Pro-197-Ser Mutation and Cytochrome P450-Mediated Metabolism Conferring Resistance to Flucarbazone-Sodium in Bromus japonicus
Abstract
:1. Introduction
2. Results
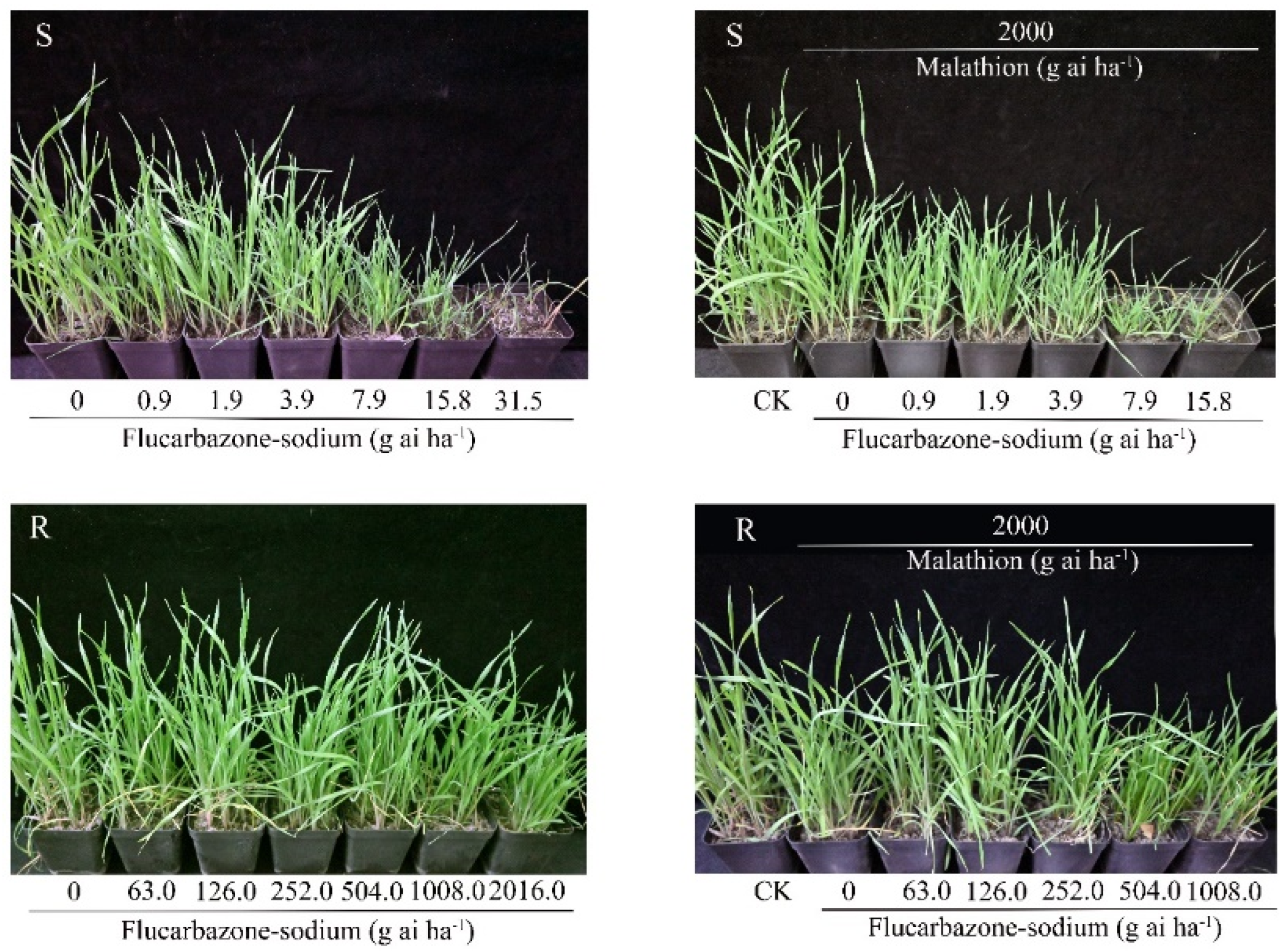
2.1. Resistance Level Evaluation
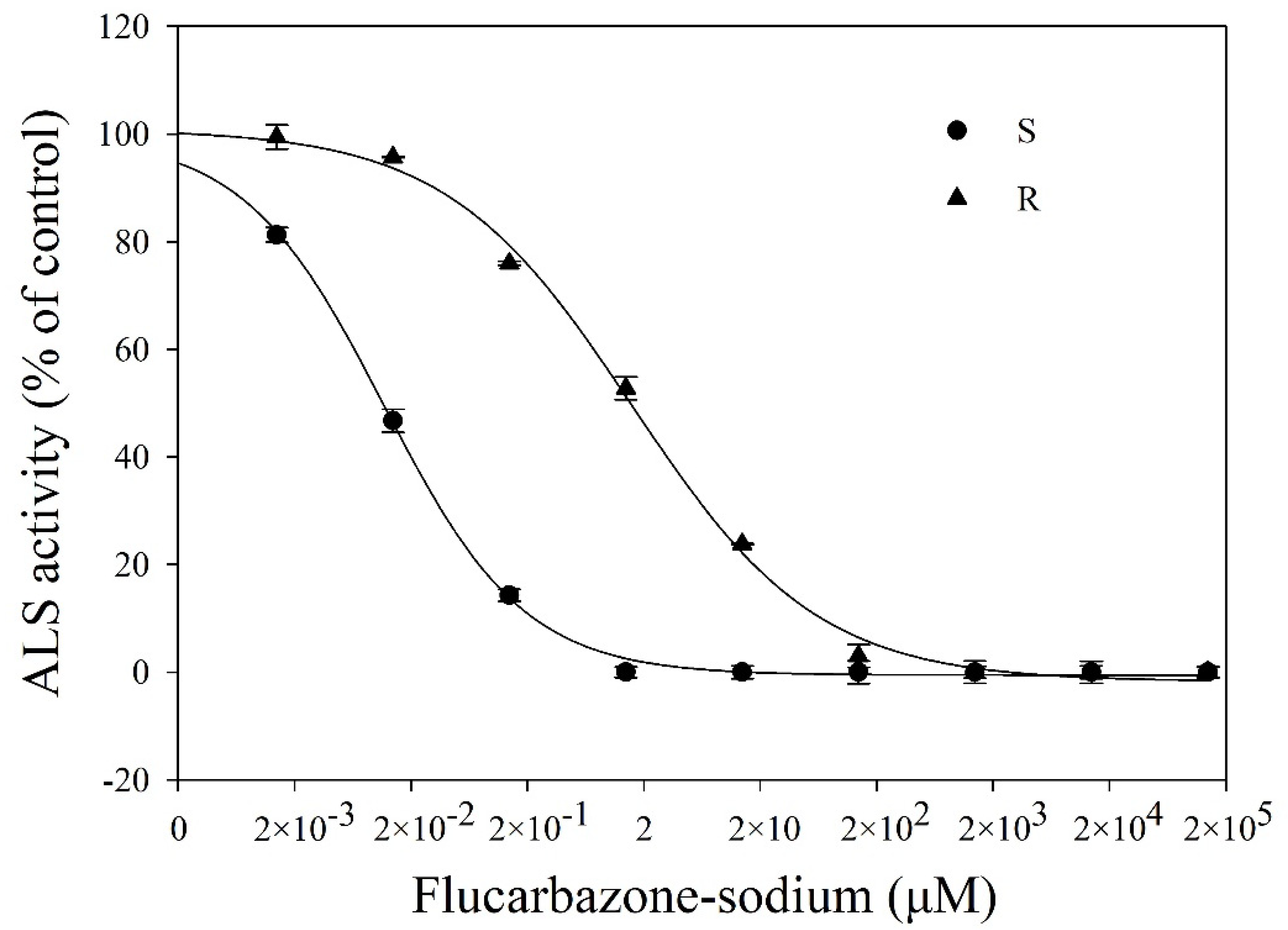
2.2. ALS Activity Assay
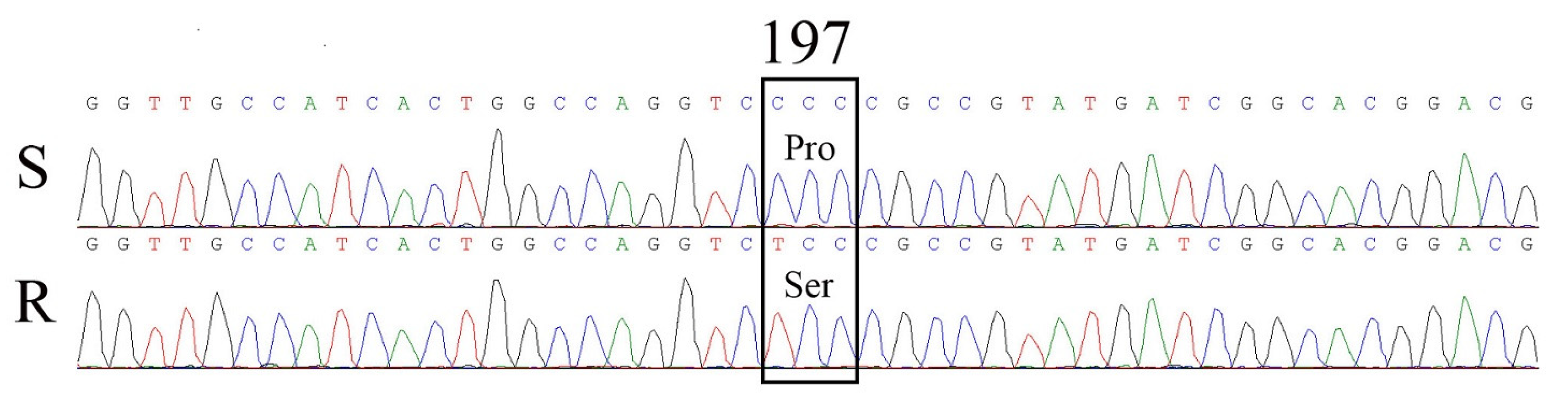
2.3. ALS Gene Sequencing
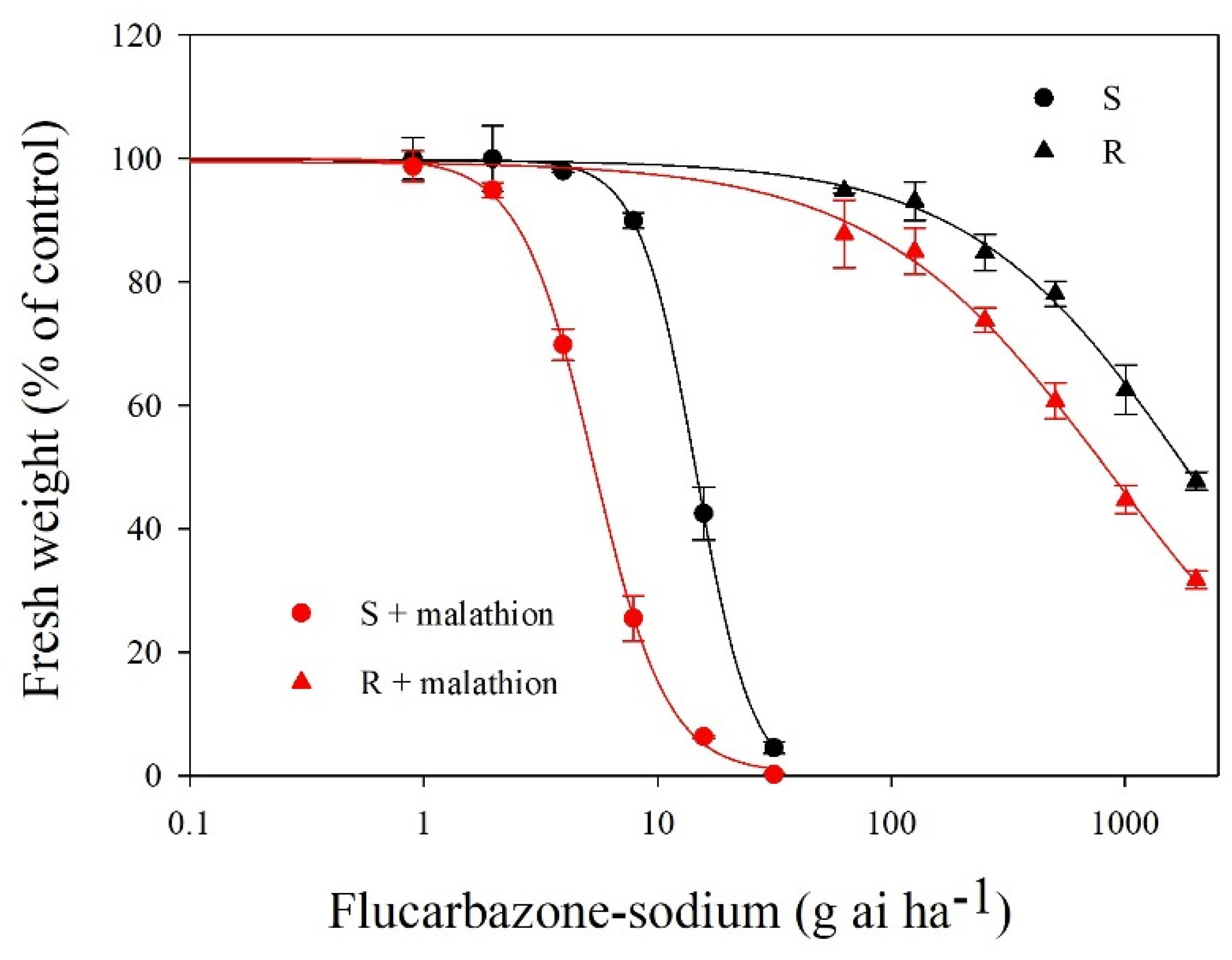
2.4. Effects of Malathion Pretreatment on Metabolic Resistance
2.5. Cross- and Multiple-Herbicide Resistance Testing
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Whole-Plant Dose–Response Tests
4.3. ALS Activity Assay
4.4. ALS Gene Cloning and Sequencing
4.5. Effects of Malathion Pretreatment on Metabolic Resistance
4.6. Cross- and Multiple-Herbicide Resistance Testing
4.7. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barkworth, M.E.; Capels, K.M.; Long, S. Flora of North America: North of Mexico; Oxford University Press: New York, NY, USA, 1993; p. 237. [Google Scholar]
- Quattrocchi, U. CRC World Dictionary of Grasses: Common Names, Scientific Names, Eponyms, Synonyms, and Etymology; CRC Press: Boca Raton, FL, USA, 2006; p. 378. [Google Scholar]
- Kumar, V.; Jha, P. First report of Ser653Asn mutation endowing high-level resistance to imazamox in downy brome (Bromus tectorum L.). Pest Manag. Sci. 2017, 73, 2585–2591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Li, Q.; Du, L.; Yuan, G.; Guo, W.; Li, W.; Wang, J. Density effect and economic threshold of Japanese brome (Bromus japonicus Houtt.) in wheat. Chil. J. Agric. Res. 2016, 76, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Johnson, E.N.; Wang, Z.; Geddes, C.M.; Coles, K.; Hamman, B.; Beres, B.L. Pyroxasulfone is effective for management of Bromus spp. in winter wheat in Western Canada. Weed Technol. 2018, 32, 739–748. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Singh, V.; Salas-Perez, R.A.; Bagavathiannan, M.V.; Lawton-Rauh, A.; Roma-Burgos, N. Target-site mutation accumulation among ALS inhibitor-resistant Palmer amaranth. Pest Manag. Sci. 2019, 75, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Anthimidou, E.; Ntoanidou, S.; Madesis, P.; Eleftherohorinos, I. Mechanisms of Lolium rigidum multiple resistance to ALS- and ACCase-inhibiting herbicides and their impact on plant fitness. Pestic. Biochem. Physiol. 2020, 164, 65–72. [Google Scholar] [CrossRef]
- Mazur, B.J.; Falco, S.C. The development of herbicide resistant crops. Ann. Rev. Plant Biol. 1989, 40, 441–470. [Google Scholar] [CrossRef]
- De Felice, M.; Guardiola, J.; Esposito, B.; Iaccarino, M. Structural genes for a newly recognized acetolactate synthase in Escherichia coli K-12. J. Bact. 1974, 120, 1068–1077. [Google Scholar] [CrossRef] [Green Version]
- Ray, T.B. Site of action of chlorsulfuron: Inhibition of valine and isoleucine biosynthesis in plants. Plant Physiol. 1984, 75, 827–831. [Google Scholar] [CrossRef] [Green Version]
- Heap, I. International Survey of Herbicide Resistant Weeds. Available online: www.weedscience.org (accessed on 1 March 2022).
- Gaines, T.A.; Duke, S.O.; Morran, S.; Rigon, C.A.G.; Tranel, P.J.; Kupper, A.; Dayan, F.E. Mechanisms of evolved herbicide resistance. J. Biol. Chem. 2020, 295, 10307–10330. [Google Scholar] [CrossRef]
- Comont, D.; Lowe, C.; Hull, R.; Crook, L.; Hicks, H.L.; Onkokesung, N.; Beffa, R.; Childs, D.Z.; Edwards, R.; Freckleton, R.P.; et al. Evolution of generalist resistance to herbicide mixtures reveals a trade-off in resistance management. Nat. Commun. 2020, 11, 3086. [Google Scholar] [CrossRef]
- Koo, D.H.; Molin, W.T.; Saski, C.A.; Jiang, J.; Putta, K.; Jugulam, M.; Friebe, B.; Gill, B.S. Extrachromosomal circular DNA-based amplification and transmission of herbicide resistance in crop weed Amaranthus palmeri. Proc. Natl. Acad. Sci. USA 2018, 115, 3332–3337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, B.P.; Tranel, P.J. Target-site mutations conferring herbicide resistance. Plants 2019, 8, 382. [Google Scholar] [CrossRef] [Green Version]
- Powles, S.B.; Yu, Q. Evolution in action: Plants resistant to herbicides. Ann. Rev. Plant Biol. 2010, 61, 317–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.W.; Mallory-Smith, C.A. Physiological and molecular basis for ALS inhibitor resistance in Bromus tectorum biotypes. Weed Res. 2004, 44, 71–77. [Google Scholar] [CrossRef]
- Tranel, P.J.; Wright, T.R.; Heap, P.J. Mutations in Herbicide-Resistant Weeds to Inhibition of Acetolactate Synthase. Available online: www.weedscience.com (accessed on 1 March 2022).
- Yu, Q.; Powles, S. Metabolism-based herbicide resistance and cross-resistance in crop weeds: A threat to herbicide sustainability and global crop production. Plant Physiol. 2014, 166, 1106–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimaano, N.G.; Iwakami, S. Cytochrome P450-mediated herbicide metabolism in plants: Current understanding and prospects. Pest Manag. Sci. 2021, 77, 22–32. [Google Scholar] [CrossRef]
- Menegat, A.; Kaiser, Y.; Stephan, A.; Ni, H.; Gerhards, R. Chlorophyll Fluorescence Microscreening as a Rapid Detection Method for Herbicide Resistance in Grass Weeds in North China Plain Winter Wheat Production Systems and Beyond. In Proceedings of the 23rd Asian-Pacific Weed Science Society Conference, Cairns, QLD, Australia, 26–29 September 2011. [Google Scholar]
- Beckie, H.J.; Tardif, F.J. Herbicide cross resistance in weeds. Crop Prot. 2012, 35, 15–28. [Google Scholar] [CrossRef]
- Zhao, N.; Bi, Y.; Wu, C.; Wang, D.; You, L.; Liu, W.; Wang, J. Cross-resistance to acetolactate synthase (ALS) inhibitors associated with different mutations in Japanese foxtail (Alopecurus japonicus). Weed Sci. 2019, 67, 389–396. [Google Scholar] [CrossRef]
- Norsworthy, J.K.; Ward, S.M.; Shaw, D.R.; Llewellyn, R.S.; Nichols, R.L.; Webster, T.M.; Bradley, K.W.; Frisvold, G.; Powles, S.B.; Burgos, N.R.; et al. Reducing the risks of herbicide resistance: Best management practices and recommendations. Weed Sci. 2017, 60, 31–62. [Google Scholar] [CrossRef] [Green Version]
- Sen, M.K.; Hamouzova, K.; Mikulka, J.; Bharati, R.; Kosnarova, P.; Hamouz, P.; Roy, A.; Soukup, J. Enhanced metabolism and target gene overexpression confer resistance against acetolactate synthase-inhibiting herbicides in Bromus sterilis. Pest Manag. Sci. 2021, 77, 2122–2128. [Google Scholar] [CrossRef] [PubMed]
- Hatami, Z.M.; Gherekhloo, J.; Rojano-Delgado, A.M.; Osuna, M.D.; Alcantara, R.; Fernandez, P.; Sadeghipour, H.R.; de Prado, R. Multiple Mechanisms increase levels of resistance in Rapistrum rugosum to ALS herbicides. Front. Plant Sci. 2016, 7, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krysiak, M.; Gawroński, S.; Adamczewski, K.; Kierzek, R. ALS gene mutations in apera spica-venti confer broad-range resistance to herbicides. J. Plant Prot. Res. 2011, 51, 261–267. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Bei, F.; Wu, C.; Zhang, L.; Jia, S.; Wang, J.; Liu, W. Investigating the mechanism of metabolic resistance to tribenuron-methyl in Capsella bursa-pastoris (L.) Medik. by full-length transcriptome assembly combined with RNA-Seq. J. Agric. Food Chem. 2021, 69, 3692–3701. [Google Scholar] [CrossRef] [PubMed]
- Mallory-Smith, C.; Hendrickson, P.; Mueller-Warrant, G. Cross-resistance of primisulfuron-resistant Bromus tectorum L. (downy brome) to sulfosulfuron. Weed Sci. 1999, 47, 256–257. [Google Scholar] [CrossRef]
- Tardif, F.J.; Powles, S.B. Effect of malathion on resistance to soil-applied herbicides in a population of rigid ryegrass (Lolium rigidum). Weed Sci 1999, 47, 258–261. [Google Scholar] [CrossRef]
- Owen, M.J.; Goggin, D.E.; Powles, S.B. Non-target-site-based resistance to ALS-inhibiting herbicides in six Bromus rigidus populations from Western Australian cropping fields. Pest Manag. Sci. 2012, 68, 1077–1082. [Google Scholar] [CrossRef]
- Soreng, R.J.; Peterson, P.M.; Romaschenko, K.; Davidse, G.; Teisher, J.K.; Clark, L.G.; Barberá, P.; Gillespie, L.J.; Zuloaga, F.O. A worldwide phylogenetic classification of the Poaceae (Gramineae) II: An update and a comparison of two 2015 classifications. J. Syst. Evol. 2017, 55, 259–290. [Google Scholar] [CrossRef] [Green Version]
- Geier, P.W.; Stahlman, P.W.; Peterson, D.E.; Miller, S.D. Application timing affects BAY MKH 6561 and MON 37500 efficacy and crop response in winter wheat. Weed Technol. 2002, 16, 800–806. [Google Scholar] [CrossRef]
- Yu, Q.; Shane Friesen, L.J.; Zhang, X.Q.; Powles, S.B. Tolerance to acetolactate synthase and acetyl-coenzyme A carboxylase inhibiting herbicides in Vulpia bromoides is conferred by two co-existing resistance mechanisms. Pestic. Biochem. Physiol. 2004, 78, 21–30. [Google Scholar] [CrossRef]
- Mei, Y.; Si, C.; Liu, M.; Qiu, L.; Zheng, M. Investigation of resistance levels and mechanisms to nicosulfuron conferred by non-target-site mechanisms in large crabgrass (Digitaria sanguinalis L.) from China. Pestic. Biochem. Physiol. 2017, 141, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Lu, Z.; Huang, H.; Li, W.; Cao, Y.; Wei, S. Target site mutations and cytochrome P450s-involved metabolism confer resistance to nicosulfuron in green foxtail (Setaria viridis). Pestic. Biochem. Physiol. 2021, 179, 104956. [Google Scholar] [CrossRef] [PubMed]
| Population | Flucarbazone-Sodium | Flucarbazone-Sodium + Malathion | I50 b (μM) | RF | ||
|---|---|---|---|---|---|---|
| GR50 a (g ai ha−1) | RF c | GR50 (g ai ha−1) | RF | |||
| S | 14.5 (0.7) | - | 6.5 (0.11) | - | 1.3 × 10−2 (9.6 × 10−3) | - |
| R | 1900.3 (29.9) | 135.7 | 840.3 (22.5) | 129.2 | 1.9 (0.4) | 135.6 |
| Herbicide | Dose a (g ai ha−1) | Population | |
|---|---|---|---|
| S (%) | R (%) | ||
| Mesosulfuron-methyl | 6.75 | 27.14 (0.16) | 99.23 (0.22) |
| 13.5 | 21.63 (0.24) | 98.71 (0.15) | |
| 27.0 | 15.42 (0.18) | 95.62 (0.11) | |
| Pyroxsulam | 7.50 | 20.39 (0.13) | 97.94 (0.21) |
| 15.0 | 17.15 (0.31) | 96.15 (0.09) | |
| 30.0 | 16.06 (0.28) | 95.32 (0.14) | |
| Clodinafop-propargyl | 33.8 | 95.84 (0.08) | 95.44 (0.36) |
| 67.5 | 90.68 (0.14) | 92.49 (0.11) | |
| 135 | 85.22 (0.35) | 90.21 (0.43) | |
| Isoproturon | 650 | 96.42 (0.27) | 96.29 (0.08) |
| 1300 | 94.23 (0.11) | 90.27 (0.14) | |
| 2600 | 85.76 (0.18) | 81.52 (0.32) | |
| Cypyrafluone | 90 | 96.20 (0.24) | 97.23 (0.15) |
| 180 | 84.83 (0.19) | 91.76 (0.22) | |
| 360 | 75.18 (0.23) | 74.28 (0.27) | |
| Population | Location | Coordinates |
|---|---|---|
| R | Wangcun Town, Baoding City, Hebei Province | 39°26′ N, 115°45′ E |
| S | Liuyuankou Town, Kaifeng City, Henan Province | 34°53′ N, 114°20′ E |
| Primer | Sequence | Tm (°C) | Amplified Mutation Sites |
|---|---|---|---|
| ALS1f | 5′-TTGATCCAGCGGAGATTGGAA-3′ | 58 | Ala122, Pro197, Ala205 |
| ALS1r | 5′- CTGGGGTCTCGAGCATCTTC-3′ | ||
| ALS2f | 5′-GCCGCATGATCGGTACG-3′ | 57 | Asp376, Arg377 |
| ALS2r | 5′-CCACCACTTGGGATCATAGG-3′ | ||
| ALS3f | 5′-ATGTGGGCGGCTCAGTATTA-3′ | 55 | Trp574, Ser653, Gly654 |
| ALS3r | 5′-TCGATCCTGCCATCACCTTC-3′ |
| Group a | Herbicide b | Doses c (g ai ha−1) |
|---|---|---|
| ALS inhibitor | Mesosulfuron-methyl 30 g L−1 OD | 6.75 13.5 27.0 |
| Pyroxsulam 4% OD | 7.50 15.0 30.0 | |
| ACCase inhibitor | Clodinafop-propargyl 15% OD | 33.8 67.5 135 |
| PS II inhibitor | Isoproturon 50% SC | 650 1300 2600 |
| HPPD inhibitor | Cypyrafluone 6% OD | 90 180 360 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, Y.; Zhou, X.; Lin, S.; Cao, Y.; Wei, S.; Huang, H.; Li, W.; Huang, Z. Pro-197-Ser Mutation and Cytochrome P450-Mediated Metabolism Conferring Resistance to Flucarbazone-Sodium in Bromus japonicus. Plants 2022, 11, 1641. https://doi.org/10.3390/plants11131641
Lan Y, Zhou X, Lin S, Cao Y, Wei S, Huang H, Li W, Huang Z. Pro-197-Ser Mutation and Cytochrome P450-Mediated Metabolism Conferring Resistance to Flucarbazone-Sodium in Bromus japonicus. Plants. 2022; 11(13):1641. https://doi.org/10.3390/plants11131641
Chicago/Turabian StyleLan, Yuning, Xinxin Zhou, Shenyuan Lin, Yi Cao, Shouhui Wei, Hongjuan Huang, Wenyu Li, and Zhaofeng Huang. 2022. "Pro-197-Ser Mutation and Cytochrome P450-Mediated Metabolism Conferring Resistance to Flucarbazone-Sodium in Bromus japonicus" Plants 11, no. 13: 1641. https://doi.org/10.3390/plants11131641
APA StyleLan, Y., Zhou, X., Lin, S., Cao, Y., Wei, S., Huang, H., Li, W., & Huang, Z. (2022). Pro-197-Ser Mutation and Cytochrome P450-Mediated Metabolism Conferring Resistance to Flucarbazone-Sodium in Bromus japonicus. Plants, 11(13), 1641. https://doi.org/10.3390/plants11131641






