Abstract
This work explored the medicinal halophyte Frankenia laevis L. (sea heath) as a potential source of bioactive natural products. In this sense, methanol and dichloromethane extracts were prepared from aerial organs containing flowers, leaves and stems, and were profiled for their chemical composition using high-performance liquid chromatography coupled with electrospray ionization mass spectrometry (HPLC-ESI-MS/MS). The extracts were evaluated for their in vitro antioxidant capacity using five complementary methods: enzyme inhibitory effects on enzymes related with neurodegeneration (acetyl (AChE) and butyrylcholinesterase (BuChE)), Type 2 diabetes (α-glucosidase and α-amylase), hyperpigmentation/food oxidation (tyrosinase), and cytotoxicity towards human hepatocarcinoma (HepG2) cells. Fifty-one molecules were identified in the extracts, including several derivatives of phenolic acids, lignans and flavonoids, monoterpenes, and hydroxylated derivatives of linoleic acid. The methanol extract was effective in DPPH and ABTS radical scavenging (EC50 = 0.25 and 0.65 mg/mL, respectively), copper chelation (EC50 = 0.78 mg/mL), and iron reduction (EC50 = 0.51 mg/mL) activities, whereas the dichloromethane extract had high iron chelating ability (EC50 = 0.76 mg/mL). Both extracts showed the capacity to inhibit α-glucosidase, especially the dichloromethane (EC50 = 0.52 mg/mL). This extract also exerted a significant selective cytotoxicity towards HepG2 cells (EC50 = 52.1 μg/mL, SI > 1.9). In conclusion, extracts from the aerial parts of sea heath were shown to be a promising source of natural products for pharmaceutical and/or food additive applications due to their high antioxidant, anti-diabetic, and cytotoxic properties.
1. Introduction
Since ancient times, nature has been a source of medicines to treat different human diseases, which were at first based on empirical aspects. Nowadays, due to the beneficial properties of plant-based products, the screening of biological activities, and the identification and isolation of bioactive compounds from plants has been increasing due to the growing demand for novel compounds to provide healthcare assistance in diverse human disorders, including inflammation, cancer, diabetes, and neurological disorders [1].
The marine environment holds high biodiversity, including numerous salt-tolerant plant species called halophytes. However, the scientific and commercial interest in marine halophytes is still in its infancy, and their high biotechnological potential is almost unexplored and underutilized. These plants can thrive in environments characterized by many abiotic stresses, such as high salinity, drought, temperature variations and light intensity. Thus, in order to survive in those harsh conditions, these plants developed strong antioxidant systems that consist of antioxidant enzymes and the synthesis of protective secondary metabolites (e.g., phenolics, alkaloids, phytosterols) which also endow important therapeutic properties for humans [2].
These plants have already provided several food and herbal supplements—such as for example, quinoa seeds (Chenopodium quinoa Willd.), sea asparagus (Salicornia sp.) and sea fennel (Crithmum maritimum L.), which have high commercial value and are highly appreciated in gourmet cuisine [3]. While other halophyte species provide botanical extracts for health applications, namely quinoa for hair loss prevention [4]; Hippophae rhamnoides L. (sea buckthorn) oil for improving immunity, supporting the gastrointestinal tract, and cardiac function [5]; Salicornia sp. as a source of minerals and amino acids [6]; the bark of Salvadora persica L. as a toothpaste for oral and dental care [7]; and Atriplex halimus L. as a food supplement to reduce menopause symptoms [8]. These plants can thus be a promising source of bioactive metabolites with potential uses in different commercial areas (e.g., food, pharma, cosmetic), finding their place in the highly demanding markets seeking innovation, while taking advantage of their suitability to be produced using saline water resources and/or saline soils [9].
Frankenia is the most extended genus in the Frankeniaceae family, which is represented by shrubby and herbaceous species that grow in arid and semi-arid environments, growing on saline, calcareous or chalky soils [10]. It includes the species Frankenia laevis L. (Figure 1), commonly known as sea heath, which is distributed along the Mediterranean region and the Atlantic coast, from Portugal (including the Azores islands), Spain, and France to the west of North Africa (Algeria, Morocco, and Tunisia) [11].

Figure 1.
Frankenia laevis L. from Southern Algarve, Portugal. Photos by Chia-Yu Chu.
Frankenia spp. is used in Asian traditional medicine in the form of an herbal tea for gargling, or for skin application due to its astringent properties, as well as in the form of tinctures to treat diverse medical conditions, such as diarrhea, dysentery, vaginal leucorrhea, gonorrhea, catarrh, and mucous problems [12]. However, there are no reports on the use of F. laevis in particular. In the last few decades, only two studies reported the in vitro biological potential of this species. Saïdana et al. [13] described the antibacterial activity of F. laevis essential oils against Staphylococcus aureus, Micrococcus luteus and Salmonella typhimurium, which they ascribed to the presence of hexadecenoic acid, benzyl benzoate, benzyl cinnamate, farnesyl acetate, methyl linoleate, eugenol and β-caryophyllene. Moreover, 80% aqueous acetone extracts from F. laevis aerial parts were described with strong in vitro radical scavenging and copper chelating activities related to their high total content in phenolic compounds [14]. Diverse sulphated phenolics were identified in whole plant aqueous–alcoholic extracts, namely sodium sulphated derivatives of acetophenone, and gallic and ellagic acids [15].
Having in mind the previously reported promising results for F. laevis, this work intended to further explore its biological activities and chemical composition, aiming at its valorization as a source of novel natural ingredients and/or products, with commercial applications in the food and pharmaceutical industries. For this purpose, hydrophilic (methanol) and lipophilic (dichloromethane) extracts were prepared from aboveground biomass containing flowers, leaves and stems, and were chemically characterized by high-performance liquid chromatography coupled with electrospray ionization mass spectrometry (HPLC-ESI-MS/MS). The extracts were then evaluated for in vitro radical scavenging activity (RSA) against 2,2-diphenyl-1-picrylhydrazyl (DPPH), and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), ferric reducing antioxidant power (FRAP), and copper (CCA)- and iron (ICA)-chelating activities. The inhibitory capacity of the extracts was also appraised on enzymes implicated in the onset of neurodegenerative diseases, especially Alzheimer’s disease, namely acetylcholinesterase (AChE) and butyrylcolinesterase (BuChE); hyperpigmentation disorders and food oxidation (tyrosinase); and type-2 diabetes (α-amylase and α-glucosidase). The cytotoxic potential of the extracts was evaluated towards tumoral human hepatocellular carcinoma (HepG2) cells versus the non-tumoral mouse stromal bone marrow (S17) cell line.
2. Results and Discussion
2.1. Chemical Composition
The efficiency of the extraction was evaluated by calculating the percentage of the extraction yield, with methanol being more effective than dichloromethane, presenting a yield almost 13 times higher (22% vs. 1.7%, respectively).
The chemical profile of extracts of sea heath was established by HPLC-ESI-MS/MS, and the results are summarized in Table 1 and Figures S1–S4 (Supplementary Materials). The compounds were identified based on their accurate m/z values, retention time, and the mass spectral fragment information by comparison with data found in the literature, and when possible, with standard compounds. A total of 51 compounds were tentatively identified in the samples; there was a prevalence of hydrophilic metabolites, as the methanol extract showed higher chemical diversity, with 45 compounds detected, whereas only 20 compounds were identified in the dichloromethane extract. Besides this, several compounds were identified in both extracts, while others were only found in one of the extracts. For instance, 14 compounds were detected in both the methanol and dichloromethane extracts, namely citric acid (1), two catechol derivatives (15,20), one O-glycoside sulfate (16), two monoterpenes (23,24), three hydroxycinnamic acid derivatives (28,32,35), one terpene (36), two ellagic acid derivatives (38,40), one fatty acid (44), and pheophytin A (51). Conversely, six compounds were found only in the dichloromethane extracts, including some hydroxylated derivatives of unsaturated fatty acids (45,46,49,50), one precursor of jasmonic acid (47), and one long-chain fatty acid (48). The remaining 31 compounds were only extracted with methanol, namely several glycosylated and sulfated phenolic acid derivatives (2–14, 17–19,25,26,29,30,33,34,39,41–43), two sulfated lignans (21,22), and three flavonoid derivatives (27,31,37).

Table 1.
HPLC-ESI-MS/MS tentative identification of the metabolites present in the methanol and dichloromethane extracts of the aerial parts of sea heath (F. laevis).
As is widely known, phenolics are one of the most important groups of plant secondary metabolites, which include flavonoids, phenolic acids and lignans, which are highly represented compounds in the sea heath extracts [16]. These molecules participate in many interactions between plants and the environment, such as, for example, herbivory, pigmentation, or allelopathy. Flavonoids possess several functions, namely plant development regulation, pigmentation, UV protection, and as microorganisms’ defense signaling molecules [17]. Lignans may have a defensive role as antifeedants against herbivores and microorganisms, as well as allelopathic effects [18]. The biosynthesis of these metabolites is usually increased when the plants are exposed to environmental stresses such as drought, UV radiation, and salinity, which characterize halophytes’ habitats. Moreover, enzymatic modifications result in many types of phenolic derivatives, mainly methylated, sulphated, and glycosylated compounds [19]. For example, an increased biosynthesis of sulphated phenolics is highly correlated with high salinity conditions, such as those to which halophyte plants are subjected. Despite the functional role of such compounds not being clear, it seems to be related to ecological adaptations such as co-pigmentation, growth regulation, molecular recognition, and detoxification, as the increased solubility and stability of these molecules enhance their interaction with biological targets [19]. Similarly, the accumulation of glycosylated derivatives may occur as a response to stress resistance mechanisms (e.g., high UV radiation, temperature, and salinity) working as osmoprotective, carbon storage, and free radical scavenging mechanisms [20]. Several sodium sulphated phenolics were already isolated from F. laevis whole-plant aqueous alcohol extract, including some gallic and ellagic acids, and acetophenone derivatives [15]. However, except for 3-O-methylgallic acid-5-O-sulfate, none of the other molecules were identified in the present work. Despite this, gallic acid has already been identified in both F. pulverulenta and F. thymifolia, linoleic acid and kaempferol were previously found in F. thymifolia, and some unidentified sulfated flavonoids were reported in F. pulverulenta [21,22].
Other major plant metabolites were found in F. laevis extracts, including citric acid, chlorophyll and jasmonic acid derivatives, which are key players in plant growth, photosynthesis, cellular respiration and photoprotection. Monoterpenes are primarily found in plant essential oils endowing allelochemical and wound-healing functions [23]. In turn, jasmonic acid is a stress-related plant hormone produced in response to environmental stresses, such as drought, salinity, and light exposure [24]. This may explain the detection of these type of metabolites in F. laevis extracts, as this species thrives in salt marshes subjected to severe abiotic stresses such as high salinity, drought, temperature variations, and high UV radiation. Overall, to the best of our knowledge, no previous studies have described the occurrence of most of the metabolites detected in this study in the same species or genus.
2.2. Biological Activities
Besides the important physiological and ecological functions of secondary plant metabolites, they are also important components for human health, contributing to the reduction of the risk of several diseases related to oxidative stress, such as inflammation, cancer, diabetes, cardiovascular disease, and neurodegeneration. In this sense, F. laevis extracts were further evaluated for their in vitro antioxidant activity, inhibition of enzymes related to diabetes, neurodegeneration, hyperpigmentation/food oxidation, and cytotoxic potential against human hepatocellular carcinoma cells.
2.2.1. Antioxidant Activity
The extracts were evaluated for their in vitro antioxidant capacity by five complementary assays, including radical scavenging, metal chelating and iron reduction assays, and the results are presented in Table 2. Only the methanol extracts showed the ability to scavenge the DPPH and ABTS radicals, reduce iron and chelate copper more than 50% at the concentration of 1 mg/mL, allowing us to calculate the half-maximal effective concentration (EC50) values (0.25, 0.65, 0.51 and 0.78 mg/mL, respectively). In turn, only the dichloromethane extract was able to chelate iron, exhibiting an EC50 value of 0.76 mg/mL.

Table 2.
Antioxidant activities of the methanol and dichloromethane extracts of sea heath’s (F. laevis) aboveground biomass. The results are expressed as half-maximal effective concentration (EC50) values (mg/mL).
As far as we know, there is only one study on the radical scavenging and metal chelation properties of F. laevis 80% aqueous acetone made from aboveground parts containing shoots and leaves, the obtained EC50 values of which were lower (DPPH: 0.12 mg/mL; ABTS: 0.18 mg/mL; CCA: 0.44 mg/mL) than those found in this work [14]; similarly to our data, the extract did not present significant ICA up to 1 mg/mL. Other species belonging to the Frankenia genus have already shown strong antioxidant properties, namely F. thymifolia, F. triandra and F. pulverulenta. In total, 80 and 96% aqueous ethanol extracts made from the aerials parts of F. triandra showed EC50 values of 37 and 15 µg/mL for ABTS radical scavenging ability and iron reducing power (FRAP), respectively [10]. Polar and non-polar fractions from F. thymifolia aerial organs were also described as having DPPH-radical scavenging activity and iron reducing power (EC50 = 99 and 120 µg/mL, correspondingly) [21]. Moreover, 80% aqueous acetone extracts from F. pulverulenta showed strong DPPH (EC50 = 0.10 mg/mL) and ABTS (EC50 = 0.15 mg/mL) reduction ability, as well as copper (EC50 = 0.50 mg/mL) and iron (EC50 = 0.30 mg/mL) chelation properties [14]. Likewise, ethyl acetate shoots and root fractions of F. pulverulenta had considerable DPPH (586 and 750 mg of TE/g) and ABTS (1453 and 1319 mg of TE/g) reducing capacity [22].
The significantly higher antioxidant activity of the methanol extracts can be explained by the highest amount of the compounds identified in these extracts, namely phenolic acids, lignans and flavonoid derivatives, as well as monoterpenes metabolites. Phenolic compounds are characterized by an aromatic ring carrying one or more hydroxyl groups, including derivatives, such as methyl, glycoside, or sulfate substituents. Their chemical structure is ideal for free radical scavenging due to the high capacity of hydroxyl groups to donate a hydrogen atom or an electron to a free radical, and the capacity of the conjugated aromatic system to delocalize unpaired electrons [25]. Moreover, some phenolics with dihydroxy groups, namely those containing catecholate and gallate groups, can conjugate transition metals (e.g., copper), preventing metal-induced free radical formation by the Fenton reaction [25]. Thus, the oxidation of biological molecules such as lipids, proteins and nucleic acids is reduced. In turn, monoterpenes are often linked to hydroxyl groups which confer them free radical and singlet oxygen scavenging properties, acting as effective antioxidants [26]. Instead, the iron chelation ability exhibited only by the dichloromethane extract can be linked with the presence of the unsaturated fatty acid derivatives, as iron can bind to low molecular weight biomolecules with chelating sites, including fatty acids such as linoleic acid, suggesting that their derivatives may also be able to chelate iron [27,28].
In addition to the antioxidant properties of the main detected metabolites (phenolic acids, lignans, flavonoids, monoterpenes, unsaturated fatty acids), they also confer important functional roles in human health and disease, exhibiting therapeutic effects against inflammation, cardiovascular, neurodegenerative diseases, cancer, diabetes, and obesity problems [29,30,31,32]. Thus, natural sources rich in these compounds, such as F. laevis, have the potential to be used to provide antioxidant supplements by free radicals’ inactivation and/or metal chelation, leading to reduced cellular damage and associated disease development. Moreover, such compounds also have applications in the food industry to prevent food oxidation, which leads to the loss of important nutritional and sensory food properties [33]. In fact, the interest in using natural antioxidants to replace their synthetic counterparts, such as BHT (E320), is rising due to many factors, including the undesirable side effects of the latter ones, which include carcinogenic properties, and the additional health benefits displayed by the former [33].
2.2.2. Enzyme Inhibition
One of the most common therapeutic tools to manage human diseases is the inhibition of key enzymes associated with those problems, contributing to symptom relief [34]. In this sense, the inhibitory capacity of F. laevis extracts was therefore evaluated on enzymes implicated in the onset of neurodegenerative diseases, especially Alzheimer’s disease (AChE and BuChE), hyperpigmentation disorders (tyrosinase) and diabetes (α-amylase and α-glucosidase) (Table 3). The most common prophylactic treatment for type 2 diabetes includes reducing carbohydrate digestibility by inhibiting two key hydrolyzing enzymes, namely α-amylase and α-glucosidase, to reduce postprandial hyperglycemia. However, the secondary effects presented by clinically used inhibitors (e.g., acarbose, miglitol, voglibose), which may include diarrhea and abdominal pain, emphasize the demand to search for novel natural therapeutic compounds with fewer harmful effects [35]. Both the methanol and dichloromethane extracts of F. laevis had the ability to inhibit the α-glucosidase enzyme, but the latter exhibited the lowest EC50 value (0.52 mg/mL), which was six-times inferior to that of the positive control, acarbose (EC50 = 3.14 mg/mL). To the best of our knowledge, there were no previous studies reporting the α-glucosidase inhibitory properties of the Frankenia genus.

Table 3.
Enzyme inhibitory activities of the methanol and dichloromethane extracts of the aerial parts of sea heath (F. laevis). The results are expressed as half-maximal effective concentration (EC50) values (mg/mL).
Many bioactive compounds, such as phenolic compounds (e.g., flavonoids, tannins, lignans), may be safer alternatives to primary pharmacological therapies, and may also contribute to the reduction of the occurrence of secondary diabetic complications linked to oxidative stress [35]. For instance, linoleic acid and its derivatives, such as those detected in F. laevis dichloromethane extract, are reported to be competitive inhibitors of α-glucosidase, and are more potent than the positive control, acarbose, and with weaker anti-α-amylase activity, which was similar to that observed in our work [36]. Loliolide, a monoterpene present in both F. laevis methanol and dichloromethane extracts, is described to have a strong inhibitory effect against α-glucosidase, with EC50 values of 388.48 µM (approx. 76 µg/mL); thus, its presence, as well as that of the structurally related molecules of isololiolide and dihydroactinidiolide, may explain the anti-α-glucosidase activity of F. laevis extracts. Furthermore, the higher abundance of these compounds in the dichloromethane extract may be related to its higher α-glucosidase inhibition [37]. Despite the lower EC50 value of the methanol extract, its α-glucosidase inhibitory activity could be due to the presence of several molecules previously reported with the capacity to inhibit carbohydrate-hydrolyzing enzymes, namely phenolic acids (e.g., gallic, ferulic, coumaric and caffeic acids) [38], lignans (e.g., lariciresinol) [39], and flavonoids (e.g., kaempferol) [40]. Overall, the F. laevis extracts were shown to hold the potential to be used as a source of α-glucosidase inhibitors, contributing to the reduction of postprandial hyperglycemia in type 2 diabetes patients. Moreover, the antioxidant ability of these extracts may also contribute to the reduction of the risk of diabetic complications associated with oxidative stress, namely microvascular and cardiovascular complications [41,42].
Conversely, F. laevis extracts did not demonstrate the ability to inhibit any of the other enzymes tested, namely AChE, BuChE, α-amylase and tyrosinase. However, methanol extracts from this species have previously shown high AChE and BuChE inhibition (approx. 80% at 1 mg/mL) [43]. In turn, similarly to our work, 80% aqueous acetone extracts of F. laevis aerial organs also did not show any capacity to inhibit tyrosinase at the concentration of 1 mg/mL [14].
2.2.3. Cytotoxicity
Hepatocellular carcinoma is one of the main liver tumors that are generally derived from chronic liver diseases, such as cirrhosis or hepatitis. It is an aggressive cancer with high metastatic capability and high resistance to cytotoxic drugs, which leads to the need to identify new drug leads for hepatocarcinoma chemotherapeutics [44]. In this respect, the cytotoxic potential of F. laevis extracts was assessed on tumoral human hepatocellular carcinoma (HepG2) cells in comparison with the non-tumoral mouse stromal bone marrow (S17) cell line. The results are presented in Table 4.

Table 4.
Cytotoxic activity of methanol and dichloromethane extracts of the aerial parts of sea heath (F. laevis) towards human hepatocarcinoma (HepG2) and mouse bone marrow stromal (S17) cell lines. The results are expressed as half-maximal effective concentration (EC50) values (µg/mL).
A significant reduction in HepG2 cell viability was observed after the application of the dichloromethane extract of F. laevis with an EC50 value of 52.1 µg/mL. Conversely, this extract did not show significant cytotoxicity towards the non-tumoral S17 cell line up to the maximum concentration tested (100 µg/mL); as such, it exhibited a selectivity index (SI) of at least 1.9 (Table 4), which means that the sample is less toxic for normal cells than tumoral ones, and that it is safer for therapeutic uses [45]. The methanol extract also did not show significant cytotoxicity up to 100 µg/mL on both HepG2 and S17 cells (Table 4).
To the best of our knowledge, this is the first report on the in vitro anti-hepatocarcinoma potential of Frankenia species. However, the monoterpene metabolites detected in higher abundance in the dichloromethane extract from F. laevis aerial parts, such as isololiolide and loliolide (Figure 2), have already been isolated from marine macro- and microalgae, respectively, and were ascribed with selective cytotoxicity against HepG2 cells [46,47].
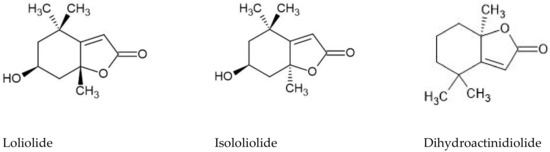
Figure 2.
Chemical structures of loliolide, isololiolide and dihydroactinidiolide.
Dihydroactinidiolide (Figure 2), a structural analog of loliolide, also showed a strong cytotoxic effect on human lung carcinoma cells (A549) [48], as well as an enriched fraction of Moringa stenopetala in loliolide and dihydroactinidiolide that was highly cytotoxic against human hepatocarcinoma (HepG2) and human breast adenocarcinoma (MCF-7) cells, with EC50 values ranging between 35 and 39 µg/mL [49]. Furthermore, the polyunsaturated fatty acid linoleic acid has been described to reduce the generation of pre-cancerous hepatic nodules in rats, demonstrating its potential chemoprotective effect on hepatocellular carcinoma [50,51]. Oxophytodienoic acid, a phytohormone with plant growth and development functions, was also described to inhibit human breast cancer cells’ proliferation by decreasing the expression of cyclin D1 [52]. Thus, the anti-hepatocarcinoma activity of F. laevis dichloromethane extract can also be ascribed to linoleic acid’s hydroxylated derivatives and the oxophytodienoic acid found in this extract. According to this, F. laevis dichloromethane extracts were shown to be a promising source of molecules with possible applications as a selective anti-hepatocarcinoma therapy, being a candidate for further studies on the identification and isolation of antitumoral lead drugs.
3. Materials and Methods
3.1. Chemicals
The DPPH and ABTS radicals, BHT, AChE from electric eels (EC 3.1.1.7), equine BuChE (EC 3.1.1.8), acetylthiocholine iodide, butyrylthiocholine iodide, 5-thio-2-nitrobenzoate (DTNB), tyrosinase from mushrooms (EC 1.14.18.1), L-tyrosine, α-amylase from porcine pancreas (EC 3.2.1.1), and α-glucosidase from Saccharomyces cerevisiae (EC 3.2.1.20) were purchased from Sigma-Aldrich (Lisbon, Portugal). Additional reagents and solvents were obtained from VWR International (Leuven, Belgium).
3.2. Plant Material
The aerial organs of F. laevis at the flowering stage, including stems, leaves, and flowers at anthesis and prior to anthesis, were collected in the southwest of Portugal (Algarve) (coordinates: 43°38′19.39″ N 116°14′28.86″ W) in June of 2019. The taxonomical classification was determined by the botanist Dr. Manuel J. Pinto (National Museum of Natural History, University of Lisbon, Botanical Garden, Portugal). The samples were oven dried for 3 days at 50 °C, powdered, and stored at −20 °C until needed.
3.3. Extraction
Dried samples were separately mixed with methanol and dichloromethane (1:40 w/v) and were extracted overnight at room temperature (RT), under stirring. The extracts were filtered (Whatman n° 4) and evaporated under a vacuum. The dried extracts were dissolved in dimethyl sulfoxide (DMSO) at the concentration of 10 mg/mL, and were stored at −20 °C.
3.4. High-Performance Liquid Chromatography Coupled with Electrospray Ionization Mass Spectrometry (Hplc-Esi-MS/Ms)
The chemical composition of the extracts was determined using a Dionex Ultimate 3000RS UHPLC instrument. The extracts were filtered through a 0.22 μm PTFE filter membrane (Labex Ltd., Hungary) before HPLC analysis. The extracts were injected onto a Thermo Accucore C18 (100 mm × 2.1 mm, i. d., 2.6 μm) column thermostated at 25 °C (± 1 °C). The solvents used were water (A) and methanol (B), both acidified with 0.1% formic acid. The flow rate was maintained at 0.2 mL/min. The elution gradient was isocratic 5% B (0–3 min), a linear gradient increasing from 5% B to 100% (3–43 min), 100% B (43–61 min), a linear gradient decreasing from 100% B to 5% (61–62 min), and 5% B (62−70 min). The column was coupled with a Thermo Q-Exactive Orbitrap mass spectrometer (Thermo Scientific, Waltham, MA, USA) equipped with an electrospray ionization source. The spectra were recorded in positive- and negative-ion mode, respectively. The trace finder 3.1 (Thermo Scientific, Waltham, MA, USA) software was applied for target screening. Most of the compounds were identified based on our previously published work or data found in the literature. In every case, the exact molecular mass, isotopic pattern, characteristic fragment ions and retention time were used for the identification of the compounds which are marked and were confirmed by standards. The difference between the measured and calculated molecular mass was less than 5 ppm in each case.
3.5. Determination of the In Vitro Biological Activities
The samples’ activities were determined at 1 mg/mL, and when the activity was higher than 50%, different concentrations (0.03125, 0.0625, 0.125, 0.25, 0.5, and 1 mg/mL) were tested for the determination of the EC50 values (mg/mL). The absorbance was measured in a microplate reader (Biotek Synergy 4), and the activity was calculated as a percentage of inhibition, relative to a control containing DMSO in place of the sample.
3.5.1. Determination of the Antioxidant Activity
RSA on DPPH and ABTS Radicals
The extracts were tested on DPPH and ABTS, as described elsewhere [53]. The samples were mixed with radical solutions (DPPH: 120 µM; ABTS: 7.4 mM) in 96-well flat-bottom microtitration plates and were incubated in darkness at RT for 30 and 6 min, respectively. The synthetic antioxidant BHT (E320), used as a preservative in food and cosmetics, was used as a positive control at the same concentrations of the samples. The absorbance was measured at 517 and 734 nm for DPPH and ABTS, respectively.
FRAP
The ability of the extracts to reduce Fe3+ was evaluated as previously described by Rodrigues et al. [53]. The samples were mixed with distilled water and 1% potassium ferricyanide in 96-well plates, and were incubated at 50 °C for 20 min. Then, 10% trichloroacetic acid and 0.1% ferric chloride solution were added. Increased absorbance at 700 nm means higher reducing activity, and the results were expressed as a percentage of inhibition relative to the positive control at the concentration of 1 mg/mL.
CCA and ICA
The CCA and ICA of the extracts and the positive control (EDTA) were assessed in 96-well microplates according to the methods described in Rodrigues et al. [53]. For CCA, the samples were mixed with 50 mM Na acetate buffer (pH 6), 4 mM pyrocatechol violet, and 50 µg/mL CuSO4 solution. For ICA, the samples were mixed with distilled water and 0.1 mg/mL FeCl2 solution. After 30 min, a 40 mM ferrozine solution was added. The change in absorbance was measured at 632 and 562 nm for CCA and ICA, correspondingly.
3.5.2. Determination of the Enzyme Inhibitory Activity
AChE and BuChE Inhibitory Activities
The inhibitory effect of the extracts and the standard (galantamine) on AChE and BuChE was assessed according to the method explained by Custódio et al. [54]. The samples were mixed with 0.02 M phosphate buffer (pH 8.0) and enzyme solution (0.28 U/mL). After 15 min at 25 °C, the substrate (acetylcholine iodide or butyrylcholine chloride, 4 mg/mL) and 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB, 1.2 mg/mL) were added and incubated for 15 min at 25 °C. The changes in the absorbances were measured at 412 nm.
Tyrosinase Inhibitory Activity
The tyrosinase inhibitory activity of the extracts and the positive control, arbutin, a commercially available tyrosinase inhibitor, were assessed as reported before [55]. The samples were mixed with enzyme solution (333 U/mL) in 25 mM potassium phosphate buffer (pH 6.5). After 5 min, the substrate L-tyrosine (2 mM) was added and incubated for an additional 30 min. period, at room temperature. The optical densities were read at 492 nm.
α-Amylase and α-Glucosidase Inhibitory Activities
The α-amylase and α-glucosidase inhibitory activities of the extracts and of the positive control, acarbose, the active ingredient of a clinically used drug for the control of type 2 diabetes (Glucobay®), were determined by the method depicted in Rodrigues et al. [56]. For α-amylase, the samples were mixed with amylase solution (100 U/mL) and 0.1% starch solution. After 10 min at 37 °C, 1 M hydrochloric acid (HCl) and 5 mM iodide solution were added. For α-glucosidase, the extracts were mixed with enzyme solution (1.0 U/mL) and incubated for 10 min. at 25 °C. Then, 5 mM of substrate solution (p-nitrophenyl-α-D-glucopyranoside) was added and incubated for a further 5 min at 25 °C. The absorbance was measured at 580 and 405 nm for α-amylase and α-glucosidase, respectively.
3.5.3. Determination of the Cytotoxic Activity
Cell Culture
The HepG2 (human hepatocellular carcinoma) and S17 (murine bone marrow stromal) cell lines were maintained in Dulbecco’s modified eagle medium (DMEM) culture medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 1% L-glutamine (2 mM), and 1% penicillin (50 U/mL)/streptomycin (50 μg/mL) and were maintained at 37 °C in humidified atmosphere with 5% CO2.
Cellular Viability Assay
HepG2 and S17 cells were plated in 96-well tissue plates at a density of 5 × 103 cells/well and were incubated for 24 h. Then, the extracts were applied at several concentrations (3.125, 6.25, 12.5, 25, 50 and 100 µg/mL) for 72 h. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric test was used to determine the cellular viability (Biotek Synergy 4), as formerly detailed [57]. The absorbance was measured at 590 nm, and the results were expressed in terms of cellular viability (%) in relation to a control containing DMSO (0.5%) and EC50 values (µg/mL). The selectivity index (SI) was obtained by dividing the EC50 value of non-tumoral cells (S17) by the EC50 of tumoral cells (HepG2).
3.6. Statistical Analysis
The results were expressed as the mean ± standard error of the mean (SEM), and the experiments were conducted at least in triplicate. Significant differences were assessed by Student’s t-test. p values lower than 0.05 were considered significant. All the statistical analysis was performed using the XLSTAT statistical package for Microsoft Excel (version 2013, Microsoft Corporation). The IC50 values were calculated by the sigmoidal fitting of the data using GraphPad Prism version 9.
4. Conclusions
This work reported the in vitro antioxidant activity, enzyme inhibition and cytotoxicity towards hepatocellular carcinoma cells of extracts made from the aerial organs of the medicinal halophyte F. laevis. The methanol extract had a high antioxidant activity against DPPH and ABTS radicals, high copper chelating ability, and iron reducing power, whilst the dichloromethane extract showed high iron chelation capacity. Both extracts had high inhibitory activity towards the α-glucosidase enzyme, and the dichloromethane extract exerted a selective cytotoxic effect on the human hepatocellular carcinoma (HepG2) cell line. However, the sea heath extracts did not display significant AChE and BuChE, α-amylase or tyrosinase inhibition. The chemical profiling of the sea heath extracts detected several derivatives of phenolic acids, lignans and flavonoids, monoterpenes, and hydroxylated derivatives of linoleic acid. These molecules have been reported to have biological activities, namely antioxidant, antidiabetic, and anti-tumor activities, which suggest that the aerial parts of F. laevis are a promising source of molecules with potential applications in pharmaceutical and/or food industries, as new drug leads, herbal health supplements, and/or food preservatives.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/plants11101353/s1, Figure S1: Total ion chromatogram (positive mode) of F. laevis methanol extract. Figure S2: Total ion chromatogram (negative mode) of F. laevis methanol extract. Figure S3: Total ion chromatogram (positive mode) of F. laevis dichloromethane extract. Figure S4: Total ion chromatogram (negative mode) of F. laevis dichloromethane extract.
Author Contributions
Conceptualization, M.J.R. and L.C.; methodology, M.J.R., C.G.P. and J.J. and Z.C.; formal analysis, M.J.R., J.J. and Z.C.; investigation, L.C., M.J.R., J.J. and Z.C.; resources, L.C. and Z.C.; writing—original draft preparation, M.J.R.; writing—review and editing, C.G.P., L.C. and Z.C.; supervision, L.C.; project administration, L.C.; funding acquisition, L.C. and Z.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Foundation for Science and Technology (FCT) and the Portuguese National Budget through projects UIDB/04326/2020, UID/DTP/04138/2020 and PTDC/BAAAGR/1391/2020 (Greenvalue). It also received funding through Fundo Azul (XtremeAquaCrops project: FA-05-2017-028) and the project HaloFarMs, which is part of the Partnership on Research and Innovation in the Mediterranean Area (PRIMA) Programme supported by the European Union and funded by the national funding bodies of Participating States (FCT in Portugal). Luísa Custódio was supported by the FCT Scientific Employment Stimulus (CEECIND/00425/2017).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The dataset is available upon request from the corresponding author.
Acknowledgments
The authors acknowledge the Marine Molecular Bioengineering group (Centre of Marine Sciences, Portugal), which provided the human hepatocellular carcinoma (HepG2) cell line, and to the Centre for Molecular and Structural Biomedicine (University of Algarve, Portugal), which kindly offered mouse bone marrow stromal (S17) cells. Furthermore, the authors thanks go to Chia-Yu Chu from the CCMAR communication and outreach office for the photo of F. laevis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Edible halophytes of the Mediterranean basin: Potential candidates for novel food products. Trends Food Sci. Technol. 2018, 74, 69–84. [Google Scholar] [CrossRef] [Green Version]
- Barreira, L.; Resek, E.; Rodrigues, M.J.; Rocha, M.I.; Pereira, H.; Bandarra, N.; da Silva, M.M.; Varela, J.; Custódio, L. Halophytes: Gourmet food with nutritional health benefits? J. Food Compos. Anal. 2017, 59, 35–42. [Google Scholar] [CrossRef]
- Presençadeluxo. 2022. Available online: https://presencadeluxo.pt/anti-queda-natural-solution-quinoa/ (accessed on 6 April 2022).
- Olas, B. The beneficial health aspects of sea buckthorn (Elaeagnus rhamnoides (L.) A.Nelson) oil. J. Ethnopharmacol. 2018, 213, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Evlash, V.; Murlykina, N.; Aksonova, O.; Hazzavi-Rogozina, L. Technology of a dietary supplement “SoleVit Mg” based on Salicornia Europaea L. for use in food technologies. In Proceedings of the BIO Web of Conferences, Belgorod, Russia, 27–28 May 2021; Volume 40, p. 02006. [Google Scholar]
- Optimah. 2022. Available online: https://optimah.com/collections/aloe-dent/products/aloedent%C2%AE-miswak-toothpaste (accessed on 6 April 2022).
- Paiskincare. 2022. Available online: https://www.paiskincare.us/products/instant-calm-redness-serum-sea-aster-wild-oat?variant=26367870855 (accessed on 6 April 2022).
- Lombardi, T.; Bertacchi, A.; Pistelli, L.; Pardossi, A.; Pecchia, S.; Toffanin, A.; Sanmartin, C. Biological and Agronomic Traits of the Main Halophytes Widespread in the Mediterranean Region as Potential New Vegetable Crops. Horticulturae 2022, 8, 195. [Google Scholar] [CrossRef]
- Torres Carro, R.; D’Almeida, R.E.; Isla, M.I.; Alberto, M.R. Antioxidant and anti-inflammatory activities of Frankenia triandra (J. Rémy) extracts. S. Afr. J. Bot. 2016, 104, 208–214. [Google Scholar] [CrossRef]
- Brightmore, D. Frankenia laevis L. J. Ecol. 1979, 67, 1097–1107. [Google Scholar] [CrossRef]
- Felter, H.W. The Eclectic Materia Medica, Pharmacology and Therapeutics; Valley Co.: Cincinnati, OH, USA, 1922. [Google Scholar]
- Saïdana, D.; Mahjoub, M.A.; Mighri, Z.; Chriaa, J.; Daamiand, M.; Helal, A.N. Studies of the essential oil composition, antibacterial and antifungal activity profiles of Frankenia laevis L. from Tunisia. J. Essent. Oil Res. 2010, 22, 349–353. [Google Scholar] [CrossRef]
- Lopes, A.; Rodrigues, M.J.; Pereira, C.; Oliveira, M.; Barreira, L.; Varela, J.; Trampetti, F.; Custódio, L. Natural products from extreme marine environments: Searching for potential industrial uses within extremophile plants. Ind. Crop. Prod. 2016, 94, 299–307. [Google Scholar] [CrossRef]
- Hussein, S.A. Phenolic sodium sulphates of Frankenia laevis L. Die Pharmazie Int. J. Pharm. Sci. 2004, 59, 304–308. [Google Scholar]
- Pereira, D.M.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From Chemistry to Biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Mathesius, U. Flavonoid Functions in Plants and Their Interactions with Other Organisms. Plants 2018, 72, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ražná, K.; Nôžková, J.; Vargaová, A.; Harenčár, Ľ.; Bjelková, M. Biological functions of lignans in plants. Agriculture 2021, 67, 155–165. [Google Scholar] [CrossRef]
- Teles, Y.C.F.; Souza, M.S.R.; Souza, M.D.F.V.D. Sulphated Flavonoids: Biosynthesis, Structures, and Biological Activities. Molecules 2018, 23, 480. [Google Scholar] [CrossRef] [Green Version]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Ksouri Wided, M.; Feten, C.; Rawya, R.; Feten, M.; Yosr, Z.; Nejla, T.; Riadh, K.; Emira, N.; Chedly, A. Antioxidant and antimicrobial properties of Frankenia thymifolia Desf. fractions and their related biomolecules identification by gas chromatography/mass spectrometry (GC/MS) and high performance liquid chromatography (HPLC). J. Med. Plant Res. 2011, 5, 5754–5765. [Google Scholar]
- Ben Mansour, R.; Wided, M.K.; Cluzet, S.; Krisa, S.; Richard, T.; Ksouri, R. LC-MS identification and preparative HPLC isolation of Frankenia pulverulenta phenolics with antioxidant and neuroprotective capacities in PC12 cell line. Pharm. Biol. 2017, 55, 880–887. [Google Scholar] [CrossRef] [Green Version]
- Ilc, T.; Parage, C.; Boachon, B.; Navrot, N.; Werck-Reichhart, D. Monoterpenol Oxidative Metabolism: Role in Plant Adaptation and Potential Applications. Front. Plant Sci. 2016, 7, 509. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of Jasmonic Acid in Plant Regulation and Response to Abiotic Stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Zielińska-Błajet, M.; Feder-Kubis, J. Monoterpenes and Their Derivatives—Recent Development in Biological and Medical Applications. Int. J. Mol. Sci. 2020, 21, 7078. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.; Kontoghiorghe, C. Iron and Chelation in Biochemistry and Medicine: New Approaches to Controlling Iron Metabolism and Treating Related Diseases. Cells 2020, 9, 1456. [Google Scholar] [CrossRef] [PubMed]
- Nalini, S.; Balasubramanian, K.A. Studies on iron binding by free fatty acids. Indian J. Biochem. Biophys. 1993, 30, 224–228. [Google Scholar]
- Nagy, K.; Tiuca, I. Importance of Fatty Acids in Physiopathology of Human Body. In Fatty Acids; IntechOpen: London, UK, 2017. [Google Scholar]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally Lignan-Rich Foods: A Dietary Tool for Health Promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, V.S.; Ferreira, F.S.; Cople, M.C.R.; Labre, T.D.S.; Augusta, I.M.; Gamallo, O.D.; Saldanha, T. Use of Natural Antioxidants in the Inhibition of Cholesterol Oxidation: A Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1465–1483. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, S.; Moreira, E.; Grosso, C.; Andrade, P.B.; Valentão, P.; Romano, A. Phenolic profile, antioxidant activity and enzyme inhibitory activities of extracts from aromatic plants used in Mediterranean diet. J. Food Sci. Technol. 2017, 54, 219–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blahova, J.; Martiniakova, M.; Babikova, M.; Kovacova, V.; Mondockova, V.; Omelka, R. Pharmaceutical Drugs and Natural Therapeutic Products for the Treatment of Type 2 Diabetes Mellitus. Pharmaceuticals 2021, 14, 806. [Google Scholar]
- Su, C.H.; Hsu, C.H.; Ng, L.T. Inhibitory potential of fatty acids on key enzymes related to type 2 diabetes. BioFactors 2013, 39, 415–421. [Google Scholar] [CrossRef]
- Thissera, B.; Visvanathan, R.; Khanfar, M.A.; Qader, M.M.; Hassan, M.H.A.; Hassan, H.M.; Bawazeer, M.; Behery, F.A.; Yaseen, M.; Liyanage, R.; et al. Sesbania grandiflora L. Poir leaves: A dietary supplement to alleviate type 2 diabetes through metabolic enzymes inhibition. S. Afr. J. Bot. 2020, 130, 282–299. [Google Scholar] [CrossRef]
- Aleixandre, A.; Gil, J.V.; Sineiro, J.; Rosell, C.M. Understanding phenolic acids inhibition of α-amylase and α-glucosidase and influence of reaction conditions. Food Chem. 2022, 372, 131231. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-X. Studies on chemical constituents and activities of lignans and terpenes from stem of Moringa oleifera. Chin. Trad. Herb. Drug 2019, 24, 5198–5205. [Google Scholar]
- Hong, H.C.; Li, S.L.; Zhang, X.Q.; Ye, W.C.; Zhang, Q.W. Flavonoids with α-glucosidase inhibitory activities and their contents in the leaves of Morus Atropurpurea. Chin. Med. 2013, 8, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress-A concise review. Saudi. Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef] [Green Version]
- Silvestre, L. Searching for Biocompounds in Algae and Seagrasses with Potential Use in the Treatment of Alzheimer’s Disease. Master’s Thesis, University of Algarve, Faro, Portugal, 2017. Available online: http://hdl.handle.net/10400.1/10712 (accessed on 5 April 2022).
- Balogh, J.; Victor, D.; Asham, E.H.; Burroughs, S.G.; Boktour, M.; Saharia, A.; Li, X.; Ghobrial, R.M.; Monsour, H.P. Hepatocellular carcinoma: A review. J. Hepatocell Carcinoma 2016, 3, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.C.; Wu, J.Y.; Liao, H.F.; Chen, Y.J.; Kuo, C.D. Comparative assessment of therapeutic safety of norcantharidin, N-farnesyloxy-norcantharimide, and N-farnesyl-norcantharimide against Jurkat T cells relative to human normal lymphoblast: A quantitative pilot study. Medicine 2016, 95, e4467. [Google Scholar] [CrossRef]
- Vizetto-Duarte, C.; Custódio, L.; Gangadhar, K.N.; Lago, J.H.G.; Dias, C.; Matos, A.M.; Neng, N.; Nogueira, J.M.F.; Barreira, L.; Albericio, F.; et al. Isololiolide, a carotenoid metabolite isolated from the brown alga Cystoseira tamariscifolia, is cytotoxic and able to induce apoptosis in hepatocarcinoma cells through caspase-3 activation, decreased Bcl-2 levels, increased p53 expression and PARP cleavage. Phytomedicine 2016, 23, 550–557. [Google Scholar]
- Gangadhar, K.N.; Rodrigues, M.J.; Pereira, H.; Gaspar, H.; Malcata, F.X.; Barreira, L.; Varela, J. Anti-Hepatocellular Carcinoma (HepG2) Activities of Monoterpene Hydroxy Lactones Isolated from the Marine Microalga Tisochrysis lutea. Mar. Drugs 2020, 18, 567. [Google Scholar] [CrossRef]
- Malek, S.N.A.; Shin, S.K.; Wahab, N.A.; Yaacob, H. Cytotoxic components of Pereskia bleo (kunth) DC. (Cactaceae) leaves. Molecules 2009, 14, 1713–1724. [Google Scholar] [CrossRef] [PubMed]
- El-Mekkawy, S.; Hassan, A.Z.; Abdelhafez, M.A.; Mahmoud, K.; Mahrous, K.F.; Meselhy, M.R.; Sendker, J.; Abdel-Sattar, E. Cytotoxicity, genotoxicity, and gene expression changes induced by methanolic extract of Moringa stenopetala leaf with LC-qTOF-MS metabolic profile. Toxicon 2021, 203, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Taketomi, A.; Harimoto, N.; Tsujita, E.; Rikimaru, T.; Shirabe, K.; Shimada, M.; Maehara, Y. Antineoplastic Effects of Gamma Linolenic Acid on Hepatocellular Carcinoma Cell Lines. J. Clin. Biochem. Nutr. 2010, 47, 81–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, H.; Han, F.; Zhang, L.; Wang, L.; Kumar, M. Gamma linolenic acid regulates PHD2 mediated hypoxia and mitochondrial apoptosis in DEN induced hepatocellular carcinoma. Drug Des. Dev. 2018, 12, 4241–4252. [Google Scholar]
- Alito, N.; Mezzadra, H.; Patel, P.; Koyuturk, M.; Altiok, S. A plant oxylipin, 12-oxo-phytodienoic acid, inhibits proliferation of human breast cancer cells by targeting cyclin D1. Breast Cancer Res. Treat. 2008, 109, 315–323. [Google Scholar]
- Rodrigues, M.J.; Soszynski, A.; Martins, A.; Rauter, A.P.; Neng, N.R.; Nogueira, J.M.F.; Varela, J.; Barreira, L.; Custódio, L. Unravelling the antioxidant potential and the phenolic composition of different anatomical organs of the marine halophyte Limonium algarvense. Ind. Crop. Prod. 2015, 77, 315–322. [Google Scholar] [CrossRef]
- Custódio, L.; Patarra, J.; Alberício, F.; Neng, N.R.; Nogueira, J.M.F.; Romano, A. Phenolic composition, antioxidant potential and in vitro inhibitory activity of leaves and acorns of Quercus suber on key enzymes relevant for hyperglycemia and Alzheimer’s disease. Ind. Crop. Prod. 2015, 64, 45–51. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Pereira, C.; Oliveira, M.; Neng, N.R.; Nogueira, J.M.F.; Zengin, G.; Mahomoodally, M.F.; Custódio, L. Sea rose (Armeria pungens (Link) Hoffmanns. & Link) as a potential source of innovative industrial products for anti-ageing applications. Ind. Crop. Prod. 2018, 121, 250–257. [Google Scholar]
- Rodrigues, M.J.; Custódio, L.; Lopes, A.; Oliveira, M.; Neng, N.R.; Nogueira, J.M.F.; Martins, A.; Rauter, A.P.; Varela, J.; Barreira, L. Unlocking the in vitro anti-inflammatory and antidiabetic potential of Polygonum maritimum. Pharm. Biol. 2017, 55, 1348–1357. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.J.; Gangadhar, K.N.; Vizetto-Duarte, C.; Wubshet, S.G.; Nyberg, N.T.; Barreira, L.; Varela, J.; Custódio, L. Maritime halophyte species from southern Portugal as sources of bioactive molecules. Mar. Drugs 2014, 12, 2228–2244. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).