Plant Volatiles and Herbivore Induced Plant Volatiles from Chili Pepper Act as Attractant of the Aphid Parasitoid Aphelinus varipes (Hymenoptera: Aphelinidae)
Abstract
1. Introduction
2. Results
2.1. Bioassay of Olfactory Response of A. varipes towards Five Host Plants
2.2. Identification of Volatiles Emitted from Healthy and Myzus Persicae-Induced Chili Pepper and Cabbage Plants
2.3. Bioassays with Synthetic Compounds
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plants and Insects
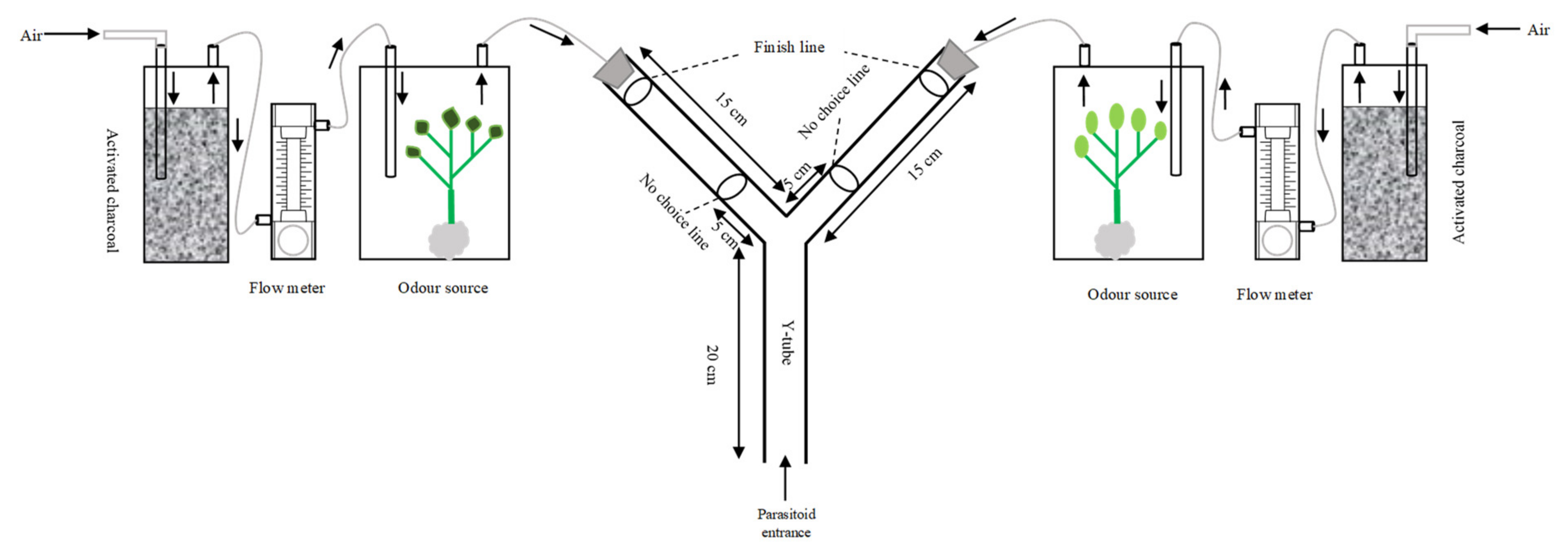
5.2. Y-Tube Olfactometer Bioassays
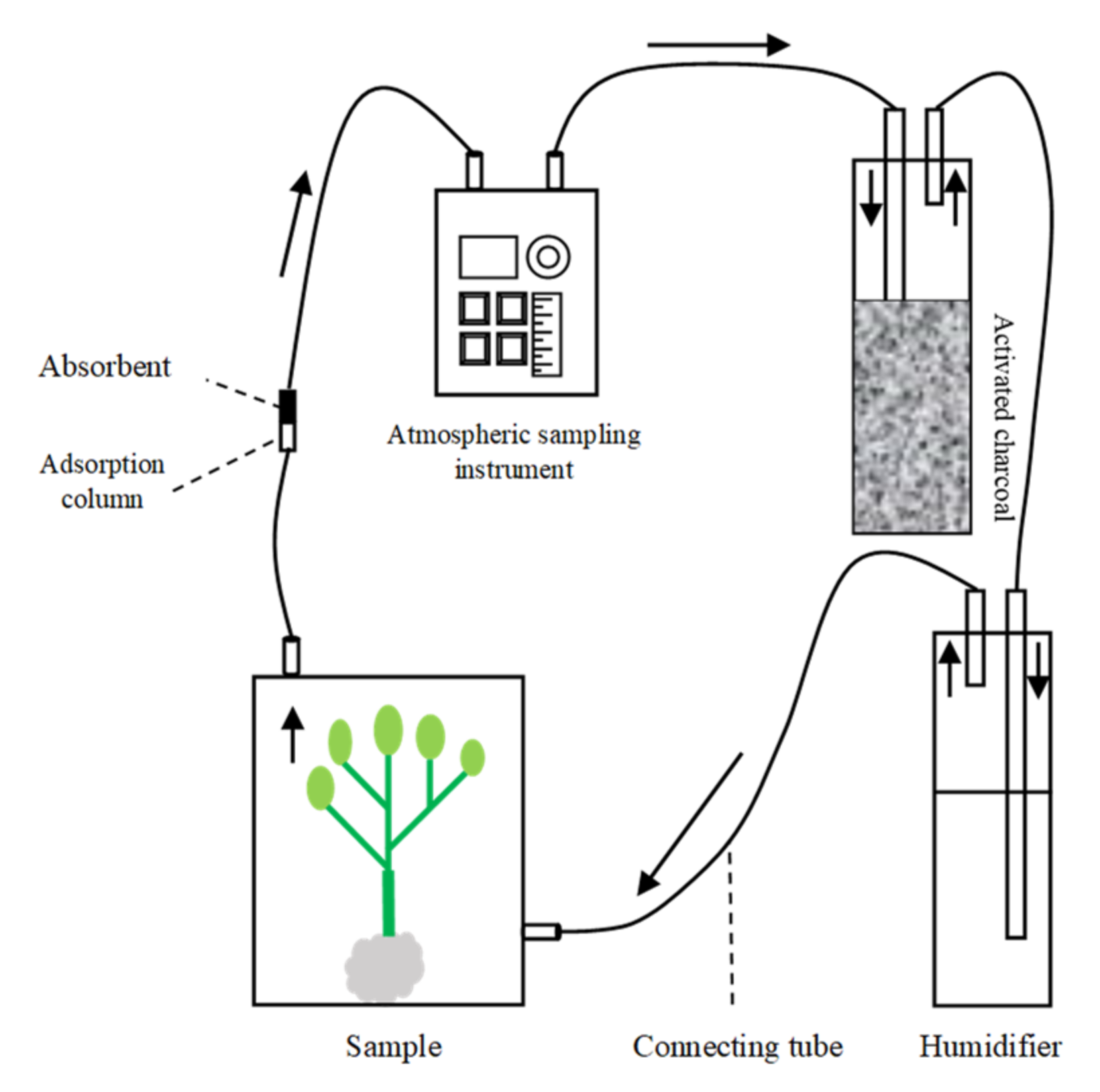
5.3. Collection of Headspace Volatiles from Plants
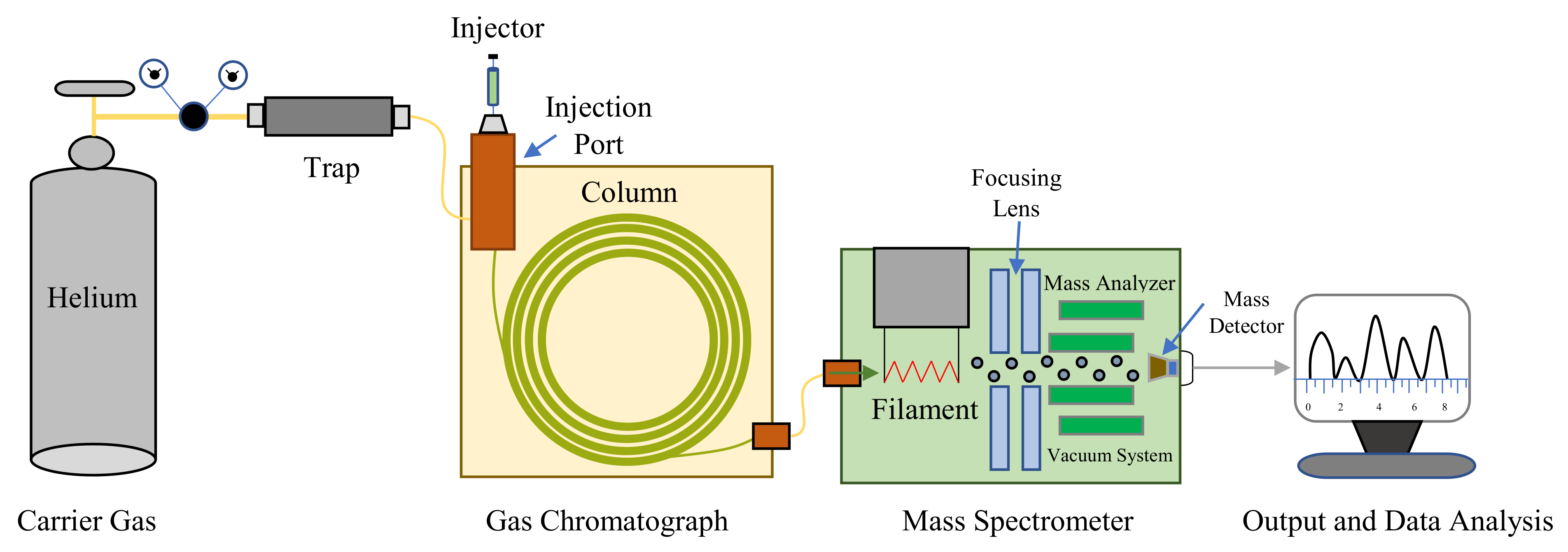
5.4. Analysis of Headspace Plant Volatiles in GC-MS
5.5. Chemicals
5.6. Bioassays with Synthetic Compounds
5.7. Statistics
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poelman, E.H. From Induced Resistance to Defence in Plant-Insect Interactions. Entomol. Exp. Appl. 2015, 157, 11–17. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Erb, M. Tritrophic Interactions Mediated by Herbivore-Induced Plant Volatiles: Mechanisms, Ecological Relevance, and Application Potential. Annu. Rev. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef]
- Gols, R. Direct and Indirect Chemical Defences against Insects in a Multitrophic Framework. Plant Cell Environ. 2014, 37, 1741–1752. [Google Scholar] [CrossRef]
- Mithöfer, A.; Boland, W. Plant Defense against Herbivores: Chemical Aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef]
- Ali, M.Y.; Naseem, T.; Arshad, M.; Ashraf, I.; Rizwan, M.; Tahir, M.; Rizwan, M.; Sayed, S.; Ullah, M.I.; Khan, R.R.; et al. Host-Plant Variations Affect the Biotic Potential, Survival, and Population Projection of Myzus persicae (Hemiptera: Aphididae). Insects 2021, 12, 375. [Google Scholar] [CrossRef]
- Halitschke, R.; Stenberg, J.A.; Kessler, D.; Kessler, A.; Baldwin, I.T. Shared Signals—“Alarm Calls” from Plants Increase Apparency to Herbivores and Their Enemies in Nature. Ecol. Lett. 2008, 11, 24–34. [Google Scholar] [CrossRef]
- Schuman, M.C.; Baldwin, I.T. Field Studies Reveal Functions of Chemical Mediators in Plant Interactions. Chem. Soc. Rev. 2018, 47, 5338–5353. [Google Scholar] [CrossRef]
- Joo, Y.; Schuman, M.C.; Goldberg, J.K.; Kim, S.G.; Yon, F.; Brütting, C.; Baldwin, I.T. Herbivore-Induced Volatile Blends with Both “Fast” and “Slow” Components Provide Robust Indirect Defence in Nature. Funct. Ecol. 2018, 32, 136–149. [Google Scholar] [CrossRef]
- Iason, G.; Dicke, M.; Hartley, S. The Ecology of Plant Secondary Metabolites: From Genes to Global Processes, 2nd ed.; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- He, J.; Fandino, R.A.; Halitschke, R.; Luck, K.; Köllner, T.G.; Murdock, M.H.; Ray, R.; Gase, K.; Knaden, M.; Baldwin, I.T.; et al. An Unbiased Approach Elucidates Variation in (S)-(+)-Linalool, a Context-Specific Mediator of a Tri-Trophic Interaction in Wild Tobacco. Proc. Natl. Acad. Sci. USA 2019, 116, 14651–14660. [Google Scholar] [CrossRef]
- Takabayashi, J.; Shiojiri, K. Multifunctionality of Herbivory-Induced Plant Volatiles in Chemical Communication in Tritrophic Interactions. Curr. Opin. Insect Sci. 2019, 32, 110–117. [Google Scholar] [CrossRef]
- De Moraes, C.M.; Lewis, W.J.; Pare, P.W.; Alborn, H.T.; Tumiinson, J.H. Herbivore-Infested Plants Selectively Attract Parasitoids. Nature 1998, 393, 570–573. [Google Scholar] [CrossRef]
- Clavijo McCormick, A.; Unsicker, S.B.; Gershenzon, J. The Specificity of Herbivore-Induced Plant Volatiles in Attracting Herbivore Enemies. Trends Plant Sci. 2012, 17, 303–310. [Google Scholar] [CrossRef]
- Ye, M.; Veyrat, N.; Xu, H.; Hu, L.; Turlings, T.C.J.; Erb, M. An Herbivore-Induced Plant Volatile Reduces Parasitoid Attraction by Changing the Smell of Caterpillars. Sci. Adv. 2018, 4, eaar4767. [Google Scholar] [CrossRef]
- Agarwala, B.K.; Das, J. Weed Host Specificity of the Aphid, Aphis Spiraecola: Developmental and Reproductive Performance of Aphids in Relation to Plant Growth and Leaf Chemicals of the Siam Weed, Chromolaena odorata. J. Insect Sci. 2012, 12, 1–13. [Google Scholar] [CrossRef]
- Minks, A.K.; Harrewijn, P. Aphids: Their Biology, Natural Enemies and Control; World Crop Pests; Elsevier: Amsterdam, The Netherlands; New York, NY, USA, 1987; Volume 10, ISBN 0444426302. [Google Scholar]
- Capinera, J.L.; Hoy, M.A.; Paré, P.W.; Farag, M.A.; Trumble, J.T.; Isman, M.B.; Adams, B.J.; Nguyen, K.B.; Panizzi, A.R.; Sánchez, N.E.; et al. Encyclopedia of Entomology; Nutrition in Insects; Springer: Dordrecht, The Netherlands, 2008; pp. 2646–2654. [Google Scholar]
- Holman, J. Host Plant Catalog of Aphids; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 9780333227794. [Google Scholar]
- Skevas, T.; Swinton, S.M.; Meehan, T.D.; Kim, T.N.; Gratton, C.; Egbendewe-Mondzozo, A. Integrating Agricultural Pest Biocontrol into Forecasts of Energy Biomass Production. Ecol. Econ. 2014, 106, 195–203. [Google Scholar] [CrossRef]
- Wenda-Piesik, A.; Piesik, D.; Nowak, A.; Wawrzyniak, M. Tribolium confusum Responses to Blends of Cereal Kernels and Plant Volatiles. J. Appl. Entomol. 2016, 140, 558–563. [Google Scholar] [CrossRef]
- Boivin, G.; Hance, T.; Brodeur, J. Aphid Parasitoids in Biological Control. Can. J. Plant Sci. 2012, 92, 1–12. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chi, H.; Liu, T.X. Demography and Parasitic Effectiveness of Aphelinus asychis Reared from Sitobion avenae as a Biological Control Agent of Myzus persicae Reared on Chili pepper and Cabbage. Biol. Control 2016, 92, 111–119. [Google Scholar] [CrossRef]
- Strong, K.L. Electrophoretic Analysis of Two Strains of Aphelinus varipes (Hymenoptera: Aphelinidae) for Use in the Biological Control of the Russian Wheat Aphid, Diuraphis noxia (Mordwilko). J. Aust. Entomol. 1993, 32, 21–22. [Google Scholar] [CrossRef]
- Bosque-Pérez, N.A.; Johnson, J.B.; Schotzko, D.J.; Unger, L. Species Diversity, Abundance, and Phenology of Aphid Natural Enemies on Spring Wheats Resistant and Susceptible to Russian Wheat Aphid. BioControl 2002, 47, 667–684. [Google Scholar] [CrossRef]
- Richardson, H.P.; Westdal, P.H. Use of Aphelinus Semiflavus Howard for Control of Aphids in a Greenhouse. Can. Entomol. 1965, 97, 1089–1106. [Google Scholar] [CrossRef]
- Ali, M.Y.; Lu, Z.; Ali, A.; Amir, M.B.; Ahmed, M.A.; Shahid, S.; Liu, T.; Pan, M. Effects of Plant-Mediated Differences in Aphid Size on Suitability of Its Parasitoid, Aphelinus varipes (Hymenoptera: Aphelinidae). J. Econ. Entomol. 2022, 115, 74–80. [Google Scholar] [CrossRef]
- Acheampong, S.; Gillespie, D.R.; Quiring, D.J.M. Survey of Parasitoids and Hyperparasitoids (Hymenoptera) of the Green Peach Aphid, Myzus persicae and the Foxglove Aphid, Aulacorthum solani (Hemiptera: Aphididae) in British Columbia. J. Entomol. Soc. Br. Columbia 2012, 109, 12–22. [Google Scholar]
- Ceballos Vázquez, M.; Duarte, L. Aphelinus abdominalis (Dalman) (Hymenoptera: Aphelinidae), Parasitoide de Áfidos en Cuba. Rev. Protección Veg. 2012, 27, 60–61. [Google Scholar]
- Poelman, E.H.; Bruinsma, M.; Zhu, F.; Weldegergis, B.T.; Boursault, A.E.; Jongema, Y.; van Loon, J.J.A.; Vet, L.E.M.; Harvey, J.A.; Dicke, M. Hyperparasitoids Use Herbivore-Induced Plant Volatiles to Locate Their Parasitoid Host. PLoS Biol. 2012, 10, e1001435. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Wäckers, F. Recruitment of Predators and Parasitoids by Herbivore-Injured Plants; Cambridge University Press: Cambridge, UK, 2004; ISBN 9780511542664. [Google Scholar]
- Mohammed, K.; Agarwal, M.; Li, B.; Newman, J.; Liu, T.; Ren, Y. Evaluation of D-Limonene and β-Ocimene as Attractants of Aphytis melinus (Hymenoptera: Aphelinidae), a Parasitoid of Aonidiella aurantii (Hemiptera: Diaspididae) on Citrus spp. Insects 2020, 11, 44. [Google Scholar] [CrossRef]
- Pan, M.; Wei, Y.; Wang, F.; Liu, T. Influence of Plant Species on Biological Control Effectiveness of Myzus persicae by Aphidius gifuensis. Crop Prot. 2020, 135, 105223. [Google Scholar] [CrossRef]
- Zhu, X.; Li, L.; Hsiang, T.; Zha, Y.; Zhou, Z.; Chen, R.; Wang, X.; Wu, Q.; Li, J. Chemical Composition and Attractant Activity of Volatiles from Rhus potaninii to the Spring Aphid Kaburagia rhusicola. Molecules 2020, 25, 3412. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Wang, R.; Yu, L.F.; Lu, P.F.; Luo, Y.Q. Identification of Caragana Plant Volatiles, Overlapping Profiles, and Olfactory Attraction to Chlorophorus caragana in the Laboratory. J. Plant Interact. 2015, 10, 41–50. [Google Scholar] [CrossRef]
- Gruber, M.Y.; Xu, N.; Grenkow, L.; Li, X.; Onyilagha, J.; Soroka, J.J.; Westcott, N.D.; Hegedus, D.D. Responses of the Crucifer Flea Beetle to Brassica Volatiles in an Olfactometer. Environ. Entomol. 2009, 38, 1467–1479. [Google Scholar] [CrossRef]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Deceptive Chemical Signals Induced by a Plant Virus Attract Insect Vectors to Inferior Hosts. Proc. Natl. Acad. Sci. USA 2010, 107, 3600–3605. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Covaci, A.D.; Roberts, J.M.; Sobhy, I.S.; Kirk, W.D.J.; Bruce, T.J.A. Effects of Cis-Jasmone Treatment of Brassicas on Interactions with Myzus persicae Aphids and Their Parasitoid Diaeretiella rapae. Front. Plant Sci. 2021, 12, 711896. [Google Scholar] [CrossRef] [PubMed]
- Pagadala Damodaram, K.J.; Gadad, H.S.; Parepally, S.K.; Vaddi, S.; Ramanna Hunashikatti, L.; Bhat, R.M. Low Moisture Stress Influences Plant Volatile Emissions Affecting Herbivore Interactions in Tomato, Solanum lycopersicum. Ecol. Entomol. 2021, 46, 637–650. [Google Scholar] [CrossRef]
- Du, X.; Witzgall, P.; Wu, K.; Yan, F.; Ma, C.; Zheng, H.; Xu, F.; Ji, G.; Wu, X. Volatiles from Prunus persica Flowers and Their Correlation with Flower-Visiting Insect Community in Wanbailin Ecological Garden. China Adv. Entomol. 2018, 6, 116–133. [Google Scholar] [CrossRef][Green Version]
- Wang, P.; Zhang, N.; Zhou, L.L.; Si, S.Y.; Lei, C.L.; Ai, H.; Wang, X.P. Antennal and Behavioral Responses of Female Maruca vitrata to the Floral Volatiles of Vigna unguiculata and Lablab purpureus. Entomol. Exp. Appl. 2014, 152, 248–257. [Google Scholar] [CrossRef]
- Coppola, M.; Cascone, P.; Madonna, V.; Di Lelio, I.; Esposito, F.; Avitabile, C.; Romanelli, A.; Guerrieri, E.; Vitiello, A.; Pennacchio, F.; et al. Plant-To-Plant Communication Triggered by Systemin Primes Anti-Herbivore Resistance in Tomato. Sci. Rep. 2017, 7, 15522. [Google Scholar] [CrossRef]
- Jiménez-Martínez, E.S.; Bosque-Pérez, N.A.; Berger, P.H.; Zemetra, R.S.; Ding, H.; Eigenbrode, S.D. Volatile Cues Influence the Response of Rhopalosiphum padi (Homoptera: Aphididae) to Barley Yellow Dwarf Virus-Infected Transgenic and Untransformed Wheat. Environ. Entomol. 2004, 33, 1207–1216. [Google Scholar] [CrossRef]
- Qiu, X.; Cao, L.; Han, R. Analysis of Volatile Components in Different Ophiocordyceps sinensis and Insect Host Products. Molecules 2020, 25, 1603. [Google Scholar] [CrossRef]
- Ganassi, S.; Grazioso, P.; De Cristofaro, A.; Fiorentini, F.; Sabatini, M.A.; Evidente, A.; Altomare, C. Long Chain Alcohols Produced by Trichoderma Citrinoviride Have Phagodeterrent Activity against the Bird Cherry-Oat Aphid Rhopalosiphum padi. Front. Microbiol. 2016, 7, 297. [Google Scholar] [CrossRef]
- Adebisi, O.; Dolma, S.K.; Verma, P.K.; Singh, B.; Reddy, S.G.E. Volatile, Non-Volatile Composition and Insecticidal Activity of Eupatorium Adenophorum spreng against Diamondback Moth, Plutella xylostella (L.), and Aphid, Aphis craccivora Koch. Toxin Rev. 2019, 38, 143–150. [Google Scholar] [CrossRef]
- Huang, K.; Shang, H.; Zhou, Q.; Wang, Y.; Shen, H.; Yan, Y. Volatiles Induced from Hypolepis punctata (Dennstaedtiaceae) by Herbivores Attract Sclomina erinacea (Hemiptera: Reduviidae): Clear Evidence of Indirect Defense in Fern. Insects 2021, 12, 978. [Google Scholar] [CrossRef] [PubMed]
- Vidal, D.M.; Moreira, M.A.B.; Coracini, M.D.A.; Zarbin, P.H.G. Isophorone Derivatives as a New Structural Motif of Aggregation Pheromones in Curculionidae. Sci. Rep. 2019, 9, 776. [Google Scholar] [CrossRef] [PubMed]
- Badra, Z.; Larsson Herrera, S.; Cappellin, L.; Biasioli, F.; Dekker, T.; Angeli, S.; Tasin, M. Species-Specific Induction of Plant Volatiles by Two Aphid Species in Apple: Real Time Measurement of Plant Emission and Attraction of Lacewings in the Wind Tunnel. J. Chem. Ecol. 2021, 47, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhu, X.; Zhou, S.; Cheng, Z.; Shi, K.; Zhang, C.; Shao, H. Phthalic Acid Esters: Natural Sources and Biological Activities. Toxins 2021, 13, 495. [Google Scholar] [CrossRef]
- Tentelier, C.; Fauvergue, X. Herbivore-Induced Plant Volatiles as Cues for Habitat Assessment by a Foraging Parasitoid. J. Anim. Ecol. 2007, 76, 1–8. [Google Scholar] [CrossRef]
- Du, Y.J.; Poppy, G.M.; Powell, W. Relative Importance of Semiochemicals from First and Second Trophic Levels in Host Foraging Behavior of Aphidius ervi. J. Chem. Ecol. 1996, 22, 1591–1605. [Google Scholar] [CrossRef]
- Powell, W.; Pennacchio, F.; Poppy, G.M.; Tremblay, E. Strategies Involved in the Location of Hosts by the Parasitoid Aphidius ervi Haliday (Hymenoptera: Braconidae: Aphidiinae). Biol. Control 1998, 11, 104–112. [Google Scholar] [CrossRef]
- Guerrieri, E.; Poppy, G.M.; Powell, W.; Tremblay, E.; Pennacchio, F. Induction and Systemic Release of Herbivore-Induced Plant Volatiles Mediating In-Flight Orientation of Aphidius ervi. J. Chem. Ecol. 1999, 25, 1247–1261. [Google Scholar] [CrossRef]
- Blande, J.D.; Pickett, J.A.; Poppy, G.M. A Comparison of Semiochemically Mediated Interactions Involving Specialist and Generalist Brassica-Feeding Aphids and the Braconid Parasitoid Diaeretiella rapae. J. Chem. Ecol. 2007, 33, 767–779. [Google Scholar] [CrossRef]
- Dahlin, I.; Vucetic, A.; Ninkovic, V. Changed Host Plant Volatile Emissions Induced by Chemical Interaction between Unattacked Plants Reduce Aphid Plant Acceptance with Intermorph Variation. J. Pest Sci. 2015, 88, 249–257. [Google Scholar] [CrossRef]
- Conboy, N.J.A.; McDaniel, T.; George, D.; Ormerod, A.; Edwards, M.; Donohoe, P.; Gatehouse, A.M.R.; Tosh, C.R. Volatile Organic Compounds as Insect Repellents and Plant Elicitors: An Integrated Pest Management (IPM) Strategy for Glasshouse Whitefly (Trialeurodes vaporariorum). J. Chem. Ecol. 2020, 46, 1090–1104. [Google Scholar] [CrossRef] [PubMed]
- Paré, P.W.; Farag, M.A. Natural Enemy Attraction to Plant Volatiles. In Encyclopedia of Entomology; Capinera, J.L., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 2567–2570. ISBN 978-1-4020-6359-6. [Google Scholar]
- Ruberti, I.; Sessa, G.; Ciolfi, A.; Possenti, M.; Carabelli, M.; Morelli, G. Plant Adaptation to Dynamically Changing Environment: The Shade Avoidance Response. Biotechnol. Adv. 2012, 30, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Garvey, M.; Creighton, C.; Kaplan, I. Pepper Domestication Enhances Parasitoid Recruitment to Herbivore-Damaged Plants. Arthropod. Plant. Interact. 2020, 14, 695–703. [Google Scholar] [CrossRef]
- Khan, M.A.M.; Ulrichs, C.; Mewis, I. Influence of Water Stress on the Glucosinolate Profile of Brassica Oleracea Var. Italica and the Performance of Brevicoryne brassicae and Myzus persicae. Entomol. Exp. Appl. 2010, 137, 229–236. [Google Scholar] [CrossRef]
- Yang, S.; Xu, R.; Yang, S.; Kuang, R. Olfactory Responses of Aphidius gifuensis to Odors of Host Plants and Aphid-Plant Complexes. Insect Sci. 2009, 16, 503–510. [Google Scholar] [CrossRef]
- Ahmed, Q.H. Evaluation of Efficacy of Fumigants and Natural Product Extracts for Management of Springtail. Ph.D. Thesis, Murdoch University, Perth, WA, Australia, 2018. [Google Scholar]
- Wei, J.; Wang, L.; Zhu, J.; Zhang, S.; Nandi, O.I.; Kang, L. Plants Attract Parasitic Wasps to Defend Themselves against Insect Pests by Releasing Hexenol. PLoS ONE 2007, 2, e852. [Google Scholar] [CrossRef]
- Du, Y.; Poppy, G.M.; Powell, W.; Wadhams, L.J. Chemically Mediated Associative Learning in the Host Foraging Behavior of the Aphid Parasitoid Aphidius ervi (Hymenoptera: Braconidae). J. Insect Behav. 1997, 10, 509–522. [Google Scholar] [CrossRef]
- van Emden, H.F.; Sponagl, B.; Wagner, E.; Baker, T.; Ganguly, S.; Douloumpaka, S. Hopkins’ ‘Host Selection Principle’, Another Nail in Its Coffin. Phvsiol. Entomol. 1996, 21, 325–328. [Google Scholar] [CrossRef]
- Storeck, A.; Poppy, G.M.; Van Emden, H.F.; Powell, W. The Role of Plant Chemical Cues in Determining Host Preference in the Generalist Aphid Parasitoid Aphidius colemani. Entomol. Exp. Appl. 2000, 97, 41–46. [Google Scholar] [CrossRef]
- Ayelo, P.M.; Yusuf, A.A.; Pirk, C.W.W.; Chailleux, A.; Mohamed, S.A.; Deletre, E. Terpenes from Herbivore-Induced Tomato Plant Volatiles Attract Nesidiocoris tenuis (Hemiptera: Miridae), a Predator of Major Tomato Pests. Pest Manag. Sci. 2021, 77, 5255–5267. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.L.; Argudín, M.F.; Powell, W. Foraging Behaviour of the Parasitoid Lysiphlebus testaceipes (Hymenoptera: Braconidae) in Response to Plant Volatiles, with Reference to Biocontrol of Aphids in Peri-Urban Vegetable Production Systems. Biocontrol Sci. Technol. 2007, 17, 677–686. [Google Scholar] [CrossRef]
- Dicke, M.; Sabelis, M.W. How Plants Obtain Preadtory Mites as Bodyguards. Neth. J. Zool. 1988, 1, 2–4. [Google Scholar]
- Turlings, T.C.J.; Tumlinson, J.H.; Lewis, W.J. Exploitation of Herbivore-Induced Plant Odors by Host-Seeking Parasitic Wasps. Science 1990, 250, 1251–1253. [Google Scholar] [CrossRef] [PubMed]
- Turlings, T.C.; Ton, J. Exploiting Scents of Distress: The Prospect of Manipulating Herbivore-Induced Plant Odours to Enhance the Control of Agricultural Pests. Curr. Opin. Plant Biol. 2006, 9, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Schuman, M.C.; Barthel, K.; Baldwin, I.T. Herbivory-Induced Volatiles Function as Defenses Increasing Fitness of the Native Plant Nicotiana attenuata in Nature. eLife 2012, 2012, e00007. [Google Scholar] [CrossRef] [PubMed]
- Frost, C.J.; Appel, H.M.; Carlson, J.E.; De Moraes, C.M.; Mescher, M.C.; Schultz, J.C. Within-Plant Signalling via Volatiles Overcomes Vascular Constraints on Systemic Signalling and Primes Responses against Herbivores. Ecol. Lett. 2007, 10, 490–498. [Google Scholar] [CrossRef]
- Veyrat, N.; Robert, C.A.M.; Turlings, T.C.J.; Erb, M. Herbivore Intoxication as a Potential Primary Function of an Inducible Volatile Plant Signal. J. Ecol. 2016, 104, 591–600. [Google Scholar] [CrossRef]
- Irmisch, S.; Clavijo McCormick, A.; Günther, J.; Schmidt, A.; Boeckler, G.A.; Gershenzon, J.; Unsicker, S.B.; Köllner, T.G. Herbivore-Induced Poplar Cytochrome P450 Enzymes of the CYP71 Family Convert Aldoximes to Nitriles Which Repel a Generalist Caterpillar. Plant J. 2014, 80, 1095–1107. [Google Scholar] [CrossRef]
- Robert, C.A.M.; Erb, M.; Duployer, M.; Zwahlen, C.; Doyen, G.R.; Turlings, T.C.J. Herbivore-Induced Plant Volatiles Mediate Host Selection by a Root Herbivore. New Phytol. 2012, 194, 1061–1069. [Google Scholar] [CrossRef]
- Gariepy, T.D.; Messing, R.H. Development and Use of Molecular Diagnostic Tools to Determine Trophic Links and Interspecific Interactions in Aphid-Parasitoid Communities in Hawaii. Biol. Control 2012, 60, 26–38. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, Q.; Yao, Q.; Chen, G.; Tong, H.; Zhang, J.; Li, C.; Su, Q.; Zhang, Y. Direct and Indirect Plant Defenses Induced by (Z)-3-Hexenol in Tomato against Whitefly Attack. J. Pest Sci. 2020, 93, 1243–1254. [Google Scholar] [CrossRef]
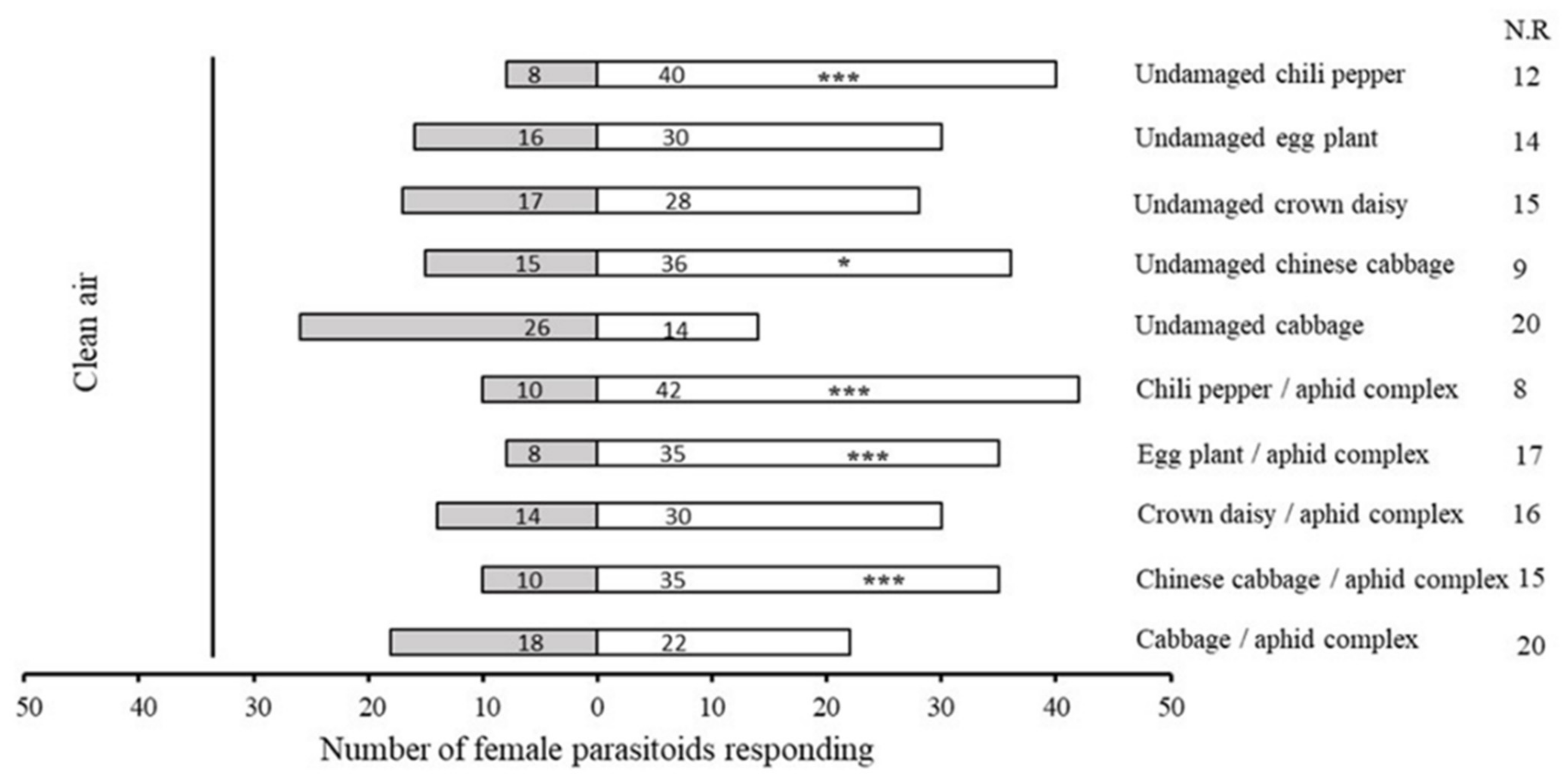
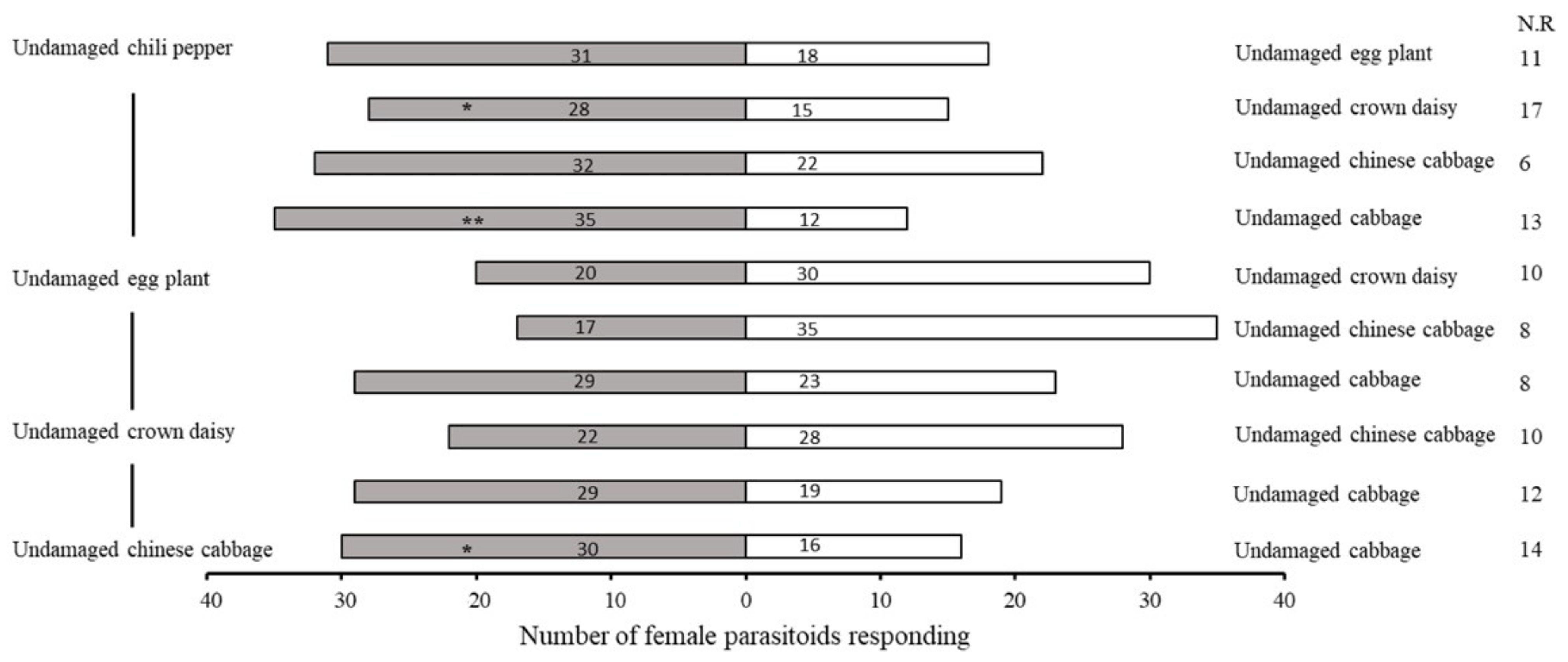
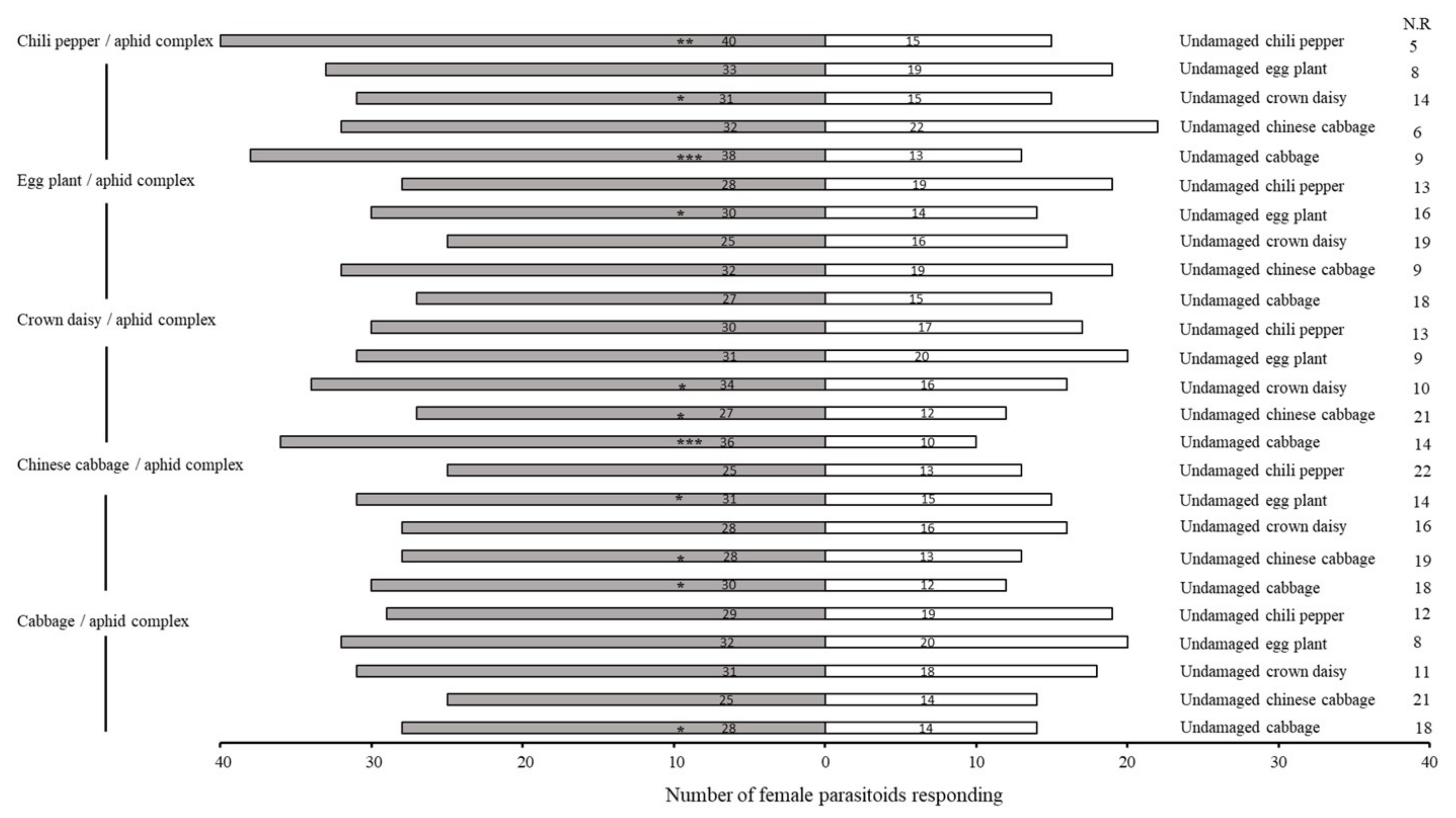
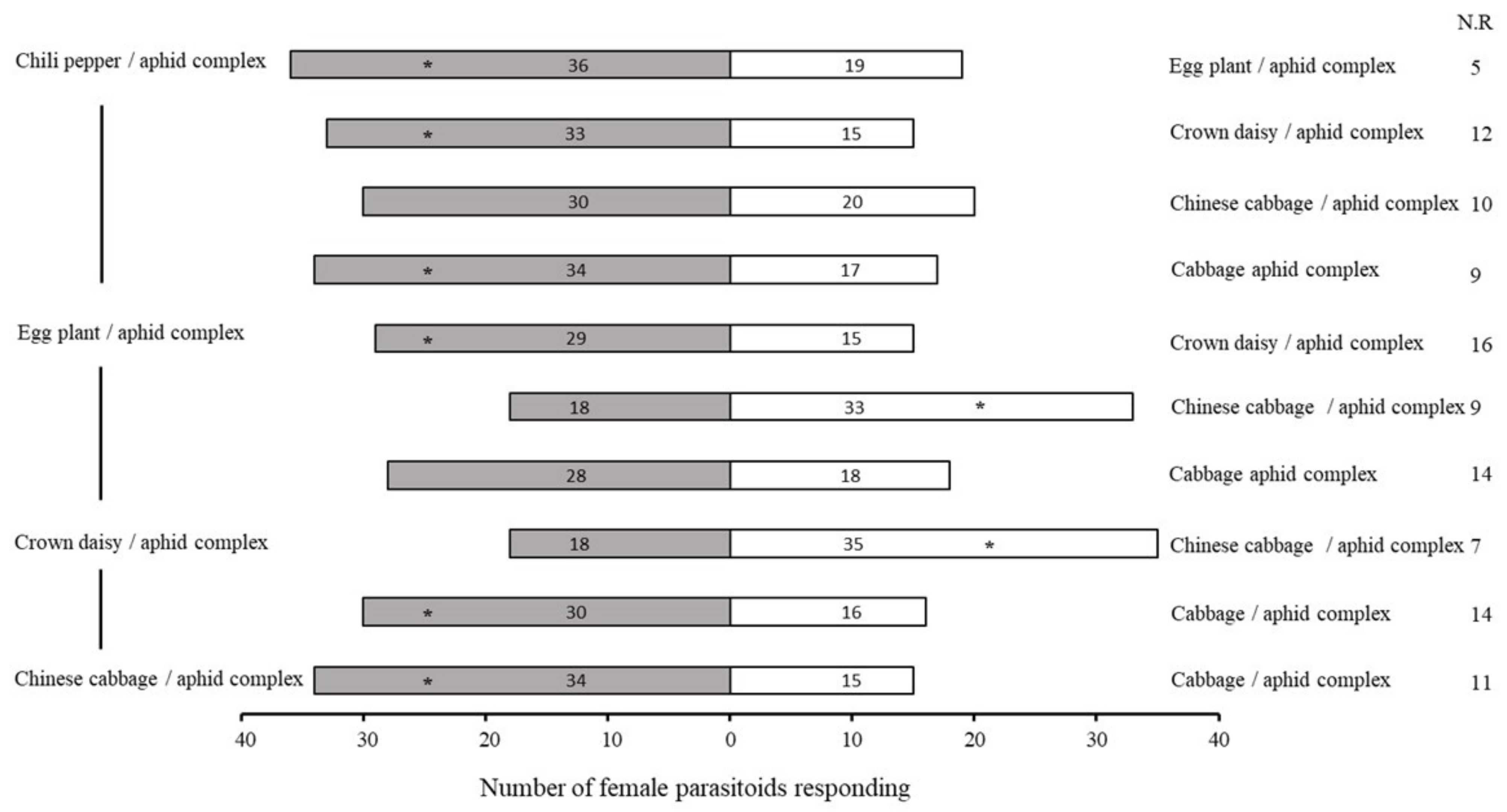
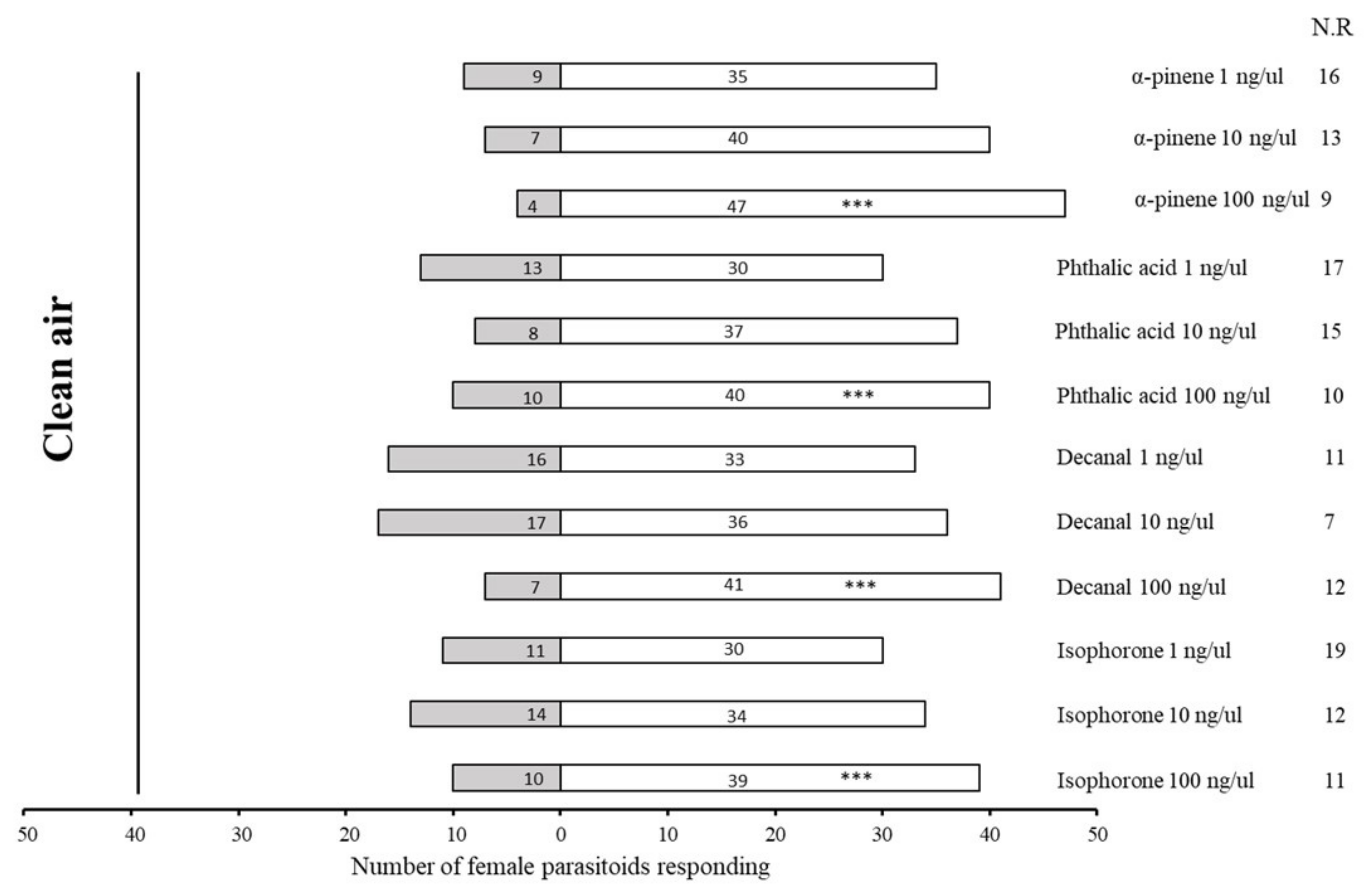
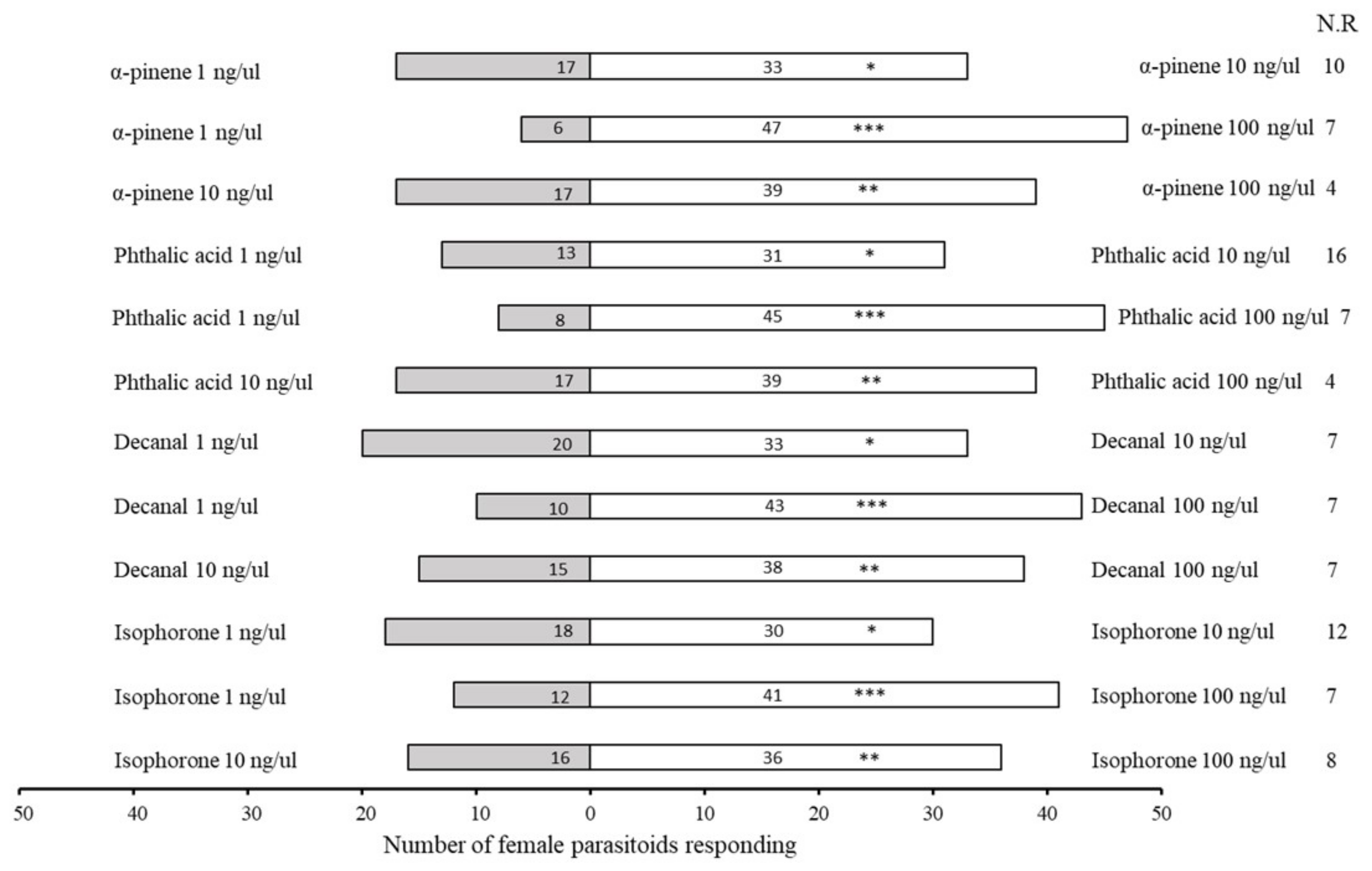
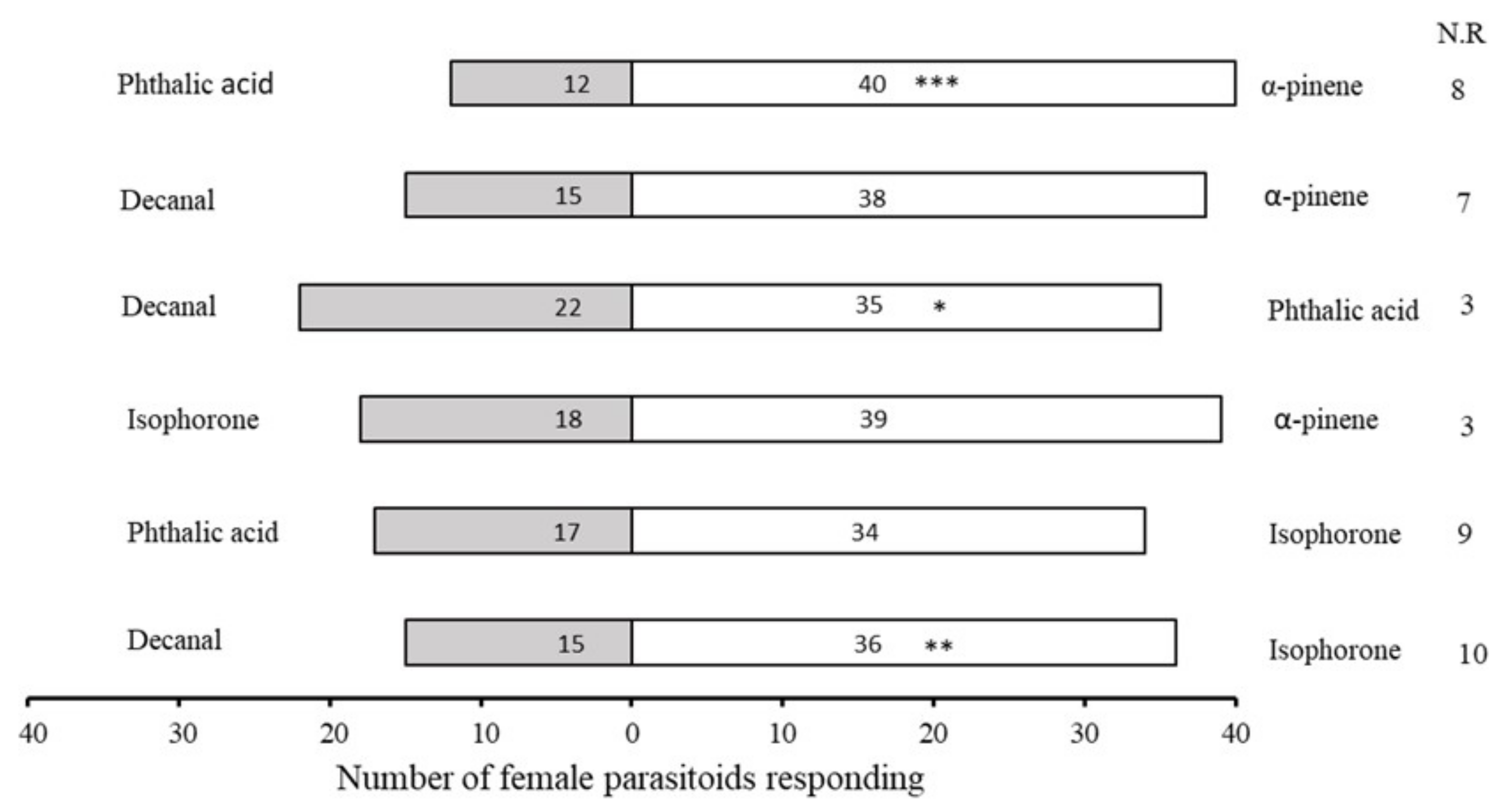
| No. | Retention Time (min) | Compound | Molecular Formula | Chemical Formula | Relative Level ± SE (%) * | Reference |
|---|---|---|---|---|---|---|
| 1 | 3.89 | p-xylene | C8H10 |  | 0.08 ± 0.006 | [33] |
| 2 | 6.59 | Decane | C10H22 |  | 0.12 ± 0.012 | [34] |
| 3 | 7.99 | Benzene, 1,4-diethyl | C10H14 | 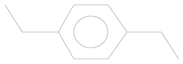 | 30.78 ± 0.577 | [35] |
| 4 | 11.10 | Benzaldehyde, 4-ethyl- | C9H10O | 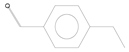 | 0.64 ± 0.012 | [36] |
| 5 | 13.77 | p-cymen-7-ol | C10H14O | 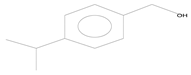 | 2.57 ± 0.028 | [37] |
| 6 | 13.97 | Tetradecane, 2,6,10-trimethyl- | C17H36 |  | 0.01 ± 0.009 | [38] |
| 7 | 19.71 | Pentadecane | C15H32 |  | 0.05 ± 0.006 | [39] |
| 8 | 25.51 | Ethanone, 1-(4-ethylphenyl)- | C10H12O |  | 0.01 ± 0.006 | [40] |
| No. | Retention Time (min) | Compound | Molecular Formula | Chemical Formula | Relative Level ± SE (%) * | Reference |
|---|---|---|---|---|---|---|
| 1 | 3.39 | 2,4-dimethyl-1-heptene | C9H18 | 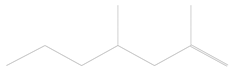 | 0.01 ± 0.010 | [41] |
| 2 | 16.50 | 3-methyl tridecane | C14H30 | 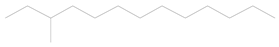 | 0.01 ± 0.006 | [42] |
| 3 | 16.62 | 2,6,11-trimethyl dodecane | C15H32 |  | 0.02 ± 0.006 | [43] |
| 4 | 21.90 | 1-hexadecanol | C16H34O |  | 0.02 ± 0.006 | [44] |
| 5 | 24.14 | 1-nonadecene | C19H38 |  | 0.01 ± 0.009 | [45] |
| No. | Retention Time (min) | Compound | Molecular Formula | Chemical Formula | Relative Level ± SE (%) * | Reference |
|---|---|---|---|---|---|---|
| 1 | 5.11 | α-pinene (chili pepper) | C10H16 | 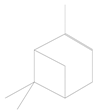 | 0.01 ± 0.009 | [46] |
| 2 | 9.99 | Isophorone (cabbage) | C9H14O | 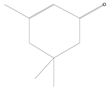 | 0.08 ± 0.006 | [47] |
| 3 | 12.19 | Decanal (chili pepper) | C10H20O |  | 0.10 ± 0.009 | [48] |
| 4 | 39.34 | Phthalic acid (chili pepper) | C24H38O4 | 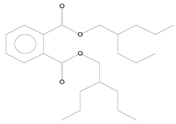 | 0.02 ± 0.006 | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.Y.; Naseem, T.; Zhang, J.; Pan, M.; Zhang, F.; Liu, T.-X. Plant Volatiles and Herbivore Induced Plant Volatiles from Chili Pepper Act as Attractant of the Aphid Parasitoid Aphelinus varipes (Hymenoptera: Aphelinidae). Plants 2022, 11, 1350. https://doi.org/10.3390/plants11101350
Ali MY, Naseem T, Zhang J, Pan M, Zhang F, Liu T-X. Plant Volatiles and Herbivore Induced Plant Volatiles from Chili Pepper Act as Attractant of the Aphid Parasitoid Aphelinus varipes (Hymenoptera: Aphelinidae). Plants. 2022; 11(10):1350. https://doi.org/10.3390/plants11101350
Chicago/Turabian StyleAli, Muhammad Yasir, Tayyaba Naseem, Jinping Zhang, Mingzhen Pan, Feng Zhang, and Tong-Xian Liu. 2022. "Plant Volatiles and Herbivore Induced Plant Volatiles from Chili Pepper Act as Attractant of the Aphid Parasitoid Aphelinus varipes (Hymenoptera: Aphelinidae)" Plants 11, no. 10: 1350. https://doi.org/10.3390/plants11101350
APA StyleAli, M. Y., Naseem, T., Zhang, J., Pan, M., Zhang, F., & Liu, T.-X. (2022). Plant Volatiles and Herbivore Induced Plant Volatiles from Chili Pepper Act as Attractant of the Aphid Parasitoid Aphelinus varipes (Hymenoptera: Aphelinidae). Plants, 11(10), 1350. https://doi.org/10.3390/plants11101350






