Breeding of Vegetable Cowpea for Nutrition and Climate Resilience in Sub-Saharan Africa: Progress, Opportunities, and Challenges
Abstract
:1. Introduction
2. Use of Cowpea as a Leafy Vegetable and Grain Crop
3. Constraints to Cowpea Production
3.1. Biotic Stresses
3.1.1. Bacterial Diseases
3.1.2. Viral Diseases
3.1.3. Root-Knot Nematode
3.1.4. Parasitic Weeds
3.1.5. Insect Pests
3.2. Abiotic Stress
3.2.1. Drought
3.2.2. Salinity
3.2.3. Heat Stress
3.2.4. Low Soil Fertility
4. Breeding Opportunities and Nutritional Profiles of Cowpea Leaves
4.1. Protein Quality and Quantity
4.2. Minerals
4.3. Vitamins
4.4. Fatty Acids/Lipids
4.5. Carbohydrates
4.6. Pharmacological Benefits of Cowpea
5. Cowpea as a Climate-Resilient Crop
6. Cowpea Genetics and Breeding Progress
6.1. Conventional Breeding
6.2. Molecular Breeding
6.2.1. Marker–Trait Associations
6.2.2. Transcriptomics
6.3. Genetic Resource Management
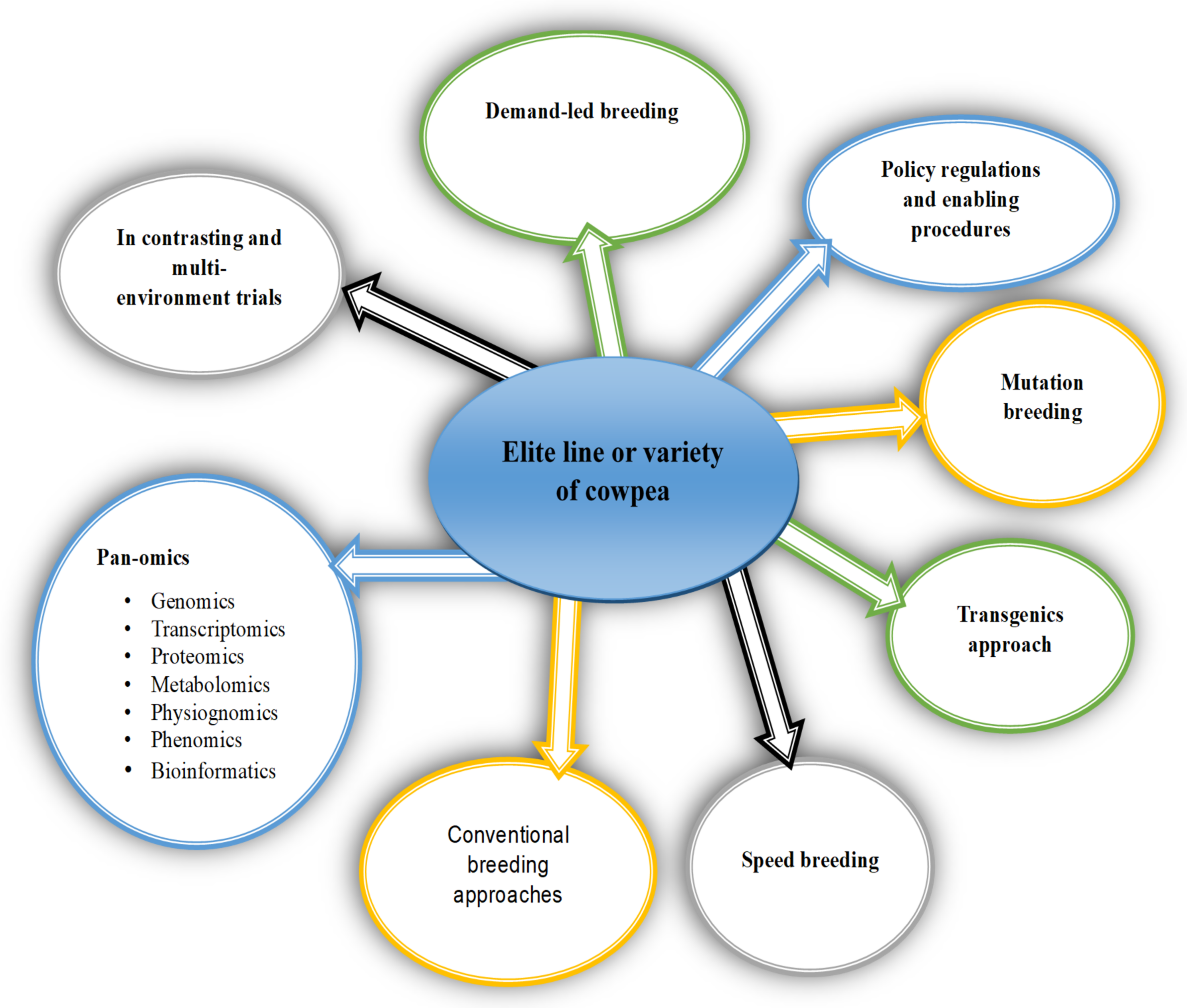
7. Breeding Strategies and Research Perspectives for Cowpea
7.1. Genomic Selection
7.2. Speed Breeding
7.3. Mutation Breeding
7.4. Genome Editing
8. Policy and Regulatory Framework
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gonçalves, A.; Goufo, P.; Barros, A.; Domínguez-Perles, R.; Trindade, H.; Rosa, E.A.; Ferreira, L.; Rodrigues, M. Cowpea [Vigna unguiculata (L.) Walp] a renewed multipurpose crop for a more sustainable agri-food system: Nutritional advantages and constraints. J. Sci. Food Agric. 2016, 96, 2941–2951. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.; Lino-Neto, T.; Rosa, E.; Carnide, V. Cowpea: A legume crop for a challenging environment. J. Sci. Food Agric. 2017, 97, 4273–4284. [Google Scholar] [CrossRef] [PubMed]
- Halilou, O.; Hamidou, F.; Taya, B.K.; Mahamane, S.; Vadez, V. Water use, transpiration efficiency and yield in cowpea (Vigna unguiculata) and peanut (Arachis hypogaea) across water regimes. Crop Pasture Sci. 2015, 66, 715–728. [Google Scholar] [CrossRef] [Green Version]
- Timko, B.B.; Singh, M.P. Cowpea, a multifunctional legume. In Genomics of Tropical Crop Plants, 1st ed.; Paul, M., Moore, H., Ray, M., Eds.; Springer Science+Business Media, LLC: New York, NY, USA, 2008; pp. 227–258. [Google Scholar]
- Sikora, R.A.; Claudius-Cole, B.; Coyne, E.J. Nematode parasites of food legumes. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture, 3rd ed.; Sikora, R.A., Coyne, D., Hallmann, J., Timper, P., Eds.; CABI Publishing: Wallingford, UK, 2018; pp. 290–345. [Google Scholar]
- Jayathilake, C.; Visvanathan, R.; Deen, A.; Bangamuwage, R.; Jayawardana, B.C.; Nammi, S.; Liyanage, R. Cowpea: An overview on its nutritional facts and health benefits Running title: Nutritional and health properties of cowpea. J. Sci. Food Agric. 2018, 98, 4793–4806. [Google Scholar] [CrossRef] [PubMed]
- Deo-Donne, P.L.; Annan, S.T.; Adarkwah, F.; Pady, F.; Anyamesem-Poku, A. Reaction of Cowpea genotypes to bacterial blight (Xanthomonas campestris pv. Vignicola) disease in Ghana. World J. Agric. Res. 2018, 6, 105–112. [Google Scholar] [CrossRef]
- De La Peña, T.C.; Pueyo, J.J. Legumes in the reclamation of marginal soils, from cultivar and inoculant selection to transgenic approaches. Agron. Sustain. Dev. 2012, 32, 65–91. [Google Scholar] [CrossRef] [Green Version]
- De Ron, A.M. Grain legumes. In Handbook of Plant Breeding, 10th ed.; Prohens-Tomás, J., Nuez, F., Carena, M.J., Eds.; Springer Science+Business Media, LLC: New York, NY, USA, 2015; pp. 1–434. [Google Scholar]
- Gerrano, A.S.; van Rensburg, W.S.J.; Adebola, P.O. Nutritional composition of immature pods in selected Cowpea [Vigna unguiculata (L.) Walp.] genotypes in South Africa. Aust. J. Crop Sci. 2017, 11, 134–141. [Google Scholar] [CrossRef]
- UNICEF/WHO/World Bank. Levels and Trends in Child Malnutrition UNICEF/WHO/World Bank Group Joint Child Malnutrition Estimates Key Findings of the 2021 Edition. 2021. Available online: https://www.who.int/publications/i/item/9789240025257 (accessed on 21 February 2022).
- Global Panel on Agriculture Food Systems for Nutrition. Future Food Systems: For People, Our Planet, and Prosperity; ANH Academy: London, UK, 2020. [Google Scholar]
- May, J.; Witten, C.; Lori, L. Challenges and Opportunities for Water, Sanitation, Hygiene (WASH) and Infant Nutrition in South Africa, 1st ed.; University of Cape Town: Cape Town, South Africa, 2020. [Google Scholar]
- National Department of Health (NDoH); Statistics South Africa (Stats SA); South African Medical Research Council (SAMRC); ICF. South Africa Demographic and Health Survey 2016; NDoH; Stats SA; SAMRC: Pretoria, South Africa; ICF: Rockville, ML, USA, 2019; pp. 1–627. [Google Scholar]
- Statistics South Africa. Poverty Trends in South Africa; An Exammination of Absolute Poverty between 2006 and 2015; Statistics South Africa: Pretoria, South Africa, 2017; p. 54. [Google Scholar]
- Thow, A.M.; Greenberg, S.; Hara, M.; Friel, S.; Sanders, D. Improving policy coherence for food security and nutrition in South Africa: A qualitative policy analysis. Food Secur. 2018, 10, 1105–1130. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, S.B.; Dzidzienyo, D.K.; Umar, M.L.; Ishiyaku, M.F.; Tongoona, P.B.; Gracen, V. Appraisal of cowpea cropping systems and farmers’ perceptions of production constraints and preferences in the dry savannah areas of Nigeria. CABI Agric. Biosci. 2021, 2, 25. [Google Scholar] [CrossRef]
- Nedumaran, S.; Abinaya, P.; Jyosthnaa, P.; Shraavya, B.; Rao, P.; Bantilan, C. Grain Legumes Production, Consumption and Trade Trends in Developing Countries; Working Paper Series 60; ICRISAT: Patancheru, India, 2015; pp. 1–64. [Google Scholar]
- FAOSTAT—Food and Agriculture Organization of the United Nations. Statistical Databases; FAOSTAT: Rome, Italy, 2022. [Google Scholar]
- Kebede, E.; Bekeko, Z. Expounding the production and importance of cowpea [Vigna unguiculata (L.) Walp.] in Ethiopia. Cogent Food Agric. 2020, 6, 1769805. [Google Scholar] [CrossRef]
- Boukar, O.; Belko, N.; Chamarthi, S.; Togola, A.; Batieno, J.; Owusu, E.; Haruna, M.; Diallo, S.; Umar, L.M.; Olufajo, O.; et al. Cowpea (Vigna unguiculata): Genetics, genomics and breeding. Plant Breed. 2019, 138, 415–424. [Google Scholar] [CrossRef] [Green Version]
- Enyiukwu, D.; Amadioha, A.; Ononuju, C. Nutritional Significance of cowpea leaves for human consumption. Greener Trends Food Sci. Nutr. 2018, 1, 1–10. [Google Scholar] [CrossRef]
- Kukal, M.S.; Irmak, S. Climate driven crop yield and yield variability and climate change impacts on the U.S. great plains agricultural production. Sci. Rep. 2018, 8, 3450. [Google Scholar] [CrossRef] [Green Version]
- FAO. Climate Change and Food Security: Risks and Responses; FAO: Rome, Italy, 2015. [Google Scholar]
- Ganiyu, S.A.; Popoola, A.R.; Owolade, O.F.; Fatona, K.A. Control of common bacterial blight disease of cowpea (Vigna unguiculata [L.] Walp) with certain plant extracts in Abeokuta, Nigeria. J. Crop Improv. 2017, 31, 280–288. [Google Scholar] [CrossRef]
- Agbicodo, E.M.; Fatokun, C.A.; Bandyopadhyay, R.; Wydra, K.; Diop, N.N.; Muchero, W.; Ehlers, J.D.; Roberts, P.A.; Close, T.J.; Visser, R.G.F.; et al. Identification of markers associated with bacterial blight resistance loci in cowpea [Vigna unguiculata (L.) Walp.]. Euphytica 2010, 75, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Adegbite, A.A.; Amusa, N.A. The major economic field diseases of cowpea in the humid agro-ecologies of south-western Nigeria. Arch. Phytopathol. Plant Prot. 2010, 43, 1608–1618. [Google Scholar] [CrossRef]
- Taiwo, M.A.; Akinjogunla, O.J. Cowpea viruses: Quantitative and qualitative effects of single and mixed viral infections. Afr. J. Biotechnol. 2006, 5, 1749–1756. [Google Scholar]
- Salem, N.M.; Ehlers, J.D.; Roberts, P.A.; Ng, J.C.K. Biological and molecular diagnosis of seedborne viruses in cowpea germplasm of geographically diverse sub-Saharan origins. Plant Pathol. 2010, 59, 773–784. [Google Scholar] [CrossRef]
- Ogunsola, K.E.; Ilori, C.; Fatokun, C.A.; Boukar, O.; Ogunsanya, P.; Kumar, P.L. Disease incidence and severity in cowpea lines evaluated for resistance to single and multiple infections of endemic viruses in Nigeria. J. Crop Improv. 2021, 35, 427–452. [Google Scholar] [CrossRef]
- Obopile, M.; Ositile, B. Life table and population parameters of cowpea aphid, Aphis craccivora Koch (Homoptera: Aphididae) on five cowpea [Vigna unguiculata (L.) Walp.] varieties. J. Pest Sci. 2010, 83, 9–14. [Google Scholar] [CrossRef]
- Oliveira, J.T.; Andrade, N.C.; Martins-Miranda, A.S.; Soares, A.A.; Gondim, D.M.; Araújo-Filho, J.H.; Freire-Filho, F.R.; Vasconcelos, I.M. Differential expression of antioxidant enzymes and PR-proteins incompatible and incompatible interactions of cowpea (Vigna unguiculata) and the root-knot nematode Meloidogyne incognita. Plant Physiol. Biochem. 2012, 51, 145–152. [Google Scholar] [CrossRef]
- Kamara, A.Y.; Ekeleme, F.; Jibrin, J.M.; Tarawali, G.; Tofa, I. Assessment of level, extent and factors influencing Striga infestation of cereals and cowpea in a Sudan Savanna ecology of northern Nigeria. Agric. Ecosyst. Environ. 2014, 188, 111–121. [Google Scholar] [CrossRef]
- Omoigui, L.O.; Kamara, A.Y.; Alunyo, G.I.; Bello, L.L.; Oluoch, M.; Timko, M.P.; Boukar, O. Identification of new sources of resistance to Striga gesnerioides in cowpea (Vigna unguiculata) accessions. Genet. Resour. Crop Evol. 2017, 64, 901–911. [Google Scholar] [CrossRef]
- Ohanmu, E.O.; Ikhajiagbe, B. Competitive effect of prominent weeds on cowpea cultivar in a typical ultisol. Am. J. Plant Physiol. 2018, 14, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Razakou, A.; Ibrahim, M.; Adamou, I.; Karimou, A.; Mahamane, S.; Adam, T.; Moumouni, S. Evaluation of cowpea lines on natural infested field of Striga gesnerioides in the Sahel Sudan of Southeast Niger. Agric. Sci. Res. J. 2017, 7, 35–41. [Google Scholar]
- Gupta, K.C.; Gupta, A.K.; Saxena, R. Weed management in cowpea [Vigna unguiculata (L.) Wasp.] under rainfed conditions. Int. J. Agric. Sci. 2016, 12, 238–240. [Google Scholar] [CrossRef]
- Prabhu, G.; Srinivasan, R.; Kantwa, S.R.; Palsaniya, D.R.; Chaudhary, M. Weed seed bank studies in the field of fodder cowpea [Vigna unguiculata (L.)]. Int. J. Appl. Pure Sci. Agric. 2015, 1, 83–87. [Google Scholar]
- Marinov-Serafimov, P. Determination of allelopathic effect of some invasive weed species on germination and initial development of grain legume crops. Pestic. Fitomed. 2010, 25, 251–259. [Google Scholar] [CrossRef]
- Fisichellaelli, N.A.; Abella, S.R.; Peters, M.; Krist, F.J. Climate, trees, pests, and weeds: Change, uncertainty, and biotic stressors in eastern U.S. national park forests. For. Ecol. Manag. 2014, 327, 31–39. [Google Scholar] [CrossRef]
- Nisha, T.Y.; Chopra, K.; Chopra, N.K.; Yadav, M.R.; Kumar, R.; Rathore, D.K.; Soni, P.G.; Makarana, G.; Tamta, A.; Kushwah, M.; et al. Weed Management in Cowpea-A Review. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1373–1385. [Google Scholar] [CrossRef] [Green Version]
- Egho, E. Management of major field insect pests and yield of cowpea [Vigna unguiculata (L) Walp] under calendar and monitored application of synthetic chemicals in Asaba, southern Nigeria. Am. J. Sci. Ind. Res. 2011, 2, 592–602. [Google Scholar] [CrossRef]
- Adelaïde, P.O.; Batieno, B.J.; Traore, F.; Jean-Baptiste, T.; Huynh, B.; Roberts, P.A.; Close, T.; Ouédraogo, J.T. Screening of cowpea [Vigna unguiculata (L.) Walp.] lines for resistance to three Aphids (Aphis craccivora Koch) strains in Burkina Faso. Afr. J. Agric. Res. 2018, 13, 1487–1495. [Google Scholar] [CrossRef] [Green Version]
- Souleymane, A.; Aken’Ova, M.E.; Fatokun, C.A.; Alabi, O.Y. Screening for resistance to cowpea aphid (Aphis craccivora Koch) in wild and cultivated cowpea [Vigna unguiculata (L.) Walp.] accessions. Int. J. Sci. Environ. Technol. 2013, 2, 611–621. [Google Scholar] [CrossRef]
- Togola, A.; Boukar, O.; Servent, A.; Chamarthi, S.; Tamò, M.; Fatokun, C. Identification of sources of resistance in cowpea mini core accessions to Aphis craccivora Koch (Homoptera: Aphididae) and their biochemical characterization. Euphytica 2020, 216, 88. [Google Scholar] [CrossRef] [PubMed]
- Mofokeng, M.A.; Gerrano, A.S. Efforts in breeding cowpea for aphid resistance: A review. Acta Agric. Scand. Sect. B Soil Plant Sci. 2021, 71, 489–497. [Google Scholar] [CrossRef]
- Stoddard, F.L.; Nicholas, A.H.; Rubiales, D.; Thomas, J.; Villegas-Fernández, A.M. Integrated pest management in faba bean. Field Crop. Res. 2010, 115, 308–318. [Google Scholar] [CrossRef] [Green Version]
- Dugje, I.Y.; Omoigui, L.O.; Ekeleme, F.; Kamara, A.Y.; Ajeigbe, H. Farmers’ Guide to Cowpea Production in West Africa; IITA: Ibadan, Nigeria, 2009; Available online: http://www.icrisat.org/tropicallegumesII/pdfs/Cowpea.pdf (accessed on 1 January 2022).
- Anjum, S.A.; Xie, X.; Wang, L.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar] [CrossRef]
- Chand, U.; Harsh, J.; Rintu, N.; Pronob, J. Heat stress and cowpea: Genetics, breeding and modern tools for improving genetic gains. Plant Physiol. Rep. 2020, 25, 645–653. [Google Scholar] [CrossRef]
- Chaudhary, S.; Devi, P.; Bhardwaj, A.; Jha, U.C.; Sperotto, R.A.; Kean, D.; Tan, Y. Identification and characterization of contrasting genotypes/cultivars for developing heat tolerance in agricultural crops: Current status and prospects. Front. Plant Sci. 2020, 11, 587264. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Jacinthe, P.A. Global synthesis of drought effects on cereal, legume, tuber and root crops production: A review. Agric. Water Manag. 2017, 179, 18–33. [Google Scholar] [CrossRef] [Green Version]
- Fatokun, C.A.; Boukar, O.; Muranaka, S. Evaluation of cowpea (Vigna unguiculata (L.) Walp.) germplasm lines for tolerance to drought. Plant Genet. Resour. 2012, 10, 171–176. [Google Scholar] [CrossRef]
- Ghonaim, M.M.; Mohamed, H.I.; Omran, A.A.A. Evaluation of wheat (Triticum aestivum L.) salt stress tolerance using physiological parameters and retrotransposon-based markers. Genet. Resour. Crop Evol. 2021, 68, 227–242. [Google Scholar] [CrossRef]
- Jemo, M.; Sulieman, S.; Bekkaoui, F.; Olomide, O.A.K.; Hashem, A.; Abd Allah, E.F.; Alqarawi, A.A.; Tran, L.S.P. Comparative analysis of the combined effects of different water and phosphate levels on growth and biological nitrogen fixation of nine cowpea varieties. Front. Plant Sci. Plant Sci. 2017, 8, 2111. [Google Scholar] [CrossRef] [Green Version]
- Korte, F.; Coulston, F.; Parlar, P.H. Mitigation the harmful effect of salt stress on physiological, biochemical and anatomical traits by foliar spray with trehalose on wheat cultivars. Fresenius Environ. Bull. 2018, 27, 6442–6446. [Google Scholar]
- Krasilnikoff, G.; Gahoonia, T.; Nielsen, N.E. Variation in phosphorus uptake efficiency by genotypes of cowpea (Vigna unguiculata) due to differences in root and root hair length and induced rhizosphere processes. Plant Soil. 2003, 251, 83–91. [Google Scholar] [CrossRef]
- Kumar, S.; Thakur, P.; Kaushal, N.; Malik, J.A.; Gaur, P. Effect of varying high temperatures during reproductive growth on reproductive function, oxidative stress and seed yield in chickpea genotypes differing in heat sensitivity. Arch. Agron. Soil Sci. 2013, 59, 823–843. [Google Scholar] [CrossRef]
- Li, B.; Gao, K.; Ren, H.; Tang, W. Molecular mechanisms governing plant responses to high temperatures. J. Integr. Plant Biol. 2018, 60, 759–779. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Zhu, Y.; Jones, A.; Rose, R.J.; Song, Y. Heat Stress in Legume Seed Setting: Effects, Causes, and Future Prospects. Front. Plant Sci. 2019, 10, 938. [Google Scholar] [CrossRef] [Green Version]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Namakka, A.; Jibrin, D.M.; Hamma, I.L. Effect of phosphorus levels on growth and yield of cowpea (Vigna unguiculata (L.) Walp.) in Zaria, Nigeria. J. Dryl. Agric. 2017, 3, 85–93. [Google Scholar]
- Nkomo, G.V.; Sedibe, M.M.; Mofokeng, M.A. Production constraints and improvement strategies of Cowpea (Vigna unguiculata L. Walp.) genotypes for drought tolerance. Int. J. Agron. 2021, 2021, 5536417. [Google Scholar] [CrossRef]
- Nunes, C.; Moreira, R.; Pais, I.; Semedo, J.; Simões, F.; Veloso, M.M.; Scotti-Campos, P. Cowpea Physiological Responses to Terminal Drought—Comparison between Four Landraces and a Commercial Variety. Plants 2022, 11, 593. [Google Scholar] [CrossRef]
- Olajide, A.A.; Ilori, C.O. Genetic variability, performance and yield potentials of ten varieties of cowpea (Vigna unguiculata (L) Walp) under drought stress. Legume Genom. Genet. 2017, 8, 817–825. [Google Scholar] [CrossRef]
- Olaleye, O.; Olajire, F.; Robert, A.C.; Nnenna, I. Phosphorus response efficiency in cowpea genotypes. J. Agric. Sci. 2011, 4, 81–90. [Google Scholar] [CrossRef]
- Priya, M.; Siddique, K.H.M.; Dhankhar, O.P.; Prasad, P.V.; Rao, B.H.; Nair, R.M.; Nayyar, H. Molecular breeding approaches involving physiological and reproductive traits for heat tolerance in food crops. Indian J. Plant Physiol. 2018, 23, 697–720. [Google Scholar] [CrossRef]
- Ravelombola, W. A Simple and Cost-effective Approach for Salt Tolerance Evaluation in Cowpea (Vigna unguiculata) Seedlings. Hortscience 2019, 54, 1280–1287. [Google Scholar] [CrossRef] [Green Version]
- Ravelombola, W.; Shi, A.; Weng, Y.; Mou, B. Association analysis of salt tolerance in cowpea (Vigna unguiculata (L.) Walp) at germination and seedling stages. Theor. Appl. Genet. 2018, 131, 79–91. [Google Scholar] [CrossRef]
- Sehgal, A.; Sita, K.; Kumar, J.; Kumar, S.; Singh, S. Effects of drought, heat and their interaction on the growth, yield and photosynthetic function of lentil (Lens culinaris Medikus) genotypes varying in heat and drought sensitivity. Front. Plant Sci. 2017, 8, 1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Baoule, A.L.; Ahmed, H.G.; Dikko, A.U.; Aliyu, U.; Sokoto, M.B.; Alhassan, J.; Musa, M.; Haliru, B. Influence of phosphorus on the performance of cowpea (Vigna unguiculata (L) Walp.) varieties in the Sudan savanna of Nigeria. Agric. Sci. 2011, 2, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Sudharani, Y.; Mohapatra, P.P.; Pattanaik, M.; Hans, H.; Maitra, S. Effect of Phosphorus on Cowpea (Vigna unguiculata L. Walp): A review. J. Pharmacogn. Phytochem. 2020, 9, 425–427. [Google Scholar] [CrossRef]
- Verbree, D.A.; Singh, B.B.; Payne, W.A. Genetics and heritability of shoot drought tolerance in cowpea seedlings. Crop Sci. 2015, 55, 146–153. [Google Scholar] [CrossRef]
- Tharanathan, R.N.; Mahadevamma, S. Grain legumes—A boon to human nutrition. Trends Food Sci. Technol. 2003, 14, 507–518. [Google Scholar] [CrossRef]
- Adebooye, O.C.; Singh, V. Tannins, phytate, amino acid, fatty acid and mineral nutrients of whole-grain and decorticated. J. Food Qual. 2007, 30, 1101–1120. [Google Scholar] [CrossRef]
- Affrifah, N.S.; Phillips, R.D.; Saalia, F.K. Cowpeas: Nutritional profile, processing methods and products—A review. Legume Sci. 2021, 131, e131. [Google Scholar] [CrossRef]
- Mumuni, A.; Shaibu, S.S.; Mohammed, H.; Adams, M.M.; Stephen, K.A. Farmer participatory pest management evaluations and variety selection in diagnostic farmer field Fora in cowpea in Ghana. Afr. J. Agric. Res. 2016, 11, 1765–1771. [Google Scholar] [CrossRef] [Green Version]
- Kamara, A.Y.; Ewansiha, S.U.; Ajeigbe, H.A.; Okechukwu, R.; Tefera, H.; Boukar, O.; Omoigui, L.O. Improvements in grain and fodder yield of cowpea (Vigna unguiculata) varieties developed in the Sudan savannas of Nigeria over the past four decades. In Proceedings of the Fifth World Cowpea Conference, Saly, Senegal, 27 September–1 October 2010; pp. 171–188. [Google Scholar] [CrossRef]
- Omomowo, O.I.; Babalola, O.O. Constraints and prospects of improving cowpea productivity to ensure food, nutritional security and environmental sustainability. Front. Plant Sci. 2021, 12, 751713. [Google Scholar] [CrossRef]
- Gerrano, A.S.; van Rensburg, W.S.J.; Ventera, S.L.; Shargie, N.G.; Amelework, B.A.; Shimelis, H.A.; Labuschagne, M.T. Selection of cowpea genotypes based on grain mineral and total protein content. Acta Agric. Scand. Sect. B Soil Plant Sci. 2019, 69, 155–166. [Google Scholar] [CrossRef]
- Elhardallou, S.B.; Khalid, I.; Gobouri, A.; Abdel-Hafez, S. Amino acid composition of cowpea [Vigna ungiculata (L.) Walp] flour and its protein isolates. Food Nutr. Sci. 2015, 6, 790–797. [Google Scholar] [CrossRef] [Green Version]
- Petchiammal, C.; Hopper, W. Antioxidant activity of proteins from fifteen varieties of legume seeds commonly consumed in India. Int. J. Pharm. Pharm. Sci. 2014, 6, 476–479. [Google Scholar] [CrossRef]
- Iqbal, A.; Khalil, I.A.; Ateeq, N.; Khan, M.S. Nutritional quality of important food legumes. Food Chem. 2006, 97, 331–335. [Google Scholar] [CrossRef]
- OECD. Safety assessment of foods and feeds derived from transgenic crops; common bean, rice, cowpea and apple compositional considerations. In Novel Food and Feed Safety; OECD Publishing: Paris, France, 2020. [Google Scholar]
- Carvalho, A.F.U.; Sousa, N.M.; Farias, D.F.; da Rocha-Bezerra, L.C.B.; da Silva, R.M.P.; Viana, M.P.; Gouveia, S.T.; Sampaio, S.S.; de Sousa, M.B.; de Lima, G.P.G.; et al. Nutritional ranking of 30 Brazilian genotypes of cowpeas including determination of antioxidant capacity and vitamins. J. Food Compos. Anal. 2012, 26, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Khattab, R.Y.; Arntfield, S.D.; Nyachoti, C.M. Nutritional quality of legume seeds as affected by some physical treatments. LWT—Food Sci. Technol. 2009, 42, 1107–1112. [Google Scholar] [CrossRef]
- Vasconcelos, I.M.; Maia, F.M.M.; Farias, D.F.; Campello, C.C.; Carvalho, A.F.U.; Moreira, R.A.; Oliveira, J.T.A. Protein fractions, amino acid composition and antinutritional constituents of high-yielding cowpea cultivars. J. Food Compos. Anal. 2010, 23, 54–60. [Google Scholar] [CrossRef]
- Dakora, F.D.; Belane, A.K. Evaluation of protein and micronutrient levels in edible cowpea [Vigna unguiculata (L.) Walp.] leaves and seeds. Front. Sustain. Food Syst. 2019, 3, 70. [Google Scholar] [CrossRef]
- Forrest, H.N. Handbook of Nutrition and Food, 3rd ed.; Taylor and Francis: London, UK, 2014. [Google Scholar]
- Belane, A.K.; Dakora, F.D. Elevated concentrations of dietarily-important [Vigna unguiculata (L.) Walp.] genotypes: Implications for human nutrition and health. Food Nutr. Sci. 2012, 3, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Belane, A.K.; Dakora, F.D. Levels of nutritionally-important trace elements and macronutrients in edible leaves and grain of 27 nodulated cowpea [Vigna unguiculata (L.) Walp.] genotypes grown in the Upper West Region of Ghana. Food Chem. 2011, 125, 99–105. [Google Scholar] [CrossRef]
- Gerrano, A.S.; Adebola, P.O.; van Rensburg, W.S.J.; Venter, S.L. Genetic variability and heritability estimates of nutritional composition in the leaves of selected cowpea genotypes [Vigna unguiculata (L.) Walp.]. HortScience 2015, 50, 1435–1440. [Google Scholar] [CrossRef]
- Boukar, O.; Massawe, F.; Muranaka, S.; Franco, J.; Maziya-Dixon, B.; Singh, B.; Fatokun, C. Evaluation of cowpea germplasm lines for protein and mineral concentrations in grains. Plant Genet. Resour. Charact. Util. 2011, 9, 515–522. [Google Scholar] [CrossRef]
- Asare, A.T.; Agbemafle, R.; Adukpo, G.E.; Diabor, E.; Adamtey, K.A. Assessment of functional properties and nutritional composition of some cowpea (Vigna unguiculata L.) genotypes in Ghana. ARPN J. Agric. Biol. Sci. 2013, 8, 465–469. [Google Scholar]
- Devi, C.B.; Kushwaha, A.; Kumar, A. Sprouting characteristics and associated changes in nutritional composition of cowpea (Vigna unguiculata). J. Food Sci. Technol. 2015, 52, 6821–6827. [Google Scholar] [CrossRef]
- USDA. Agricultural Research Service Food Data Central: Foundation Foods; USDA: Washington, DC, USA, 2021. Available online: fdc.nal.usda.gov (accessed on 24 March 2022).
- Antova, G.A.; Stoilova, T.D.; Ivanova, M.M. Proximate and lipid composition of cowpea (Vigna unguiculata L.) cultivated in Bulgaria. J. Food Compos. Anal. 2014, 33, 146–152. [Google Scholar] [CrossRef]
- Owade, J.O.; Abong, G.; Okoth, M.; Mwang’ombe, A.W. A review of the contribution of cowpea leaves to food and nutrition security in East Africa. Food Sci. Nutr. 2020, 8, 36–47. [Google Scholar] [CrossRef] [Green Version]
- Khalid, I.I.; Elharadallou, S.B. Functional properties of cowpea [Vigna ungiculata (L.) Walp] and Lupin (Lupinus termis) Flour and Protein Isolates. J. Nutr. Food Sci. 2013, 3, 234. [Google Scholar] [CrossRef] [Green Version]
- Chikwendu, J.N.; Igbatim, A.C.; Obizoba, I.C. Chemical composition of processed cowpea tender leaves and husks. Int. J. Sci. Res. Publ. 2014, 4, 1–5. [Google Scholar]
- Baptista, A.; Pinho, O.; Pinto, E.; Casal, S.; Mota, C.; Ferreira, L.V.O. Characterization of protein and fat composition of seeds from common beans (Phaseolus vulgaris L.), cowpea [Vigna unguiculata (L.) Walp] and bambara groundnuts [Vigna subterranea (L.) Verdc] from Mozambique. J. Food Meas. Charact. 2017, 11, 442–450. [Google Scholar] [CrossRef]
- Kapravelou, G.; Martínez, R.; Andrade, A.M.; Chaves, C.L.; López-Jurado, M.; Aranda, P.; Arrebola, F.; Cañizares, F.J.; Galisteo, M.; Porres, J.M. Improvement of the antioxidant and hypolipidaemic effects of cowpea flours (Vigna unguiculata) by fermentation: Results of in vitro and in vivo experiments. J. Sci. Food Agric. 2015, 95, 1207–1216. [Google Scholar] [CrossRef]
- Bello, S.K.; Yusuf, A.A.; Cargele, M. Performance of cowpea as influenced by native strain of rhizobia, lime and phosphorus in Samaru, Nigeria. Symbiosis 2018, 75, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Chang, S.K.C. Comparative study on antiproliferation properties and cellular antioxidant activities of commonly consumed food legumes against nine human cancer cell lines. Food Chem. 2012, 134, 1287–1296. [Google Scholar] [CrossRef]
- Xiong, S.; Yao, X.; Li, A. Antioxidant properties of peptide from cowpea seed. Int. J. Food Prop. 2013, 16, 1245–1256. [Google Scholar] [CrossRef]
- Santos, C.A.F.; Boiteux, L.S. Breeding biofortifed cowpea lines for semi-arid tropical areas by combining higher seed protein and mineral levels. Genet. Mol. Res. 2013, 12, 6782–6789. [Google Scholar] [CrossRef]
- Duranti, M. Grain legume proteins and nutraceutical properties. Fitoterapia 2006, 77, 67–82. [Google Scholar] [CrossRef]
- Uruakp, F. Influence of cowpea (Vigna unguiculata) peptides on insulin resistance. J. Nutr. Health Food Sci. 2015, 3, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Apea-Bah, F.B.; Minnaar, A.; Bester, M.J.; Duodu, K.G. Does a sorghum-cowpea composite porridge hold promise for contributing to alleviating oxidative stress? Food Chem. 2014, 157, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Ojwang, L.O.; Banerjee, N.; Noratto, G.D.; Angel-Morales, G.; Hachibamba, T.; Awika, J.M.; Mertens-Talcott, S.U. Polyphenolic extracts from cowpea (Vigna unguiculata) protect colonic myofibroblasts (CCD18Co cells) from lipopolysaccharide (LPS)-induced inflammation-modulation of microRNA 126. Food Funct. 2015, 6, 146–154. [Google Scholar] [CrossRef]
- Frota, K.M.G.; Mendonça, S.; Saldiva, P.H.N.; Cruz, R.J.; Arêas, J.A.G. Cholesterol-lowering properties of whole cowpea seed and its protein isolate in hamsters. J. Food Sci. 2008, 73, 235–240. [Google Scholar] [CrossRef] [Green Version]
- Sinha, R.; Kawatra, A. Effect of processing on phytic acid and polyphenol contents of cowpeas [Vigna unguiculata (L) Walp]. Plant Foods Hum. Nutr. 2003, 58, 1–8. [Google Scholar] [CrossRef]
- Feulner, G. Global Challenges: Climate Change. Glob. Chall. 2017, 1, 5–6. [Google Scholar] [CrossRef]
- Vermeulen, S.J.; Campbell, B.M.; Ingram, J.S.I. Climate change and food systems. Annu. Rev. Environ. Resour. 2012, 37, 195–222. [Google Scholar] [CrossRef] [Green Version]
- Schmidhuber, J.; Tubiello, F.N. Global food security under climate change. Proc. Natl. Acad. Sci. USA 2007, 104, 19703–19708. [Google Scholar] [CrossRef] [Green Version]
- Kumar, B.; Bhalothia, P. Orphan crops for future food security. J. Biosci. 2020, 45, 131. [Google Scholar] [CrossRef]
- Tadele, Z. Orphan crops: Their importance and the urgency of improvement. Planta 2019, 250, 677–694. [Google Scholar] [CrossRef] [Green Version]
- Talabi, A.O.; Vikram, P.; Thushar, S.; Rahman, H.; Ahmadzai, H.; Nhamo, N.; Shahid, M.; Singh, R.K. Orphan Crops: A best fit for dietary enrichment and diversification in highly deteriorated marginal environments. Front. Plant Sci. 2022, 13, 839704. [Google Scholar] [CrossRef] [PubMed]
- Mabhaudhi, T.; Chimonyo, V.G.P.; Hlahla, S.; Massawe, F.; Mayes, S.; Nhamo, L.; Modi, T.A. Prospects of orphan crops in climate change. Planta 2019, 250, 695–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. Future Smart Food-Rediscovering Hidden Treasures of Neglected and Underutilized Species for Zero Hunger in Asia; FAO: Rome, Italy, 2018. [Google Scholar]
- Masipa, T.S. The impact of climate change on food security in South Africa: Current realities and challenges ahead. Jàmbá—J. Disaster Risk Stud. 2017, 9, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egbadzor, K.F.; Danquah, E.Y.; Ofori, K.; Yeboah, M.; Offei, S.K. Diversity in 118 Cowpea [Vigna unguiculate (L.) Walp] Accessions Assessed with 16 Morphological Traits. Int. J. Plant Breed. Genet. 2014, 8, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Amatriaín, M.; Mirebrahim, H.; Xu, P.; Wanamaker, S.I.; Luo, M.; Alhakami, H.; Alpert, M.; Atokple, I.; Batieno, B.J.; Boukar, O.; et al. Genome resources for climate-resilient cowpea, an essential crop for food security. Plant J. 2017, 89, 1042–1054. [Google Scholar] [CrossRef] [Green Version]
- Horn, L.N.; Shimelis, H. Production constraints and breeding approaches for cowpea improvement for drought prone agro-ecologies in Sub-Saharan Africa. Ann. Agric. Sci. 2020, 65, 83–91. [Google Scholar] [CrossRef]
- Singh, A.; Singh, Y.V.; Sharma, A.; Visen, A.; Singh, M.K.; Singh, S. Genetic analysis of quantitative traits in cowpea [Vigna unguiculata (L.) Walp.] using six parameter genetic model. Legume Res. 2016, 39, 502–509. [Google Scholar] [CrossRef] [Green Version]
- Hall, A.E. Phenotyping cowpeas for adaptation to drought. Front. Physiol. 2012, 3, 155. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, R.E.; Debernardi, J.M.; Palatnik, J.F. Morphogenesis of simple leaves: Regulation of leaf size and shape. Wiley Interdiscip. Rev. Dev. Biol. 2014, 3, 41–57. [Google Scholar] [CrossRef]
- Fritz, M.A.; Rosa, S.; Sicard, A. Mechanisms underlying the environmentally induced plasticity of leaf morphology. Front. Genet. 2018, 478, 478. [Google Scholar] [CrossRef]
- Scoffoni, C.; Rawls, M.; Mckown, A.; Cochard, H.; Sack, L. Decline of leaf hydraulic conductance with dehydration: Relationship to leaf size and venation architecture. Plant Physiol. 2011, 156, 832–843. [Google Scholar] [CrossRef] [Green Version]
- Chitwood, D.H.; Sinha, N.R. Review Evolutionary and Environmental Forces Sculpting Leaf Development. Curr. Biol. 2016, 26, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Okonya, J.; Maass, B. Protein and iron composition of cowpea leaves: An evaluation of six cowpea varieties grown in Eastern Africa. Afr. J. Food Agric. Nutr. Dev. 2014, 14, 9329–9340. [Google Scholar] [CrossRef]
- El-Shaieny, A.A.H.; Abdel-Ati, Y.Y.; El-Damarany, A.M.; Rashwan, A.M. Stability analysis of components characters in cowpea [Vigna unguiculata (L.) Walp]. J. Hortic. For. 2015, 7, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Sousa, M.B.E.; Damasceno-Silva, K.J.; Rocha, M.D.M.; De Menezes, J.Â.N., Jr.; Lima, L.R.L. Genotype by environment interaction in cowpea lines using GGE biplot method. Rev. Caatinga 2018, 31, 64–71. [Google Scholar] [CrossRef]
- Mekonnen, T.W.; Mekbib, F.; Amsalu, B.; Gedil, M.; Labuschagne, M. Genotype by environment interaction and grain yield stability of drought tolerant cowpea landraces in Ethiopia. Euphytica 2022, 218, 57. [Google Scholar] [CrossRef]
- Matova, P.M.; Gasura, E. Yield and stability of new cowpea varieties in Zimbabwe. Afr. Crop Sci. J. 2018, 26, 277–289. [Google Scholar] [CrossRef] [Green Version]
- Gerrano, A.S.; van Rensburg, W.S.J.; Mathew, I.; Shayanowako, A.I.T.; Bairu, M.W.; Venter, S.L.; Swart, W.; Mofokeng, A.; Mellem, J.; Labuschagne, M. Genotype and genotype × environment interaction effects on the grain yield performance of cowpea genotypes in dryland farming system in South Africa. Euphytica 2020, 216, 80. [Google Scholar] [CrossRef]
- Mbuma, N.W.; Gerrano, A.S.; Lebaka, N.; Mofokeng, A.; Labuschagne, M. The evaluation of a southern African cowpea germplasm collection for seed yield and yield components. Crop Sci. 2021, 61, 466–489. [Google Scholar] [CrossRef]
- Gerrano, A.S.; Thungo, Z.G.; Shimelis, H.; Mashilo, J.; Mathew, I. Genotype-by-environment Interaction for the contents of micro-nutrients and protein in the green pods of cowpea. Agriculture 2022, 12, 531. [Google Scholar] [CrossRef]
- Herniter, I.A.; Muñoz-Amatriaín, M.; Lo, S.; Guo, Y.N.; Close, T.J. Identification of candidate genes controlling black seed coat and pod tip color in cowpea [Vigna unguiculata (L.) Walp]. G3 Genes Genomes Genet. 2018, 8, 3347–3355. [Google Scholar] [CrossRef] [Green Version]
- Herniter, I.A.; Lo, R.; Muñoz-Amatriaín, M.; Lo, S.; Guo, Y.N.; Huynh, B.L.; Lucas, M.; Jia, Z.; Roberts, P.A.; Lonardi, S.; et al. Seed coat pattern QTL and development in cowpea [Vigna unguiculata (L.) Walp]. Front. Plant Sci. 2019, 10, 1346. [Google Scholar] [CrossRef] [Green Version]
- Huynh, B.L.; Matthews, W.C.; Ehlers, J.D.; Lucas, M.R.; Santos, J.R.; Ndeve, A.; Close, T.J.; Roberts, P.A. A major QTL corresponding to the Rk locus for resistance to root-knot nematodes in cowpea [Vigna unguiculata (L.) Walp]. Theor. Appl. Genet. 2016, 129, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Lo, S.; Muñoz-Amatriaín, M.; Boukar, O.; Herniter, I.; Cisse, N.; Guo, Y.N.; Roberts, P.A.; Xu, S.; Fatokun, C.; Close, T.J. Identification of QTL controlling domestication-related traits in cowpea [Vigna unguiculata (L.) Walp]. Sci. Rep. 2018, 8, 6261. [Google Scholar] [CrossRef] [Green Version]
- Lonardi, S.; Muñoz-Amatriaín, M.; Liang, Q.; Shu, S.; Wanamaker, S.I.; Lo, S.; Tanskanen, J.; Schulman, A.H.; Zhu, T.; Luo, M.C.; et al. The genome of cowpea [Vigna unguiculata (L.) Walp]. Plant J. 2019, 98, 767–782. [Google Scholar] [CrossRef] [Green Version]
- Elakhdar, A.; EL-Sattar, M.A.; Amer, K.; Rady, A.; Kumamaru, T. Population structure and marker–trait association of salt tolerance in barley (Hordeum vulgare L.). Comptes Rendus. Biol. 2016, 339, 454–461. [Google Scholar] [CrossRef] [PubMed]
- González, A.M.; Yuste-Lisbona, F.J.; Saburido, S.; Bretones, S.; De Ron, A.M.; Lozano, R.; Santalla, M. Major contribution of flowering time and vegetative growth to plant production in common bean as deduced from a comparative genetic mapping. Front. Plant Sci. 2016, 7, 1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, P.; Wu, X.; Wang, B.; Hu, T.; Lu, Z.; Liu, Y.; Qin, D.; Wang, S.; Li, G. QTL mapping and epistatic interaction analysis in asparagus bean for several characterized and novel horticulturally important traits. BMC Genet. 2013, 14, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, M.A.; Santos, C.A.; Santana, J.R. Mapping of AFLP loci linked to tolerance to cowpea golden mosaic virus. Genet. Mol. Res. 2012, 11, 3789–3797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pottorff, M.; Roberts, P.A.; Close, T.J.; Lonardi, S.; Wanamaker, S.; Ehlers, J.D. Identification of candidate genes and molecular markers for heat-induced brown discoloration of seed coats in cowpea [Vigna unguiculata (L.) Walp]. BMC Genom. 2014, 15, 328. [Google Scholar] [CrossRef] [Green Version]
- Lucas, M.R.; Ehlers, J.D.; Roberts, P.A.; Close, T.J. Markers for the quantitative inheritance of resistance to foliar thrips in cowpea. Crop Sci. 2012, 52, 2075–2081. [Google Scholar] [CrossRef]
- Muchero, W.; Ehlers, J.D.; Close, T.J.; Roberts, P.A. Genic SNP markers and legume synteny reveal candidate genes underlying QTL for Macrophomina phaseolina resistance and maturity in cowpea [Vigna unguiculata (L) Walp.]. BMC Genom. 2011, 12, 8. [Google Scholar] [CrossRef] [Green Version]
- Ouédraogo, J.T.; Maheshwari, V.; Berner, D.K.; St-Pierre, C.A.; Belzile, F.; Timko, M.P. Identification of AFLP markers linked to the resistance of cowpea (Vigna unguiculata L.) to parasitism by Striga gesnerioides. Theor. Appl. Genet. 2001, 102, 1029–1036. [Google Scholar] [CrossRef]
- Andargie, M.; Pasquet, R.S.; Muluvi, G.M.; Timko, M.P. Quantitative trait loci analysis of flowering time related traits identified in recombinant inbred lines of cowpea (Vigna unguiculata). Genome 2013, 294, 289–294. [Google Scholar] [CrossRef]
- Kongjaimun, A.; Kaga, A.; Tomooka, N.; Somta, P.; Shimizu, T.; Shu, Y.; Isemura, T.; Vaughan, D.A.; Srinives, P. An SSR-based linkage map of yardlong bean (Vigna unguiculata (L.) Walp. subsp. unguiculata Sesquipedalis Group) and QTL analysis of pod length. Genome 2012, 55, 81–92. [Google Scholar] [CrossRef]
- Kongjaimun, A.; Somta, P.; Tomooka, N.; Kaga, A.; Vaughan, D.A.; Srinives, P. QTL mapping of pod tenderness and total soluble solid in yardlong bean [Vigna unguiculata (L.) Walp. subsp. unguiculata cv.-gr. sesquipedalis]. Euphytica 2013, 189, 217–223. [Google Scholar] [CrossRef]
- Muchero, W.; Ehlers, J.D.; Roberts, P.A. QTL analysis for resistance to foliar damage caused by Thrips tabaci and Frankliniella schultzei (Thysanoptera: Thripidae) feeding in cowpea [Vigna unguiculata (L.) Walp.]. Mol. Breed. 2010, 25, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Andargie, M.; Pasquet, R.S.; Gowda, B.S.; Muluvi, G.M.; Timko, M.P. Construction of a SSR-based genetic map and identification of QTL for domestication traits using recombinant inbred lines from a cross between wild and cultivated cowpea [V. unguiculata (L.) Walp.]. Mol. Breed. 2011, 28, 413–420. [Google Scholar] [CrossRef]
- Guo, J.; Huang, Z.; Sun, J.; Cui, X.; Liu, Y. Research progress and future development trends in medicinal plant transcriptomics. Front. Plant Sci. 2021, 12, 691838. [Google Scholar] [CrossRef]
- Chen, X.; Laudeman, T.W.; Rushton, P.J.; Spraggins, T.A.; Timko, M.P. CGKB: An annotation knowledge base for cowpea (Vigna unguiculata L.) methylation filtered genomic gene space sequences. BMC Bioinform. 2007, 8, 129. [Google Scholar] [CrossRef] [Green Version]
- Xia, Q.; Pan, L.; Zhang, R.; Ni, X.; Wang, Y.; Dong, X.; Gao, Y.; Zhang, Z.; Kui, L.; Li, Y.; et al. The genome assembly of asparagus bean (Vigna unguiculata ssp. sesquipedialis). Sci. Data 2019, 6, 124. [Google Scholar] [CrossRef] [Green Version]
- Spriggs, A.; Henderson, S.T.; Hand, M.L.; Johnson, S.D.; Taylor, J.M.; Koltunow, A. Assembled genomic and tissue-specific transcriptomic data resources for two genetically distinct lines of cowpea [Vigna unguiculata (L.) Walp]. Gates Open Res. 2018, 2, 7. [Google Scholar] [CrossRef]
- Huang, K.; Mellor, K.E.; Paul, S.N.; Lawson, M.J.; Mackey, A.J.; Timko, M.P. Global changes in gene expression during compatible and incompatible interactions of cowpea (Vigna unguiculata L.) with the root parasitic angiosperm Striga gesnerioides. BMC Genom. 2012, 13, 402. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.; Gupta, K.; Tyagi, V.; Rajkumar, S. Plant genetic resources in India: Management and utilization. Vavilov J. Genet. Breed. 2020, 24, 306–314. [Google Scholar] [CrossRef]
- Mahalakshmi, V.; Ng, Q.; Lawson, M.; Ortiz, R. Cowpea [Vigna unguiculata (L.) Walp.] core collection defined by geographical, agronomical and botanical descriptors. Plant Genet. Resour. Charact. Util. 2007, 5, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Fatokun, C.; Girma, G.; Abberton, M.; Gedil, M.; Unachukwu, N.; Oyatomi, O.; Yusuf, M.; Rabbi, I.; Boukar, O. Genetic diversity and population structure of a mini-core subset from the world cowpea [Vigna unguiculata (L.) Walp] germplasm collection. Sci. Rep. 2018, 8, 16035. [Google Scholar] [CrossRef] [Green Version]
- Xiong, H.; Shi, A.; Mou, B.; Qin, J.; Motes, D.; Lu, W.; Ma, J.; Weng, Y.; Yang, W.; Wu, D. Genetic diversity and population structure of cowpea [Vigna unguiculata (L.) Walp]. PLoS ONE 2016, 11, e0160941. [Google Scholar] [CrossRef] [Green Version]
- Huynh, B.L.; Close, T.J.; Roberts, P.A.; Hu, Z.; Wanamaker, S.; Lucas, M.R.; Chiulele, R.; Cissé, N.; David, A.; Hearne, S.; et al. Gene pools and the genetic architecture of domesticated cowpea. Plant Genome 2013, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Shegro, A.; Lubinga, M.H.; Wolday, M. Genetic resources management, seed production constraints and trade performance of orphan crops in Southern Africa: A case of Cowpea. S. Afr. J. Bot. 2022, 146, 340–347. [Google Scholar] [CrossRef]
- Tadele, Z. African orphan crops under abiotic stresses. Scientifica 2018, 2018, 1451894. [Google Scholar] [CrossRef] [Green Version]
- Kamenya, S.N.; Mikwa, E.O.; Song, B.; Odeny, D.A. Genetics and breeding for climate change in Orphan crops. Theor. Appl. Genet. 2021, 134, 1787–1815. [Google Scholar] [CrossRef] [PubMed]
- Bhat, J.A.; Ali, S.; Salgotra, R.K.; Mir, Z.A.; Dutta, S.; Jadon, V.; Tyagi, A.; Mushtaq, M.; Jain, N.; Singh, P.K.; et al. Genomic selection in the era of next generation sequencing for complex traits in plant breeding. Front. Genet. 2016, 7, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marulanda, J.J.; Mi, X.; Utz, H.F.; Melchinger, A.E.; Würschum, T.; Longin, C.F.H. Optimum breeding strategies using genomic and phenotypic selection for the simultaneous improvement of two traits. Theor. Appl. Genet. 2021, 134, 4025–4042. [Google Scholar] [CrossRef] [PubMed]
- Varshney, R.K.; Thudi, M.; Pandey, M.K.; Tardieu, F.; Ojiewo, C.; Vadez, V.; Whitbread, A.M.; Siddique, K.H.M.; Nguyen, H.T.; Carberry, P.S.; et al. Accelerating genetic gains in legumes for the development of prosperous smallholder agriculture: Integrating genomics, phenotyping, systems modelling and agronomy. J. Exp. Bot. 2018, 69, 3293–3312. [Google Scholar] [CrossRef] [Green Version]
- Pandey, M.K.; Roorkiwal, M.; Singh, V.K.; Ramalingam, A.; Kudapa, H.; Thudi, M.; Chitikineni, A.; Rathore, A.; Varshney, R.K. Emerging genomic tools for legume breeding: Current status and future prospects. Front. Plant Sci. 2016, 7, 455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, C.Y.; Fan, L. Orphan crops and their wild relatives in the genomic era. Mol. Plants 2021, 14, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Leridon, H. World population outlook: Explosion or implosion? Popul. Soc. 2020, 573, 1–4. [Google Scholar] [CrossRef]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [Green Version]
- Razzaq, A.; Kaur, P.; Akhter, N.; Wani, S.H.; Saleem, F. Next-generation breeding strategies for climate-ready crops. Front. Plant Sci. 2021, 12, 620420. [Google Scholar] [CrossRef]
- Hussain, B. Modernization in plant breeding approaches for improving biotic stress resistance in crop plants. Turk. J. Agric. For. 2015, 39, 515–530. [Google Scholar] [CrossRef]
- Chiurugwi, T.; Kemp, S.; Powell, W.; Hickey, L.T. Speed breeding orphan crops. Theor. Appl. Genet. 2019, 132, 607–616. [Google Scholar] [CrossRef]
- Fiyaz, R.A.; Ajay, B.C.; Ramya, K.T.; Kumar, A.; Sundaram, R.M.; Rao, L.V.S. Speed Breeding: Methods and Applications in Accelerated Plant Breeding, 1st ed.; Singh, G.S., Hussain, W.S., Eds.; Springer: Cham, Switzerland, 2022; p. 455. [Google Scholar]
- Wanga, M.A.; Shimelis, H.; Mashilo, J.; Laing, M.D. Opportunities and challenges of speed breeding: A review. Plant Breed. 2021, 140, 185–194. [Google Scholar] [CrossRef]
- Sarsu, F. Contribution of induced mutation in crops to global food security. ACI Av. Cienc. Ing. 2021, 12, 2–11. [Google Scholar] [CrossRef]
- Sangle, S.M.; Lad, J.S. Review on mutation breeding for improvement of food legumes-past and recent. Int. J. Res. Anal. Rev. 2020, 7, 476–481. [Google Scholar]
- Chaudhary, J.; Deshmukh, R.; Sonah, H. Mutagenesis approaches and their role in crop improvement. Plants 2019, 8, 467. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, A.; Guiderdoni, E.; An, G.; Hsing, Y.C.; Han, C.; Lee, M.C.; Yu, S.M.; Upadhyaya, N.; Ramachandran, S.; Zhang, Q.; et al. Mutant resources in rice for functional genomics of the grasses. Plant Physiol. 2009, 149, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Horn, L.N.; Ghebrehiwot, H.M.; Shimelis, H.A. Selection of novel cowpea genotypes derived through gamma irradiation. Front. Plant Sci. 2016, 7, 262. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Li, X.; Ma, B.; Gao, Q.; Du, H.; Han, Y.; Li, Y.; Cao, Y.; Qi, M.; Zhu, Y.; et al. The tartary buckwheat genome provides insights into rutin biosynthesis and abiotic stress tolerance. Mol. Plant 2017, 10, 1224–1237. [Google Scholar] [CrossRef] [Green Version]
- Venezia, M.; Krainer, K.M. Current advancements and limitations of gene editing in orphan crops. Front. Plant Sci. 2021, 12, 1987. [Google Scholar] [CrossRef]
- Østerberg, J.T.; Xiang, W.; Olsen, L.I.; Edenbrandt, A.K.; Vedel, S.E.; Christiansen, A.; Landes, X.; Andersen, M.M.; Pagh, P.; Sandøe, P.; et al. Accelerating the domestication of new crops: Feasibility and approaches. Trends Plant Sci. 2017, 22, 373–384. [Google Scholar] [CrossRef]
- Kang, Y.J.; Lee, T.; Lee, J.; Shim, S.; Jeong, H.; Satyawan, D.; Kim, M.Y.; Lee, S.H. Translational genomics for plant breeding with the genome sequence explosion. Plant Biotechnol. J. 2016, 14, 1057–1069. [Google Scholar] [CrossRef]
- Michael, T.P.; Van Buren, R. Progress, challenges and the future of crop genomes. Curr. Opin. Plant Biol. 2015, 24, 71–81. [Google Scholar] [CrossRef]
- Bao, A.; Zhang, C.; Huang, Y.; Chen, H.; Zhou, X.; Cao, D. Genome editing technology and application in soybean improvement. Oil Crop Sci. 2020, 5, 31–40. [Google Scholar] [CrossRef]
- Pacher, M.; Puchta, H. From classical mutagenesis to nuclease-based breeding—directing natural DNA repair for a natural end-product. Plant J. 2017, 90, 819–833. [Google Scholar] [CrossRef] [Green Version]
- Gbash, S.; Ade, O.; Adebiyi, J.A.; Targuma, S.; Tebele, S.; Areo, O.M.; Olopade, B.; Odukoya, J.O.; Njobeh, P. Food safety, food security and genetically modified organisms in Africa: A current perspective. Biotechnol. Genet. Eng. Rev. 2021, 37, 30–63. [Google Scholar] [CrossRef]
- Huesing, J.E.; Romeis, J.; Ellstrand, N.C.; Raybould, A.; Hellmich, R.L.; Wolt, J.D.; Dabiré-Binso, L.C.; Fatokun, C.A.; Hokanson, K.E.; Ishiyaku, M.F.; et al. Regulatory considerations surrounding the report of the deliberations of an expert. GM Crops 2011, 2, 211–224. [Google Scholar] [CrossRef]


| Amino Acid | Leaves (g/100 g Protein) | Grain (g/100 g Protein) | ||
|---|---|---|---|---|
| Mean Range | References | Mean Range | References | |
| Aspartic acid | 10.8–26.7 | [86,88] | 6.0–13 | [86,89,90] |
| Arginine | 7.4–17.3 | [88,91] | 5.0–10.8 | [86,90] |
| Alanine | 4.2–9.8 | [91,92,93] | 3.4–5.1 | [10] |
| Methionine | 1.0–4.5 | [91,94] | 0.9–3.5 | [91,94] |
| Glutamic acid | 17.2–45.3 | [91,93] | 8.5–19 | [91,95] |
| Glycine | 3.8–12.6 | [91,93] | 3.1–4.8 | [94,96] |
| Cysteine | 0.5–2.9 | [86,93] | 0.3–2.4 | [91,96] |
| Histidine | 1.8–8.6 | [91,94] | 2.0–4.41 | [94,97] |
| Isoleucine | 4.1–11.1 | [84,91] | 2.8–5.4 | [94,97] |
| Leucine | 7.4–19.6 | [91,94] | 5.7–11.3 | [94,97] |
| Lysine | 3.0–16.3 | [91,94] | 3.5–8.0 | [5] |
| Phenylalanine | 4.6–14.4 | [91,94] | 4.4–9.9 | [94,95] |
| Proline | 4.0–15.9 | [91,93] | 3.1–8.9 | [91,95] |
| Serine | 3.0–11.6 | [91,93] | 3.8–5.8 | [94,95] |
| Threonine | 3.2–10.8 | [84,91] | 3.0–5.9 | [94,97] |
| Tryptophan | 1.3–4.1 | [91,93] | 0.9–1.5 | [94,95] |
| Tyrosine | 3.0–9.3 | [91,93] | 2.6–4.5 | [5] |
| Valine | 5.0–12.8 | [91,93] | 3.4–6.2 | [91,94] |
| Leaves | Immature Pod | Grain | ||||
|---|---|---|---|---|---|---|
| Minerals | Mean Range | References | Mean Range | References | Mean Range | References |
| Macro-minerals (mg/100 g dry matter) | ||||||
| Calcium | 15.2–46.20 | [15] | 223.67–867.77 | [16] | 0.07–2.7 | [89,94] |
| Phosphorus | 2.3–6.10 | [15] | 383.43–537.53 | [16] | 2.1–592.4 | [89,94] |
| Potassium | 9.30–35.60 | [15] | 170.74–240.78 | [10,85] | 9.57–1445.2 | [89,95] |
| Magnesium | 4.3–8.4 | [15] | 297.97–426.20 | [16] | 1.3–227.4 | [89,99,100,101] |
| Sulfur | 153.3–200.0 | [24] | 120.0–147.3 | [42] | ||
| Micro-minerals (mg/100 g dry matter) | ||||||
| Copper | 0.15–2.2 | [101,102,103] | 0.48–0.95 | [16] | 0.5–2.2 | [95,101] |
| Iron | 26.76–182.33 | [25] | 6.01–9.78 | [16] | 3.4–10.6 | [89,104] |
| Manganese | 10.57–204 | [101,103] | 2.11–4.77 | [16] | 1.38–4.3 | [89,101] |
| Sodium | 11.59–43.95 | [25] | 13.70–32.93 | [16] | 8.4–79.81 | [89,95] |
| Zinc | 2.78–22.3 | [101,103] | 1.42–5.63 | [10,85] | 2.4–5.11 | [89,94] |
| Aluminum | 1.84–7.86 | [16] | ||||
| Boron | 3.14–5.01 | [27] | 2.13–4.03 | [16] | 1.47–2.14 | [27] |
| Selenium | 2.5–3.4 | [23] | ||||
| Vitamins | Mean Range/Mean (%) | References |
|---|---|---|
| Vitamin A | 0.00–0.07 | [5] |
| Vitamin B1 | 0.2–1.7 | [5] |
| Vitamin B2 | 0.1–76 | [91,107] |
| Vitamin B3 | 0.7–4.0 | [5] |
| Vitamin B5 | 1.7–2.2 | [5] |
| Vitamin B6 | 0.2–0.41 | [5] |
| Vitamin B7 | 0.02–0.03 | [5] |
| Vitamin B9 | 0.1–0.4 | [5] |
| Vitamin B12 | 0 or trace | [5] |
| Vitamin C | 1.5–1.69 | [28] |
| Vitamin D | 0.00 | [28] |
| Vitamin E | 0.07–20 | [5] |
| Nutrient | Leaves | Grain | ||
|---|---|---|---|---|
| Mean Range (%) | References | Mean Range (%) | References | |
| Moisture | 8–9 | [30] | 11.81–13.24 | [31] |
| Ash | 8.1–14.4 | [10] | 3.1–5.8 | [10] |
| Crude protein | 27–43 | [32] | 21–33 | [32] |
| Crude lipid | 1.3–4.1 | [10] | 0.5–3.9 | [10] |
| Crude fiber | 10.09–35.9 | [94,108,109] | 18–32 | [11] |
| Carbohydrate | 59.7–65.2 | [33] | 50–60 | [34] |
| Traits | Population | Type | Marker Type | QTLs | References |
|---|---|---|---|---|---|
| Cowpea golden mosaic virus | IT97 K-499-35 × Canapu T16 | F2 | AFLP | 3 | [147] |
| Fusarium wilt resistance | CB27 × 24–125B-1 | RIL | SNP | 1 | [148] |
| Days to flowering | 524B × 219-01 | RIL | SSR | 3 | [152] |
| Pod length | (JP81610 × TVNU457) × JP81610 | BC1F1 | SSR | 9 | [153] |
| Pod tenderness | (JP81610 × JP89083) × JP81610 | BC1F1 | SSR | 3 | [154] |
| Foliar thrips | CB46 × IT93 K-503-1 and CB27 × IT82E-18 | RILs | SNP | 3 | [155] |
| Cowpea bacterial blight resistance | Danlla × TVu7778 | RIL | SNP | 3 | [155] |
| Charcoal rot resistance | IT93 K-503-1 × CB46 | RIL | SNP | 9 | [150] |
| Striga gesnerioides | TVx 3236 × IT82D-849 | F2 | AFLP | 3 | [151] |
| Hastate leaf shape | Sanzi × Vita 7 | RIL | SNP | 1 | [148] |
| Pod fiber layer thickness | 524B × 219-01 | RIL | SSR | 4 | [156] |
| Pod number per plant | ZN016 × ZJ282 | RIL | SSR | 3 | [146] |
| Pod tenderness | (JP81610 × JP89083) × JP81610 | BC1F1 | SSR | 3 | [154] |
| Nodes to the first flower | ZN016 × ZJ282 | RIL | SNP | 4 | [146] |
| Days to first flowering | ZN016 × ZJ282 | RIL | SNP | 3 | [146] |
| Days to maturity | IT93K503-1 × CB46 | RIL | AFLP | 2 | [155] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mekonnen, T.W.; Gerrano, A.S.; Mbuma, N.W.; Labuschagne, M.T. Breeding of Vegetable Cowpea for Nutrition and Climate Resilience in Sub-Saharan Africa: Progress, Opportunities, and Challenges. Plants 2022, 11, 1583. https://doi.org/10.3390/plants11121583
Mekonnen TW, Gerrano AS, Mbuma NW, Labuschagne MT. Breeding of Vegetable Cowpea for Nutrition and Climate Resilience in Sub-Saharan Africa: Progress, Opportunities, and Challenges. Plants. 2022; 11(12):1583. https://doi.org/10.3390/plants11121583
Chicago/Turabian StyleMekonnen, Tesfaye Walle, Abe Shegro Gerrano, Ntombokulunga Wedy Mbuma, and Maryke Tine Labuschagne. 2022. "Breeding of Vegetable Cowpea for Nutrition and Climate Resilience in Sub-Saharan Africa: Progress, Opportunities, and Challenges" Plants 11, no. 12: 1583. https://doi.org/10.3390/plants11121583
APA StyleMekonnen, T. W., Gerrano, A. S., Mbuma, N. W., & Labuschagne, M. T. (2022). Breeding of Vegetable Cowpea for Nutrition and Climate Resilience in Sub-Saharan Africa: Progress, Opportunities, and Challenges. Plants, 11(12), 1583. https://doi.org/10.3390/plants11121583







