Antidiabetic Potential of Plants from the Caribbean Basin
Abstract
1. Introduction
2. The Caribbean Flora: A Unique and Diverse Source of Pharmacological Compounds
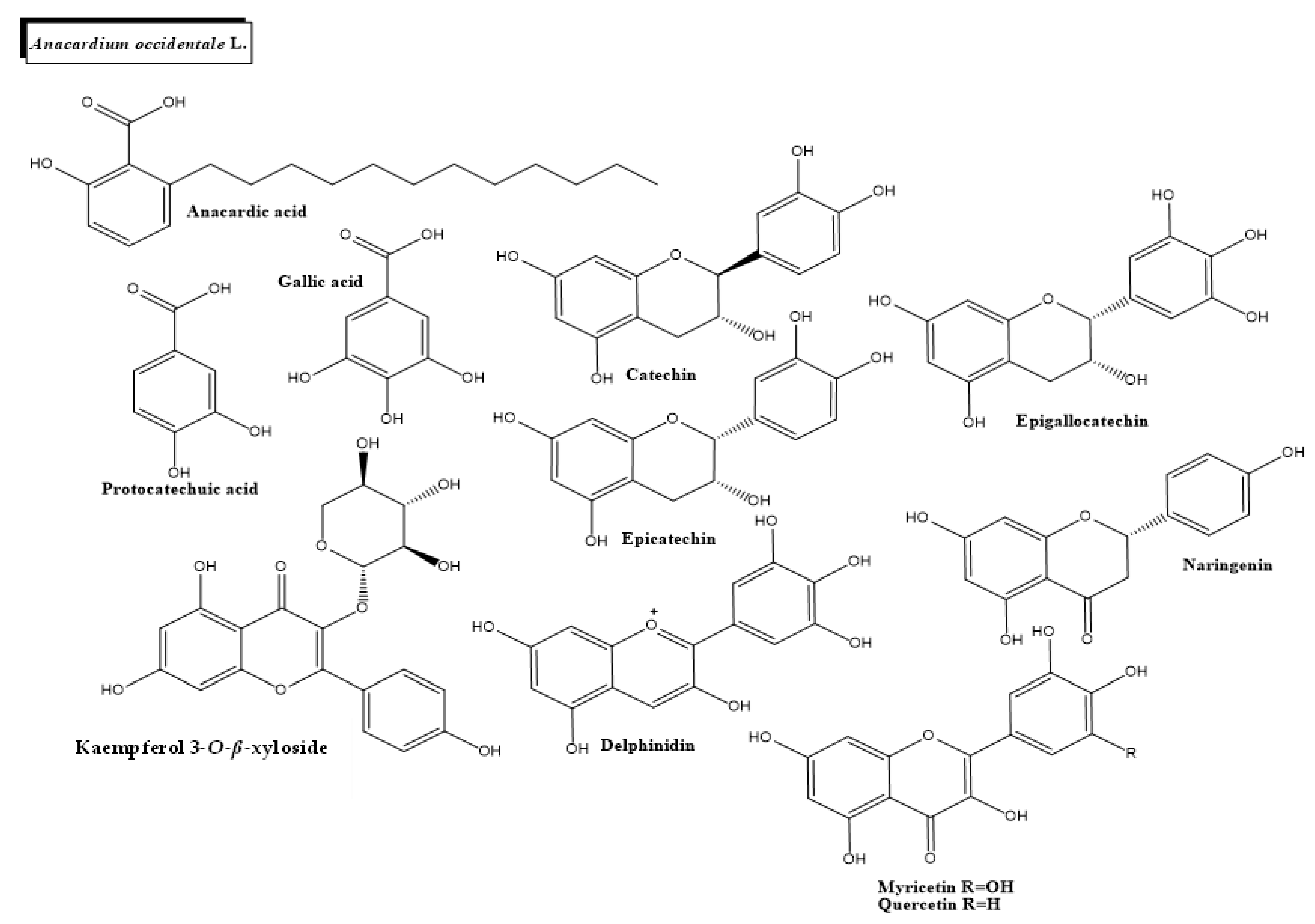
2.1. Anacardium occidentale L.
2.2. Hyptis suaveolens (L.) Poit.
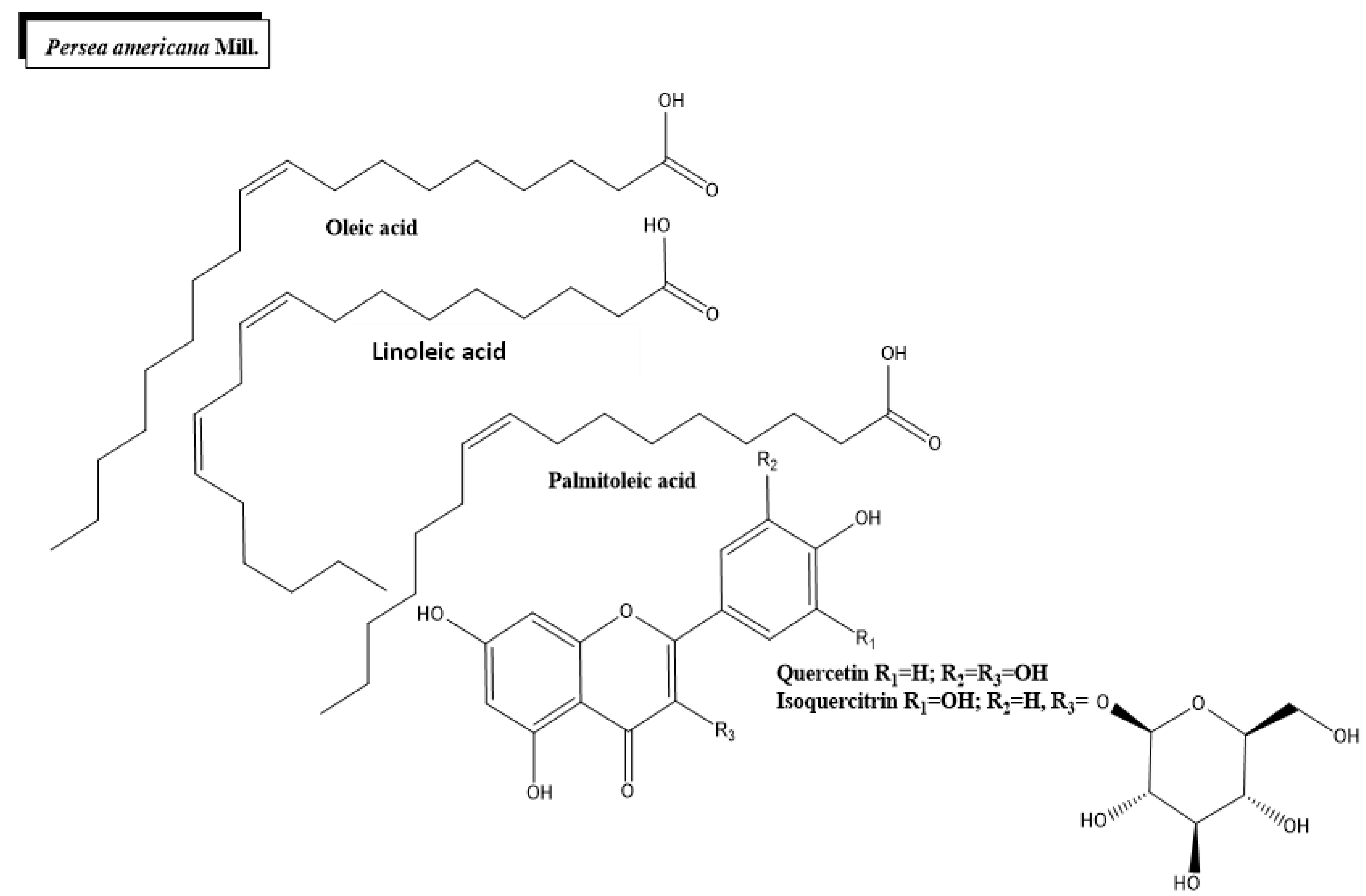
2.3. Persea americana Mill.
2.4. Psidium guajava L.
2.5. Tecoma stans (L.) Juss. ex Kunth
2.6. Momordica charantia L.
2.7. Phyllanthus niruri L.
3. Conclusions and Further Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, Å. Type 1 diabetes mellitus. Nat. Rev. Dis. Primers 2017, 3, 17016. [Google Scholar] [CrossRef] [PubMed]
- Roden, M.; Shulman, G.I. The integrative biology of type 2 diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Report on Diabetes. 2016. Available online: http://apps.who.int/iris/bitstream/handle/10665/254648/9789242565256-fre.pdf?sequence=1 (accessed on 1 March 2022).
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Rincón, H.; Cantoral, A.; Arrieta, A.; Espinal, C.; Magnus, M.H.; Palacios, C.; Tapia-Conyer, R. Review: Type 2 diabetes in Latin America and the Caribbean: Regional and country comparison on prevalence, trends, costs and expanded prevention. Prim. Care Diabetes 2021, 15, 352–359. [Google Scholar] [CrossRef]
- Barcelo, A.; Arredondo, A.; Gordillo-Tobar, A.; Segovia, J.; Qiang, A. The cost of diabetes in Latin America and the Caribbean in 2015: Evidence for decision and policy makers. J. Glob. Health 2017, 7, 020410. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Glyoxalase in diabetes, obesity and related disorders. Semin. Cell Dev. Biol. 2011, 22, 309–317. [Google Scholar] [CrossRef]
- Rabbani, N.; Xue, M.; Thornalley, P.J. Dicarbonyl stress, protein glycation and the unfolded protein response. Glycoconj. J. 2021, 38, 331–340. [Google Scholar] [CrossRef]
- Lotfy, M.; Adeghate, J.; Kalasz, H.; Singh, J.; Adeghate, E. Chronic Complications of Diabetes Mellitus: A Mini Review. Curr. Diabetes Rev. 2017, 13, 3–10. [Google Scholar] [CrossRef]
- Blaslov, K.; Naranđa, F.S.; Kruljac, I.; Renar, I.P. Treatment approach to type 2 diabetes: Past, present and future. World J. Diabetes 2018, 9, 209–219. [Google Scholar] [CrossRef]
- Mudaliar, S.; Henry, R.R. Combination therapy for type 2 diabetes. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 1999, 5, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Ata, A.; Anil Kumar, N.V.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Abdulmajid Ayatollahi, S.; Tsouh Fokou, P.V.; Kobarfard, F.; Amiruddin Zakaria, Z.; et al. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Blahova, J.; Martiniakova, M.; Babikova, M.; Kovacova, V.; Mondockova, V.; Omelka, R. Pharmaceutical Drugs and Natural Therapeutic Products for the Treatment of Type 2 Diabetes Mellitus. Pharmaceuticals 2021, 14, 806. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.A.; Stafford, J.M.; Arcury, T.A.; Snively, B.M.; Smith, S.L.; Grzywacz, J.G.; Quandt, S.A. Complementary and Alternative Medicine Use and Diabetes Self-Management Among Rural Older Adults. Complementary Health Pract. Rev. 2006, 11, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Garrow, D.; Egede, L.E. Association between complementary and alternative medicine use, preventive care practices, and use of conventional medical services among adults with diabetes. Diabetes Care 2006, 29, 15–19. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alarcon-Aguilara, F.J.; Roman-Ramos, R.; Perez-Gutierrez, S.; Aguilar-Contreras, A.; Contreras-Weber, C.C.; Flores-Saenz, J.L. Study of the anti-hyperglycemic effect of plants used as antidiabetics. J. Ethnopharmacol. 1998, 61, 101–110. [Google Scholar] [CrossRef]
- Farooqi, H.; Siraj, S.; Adhami, S. Unexplored Medicinal Plants of Potential Therapeutic Importance: A Review. Trop. J. Nat. Prod. Res. 2018, 2, 3–11. [Google Scholar] [CrossRef]
- Zhang, A.L.; Xue, C.C.; Fong, H. Integration of Herbal Medicine into Evidence-Based Clinical Practice: Current Status and Issues. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Posadzki, P.; Watson, L.K.; Ernst, E. Adverse effects of herbal medicines: An overview of systematic reviews. Clin. Med. 2013, 13, 7–12. [Google Scholar] [CrossRef]
- Picking, D.; Younger, N.; Mitchell, S.; Delgoda, R. The prevalence of herbal medicine home use and concomitant use with pharmaceutical medicines in Jamaica. J. Ethnopharmacol. 2011, 137, 305–311. [Google Scholar] [CrossRef]
- Boulogne, I.; Germosén-Robineau, L.; Ozier-Lafontaine, H.; Fleury, M.; Loranger-Merciris, G. TRAMIL ethnopharmalogical survey in Les Saintes (Guadeloupe, French West Indies): A comparative study. J. Ethnopharmacol. 2011, 133, 1039–1050. [Google Scholar] [CrossRef]
- Bahall, M.; Edwards, M. Perceptions of complementary and alternative medicine among cardiac patients in South Trinidad: A qualitative study. BMC Complementary Altern. Med. 2015, 15, 99. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Valentín, E.; Francisco-Ortega, J. Plant Evolution and Biodiversity in the Caribbean Islands–Perspectives from Molecular Markers. Bot. Rev. 2008, 74, 1–4. [Google Scholar] [CrossRef]
- Maunder, M.; Leiva, A.; Santiago-Valentín, E.; Stevenson, D.W.; Acevedo-Rodríguez, P.; Meerow, A.W.; Mejía, M.; Clubbe, C.; Francisco-Ortega, J. Plant Conservation in the Caribbean Island Biodiversity Hotspot. Bot. Rev. 2008, 74, 197–207. [Google Scholar] [CrossRef]
- Rana, Z.H.; Alam, M.K.; Akhtaruzzaman, M. Nutritional Composition, Total Phenolic Content, Antioxidant and α-Amylase Inhibitory Activities of Different Fractions of Selected Wild Edible Plants. Antioxidants 2019, 8, 203. [Google Scholar] [CrossRef]
- Alam, M.K.; Rana, Z.H.; Islam, S.N. Comparison of the Proximate Composition, Total Carotenoids and Total Polyphenol Content of Nine Orange-Fleshed Sweet Potato Varieties Grown in Bangladesh. Foods 2016, 5, 64. [Google Scholar] [CrossRef]
- Chung, S.; Shin, E.J.; Choi, H.K.; Park, J.H.; Hwang, J.T. Anacardic acid mitigates liver fat accumulation and impaired glucose tolerance in mice fed a high-fat and high-sucrose diet. Food Sci. Nutr. 2020, 8, 796–804. [Google Scholar] [CrossRef]
- Tedong, L.; Madiraju, P.; Martineau, L.C.; Vallerand, D.; Arnason, J.T.; Desire, D.D.; Lavoie, L.; Kamtchouing, P.; Haddad, P.S. Hydro-ethanolic extract of cashew tree (Anacardium occidentale) nut and its principal compound, anacardic acid, stimulate glucose uptake in C2C12 muscle cells. Mol. Nutr. Food Res. 2010, 54, 1753–1762. [Google Scholar] [CrossRef]
- Begum, A.U.; Venkatesh, S.; Prakash, J.; Alvala, R. Evaluation of glucose utilization capacity of bioactivity-guided fractions of Barleria prionitis Linn and Hyptis suaveolens (L.) Poit in isolated rat hemidiaphragm. Ayu 2016, 37, 145–150. [Google Scholar] [CrossRef]
- Beidokhti, M.N.; Eid, H.M.; Villavicencio, M.L.S.; Jäger, A.K.; Lobbens, E.S.; Rasoanaivo, P.R.; McNair, L.M.; Haddad, P.S.; Staerk, D. Evaluation of the antidiabetic potential of Psidium guajava L. (Myrtaceae) using assays for α-glucosidase, α-amylase, muscle glucose uptake, liver glucose production, and triglyceride accumulation in adipocytes. J. Ethnopharmacol. 2020, 257, 112877. [Google Scholar] [CrossRef]
- Wang, H.; Du, Y.-J.; Song, H.-C. α-Glucosidase and α-amylase inhibitory activities of guava leaves. Food Chem. 2010, 123, 6–13. [Google Scholar] [CrossRef]
- Griffiths, D.W. The inhibition of digestive enzymes by polyphenolic compounds. Adv. Exp. Med. Biol. 1986, 199, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-W.; Hsieh, C.-L.; Wang, H.-Y.; Chen, H.-Y. Inhibitory effects of guava (Psidium guajava L.) leaf extracts and its active compounds on the glycation process of protein. Food Chem. 2009, 113, 78–84. [Google Scholar] [CrossRef]
- Hsieh, C.-L.; Lin, Y.-C.; Yen, G.-C.; Chen, H.-Y. Preventive effects of guava (Psidium guajava L.) leaves and its active compounds against α-dicarbonyl compounds-induced blood coagulation. Food Chem. 2007, 103, 528–535. [Google Scholar] [CrossRef]
- Ramirez, G.; Zamilpa, A.; Zavala, M.; Perez, J.; Morales, D.; Tortoriello, J. Chrysoeriol and other polyphenols from Tecoma stans with lipase inhibitory activity. J. Ethnopharmacol. 2016, 185, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Castro, A.J.; Zapata-Bustos, R.; Romo-Yañez, J.; Camarillo-Ledesma, P.; Gómez-Sánchez, M.; Salazar-Olivo, L.A. The antidiabetic plants Tecoma stans (L.) Juss. ex Kunth (Bignoniaceae) and Teucrium cubense Jacq (Lamiaceae) induce the incorporation of glucose in insulin-sensitive and insulin-resistant murine and human adipocytes. J. Ethnopharmacol. 2010, 127, 1–6. [Google Scholar] [CrossRef]
- Najari Beidokhti, M.; Andersen, M.V.; Eid, H.M.; Sanchez Villavicencio, M.L.; Staerk, D.; Haddad, P.S.; Jäger, A.K. Investigation of antidiabetic potential of Phyllanthus niruri L. using assays for α-glucosidase, muscle glucose transport, liver glucose production, and adipogenesis. Biochem. Biophys. Res. Commun. 2017, 493, 869–874. [Google Scholar] [CrossRef]
- Giribabu, N.; Rao, P.V.; Kumar, K.P.; Muniandy, S.; Swapna Rekha, S.; Salleh, N. Aqueous Extract of Phyllanthus niruri Leaves Displays In Vitro Antioxidant Activity and Prevents the Elevation of Oxidative Stress in the Kidney of Streptozotocin-Induced Diabetic Male Rats. Evid. Based Complementary Altern. Med. 2014, 2014, 834815. [Google Scholar] [CrossRef]
- Jaiswal, Y.S.; Tatke, P.A.; Gabhe, S.Y.; Vaidya, A.B. Antidiabetic activity of extracts of Anacardium occidentale Linn. leaves on n-streptozotocin diabetic rats. J. Tradit. Complementary Med. 2017, 7, 421–427. [Google Scholar] [CrossRef]
- Kamtchouing, P.; Sokeng, S.D.; Moundipa, P.F.; Watcho, P.; Jatsa, H.B.; Lontsi, D. Protective role of Anacardium occidentale extract against streptozotocin-induced diabetes in rats. J. Ethnopharmacol. 1998, 62, 95–99. [Google Scholar] [CrossRef]
- Ojewole, J.A. Laboratory evaluation of the hypoglycemic effect of Anacardium occidentale Linn (Anacardiaceae) stem-bark extracts in rats. Methods Find. Exp. Clin. Pharmacol. 2003, 25, 199–204. [Google Scholar] [CrossRef]
- Alexander-Lindo, R.L.; Morrison, E.Y.; Nair, M.G. Hypoglycaemic effect of stigmast-4-en-3-one and its corresponding alcohol from the bark of Anacardium occidentale (cashew). Phytother. Res. PTR 2004, 18, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Fusco, R.; Peritore, A.F.; Cordaro, M.; D’Amico, R.; Genovese, T.; Gugliandolo, E.; Crupi, R.; Smeriglio, A.; Mandalari, G.; et al. The Antioxidant and Anti-Inflammatory Properties of Anacardium occidentale L. Cashew Nuts in a Mouse Model of Colitis. Nutrients 2020, 12, 834. [Google Scholar] [CrossRef]
- Castro, A.J.; Frederico, M.J.; Cazarolli, L.H.; Mendes, C.P.; Bretanha, L.C.; Schmidt, É.C.; Bouzon, Z.L.; de Medeiros Pinto, V.A.; da Fonte Ramos, C.; Pizzolatti, M.G. The mechanism of action of ursolic acid as insulin secretagogue and insulinomimetic is mediated by cross-talk between calcium and kinases to regulate glucose balance. Biochim. Et Biophys. Acta 2015, 1850, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.B.; Verma, A.; Mukerjee, A.; Vijayakumar, M. Anti-hyperglycemic activity of leaves extract of Hyptis suaveolens L. Poit in streptozotocin induced diabetic rats. Asian Pac. J. Trop. Med. 2011, 4, 689–693. [Google Scholar] [CrossRef]
- Kouamé, N.M.; Koffi, C.; N’Zoué, K.S.; Yao, N.A.R.; Doukouré, B.; Kamagaté, M. Comparative Antidiabetic Activity of Aqueous, Ethanol, and Methanol Leaf Extracts of Persea americana and Their Effectiveness in Type 2 Diabetic Rats. Evid. Based Complementary Altern. Med. 2019, 2019, 5984570. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.R.; Vasconcelos, C.F.; Costa-Silva, J.H.; Maranhão, C.A.; Costa, J.; Batista, T.M.; Carneiro, E.M.; Soares, L.A.; Ferreira, F.; Wanderley, A.G. Anti-diabetic activity of extract from Persea americana Mill. leaf via the activation of protein kinase B (PKB/Akt) in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2012, 141, 517–525. [Google Scholar] [CrossRef]
- Ezejiofor, A.N.; Okorie, A.; Orisakwe, O.E. Hypoglycaemic and tissue-protective effects of the aqueous extract of persea americana seeds on alloxan-induced albino rats. Malays. J. Med. Sci. 2013, 20, 31–39. [Google Scholar]
- Oboh, G.; Isaac, A.T.; Akinyemi, A.J.; Ajani, R.A. Inhibition of key enzymes linked to type 2 diabetes and sodium nitroprusside induced lipid peroxidation in rats’ pancreas by phenolic extracts of avocado pear leaves and fruit. Int. J. Biomed. Sci 2014, 10, 208–216. [Google Scholar]
- Xu, C.; Li, X.; Zeng, D.; Liu, Y.; Gao, Y.; Tsunoda, M.; Deng, S.; Xie, X.; Wang, R.; Li, L.-S.; et al. Amino Acid Profiling Study of Psidium guajava L. Leaves as an Effective Treatment for Type 2 Diabetic Rats. Evid. Based Complementary Altern. Med. 2020, 2020, 9784382. [Google Scholar] [CrossRef]
- Vinayagam, R.; Jayachandran, M.; Chung, S.S.M.; Xu, B. Guava leaf inhibits hepatic gluconeogenesis and increases glycogen synthesis via AMPK/ACC signaling pathways in streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2018, 103, 1012–1017. [Google Scholar] [CrossRef]
- Shen, S.C.; Cheng, F.C.; Wu, N.J. Effect of guava (Psidium guajava Linn.) leaf soluble solids on glucose metabolism in type 2 diabetic rats. Phytother. Res. 2008, 22, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.K.; Lee, C.H.; Lee, M.S.; Bae, E.Y.; Sohn, C.B.; Oh, H.; Kim, B.Y.; Ahn, J.S. Antidiabetic effects of extracts from Psidium guajava. J. Ethnopharmacol. 2005, 96, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Peng, B.; Wei, W.; Tian, X.; Wu, Z. Antioxidant and Anti-Diabetic Activities of Polysaccharides from Guava Leaves. Molecules 2019, 24, 1343. [Google Scholar] [CrossRef] [PubMed]
- Soman, S.; Rajamanickam, C.; Rauf, A.A.; Madambath, I. Molecular mechanisms of the antiglycative and cardioprotective activities of Psidium guajava leaves in the rat diabetic myocardium. Pharm. Biol. 2016, 54, 3078–3085. [Google Scholar] [CrossRef]
- Kuang, Q.T.; Zhao, J.J.; Ye, C.L.; Wang, J.R.; Ye, K.H.; Zhang, X.Q.; Wang, Y.; Ye, W.C. Nephro-protective effects of total triterpenoids from Psidium guajava leaves on type 2 diabetic rats. Zhong Yao Cai Zhongyaocai J. Chin. Med. Mater. 2012, 35, 94–97. [Google Scholar]
- Aguilar-Santamaría, L.; Ramírez, G.; Nicasio, P.; Alegría-Reyes, C.; Herrera-Arellano, A. Antidiabetic activities of Tecoma stans (L.) Juss. ex Kunth. J. Ethnopharmacol. 2009, 124, 284–288. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Ren, B.; Qin, W.; Wu, F.; Wang, S.; Pan, C.; Wang, L.; Zeng, B.; Ma, S.; Liang, J. Apigenin and naringenin regulate glucose and lipid metabolism, and ameliorate vascular dysfunction in type 2 diabetic rats. Eur. J. Pharmacol. 2016, 773, 13–23. [Google Scholar] [CrossRef]
- Rodriguez de Sotillo, D.V.; Hadley, M. Chlorogenic acid modifies plasma and liver concentrations of: Cholesterol, triacylglycerol, and minerals in (fa/fa) Zucker rats. J. Nutr. Biochem. 2002, 13, 717–726. [Google Scholar] [CrossRef]
- Pascoe-González, S.; Ramos-Zavala, M.G.; Buenrostro Ahued, M.A.; Hernández-González, S.O.; Cardona-Muñoz, E.G.; García-Benavides, L.; Grover-Páez, F. Administration of Herbarium Mixture (Guazuma ulmifolia/Tecoma stans) on Metabolic Profile in Type 2 Diabetes Mellitus Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Med. Food 2021, 24, 527–532. [Google Scholar] [CrossRef]
- Fernandes, N.P.; Lagishetty, C.V.; Panda, V.S.; Naik, S.R. An experimental evaluation of the antidiabetic and antilipidemic properties of a standardized Momordica charantia fruit extract. BMC Complementary Altern. Med. 2007, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Virdi, J.; Sivakami, S.; Shahani, S.; Suthar, A.C.; Banavalikar, M.M.; Biyani, M.K. Antihyperglycemic effects of three extracts from Momordica charantia. J. Ethnopharmacol. 2003, 88, 107–111. [Google Scholar] [CrossRef]
- Chaturvedi, P.; George, S.; Milinganyo, M.; Tripathi, Y.B. Effect of Momordica charantia on lipid profile and oral glucose tolerance in diabetic rats. Phytother. Res. 2004, 18, 954–956. [Google Scholar] [CrossRef] [PubMed]
- Hartajanie, L.; Fatimah-Muis, S.; Heri-Nugroho Hs, K.; Riwanto, I.; Sulchan, M. Probiotics Fermented Bitter Melon Juice as Promising Complementary Agent for Diabetes Type 2: Study on Animal Model. J. Nutr. Metab. 2020, 2020, 6369873. [Google Scholar] [CrossRef] [PubMed]
- Bhat, G.A.; Khan, H.A.; Alhomida, A.S.; Sharma, P.; Singh, R.; Paray, B.A. GLP-I secretion in healthy and diabetic Wistar rats in response to aqueous extract of Momordica charantia. BMC Complementary Altern. Med. 2018, 18, 162. [Google Scholar] [CrossRef] [PubMed]
- Saeed, F.; Sultan, M.T.; Riaz, A.; Ahmed, S.; Bigiu, N.; Amarowicz, R.; Manea, R. Bitter Melon (Momordica charantia L.) Fruit Bioactives Charantin and Vicine Potential for Diabetes Prophylaxis and Treatment. Plants 2021, 10, 730. [Google Scholar]
- Offor, U.; Edwin, C.S.N.; Ogedengbe, O.O.; Jegede, A.I.; Peter, A.I.; Onyemaechi, O.A. Renal histopathological and biochemical changes following adjuvant intervention of Momordica charantia and antiretroviral therapy in diabetic rats. Iran. J. Basic Med. Sci. 2019, 22, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Mediani, A.; Abas, F.; Maulidiani, M.; Khatib, A.; Tan, C.P.; Ismail, I.S.; Shaari, K.; Ismail, A.; Lajis, N.H. Metabolic and biochemical changes in streptozotocin induced obese-diabetic rats treated with Phyllanthus niruri extract. J. Pharm. Biomed. Anal. 2016, 128, 302–312. [Google Scholar] [CrossRef]
- Okoli, C.O.; Obidike, I.C.; Ezike, A.C.; Akah, P.A.; Salawu, O.A. Studies on the possible mechanisms of antidiabetic activity of extract of aerial parts of Phyllanthus niruri. Pharm. Biol. 2011, 49, 248–255. [Google Scholar] [CrossRef]
- Moreno-Valdespino, C.A.; Luna-Vital, D.; Camacho-Ruiz, R.M.; Mojica, L. Bioactive proteins and phytochemicals from legumes: Mechanisms of action preventing obesity and type-2 diabetes. Food Res. Int. 2020, 130, 108905. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Y.; Rasool, S.; Geetha, T.; Babu, J.R. Effects and Underlying Mechanisms of Bioactive Compounds on Type 2 Diabetes Mellitus and Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2019, 2019, 8165707. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.H.; Ngoh, G.C.; Yusoff, R. A brief review on anti diabetic plants: Global distribution, active ingredients, extraction techniques and acting mechanisms. Pharmacogn. Rev. 2012, 6, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.J.; Nicholls, S.J. Treating Dyslipidemia in Type 2 Diabetes. Cardiol. Clin. 2018, 36, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar]
- Dahibhate, L.N.; Saddhe, A.A.; Kumar, K. Mangrove Plants as a Source of Bioactive Compounds: A Review. Nat. Prod. J. 2019, 9, 86–97. [Google Scholar] [CrossRef]
- Durán, R.; Cebrián-Torrejón, G.; Nossin, E.; Gómez-Estrada, H.; Costaguta, M. Medicina popular y atención primaria de la salud (APS): 35 años de experiencia TRAMIL en el Caribe. Steviana 2021, 10, 41–47. [Google Scholar]
- Salehi, B.; Gültekin-Özgüven, M.; Kirkin, C.; Özçelik, B.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Bezerra, C.F.; da Silva, T.G.; Coutinho, H.D.M.; Amina, B.; et al. Antioxidant, Antimicrobial, and Anticancer Effects of Anacardium Plants: An Ethnopharmacological Perspective. Front. Endocrinol. 2020, 11, 295. [Google Scholar] [CrossRef]
- Ukwenya, V.O.; Adelakun, S.A.; Elekofehinti, O.O. Exploring the antidiabetic potential of compounds isolated from Anacardium occidentale using computational aproach: Ligand-based virtual screening. Silico Pharmacol. 2021, 9, 25. [Google Scholar] [CrossRef]
- Okpashi, V.E.; Bayim, B.P.; Obi-Abang, M. Comparative Effects of Some Medicinal Plants: Anacardium occidentale, Eucalyptus globulus, Psidium guajava, and Xylopia aethiopica Extracts in Alloxan-Induced Diabetic Male Wistar Albino Rats. Biochem. Res. Int. 2014, 2014, 203051. [Google Scholar] [CrossRef]
- Singh, R. Antihyperglycemic effect of ethanolic extract and fractions of anacardium occidentale L. Stem bark in streptozotocin-induced diabetic rats. J. Basic Clin. Pharm. 2009, 1, 16–19. [Google Scholar]
- Oliveira, A.S.; Nascimento, J.R.; Trovão, L.O.; Alves, P.C.S.; Maciel, M.C.G.; Silva, L.D.M.; Marques, A.A.; Santos, A.; Silva, L.A.; Nascimento, F.R.F.; et al. The anti-inflammatory activity of Anacardium occidentale L. increases the lifespan of diabetic mice with lethal sepsis. J. Ethnopharmacol. 2019, 236, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Tédong, L.; Dzeufiet, P.D.; Dimo, T.; Asongalem, E.A.; Sokeng, S.N.; Flejou, J.F.; Callard, P.; Kamtchouing, P. Acute and subchronic toxicity of Anacardium occidentale Linn (Anacardiaceae) leaves hexane extract in mice. Afr. J. Tradit. Complementary Altern. Med. AJTCAM 2006, 4, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Lima Júnior, J.P.; Franco, R.R.; Saraiva, A.L.; Moraes, I.B.; Espindola, F.S. Anacardium humile St. Hil as a novel source of antioxidant, antiglycation and α-amylase inhibitors molecules with potential for management of oxidative stress and diabetes. J. Ethnopharmacol. 2021, 268, 113667. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Sohrab, S.; Mishra, S.K. A review on the phytochemical and pharmacological properties of Hyptis suaveolens (L) Poit. Future J. Pharm. Sci. 2021, 7, 65. [Google Scholar] [CrossRef]
- Seo, D.Y.; Lee, S.R.; Heo, J.W.; No, M.H.; Rhee, B.D.; Ko, K.S.; Kwak, H.B.; Han, J. Ursolic acid in health and disease. Korean J. Physiol. Pharmacol. 2018, 22, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Nayak, P.; Kar, D.M.; Nayak, S. In Vitro α-Amylase Inhibition and Antioxidant potential of Chloroform Fraction of Hydroalcoholic Extract Obtained from Hyptis Suaveolens. J. App. Pharm. Sci. 2014, 4, 046–051. [Google Scholar] [CrossRef]
- Ogar, I.; Egbung, G.E.; Nna, V.U.; Iwara, I.A.; Itam, E. Anti-hyperglycemic potential of Hyptis verticillata jacq in streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2018, 107, 1268–1276. [Google Scholar] [CrossRef]
- Ogar, I.; Egbung, G.E.; Nna, V.U.; Atangwho, I.J.; Itam, E.H. Hyptis verticillata attenuates dyslipidaemia, oxidative stress and hepato-renal damage in streptozotocin-induced diabetic rats. Life Sci. 2019, 219, 283–293. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Alsherbiny, M.A.; Perera, S.; Low, M.; Basu, A.; Devi, O.A.; Barooah, M.S.; Li, C.G.; Papoutsis, K. The Odyssey of Bioactive Compounds in Avocado (Persea americana) and Their Health Benefits. Antioxidants 2019, 8, 426. [Google Scholar] [CrossRef]
- Salazar-López, N.J.; Domínguez-Avila, J.A.; Yahia, E.M.; Belmonte-Herrera, B.H.; Wall-Medrano, A.; Montalvo-González, E.; González-Aguilar, G.A. Avocado fruit and by-products as potential sources of bioactive compounds. Food Res. Int. 2020, 138, 109774. [Google Scholar] [CrossRef]
- Ochoa-Zarzosa, A.; Báez-Magaña, M.; Guzmán-Rodríguez, J.J.; Flores-Alvarez, L.J.; Lara-Márquez, M.; Zavala-Guerrero, B.; Salgado-Garciglia, R.; López-Gómez, R.; López-Meza, J.E. Bioactive Molecules from Native Mexican Avocado Fruit (Persea americana var. drymifolia): A Review. Plant Foods Hum. Nutr. 2021, 76, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.; Eck, P.; Chen, S.; Corpe, C.P.; Lee, J.H.; Kruhlak, M.; Levine, M. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J. 2007, 21, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Rey, D.; Fernandes, T.A.; Sulis, P.M.; Gonçalves, R.; Sepúlveda, R.M.; Silva Frederico, M.J.; Aragon, M.; Ospina, L.F.; Costa, G.M.; Silva, F. Cellular target of isoquercetin from Passiflora ligularis Juss for glucose uptake in rat soleus muscle. Chem. Biol. Interact. 2020, 330, 109198. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, P.; Garcia, P.; Quitral, V.; Vasquez, K.; Parra-Ruiz, C.; Reyes-Farias, M.; Garcia-Diaz, D.F.; Robert, P.; Encina, C.; Soto-Covasich, J. Pulp, Leaf, Peel and Seed of Avocado Fruit: A Review of Bioactive Compounds and Healthy Benefits. Food Rev. Int. 2021, 37, 619–655. [Google Scholar] [CrossRef]
- Hanada, M.; Feng, J.; Hemmings, B.A. Structure, regulation and function of PKB/AKT--a major therapeutic target. Biochim. Et Biophys. Acta 2004, 1697, 3–16. [Google Scholar] [CrossRef]
- Zdychová, J.; Komers, R. Emerging role of Akt kinase/protein kinase B signaling in pathophysiology of diabetes and its complications. Physiol. Res. 2005, 54, 1–16. [Google Scholar]
- Gondwe, M.; Kamadyaapa, D.R.; Tufts, M.A.; Chuturgoon, A.A.; Ojewole, J.A.; Musabayane, C.T. Effects of Persea americana Mill (Lauraceae) [“Avocado”] ethanolic leaf extract on blood glucose and kidney function in streptozotocin-induced diabetic rats and on kidney cell lines of the proximal (LLCPK1) and distal tubules (MDBK). Methods Find. Exp. Clin. Pharmacol. 2008, 30, 25–35. [Google Scholar] [CrossRef]
- Sultan, K.; Zakir, M.; Khan, H.; Khan, I.U.; Ayaz, S.; Khan, I.; Khan, J.; Khan, M.A. Antihyperglycemic effect of Persea duthieion blood glucose levels and body weight in alloxan induced diabetic rabbits. Pak. J. Pharm. Sci. 2016, 29, 837–842. [Google Scholar]
- Spínola, V.; Castilho, P.C. Assessing the In Vitro Inhibitory Effects on Key Enzymes Linked to Type-2 Diabetes and Obesity and Protein Glycation by Phenolic Compounds of Lauraceae Plant Species Endemic to the Laurisilva Forest. Molecules 2021, 26, 2023. [Google Scholar] [CrossRef]
- Correa, M.G.; Couto, J.S.; Teodoro, A.J. Anticancer Properties of Psidium guajava—A Mini-Review. Asian Pac. J. Cancer Prev. 2016, 17, 4199–4204. [Google Scholar]
- Hirudkar, J.R.; Parmar, K.M.; Prasad, R.S.; Sinha, S.K.; Jogi, M.S.; Itankar, P.R.; Prasad, S.K. Quercetin a major biomarker of Psidium guajava L. inhibits SepA protease activity of Shigella flexneri in treatment of infectious diarrhoea. Microb. Pathog. 2020, 138, 103807. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Aldesouki, H.M.; Badria, F.A. Effect of phenolic compounds from the leaves of Psidium guajava on the activity of three metabolism-related enzymes. Biotechnol. Appl. Biochem. 2021, 68, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.C.; Shen, S.C.; Wu, J.S. Effect of guava (Psidium guajava L.) leaf extract on glucose uptake in rat hepatocytes. J. Food Sci. 2009, 74, H132–H138. [Google Scholar] [CrossRef] [PubMed]
- Eidenberger, T.; Selg, M.; Krennhuber, K. Inhibition of dipeptidyl peptidase activity by flavonol glycosides of guava (Psidium guajava L.): A key to the beneficial effects of guava in type II diabetes mellitus. Fitoterapia 2013, 89, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Combs, A.P. Recent advances in the discovery of competitive protein tyrosine phosphatase 1B inhibitors for the treatment of diabetes, obesity, and cancer. J. Med. Chem. 2010, 53, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Bakr, R.O.; Fayed, M.A.A.; Salem, M.A.; Hussein, A.S. Tecoma stans: Alkaloid Profile and Antimicrobial Activity. J. Pharm. Bioallied Sci. 2019, 11, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Marzouk, M.; Gamal-Eldeen, A.; Mohamed, M.; El-Sayed, M. Anti-proliferative and antioxidant constituents from Tecoma stans. Zeitschrift fur Naturforschung C J. Biosci. 2006, 61, 783–791. [Google Scholar]
- Anand, M.; Basavaraju, R. A review on phytochemistry and pharmacological uses of Tecoma stans (L.) Juss. ex Kunth. J. Ethnopharmacol. 2021, 265, 113270. [Google Scholar] [CrossRef]
- Nickavar, B.; Abolhasani, L. Bioactivity-Guided Separation of an α-Amylase Inhibitor Flavonoid from Salvia virgata. Iran. J. Pharm. Res. 2013, 12, 57–61. [Google Scholar]
- Constantino, L.; Raimondi, L.; Pirisino, R.; Brunetti, T.; Pessotto, P.; Giannessi, F.; Lins, A.P.; Barlocco, D.; Antolini, L.; El-Abady, S.A. Isolation and Pharmacological Activities of the Tecoma Stans Alkaloids. Farmaco 2003, 58, 781–785. [Google Scholar] [CrossRef]
- Hammouda, Y.; Rashid, A.K.; Amer, M.S. hypoglycaemic properties of tecomine and tecostanine. J. Pharm. Pharmacol. 1964, 16, 833–834. [Google Scholar] [CrossRef] [PubMed]
- Larbie, C.; Owusu Nyarkoh, C.; Owusu Adjei, C. Phytochemical and Safety Evaluation of Hydroethanolic Leaf Extract of Tecoma stans (L.) Juss. ex Kunth. Evid. Based Complementary Altern. Med. 2019, 2019, 7417624. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Kim, E.K.; Choi, Y.J.; Tang, Y.; Moon, S.H. The Role of Momordica charantia in Resisting Obesity. Int. J. Environ. Res. Public Health 2019, 16, 3251. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.W.; Yung, M.M.; Chan, Y.S.; Xuan, Y.; Yang, H.; Xu, D.; Zhan, J.B.; Chan, K.K.; Ng, T.B.; Ngan, H.Y. MAP30 protein from Momordica charantia is therapeutic and has synergic activity with cisplatin against ovarian cancer in vivo by altering metabolism and inducing ferroptosis. Pharmacol. Res. 2020, 161, 105157. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Wong, W.F.; Dong, J.; Cheng, K.-K. Momordica charantia Suppresses Inflammation and Glycolysis in Lipopolysaccharide-Activated RAW264.7 Macrophages. Molecules 2020, 25, 3783. [Google Scholar] [CrossRef] [PubMed]
- Raman, A.; Lau, C. Anti-diabetic properties and phytochemistry of Momordica charantia L. (Cucurbitaceae). Phytomedicine Int. J. Phytother. Phytopharm. 1996, 2, 349–362. [Google Scholar] [CrossRef]
- Harinantenaina, L.; Tanaka, M.; Takaoka, S.; Oda, M.; Mogami, O.; Uchida, M.; Asakawa, Y. Momordica charantia constituents and antidiabetic screening of the isolated major compounds. Chem. Pharm. Bull. 2006, 54, 1017–1021. [Google Scholar] [CrossRef]
- Tran, K.L.; Park, Y.I.; Pandya, S.; Muliyil, N.J.; Jensen, B.D.; Huynh, K.; Nguyen, Q.T. Overview of Glucagon-Like Peptide-1 Receptor Agonists for the Treatment of Patients with Type 2 Diabetes. Am. Health Drug Benefits 2017, 10, 178–188. [Google Scholar]
- Ma, C.; Yu, H.; Xiao, Y.; Wang, H. Momordica charantia extracts ameliorate insulin resistance by regulating the expression of SOCS-3 and JNK in type 2 diabetes mellitus rats. Pharm. Biol. 2017, 55, 2170–2177. [Google Scholar] [CrossRef]
- Torisu, T.; Sato, N.; Yoshiga, D.; Kobayashi, T.; Yoshioka, T.; Mori, H.; Iida, M.; Yoshimura, A. The dual function of hepatic SOCS3 in insulin resistance in vivo. Genes Cells Devoted Mol. Cell. Mech. 2007, 12, 143–154. [Google Scholar] [CrossRef]
- Li, H.; Yu, X. Emerging role of JNK in insulin resistance. Curr. Diabetes Rev. 2013, 9, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Kaur, B.; Sirhindi, G. Phytochemistry and Pharmacology of Phyllanthus niruri L.: A Review. Phytother. Res. 2017, 31, 980–1004. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Parmar, J.; Verma, P.; Sharma, P.; Goyal, P.K. Anti-tumor activity of Phyllanthus niruri (a medicinal plant) on chemical-induced skin carcinogenesis in mice. Asian Pac. J. Cancer Prev. 2009, 10, 1089–1094. [Google Scholar] [PubMed]
- Lee, S.H.; Jaganath, I.B.; Wang, S.M.; Sekaran, S.D. Antimetastatic effects of Phyllanthus on human lung (A549) and breast (MCF-7) cancer cell lines. PLoS ONE 2011, 6, e20994. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyah, V.; Chan, K.L. Mechanisms of antihyperuricemic effect of Phyllanthus niruri and its lignan constituents. J. Ethnopharmacol. 2009, 124, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Joshi, T.; Joshi, T.; Chandra, S.; Tamta, S. In silico screening of potential antidiabetic phytochemicals from Phyllanthus emblica against therapeutic targets of type 2 diabetes. J. Ethnopharmacol. 2020, 248, 112268. [Google Scholar] [CrossRef] [PubMed]
- Srividya, N.; Periwal, S. Diuretic, hypotensive and hypoglycaemic effect of Phyllanthus amarus. Indian J. Exp. Biol. 1995, 33, 861–864. [Google Scholar]
- Tan, S.P.; Tan, E.N.; Lim, Q.Y.; Nafiah, M.A. Phyllanthus acidus (L.) Skeels: A review of its traditional uses, phytochemistry, and pharmacological properties. J. Ethnopharmacol. 2020, 253, 112610. [Google Scholar] [CrossRef]







| Plant | Origin | Part Used | Extract | Activity | References |
|---|---|---|---|---|---|
| Anacardium occidentale L. (Anacardiaceae) | Indigenous | Cashew seed Cashew nuts | Hydroethanolic | Hypolipidemic | [28,29] |
| Hyptis suaveolens (L.) Poit. (Lamiaceae) | Indigenous | Aerial parts | Ethanolic Aqueous-ethanolic Petroleum ether Chloroform fraction | Hypoglycemic | [30] |
| P. guajava L. (Myrtaceae) | Indigenous | Leaves Bark | Aqueous | Hypoglycemic α-amylase inhibitor α-glucosidase inhibitor Hypolipidemic Antiglycation | [31,32,33,34,35] |
| Tecoma stans (L.) Juss. ex Kunth (Bignoniaceae) | Indigenous | Leaves | Hydroalcoholic Aqueous | Antihyperlipidemic | [36,37] |
| Phyllanthus niruri L. (Phyllanthaceae) | Exotic | Leaves | Ethanolic Aqueous | Antihyperlipidemic Antihyperglycemic α-glucosidase inhibitor Antioxidant | [38,39] |
| Plant | Origin | Part Used | Extract | Activity | References |
|---|---|---|---|---|---|
| Anacardium occidentale L. (Anacardiaceae) | Indigenous | Leaves Bark Cashew nuts | Hexane Aqueous Methanolic Ethanolic | Hypoglycemic Hypolipidemic Anti-inflammatory antioxidant | [39,40,41,42,43,44] |
| Hyptissuaveolens (L.) Poit. (Lamiaceae) | Indigenous | Leaves | Ethanolic Aqueous-ethanolic | Insulin-mimetism Insulin secretagogue Hypoglycemic hypolipidemic | [45,46] |
| Persea americana Mill (Lauraceae) | Indigenous | Leaves Fruit Seeds | Hydroalcoholic Phenolic Aqueous Ethanolic Methanolic | Hypoglycemic α-amylase inhibitor α-glucosidase inhibitor DM-associated complication protection (nephroprotection and hepatoprotection) Hypolipidemic Pancreatic protector | [47,48,49,50] |
| P. guajava L. (Myrtaceae) | Indigenous | Leaves | Aqueous Ethanolic | Hypoglycemic Hypolipidemic Antiglycation DM-associated complication protection (cardioprotection and nephroprotection) Antioxidant | [51,52,53,54,55,56,57] |
| Tecoma stans (L.) Juss. ex Kunth (Bignoniaceae) | Indigenous | Leaves | Aqueous | Antihyperlipidemic Antihyperglycemic antioxidant α-glucosidase inhibitor Antiglycation | [58,59,60,61,62] |
| Momordica charantia L. (Cucurbitaceae) | Exotic | Fruit | Aqueous Methanolic Ethanolic | Antihyperglycemic Antihyperlipidemic Antioxidant DM-associated complication protection (nephroprotection) | [63,64,65,66,67,68,69] |
| Phyllanthus niruri L. (Phyllanthaceae) | Exotic | Aerial parts Leaves | Ethanolic Aqueous | Antihyperlipidemic Antihyperglycemic Antiglycation DM-associated complication protection (nephroprotection) Antioxidant | [39,70,71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méril-Mamert, V.; Ponce-Mora, A.; Sylvestre, M.; Lawrence, G.; Bejarano, E.; Cebrián-Torrejón, G. Antidiabetic Potential of Plants from the Caribbean Basin. Plants 2022, 11, 1360. https://doi.org/10.3390/plants11101360
Méril-Mamert V, Ponce-Mora A, Sylvestre M, Lawrence G, Bejarano E, Cebrián-Torrejón G. Antidiabetic Potential of Plants from the Caribbean Basin. Plants. 2022; 11(10):1360. https://doi.org/10.3390/plants11101360
Chicago/Turabian StyleMéril-Mamert, Vanessa, Alejandro Ponce-Mora, Muriel Sylvestre, Genica Lawrence, Eloy Bejarano, and Gerardo Cebrián-Torrejón. 2022. "Antidiabetic Potential of Plants from the Caribbean Basin" Plants 11, no. 10: 1360. https://doi.org/10.3390/plants11101360
APA StyleMéril-Mamert, V., Ponce-Mora, A., Sylvestre, M., Lawrence, G., Bejarano, E., & Cebrián-Torrejón, G. (2022). Antidiabetic Potential of Plants from the Caribbean Basin. Plants, 11(10), 1360. https://doi.org/10.3390/plants11101360








