Plant Growth Regulators Improve Grain Production and Water Use Efficiency of Foeniculum vulgare Mill. under Water Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site Characteristics
2.2. Experimental Design
2.3. Determination of Free Proline and Total Soluble Sugar Concentrations
2.4. Analysis of Relative Water Content (RWC) in Leaves
2.5. Biomass, Yield, and Yield Components
2.6. Extraction and Determination of Essential Oils
2.7. Calculation of Water Use Efficiency (WUE)
2.8. Calculation of Spraying Use Efficiency (SUE) and Benefit to Cost Ratio (BCR)
2.9. Statistical Analysis
3. Results
3.1. Free Proline and Total Soluble Sugar Levels
3.2. Relative Water Content in Leaves
3.3. Biomass, Grain Yield, and Selected Yield Components
3.4. Essential Oil Content and Yield
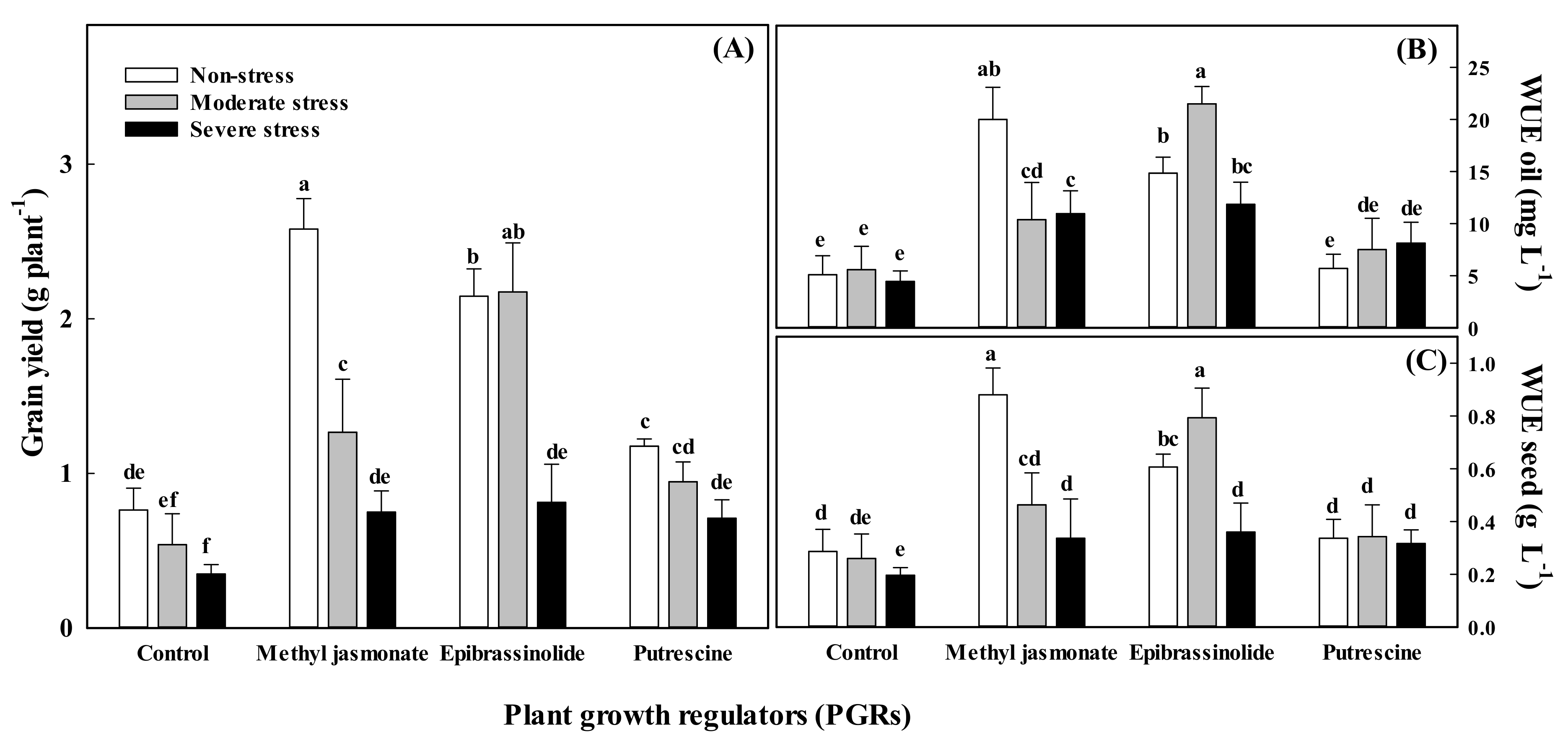
3.5. Water Use Efficiency
3.6. Analysis of Spraying Use Efficiency and Benefit-to-Cost Ratio
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Backhaus, S.; Kreyling, J.; Grant, K.; Beierkuhnlein, C.; Walter, J.; Jentsch, A. Recurrent Mild Drought Events Increase Resistance Toward Extreme Drought Stress. Ecosystems 2014, 17, 1068–1081. [Google Scholar] [CrossRef]
- McDowell, N.G. Mechanisms Linking Drought, Hydraulics, Carbon Metabolism, and Vegetation Mortality. Plant Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Tan, Y.; Liang, Z.; Shao, H.; Du, F. Effect of Water Deficits on the Activity of Anti-Oxidative Enzymes and Osmoregulation among Three Different Genotypes of Radix Astragali at Seeding Stage. Colloids Surf. B Biointerfaces 2006, 49, 60–65. [Google Scholar] [CrossRef]
- Mafakheri, A.; Siosemardeh, A.; Bahramnejad, B.; Struik, P.C.; Sohrabi, Y. Effect of Drought Stress on Yield, Proline and Chlorophyll Contents in Three Chickpea Cultivars. Aust. J. Crop Sci. 2010, 4, 580–585. [Google Scholar]
- Kolenc, Z.; Vodnik, D.; Mandelc, S.; Javornik, B.; Kastelec, D.; Čerenak, A. Hop (Humulus lupulus L.) Response Mechanisms in Drought Stress: Proteomic Analysis with Physiology. Plant Physiol. Biochem. 2016, 105, 67–78. [Google Scholar] [CrossRef]
- Barnabás, B.; Jäger, K.; Fehér, A. The Effect of Drought and Heat Stress on Reproductive Processes in Cereals. Plant. Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef]
- Gruszka, D. The Brassinosteroid Signaling Pathway—New Key Players and Interconnections with Other Signaling Networks Crucial for Plant Development and Stress Tolerance. Int. J. Mol. Sci. 2013, 14, 8740–8774. [Google Scholar] [CrossRef] [Green Version]
- Xi, Z.; Zhang, Z.; Huo, S.; Luan, L.; Gao, X.; Ma, L.; Fang, Y. Regulating the Secondary Metabolism in Grape Berry Using Exogenous 24-Epibrassinolide for Enhanced Phenolics Content and Antioxidant Capacity. Food Chem. 2013, 141, 3056–3065. [Google Scholar] [CrossRef]
- Swamy, K.N.; Rao, S.S.R. Influence of 28-Homobrassinolide on Growth, Photosynthesis Metabolite and Essential Oil Content of Geranium [Pelargonium graveolens (L.) Herit]. Am. J. Plant Physiol. 2008, 3, 173–179. [Google Scholar] [CrossRef] [Green Version]
- Fariduddin, Q.; Hasan, S.A.; Ali, B.; Hayat, S.; Ahmad, A. Effect of Modes of Application of 28-Homobrassinolide on Mung Bean. Turkish J. Biol. 2008, 32, 17–21. [Google Scholar]
- Bera, A.K.; Pramanik, K. Response of Hybrid Rice (Oryza sativa L.) to Varying Levels of Nitrogen and Homo-Brassinosteroids in Lateritic Zone of West Bengal. Int. J. Bio-Resour. Stress Manag. 2012, 3, 165–168. [Google Scholar]
- Hnilička, F.; Hniličková, H.; Martinková, J.; Bláha, L. The Influence of Drought and the Application of 24-Epibrassinolide on the Formation of Dry Matter and Yield in Wheat. Cereal Res. Commun. 2007, 35, 457–460. [Google Scholar] [CrossRef]
- Kamiab, F.; Talaie, A.; Khezri, M.; Javanshah, A. Exogenous Application of Free Polyamines Enhance Salt Tolerance of Pistachio (Pistacia vera L.) Seedlings. Plant Growth Regul. 2014, 72, 257–268. [Google Scholar] [CrossRef]
- Silveira, V.; de Vita, A.M.; Macedo, A.F.; Dias, M.F.R.; Floh, E.I.S.; Santa-Catarina, C. Morphological and Polyamine Content Changes in Embryogenic and Non-Embryogenic Callus of Sugarcane. Plant Cell Tissue Organ Cult. 2013, 114, 351–364. [Google Scholar] [CrossRef]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine Function in Plants: Metabolism, Regulation on Development, and Roles in Abiotic Stress Responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef]
- Rohwer, C.L.; Erwin, J.E. Spider Mites (Tetranychus urticae) Perform Poorly on and Disperse from Plants Exposed to Methyl Jasmonate. Entomol. Exp. Appl. 2010, 137, 143–152. [Google Scholar] [CrossRef]
- Khatun, S.; Roy, T.S.; Haque, M.N.; Alamgir, B. Effect of Plant Growth Regulators and Their Time of Application on Yield Attributes and Quality of Soybean. Int. J. Plant Soil Sci. 2016, 11, 1–9. [Google Scholar] [CrossRef]
- Bakhsh, I.; Khan, H.; Usman, K.; Qasim, M.; Anwar, S.; Javaria, S. Effect of Plant Growth Regulator Application at Different Growth Stages on the Yield Potential of Coarse Rice. Sarhad J. Agric. 2011, 27, 513–518. [Google Scholar]
- Waqas, M.A.; Khan, I.; Akhter, M.J.; Noor, M.A.; Ashraf, U. Exogenous Application of Plant Growth Regulators (PGRs) Induces Chilling Tolerance in Short-Duration Hybrid Maize. Environ. Sci. Pollut. Res. 2017, 24, 11459–11471. [Google Scholar] [CrossRef]
- Bistgani, Z.E.; Siadat, S.A.; Bakhshandeh, A.; Pirbalouti, A.G.; Hashemi, M. Interactive Effects of Drought Stress and Chitosan Application on Physiological Characteristics and Essential Oil Yield of Thymus daenensis Celak. Crop J. 2017, 5, 407–415. [Google Scholar] [CrossRef]
- Keshavarz Afshar, R.; Hashemi, M.; DaCosta, M.; Spargo, J.; Sadeghpour, A. Biochar Application and Drought Stress Effects on Physiological Characteristics of Silybum marianum. Commun. Soil Sci. Plant Anal. 2016, 47, 743–752. [Google Scholar] [CrossRef]
- Hassanpour, H.; Khavari-Nejad, R.A.; Niknam, V.; Razavi, K.; Najafi, F. Effect of Penconazole and Drought Stress on the Essential Oil Composition and Gene Expression of Menthapulegium L.(Lamiaceae) at Flowering Stage. Acta Physiol. Plant. 2014, 36, 1167–1175. [Google Scholar] [CrossRef]
- Selmar, D.; Kleinwächter, M. Influencing the Product Quality by Deliberately Applying Drought Stress during the Cultivation of Medicinal Plants. Ind. Crops Prod. 2013, 42, 558–566. [Google Scholar] [CrossRef]
- Bahmani, K.; Darbandi, A.I.; Ramshini, H.A.; Moradi, N.; Akbari, A. Agro-Morphological and Phytochemical Diversity of Various Iranian Fennel Landraces. Ind. Crops Prod. 2015, 77, 282–294. [Google Scholar] [CrossRef]
- Díaz-Maroto, M.C.; Pérez-Coello, M.S.; Esteban, J.; Sanz, J. Comparison of the Volatile Composition of Wild Fennel Samples (Foeniculum vulgare Mill.) from Central Spain. J. Agric. Food Chem. 2006, 54, 6814–6818. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, E.; Hashiguchi, A.; Fakheri, B.A.; Aharizad, S.; Emamjomeh, A.; Norouzi, M.; Komatsu, S. Differences in Fennel Seed Responses to Drought Stress at the Seed Formation Stage in Sensitive and Tolerant Genotypes. J. Plant Biochem. Biotechnol. 2019, 28, 35–49. [Google Scholar] [CrossRef]
- Nourimand, M.; Mohsenzadeh, S.; Teixeira da Silva, J.A. Physiological Responses of Fennel Seedling to Four Environmental Stresses. Iran. J. Sci. Technol. 2012, 36, 37–46. [Google Scholar]
- Gheisari Zardak, S.; Movahhedi Dehnavi, M.; Salehi, A.; Gholamhoseini, M. Responses of Field Grown Fennel (Foeniculum vulgare Mill.) to Different Mycorrhiza Species under Varying Intensities of Drought Stress. J. Appl. Res. Med. Aromat. Plants 2017, 5, 16–25. [Google Scholar] [CrossRef]
- Nikbakht, A.; Kafi, M.; Haghighi, M. The Abilities and Potentials of Medicinal Plants Production and Herbal Medicine in Iran. In VIII International People-Plant Symposium on Exploring Therapeutic Powers of Flowers, Greenery and Nature, Proceedings of the 8th International People-Plant Symposium on Exploring Therapeutic Powers of Flowers, Greenery and Nature, Awaji, Japan, 4–6 June 2004; International Society for Horticultural Science: Leuven, Belgium, 2008; pp. 259–262. [Google Scholar]
- Liu, Y.; Li, P.; Xu, G.C.; Xiao, L.; Ren, Z.P.; Li, Z.B. Growth, Morphological, and Physiological Responses to Drought Stress in Bothriochloa ischaemum. Front. Plant Sci. 2017, 8, 230. [Google Scholar] [CrossRef] [Green Version]
- Sheokand, S.; Kumari, A.; Sawhney, V. Effect of Nitric Oxide and Putrescine on Antioxidative Responses under NaCl Stress in Chickpea Plants. Physiol. Mol. Biol. Plants 2008, 14, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Abdelgawad, Z.A.; Khalafaallah, A.A.; Abdallah, M.M. Impact of Methyl Jasmonate on Antioxidant Activity and Some Biochemical Aspects of Maize Plant Grown under Water Stress Condition. Agric. Sci. 2014, 5, 1077–1088. [Google Scholar] [CrossRef] [Green Version]
- Anjum, S.A.; Xie, X.Y.; Farooq, M.; Wang, L.C.; Xue, L.I.; Shahbaz, M.; Salhab, J. Effect of Exogenous Methyl Jasmonate on Growth, Gas Exchange and Chlorophyll Contents of Soybean Subjected to Drought. Afr. J. Biotechnol. 2011, 10, 9647–9656. [Google Scholar]
- Jiménez, S.; Fattahi, M.; Bedis, K.; Nasrolahpour-Moghadam, S.; Irigoyen, J.J.; Gogorcena, Y. Interactional Effects of Climate Change Factors on the Water Status, Photosynthetic Rate, and Metabolic Regulation in Peach. Front. Plant Sci. 2020, 11, 43–56. [Google Scholar] [CrossRef] [Green Version]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Irogoyen, J.J.; Emerich, D.W.; Sanchez-Diaz, M. Water Stress Induced Changes in Concentration of Proline and Total Soluble Sugars in Nodulated Alfalfa (Medicago sativa) Plants. Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Horgan, T.; Astatkie, T.; Schlegel, V. Distillation Time Modifies Essential Oil Yield, Composition, and Antioxidant Capacity of Fennel (Foeniculum vulgare Mill). J. Oleo Sci. 2013, 62, 665–672. [Google Scholar] [CrossRef] [Green Version]
- Rashidi, S.; Ebadi, A.; Parmoon, G.; Jahanbakhsh, S.; Haghighat, Z. Effect of Nitrogen Source on Bean Growth under Water Deficit Conditions. Philipp. Agric. Sci. 2015, 98, 279–285. [Google Scholar]
- Meena, A.K.; Singh, A.K.; Singh, B. Estimation of Benefit/Cost Ratio of Various Plant Growth Regulators in Phalsa (Grewia subinequalis DC). In Proceedings of the International Symposium on Horticulture: Priorities and Emerging Trends, Bangalore, India, 5–8 September 2017; pp. 145–148. [Google Scholar]
- Askari, E.; Ehsanzadeh, P. Drought Stress Mitigation by Foliar Application of Salicylic Acid and Their Interactive Effects on Physiological Characteristics of Fennel (Foeniculum vulgare Mill.) Genotypes. Acta Physiol. Plant. 2015, 37, 4. [Google Scholar] [CrossRef]
- Coban, F.; Ozer, H.; Ors, S.; Sahin, U.; Yildiz, G.; Cakmakci, T. Effects of Deficit Irrigation on Essential Oil Composition and Yield of Fennel (Foeniculum vulgare Mill) in a High-Altitude Environment. J. Essent. Oil Res. 2018, 30, 457–463. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of Proline under Changing Environments: A Review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alqudah, A.M.; Samarah, N.H.; Mullen, R.E. Drought Stress Effect on Crop Pollination, Seed Set, Yield and Quality. In Alternative Farming Systems, Biotechnology, Drought Stress and Ecological Fertilisation; Springer: Berlin/Heidelberg, Germany, 2011; pp. 193–213. [Google Scholar]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M.; Aroca, R. Plant Responses to Drought Stress: From Morphological to Molecular Features; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–33. [Google Scholar]
- Wang, Y.; Xu, C.; Zhang, B.; Wu, M.; Chen, G. Physiological and Proteomic Analysis of Rice (Oryza sativa L.) in Flag Leaf during Flowering Stage and Milk Stage under Drought Stress. Plant Growth Regul. 2017, 82, 201–218. [Google Scholar] [CrossRef]
- Anjum, S.A.; Xie, X.; Wang, L.; Saleem, M.F.; Man, C.; Lei, W. Morphological, Physiological and Biochemical Responses of Plants to Drought Stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Hlaváčová, M.; Klem, K.; Rapantová, B.; Novotná, K.; Urban, O.; Hlavinka, P.; Smutná, P.; Horáková, V.; Škarpa, P.; Pohanková, E. Interactive Effects of High Temperature and Drought Stress during Stem Elongation, Anthesis and Early Grain Filling on the Yield Formation and Photosynthesis of Winter Wheat. Field Crop. Res. 2018, 221, 182–195. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Murali, P.V. Exogenous Jasmonic Acid Alleviates Adverse Effects of Drought Stress in Allium cepa L. Int. J. Geol. Agric. Environ. Sci. 2015, 3, 10–18. [Google Scholar]
- Shahzad, B.; Tanveer, M.; Che, Z.; Rehman, A.; Cheema, S.A.; Sharma, A.; Song, H.; ur Rehman, S.; Zhaorong, D. Role of 24-Epibrassinolide (EBL) in Mediating Heavy Metal and Pesticide Induced Oxidative Stress in Plants: A Review. Ecotoxicol. Environ. Saf. 2018, 147, 935–944. [Google Scholar] [CrossRef]
- Kaya, A.; Doganlar, Z.B. Exogenous Jasmonic Acid Induces Stress Tolerance in Tobacco (Nicotiana tabacum) Exposed to Imazapic. Ecotoxicol. Environ. Saf. 2016, 124, 470–479. [Google Scholar] [CrossRef]
- Albacete, A.A.; Martínez-Andújar, C.; Pérez-Alfocea, F. Hormonal and Metabolic Regulation of Source Sink Relations under Salinity and Drought: From Plant Survival to Crop Yield Stability. Biotechnol. Adv. 2014, 32, 12–30. [Google Scholar] [CrossRef]
- Ye, Q.; Zhu, W.; Li, L.; Zhang, S.; Yin, Y.; Ma, H.; Wang, X. Brassinosteroids Control Male Fertility by Regulating the Expression of Key Genes Involved in Arabidopsis Anther and Pollen Development. Proc. Natl. Acad. Sci. USA 2010, 107, 6100–6105. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.-B.; Huang, H.-Y.; Hu, Y.-W.; Zhu, S.-W.; Wang, Z.-Y.; Lin, W.-H. Brassinosteroid Regulates Seed Size and Shape in Arabidopsis. Plant Physiol. 2013, 162, 1965–1977. [Google Scholar] [CrossRef]
- Khanam, D.; Mohammad, F. Effect of Structurally Different Plant Growth Regulators (PGRs) on the Concentration, Yield, and Constituents of Peppermint Essential Oil. J. Herbs. Spices Med. Plants 2017, 23, 26–35. [Google Scholar] [CrossRef]
- Bahreininejad, B.; Razmjoo, J.; Mirza, M. Effect of Water Stress on Productivity and Essential Oil Content and Composition of Thymus carmanicus. J. Essent. Oil Bear. Plants 2014, 17, 717–725. [Google Scholar] [CrossRef]
- Monclus, R.; Dreyer, E.; Villar, M.; Delmotte, F.M.; Delay, D.; Petit, J.; Barbaroux, C.; Le Thiec, D.; Bréchet, C.; Brignolas, F. Impact of Drought on Productivity and Water Use Efficiency in 29 Genotypes of Populus Deltoides× Populus Nigra. New Phytol. 2006, 169, 765–777. [Google Scholar] [CrossRef]
- Javadipour, Z.; Balouchi, H.; Dehnavi, M.M.; Yadavi, A. Roles of Methyl Jasmonate in Improving Growth and Yield of Two Varieties of Bread Wheat (Triticum aestivum) under Different Irrigation Regimes. Agric. Water Manag. 2019, 222, 336–345. [Google Scholar] [CrossRef]
- Accamando, A.K.; Cronin, J.T. Costs and Benefits of Jasmonic Acid Induced Responses in Soybean. Environ. Entomol. 2012, 41, 551–561. [Google Scholar] [CrossRef]
- Patel, M.J.; Patel, H.C.; Chavda, J.C. Effect of Plant Growth Regulators and Their Application Methods on Growth and Yield of Onion (Allium cepa L.) Cv. Gujarat White Onion-1. Adv. Res. J. Crop Improv. 2010, 1, 85–87. [Google Scholar]
- Coste, A.; Vlase, L.; Halmagyi, A.; Deliu, C.; Coldea, G. Effects of Plant Growth Regulators and Elicitors on Production of Secondary Metabolites in Shoot Cultures of Hypericum hirsutum and Hypericum maculatum. Plant Cell Tissue Organ Cult. 2011, 106, 279–288. [Google Scholar] [CrossRef]


| Treatments | Proline (µg g FW−1) | Total Soluble Sugars (mg g FW−1) | RWC (%) | |||
|---|---|---|---|---|---|---|
| Vegetative | Generative | Vegetative | Generative | Vegetative | Generative | |
| Non-stress | † 0.55 ± 0.06 b | 1.32 ± 0.22 b | 0.97 ± 0.08 b | 0.36 ± 0.06 b | 84.0 ± 1.6 a | 81.5 ± 1.4 a |
| Moderate stress | 1.39 ± 0.24 a | 2.11 ± 0.21 a | 1.55 ± 0.10 a | 0.55 ± 0.05 a | 80.1 ± 2.8 b | 79.4 ± 2.4 b |
| Severe stress | 1.58 ± 0.20 a | 2.19 ± 0.25 a | 1.60 ± 0.10 a | 0.41 ± 0.05 ab | 78.9 ± 3.0 b | 77.9 ± 2.5 b |
| LSD (0.05) | 0.50 | 0.53 | 0.19 | 0.14 | 2.69 | 2.69 |
| PGRs | ||||||
| Control | 1.28 ± 0.20 ab | 1.84 ± 0.31 b | 1.64 ± 0.11 a | 0.50 ± 0.03 a | 79.9 ± 1.3 a | 77.9 ± 1.2 a |
| Methyl jasmonate | 0.74 ± 0.15 b | 2.32 ± 0.29 a | 1.46 ± 0.13 b | 0.30 ± 0.06 c | 80.8 ± 1.5 a | 78.4 ± 1.5 a |
| Epibrassinolide | 1.59 ± 0.35 a | 2.03 ± 0.27 ab | 0.95 ± 0.14 c | 0.42 ± 0.10 b | 82.8 ± 1.2 a | 81.4 ± 0.9 a |
| Putrescine | 1.09 ± 0.25 ab | 1.30 ± 0.21 c | 1.45 ± 0.12 b | 0.54 ± 0.06 a | 83.0 ± 1.1 a | 80.7 ± 1.0 a |
| LSD (0.05) | 0.58 | 0.61 | 0.22 | 0.16 | 3.1 | 3.1 |
| †† F test | ||||||
| Water stress (WS) | ** | ** | ** | * | ** | * |
| PGR | * | * | ** | * | ns | ns |
| WS × PGR | ns | ns | ns | ns | ns | ns |
| CV (%) | 15.6 | 17.9 | 16.8 | 23.5 | 3.89 | 4.65 |
| Treatments | Grain Number (per Plant) | 1000-Grain wt. (g) | Grain Yield (g Plant−1) | Shoot Biomass (g Plant−1) | ||||
|---|---|---|---|---|---|---|---|---|
| Vegetative | Generative | Vegetative | Generative | Vegetative | Generative | Vegetative | Generative | |
| Non-stress | † 539.6 ± 99.0 a | 529.2 ± 104.1 a | 3.17 ± 0.26 a | 3.25 ± 0.40 a | 1.68 ± 0.32 a | 1.72 ± 0.31 a | 13.5 ± 0.95 a | 13.8 ± 0.60 a |
| Moderate stress | 459.2 ± 117.0 b | 470.0 ± 98.2 ab | 2.28 ± 0.38 ab | 3.11 ± 0.32 a | 1.23 ± 0.22 a | 1.43 ± 0.26 a | 8.6 ± 0.81 b | 13.8 ± 0.90 a |
| Severe stress | 237.3 ± 48.1 c | 389.1 ± 77.4 b | 1.82 ± 0.30 b | 2.01 ± 0.21 b | 0.66 ± 0.11 b | 0.91 ± 0.20 b | 5.6 ± 0.27 c | 9.2 ± 1.70 b |
| LSD (0.05) | 241.0 | 228.7 | 0.85 | 0.73 | 0.37 | 0.68 | 1.7 | 3.6 |
| PGRs | ||||||||
| Control | 220.6 ± 35.4 c | 307.5 ± 65.5 c | 2.01 ± 0.18 c | 1.92 ± 0.14 c | 0.55 ± 0.11 c | 0.61 ± 0.10 c | 6.3 ± 0.9 b | 10.8 ± 1.16 a |
| Methyl jasmonate | 566.1 ± 104.1 a | 561.8 ± 106.1 a | 2.20 ± 0.43 ab | 3.44 ± 0.50 a | 1.53 ± 0.34 a | 1.93 ± 0.29 ab | 9.3 ± 1.5 a | 14.9 ± 1.39 a |
| Epibrassinolide | 540.2 ± 105.1 ab | 509.4 ± 139.2 ab | 3.35 ± 0.39 a | 3.29 ± 0.38 a | 1.71 ± 0.36 a | 1.65 ± 0.33 a | 9.9 ± 1.4 a | 11.8 ± 1.24 a |
| Putrescine | 320.3 ± 81.2 b | 469.8 ± 99.8 b | 2.41 ± 0.44 b | 2.79 ± 0.24 b | 0.94 ± 0.14 b | 1.23 ± 0.32 b | 10.1 ± 1.7 a | 11.8 ± 1.12 a |
| LSD (0.05) | 248.3 | 239.5 | 0.98 | 0.85 | 0.42 | 0.79 | 1.95 | 4.2 |
| †† F test | ||||||||
| Water stress (WS) | * | ns | * | * | ** | * | ** | ** |
| PGR | * | * | * | ** | ** | * | ** | ns |
| WS × PGR | ns | ns | ns | ns | * | ns | ns | ns |
| CV (%) | 16.90 | 19.1 | 18.2 | 13.2 | 19.62 | 20.15 | 13.15 | 15.6 |
| Treatments | Essential Oil Content (mg g−1 of Seeds) | Essential Oil Yield (mg Plant−1) | WUE for Grain (g L−1) | WUE for Essential Oil (mg L−1) | ||||
|---|---|---|---|---|---|---|---|---|
| Vegetative | Generative | Vegetative | Generative | Vegetative | Generative | Vegetative | Generative | |
| Non-stress | † 19.79 ± 1.2 b | 18.53 ± 1.0 b | 36.6 ± 8.7 a | 34.6 ± 6.0 a | 0.47 ± 0.09 ab | 0.46 ± 0.06 ab | 11.4 ± 2.5 a | 11.04 ± 1.2 a |
| Moderate stress | 22.16 ± 1.1 b | 20.79 ± 1.1 a | 29.9 ± 6.7 b | 26.7 ± 5.1 ab | 0.53 ± 0.08 a | 0.54 ± 0.05 a | 11.3 ± 2.4 a | 12.23 ± 1.5 a |
| Severe stress | 27.13 ± 1.5 a | 21.11 ± 1.5 a | 19.3 ± 4.0 c | 21.4 ± 5.3 b | 0.30 ± 0.05 b | 0.32 ± 0.06 b | 8.9 ± 1.7 a | 9.56 ± 0.6 a |
| LSD (0.05) | 0.60 | 1.43 | 9.8 | 12.5 | 0.12 | 0.13 | 3.02 | 3.12 |
| PGRs | ||||||||
| Control | 19.69 ± 1.1 c | 18.12 ± 1.4 b | 11.0 ± 2.3 c | 11.5 ± 2.3 b | 0.25 ± 0.04 b | 0.24 ± 0.04 c | 5.1 ± 0.9 b | 5.35 ± 0.5 c |
| Methyl jasmonate | 24.82 ± 1.8 ab | 19.59 ± 1.6 b | 37.8 ± 8.6 b | 37.3 ± 5.9 a | 0.56 ± 0.10 a | 0.55 ± 0.06 a | 13.8 ± 2.5 a | 14.94 ± 1.5 a |
| Epibrassinolide | 26.74 ± 1.7 a | 21.58 ± 1.2 a | 45.9 ± 9.5 a | 34.6 ± 5.8 a | 0.59 ± 0.11 a | 0.53 ± 0.06 a | 16.1 ± 2.9 a | 12.88 ± 1.3 ab |
| Putrescine | 20.86 ± 1.5 bc | 21.28 ± 1.3 a | 19.6 ± 3.0 c | 26.9 ± 7.4 a | 0.33 ± 0.04 b | 0.44 ± 0.07 b | 7.1 ± 1.2 b | 10.60 ± 1.7 bc |
| LSD (0.05) | 0.77 | 1.65 | 11.3 | 15.7 | 0.13 | 0.15 | 3.5 | 3.6 |
| †† F test | ||||||||
| Water stress (WS) | ** | ** | ** | * | ** | * | ns | ns |
| PGR | ** | ** | ** | ** | ** | * | ** | * |
| WS × PGR | ** | ** | * | ns | * | ns | * | ns |
| CV (%) | 3.11 | 8.38 | 18.2 | 13.2 | 18.65 | 20.2 | 18.5 | 19.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parmoon, G.; Ebadi, A.; Hashemi, M.; Hawrylak-Nowak, B.; Baskin, C.; Jahanbakhsh, S. Plant Growth Regulators Improve Grain Production and Water Use Efficiency of Foeniculum vulgare Mill. under Water Stress. Plants 2022, 11, 1718. https://doi.org/10.3390/plants11131718
Parmoon G, Ebadi A, Hashemi M, Hawrylak-Nowak B, Baskin C, Jahanbakhsh S. Plant Growth Regulators Improve Grain Production and Water Use Efficiency of Foeniculum vulgare Mill. under Water Stress. Plants. 2022; 11(13):1718. https://doi.org/10.3390/plants11131718
Chicago/Turabian StyleParmoon, Ghasem, Ali Ebadi, Masoud Hashemi, Barbara Hawrylak-Nowak, Carol Baskin, and Soodabe Jahanbakhsh. 2022. "Plant Growth Regulators Improve Grain Production and Water Use Efficiency of Foeniculum vulgare Mill. under Water Stress" Plants 11, no. 13: 1718. https://doi.org/10.3390/plants11131718
APA StyleParmoon, G., Ebadi, A., Hashemi, M., Hawrylak-Nowak, B., Baskin, C., & Jahanbakhsh, S. (2022). Plant Growth Regulators Improve Grain Production and Water Use Efficiency of Foeniculum vulgare Mill. under Water Stress. Plants, 11(13), 1718. https://doi.org/10.3390/plants11131718










