Ethnobotanical Uses, Phytochemical Composition, Biosynthesis, and Pharmacological Activities of Carpesium abrotanoides L. (Asteraceae)
Abstract
:1. Introduction
2. Methodology
3. Traditional Uses and Clinical Trials
4. Pharmacological Activities of Crude Extracts
4.1. Cytotoxic Activity
4.2. Antiparasitic Activity
4.3. Insecticidal Activity
4.4. Antidiabetic Activity
4.5. Antioxidant Activity
4.6. Anti-Inflammatory Activity
5. Phytochemicals and Their Pharmacological Activities
5.1. Monoterpenes
5.2. Sesquiterpene Lactones (STLs)
STLs Isolation and Identification
5.3. Other Metabolites and Volatile Constituents
6. Toxicity Studies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-Azinobis-3-ethylbenzothiazoline-6-sulfonic acid |
| C452 | Cysteine 452 |
| CAT | Catalase |
| CH2Cl2 | Dichloromethane |
| CHCl3 | Chloroform |
| COX-2 | Cyclooxygenase |
| DEET | N,N-Diethyl-meta-toluamide |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl |
| DTT | Dithiothreitol |
| ECD | Electronic circular dichroism |
| EtOH | Ethanol |
| EtOAc | Ethyl acetate |
| FDI | Feeding-deterrence index |
| FIC | Ferrous ion chelating |
| GC-MS | Gas chromatography–mass spectrometry |
| GLUT1 | Glucose transporter-1 |
| Gpx | Glutathione peroxidase |
| GSH | Glutathione |
| HIF-1α | Hypoxia-inducible factor-1α |
| HINAE | Hirame natural embryo |
| HK2 | Hexokinase-2 |
| HPLC-ESI-Q-TOF-MS/MS | Electrospray ionization-quadrupole time-of-flight mass spectrometry |
| HSCCC | Highspeed counter-current chromatography |
| IC50 | Half-maximal inhibitory concentration |
| IL-6 | Interleukin-6 |
| IL-4 | Interleukin-4 |
| IL-13 | Interleukin-13 |
| iNOS | Inducible nitric oxide synthase |
| IR | Infrared |
| LD50 | Half maximal lethal concentration |
| LDHA | Lactate dehydrogenase A |
| MDA | Malondialdehyde |
| MeOH | Methanol |
| MyD-88 | Myeloid-differential-factor-88 |
| NMR | Nuclear magnetic resonance |
| NOS | Nitric oxide scavenging |
| NQO1 | NAD(P)H:quinone oxidoreductase |
| Nrf2 | NF-E2-related factor 2 |
| PARP | Poly(ADPribose) polymerase |
| PE | Petroleum ether |
| PGE2 | Prostaglandin E2 |
| PKM2 | Pyruvate kinase M2 |
| RP-18 | Reversed phase-18 |
| SiO2 CC | Silica gel column chromatography |
| SOD | Superoxide dismutase |
| TDDFT-ECD | Time-dependent density-functional-theory electronic circular dichroism |
| TLC | Thin layer chromatography |
| TLRs | Toll-like receptors |
| TRIF | Toll-interleukin-1 receptor domain-containing adapter inducing interferon-β |
References
- Chaachouay, N.; Douira, A.; Zidane, L. Herbal medicine used in the treatment of human diseases in the rif, Northern Morocco. Arab. J. Sci. Eng. 2022, 47, 131–153. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.H.; Sadeghi, Z.; Hosseini, S.V.; Bussmann, R.W. Ethnopharmacological study of medicinal plants in Sarvabad, Kurdistan, Iran. J. Ethnopharmacol. 2022, 288, 114985. [Google Scholar] [CrossRef] [PubMed]
- Jahangir, M.A.; Muheem, A.; Imam, S.S.; Gilani, S.J.; Zafar, A.; Alshehri, S.; Jafar, M. High altitude edible plants: A great resource for human health and their socio-economic significance. In Edible Plants in Health and Diseases; Springer: Berlin/Heidelberg, Germany, 2022; pp. 161–180. [Google Scholar]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.R.M.; Bagalagel, A.A.; Diri, R.M.; Noor, A.O.; Bakhsh, H.T.; Mohamed, G.A. Phytoconstituents and pharmacological activities of Indian Camphorweed (Pluchea Indica): A multi-potential medicinal plant of nutritional and ethnomedicinal importance. Molecules 2022, 27, 2383. [Google Scholar] [CrossRef]
- Abdallah, H.M.; Mohamed, G.A.; Ibrahim, S.R.M. Lansium domesticum—A fruit with multi-benefits: Traditional uses, phytochemicals, nutritional value, and bioactivities. Nutrients 2022, 14, 1531. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Omar, A.M.; Bagalagel, A.A.; Diri, R.M.; Noor, A.O.; Almasri, D.M.; Mohamed, S.G.A.; Mohamed, G.A. Thiophenes—naturally occurring plant metabolites: Biological activities and in silico evaluation of their potential as cathepsin D inhibitors. Plants 2022, 11, 539. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lu, Y. Noteworthy plants in Asteraceae from China. J. Zhejiang Univ. 2019, 46, 209–214. [Google Scholar]
- Zhang, J.P.; Wang, G.W.; Tian, X.H.; Yang, Y.X.; Liu, Q.X.; Chen, L.P.; Li, H.L.; Zhang, W.D. The genus Carpesium: A review of its ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2015, 163, 173–191. [Google Scholar] [CrossRef] [PubMed]
- Abid, R.; Zehra, N. Micromorphology of cypsela and its taxonomic significance of some genera in the tribe Inuleae (Asteraceae) from Pakistan. Pak. J. Bot 2007, 39, 1407–1416. [Google Scholar]
- Ye, H.; Li, C.; Ye, W.; Zeng, F.; Liu, F.; Liu, Y.; Wang, F.; Ye, Y.; Fu, L.; Li, J. Medicinal angiosperms of Compositae (cont. I). In Common Chinese Materia Medica; Springer: Berlin/Heidelberg, Germany, 2022; pp. 287–362. [Google Scholar]
- The Plant List. Retrived 10 March 2022. Available online: http://www.theplantlist.org/ (accessed on 3 March 2022).
- Wang, F.; Yang, K.; Ren, F.; Liu, J. Sesquiterpene lactones from Carpesium abrotanoides. Fitoterapia 2009, 80, 21–24. [Google Scholar] [CrossRef]
- Editorial Board of Chinese Materia Medica. Chinese Materia Medica; Shanghai Science & Technology Press: Shanghai, China, 1999; pp. 756–763. [Google Scholar]
- Wang, Q.; Pan, L.; Lin, L.; Zhang, R.; Du, Y.; Chen, H.; Huang, M.; Guo, K.; Yang, X. Essential oil from Carpesium abrotanoides L. induces apoptosis via activating mitochondrial pathway in hepatocellular carcinoma cells. Curr. Med. Sci. 2018, 38, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Yang, Y.; Wan, D.; Yu, H.; Cai, Y.; Wang, X.; Tang, D.; Tang, C.; Zhang, S. Sesquiterpene lactones isolated from Carpesium abrotanoides L. by LC–MS combined with HSCCC inhibit liver cancer through suppression of the JAK2/STAT3 signaling pathway. Med. Chem. Res. 2022, 31, 436–445. [Google Scholar] [CrossRef]
- Chai, X.X.; Le, Y.F.; Wang, J.C.; Mei, C.X.; Feng, J.F.; Zhao, H.; Wang, C.; Lu, D.Z. Carpesium abrotanoides (L.) root as a potential source of natural anticancer compounds: Targeting glucose metabolism and PKM2/HIF-1α axis of breast cancer cells. J. Food Sci. 2019, 84, 3825–3832. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Tashi, N.; Singh, B.; Dutt, H.C. Ethnobotanical plants used for gastrointestinal ailments by the inhabitants of Kishtwar plateau in Northwestern Himalaya. Indian J. Tradit. Knowl. 2020, 19, 288–298. [Google Scholar]
- Lee, S.B.; Kang, K.; Lee, H.J.; Yun, J.H.; Jho, E.H.; Kim, C.Y.; Nho, C.W. The chemopreventive effects of carpesium abrotanoides are mediated by induction of phase II detoxification enzymes and apoptosis in human colorectal cancer cells. J. Med. Food 2010, 13, 39–46. [Google Scholar] [CrossRef]
- Lee, J.H.; Hwang, K.H.; Kim, G.H. In vitro evaluation of anti-inflammatory activity for salad-food material Carpesium abrotanoides. J. Food Biochem. 2013, 37, 18–25. [Google Scholar] [CrossRef]
- Guochao, J.; Wy, M.; Tshingh, T.W. Oral Traditional Chinese Medicine for Treating Intestinal Ascariasis. CN 200610172207, 25 December 2006. [Google Scholar]
- Bae, K.H.; Lee, J.S.; Min, B.S. Screening on the cytotoxicity of medicinal plants against L1210 and HL60 cancer Cells. Korean J. Pharmacogn. 1996, 27, 173–177. [Google Scholar]
- Wang, C.; Jiang, J.; Ji, J.; Cai, Q.; Chen, X.; Yu, Y.; Zhu, Z.; Zhang, J. PKM2 promotes cell migration and inhibits autophagy by mediating PI3K/AKT activation and contributes to the malignant development of gastric cancer. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Woo, S.J.; Jeong, M.G.; Jeon, E.J.; Do, M.Y.; Kim, N.Y. Antiparasitic potential of ethanolic extracts of carpesii fructus against Miamiensis avidus in Hirame natural embryo cell line and their effects on immune response-and biotransformation-related genes. Comp. Biochem. Physiol. Part-C Toxicol. Pharmacol. 2022, 251, 109214. [Google Scholar] [CrossRef]
- Moustafa, E.M.M.; Naota, M.; Morita, T.; Tange, N.; Shimada, A. Pathological study on the scuticociliatosis affecting farmed Japanese flounder (Paralichthys olivaceus) in Japan. J. Vet. Med. Sci. 2010, 72, 1359–1362. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.H.; Sim, D.S.; Park, M.S.; Jo, Q.; Kim, Y. Effects of formalin on haematological and blood chemistry in olive flounder, Paralichthys olivaceus (Temminck et Schlegel). Aquacult. Res. 2003, 34, 1269–1275. [Google Scholar] [CrossRef]
- Liu, X.Y.; Guo, G.W.; Wang, H. KillingeEffect of Carpesium abrotanoides on Taenia asiatica cysticercus. Chin. J. Parasitol. Parasit. Dis. 2015, 33, 18. [Google Scholar]
- Ahmad, M.; Farid, A.; Saeed, M. Resistance to new insecticides and their synergism in Spodoptera exigua (Lepidoptera: Noctuidae) from Pakistan. Crop. Prot. 2018, 107, 79–86. [Google Scholar] [CrossRef]
- De Castro, A.A.; Legaspi, J.C.; Tavares, W.D.S.; Meagher, R.L., Jr.; Miller, N.; Kanga, L.; Haseeb, M.; Serrao, J.E.; Wilcken, C.F.; Zanuncio, J.C. Lethal and behavioral effects of synthetic and organic insecticides on Spodoptera exigua and its Predator Podisus maculiventris. PLoS ONE 2018, 13, e0206789. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Jiang, H.; Zhang, Y.; He, W.; Zhang, L. Insecticidal activities of ethanol extracts from thirty Chinese medicinal plants against Spodoptera Exigua (Lepidoptera: Noctuidae). J. Med. Plants Res. 2012, 6, 1263–1267. [Google Scholar]
- Jansen, C.C.; Beebe, N.W. The dengue vector Aedes aegypti: What comes next. Microb. Infect. 2010, 12, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Haris, A.; Azeem, M.; Binyameen, M. Mosquito repellent potential of Carpesium abrotanoides essential oil and its main components against a Dengue vector, Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2022, 59, 801–809. [Google Scholar] [CrossRef]
- Mazzarri, M.B.; Georghiou, G.P. Characterization of resistance to organophosphate, carbamate, and pyrethroid insecticides in field populations of Aedes aegypti from Venezuela. J. Am. Mosq. Control Assoc. 1995, 11, 315–322. [Google Scholar]
- Arslan, A.; Rathor, H.R.; Mukhtar, M.U.; Mushtaq, S.; Bhatti, A.; Asif, M.; Arshad, I.; Ahmad, J.F. Spatial Distribution and insecticide susceptibility status of Aedes aegypti and Aedes albopictus in dengue affected Urban areas of Rawalpindi, Pakistan. J. Vector Borne Dis. 2016, 53, 136. [Google Scholar]
- World Health Organization. Diabetes 2021. Retrived 15 March 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 3 March 2022).
- Ibrahim, S.R.M.; Mohamed, G.A.; Zayed, M.F.; Ross, S.A. 8-Hydroxyirilone 5-methyl ether and 8-hydroxyirilone, new antioxidant and α-amylase inhibitors isoflavonoids from Iris germanica rhizomes. Bioorg. Chem. 2017, 70, 192–198. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A.; Elfaky, M.A.; Al Haidari, R.A.; Zayed, M.F.; El-Kholy, A.A.; Khedr, A.I.M. Garcixanthone A, a new cytotoxic xanthone from the pericarps of Garcinia mangostana. J. Asian Nat. Prod. Res. 2019, 21, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Alhakamy, N.A.; Mohamed, G.A.; Fahmy, U.A.; Eid, B.G.; Ahmed, O.A.A.; Al-Rabia, M.W.; Khedr, A.I.M.; Nasrullah, M.Z.; Ibrahim, S.R.M. New alpha-amylase inhibitory metabolites from pericarps of Garcinia mangostana. Life 2022, 12, 384. [Google Scholar] [CrossRef] [PubMed]
- Mayur, B.; Sancheti, S.; Shruti, S.; Sung-Yum, S. Antioxidant and alpha-glucosidase inhibitory properties of Carpesium Abrotanoides L. J. Med. Plants Res. 2010, 4, 1547–1553. [Google Scholar]
- Kang, H.J.; Kim, H.J.; Jeong, S.I.; Kim, H.S.; Jeon, I.H.; Mok, J.Y.; Shim, J.S.; Jang, S.I. Antioxidant and antihemolytic activities of ethanol extracts of Carpesii Fructus and Farfarae Flos. Korea J. Herbol. 2013, 28, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Jeong, D.W.; Lee, E.K.; Lee, C.H.; Lim, S.J.; Gu, G.J.; Paek, J.H.; Kim, S.; Lim, S.S.; Youn, H.S. Carpesium Abrotanoides extract inhibits cyclooxygenase-2 Expression Induced by Toll-Like Receptor Agonists. Toxicol. Environ. Health Sci. 2013, 5, 92–96. [Google Scholar] [CrossRef]
- Lee, E.K.; Jeong, D.W.; Lim, S.J.; Gu, G.J.; Ahn, S.I.; Kim, J.S.; Paek, J.H.; Kim, S.; Hong, J.S.; Lim, S.S. Carpesium Abrotanoides extract inhibits inducible nitric oxide synthase expression induced by Toll-like receptor agonists. Food Sci. Biotechnol. 2014, 23, 1637–1641. [Google Scholar] [CrossRef]
- Ko, Y.E.; Oh, S.R.; Song, H.H.; Ryu, H.W.; Ly, S.Y.; Kim, J.W. The effect of 4α, 5α-epoxy-10α, 14-dihydro-inuviscolide, a novel immunosuppressant isolated from Carpesium abrotanoides, on the cytokine profile in vitro and in vivo. Int. Immunopharmacol. 2015, 25, 121–129. [Google Scholar] [CrossRef]
- Wu, H.; Wu, H.; Wang, W.; Liu, T.; Qi, M.; Feng, J.; Li, X.; Liu, Y. Insecticidal activity of sesquiterpene lactones and monoterpenoid from the fruits of Carpesium Abrotanoides. Ind. Crop. Prod. 2016, 92, 77–83. [Google Scholar] [CrossRef]
- Yang, B.J.; Zeng, Z.Q.; Song, Y.; Hao, X.J.; Li, S.L. Terpenes from Carpesium abrotanoides L. and their anti-tumor activity. Nat. Prod. Res. Develop. 2021, 33, 951. [Google Scholar]
- Kariyone, T.; Kawano, N. Components of Carpesium abrotanoides. I. Yakugaku Zasshi 1949, 69, 317–318. [Google Scholar] [CrossRef] [Green Version]
- Kariyone, T.; Naito, S.; Chatani, J. Studies on the component of Carpesium abrotanoides. II.: Chemical constitution of Carpesia Lactone.(1). Pharm. Bull. 1954, 2, 339–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kariyone, T.; Naito, S. Studies on the components of Carpesium abrotanoides. III. Chemical structure of carpesia lactone. (2). Yakugaku Zasshi 1955, 75, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Naito, S. Studies on the components of Carpesium abrotanoides. IV. Chemical structure of carpesia lactone. (3). Yakugaku Zasshi 1955, 75, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Naito, S. Studies on the components of Carpesium abrotanoides. V. Chemical structure of carpesia lactone. (4). Yakugaku Zasshi 1955, 75, 325–329. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Qin, W.; Tian, L.; Zhang, X.; Lin, F.; Cheng, F.; Chen, J.; Liu, C.; Guo, Z.; Proksch, P. Caroguaianolide A–E, five new cytotoxic sesquiterpene lactones from Carpesium abrotanoides L. Fitoterapia 2018, 127, 349–355. [Google Scholar] [CrossRef]
- Yang, B.; Wang, J.; Zeng, Z.; Yang, X.; Huang, A.; Hao, X.; Ding, X.; Li, S. Sesquiterpene lactones from Carpesium abrotanoides L. and their activity in inducing protective autophagy. Nat. Prod. Res. 2021, 1–4. [Google Scholar] [CrossRef]
- Hu, Q.; Wu, P.; Liu, Y.; Qi, F.; Yu, C.; Zhao, Y.; Yu, Y.; Fei, D.; Zhang, Z. Three new sesquiterpene lactones from Carpesium abrotanoides. Phytochem. Lett. 2018, 27, 154–159. [Google Scholar] [CrossRef]
- He, Y.; Cai, L.; Qian, Q.; Yang, S.; Chen, D.; Zhao, B.; Zhong, Z.; Zhou, X. Anti-influenza A (H1N1) viral and cytotoxic sesquiterpenes from Carpesium abrotanoides. Phytochem. Lett. 2020, 35, 41–45. [Google Scholar] [CrossRef]
- Maruyama, M.; Omura, S. Carpesiolin from Carpesium Abrotanoides. Phytochemistry 1977, 16, 782–783. [Google Scholar] [CrossRef]
- Wang, J.; He, W.; Zhang, X.; Zhao, B.; Liu, Y.; Zhou, X. Dicarabrol, a new dimeric sesquiterpene from Carpesium abrotanoides L. Bioorg. Med. Chem. Lett. 2015, 25, 4082–4084. [Google Scholar] [CrossRef]
- Maruyama, M.; Shibata, F. Stereochemistry of granilin isolated from Carpesium abrotanoides. Phytochemistry 1975, 14, 2247–2248. [Google Scholar] [CrossRef]
- Qian, Q.; Gong, L.; Yang, S.; Zhao, B.; Cai, J.; Zhang, Z.; Wang, Y.; Zhou, X. Two new compounds from Carpesium abrotanoides. Phytochem. Lett. 2020, 40, 5–9. [Google Scholar] [CrossRef]
- Maruyama, M.; Karube, A.; Sato, K. Sesquiterpene lactones from Carpesium abrotanoides. Phytochemistry 1983, 22, 2773–2774. [Google Scholar] [CrossRef]
- Wang, L.; Jin, G.; Tian, L.; Ebrahim, W.; Höfert, S.; Janiak, C.; Chen, J.; Guo, Z.; Schäberle, T.F.; Liu, Z. New eremophilane-type sesquiterpenes and maleimide-bearing compounds from Carpesium abrotanoides L. Fitoterapia 2019, 138, 104294. [Google Scholar] [CrossRef] [PubMed]
- Minato, H.; Nosaka, S.; Horibe, I. 1057. Studies on Sesquiterpenoids. Part VIII. The Structure of Carabrone, a New Component of Carpesium abrotanoides, Linn. J. Chem. Soc. 1964, pp. 5503–5510. Available online: https://pubs.rsc.org/en/content/articlelanding/1964/jr/jr9640005503 (accessed on 3 March 2022).
- Jie-Wei, W.; Chun-Ping, T.; Sheng, Y.; Chang-Qiang, K.E.; Yang, Y.E. Three new carabrane sesquiterpenoid derivatives from the whole plant of Carpesium abrotanoides L. Chin. J. Nat. Med. 2021, 19, 868–873. [Google Scholar]
- Wu, J.; Tang, C.; Ke, C.; Yao, S.; Liu, H.; Lin, L.; Ye, Y. Dicarabrol A, dicarabrone c and dipulchellin a, unique sesquiterpene lactone dimers from Carpesium abrotanoides. RSC Adv. 2017, 7, 4639–4644. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Tang, C.; Chen, L.; Qiao, Y.; Geng, M.; Ye, Y. Dicarabrones A and B, a pair of new epimers dimerized from sesquiterpene lactones via a [3+2] cycloaddition from Carpesium abrotanoides. Org. Lett. 2015, 17, 1656–1659. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Li, H.; Mo, Q.; Huang, H.; Tao, M.; Luo, Q.; Liu, H. Five new C17/C15 sesquiterpene lactone dimers from Carpesium abrotanoides. Fitoterapia 2020, 145, 104630. [Google Scholar] [CrossRef]
- Naito, S. Studies on the components of Carpesium abrotanoides. VI Non-acidic components. Yakugaku Zasshi 1955, 75, 355–356. [Google Scholar] [CrossRef] [Green Version]
- Kameoka, H.; Sagara, K.; Miyazawa, M. Components of essential oils of kakushitsu (Daucus carota L. and Carpesium abrotanoides L.). J. Agric. Chem. Soc. JPN 1989, 63, 185–188. [Google Scholar]
- Lee, J.S.; Min, B.S.; Lee, S.M.; Na, M.K.; Kwon, B.M.; Lee, C.O.; Kim, Y.H.; Bae, K.H. Cytotoxic sesquiterpene lactones from Carpesium abrotanoides. Planta Med. 2002, 68, 745–747. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Ma, Z.; Li, J.; He, J.; Xu, H.; Zhang, X. Synthesis and antifungal activity of carabrone derivatives. Molecules 2010, 15, 6485–6492. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Gao, S.; Zu, X.; Shen, Y.; Shan, L.; Li, H.; Zhang, W. Cytotoxic 2, 4-linked sesquiterpene lactone dimers from Carpesium faberi exhibiting NF-κB inhibitory activity. RSC Adv. 2015, 5, 55285–55289. [Google Scholar] [CrossRef]
- Yang, Y.; Shan, L.; Liu, Q.; Shen, Y.; Zhang, J.; Ye, J.; Xu, X.; Li, H.; Zhang, W. Carpedilactones A–D, four new isomeric sesquiterpene lactone dimers with potent cytotoxicity from Carpesium faberi. Org. Lett. 2014, 16, 4216–4219. [Google Scholar] [CrossRef] [PubMed]
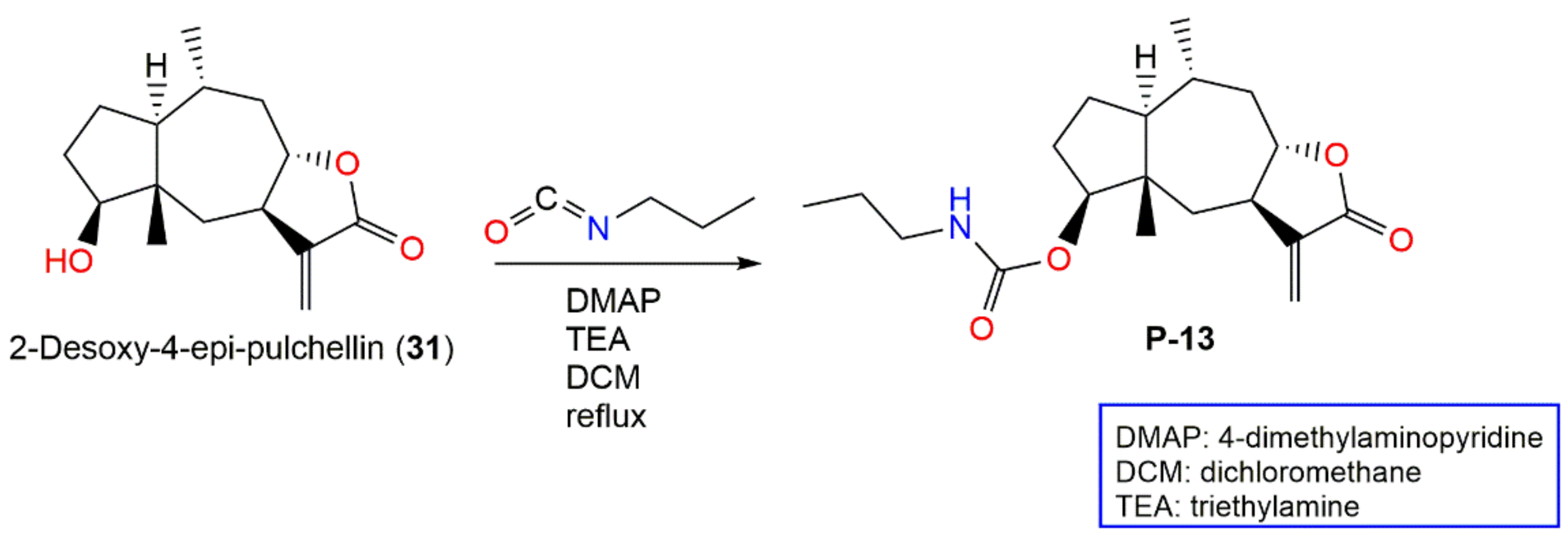
- Huang, H.; Niu, J.; Wang, F.; Hu, L.; Yu, Q. A natural compound derivative P-13 inhibits STAT3 signaling by covalently inhibiting janus kinase 2. Invest New Drugs 2019, 37, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Cheng, F.; Wang, L.; Qin, W.; Zou, K.; Chen, J. CLE-10 from Carpesium abrotanoides L. suppresses the growth of human breast cancer cells (MDA-MB-231) in vitro by inducing apoptosis and pro-death autophagy via the PI3K/Akt/mTOR signaling pathway. Molecules 2019, 24, 1091. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Liu, F.; Mu, W.; Wang, Q.; Li, H.; Chen, C. Life table study of the effects of sublethal concentrations of thiamethoxam on Bradysia Odoriphaga Yang and Zhang. Pestic. Biochem. Physiol. 2014, 111, 31–37. [Google Scholar] [CrossRef]
- Liu, J.; Wang, W.; Xu, K. G1san improved detection of pesticide residues in sulfur-containing vegetables based on acetylcholineesterase inhibition effects. Agro Food Ind. Hi-Tech 2013, 24, 47–51. [Google Scholar]
- Kuo, Y.H.; Huang, S.L.; Chang, C.I. A Phenolic and an aliphatic lactone from Diospyros maritima. Phytochemistry 1998, 49, 2505–2507. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Song, G.; Zhang, G.; Ye, Z.; Williamson, E.M. The classification and application of toxic Chinese materia medica. Phytother. Res. 2014, 28, 334–347. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, J.J.; Seo, B. A philological study on poisoning and side effects of Carpesii Fructus. J. East West Med. 2014, 39, 17–22. [Google Scholar]
- Maclean, W. Clinical Handbook of Chinese Herbs: Desk Reference, Revised Edition; Singing Dragon: Philadelphia, PA, USA, 2017. [Google Scholar]
- Shi, H.W.; Wang, Y.X.; Wu, J.; Lin, M.C. Effects of four chinese medicinal herbs on adenosine deaminase (ADA) in blood-serum of goldfish. Shandong Fish 2008, 25, 4–6. [Google Scholar]
- Zhou, L.; Chen, X.; Zheng, T. Study on the ecological safety of algacides: A comprehensive strategy for their screening. J. Appl. Phycol. 2010, 22, 803–811. [Google Scholar] [CrossRef] [Green Version]
- Xia, Q.; Luo, J.; Mei, X.; Wang, Y.; Huang, W.; Wang, J.; Yang, R.; Ma, Z.; Lin, R. A developmental toxicity assay of Carpesii Fructus on zebrafish embryos/larvae. Toxicol. Res. 2017, 6, 460–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Liu, X.; Liu, T.; Wang, Y.; Ahmed, N.; Li, Z.; Jiang, H. Synthetic biology of plant natural products: From pathway elucidation to engineered biosynthesis in plant cells. Plant Commun. 2021, 2, 100229. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, S.; Tsunematsu, Y.; Sato, M.; Watanabe, K. Elucidation of biosynthetic pathways of natural products. Chem. Rec. 2017, 17, 1095–1108. [Google Scholar] [CrossRef]
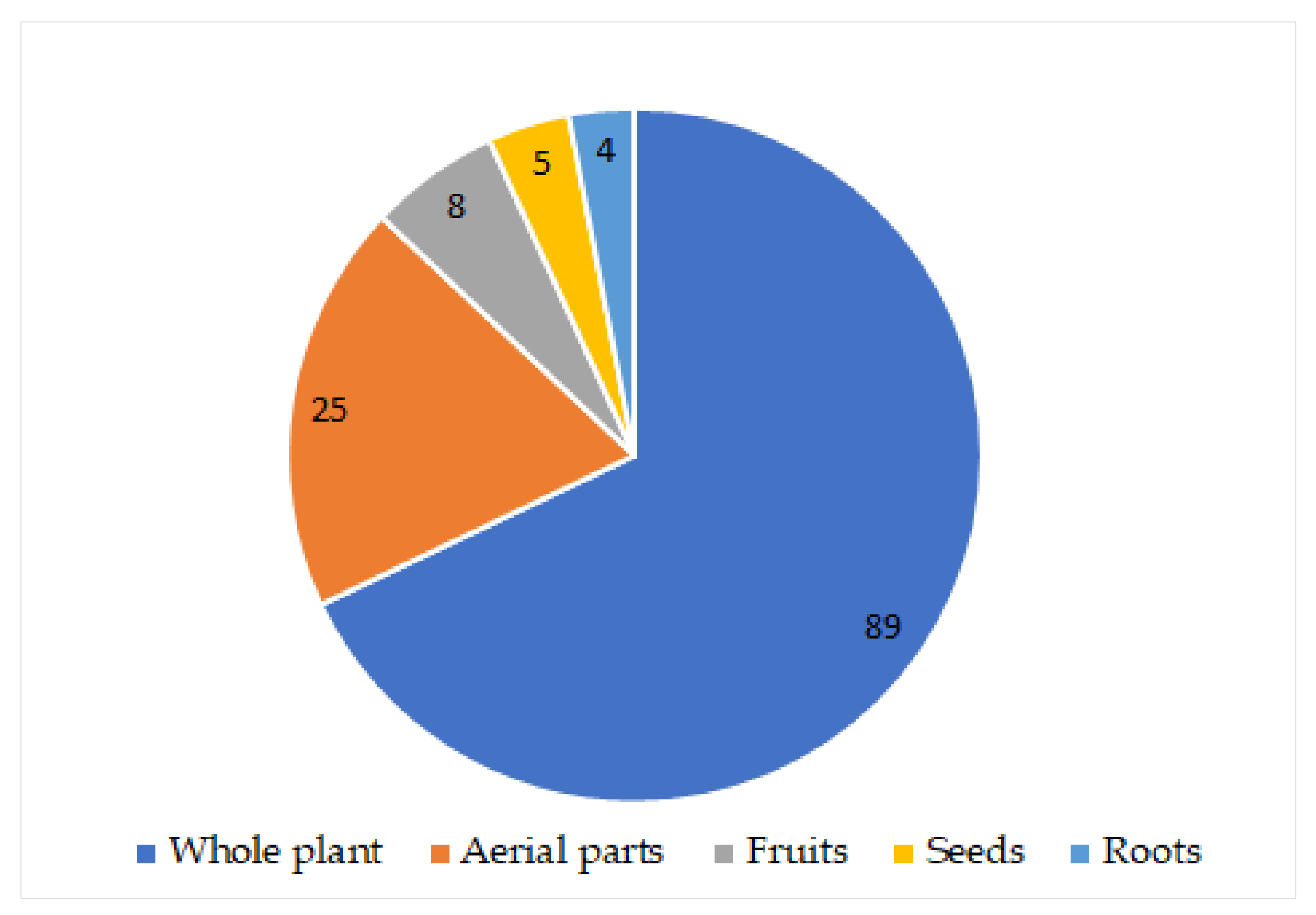
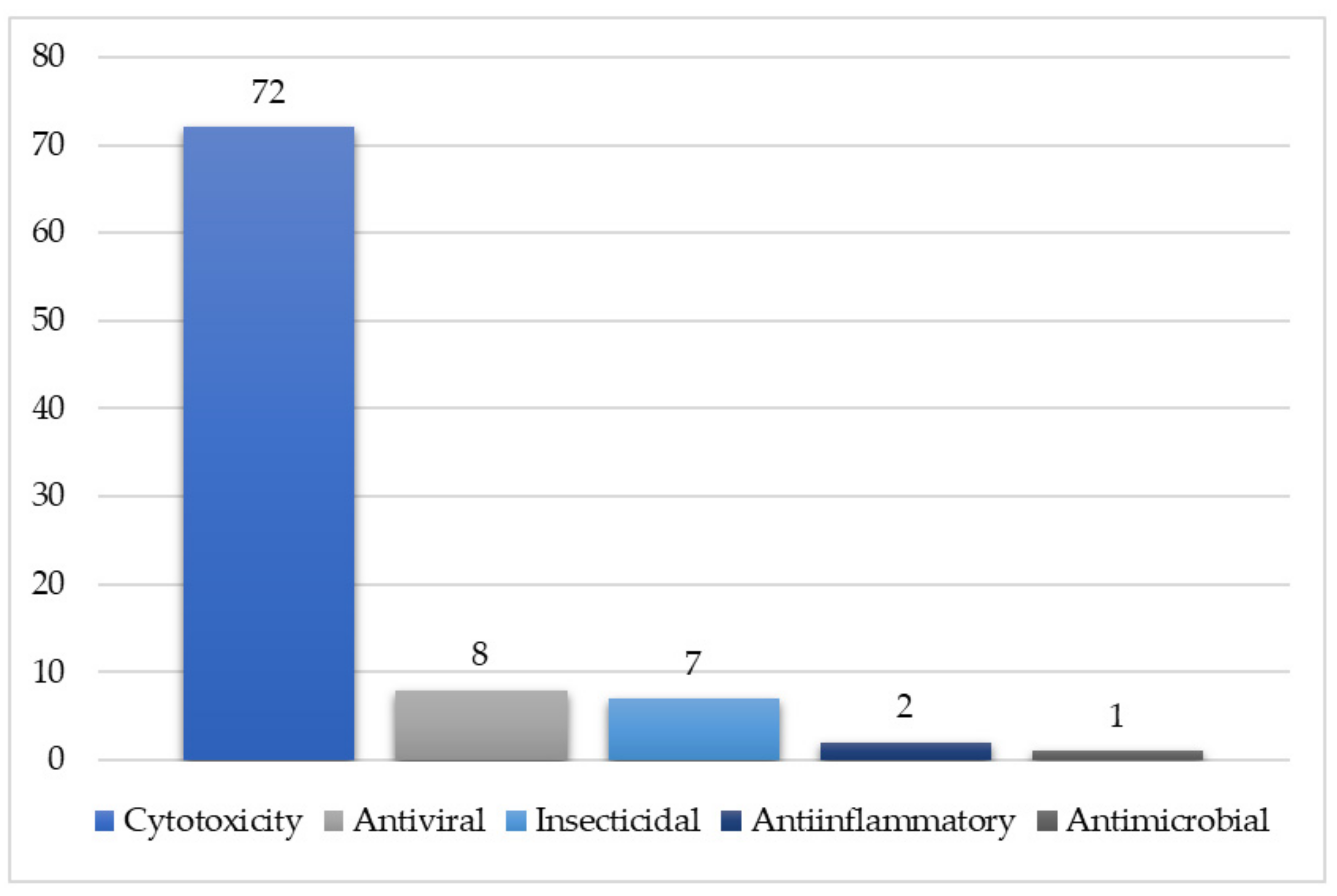

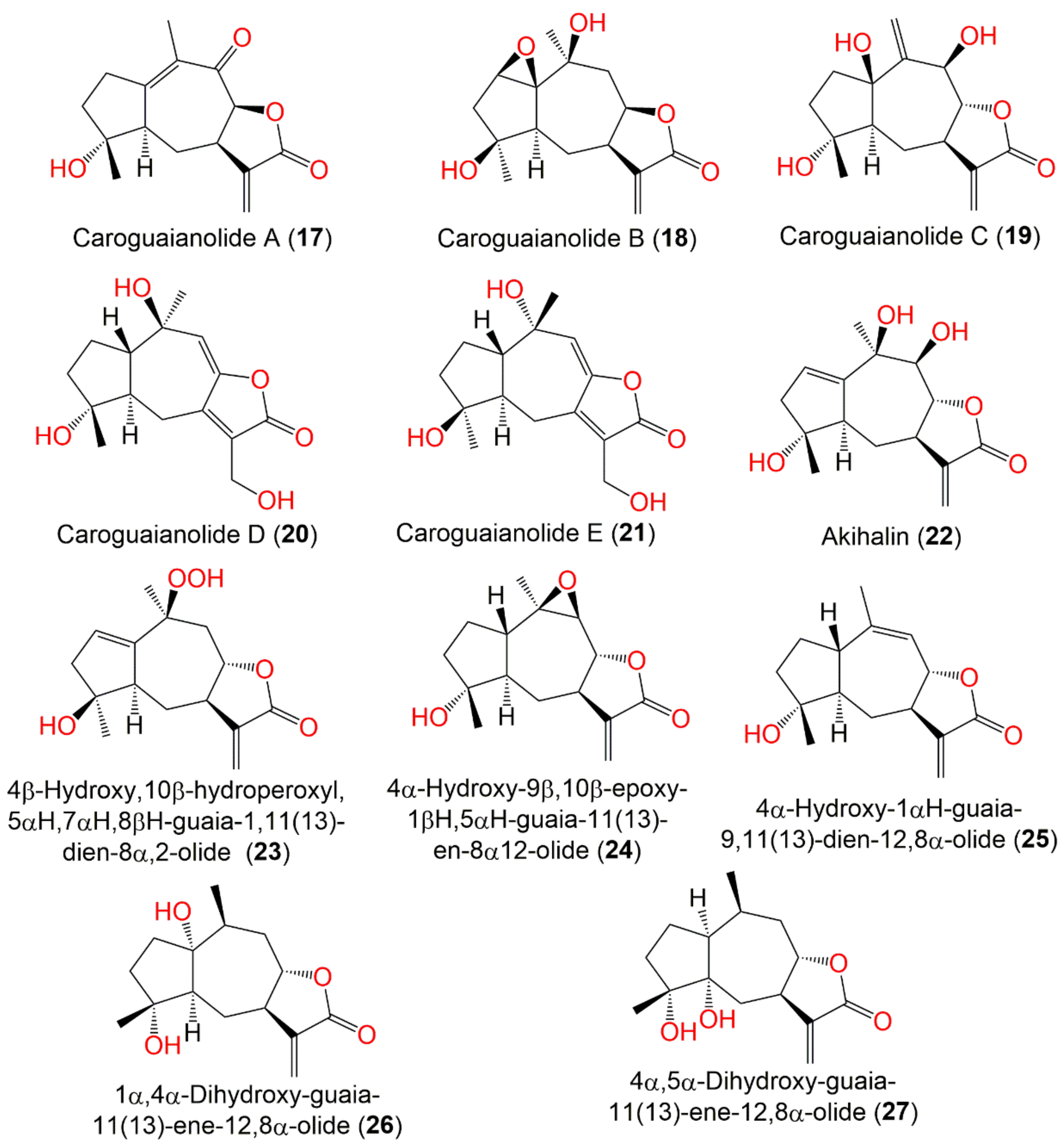
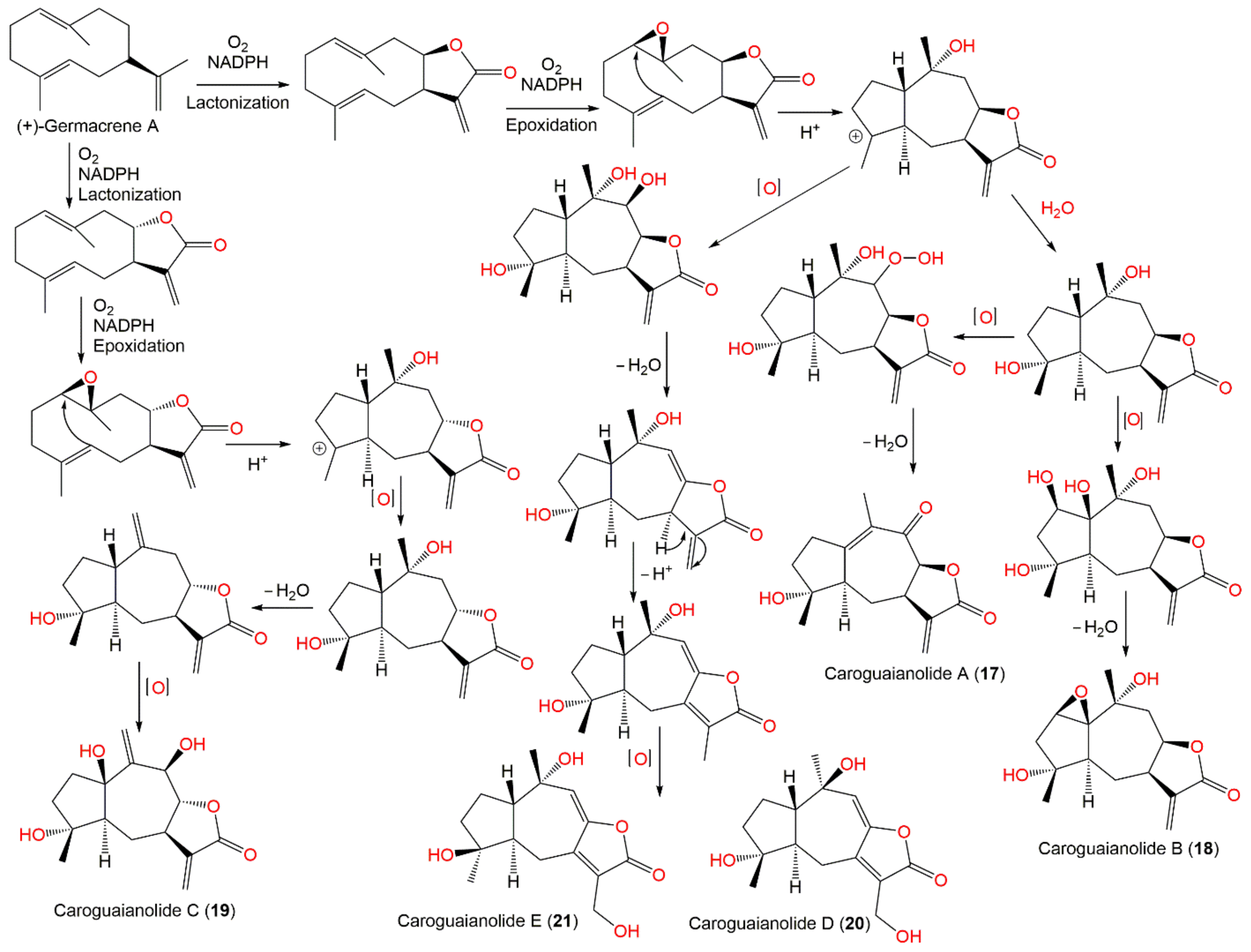
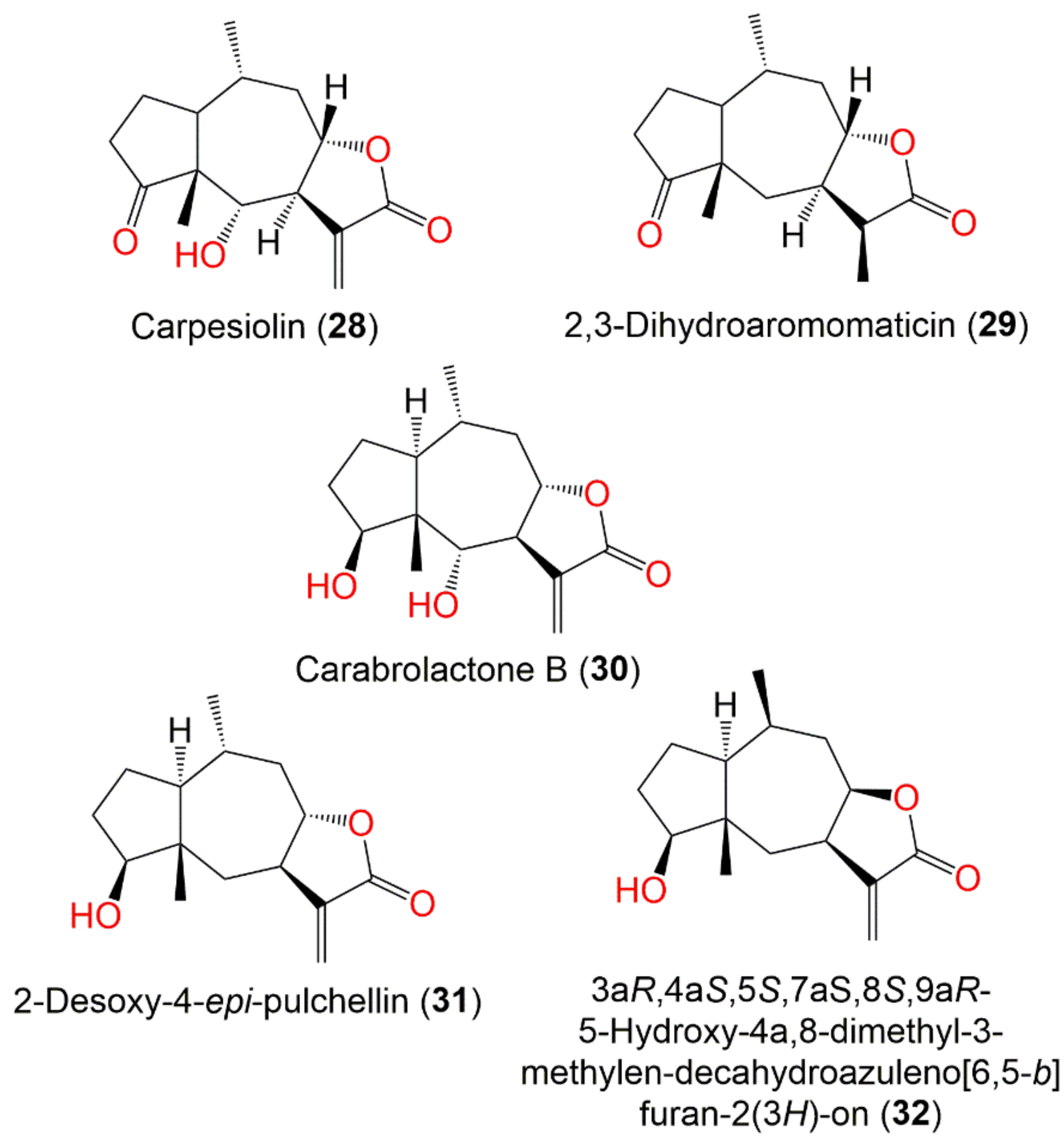
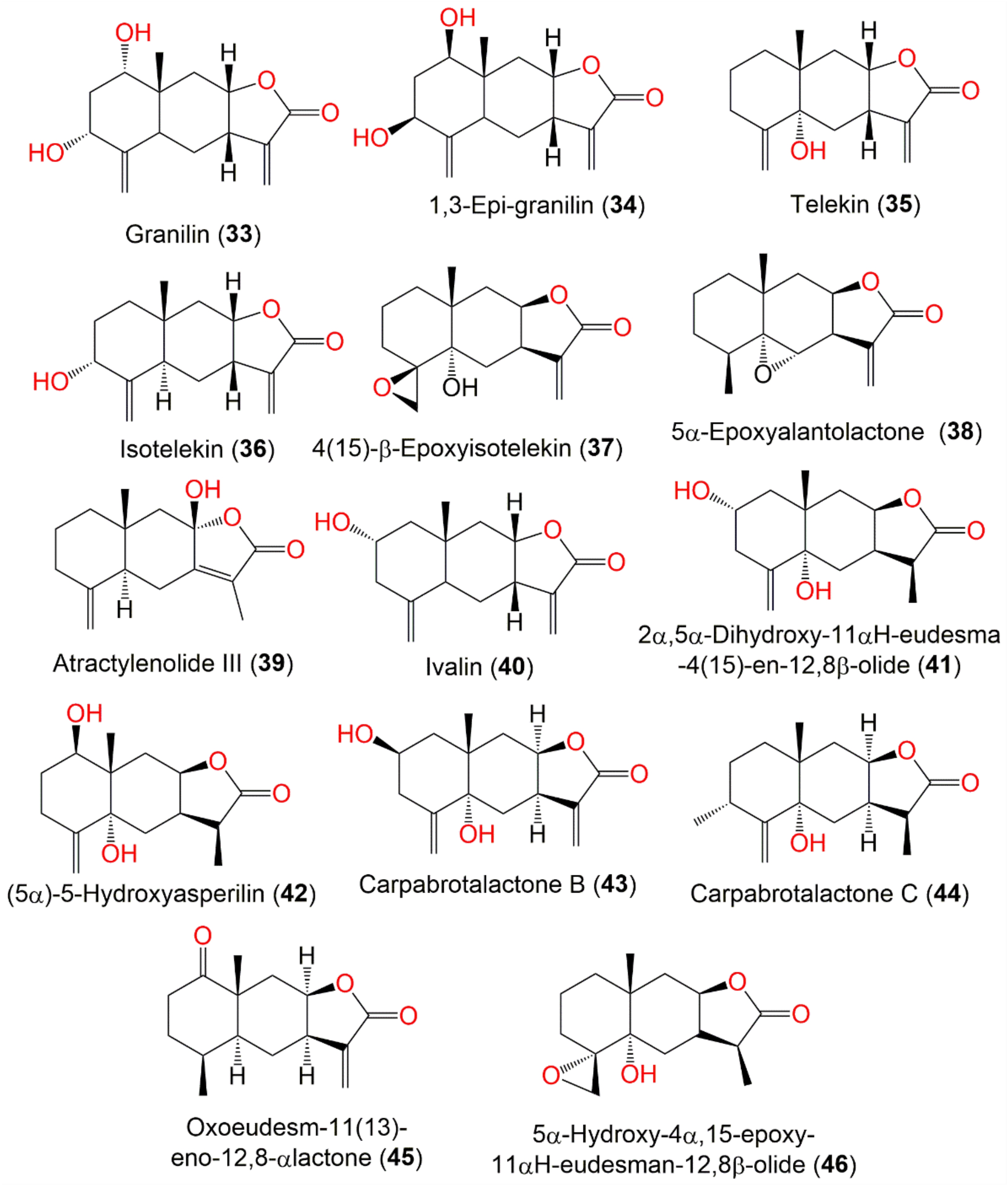

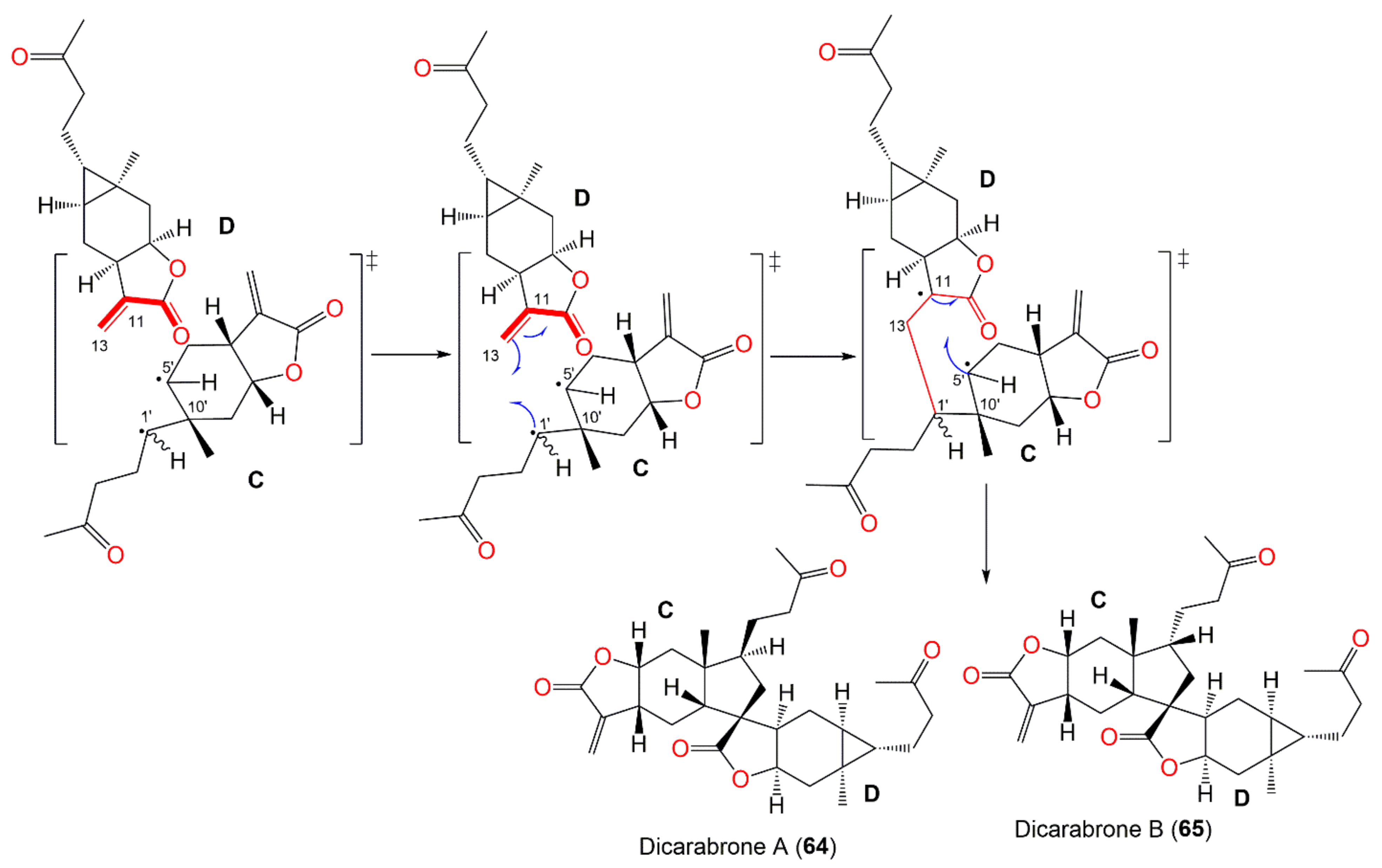
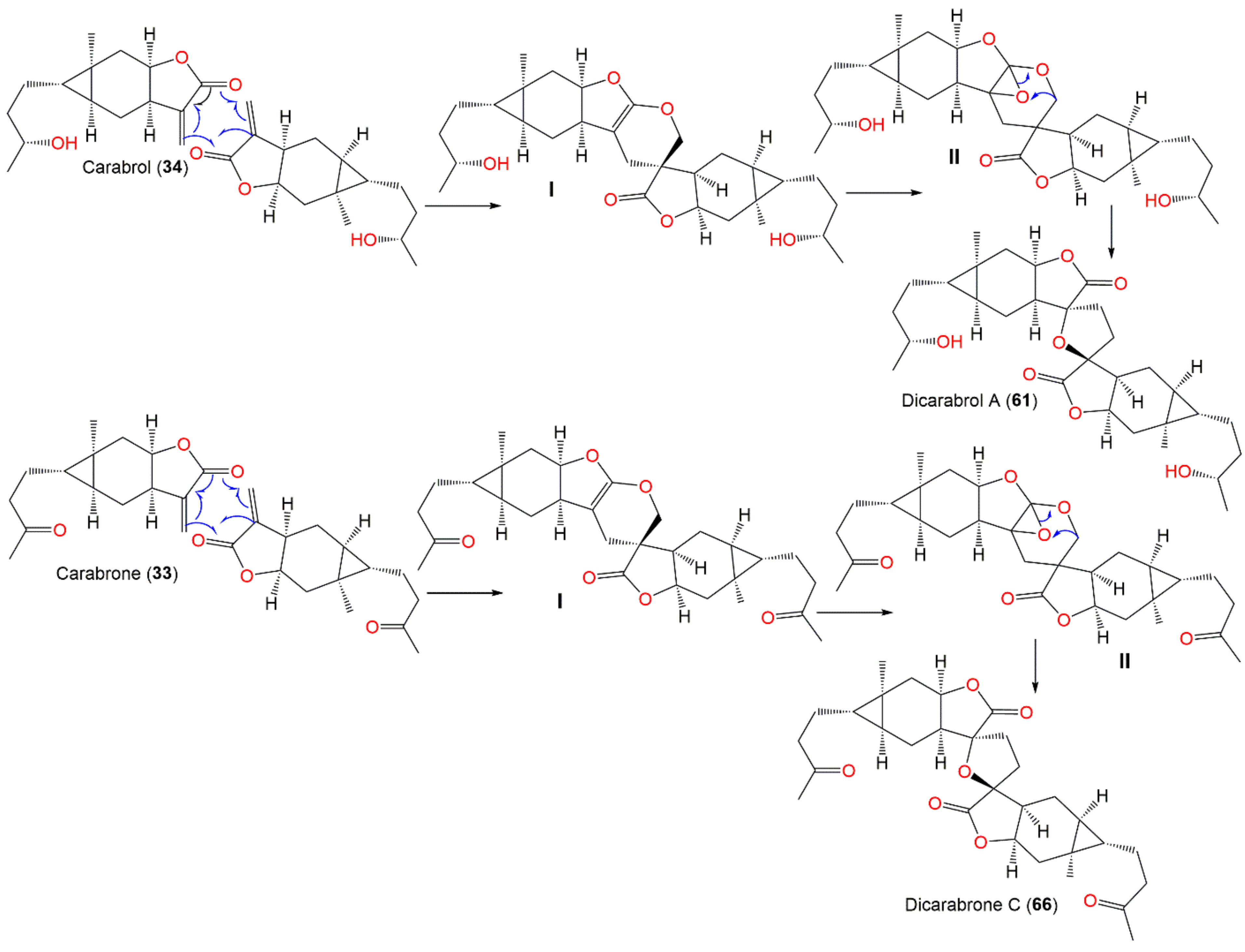

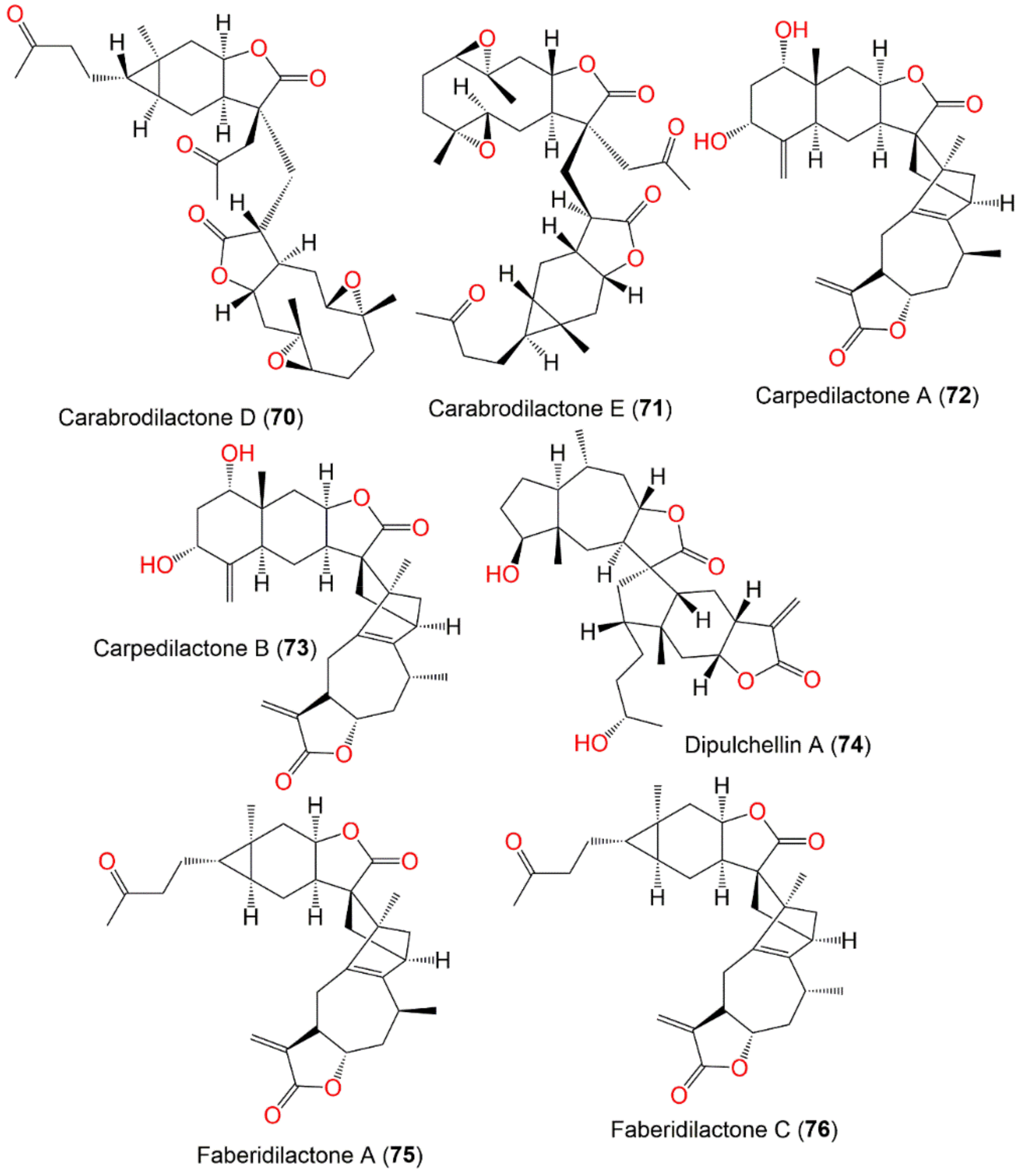
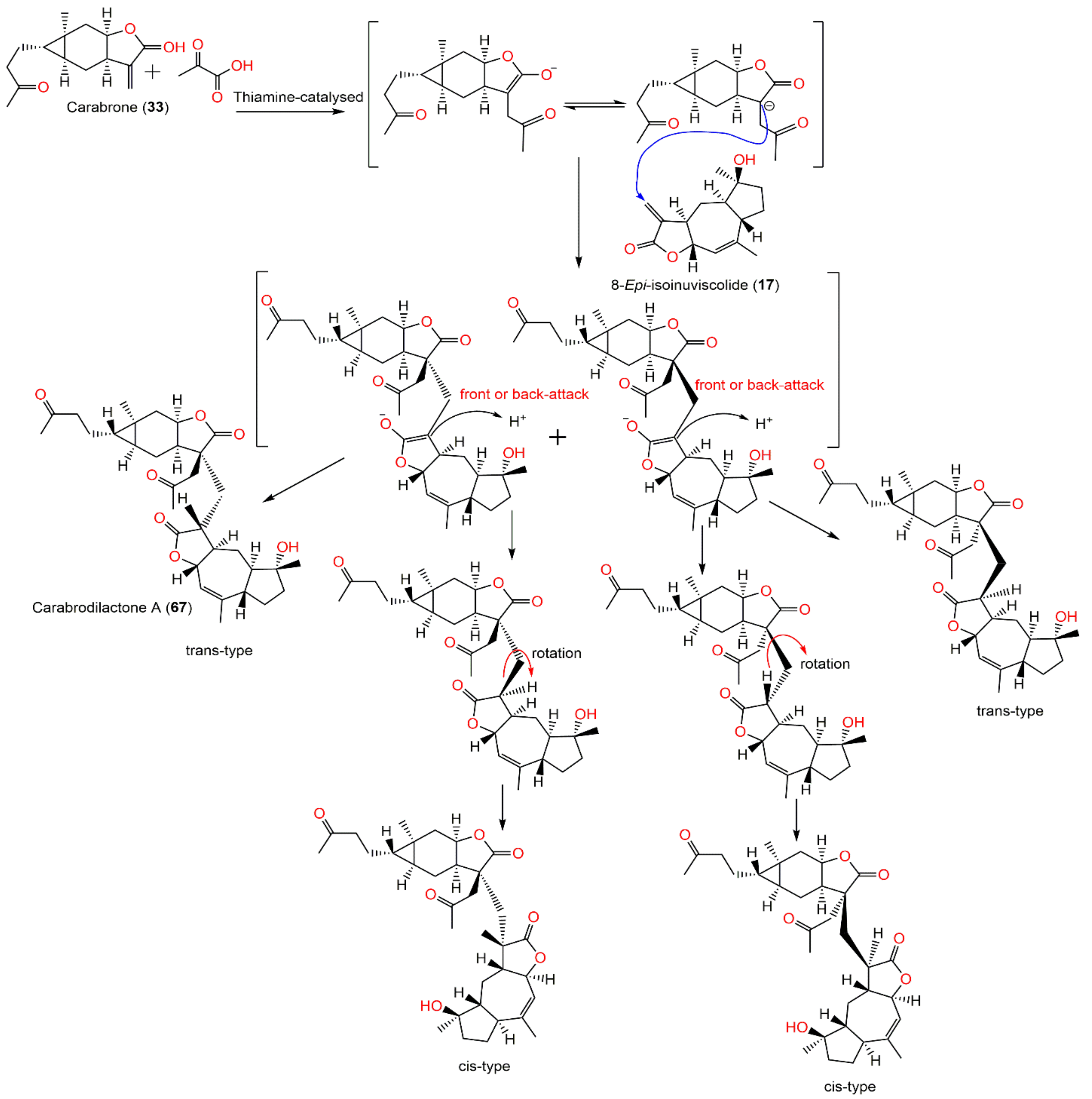

| Compound Name | Plant Part | Extract/Fraction | Mol. Wt | Mol. Formula | City, Country | Ref. |
|---|---|---|---|---|---|---|
| Monoterpenes | ||||||
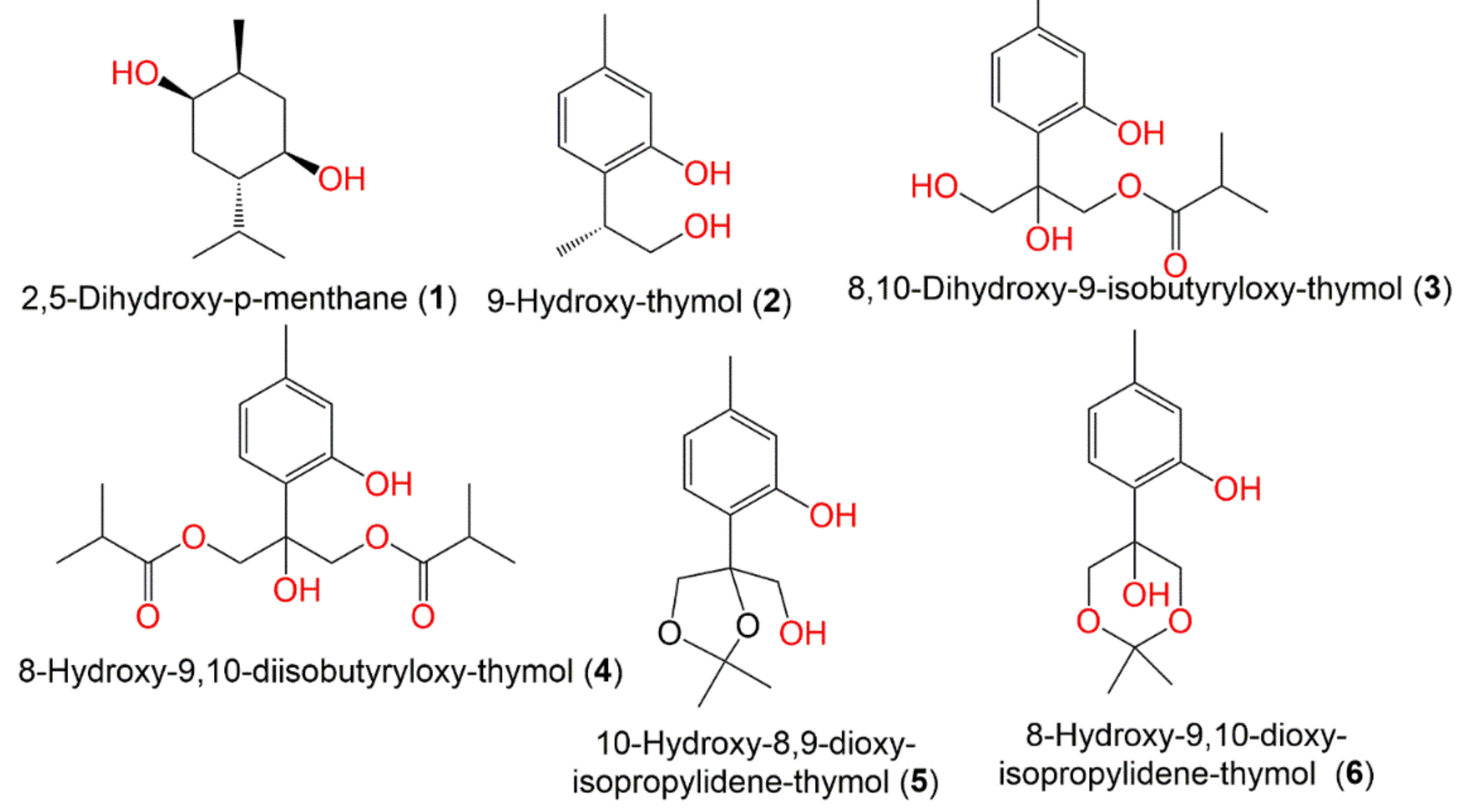
| 2,5-Dihydroxy-p-menthane (1) | Fruits | PE/acetone fraction of MeOH extract | 172 | C10H20O2 | Tianquan, Sichuan, China | [44] |
| 9-Hydroxy-thymol (2) | Whole plant | PE fraction of 95% EtOH extract | 166 | C10H14O2 | China | [45] |
| 8-Hydroxy-9,10-diisobutyryloxy-thymol (3) | Whole plant | PE fraction of 95% EtOH extract | 338 | C18H26O6 | China | [45] |
| 8,10-Dihydroxy-9-isobutyryloxy-thymol (4) | Whole plant | PE fraction of 95% EtOH extract | 268 | C14H20O5 | China | [45] |
| 10-Hydroxy-8,9-dioxy-isopropylidene-thymol (5) | Whole plant | PE fraction of 95% EtOH extract | 238 | C13H18O4 | China | [45] |
| 8-Hydroxy-9,10-dioxy-isopropylidene-thymol (6) | Whole plant | PE fraction of 95% EtOH extract | 238 | C13H18O4 | China | [45] |
| Guaianolide sesquiterpenes | ||||||
| Carpesia lactone (7) | Ripe seeds | Ether extract | 248 | C15H20O3 | Japan | [46,47,48,49,50] |
| Tetrahydro-carpesia lactone (8) | Ripe seeds | Ether extract | 252 | C15H24O3 | Japan | [46] |
| Dihydro-carpesia lactone (9) | Ripe seeds | Ether extract | 250 | C15H22O3 | Japan | [48] |
| Inuviscolide (10) | Whole plant | EtOAc fraction of 95% EtOH extract | 248 | C15H20O3 | Changyang, Hubei, China | [51] |
| Whole plant | EtOAc fraction of 95% MeOH extract | - | - | Bozhou, Anhui, China | [52] | |
| 4-Epi-isoinuviscolide (11) | Aerial parts | PE/acetone fraction of 95% EtOH extract | 248 | C15H20O3 | Tiger Leaping Gorge, Yunnan, China | [13] |
| 8-Epi-isoinuviscolide (12) | Whole plant | EtOAc fraction of 95% EtOH extract | 248 | C15H20O3 | Changyang, Hubei, China | [51] |
| 4β,10β-dihydroxy-5αH-1,11(13)-guaidien-8α,12-olide (13) | Whole plant | EtOAc fraction of 95% EtOH extract | 264 | C15H20O4 | Hebei, China | [53] |
| 4α,5α-Epoxy-10α,14-dihydro-inuviscolide (14) | Aerial parts | CHCl3-soluble fraction of MeOH extract | 248 | C15H20O3 | Korea | [51] |
| 9β-Hydroxy-1βH,11αH-guaia-4,10(14)-dien-12,8α-olide (15) | Fruits | PE/acetone fraction of MeOH extract | 248 | C15H20O3 | Tianquan, Sichuan, China | [44] |
| 9β-Hydroxy-1βH,11βH-guaia-4,10(14)-dien-12,8α-olide (16) | Fruits | PE/acetone fraction of MeOH extract | 248 | C15H20O3 | Tianquan, Sichuan, China | [44] |
| Caroguaianolide A (17) | Whole plant | EtOAc fraction of 95% EtOH extract | 262 | C15H18O4 | Changyang, Hubei, China | [51] |
| Caroguaianolide B (18) | Whole plant | EtOAc fraction of 95% EtOH extract | 280 | C15H20O5 | Changyang, Hubei, China | [51] |
| Caroguaianolide C (19) | Whole plant | EtOAc fraction of 95% EtOH extract | 280 | C15H20O5 | Changyang, Hubei, China | [51] |
| Caroguaianolide D (20) | Whole plant | EtOAc fraction of 95% EtOH extract | 280 | C15H20O5 | Changyang, Hubei, China | [51] |
| Caroguaianolide E (21) | Whole plant | EtOAc fraction of 95% EtOH extract | 280 | C15H20O5 | Changyang, Hubei, China | [51] |
| Akihalin (22) | Whole plant | EtOAc fraction of 95% EtOH extract | 280 | C15H20O5 | Changyang, Hubei, China | [51] |
| 4β-Hydroxy,10β-hydroperoxyl,5αH,7αH,8βH-guaia-1,11(13)- dien-8α,12-olide (23) | Whole plant | EtOAc fraction of 95% EtOH extract | 280 | C15H20O5 | Changyang, Hubei, China | [51] |
| 4α-Hydroxy-9β,10β-epoxy-1βH,5αH-guaia-11(13)-en-8α,12-olide (24) | Whole plant | EtOAc fraction of 95% EtOH extract | 264 | C15H20O4 | Changyang, Hubei, China | [51] |
| 4α-Hydroxy-1βH-guaia-9,11(13)-dien-12,8α-olide (25) | Whole plant | EtOAc fraction of 95% EtOH extract | 248 | C15H20O3 | Changyang, Hubei, China | [51] |
| 1α,4α-Dihydroxy-guaia-11(13)-ene-12,8α-olide (26) | Whole plant | EtOAc fraction of 70% EtOH extract | 266 | C15H22O4 | Changsha, Hunan, China | [54] |
| 4α,5α-Dihydroxy-guaia-11(13)-en-12,8α-lactone (27) | Whole plant | EtOAc fraction of 70% EtOH extract | 266 | C15H22O4 | Changsha, Hunan, China | [54] |
| Pseudo-guaianolide sesquiterpenes | ||||||
| Carpesiolin (28) | Whole plant | CHCl3-soluble fraction of MeOH extract | 264 | C15H20O4 | Japan | [55] |
| Aerial parts | CHCl3-soluble fraction of MeOH extract | - | - | Korea | [51] | |
| 2,3-Dihydroaromomaticin (29) | Aerial parts | CHCl3-soluble fraction of MeOH extract | 250 | C15H22O3 | Korea | [51] |
| Whole plant | PE fraction of 95% MeOH extract | - | - | Bozhou, Anhui, China | [52] | |
| Carabrolactone B (30) | Aerial parts | PE/acetone fraction of 95% EtOH extract | 266 | C15H22O4 | Tiger Leaping Gorge, Yunnan, China | [13] |
| Whole plant | EtOAc fraction of 95% EtOH extract | - | - | Changyang, Hubei, China | [51] | |
| Whole plant | EtOAc fraction of 95% MeOH extract | - | - | Bozhou, Anhui, China | [52] | |
| 2-Desoxy-4-epi-pulchellin (31) | Aerial parts | PE/acetone fraction of 95% EtOH extract | 250 | C15H22O3 | Tiger Leaping Gorge, Yunnan, China | [13] |
| Whole plant | EtOAc fraction of 95% EtOH extract | - | - | Changsha County of Hunan, China | [56] | |
| Whole plant | EtOAc fraction of 95% EtOH extract | - | - | Changyang, Hubei, China | [51] | |
| Whole plant | EtOAc fraction of 95% EtOH extract | - | - | Hebei, China | [53] | |
| Whole plant | EtOAc fraction of 95% MeOH extract | - | - | Bozhou, Anhui, China | [52] | |
| 3aR,4aS,5S,7aS,8S,9aR)-5-Hydroxy-4a,8-dimethyl-3-methylen-decahydroa-zuleno [6,5-b]furan-2(3H)-on (32) | Whole plant | EtOAc fraction of 95% EtOH extract | 250 | C15H22O3 | Changyang, Hubei, China | [51] |
| Eudesmanolide sesquiterpenes | ||||||
| Granilin (33) | Whole plant | CHCl3-soluble fraction of MeOH extract | 264 | C15H20O4 | Japan | [57] |
| 1.3-Epi-granilin (34) | Whole plant | EtOAc fraction of 95% MeOH extract | 264 | C15H20O4 | Bozhou, Anhui, China | [52] |
| Fruits | PE/acetone fraction of MeOH extract | - | - | Tianquan, Sichuan, China | [44] | |
| Telekin (35) | Aerial parts | CHCl3-soluble fraction of MeOH extract | 248 | C15H20O3 | Korea | [51] |
| Whole plant | EtOAc fraction of 70% EtOH extract | - | - | Dao County, Hunan, China | [16] | |
| Whole plant | PE fraction of 95% MeOH extract | - | - | Bozhou, Anhui, China | [52] | |
| Isotelekin (36) | Whole plant | EtOAc fraction of 95% MeOH extract | 248 | C15H20O3 | Bozhou, Anhui, China | [52] |
| 4(15)-β-Epoxyisotelekin (37) | Whole plant | EtOAc fraction of 95% EtOH extract | 264 | C15H20O4 | Hebei, China | [53] |
| Whole plant | EtOAc fraction of 95% MeOH extract | - | - | Bozhou, Anhui, China | [52] | |
| 5α-Epoxyalantolactone (38) | Whole plant | EtOAc fraction of 95% MeOH extract | 248 | C15H20O3 | Bozhou, Anhui, China | [52] |
| Atractylenolide III (39) | Whole plant | EtOAc fraction of 95% MeOH extract | 248 | C15H20O3 | Bozhou, Anhui, China | [52] |
| Ivalin (40) | Aerial parts | CHCl3-soluble fraction of MeOH extract | 248 | C15H20O3 | Korea | [51] |
| Whole plant | EtOAc fraction of 95% MeOH extract | - | - | Bozhou, Anhui, China | [52] | |
| 2α,5α-Dihydroxy-11αH-eudesma-4(15)-en-12,8β-olide (41) | Whole plant | EtOAc fraction of 70% EtOH extract | 266 | C15H22O4 | Changsha, Hunan, China | [54] |
| Whole plant | EtOAc fraction of 70% EtOH extract | - | - | Dao County, Hunan, China | [16] | |
| (5α)-5-Hydroxyasperilin (42) | Whole plant | EtOAc fraction of 70% EtOH extract | 266 | C15H22O4 | Changsha, Hunan, China | [54] |
| Whole plant | PE fraction of 95% MeOH extract | - | - | Bozhou, Anhui, China | [52] | |
| Carpabrotalactone B (43) | Whole plant | EtOAc fraction of 70% EtOH extract | 264 | C15H20O4 | Changsha, Hunan, China | [54] |
| Carpabrotalactone C (44) | Whole plant | EtOAc fraction of 70% EtOH extract | 264 | C15H20O4 | Changsha, Hunan, China | [58] |
| Oxoeudesm-11(13)-eno-12,8α-lactone (45) | Whole plant | EtOAc fraction of 70% EtOH extract | 248 | C15H20O3 | Dao County, Hunan, China | [16] |
| 5α-Hydroxy-4α,15-epoxy-11αH-eudesman-12,8β-olide (46) | Whole plant | EtOAc fraction of 95% EtOH extract | 266 | C15H22O4 | Hebei, China | [53] |
| Germacranolide sesquiterpenes | ||||||
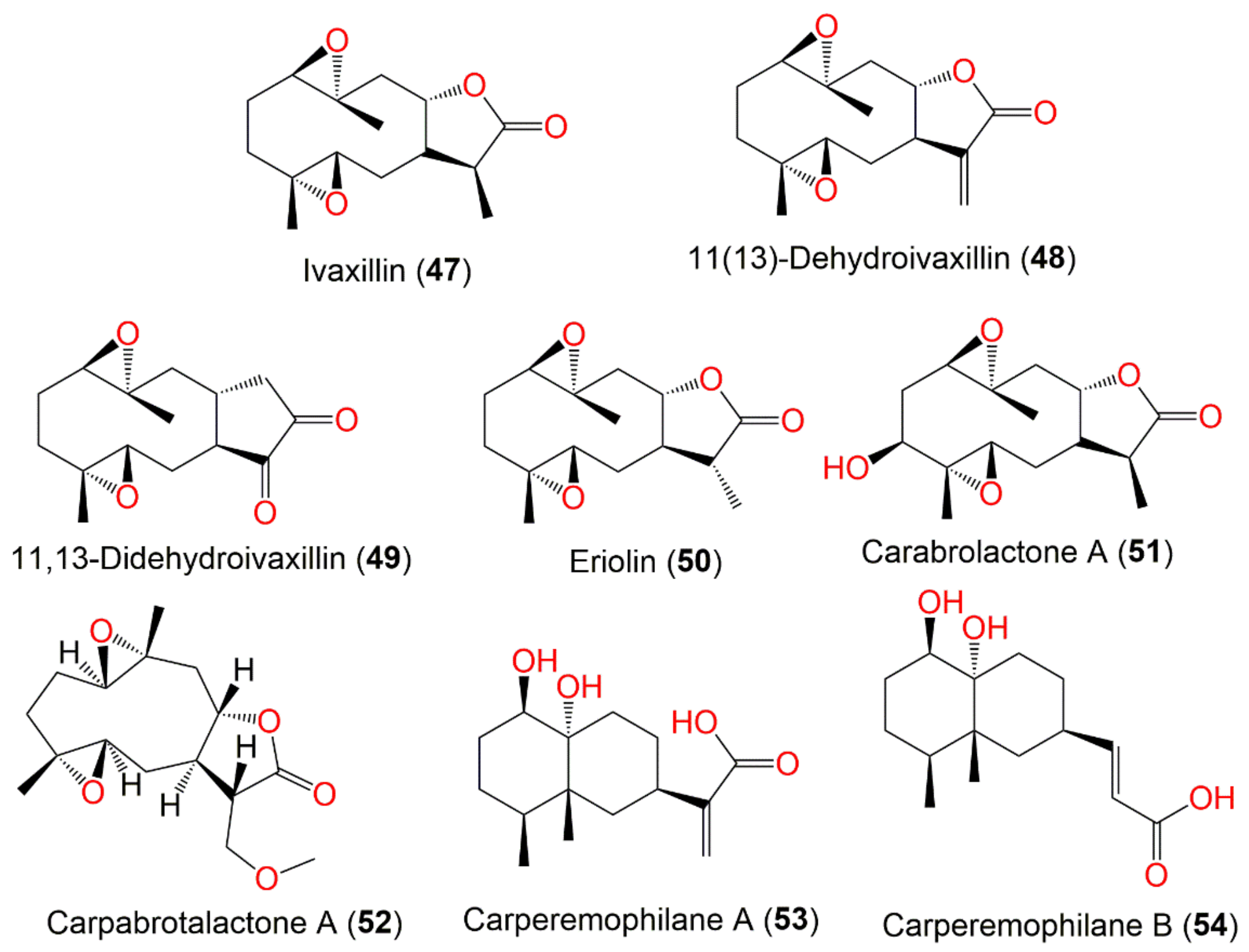
| Ivaxillin (47) | Whole plant | CHCl3 fraction of MeOH extract | 266 | C15H22O4 | Japan | [59] |
| Aerial parts | PE/acetone fraction of 95% EtOH extract | - | - | Tiger Leaping Gorge, Yunnan, China | [13] | |
| Fruits | PE/acetone fraction of MeOH extract | - | - | Tianquan, Sichuan, China | [44] | |
| 11(13)-Dehydroivaxillin (48) | Whole plant | CHCl3 fraction of MeOH extract | 264 | C15H20O4 | Japan | [59] |
| Whole plant | EtOAc fraction of 95% EtOH extract | - | - | Changsha, Hunan, China | [56] | |
| Whole plant | EtOAc fraction of 70% EtOH extract | - | - | Changsha, Hunan, China | [54] | |
| Whole plant | PE fraction of 95% MeOH extract | - | - | Bozhou, Anhui, China | [52] | |
| 11,13-Didehydroivaxillin (49) | Aerial parts | CHCl3 fraction of MeOH extract | 264 | C15H20O4 | Korea | [51] |
| Eriolin (50) | Whole plant | CHCl3-soluble fraction of MeOH extract | 266 | C15H22O4 | Japan | [59] |
| Aerial parts | PE/acetone fraction of 95% EtOH extract | - | - | Tiger Leaping Gorge, Yunnan, China | [13] | |
| Fruits | PE/acetone fraction of MeOH extract | - | - | Tianquan, Sichuan, China | [44] | |
| Whole plant | EtOAc fraction of 70% EtOH extract | - | - | Changsha, Hunan, China | [54] | |
| Carabrolactone A (51) | Aerial parts | PE/acetone fraction of 95% EtOH extract | 282 | C15H22O5 | Tiger Leaping Gorge, Yunnan, China | [13] |
| Carpabrotalactone A (52) | Whole plant | EtOAc fraction of 70% EtOH extract | 296 | C16H24O5 | Changsha, Hunan, China | [54] |
| Eremophilanoide sesquiterpenes | ||||||
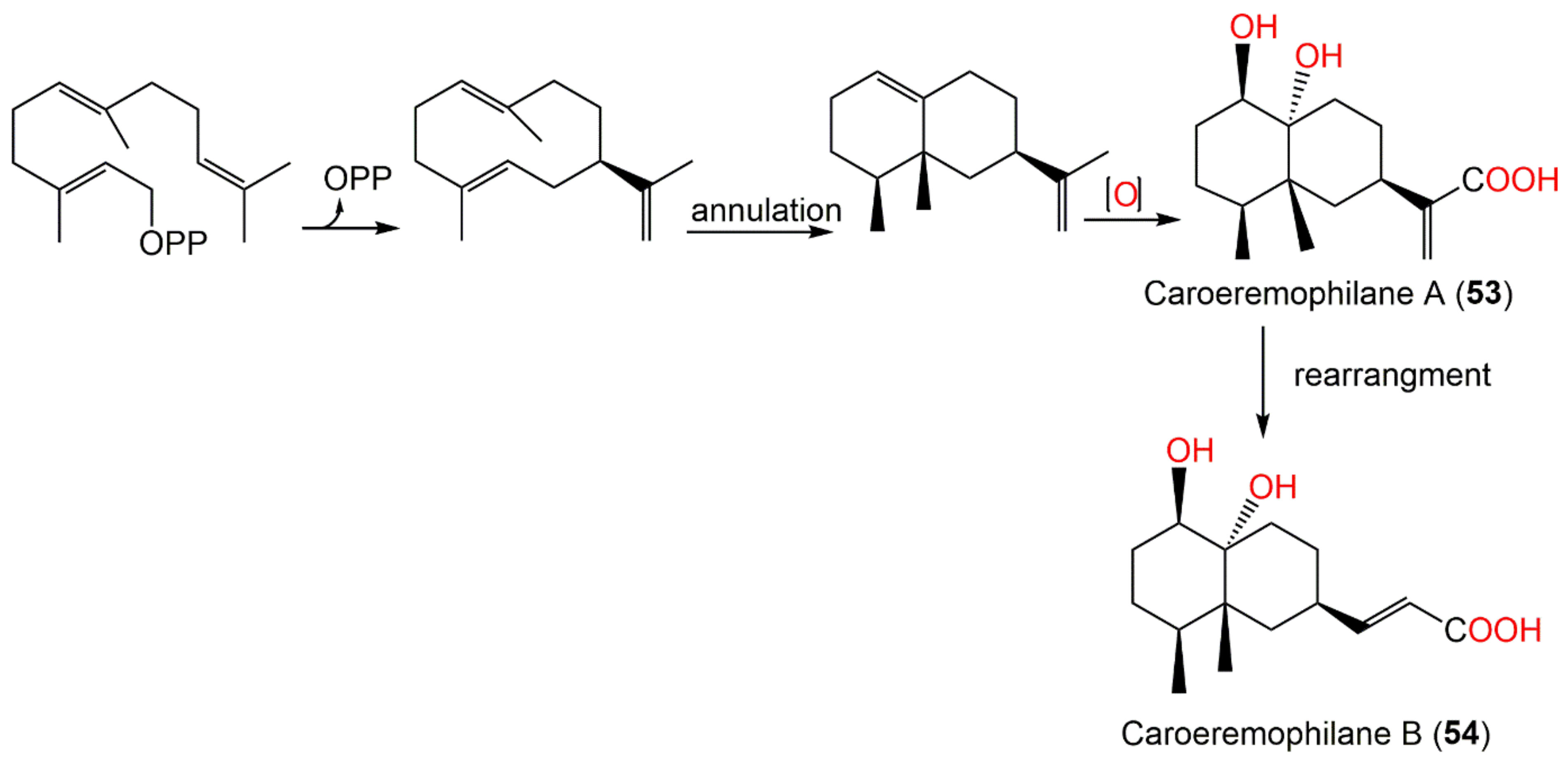
| Carperemophilane A (53) | Whole plant | EtOAc fraction of 95% EtOH extract | 268 | C15H24O4 | Changyang, Hubei, China | [60] |
| Carperemophilane B (54) | Whole plant | EtOAc fraction of 95% EtOH extract | 268 | C15H24O4 | Changyang, Hubei, China | [60] |
| Sesquiterpenes with cyclopropane ring | ||||||
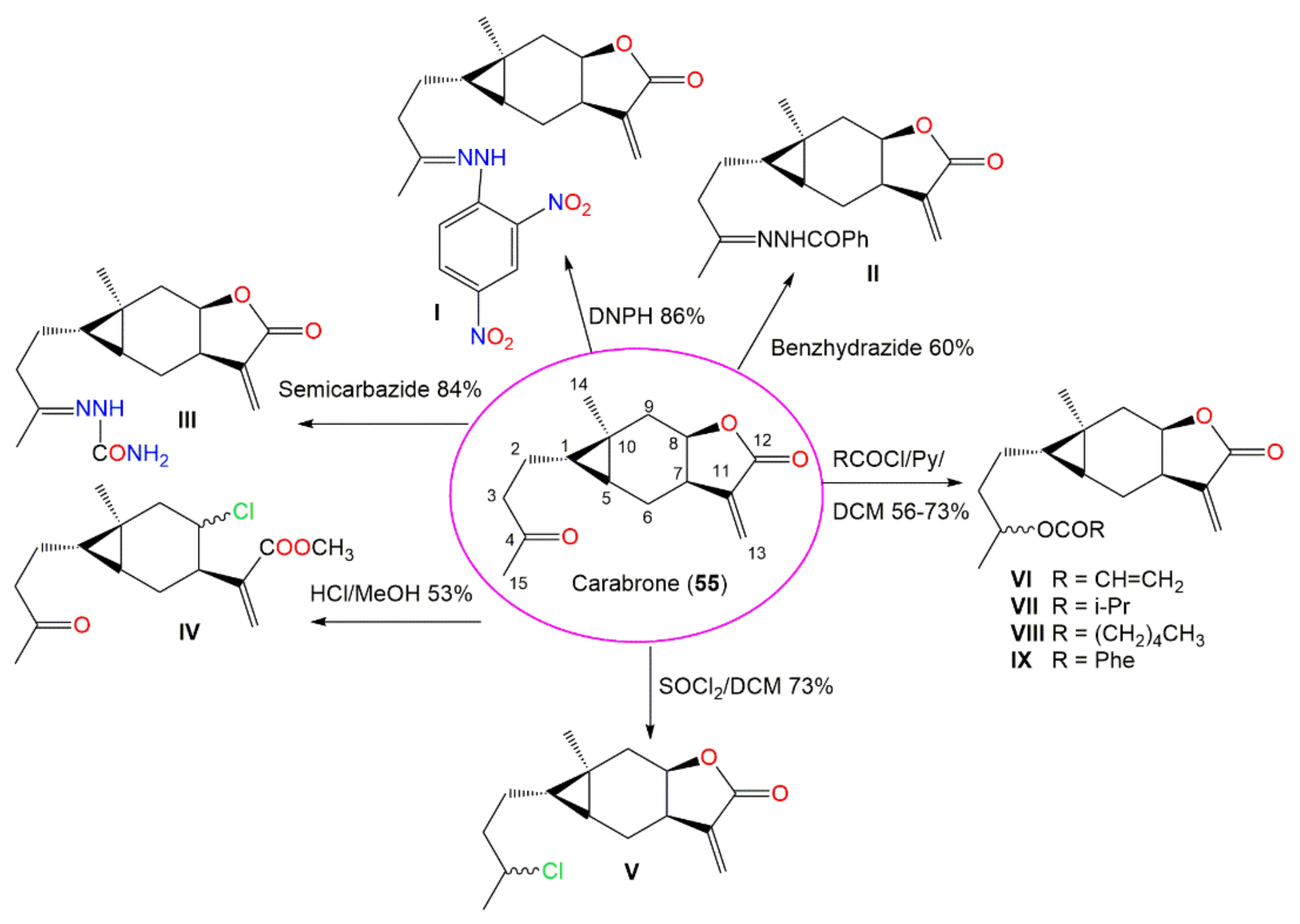
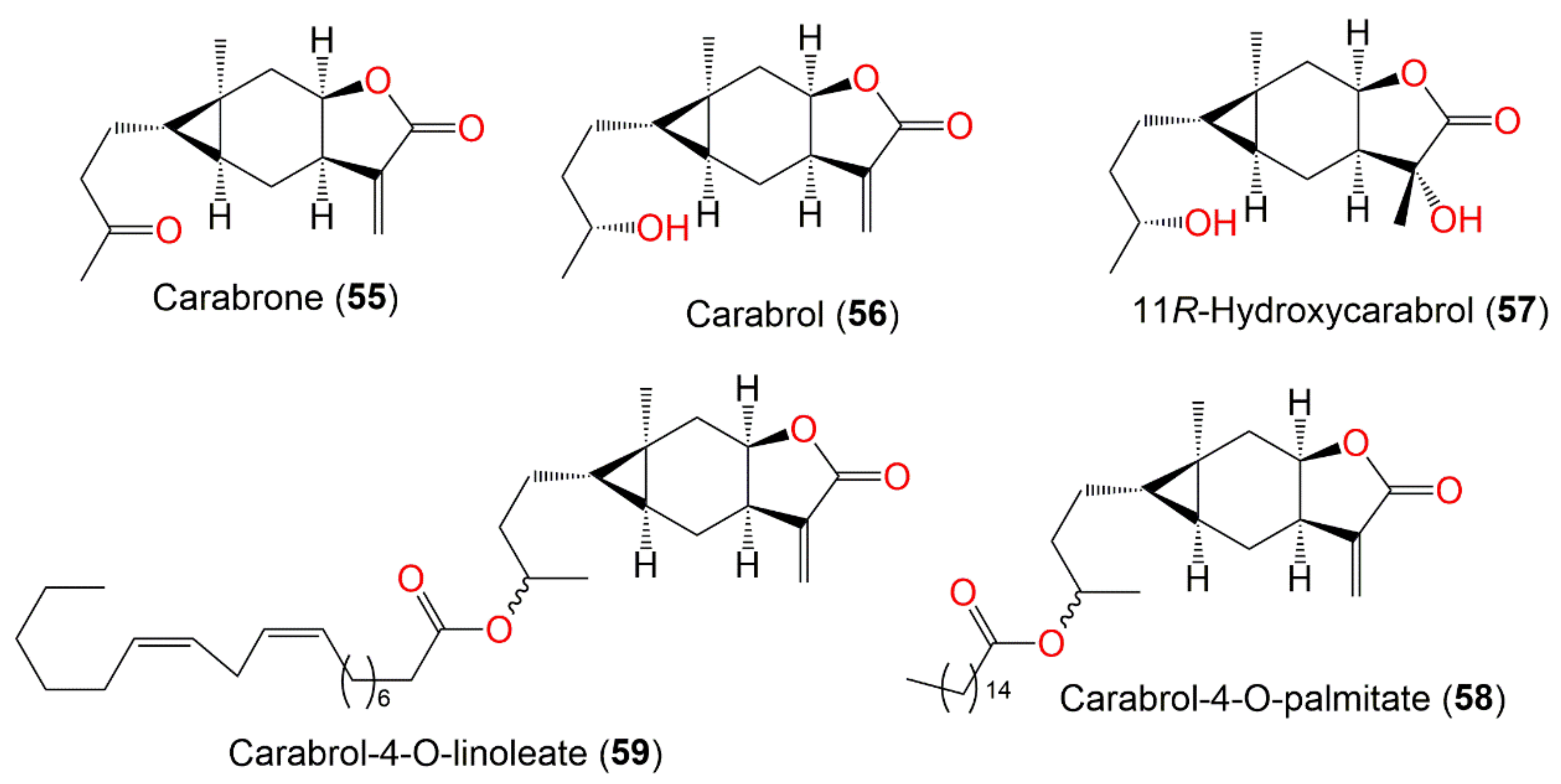
| Carabrone (55) | Fruits | Ether extract | 248 | C15H20O3 | Japan | [61] |
| Whole plant | CHCl3-soluble fraction of MeOH extract | - | - | Japan | [59] | |
| Aerial parts | PE/acetone fraction of 95% EtOH extract | - | - | Tiger Leaping Gorge, Yunnan, China | [13] | |
| Aerial parts | CHCl3 fraction of MeOH extract | - | - | Korea | [51] | |
| Fruits | PE/acetone fraction of MeOH extract | - | - | Tianquan, Sichuan, China | [44] | |
| Whole plant | EtOAc fraction of 95% EtOH extract | - | - | Hebei, China | [53] | |
| Carabrol (56) | Whole plant | CHCl3-soluble fraction of MeOH extract | 250 | C15H22O3 | Japan | [59] |
| Aerial parts | CHCl3-soluble fraction of MeOH extract | - | - | Korea | [51] | |
| Whole plant | EtOAc fraction of 95% EtOH extract | - | - | Changsha, Hunan, China | [56] | |
| Whole plant | EtOAc fraction of 95% EtOH extract | - | - | Hebei, China | [53] | |
| Whole plant | EtOAc fraction of 95% EtOH extract | - | - | Changyang, Hubei, China | [60] | |
| Whole plant | EtOAc fraction of 95% MeOH extract | - | - | Bozhou, Anhui, China | [52] | |
| 11R-Hydroxycarabrol (57) | Whole plant | CHCl3-soluble fraction of 95% EtOH | 268 | C15H24O4 | Henan, China | [62] |
| Carabrol-4-O-palmitate (58) | Whole plant | EtOAc fraction of 95% EtOH extract | 488 | C31H52O4 | Hebei, China | [53] |
| Carabrol-4-O-linoleate (59) | Whole plant | EtOAc fraction of 95% EtOH extract | 516 | C33H56O4 | Hebei, China | [53] |
| Dimeric sesquiterpenes | ||||||
| Dicarabrol (60) | Whole plant | EtOAc fraction of EtOH extract | 500 | C30H44O6 | Changsha, Hunan, China | [56] |
| Dicarabrol A (61) | Whole plant | CH2Cl2-soluble fraction of 95% EtOH extract | 516 | C30H44O7 | Henan, China | [63] |
| Dicarabrol B (62) | Whole plant | CHCl3-soluble fraction of 95% EtOH | 500 | C30H44O6 | Henan, China | [62] |
| Dicarabrol C (63) | Whole plant | CHCl3-soluble fraction of 95% EtOH | 500 | C30H44O6 | Henan, China | [62] |
| Dicarabrone A (64) | Whole plant | CHCl3-soluble fraction of 95% EtOH extract | 496 | C30H40O6 | Henan, China | [64] |
| Dicarabrone B (65) | Whole plant | CHCl3-soluble fraction of 95% EtOH extract | 496 | C30H40O6 | Henan, China | [64] |
| Dicarabrone C (66) | Whole plant | CH2Cl2-soluble fraction of 95% EtOH extract | 512 | C30H40O7 | Henan, China | [63] |
| Carabrodilactone A (67) | Whole plant | EtOAc fraction of 95% EtOH extract | 540 | C32H44O7 | Pu’an, GuiZhou, China | [65] |
| Carabrodilactone B (68) | Whole plant | EtOAc fraction of 95% EtOH extract | 542 | C32H46O7 | Pu’an, GuiZhou, China | [65] |
| Carabrodilactone C (69) | Whole plant | EtOAc fraction of 95% EtOH extract | 556 | C32H44O8 | Pu’an, GuiZhou, China | [65] |
| Carabrodilactone D (70) | Whole plant | EtOAc fraction of 95% EtOH extract | 556 | C32H44O8 | Pu’an, GuiZhou, China | [65] |
| Carabrodilactone E (71) | Whole plant | EtOAc fraction of 95% EtOH extract | 556 | C32H44O8 | Pu’an, GuiZhou, China | [65] |
| Carpedilactone A (72) | Whole plant | EtOAc fraction of 95% EtOH extract | 494 | C30H38O6 | Pu’an, GuiZhou, China | [65] |
| Carpedilactone B (73) | Whole plant | EtOAc fraction of 95% EtOH extract | 494 | C30H38O6 | Pu’an, GuiZhou, China | [65] |
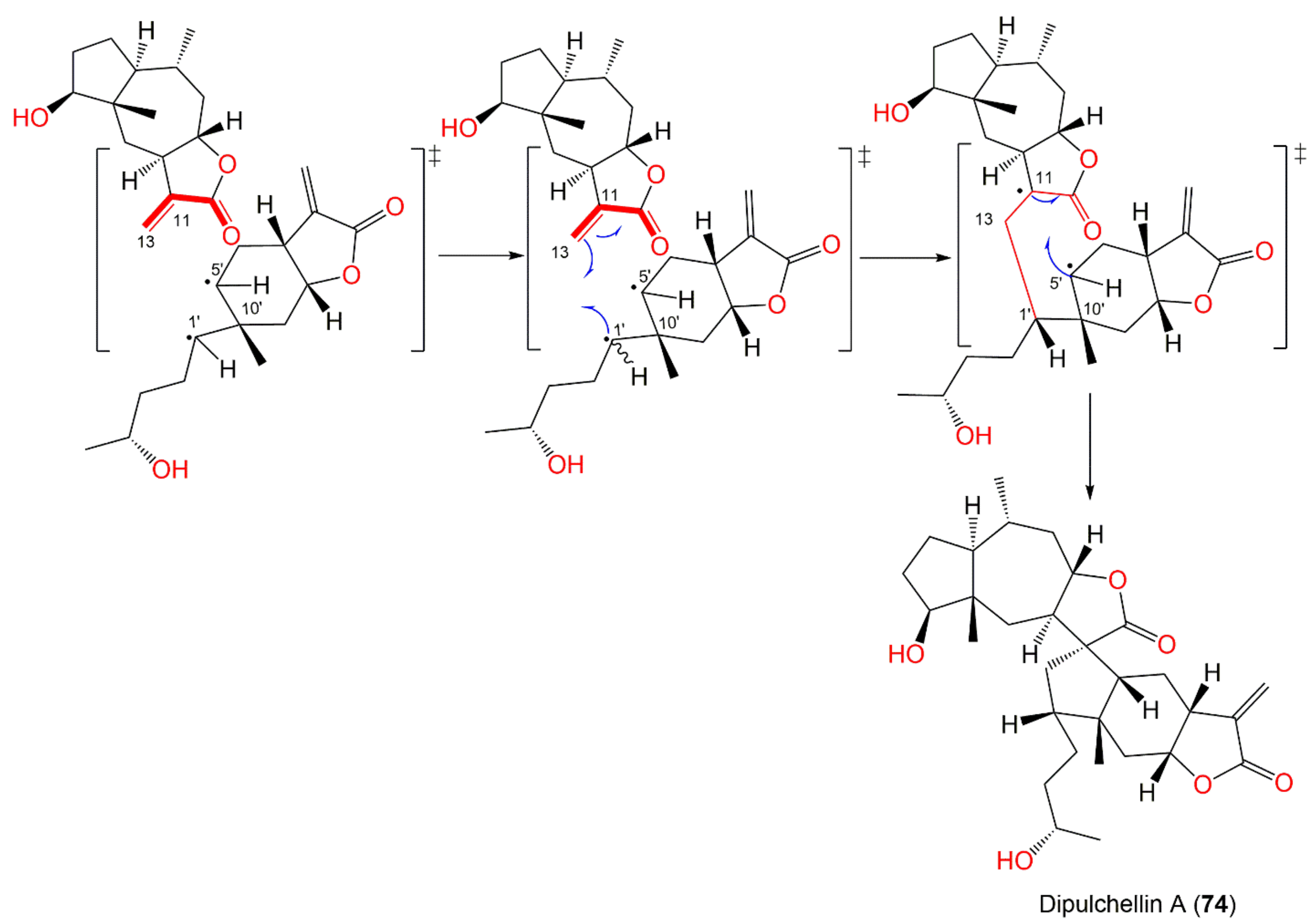
| Dipulchellin A (74) | Whole plant | CH2Cl2-soluble fraction of 95% EtOH extract | 500 | C30H44O6 | Henan, China | [63] |
| Faberidilactone A (75) | Whole plant | EtOAc fraction of 95% EtOH extract | 478 | C30H38O5 | Pu’an, GuiZhou, China | [65] |
| Faberidilactone C (76) | Whole plant | EtOAc fraction of 95% EtOH extract | 478 | C30H38O5 | Pu’an, GuiZhou, China | [65] |
| Other metabolites | ||||||
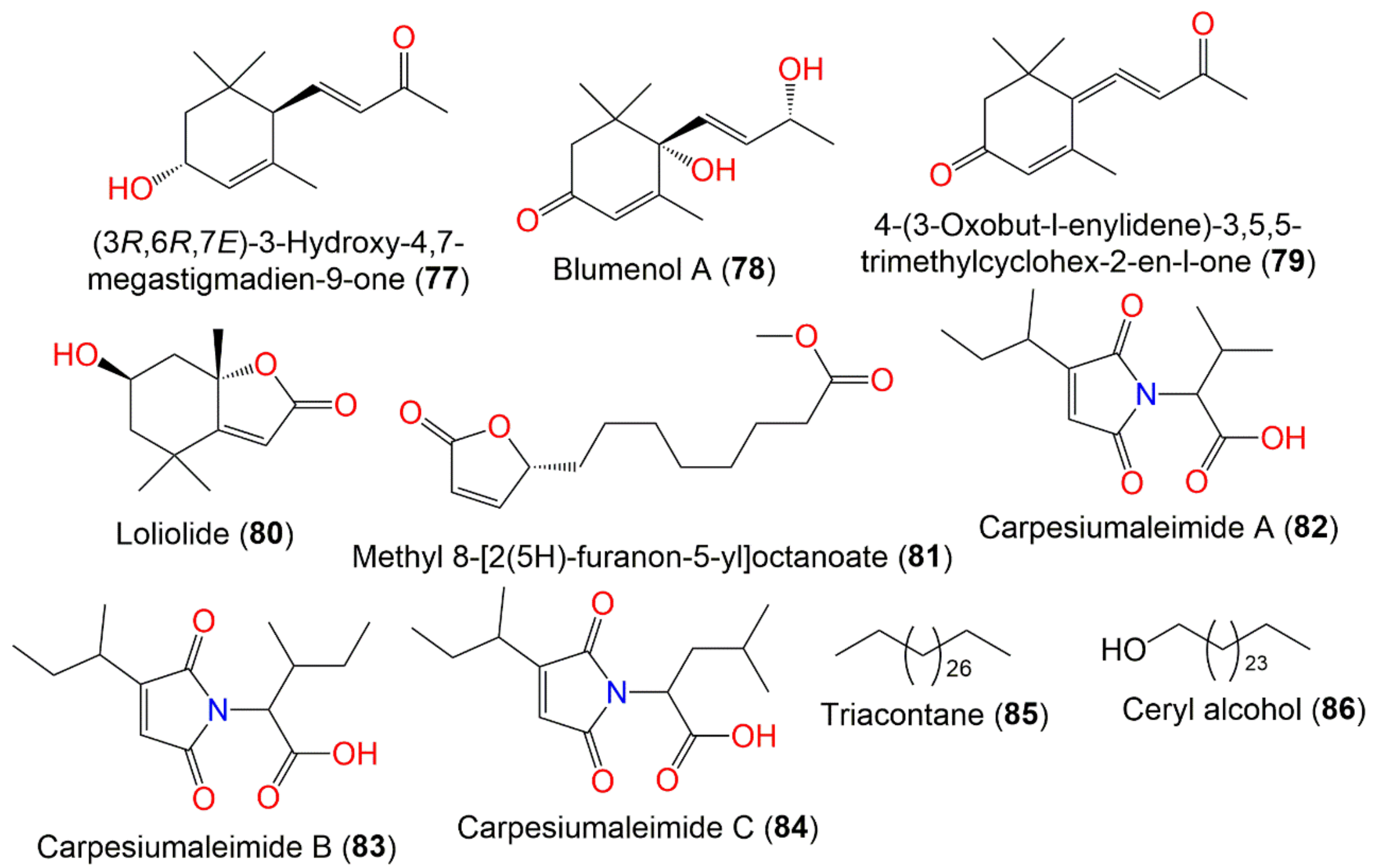
| (3R,6R,7E)-3-Hydroxy-4,7-megastigmadien-9-one (77) | Whole plant | PE fraction of 95% EtOH extract | 208 | C13H20O2 | China | [45] |
| Blumenol A (78) | Whole plant | PE fraction of 95% EtOH extract | 224 | C13H20O3 | China | [45] |
| 4-(3-Oxobut-l-enylidene)-3,5,5-trimethylcyclohex-2-en-l-one (79) | Whole plant | PE fraction of 95% EtOH extract | 208 | C13H16O2 | China | [45] |
| Loliolide (80) | Whole plant | PE fraction of 95% EtOH extract | 196 | C11H16O3 | China | [45] |
| Methyl 8-[2(5H)-furanon-5-yl]octanoate (81) | Whole plant | EtOAc fraction of 70% EtOH extract | 240 | C13H20O4 | Changsha, Hunan, China | [58] |
| Carpesiumaleimide A (82) | Whole plant | EtOAc fraction of 95% EtOH extract | 253 | C13H19NO4 | Changyang, Hubei, China | [60] |
| Carpesiumaleimide C (83) | Whole plant | EtOAc fraction of 95% EtOH extract | 267 | C14H21NO4 | Changyang, Hubei, China | [60] |
| Carpesiumaleimide B (84) | Whole plant | EtOAc fraction of 95% EtOH extract | 267 | C14H21NO4 | Changyang, Hubei, China | [60] |
| Triacontane (85) | Ripe seeds | Ether extract | 422 | C30H62 | Japan | [46] |
| Ceryl alcohol (86) | Ripe seeds | Ether extract | 382 | C26H54O | Japan | [66] |
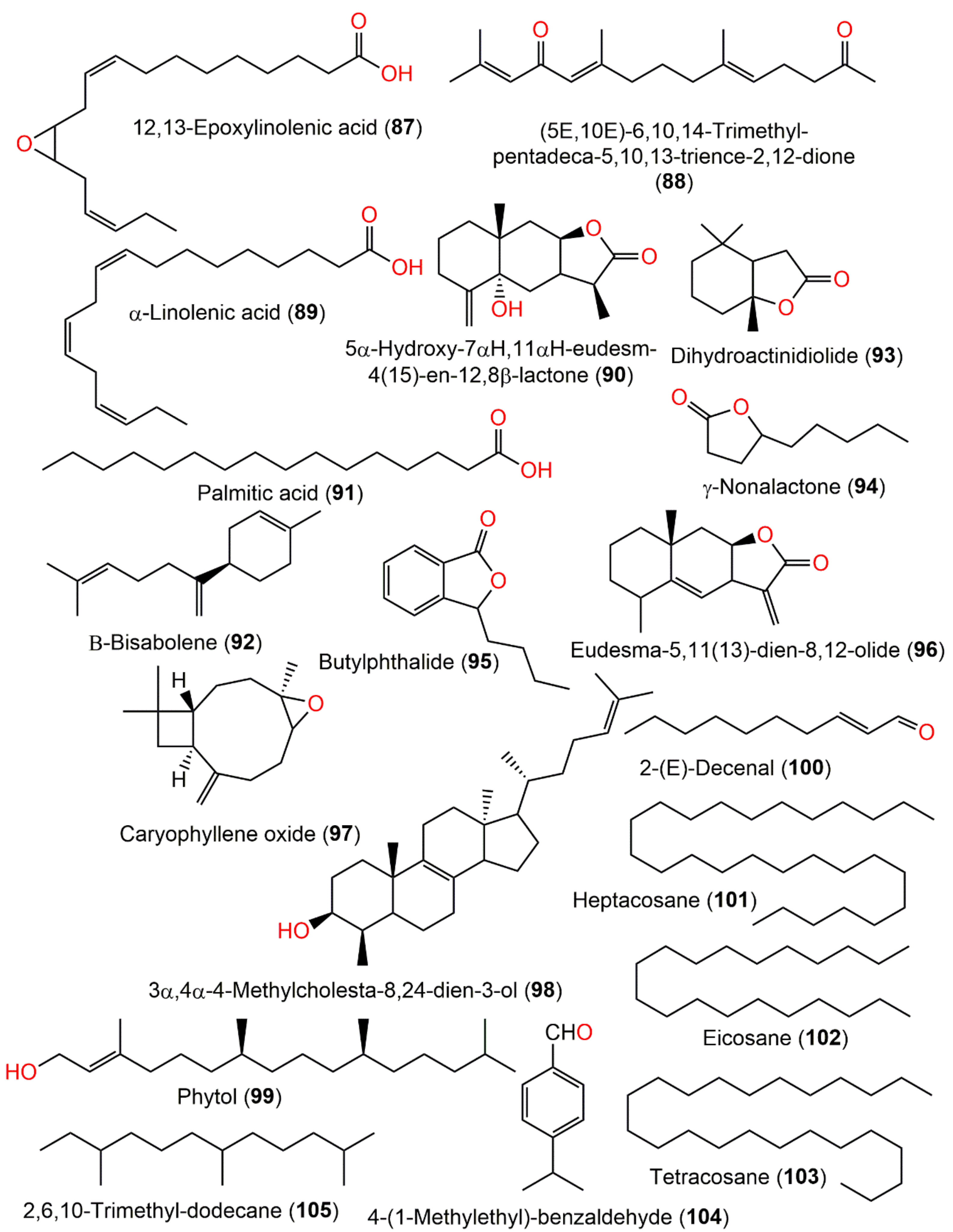
| 12,13-Epoxylinolenic acid (87) | Roots | PE fraction of 80% EtOH extract | 294 | C18H30O3 | Fuyang, Zhejinag, China | [17] |
| (5E,10E)-6,10,14-trimethyl-pentadeca-5,10,13-trience-2,12-dione (88) | Roots | PE fraction of 80% EtOH extract | 276 | C18H28O2 | Fuyang, Zhejinag, China | [17] |
| 5α-hydroxy-7αH,11αH-eudesm-4(15)-en-12,8β-lactone (90) | Roots | PE fraction of 80% EtOH extract | 250 | C15H22O3 | Fuyang, Zhejinag, China | [17] |
| Palmitic acid (91) | Roots | PE fraction of 80% EtOH extract | 256 | C16H32O2 | Fuyang, Zhejinag, China | [17] |
| β-Bisabolene (92) | Whole plant | Essential oil | 204 | C15H24 | Japan | [67] |
| Whole plant | Essential oil | - | - | Hubei, China | [15] | |
| Dihydroactinidiolide (93) | Whole plant | Essential oil | 182 | C11H18 | Japan | [67] |
| γ-Nonalactone (94) | Whole plant | Essential oil | 156 | C9H16O2 | Japan | [67] |
| Butylphthalide (95) | Whole plant | Essential oil | 190 | C9H16O2 | Japan | [67] |
| Eudesma-5,11(13)-dien-8,12-olide (96) | Whole plant | Essential oil | 232 | C15H20O2 | Hubei, China | [15] |
| Caryophyllene oxide (97) | Whole plant | Essential oil | 220 | C15H24O | Hubei, China | [15] |
| Aerial parts | Essential oil | Abbottabad, Pakistan | [32] | |||
| 3α,4α-4-Methylcholesta-8,24-dien-3-ol (98) | Whole plant | Essential oil | 398 | C28H46O | Hubei, China | [15] |
| Phytol (99) | Whole plant | Essential oil | 296 | C20H40O | Hubei, China | [15] |
| 2-(E)-Decenal (100) | Whole plant | Essential oil | 154 | C10H18O | Hubei, China | [15] |
| Heptacosane (101) | Whole plant | Essential oil | 380 | C27H56 | Hubei, China | [15] |
| Eicosane (102) | Whole plant | Essential oil | 280 | C20H42 | Hubei, China | [15] |
| Tetracosane (103) | Whole plant | Essential oil | 338 | C24H50 | Hubei, China | [15] |
| 4-(1-Methylethyl)-benzaldehyde (104) | Whole plant | Essential oil | 148 | C10H12O | Hubei, China | [15] |
| 2,6,10-Trimethyl-dodecane (105) | Whole plant | Essential oil | 212 | β-cedrene | Hubei, China | [15] |
| Torreyol (106) | Whole plant | Essential oil | 222 | C15H26O | Hubei, China | [15] |
| Androst-5,7-dien-3-ol-17-one (107) | Whole plant | Essential oil | 286 | C19H26O2 | Hubei, China | [15] |
| β-Cedrene (108) | Whole plant | Essential oil | 204 | C15H24 | Hubei, China | [15] |
| α-Curcumene (109) | Whole plant | Essential oil | 202 | C15H22 | Hubei, China | [15] |
| 2,6,10-Trimethyl-tetradecane (110) | Whole plant | Essential oil | 240 | C17H36 | Hubei, China | [15] |
| Caryophyllene (111) | Aerial parts | Essential oil | 204 | C15H24 | Abbottabad, Pakistan | [32] |
| Trans-nerolidol (112) | Aerial parts | Essential oil | 222 | C15H26O | Abbottabad, Pakistan | [32] |
| Geranyl isobutyrate (113) | Aerial parts | Essential oil | 224 | C14H24O2 | Abbottabad, Pakistan | [32] |
| δ-cadinene (114) | Aerial parts | Essential oil | 204 | C15H24 | Abbottabad, Pakistan | [32] |
| β-Eudesmene (115) | Aerial parts | Essential oil | 204 | C15H24 | Abbottabad, Pakistan | [32] |
| Trans-lachnophyllum ester (116) | Aerial parts | Essential oil | 176 | C11H12O2 | Abbottabad, Pakistan | [32] |
| α-Caryophyllene (117) | Aerial parts | Essential oil | 204 | C15H24 | Abbottabad, Pakistan | [32] |
| Neryl 3-methylbutyrate (118) | Aerial parts | Essential oil | 238 | C15H26O2 | Abbottabad, Pakistan | [32] |
| Compound Name | Biological Activity | Assay/Organism/ Cell Line | Biological Results | Ref. | |
|---|---|---|---|---|---|
| Compound | Positive Control | ||||
| Inuviscolide (10) | Cytotoxicity | MTT/MDA-MB-231 | 17.26 μM (IC50) | Mitomycin C 4.56 μM (IC50) | [51] |
| 8-Epi-isoinuviscolide (12) | Cytotoxicity | MTT/MDA-MB-231 | 16.87 μM (IC50) | Mitomycin C 4.56 μM (IC50) | [51] |
| 4α,5α-Epoxy-10α,14-dihydro-inuviscolide (14) | Cytotoxicity | SRB/L1210 | 1.8 μM (ED50) | Cisplatin 0.07 μM (ED50) | [51] |
| SRB/A549 | 5.5 μM (ED50) | Cisplatin 4.1 μM (ED50) | [51] | ||
| SRB/SK-OV-3 | 3.4 μM (ED50) | Cisplatin 2.8 μM (ED50) | [51] | ||
| SRB/SK-MEL-2 | 3.3 μM (ED50) | Cisplatin 2.6 μM (ED50) | [51] | ||
| SRB/XF-498 | 4.4 μM (ED50) | Cisplatin 2.9 μM (ED50) | [51] | ||
| SRB/HCT-15 | 5.8 μM (ED50) | Cisplatin 7.1 μM (ED50) | [51] | ||
| Caroguaianolide A (17) | Cytotoxicity | MTT/MDA-MB-231 | 7.96 μM (IC50) | Mitomycin C 4.56 μM (IC50) | [51] |
| MTT/HGC-27 | 10.47 μM (IC50) | Mitomycin C 6.68 μM (IC50) | [51] | ||
| Caroguaianolide B (18) | Cytotoxicity | MTT/MDA-MB-231 | 4.25 μM (IC50) | Mitomycin C 4.56 μM (IC50) | [51] |
| MTT/HGC-27 | 6.47 μM (IC50) | Mitomycin C 6.68 μM (IC50) | [51] | ||
| Caroguaianolide C (19) | Cytotoxicity | MTT/MDA-MB-231 | 2.67 μM (IC50) | Mitomycin C 4.56 μM (IC50) | [51] |
| MTT/HGC-27 | 4.83 μM (IC50) | Mitomycin C 6.68 μM (IC50) | [51] | ||
| Caroguaianolide D (20) | Cytotoxicity | MTT/MDA-MB-231 | 17.21 μM (IC50) | Mitomycin C 4.56 μM (IC50) | [51] |
| Caroguaianolide E (21) | Cytotoxicity | MTT/MDA-MB-231 | 18.37 μM (IC50) | Mitomycin C 4.56 μM (IC50) | [51] |
| Akihalin (22) | Cytotoxicity | MTT/MDA-MB-231 | 4.83 μM (IC50) | Mitomycin C 4.56 μM (IC50) | [51] |
| MTT/HGC-27 | 7.35 μM (IC50) | Mitomycin C 6.68 μM (IC50) | [51] | ||
| 4β-Hydroxy,10β-hydroperoxyl,5αH,7αH,8βH-guaia-1,11(13)- dien-8α,12-olide (23) | Cytotoxicity | MTT/MDA-MB-231 | 5.79 μM (IC50) | Mitomycin C 4.56 μM (IC50) | [51] |
| MTT/HGC-27 | 12.34 μM (IC50) | Mitomycin C 6.68 μM (IC50) | [51] | ||
| 4α-Hydroxy-1βH-guaia-9,11(13)-dien-12,8α-olide (25) | Cytotoxicity | MTT/MDA-MB-231 | 4.07 μM (IC50) | Mitomycin C 4.56 μM (IC50) | [51] |
| MTT/HGC-27 | 8.95 μM (IC50) | Mitomycin C 6.68 μM (IC50) | [51] | ||
| 1α,4α-Dihydroxy-guaia-11(13)-ene-12,8α-olide (26) | Cytotoxicity | CCK-8/HeLa | 15.6 μM (IC50) | Cisplatin 11.1 μM (IC50) | [54] |
| CCK-8/Caco-2 | 28.5 μM (IC50) | Cisplatin 22.1 μM (IC50) | [54] | ||
| Anti-influenza A | CCK-8/H1N1 | 1.3 μM (IC50) | Oseltamivir 0.05 μM (IC50) | [54] | |
| 4α,5α-Dihydroxy-guaia-11(13)-en-12,8α-lactone (27) | Cytotoxicity | CCK-8/ISK | 30.3 μM (IC50) | Cisplatin 31.2 μM (IC50) | [54] |
| CCK-8/HeLa | 9.9 μM (IC50) | Cisplatin 11.1 μM (IC50) | [54] | ||
| CCK-8/A549 | 47.4 μM (IC50) | Cisplatin 40.1 μM (IC50) | [54] | ||
| CCK-8/Caco-2 | 16.4 μM (IC50) | Cisplatin 22.1 μM (IC50) | [54] | ||
| Anti-influenza A | CCK-8/H1N1 | 0.4 μM (IC50) | Oseltamivir 0.05 μM (IC50) | [54] | |
| Carpesiolin (28) | Cytotoxicity | SRB/L1210 | 9.6 μM (ED50) | Cisplatin 0.07 μM (ED50) | [51] |
| SRB/A549 | 13.2 μM (ED50) | Cisplatin 4.1 μM (ED50) | [51] | ||
| SRB/SK-OV-3 | 9.8 μM (ED50) | Cisplatin 2.8 μM (ED50) | [51] | ||
| SRB/SK-MEL-2 | 7.7 μM (ED50) | Cisplatin 2.6 μM (ED50) | [51] | ||
| SRB/XF-498 | 9.3 μM (ED50) | Cisplatin 2.9 μM (ED50) | [51] | ||
| SRB/HCT-15 | 6.7 μM (ED50) | Cisplatin 7.1 μM (ED50) | [51] | ||
| 2,3-Dihydroaromomaticin (29) | Cytotoxicity | SRB/L1210 | 5.0 μM (ED50) | Cisplatin 0.07 μM (ED50) | [51] |
| SRB/A549 | 7.1 μM (ED50) | Cisplatin 4.1 μM (ED50) | [51] | ||
| SRB/SK-OV-3 | 3.9 μM (ED50) | Cisplatin 2.8 μM (ED50) | [51] | ||
| SRB/SK-MEL-2 | 4.0 μM (ED50) | Cisplatin 2.6 μM (ED50) | [51] | ||
| SRB/XF-498 | 3.3 μM (ED50) | Cisplatin 2.9 μM (ED50) | [51] | ||
| SRB/HCT-15 | 8.1 μM (ED50) | Cisplatin 7.1 μM (ED50) | [51] | ||
| MTT/A549 | 7.09 μM (IC50) | Vinblastine 18.5 μM (IC50) | [52] | ||
| MTT/HepG2 | 9.03 μM (IC50) | Vinblastine 9.29 μM (IC50) | [52] | ||
| MTT/MDA-MB-231 | 5.16 μM (IC50) | Vinblastine 42.51 μM (IC50) | [52] | ||
| MTT/HCT116 | 10.15 μM (IC50) | Vinblastine 30.62 μM (IC50) | [52] | ||
| MTT/CNE2 | 12.99 μM (IC50) | Vinblastine 1.16 μM (IC50) | [52] | ||
| 2-Desoxy-4-epi-pulchellin (31) | Cytotoxicity | CCK-8/K562 | 17.0 μM (IC50) | Taxol 3.8 μM (IC50) | [56] |
| CCK-8/MCF-7 | 11.0 μM (IC50) | Taxol 2.3 μM (IC50) | [56] | ||
| CCK-8/HeLa | 6.0 μM (IC50) | Taxol 2.9 μM (IC50) | [56] | ||
| CCK-8/DU145 | 3.1 μM (IC50) | Taxol 3.1 μM (IC50) | [56] | ||
| CCK-8/U937 | 2.2 μM (IC50) | Taxol 2.1 μM (IC50) | [56] | ||
| CCK-8/H1975 | 13.0 μM (IC50) | Taxol 2.8 μM (IC50) | [56] | ||
| CCK-8/SGC-7901 | 22.0 μM (IC50) | Taxol 9.8 μM (IC50) | [56] | ||
| CCK-8/A549 | 6.2 μM (IC50) | Taxol 2.1 μM (IC50) | [56] | ||
| CCK-8/MOLT-4 | 5.5 μM (IC50) | Taxol 2.7 μM (IC50) | [56] | ||
| CCK-8/HL60 | 2.4 μM (IC50) | Taxol 3.8 μM (IC50) | [56] | ||
| MTT/MDA-MB-231 | 18.67 μM (IC50) | Mitomycin C 4.56 μM (IC50) | [51] | ||
| MTT/A549 | 6.04 μM (IC50) | Vinblastine 18.5 μM (IC50) | [52] | ||
| MTT/HepG2 | 18.25 μM (IC50) | Vinblastine 9.29 μM (IC50) | [52] | ||
| MTT/MDA-MB-231 | 21.55 μM (IC50) | Vinblastine 42.51 μM (IC50) | [52] | ||
| Antiviral | CPE inhibition/H1N1 | 29.3 μM (IC50) | Osehamivir 0.025 μM (IC50) | [56] | |
| CPE inhibition/H3N2 | 47.3 μM (IC50) | Osehamivir 0.015 μM (IC50) | [56] | ||
| Antimycobacterial | GFPMA/Mycobacterium tuberculosis strains H37Rv, H37Ra | 7.6 μM (MIC) | Isoniazid, 2.0 μM (MIC) | [56] | |
| 3aR,4aS,5S,7aS,8S,9aR)-5-Hydroxy-4a,8-dimethyl-3-methylen-decahydroa-zuleno [6,5-b]furan-2(3H)-on (32) | Cytotoxicity | MTT/MDA-MB-231 | 5.32 μM (IC50) | Mitomycin C 4.56 μM (IC50) | [51] |
| MTT/HGC-27 | 11.44 μM (IC50) | Mitomycin C 6.68 μM (IC50) | [51] | ||
| Telekin (35) | Cytotoxicity | SRB/L1210 | 7.5 μM (ED50) | Cisplatin 0.07 μM ED50) | [51] |
| SRB/A549 | 7.5 μM (ED50) | Cisplatin 4.1 μM (ED50) | [51] | ||
| SRB/SK-OV-3 | 4.1 μM (ED50) | Cisplatin 2.8 μM (ED50) | [51] | ||
| SRB/SK-MEL-2 | 4.9 μM (ED50) | Cisplatin 2.6 μM (ED50) | [51] | ||
| SRB/XF-498 | 6.6 μM (ED50) | Cisplatin 2.9 μM (ED50) | [51] | ||
| SRB/HCT-15 | 6.7 μM (ED50) | Cisplatin 7.1 μM (ED50) | [51] | ||
| HepG2/CCK-8 | 2.95 μM (IC50) | Paclitaxel | [16] | ||
| MTT/A549 | 11.08 μM (IC50) | Vinblastine 18.5 μM (IC50) | [52] | ||
| MTT/HepG2 | 7.89 μM (IC50) | Vinblastine 9.29 μM (IC50) | [52] | ||
| MTT/MDA-MB-231 | 9.46 μM (IC50) | Vinblastine 42.51 μM (IC50) | [52] | ||
| MTT/HCT116 | 9.69 μM (IC50) | Vinblastine 30.62 μM (IC50) | [52] | ||
| MTT/CNE2 | 13.66 μM (IC50) | Vinblastine 1.16 μM (IC50) | [52] | ||
| 5α-Epoxyalantolactone (38) | Cytotoxicity | MTT/A549 | 3.51 μM (IC50) | Vinblastine 18.5 μM (IC50) | [52] |
| MTT/HepG2 | 2.73 μM (IC50) | Vinblastine 9.29 μM (IC50) | [52] | ||
| MTT/MDA-MB-231 | 4.18 μM (IC50) | Vinblastine 42.51 μM (IC50) | [52] | ||
| MTT/HCT116 | 3.75 μM (IC50) | Vinblastine 30.62 μM (IC50) | [52] | ||
| MTT/CNE2 | 7.21 μM (IC50) | Vinblastine 1.16 μM (IC50) | [52] | ||
| MTT/NCM460 | 13.66 μM (IC50) | Vinblastine | [52] | ||
| Ivalin (40) | Cytotoxicity | SRB/L1210 | 7.5 μM (ED50) | Cisplatin 0.07 μM ED50) | [51] |
| SRB/A549 | 9.9 μM (ED50) | Cisplatin 4.1 μM (ED50) | [51] | ||
| SRB/SK-OV-3 | 3.5 μM (ED50) | Cisplatin 2.8 μM (ED50) | [51] | ||
| SRB/SK-MEL-2 | 3.0 μM (ED50) | Cisplatin 2.6 μM (ED50) | [51] | ||
| SRB/XF-498 | 2.9 μM (ED50) | Cisplatin 2.9 μM (ED50) | [51] | ||
| SRB/HCT-15 | 2.6 μM (ED50) | Cisplatin 7.1 μM (ED50) | [51] | ||
| MTT/A549 | 7.77 μM (IC50) | Vinblastine 18.5 μM (IC50) | [52] | ||
| MTT/HepG2 | 6.1 μM (IC50) | Vinblastine 9.29 μM (IC50) | [52] | ||
| MTT/MDA-MB-231 | 7.42 μM (IC50) | Vinblastine 42.51 μM (IC50) | [52] | ||
| MTT/HCT116 | 13.12 μM (IC50) | Vinblastine 30.62 μM (IC50) | [52] | ||
| MTT/CNE2 | 23.6 μM (IC50) | Vinblastine 1.16 μM (IC50) | [52] | ||
| 2α,5α-Dihydroxy-11αH-eudesma-4(15)-en-12,8β-olide (41) | Cytotoxicity | CCK-8/ISK | 21.8 μM (IC50) | Cisplatin 31.2 μM (IC50) | [54] |
| CCK-8/HeLa | 8.5 μM (IC50) | Cisplatin 11.1 μM (IC50) | [54] | ||
| CCK-8/Sw620 | 42.3 μM (IC50) | Cisplatin 37.9 μM (IC50) | [54] | ||
| CCK-8/RBE | 45.1 μM (IC50) | Cisplatin 42.5 μM (IC50) | [54] | ||
| CCK-8/Caco-2 | 42.6 μM (IC50) | Cisplatin 22.1 μM (IC50) | [54] | ||
| CCK-8/HepG2 | 9.83 μM (IC50) | Paclitaxel | [16] | ||
| (5α)-5-Hydroxyasperilin (42) | Cytotoxicity | CCK-8/ISK | 25.8 μM (IC50) | Cisplatin 31.2 μM (IC50) | [54] |
| CCK-8/HeLa | 10.7 μM (IC50) | Cisplatin 11.1 μM (IC50) | [54] | ||
| CCK-8/Sw620 | 28.1 μM (IC50) | Cisplatin 37.9 μM (IC50) | [54] | ||
| CCK-8/RBE | 28.4 μM (IC50) | Cisplatin 42.5 μM (IC50) | [54] | ||
| CCK-8/Caco-2 | 21.5 μM (IC50) | Cisplatin 22.1 μM (IC50) | [54] | ||
| Carpabrotalactone B (43) | Cytotoxicity | CCK-8/ISK | 33.8 μM (IC50) | Cisplatin 31.2 μM (IC50) | [54] |
| CCK-8/HeLa | 25.6 μM (IC50) | Cisplatin 11.1 μM (IC50) | [54] | ||
| CCK-8/Caco-2 | 45.4 μM (IC50) | Cisplatin 22.1 μM (IC50) | [54] | ||
| Anti-influenza A | CCK-8/H1N1 | 6.2 μM (IC50) | Oseltamivir 0.05 μM (IC50) | [54] | |
| Carpabrotalactone C (44) | Cytotoxicity | CCK-8/A549 | 19.1 μM (IC50) | Cisplatin 12.77 μM (IC50) | [58] |
| CCK-8/SMMC-7721 | 23.68 μM (IC50) | Cisplatin 5.1 μM (IC50) | [58] | ||
| CCK-8/MCF-7 | 20.32 μM (IC50) | Cisplatin 12.6 μM (IC50) | [58] | ||
| CCK-8/SW480 | 11.46 μM (IC50) | Cisplatin 6.67 μM (IC50) | [58] | ||
| Oxoeudesm-11(13)-eno-12,8α-lactone (45) | Cytotoxicity | CCK-8/HepG2 | 4.15 μM (IC50) | Paclitaxel | [16] |
| Anti-inflammatory | NO inhibition/LPS | 63.23 μM (IC50) | Dexamethasone 3.61 μM (IC50) | [58] | |
| 11(13)-Dehydroivaxillin (48) | Cytotoxicity | CCK-8/K562 | 1.7 μM (IC50) | Taxol 3.8 μM (IC50) | [56] |
| CCK-8/MCF-7 | 4.1 μM (IC50) | Taxol 2.3 μM (IC50) | [56] | ||
| CCK-8/HeLa | 1.0 μM (IC50) | Taxol 2.9 μM (IC50) | [56] | ||
| CCK-8/DU145 | 0.38 μM (IC50) | Taxol 3.1 μM (IC50) | [56] | ||
| CCK-8/U937 | 0.21 μM (IC50) | Taxol 2.1 μM (IC50) | [56] | ||
| CCK-8/H1975 | 5.4 μM (IC50) | Taxol 2.8 μM (IC50) | [56] | ||
| CCK-8/SGC-7901 | 6.4 μM (IC50) | Taxol 9.8 μM (IC50) | [56] | ||
| CCK-8/A549 | 2.0 μM (IC50) | Taxol 2.1 μM (IC50) | [56] | ||
| CCK-8/MOLT-4 | 1.2 μM (IC50) | Taxol 2.7 μM (IC50) | [56] | ||
| CCK-8/ISK | 13.7 μM (IC50) | Cisplatin 31.2 μM (IC50) | [54] | ||
| CCK-8/HeLa | 12.3 μM (IC50) | Cisplatin 11.1 μM (IC50) | [54] | ||
| CCK-8/Sw620 | 12.1 μM (IC50) | Cisplatin 37.9 μM (IC50) | [54] | ||
| CCK-8/RBE | 7.8 μM (IC50) | Cisplatin 42.5 μM (IC50) | [54] | ||
| CCK-8/Caco-2 | 20.4 μM (IC50) | Cisplatin 22.1 μM (IC50) | [54] | ||
| MTT/A549 | 17.6 μM (IC50) | Vinblastine 18.5 μM (IC50) | [52] | ||
| MTT/HepG2 | 14.59 μM (IC50) | Vinblastine 9.29 μM (IC50) | [52] | ||
| MTT/MDA-MB-231 | 10.88 μM (IC50) | Vinblastine 42.51 μM (IC50) | [52] | ||
| CCK-8/HL60 | 0.18 μM (IC50) | Taxol 3.8 μM (IC50) | [60] | ||
| Anti-influenza A | CCK-8/H1N1 | 11.6 μM (IC50) | Oseltamivir 0.05 μM (IC50) | [54] | |
| Antiviral | CPE inhibition/H1N1 | 10.8 μM (IC50) | Osehamivir 0.025 μM (IC50) | [56] | |
| CPE inhibition/H3N2 | 11.6 μM (IC50) | Osehamivir 0.015 μM (IC50) | [56] | ||
| Antimycobacterial | GFPMA/Mycobacterium tuberculosis strains H37Rv, H37Ra | 6.0 μM (MIC) | Isoniazid, 2.0 μM (MIC) | [56] | |
| 11,13-Didehydroivaxillin (49) | Cytotoxicity | SRB/L1210 | 11.4 μM (ED50) | Cisplatin 0.07 μM ED50) | [51] |
| SRB/A549 | 18.4 μM (ED50) | Cisplatin 4.1 μM (ED50) | [51] | ||
| SRB/SK-OV-3 | 9.7 μM (ED50) | Cisplatin 2.8 μM (ED50) | [51] | ||
| SRB/SK-MEL-2 | 9.5 μM (ED50) | Cisplatin 2.6 μM (ED50) | [51] | ||
| SRB/XF-498 | 10.6 μM (ED50) | Cisplatin 2.9 μM (ED50) | [51] | ||
| SRB/HCT-15 | 7.4 μM (ED50) | Cisplatin 7.1 μM (ED50) | [51] | ||
| Eriolin (50) | Cytotoxicity | CCK-8/ISK | 41.6 μM (IC50) | Cisplatin 31.2 μM (IC50) | [54] |
| CCK-8/HeLa | 14.8 μM (IC50) | Cisplatin 11.1 μM (IC50) | [54] | ||
| CCK-8/Sw620 | 46.4 μM (IC50) | Cisplatin 37.9 μM (IC50) | [54] | ||
| CCK-8/Caco-2 | 12.7 μM (IC50) | Cisplatin 22.1 μM (IC50) | [54] | ||
| Anti-influenza A | CCK-8/H1N1 | 16.4 μM (IC50) | Oseltamivir 0.05 μM (IC50) | [54] | |
| Carpabrotalactone A (52) | Cytotoxicity | CCK-8/ISK | 21.0 μM (IC50) | Cisplatin 31.2 μM (IC50) | [54] |
| CCK-8/HeLa | 9.6 μM (IC50) | Cisplatin 11.1 μM (IC50) | [54] | ||
| CCK-8/Sw620 | 44.5 μM (IC50) | Cisplatin 37.9 μM (IC50) | [54] | ||
| CCK-8/Caco-2 | 13.4 μM (IC50) | Cisplatin 22.1 μM (IC50) | [54] | ||
| Carperemophilane A (53) | Cytotoxicity | MTT/MDA-MB-231 | 22.67 μM (IC50) | Mitomycin C 4.89 μM (IC50) | [60] |
| MTT/HGC-27 | 24.83 μM (IC50) | Mitomycin C 6.73 μM (IC50) | [60] | ||
| Carperemophilane B (54) | Cytotoxicity | MTT/MDA-MB-231 | 34.83 μM (IC50) | Mitomycin C 4.89 μM (IC50) | [60] |
| MTT/HGC-27 | 37.35 μM (IC50) | Mitomycin C 6.73 μM (IC50) | [60] | ||
| Carabrone (55) | Antifungal | Spore germination/Colletotrichum lagenarium | 7.1 μg/mL (EC50) | Chlorothalonil 0.75 μg/mL (EC50) | [69] |
| Cytotoxicity | SRB/L1210 | 6.2 μM (ED50) | Cisplatin 0.07 μM ED50) | [51] | |
| SRB/A549 | 17.1 μM (ED50) | Cisplatin 4.1 μM (ED50) | [51] | ||
| SRB/SK-OV-3 | 11.3 μM (ED50) | Cisplatin 2.8 μM (ED50) | [51] | ||
| SRB/SK-MEL-2 | 8.5 μM (ED50) | Cisplatin 2.6 μM (ED50) | [51] | ||
| SRB/XF-498 | 19.8 μM (ED50) | Cisplatin 2.9 μM (ED50) | [51] | ||
| SRB/HCT-15 | 7.4 μM (ED50) | Cisplatin 7.1 μM (ED50) | [51] | ||
| MTT/HCT117 | 17.13 μM (IC50) | Doxorubicin 0.20 μM (IC50) | [70] | ||
| MTT/CCRF-CEM | 43.66 μM (IC50) | Doxorubicin 0.003 μM (IC50) | [70] | ||
| MTT/K562 | 19.42 μM (IC50) | Doxorubicin 0.04 μM (IC50) | [70] | ||
| MTT/HL60 | 23.29 μM (IC50) | Doxorubicin < 0.001 μM (IC50) | [70] | ||
| Carabrol (56) | Cytotoxicity | SRB/L1210 | 11.4 μM (ED50) | Cisplatin 0.07 μM ED50) | [51] |
| SRB/A549 | 13.1 μM (ED50) | Cisplatin 4.1 μM (ED50) | [51] | ||
| SRB/SK-OV-3 | 10.6 μM (ED50) | Cisplatin 2.8 μM (ED50) | [51] | ||
| SRB/SK-MEL-2 | 11.2 μM (ED50) | Cisplatin 2.6 μM (ED50) | [51] | ||
| SRB/XF-498 | 13.5 μM (ED50) | Cisplatin 2.9 μM (ED50) | [51] | ||
| SRB/HCT-15 | 10.8 μM (ED50) | Cisplatin 7.1 μM (ED50) | [51] | ||
| CCK-8/K562 | 4.7 μM (IC50) | Taxol 3.8 μM (IC50) | [56] | ||
| CCK-8/MCF-7 | 47.0 μM (IC50) | Taxol 2.3 μM (IC50) | [56] | ||
| CCK-8/HeLa | 6.6 μM (IC50) | Taxol 2.9 μM (IC50) | [56] | ||
| CCK-8/DU145 | 2.6 μM (IC50) | Taxol 3.1 μM (IC50) | [56] | ||
| CCK-8/U937 | 0.94 μM (IC50) | Taxol 2.1 μM (IC50) | [56] | ||
| CCK-8/H1975 | 4.0 μM (IC50) | Taxol 2.8 μM (IC50) | [56] | ||
| CCK-8/MOLT-4 | 8.9 μM (IC50) | Taxol 2.7 μM (IC50) | [56] | ||
| CCK-8/HL60 | 0.36 μM (IC50) | Taxol 3.8 μM (IC50) | [56] | ||
| MTT/MDA-MB-231 | 7.45 μM (IC50) | Mitomycin C 4.89 μM (IC50) | [60] | ||
| MTT/HGC-27 | 10.27 μM (IC50) | Mitomycin C 6.73 μM (IC50) | [60] | ||
| MTT/HCT117 | 29.95 μM (IC50) | Doxorubicin 0.20 μM (IC50) | [70] | ||
| MTT/CCRF-CEM | 32.26 μM (IC50) | Doxorubicin 0.003 μM (IC50) | [70] | ||
| MTT/K562 | 9.1 μM (IC50) | Doxorubicin 0.04 μM (IC50) | [70] | ||
| MTT/HL60 | 23.73 μM (IC50) | Doxorubicin < 0.001 μM (IC50) | [70] | ||
| Antiviral | CPE inhibition/H1N1 | 45.5 μM (IC50) | Osehamivir 0.025 μM (IC50) | [56] | |
| Carabrol-4-O-palmitate (58) | Cytotoxicity | MTA/HL60 | 45.85 μM (IC50) | Cisplatin 2.32 μM (IC50) | [53] |
| Dicarabrol (60) | Cytotoxicity | CCK-8/K562 | 1.2 μM (IC50) | Taxol 3.8 μM (IC50) | [56] |
| CCK-8/MCF-7 | 3.3 μM (IC50) | Taxol 2.3 μM (IC50) | [56] | ||
| CCK-8/HeLa | 0.61 μM (IC50) | Taxol 2.9 μM (IC50) | [56] | ||
| CCK-8/DU145 | 0.31 μM (IC50) | Taxol 3.1 μM (IC50) | [56] | ||
| CCK-8/U937 | 0.15 μM (IC50) | Taxol 2.1 μM (IC50) | [56] | ||
| CCK-8/H1975 | 1.4 μM (IC50) | Taxol 2.8 μM (IC50) | [56] | ||
| CCK-8/SGC-7901 | 0.71 μM (IC50) | Taxol 9.8 μM (IC50) | [56] | ||
| CCK-8/A549 | 2.7 μM (IC50) | Taxol 2.1 μM (IC50) | [56] | ||
| CCK-8/MOLT-4 | 1.3 μM (IC50) | Taxol 2.7 μM (IC50) | [56] | ||
| CCK-8/HL60 | 0.10 μM (IC50) | Taxol 3.8 μM (IC50) | [56] | ||
| Antiviral | CPE inhibition/H1N1 | 15.9 μM (IC50) | Osehamivir 0.025 μM (IC50) | [56] | |
| CPE inhibition/H3N2 | 30.0 μM (IC50) | Osehamivir 0.015 μM (IC50) | [56] | ||
| Antimycobacterial | GFPMA/Mycobacterium tuberculosis strains H37Rv, H37Ra | 3.7 μM (MIC) | Isoniazid, 2.0 μM (MIC) | [56] | |
| Dicarabrol A (61) | Cytotoxicity | MTT/HL60 | 8.7 μM (IC50) | Doxorubicin | [63] |
| Dicarabrol B (62) | Cytotoxicity | MTT/HL60 | 20.0 μM (IC50) | Doxorubicin | [62] |
| MTT/A549 | 20.0 μM (IC50)) | Doxorubicin | [62] | ||
| Dicarabrol C (63) | Cytotoxicity | MTT/HL60 | 3.6 μM (IC50) | Doxorubicin | [62] |
| Dicarabrone A (64) | Cytotoxicity | MTT/HL60 | 9.1 μM (IC50) | Doxorubicin | [64] |
| Dicarabrone B (65) | Cytotoxicity | MTT/HL60 | 8.2 μM (IC50) | Doxorubicin | [64] |
| Dicarabrone C (66) | Cytotoxicity | MTT/HL60 | 8.2 μM (IC50) | Doxorubicin | [63] |
| Carabrodilactone A (67) | Cytotoxicity | MTT/A549 | 4.34 μM (IC50) | Doxorubicin 0.05 μM (IC50) | [65] |
| MTT/HCT117 | 3.08 μM (IC50) | Doxorubicin 0.05 μM (IC50) | [65] | ||
| MTT/MDA-MB 231 | 8.05 μM (IC50) | Doxorubicin 0.05 μM (IC50) | [65] | ||
| MTT/BEL 7404 | 3.2 μM (IC50) | Doxorubicin 0.05 μM (IC50) | [65] | ||
| Carpedilactone A (72) | Cytotoxicity | MTT/A549 | 2.63 μM (IC50) | Doxorubicin 0.05 μM (IC50) | [71] |
| MTT/BEL 7404 | 5.53 μM (IC50) | Doxorubicin 0.22 μM (IC50) | [71] | ||
| MTT/HLF | 1.45 μM (IC50) | Doxorubicin 0.07 μM (IC50) | [71] | ||
| MTT/CCRF-CEM | 0.14 μM (IC50) | Doxorubicin 0.01 μM (IC50) | [71] | ||
| Carpedilactone B (73) | Cytotoxicity | MTT/A549 | 9.53 μM (IC50) | Doxorubicin 0.05 μM (IC50) | [71] |
| MTT/BEL 7404 | 14.19 μM (IC50) | Doxorubicin 0.22 μM (IC50) | [71] | ||
| MTT/HLF | 3.31 μM (IC50) | Doxorubicin 0.07 μM (IC50) | [71] | ||
| MTT/CCRF-CEM | 0.32 μM (IC50) | Doxorubicin 0.01 μM (IC50) | [71] | ||
| Dipulchellin A (74) | Cytotoxicity | MTT/HL60 | 8.9 μM (IC50) | Doxorubicin | [63] |
| Faberidilactone A (75) | Cytotoxicity | MTT/HCT117 | 3.44 μM (IC50) | Doxorubicin 0.20 μM (IC50) | [70] |
| MTT/CCRF-CEM | 2.71 μM (IC50) | Doxorubicin 0.003 μM (IC50) | [70] | ||
| MTT/K562 | 2.63 μM (IC50) | Doxorubicin 0.04 μM (IC50) | [70] | ||
| MTT/HL60 | 4.95 μM (IC50) | Doxorubicin < 0.001 μM (IC50) | [70] | ||
| Faberidilactone C (76) | Cytotoxicity | MTT/HCT117 | 4.00 μM (IC50) | Doxorubicin 0.20 μM (IC50) | [70] |
| MTT/CCRF-CEM | 2.66 μM (IC50) | Doxorubicin 0.003 μM (IC50) | [70] | ||
| MTT/K562 | 4.92 μM (IC50) | Doxorubicin 0.04 μM (IC50) | [70] | ||
| MTT/HL60 | 8.12 μM (IC50) | Doxorubicin < 0.001 μM (IC50) | [70] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, S.R.M.; Fadil, S.A.; Fadil, H.A.; Hareeri, R.H.; Abdallah, H.M.; Mohamed, G.A. Ethnobotanical Uses, Phytochemical Composition, Biosynthesis, and Pharmacological Activities of Carpesium abrotanoides L. (Asteraceae). Plants 2022, 11, 1598. https://doi.org/10.3390/plants11121598
Ibrahim SRM, Fadil SA, Fadil HA, Hareeri RH, Abdallah HM, Mohamed GA. Ethnobotanical Uses, Phytochemical Composition, Biosynthesis, and Pharmacological Activities of Carpesium abrotanoides L. (Asteraceae). Plants. 2022; 11(12):1598. https://doi.org/10.3390/plants11121598
Chicago/Turabian StyleIbrahim, Sabrin R. M., Sana A. Fadil, Haifa A. Fadil, Rawan H. Hareeri, Hossam M. Abdallah, and Gamal A. Mohamed. 2022. "Ethnobotanical Uses, Phytochemical Composition, Biosynthesis, and Pharmacological Activities of Carpesium abrotanoides L. (Asteraceae)" Plants 11, no. 12: 1598. https://doi.org/10.3390/plants11121598
APA StyleIbrahim, S. R. M., Fadil, S. A., Fadil, H. A., Hareeri, R. H., Abdallah, H. M., & Mohamed, G. A. (2022). Ethnobotanical Uses, Phytochemical Composition, Biosynthesis, and Pharmacological Activities of Carpesium abrotanoides L. (Asteraceae). Plants, 11(12), 1598. https://doi.org/10.3390/plants11121598









