibitors. Nat. Prod. Res. 2021, 35, 2608–2611. [Google Scholar] [CrossRef]
- Sigma-Aldrich Product. α-Glucosidase from Saccharomyces cerevisiae. Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/LT/en/product/sigma/g5003 (accessed on 20 April 2022).
- Sigma-Aldrich Product. α-Amylase from Porcine Pancreas. Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/product/sigma/a6255 (accessed on 19 April 2022).
- Sigma-Aldrich Product. Lung Acetone Powder from Rabbit. Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/product/sigma/l0756 (accessed on 20 April 2022).
- Sigma-Aldrich Product. Acetylcholinesterase from Electrophorus electricus. Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/product/sigma/c3389 (accessed on 20 April 2022).
- Vytautas Magnus University Botanical Garden in Kaunas, Lithuania. Available online: http://www.bgci.org/garden.php?id=119 (accessed on 23 April 2022).
- Waters ACQUITY UPLC System. Ultra Perfomance LC Separation Science Redefined. (Part Number 720001136EN). Available online: https://www.waters.com/webassets/cms/library/docs/720001136en.pdf (accessed on 24 April 2022).
- Swartz, M.E. UPLC: An introduction and review. J. Liq. Chrom. 2005, 28, 1253–1263. [Google Scholar] [CrossRef]
- Waters Corporation. Waters, the Science of What’s Possible. ACQUITY UPLC and ACQUITY PREMIER BEH Columns (Part Number 715001371). Available online: www.waters.com (accessed on 24 April 2022).
- Vermeirssen, V.; Van Camp, J.; Verstraete, W. Optimisation and validation of an angiotensin-converting enzyme inhibition assay for the screening of bioactive peptides. J. Biochem. Biophys. Methods 2002, 51, 75–87. [Google Scholar] [CrossRef]
- Donath-Nagy Gabriella, Vancea Szende, Imre Silvia Comparative study if captopril Derivatization Reaction by LC-UV, LC-MS and CE-UV methods. Croatica Chemica Acta 2011, 84, 423–427. [CrossRef]
- Sigma-Aldrich Protocol. Enzymatic Assay of α-Amylase (EC 3.2.1.1) Sigma-Aldrich. Available online: http://www.sigmaaldric.com/technical-documents/protocol/enzymatic-assay-of-a-amylase.html (accessed on 19 April 2022).
- Eyer, P.; Worek, F.; Kiderlen, D.; Sinko, G.; Stuglin, A.; Simeon-Rudolf, V.; Reiner, E. Molar absorption coefficients for the reduced Ellman reagent: Reassessment. Anal. Biochem. 2003, 312, 224–227. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).