Physical Characteristics, Mineral Content, and Antioxidant and Antibacterial Activities of Punica granatum or Citrus sinensis Peel Extracts and Their Applications to Improve Cake Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruits
2.2. Preparation of P. granatum and C. sinensis L. Peel Powders
2.3. Physical Analysis of P. granatum and C. sinensis L. Peel Powders
2.4. Chemical Composition of P. granatum and C. sinensis L. Peel Powders
2.5. Mineral Content of Fruit Peel Powders
2.6. Preparation of Extracts
2.7. Phytochemical Screening of Peel Extracts
2.8. Total Phenolic Content
2.9. Total Flavonoid Content
2.10. Extraction of Polyphenolics from Orange and Pomegranate
2.11. UPLC-MS Analysis Conditions
2.12. GC-MS Qualitative Identification
2.13. DPPH Assay
2.14. Scavenging of Hydrogen Peroxide
2.15. Determination of Extraction Yield
2.16. Isolation and Identification of Bacterial Pathogens
2.17. Morphological or Biochemical Test
2.18. S rDNA Sequencing or Phylogenetic Analysis
2.19. The Activity of Antibacterial Extract
2.20. Sensitivity Test of Antibiotics
2.21. Minimum Inhibitory Concentration (MIC)
2.22. Preparation of Cakes
2.23. Shelf Life Effect upon Sensory Evaluation of Cake Supplemented with C. sinensis and P. granatum Peel Powder at Room Temperature (30 °C) and Refrigeration Temperature (3 °C)
2.24. Analysis of the Microbial Load in Cake during the Storage Period
2.25. Statistical Analysis
3. Results and Discussion
3.1. Physical Characteristics of Fruit Peel Powders
3.2. Chemical Composition and Mineral Content of Fruit Peel Powders
3.3. Phytochemical Screening
3.4. Total Phenolic and Flavonoid Content
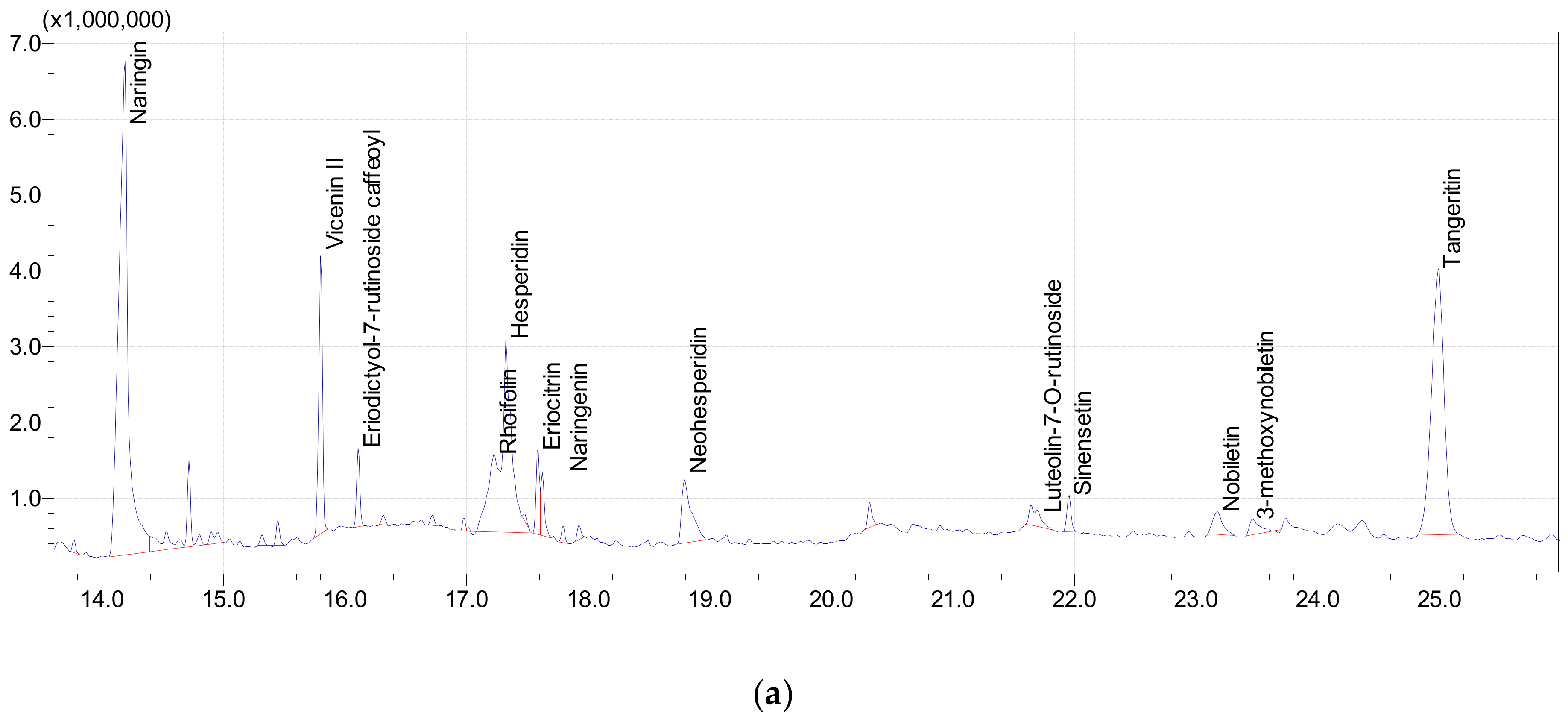
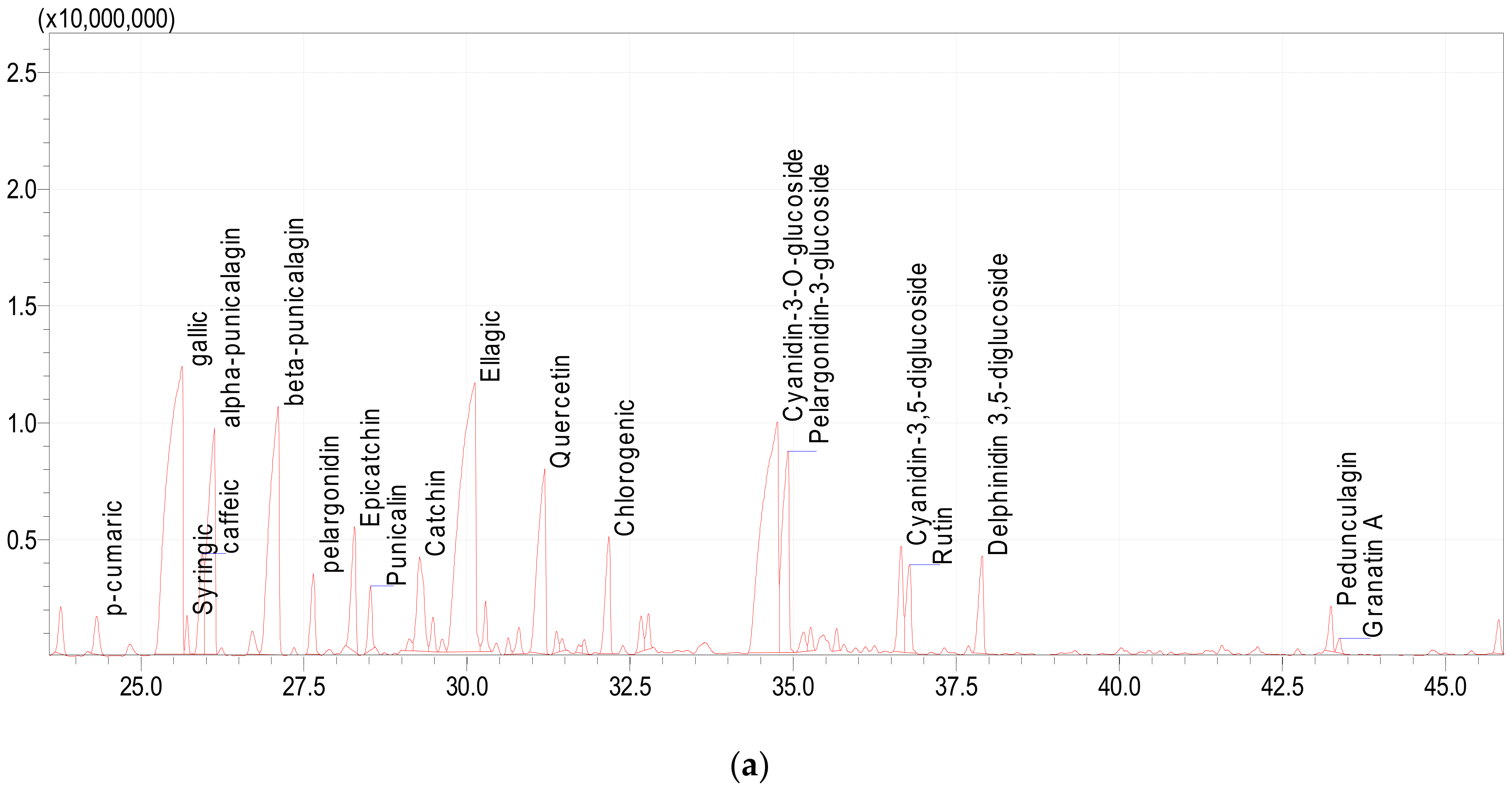
3.5. UPLC-MS Analysis of Polyphenols
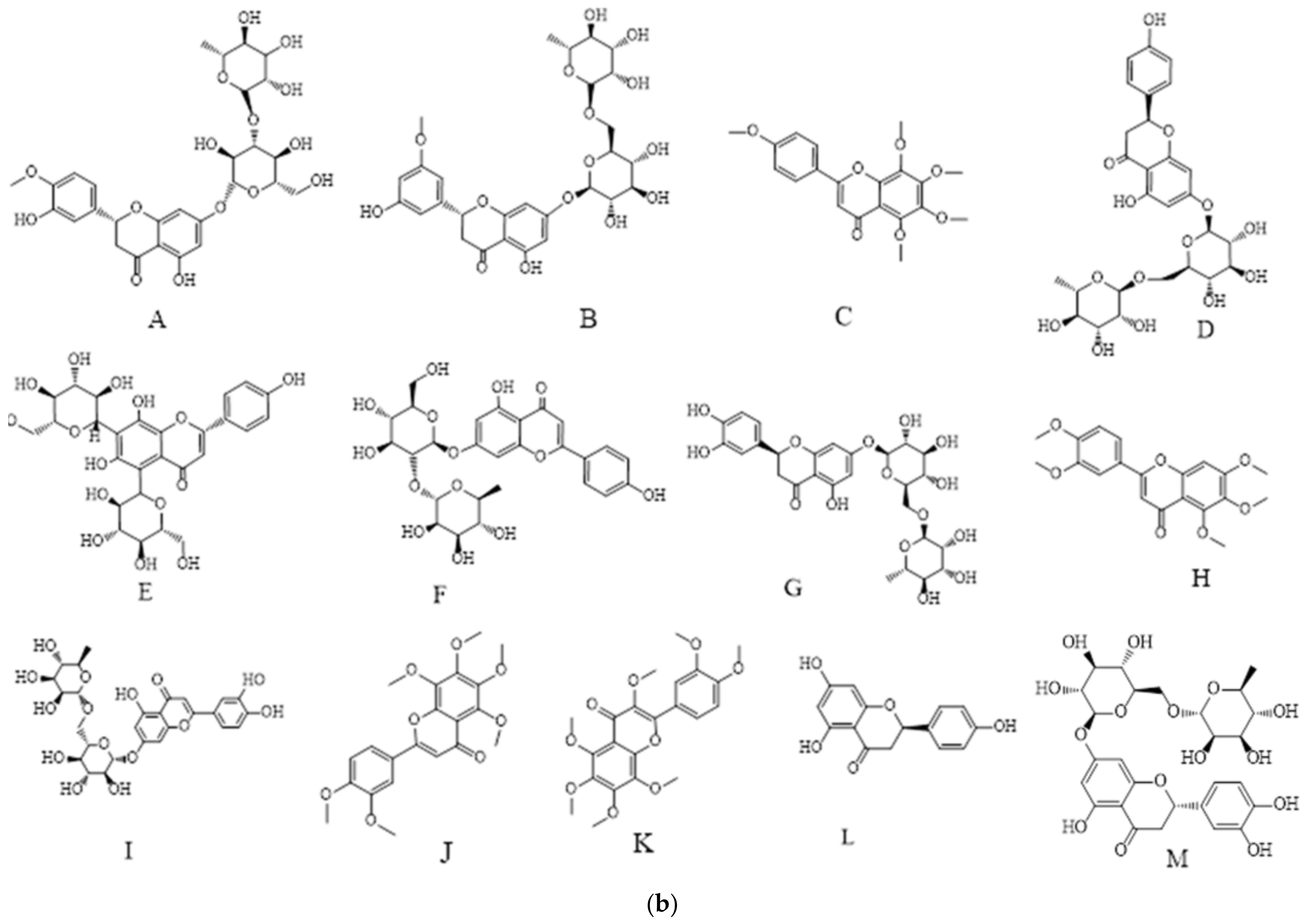
3.5.1. UPLC-MS Analysis of Polyphenols in Orange Peel
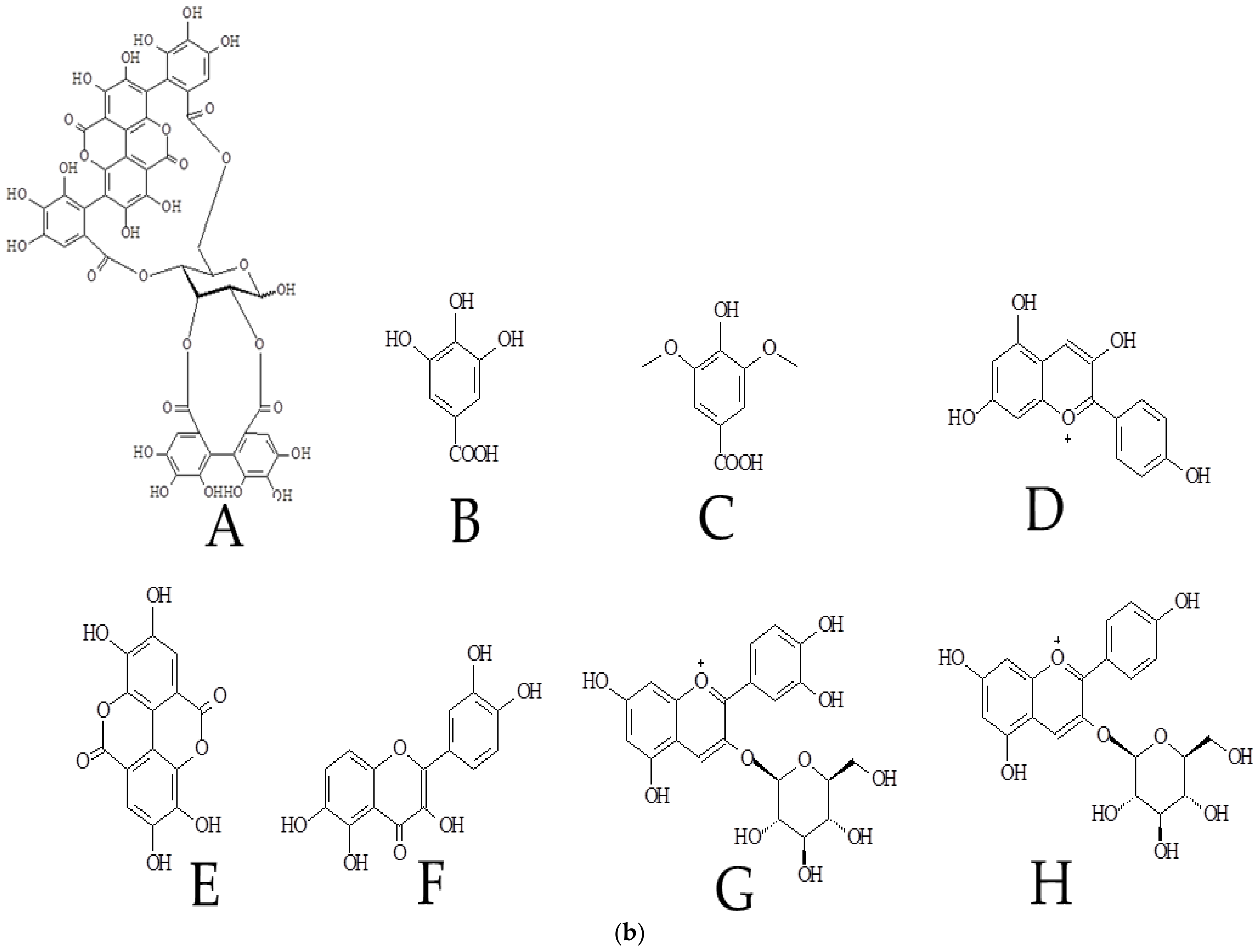
3.5.2. UPLC-MS Analysis of Polyphenols in Pomegranate Peel
3.6. Essential Oil Compounds in C. sinensis Peels by GC-MS
3.7. Antioxidant Activity
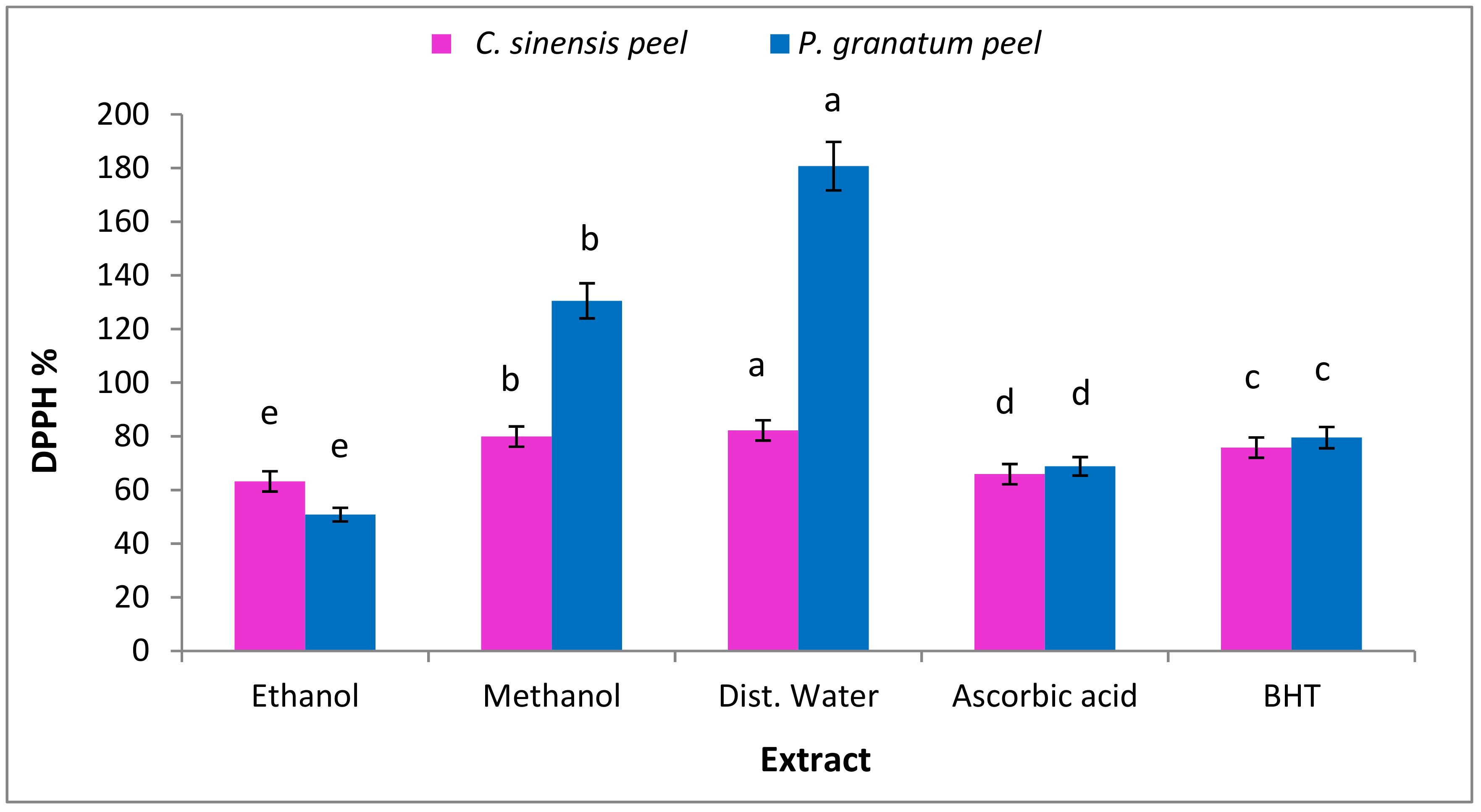
3.7.1. Radical Scavenging Activities (DPPH)
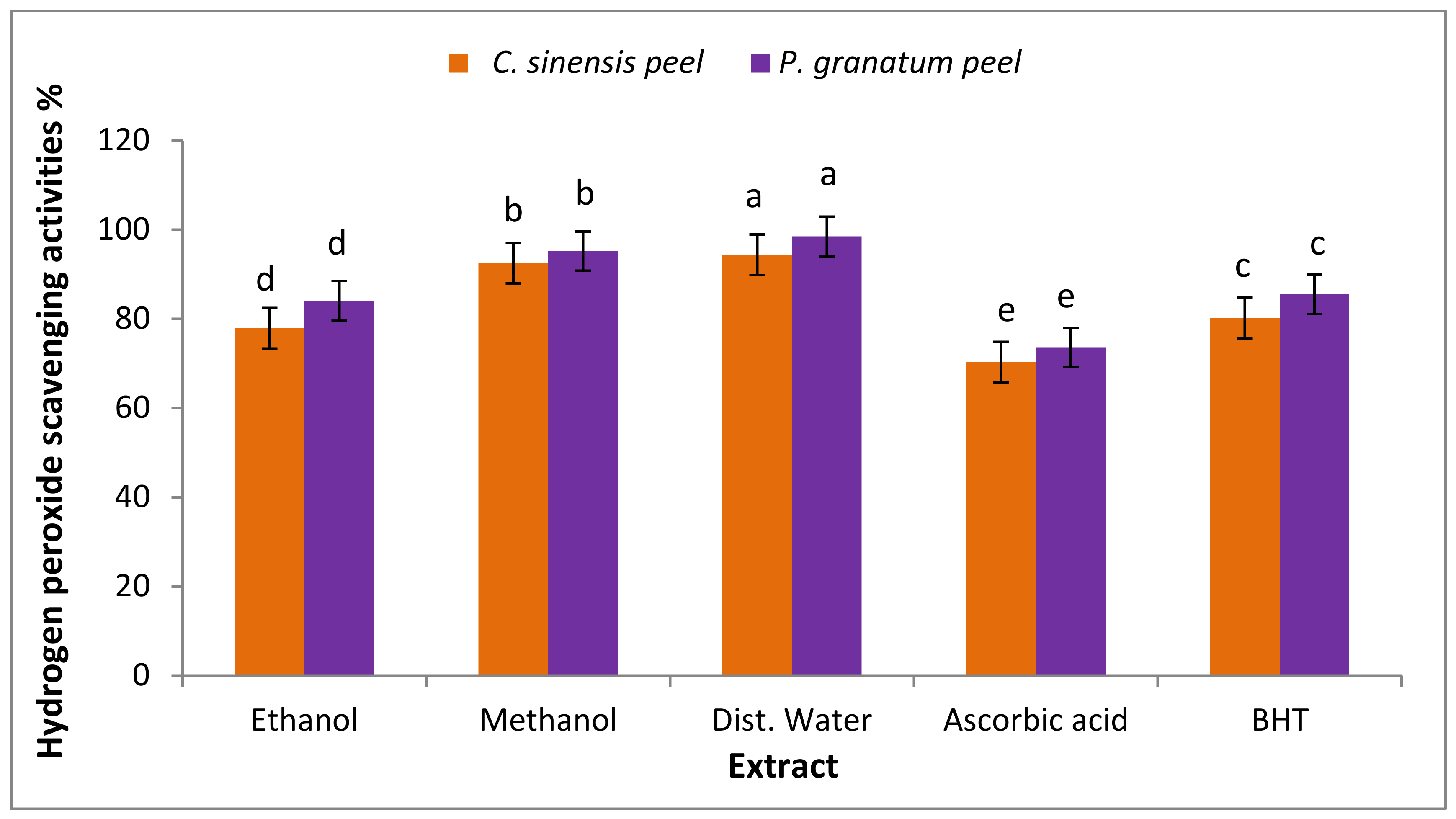
3.7.2. Hydrogen Peroxide Scavenging Activity
3.8. Extraction Yield
3.9. Identification of Bacterial Isolates
3.10. Antibacterial Activity of Fruits Peel Extracts
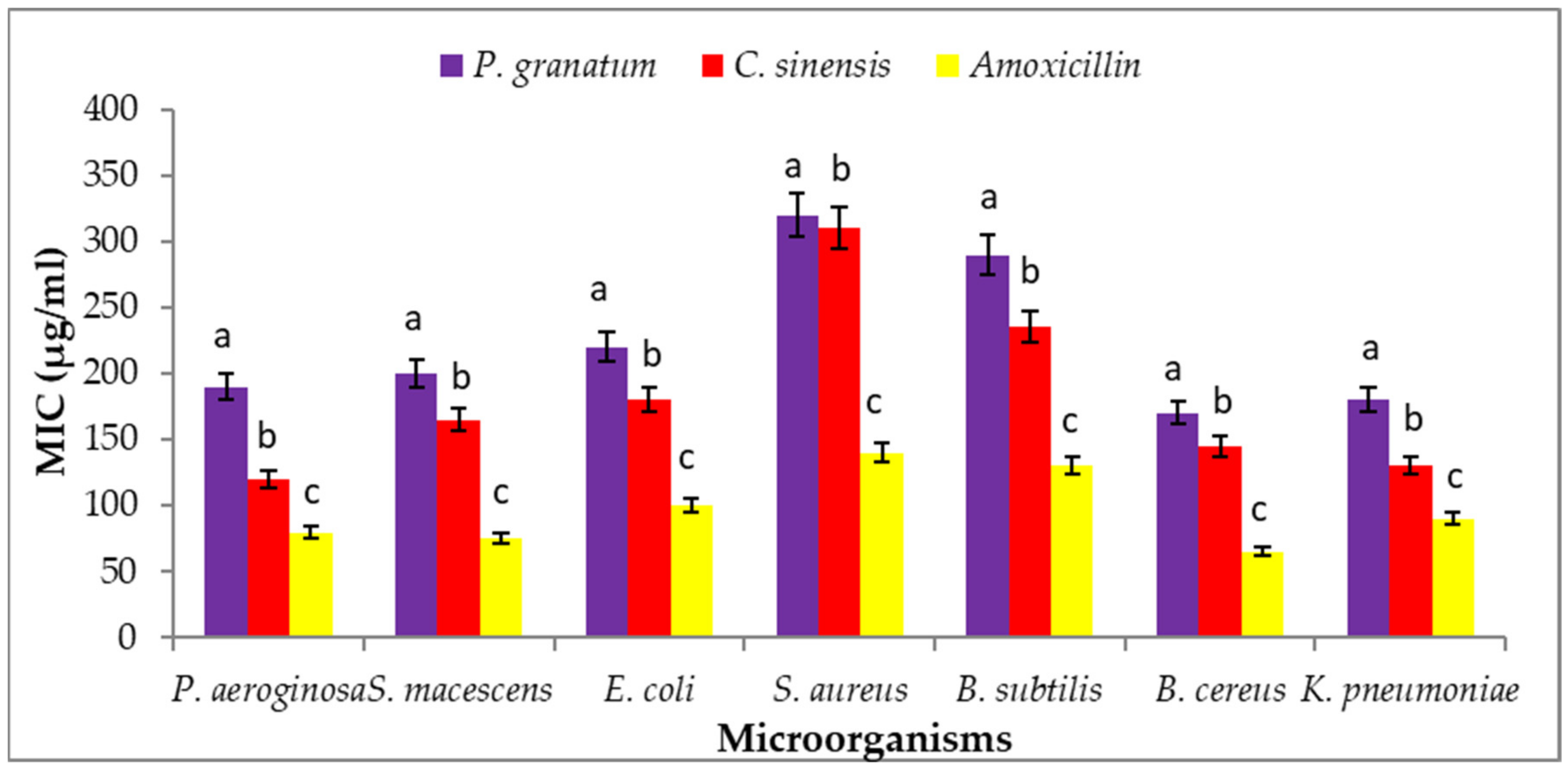
3.11. Minimum Inhibitory Concentration (MIC)
3.12. Correlation Study
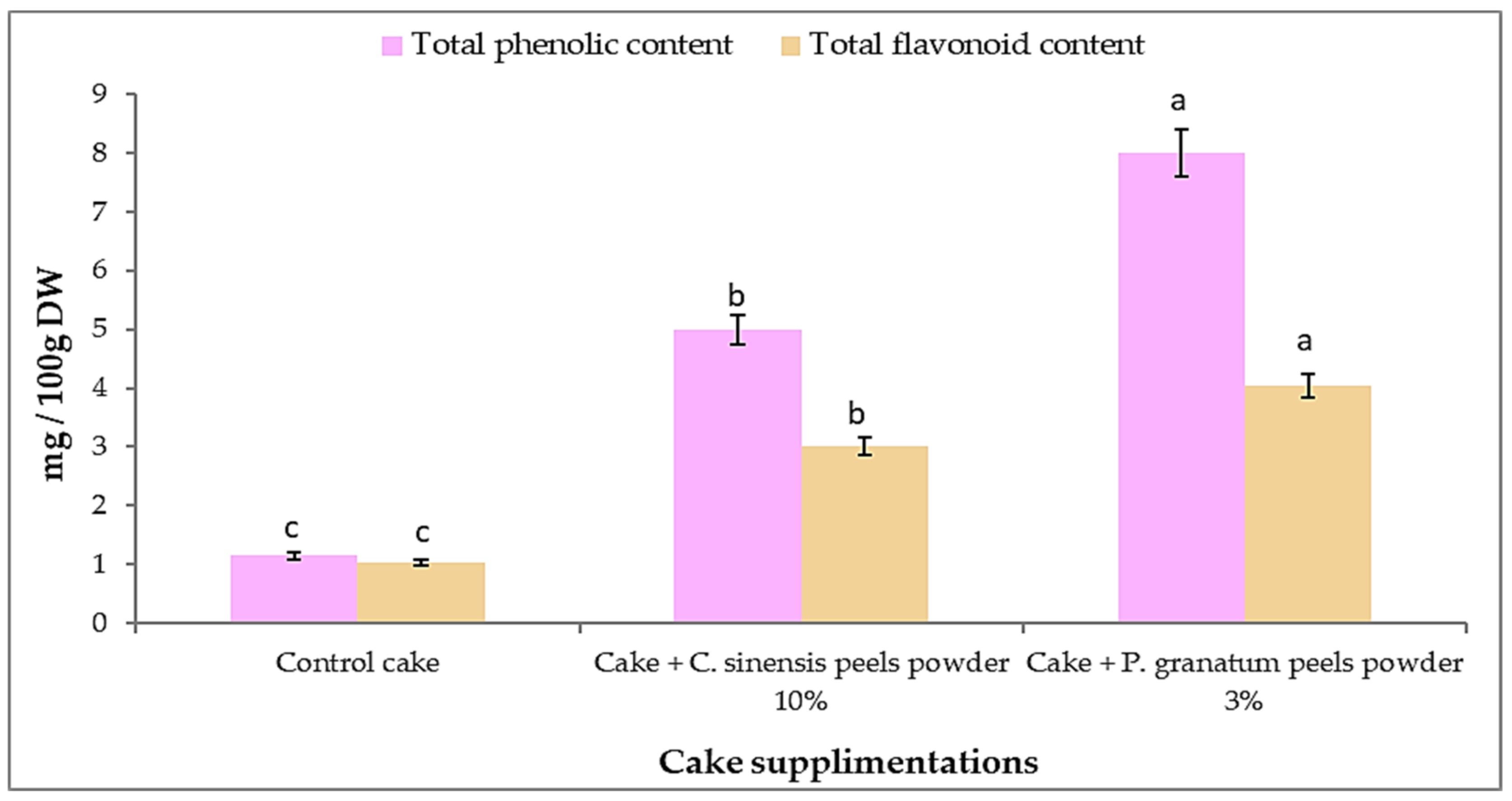
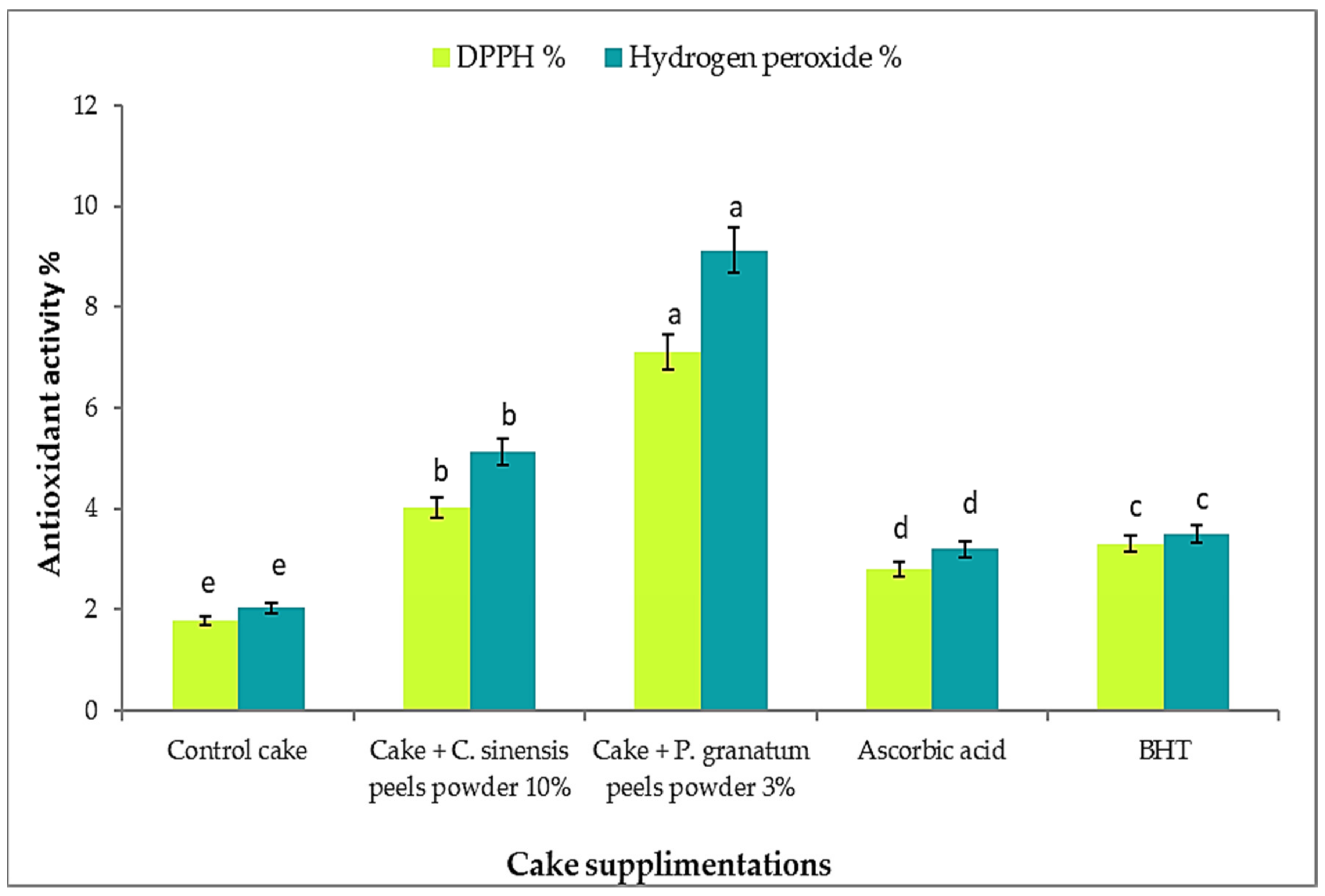
3.13. Total Phenolic, Flavonoids, and Antioxidant Activity in Cakes after Addition of P. granatum and C. sinensis Peel Powder
3.14. The Effect of Shelf Life on Sensory Evaluation of Cake Supplemented with C. sinensis and P. granatum Peel Powder
3.15. Analysis of Microbial Load in Cake
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7. [Google Scholar] [CrossRef]
- Ullah, F.; Ayaz, M.; Sadiq, A.; Ullah, F.; Hussain, I.; Shahid, M.; Yessimbekov, Z.; Adhikari-Devkota, A.; Devkota, H.P. Potential role of plant extracts and phytochemicals against foodborne pathogens. Appl. Sci. 2020, 10, 4597. [Google Scholar] [CrossRef]
- Coburn, B.; Grassl, G.A.; Finlay, B.B. Salmonella, the host and disease: A brief review. Immunol. Cell Biol. 2007, 85, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Cortez, C.; Palma-Martínez, I.; Gonzalez-Avila, L.U.; Guerrero-Mandujano, A.; Solís, R.C.; Castro-Escarpulli, G. Food Poisoning Caused by Bacteria (Food Toxins). In Poisoning—From Specific Toxic Agents to Novel Rapid and Simplified Techniques for Analysis; IntechOpen Limited: London, UK, 2017; pp. 33–72. [Google Scholar]
- Castillo, S.L.; Heredia, N.; Contreras, J.F.; Garcia, S. Extracts of edible and medicinal plants in inhibition of growth, adherence, and cytotoxin production of Campylobacter jejuni and Campylobacter coli. J. Food Sci. 2011, 76, M421–M426. [Google Scholar] [CrossRef]
- Harris, J.C.; Plummer, S.; Turner, M.P.; Lloyd, D. The microaerophilic flagellate Giardia intestinalis: Allium sativum (garlic) is an effective antigiardial. Microbiogy 2000, 146, 3119–3127. [Google Scholar] [CrossRef] [Green Version]
- Silver, L.; Bostian, K. Screening of natural products for antimicrobial agents. Eur. J. Clin. Microbiol. Infect. Dis. 1990, 9, 455–461. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Elmelegy, A.A.; Eldesoky, S.E.; Safwat, G. Phytochemical screening, antimicrobial, antioxidant, anticancer activities and nutritional values of cactus (Opuntia ficus Indicia) pulp and peel. Fresen. Environ. Bull. 2019, 28, 1534–1551. [Google Scholar]
- El-Beltagi, H.S.; Dawi, F.; Aly, A.A.; El-Ansary, A.E. Chemical compositions and biological activities of the essential oils from gamma irradiated celery (Apium graveolens L.) seeds. Not. Bot. Hort. Agrobot. Cluj Napoca 2020, 48, 2114–2133. [Google Scholar] [CrossRef]
- Dawi, F.; El-Beltagi, H.S.; Abdel-Mobdy, Y.E.; Salah, S.M.; Ghaly, I.S.; Abdel-Rahim, E.A.; Mohamed, H.I.; Soliman, A.M. Synergistic impact of the pomegranate peels and its nanoparticles against the infection of tobacco mosaic virus (TMV). Fresen. Environ. Bull. 2021, 30, 731–746. [Google Scholar]
- Rajendrasozhan, S.; Moll, H.E.; Snoussi, M.; Romeilah, R.M.; El-Beltagi, H.S.; Shalaby, E.A.; Younes, K.M.; El-Beltagi, H.S. Phytochemical screening and antimicrobial activity of various extracts of aerial parts of Rhanterium Epapposum. Processes 2021, 9, 1351. [Google Scholar] [CrossRef]
- Younes, K.M.; Romeilah, R.; El-Beltagi, H.S.; El Moll, H.; Rajendrasozhan, S.; El-Shemy, H.A.; Shalaby, E.A. In-vitro evaluation of antioxidant and antiradical potential of successive extracts, semi-purified fractions and biosynthesized silver nanoparticles of Rumex vesicarius. Not. Bot. Horti Agrobo. Cluj Napoca 2021, 49, 12293. [Google Scholar] [CrossRef]
- El-desoky, A.H.; Abdel-Rahman, R.F.; Ahmed, O.K.; El-Beltagi, H.S.; Hattori, M. Anti-inflammatory and antioxidant activities of naringin isolated from Carissa carandas L.: In vitro and in vivo evidence. Phytomedicine 2018, 42, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Arias, A.; Feijoo, G.; Moreira, M.T. Exploring the potential of antioxidants from fruits and vegetables and strategies for their recovery. Innov. Food Sci. Emerg. Technol. 2022, 102974. [Google Scholar] [CrossRef]
- Laranjeira, T.; Costa, A.; Faria-Silva, C.; Ribeiro, D.; de Oliveira, J.M.P.F.; Simões, S.; Ascenso, A. Sustainable Valorization of Tomato By-Products to Obtain Bioactive Compounds: Their Potential in Inflammation and Cancer Management. Molecules 2022, 27, 1701. [Google Scholar] [CrossRef]
- Chacko, C.M.; Estherlydia, D. Antimicrobial evaluation of jams made from indigenous fruit peels. Int. J. Adv. Res. 2014, 2, 202–207. [Google Scholar]
- Girotto, F.; Alibardi, L.; Cossu, R. Food waste generation and industrial uses: A review. Waste Manag. 2015, 45, 32–41. [Google Scholar] [CrossRef]
- Chen, X.M.; Tait, A.R.; Kitts, D.D. Flavonoid composition of orange peel and its association with antioxidant and anti-inflammatory activities. Food Chem. 2017, 218, 15–21. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Abdelazeem, A.S.; Youssef, R.; Safwat, G. GC-MS analysis, antioxidant, antimicrobial and anticancer activities of extracts from Ficus sycomorus fruits and leaves. Not. Bot. Horti Agrobo. Cluj Napoca 2019, 47, 493–505. [Google Scholar] [CrossRef] [Green Version]
- Afify, A.E.-M.M.R.; El-Beltagi, H.S.; Aly, A.A.; El-Ansary, A.E. Antioxidant enzyme activities and lipid peroxidation as biomarker for potato tuber stored by two essential oils from Caraway and Clove and its main component carvone and eugenol. Asian Pac. J. Trop. Biomed. 2012, 2, S772–S780. [Google Scholar] [CrossRef]
- Qin, G.; Xu, C.; Ming, R.; Tang, H.; Guyot, R.; Kramer, E.M.; Hu, Y.; Yi, X.; Qi, Y.; Xu, X.; et al. The pomegranate (Punica granatum L.) genome and the genomics of punicalagin biosynthesis. Plant J. 2017, 91, 1108–1128. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.; Wang, H.; Zhou, Q.; Wu, C.; Gao, X.; Cheng, X.; Tian, L.; Wang, C. Simultaneous determination of polyphenols and triterpenes in pomegranate peel based on high-performance liquid chromatography fingerprint by solvent extraction and ratio blending method in tandem with wavelength switching. Biomed. Chromatogr. 2019, 33, e4690. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Pomegranate peel and peel extracts: Chemistry and food features. Food Chem. 2015, 174, 417–425. [Google Scholar] [CrossRef] [PubMed]
- El-Hadary, A.E.; Ramadan, M.F. Phenolic profiles, antihyperglycemic, antihyperlipidemic, and antioxidant properties of pomegranate (Punica granatum) peel extract. J. Food Biochem. 2019, 43, e12803. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J.A. Pomegranate and its many functional components as related to human health: A review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 635–654. [Google Scholar] [CrossRef]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD-ESI/MSn. Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef]
- Melgarejo-Sánchez, P.; Núñez-Gómez, D.; Martínez-Nicolás, J.J.; Hernández, F.; Legua, P.; Melgarejo, P. Pomegranate variety and pomegranate plant part, relevance from bioactive point of view: A review. Bioresour. Bioprocess. 2021, 8, 2. [Google Scholar] [CrossRef]
- Caruso, A.; Barbarossa, A.; Tassone, A.; Ceramella, J.; Carocci, A.; Catalano, A.; Basile, G.; Fazio, A.; Iacopetta, D.; Franchini, C.; et al. Pomegranate: Nutraceutical with promising benefits on human health. Appl. Sci. 2020, 10, 6915. [Google Scholar] [CrossRef]
- Fawole, O.A.; Makunga, N.P.; Opara, U.L. Antibacterial, antioxidant and tyrosinase inhibition activities of pomegranate fruit peel methanolic extract. BMC Complement Altern. Med. 2012, 12, 200–225. [Google Scholar] [CrossRef] [Green Version]
- Qusty, N.F.; Alghamdi, A.A.; Al-Hazmi, A.; Bandah, B.J.; Mukhtar, M.H.; Alhadrami, M.; Halawani, I.F.; Almalki, A.A.; Assaggaf, H.M.; Aldairi, A.F. Antioxidant activity derived from Punica granatum L. peels extract in micetoxicity induced by a mixture of Nerium oleander extract, acetaminophen and gentamicin. Med. Sci. 2021, 25, 3541–3550. [Google Scholar]
- Fahmy, H.; Hegazi, N.; El-Shamy, S.; Farag, M.A. Pomegranate juice as a functional food: A comprehensive review of its polyphenols, therapeutic merits, and recent patents. Food Funct. 2020, 11, 5768–5781. [Google Scholar] [CrossRef]
- Howell, A.B.; D’Souza, D.H. The pomegranate: Effects on bacteria and viruses that influence human health. Evid. Based Complement. Alternat. Med. 2013, 2013, 606212. [Google Scholar] [CrossRef] [Green Version]
- Mousa, R.M.A.; Danial, A.W. Developing quality and safety of cloudy mango juices by gum Arabic encapsulated a droplet of vanillin embedded in mango oil along with ultrasonic for longer shelf-life. J. Food Process. Preserv. 2021, 46, e16223. [Google Scholar] [CrossRef]
- Abdel-Rahim, E.A.; El-Beltagi, H.S.; Romela, R.M. White Bean seeds and Pomegranate peel and fruit seeds as hypercholesterolemic and hypolipidemic agents in albino rats. Grasas Aceites 2013, 64, 50–58. [Google Scholar] [CrossRef]
- Zema, D.A.; Calabro, P.S.; Folino, A.; Tamburino, V.; Zappia, G.; Zimbone, S.M. Valorisation of citrus processing waste: A review. Waste Manag. 2018, 80, 252–273. [Google Scholar] [CrossRef] [PubMed]
- Berk, Z. By-products of the citrus processing industry. In Citrus Fruit Processing; Berk, Z., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 219–233. [Google Scholar]
- Rafiq, S.; Kaul, R.; Sofi, S.A.; Bashir, N.; Nazir, F.; Nayik, G.A. Citrus peel as a source of functional ingredient: A review. J. Saudi Soc. Agric. Sci. 2018, 17, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Di Donna, L.; Bartella, L.; De Vero, L.; Gullo, M.; Giuffre, A.M.; Zappia, C.; Capocasale, M.; Poiana, M.; D’Urso, S.; Caridi, A. Vinegar production from citrus bergamia by-products and preservation of bioactive compounds. Eur. Food Res. Technol. 2020, 246, 1981–1990. [Google Scholar] [CrossRef]
- Espinosa-Pardo, F.A.; Nakajima, V.M.; Macedo, G.A.; Macedo, J.A.; Martinez, J. Extraction of phenolic compounds from dry and fermented orange pomace using supercritical CO2 and cosolvents. Food Bioprod. Proc. 2017, 101, 1–10. [Google Scholar] [CrossRef]
- Danial, A.; Abdel Basset, R. Orange peel inhibited hup and enhanced hydrogen evolution in some purple non-sulfur bacteria. Int. J. Hydrogen Energy 2015, 40, 941–947. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; Gabra, F.A.; Danial, A.W.; Abdel-Wahab, A.M. Enhancement of biohydrogen production from sustainable orange peel wastes using Enterobacter species isolated from domestic wastewater. Inter. J. Energy Res. 2019, 43, 391–404. [Google Scholar] [CrossRef] [Green Version]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibers in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, A.S.; Joshi, D.C. Effect of replacement of wheat flour with pumpkin powder on textural and sensory qualities of biscuit. Int. Food Res. J. 2013, 20, 587–591. [Google Scholar]
- Hafez, A.A. Physico-Chemical and Sensory Properties of Cakes Supplemented with Different Concentration of Marjoram. Aust. J. Basic Appl. Sci. 2012, 6, 463–470. [Google Scholar]
- Ahmed, A.R. Influence of Chemical Properties of Wheat-Lupine Flour Blends on Cake Quality. Am. J. Food Sci. Technol. 2014, 2, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, R.; Jimenez, A.; Fernandez-Bolanos, J.; Guillen, R.; Heredia, A. Dietary Fibre from Vegetable Products as Source of Functional Ingredients. Trends Food Sci. Technol. 2006, 17, 3–15. [Google Scholar] [CrossRef]
- King, D.E. Dietary Fiber, Inflammation, and Cardiovascular Disease. Mol. Nutr. Food Res. 2005, 49, 594–600. [Google Scholar] [CrossRef]
- Gomez, M.; Moraleja, A.; Oliete, B.; Ruiz, E.; Caballero, P.A. Effect of Fibre Size on the Quality of Fibre-Enriched Layer Cakes. LWT Food Sci. Technol. 2010, 43, 33–38. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 17th ed.; Horwitz, W., Ed.; AOAC: Washington, DC, USA, 2000. [Google Scholar]
- AOAC, Association of Official Analytical Chemists. Official Methods of Analysis, 18th ed.; AOAC International: Arlington, VA, USA, 1996. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis. Food Composition, Additives and Natural Contaminants, 15th ed.; Association of Official Analytical Chemists Inc.: Aldric, RC, USA, 2005. [Google Scholar]
- Hashim, N.; Razak, S.; Mamat, A.S.; Ahmad, S. Effect of Differences Methanol Concentration and Extraction Time on the Antioxidant Capacity, Phenolics Content and Bioactive Constituents of Orthosiphon Stamineus Extracts. Matec. Web Conf. 2016, 7, 01004. [Google Scholar] [CrossRef] [Green Version]
- Salem, M.Z.; Zayed, M.Z.; Ali, H.M.; Abd El-Kareem, S.M. Chemical composition, antioxidant and antibacterial activities of extracts from schinusmolle wood branch growing in Egypt. J. Wood Sci. 2016, 62, 548–561. [Google Scholar] [CrossRef] [Green Version]
- Trease, E.; Evans, W.C. Pharmacognosy, 15th ed.; Saunder Publisher: London, UK, 2004; pp. 137–440. [Google Scholar]
- Zhou, K.; Yu, L. Total phenolic contents and antioxidant properties of commonly consumed vegetables grown in Colorado. LWT—Food Sci. Technol. 2006, 39, 1155–1162. [Google Scholar] [CrossRef]
- Zou, Y.; Lu, Y.; Wei, D. Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J. Agric. Food Chem. 2004, 52, 5032–5039. [Google Scholar] [CrossRef]
- Abid, M.; Yaich, H.; Cheikhrouhou, S.; Khemakhem, I.; Bouaziz, M.; Attia, H.; Ayadi, M.A. Antioxidant properties and phenolic profile characterization by LC-MS/MS of selected Tunisian pomegranate peels. J. Food Sci. Technol. 2017, 54, 2890–2901. [Google Scholar] [CrossRef]
- Amin, I.; Norazaidah, Y.; Emmy Hainida, K.I. Antioxidant activity and phenolic content of raw and blanched Amaranthus species. Food Chem. 2006, 94, 47–52. [Google Scholar] [CrossRef]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chemist. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Bergey, D.H.; Krieg, N.R.; Holt, J.G. Bergey’s Manual of Systematic Bacteriology. Endospore-Forming Bact; Williams & Wilkins: Baltimore, MD, USA, 1984; p. 2. [Google Scholar]
- Brenner, D.J.; Krieg, N.R.; Staley, J.T. Bergey’s Manual of Systematic Bacteriology. Proteobacteria; Springer: Berlin/Heidelberg, Germany, 2005; Volume 2. [Google Scholar]
- Shahbazi, Y. Chemical composition and in vitro antibacterial activity of Mentha spicata essential oil against common food-borne pathogenic bacteria. J. Pathog. 2015, 2015, 3491–3503. [Google Scholar] [CrossRef]
- Seeley, H.W.; Vandemark, P.J.; Lee, J.J. Microbes in action: A Laboratory Manual of Microbiology, 4th ed.; W.H. Freeman and Co.: New York, NY, USA, 2001; pp. 57–130. [Google Scholar]
- Baker, F.J.; Pallister, C.J. Baker and the Siverton’s Introduction to Medical Labotatory Technology, 7th ed.; Butterworth Heinous Main: Oxford, UK, 1998; pp. 241–440. [Google Scholar]
- Sharoba, A.M.; Farrag, M.A.; Abd El-Sala, A.M. Utilization of some fruits and vegetables waste as a source of dietary fibre and its effect on the cake making and its quality attributes. J. Agroaliment. Process. Technol. 2013, 19, 429–444. [Google Scholar]
- Zaker, M.A.; Sawate, A.R.; Patil, B.M.; Sadawarte, S.K.; Kshirsagar, R.B. Utilization of orange (Citrus sinesis) peel powder as a source of dietary fiber and its effect on the cake quality attributes. Int. J. Agric. Sci. 2017, 13, 56–61. [Google Scholar]
- Vanderzant, C.; Splittstoesser, D. Compendium of Methods for the Microbial Examination of Foods; APHA: Washington, DC, USA, 1992. [Google Scholar]
- Kaur, P.; Kataria, S.K.; Singh, B.; Arora, S. Pharmacognostic Investigation of Punica granatum L. Peel. Int. J. Pharm. Drug Anal. 2018, 6, 116–121. [Google Scholar]
- Hanafy, S.M.; Abd El-Shafea, Y.M.; Saleh, W.D.; Fathy, H.M. Chemical profiling, in vitro antimicrobial and antioxidant activities of pomegranate, orange and banana peel-extracts against pathogenic microorganisms. J. Gene. Enginer. Biotechnol. 2021, 19, 80. [Google Scholar] [CrossRef]
- Romelle, F.D.; Rani, A.; Manohar, R.S. Chemical composition of some selected fruit peels. Europ. J. Food Sci. Technol. 2016, 4, 12–21. [Google Scholar]
- Rowayshed, G.; Salama, A.; Abul-Fadl, M.; Akila-Hamza, S.; Emad, A.M. Nutritional and chemical evaluation for pomegranate (Punica granatum L.) fruit peel and seeds powders by products. Middle East J. Appl. Sci. 2013, 3, 169–179. [Google Scholar]
- de la Torre, I.; Martin-Dominguez, V.; Acedos, M.; Esteban, J.; Santos, V.; Ladero, M. Utilisation/upgrading of orange peel waste from a biological biorefinery perspective. App. Microbiol. Biotechnol. 2019, 103, 5975–5991. [Google Scholar] [CrossRef] [PubMed]
- Abdel Wahab, A.S.; Abou Elyazeed, A.M.; Abdalla, A.E. Bioactive Compounds in Some Citrus Peels as Affected by Drying Processes and Quality Evaluation of Cakes Supplemented with Citrus Peels Powder. J. Adv. Agric. Res. 2018, 23, 44–67. [Google Scholar]
- Spanova, M.; Daum, G. Squalene: Biochemistry, molecular biology, process biotechnology, and applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1299–1320. [Google Scholar] [CrossRef]
- Mohamed, H.I.; Abd-El Hameed, A.G. Molecular and biochemical markers of some Vicia faba L. genotype in response to storage insect pests infestation. J. Plant Interact. 2014, 9, 618–626. [Google Scholar] [CrossRef]
- Mohamed, H.I.; Akladious, S.A. Influence of garlic extract on enzymatic and non-enzymatic antioxidants in soybean plants (Glycine max) grown under drought stress. Life Sci. J. 2014, 11, 46–58. [Google Scholar]
- Gul, R.; Jan, S.U.; Syed, F.; Sherani, F.; Nusrat, J. Preliminary phytochemical screening, quantitative analysis of alkaloids, and antioxidant activity of crude plant extracts from Ephedra intermedia Indigenous to Balochistan. Sci. World J. 2017, 2017, 5873648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manivannan, A.; Soundararajan, P.; Park, Y.G.; Jeong, B.R. Chemical elicitor induced modulation of antioxidant metabolism and enhancement of secondary metabolite accumulation in cell suspension cultures of Scrophularia kakudensis Franch. Int. J. Mol. Sci. 2016, 17, 399. [Google Scholar] [CrossRef]
- Omoregie, E.; Osagie, A. Antioxidant Properties of methanolic extracts of some Nigerian Plants on Nutritionally-Stressed Rats. Nig. J. Basic Appl. Sci. 2012, 20, 7–20. [Google Scholar]
- Emran, T.B.; Mir, M.N.; Rahman, A.; Zia, U.; Islam, M. Phytochemical, Antimicrobial, Cytotoxic, Analgesic and Anti-inflammatory Properties of Azadirachta indiaca: A Therapeutic Study. J. Bioana Biomed. 2015, 12, S12. [Google Scholar]
- Mathew, B.B.; Jatawa, S.K.; Tiwari, A. Phytochemical Analysis of Citrus Limonum pulp and peel. Int. J. Pharm. Pharm. Sci. 2012, 4, 269–371. [Google Scholar]
- Blanco-Salas, J.; Vazquez, F.M.; Hortigón-Vinagre, M.P.; Ruiz-Tellez, T. Bioactive Phytochemicals from Mercurialis spp. Used in Traditional Spanish Medicine. Plants 2019, 8, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamed, M.M.; Abd El-Mobdy, M.A.; Kamel, M.T.; Mohamed, H.I.; Bayoumi, A.E. Phytochemical and biological activities of two asteraceae plants Senecio vulgaris and Pluchea dioscoridis L. Pharmacol. Online 2019, 2, 101–121. [Google Scholar]
- Sofy, A.R.; Sofy, M.R.; Hmed, A.A.; Dawoud, R.A.; Refaey, E.E.; Mohamed, H.I.; El-Dougdoug, N.K. Molecular characterization of the Alfalfa mosaic virus infecting Solanum melongena in Egypt and control of its deleterious effects with melatonin and salicylic acid. Plants 2021, 28, 459. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, N.; Sari, F.; Velioglu, Y. Effects of extraction solvents on concentration and antioxidant activity of black and black Mate tea polyphenols determined by ferrous tartrate and Folin-Ciocalteu methods. Food Chem. 2006, 99, 835–841. [Google Scholar] [CrossRef]
- Selahvarzi, A.; Ramezan, Y.; Sanjabi, M.R.; Mirsaeedghazi, H.; Azarikia, F.; Abedinia, A. Investigation of antimicrobial activity of orange and pomegranate peels extracts and their use as a natural preservative in a functional beverage. J. Food Meas. Charact. 2021, 15, 5683–5694. [Google Scholar] [CrossRef]
- Romeilah, R.M.; El-Beltagi, H.S.; Shalaby, E.A.; Younes, K.M.; El Moll, H.; Rajendrasozhan, S.; Mohamed, H.I. Antioxidant and cytotoxic activities of Artemisia monosperma L. and Tamarix aphylla essential oils. Not. Bot. Horti. Agrobot. Cluj Napoca 2021, 9, 12233. [Google Scholar] [CrossRef]
- Kharchoufi, S.; Licciardello, F.; Siracusa, L.; Muratore, G.; Hamdi, M.; Restuccia, C. Antimicrobial and antioxidant features of ‘Gabsi’ pomegranate peel extracts. Ind. Crops Prod. 2018, 111, 345–352. [Google Scholar] [CrossRef]
- Suryawanshi, J.A.S. An overview of Citrus aurantium used in treatment of various diseases. Afr. J. Plant Sci. 2011, 5, 390–395. [Google Scholar]
- Bacanlı, M.; Başaran, A.A.; Başaran, N. The antioxidant and antigenotoxic properties of citrus phenolics limonene and naringin. Food Chem. Toxicol. 2015, 81, 160–170. [Google Scholar] [CrossRef]
- Shehata, M.G.; Awad, T.S.; Asker, D.; El Sohaimy, S.A.; Abd El-Aziz, N.M.; Youssef, M.M. Antioxidant and antimicrobial activities and UPLC-ESI-MS/MS polyphenolic profile of sweet orange peel extracts. Curr. Res. Food Sci. 2021, 1, 326–335. [Google Scholar] [CrossRef]
- Safdar, M.N.; Kausar, T.; Jabbar, S.; Mumtaz, A.; Ahad, K.; Saddozai, A.A. Extraction and quantification of polyphenols from kinnow (Citrus reticulate L.) peel using ultrasound and maceration techniques. J. Food Drug Anal. 2017, 25, 488–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; He, X.; Li, M.; Zhao, W.; Liu, L.; Kong, X. Chemical fingerprint and quantitative analysis for quality control of polyphenols extracted from pomegranate peel by HPLC. Food Chem. 2015, 176, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Gigliobianco, M.R.; Cortese, M.; Nannini, S.; Di Nicolantonio, L.; Peregrina, D.V.; Lupidi, G.; Vitali, L.A.; Bocchietto, E.; Di Martino, P.; Censi, R. Chemical, antioxidant, and antimicrobial properties of the peel and male flower by-products of four varieties of Punica granatum L. cultivated in the Marche region for their use in cosmetic products. Antioxidants 2022, 11, 768. [Google Scholar] [CrossRef] [PubMed]
- Cholke, P.B.; Bhor, A.K.; Shete, A.M.; Sonawane, R.K. Extraction and GC-Ms analysis of orange (Citrus sinensis) peel oil. Res. J. Agric. Biol. Sci. 2017, 2, 41–51. [Google Scholar] [CrossRef]
- Ngele, K.K.; Olugbue, V.U.; Okorie, U.V. Phytochemical Constituents Antimicrobial Effect of Unripe Epicarp Of orange friuts (Citrus sinensis) against Escherichia coli and Staphylococcus aureus. Int. J. Sci. Nat. 2014, 5, 418–422. [Google Scholar]
- Nasr, R.B.; Baudelaire, E.D.; Dicko, A.; Ouarda, H. Phytochemicals, Antioxidant Attributes and Larvicidal Activity of Mercurialis annua L. (Euphorbiaceae) Leaf Extracts against Tribolium confusum (Du Val) Larvae (Coleoptera; Tenebrionidae). Biology 2021, 10, 344. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Nabavi, S.M.; Setzer, W.N.; Nabavi, S.A.; Nabavi, S.A.; Ebrahimzadeh, M.A. Antioxidant and anti-hemolytic activity of lipid-soluble bioactive substances in avocado fruits. Fruits 2013, 68, 185–193. [Google Scholar] [CrossRef] [Green Version]
- El-Beltagi, H.S.; El-Mogy, M.M.; Parmar, A.; Mansour, A.T.; Shalaby, T.A.; Ali, M.R. Phytochemical characterization and utilization of dried red beetroot (Beta vulgaris) peel extract in maintaining the quality of Nile Tilapia Fish Fillet. Antioxidants 2022, 11, 906. [Google Scholar] [CrossRef]
- Karoui, I.J.; Marzouk, B. Characterization of bioactive compounds in Tunisian bitter orange (Citrus aurantium L.) peel and juice and determination of their antioxidant activities. BioMed. Res. Int. 2013, 2013, 345415–345427. [Google Scholar]
- Maksoud, S.; Abdel-Massih, R.M.; Rajha, H.N.; Louka, N.; Chemat, F.; Barba, F.; Debs, E. Citrus aurantium L. Active Constituents, Biological Effects and Extraction Methods. An Updated Review. Molecules 2021, 26, 5832. [Google Scholar] [CrossRef]
- Sousa, C.; Pereira, D.M.; Valentao, P.; Ferreres, F.; Pereira, J.A.; Seabra, R.M. Pieris brassicae inhibits xanthine oxidase. J. Agricul. Food Chemist. 2009, 57, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Abd El- Rahman, S.S.; Mohamed, H.I. Application of benzothiadiazole and Trichoderma harzianum to control faba bean chocolate spot disease and their effect on some physiological and biochemical traits. Acta Physiol. Plant. 2014, 36, 343–354. [Google Scholar] [CrossRef]
- Singh, S.; Immanuel, G. Extraction of Antioxidants from Fruit Peels and its Utilization in Paneer. J. Food Process Technol. 2014, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Pal, J.; Raju, C.V.; Pandey, G.; Shukla, B.N. Antimicrobial activity of pomegranate and orange peels extracts against selected food borne pathogens. Pharma Innov. J. 2018, 7, 176–178. [Google Scholar]
- Qu, W.; Breksa, A.P., III; Pan, Z.; Ma, H. Quantitative determination of major polyphenol constituents in pomegranate products. Food Chem. 2012, 132, 1585–1591. [Google Scholar] [CrossRef]
- Estabraghi, E.; Sadeghpour, M.; Mehrabani, A. Study of pomegranate hydromethanol extract on Staphylococcus aureus and Escherichia coli by microplate in laboratory conditions. J. Payavard Salamat 2018, 12, 183–192. [Google Scholar]
- Ali, A.; Chen, Y.; Liu, H.; Yu, L.; Baloch, Z.; Khalid, S.; Zhu, J.; Chen, L. Starch-based antimicrobial films functionalized by pomegranate peel. Int. J. Biol. Macromol. 2019, 129, 1120–1126. [Google Scholar] [CrossRef]
- Rakholiya, K.; Kaneria, M.; Chanda, S. Inhibition of microbial pathogens using fruit and vegetable peel extracts. Int. J. Food Sci. Nutr. 2014, 65, 733–739. [Google Scholar] [CrossRef]
- Álvarez-Ordóñez, A.; Carvajal, A.; Arguello, H.; Martínez-Lobo, F.J.; Naharro, G.; Rubio, P. Antibacterial activity and mode of action of a commercial citrus fruit extract. J. Appl. Microbiol. 2013, 115, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Ramadan, H.; Min, B.; Tiwari, A.K.; Reddy, G.; Adesiyun, A.; Hinton, A.; Abdela, W. Antibacterial activity of Pomegranate, Orange and Lemon peel extracts against food-borne pathogens and spoilage bacteria In vitro and on poultry skin. Int. J. Poult. Sci. 2015, 14, 229–239. [Google Scholar] [CrossRef] [Green Version]
- Sivaraj, A.; Devagnanaroopan, J.; Palani, B.; Senthilkumar, B. Antibacterial activity of Eucalyptus oblique aqueous leaf extract on selective Vibrio species of Penaeus monodon culture hatchery. World J. Pharm. Sci. 2017, 5, 36–39. [Google Scholar]
- Karthikeyan, G.; Vidya, A. Phytochemical analysis, antioxidant and antibacterial activity of pomegranate peel. Res. J. Life Sci. Bioinform. Pharm. Chem. Sci. 2019, 5, 218–231. [Google Scholar]
- Saleem, M.; Saeed, M.T. Potential application of waste fruit peels (orange, yellow lemon and banana) as wide range natural antimicrobial agent. J. King Saud Univ. Sci. 2020, 32, 805–810. [Google Scholar] [CrossRef]
- Malviya, S.; Jha, A.; Hettiarachchy, N. Antioxidant and antibacterial potential of pomegranate peel extracts. J. Food Sci. Technol. 2014, 51, 4132–4137. [Google Scholar] [CrossRef] [Green Version]
- Wafa, B.A.; Makni, M.; Ammar, S.; Khannous, L.; Hassana, A.B.; Bouaziz, M.; Es-Safi, N.E.; Gdoura, R. Antimicrobial effect of the Tunisian Nana variety Punica granatum L. extracts against Salmonella enterica (serovars Kentucky and Enteritidis) isolated from chicken meat and phenolic composition of its peel extract. Int. J. Food Microbiol. 2017, 241, 123–131. [Google Scholar] [CrossRef]
- Oboh, G.; Adefegha, S.A. Inhibitory properties of phenolic enriched in wheat biscuits on Fe2+ induced Lipid peroxidation in Rat’s brain in vitro. Adv. Food Sci. 2010, 32, 162–169. [Google Scholar]
- Lotfy, L.M.; Barakat, E.H. Utilization of Pomegranate Peels Flour to Improve Sponge Cake Quality. J. Food Dairy Sci. 2018, 3, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Sreekumar, S.; Sithul, H.; Muraleedharan, P.; Azeez, J.M.; Sreeharshan, S. Pomegranate Fruit as a Rich Source of Biologically Active Compounds. Hindawi Publ. Corp. BioMed Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Ismail, T.; Akhtar, S.; Riaz, M.; Ismail, A. Effect of pomegranate peel supplementation on nutritional, organoleptic and stability properties of cookies. Int. J. Food Sci. Nutr. 2014, 65, 661–666. [Google Scholar] [CrossRef]
- Prithwa, P.; Sauryya, B. Antioxidant profile and sensory evaluation of cookies fortified with juice and peel powder of fresh pomegranate (Punica granatum). Int. J. Agric. Food Sci. 2015, 5, 85–91. [Google Scholar]
- Magda, R.A.; Awad, A.M.; Selim, K.A. Evaluation of mandarin and navel orange peels as natural sources of antioxidant in biscuits. Alex. J. Food Sci. Technol. 2008, 5, 75–82. [Google Scholar] [CrossRef]
- Urganci, U.; Fatma, I.S. Quality Characteristics of Biscuits Fortified with Pomegranate Peel. Akad. Gıda. 2021, 19, 10–20. [Google Scholar] [CrossRef]
- Abd El-Galeel, M.A.; Shoughy, M.I. Investigation the drying characteristics of some citrus peels to utilize preparation of cakes. J. Food Dairy Sci. Mansoura Univ. 2013, 4, 343–358. [Google Scholar] [CrossRef]
- Ahenkora, K.; Kyei, M.A.; Marfo, E.K.; Bonfue, B. Nutritional composition of false horn Appantu plantain during ripening and processing. Afr. Crop. Sci. Res. 1996, 4, 243–247. [Google Scholar]
- Shallan, M.A.; El-Beltagi, H.S.; Mona, A.M.; Amera, T.M.; Sohir, N.A. Effect of amylose content and pre-germinated brown rice on serum blood glucose and lipids in experimental animal. Aust. J. Basic Appl. Sci. 2010, 4, 114–121. [Google Scholar]
- Abdel-Rahim, E.A.; El-Beltagi, H.S. Constituents of apple, parsley and lentil edible plants and their therapy treatments for blood picture as well as liver and kidneys functions against lipidemic disease. EJEAFChe 2010, 9, 1117–1127. [Google Scholar]
- Izonfuo, W.A.L.; Omuoru, V.O.T. Effect of ripening on the chemical composition of plant peels and pulps (Musa paradisiacal). J. Sci. Food Agric. 1998, 45, 333–336. [Google Scholar] [CrossRef]
- Al-Saab, A.H.; Gadallah, M.G.E. Phytochemicals, antioxidant activity and quality properties of fibre enriched cookies incorporated with orange peel powder. Food Res. 2021, 5, 72–79. [Google Scholar] [CrossRef]
- Zaker, M.A.; Sawate, A.R.; Patil, B.M.; Sadawarte, S.K. Studies on Effect of Orange Peel Powder Incorporation on Physical, Nutritional and Sensorial Quality of Cookies. Int. J. Eng. Res. Technol. 2016, 5, 78–82. [Google Scholar] [CrossRef]
- Haque, E.; Hanif, M.S.; Nadeem, M.; Mehmood, A.; Ibrar, M. Physicochemical and rheological study of orange pulp fortified cookies. Sci. Lett. 2015, 3, 64–67. [Google Scholar]
- Chikezie, E.; Agomuo, N.; Amadi, B.A. Biochemistry, Practical/Research Method, a Fundamental Approach; Mega Soft Publishers: Owerri, Nigeria, 2008; Volume 2, pp. 51–53. [Google Scholar]
- Mahmoud, M.H.; Abou-Arab, A.A.; Abu-Salem, F.M. Preparation of orange peel biscuits enrich with phenolic compounds as natural antioxidants. Res. J. Pharm. Biol. Chem. Sci. 2017, 8, 798–807. [Google Scholar]
- Khule, G.D.; Khupase, S.P.; Giram, K.K. Development and quality evaluation of orange pomace fortified biscuits. J. Pharmacogn. Phytochem. 2019, 8, 3695–3701. [Google Scholar]
- Bourekoua, H.; Rózyło, R.; Gawlik-Dziki, U.; Benatallah, L.; Zidoune, M.N.; Dziki, D. Pomegranate Seed Powder as a Functional Component of Gluten-Free Bread (Physical, Sensorial and Antioxidant Evaluation). Int. J. Food Sci. Technol. 2018, 53, 1906–1913. [Google Scholar] [CrossRef]
- Dimic, G.; Tanackov, S.I.; Mojovic, L.; Pejin, J. Antifungal activity of lemon essential oil, coriander and cinnamon extracts on foodborne molds in direct contact and the vapor phase. J. Food Process. Pres. 2015, 39, 1778–1787. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential oils: Extraction, bioactivities, and their uses for food preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef] [PubMed]
- Eshak, N.S. Sensory evaluation and nutritional value of balady flat bread supplemented with banana peels as a natural source of dietary fiber. Ann. Agric. Sci. 2016, 61, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Kobeasy, M.I.; El-Beltagi, H.S.; El-Shazly, M.A.; Khattab, E.A.H. Induction of resistance in Arachis hypogaea L. Against Peanut mottle virus by nitric oxide and salicylic acid. Physiol. Mol. Plant Pathol. 2011, 76, 112–118. [Google Scholar] [CrossRef]





| Ingredients | Weight (g) |
|---|---|
| Wheat flour (72% extraction) | 250 |
| Sugar | 125 |
| Milk powder | 25 |
| Fat | 53.5 |
| Fresh whole egg | 110 |
| Baking powder | 12.5 |
| Vanilla | 2.0 |
| Peels | P. granatum | C. sinensis L. |
|---|---|---|
| Color | Dark brown color | Light brown color |
| Odor | Characteristic with tannin odor | Characteristic with pleasant odor |
| Appearance | Dark brown colored granular powder | Light brown colored granular powder |
| Peels | Ca | Mg | Fe | Cu | P | K |
|---|---|---|---|---|---|---|
| C. sinensis | 317 ± 5.0 b | 80.3 ± 3.0 b | 16.0 ± 0.8 b | 1.3 ± 0.1 b | 60.4 ± 3.0 a | 163.4 ± 6.0 a |
| P. granatum | 757.7 ± 7.0 a | 168.1 ± 4.0 a | 21.5 ± 0.9 a | 1.7 ± 0.2 a | 58.8 ± 4.0 b | 83.4 ± 5.0 b |
| Phytochemicals | C. sinensis Peel | P. granatum Peel | ||||
|---|---|---|---|---|---|---|
| Ethanol | Methanol | Dist. Water | Ethanol | Methanol | Dist. Water | |
| Alkaloids | + | ++ | ++ | ++ | +++ | +++ |
| Tannins | + | ++ | ++ | ++ | +++ | +++ |
| Cardiac glycosides | + | + | + | + | + | + |
| Flavonoids | ++ | ++ | ++ | ++ | +++ | +++ |
| Glycosides | + | ++ | ++ | + | − | − |
| Phenols | + | ++ | ++ | ++ | +++ | +++ |
| Saponins | + | ++ | − | ++ | ++ | − |
| Steroids | + | ++ | ++ | ++ | +++ | +++ |
| Terpenoids | + | ++ | ++ | ++ | ++ | +++ |
| Peel Powder | C. sinensis | P. granatum | ||
|---|---|---|---|---|
| Extract | Total Phenolic Content mg GAE/100 g DW | Total Flavonoid Content mg QE/g DW | Total Phenolic Content mg GAE/100 g DW | Total Flavonoid Content mg QE/g DW |
| Ethanol | 143.7 ± 2.0 c | 10.2 ± 0.3 c | 350.4 ± 3.0 c | 20.5 ± 0.2 c |
| Methanol | 155.4 ± 2.0 b | 15.7 ± 0.3 b | 490.6 ± 4.0 b | 40.3 ± 0.3 b |
| Dist. Water | 160.3 ± 3.0 a | 22.2 ± 0.5 a | 513.8 ± 4.0 a | 45.3 ± 0.5 a |
| Compounds Class | Phenolic Compounds | Base Peak m/z | [M-H]− | Retention Time (min) | Relative Percentage |
|---|---|---|---|---|---|
| Flavonoid | Naringin | 271 | 579 | 14.189 | 31.75 |
| Flavonoid | Vicenin II | 299 | 593 | 15.802 | 6.61 |
| Flavonoid | Eriodictyol-7-rutinoside | 449 | 595 | 16.11 | 1.63 |
| Flavonoid | Apigenin 7-O-neohesperidoside (Rhoifolin) | 269 | 577 | 17.229 | 5.91 |
| Flavonoid | Hesperidin | 301 | 609 | 17.326 | 10.13 |
| Flavonoid | Eriocitrin | 289 | 595 | 17.587 | 1.96 |
| Flavonoid | Naringenin | 271 | 271 | 17.625 | 1.31 |
| Flavonoid | Neohesperidin | 301 | 609 | 18.793 | 3.9 |
| Flavonoid | Luteolin-7-O-rutinoside | 271 | 593 | 21.708 | 0.7 |
| Flavonoid | Sinensetin | 371 | 371 | 21.956 | 0.98 |
| Flavonoid | Nobiletin | 387 | 401 | 23.175 | 1.42 |
| Flavonoid | 3-methoxynobiletin | 417 | 431 | 23.458 | 0.96 |
| Flavonoid | Tangeritin | 353 | 371 | 24.994 | 21.11 |
| Compounds Class | Phenolic Compounds | Base Peak m/z | [M-H]− | Retention Time (min) | Relative Percentage |
|---|---|---|---|---|---|
| Phenolic acid | p-cumaric | 164 | 164 | 24.324 | 0.74 |
| Tanines | Gallic | 170 | 170 | 25.634 | 17.44 |
| Phenolic acid | Syringic | 198 | 198 | 25.709 | 0.43 |
| Phenolic acid | Caffeic | 180 | 180 | 25.944 | 2.05 |
| Tannins | Alpha-punicalagin | 782 | 1084 | 26.127 | 6.34 |
| Tannins | Beta-punicalagin | 782 | 1084 | 27.106 | 9.25 |
| Flavonoid | Pelargonidin | 271 | 271 | 27.646 | 1.41 |
| Flavonoid | Epicatchin | 139 | 290 | 28.277 | 2.35 |
| Tannins | Punicalin | 601 | 782 | 28.521 | 0.96 |
| Flavonoid | Catchin | 290 | 290 | 29.275 | 2.67 |
| Phenolic acid | Ellagic | 302 | 302 | 30.12 | 16.54 |
| Flavonoid | Quercetin | 302 | 302 | 31.191 | 5.64 |
| Phenolic acid | Chlorogenic | 191 | 354 | 32.175 | 2.3 |
| Flavonoid | Cyanidin-3-O-glucoside | 317 | 484 | 34.757 | 14.54 |
| Flavonoid | Pelargonidin-3-glucoside | 359 | 433 | 34.922 | 5.44 |
| Flavonoid | Cyanidin-3,5-diglucoside | 301 | 611 | 36.654 | 2.04 |
| Flavonoid | Rutin | 580 | 610 | 36.782 | 1.72 |
| Flavonoid | Delphinidin 3,5-diglucoside | 527 | 627 | 37.898 | 1.94 |
| Tannins | Pedunculagin | 782 | 1084 | 43.245 | 0.71 |
| Tannins | Granatin A | 784 | 951 | 43.375 | 0.24 |
| Essential Oil Compounds | Retention Time (min) | Relative Percentage |
|---|---|---|
| β-Myrcene | 5.418 | 1.42 |
| D-Limonene | 6.254 | 95.7 |
| β-Linalool | 7.823 | 1.73 |
| Decanal | 10.393 | 0.58 |
| Valencene | 17.815 | 0.57 |
| Microorganisms | Gram Stain | Motility | Spore Formation | Shape | Catalase | Oxidase | Nitrate Reductase | Gelatine Hydrolysis | Indole | MR | VP | Urease |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pseudomonas aeroginosa | − | + | − | Short rod | + | + | + | + | − | − | − | − |
| Serratia macescens | − | + | − | Short rod | + | − | + | + | − | − | + | + |
| E. coli | − | + | − | Short rod | + | − | + | − | + | + | − | − |
| Staphylococcus aureus | + | − | − | Cocci | + | − | + | + | − | + | + | + |
| Bacillus subtilis | + | + | + | Rod | + | − | + | + | − | − | + | − |
| Bacillus cereus | + | + | + | Rod | + | − | − | − | − | − | + | − |
| Klebsiella pneumonia | − | − | − | Short rod | + | − | + | − | − | + | + | + |
| Zone of Inhibition (mm) | |||||||
|---|---|---|---|---|---|---|---|
| Peel Extract | P. granatum | C. sinensis | Antibiotic | ||||
| Solvent | Eth. | Meth. | DW | Eth. | Meth. | DW | Amoxicillin |
| Microorganism | |||||||
| P. aeruginosa (−ve) | 19 ± 0.1 d | 27 ± 0.3 b | 30 ± 0.3 a | 18 ± 0.1 e | 19 ± 0.1 d | 21 ± 0.1 c | 30 ± 0.5 a |
| S. marcescens (−ve) | 23 ± 0.2 f | 28 ± 0.3 c | 33 ± 0.3 a | 24 ± 0.2 e | 27 ± 0.3 d | 27 ± 0.2 d | 32 ± 0.5 b |
| E. coli (−ve) | 20 ± 0.2 d | 21 ± 0.2 c | 25 ± 0.2 b | 16 ± 0.1 f | 19 ± 0.1 e | 21 ± 0.1 c | 34 ± 0.4 a |
| S. aureus (+ve) | 18 ± 0.1 f | 20 ± 0.2 e | 22 ± 0.1 d | 22 ± 0.2 d | 26 ± 0.2 c | 32 ± 0.3 a | 29 ± 0.4 b |
| B. subtilis (+ve) | 17 ± 0.1 f | 18 ± 0.1 e | 20 ± 0.1 c | 18 ± 0.1 e | 19 ± 0.1 d | 23 ± 0.1 b | 28 ± 0.4 a |
| B. cereus (+ve) | 18 ± 0.1 e | 19 ± 0.1 d | 22 ± 0.1 b | 15 ± 0.1 f | 21 ± 0.1 c | 24 ± 0.1 a | 22 ± 0.3 b |
| K. pneumoniae (−ve) | 19 ± 0.1 f | 25 ± 0.2 e | 28 ± 0.2 c | 18 ± 0.1 g | 27 ± 0.2 d | 30 ± 0.3 a | 29 ± 0.4 b |
| TPC (mg GAE/100 g DW) | TFC (mg QE/g DW) | DPPH % | H2O2% | |
|---|---|---|---|---|
| Orange peel | ||||
| TPC (mg GAE/100 g DW) | 1 | −0.241 | −0.647 | −0.796 |
| TFC (mgQE/g DW) | −0.241 | 1 | 0.896 | 0.779 |
| DPPH % | −0.647 | 0.896 | 1 | 0.977 |
| H2O2 | −0.796 | 0.779 | 0.977 | 1 |
| Pomegranate peel | ||||
| TPC (mg GAE/100 g DW) | 1 | 0.998 | −0.259 | −0.171 |
| TFC (mgQE/g DW) | 0.998 | 1 | −0.317 | −0.111 |
| DPPH % | −0.259 | −0.317 | 1 | −0.908 |
| H2O2 | −0.171 | −0.111 | −0.908 | 1 |
| Samples | Storage Time (Days) | Color | Appearance | Taste | Texture | Aroma | Overall Acceptability |
|---|---|---|---|---|---|---|---|
| Control cake | Day 0 | 8.19 ± 0.26 a | 8.57 ± 0.12 a | 8.35 ± 0.27 a | 8.62 ± 0.23 a | 8.90 ± 0.31 a | 8.03 ± 0.45 a |
| Day 5 | 7.85 ± 0.31 b | 6.98 ± 0.24 d | not edible | 6.02 ± 0.30 e | 6.43 ± 0.27 d | 6.05 ± 0.31 e | |
| Day 10 | 6.30 ± 0.13 e | 5.62 ± 0.11 h | not edible | 4.31 ± 0.13 g | 4.69 ± 0.21 f | 3.46 ± 0.11 g | |
| C. sinensis peels fortified cake 10% | Day 0 | 6.01 ± 0.26 f | 6.01 ± 0.16 g | 6.69 ± 0.34 e | 6.98 ± 0.28 d | 8.03 ± 0.22 b | 6.99 ± 0.16 d |
| Day 5 | 7.01 ± 0.29 d | 7.14 ± 0.12 c | 7.01 ± 0.10 d | 7.03 ± 0.29 d | 7.57 ± 0.37 c | 6.89 ± 0.15 d | |
| Day 10 | 6.02 ± 0.21 f | 5.42 ± 0.18 i | not edible | 4.05 ± 0.17 h | 4.27 ± 0.22 g | 4.01 ± 0.13 f | |
| P. granatum peel-fortified cake 3% | Day 0 | 6.12 ± 0.19 f | 6.67 ± 0.17 e | 7.03 ± 0.15 c | 7.53 ± 0.28 c | 8.92 ± 0.47 a | 7.17 ± 0.10 c |
| Day 5 | 8.09 ± 0.20 a | 8.43 ± 0.11 b | 7.89 ± 0.21 b | 8.03 ± 0.20 b | 8.01 ± 0.19 b | 7.69 ± 0.34 b | |
| Day 10 | 7.53 ± 0.38 c | 6.24 ± 0.11 f | not edible | 5.34 ± 0.24 f | 5.63 ± 0.26 e | 4.15 ± 0.14 f |
| Samples | Storage Time (Days) | Color | Appearance | Taste | Texture | Aroma | Overall Acceptability |
|---|---|---|---|---|---|---|---|
| Control cake | Day 0 | 8.19 ± 0.14 a | 8.57 ± 0.13 a | 8.35 ± 0.18 a | 8.62 ± 015 a | 8.90 ± 0.31 a | 8.03 ± 0.17 a |
| Day 5 | 8.18 ± 0.18 a | 8.50 ± 0.14 a | 8.01 ± 0.11 b | 7.54 ± 0.18 b | 8.01 ± 0.33 c | 7.53 ± 0.16 b | |
| Day 10 | 8.01 ± 0.15 b | 8.43 ± 0.16 a | 7.04 ± 0.17 c | 6.43 ± 0.16 f | 7.68 ± 0.11 e | 6.54 ± 0.21 e | |
| Day 15 | 7.52 ± 0.19 c | 7.89 ± 0.24 b | 6.31 ± 0.29 e | 5.37 ± 0.11 i | 6.89 ± 0.23 g | 6.01 ± 0.21 f | |
| Day 20 | 6.63 ± 0.30 d | 6.55 ± 0.14 c | not edible | 4.99 ± 0.18 j | 5.87 ± 0.14 j | 5.03 ± 0.34 i | |
| Day 25 | 6.57 ± 0.21 d | 5.45 ± 0.18 h | not edible | 4.01 ± 0.11 l | 5.01 ± 0.15 m | 4.02 ± 0.18 k | |
| C. sinensis peel-fortified cake 10% | Day 0 | 6.01 ± 0.26 e | 6.01 ± 0.17 f | 6.69 ± 0.16 d | 6.98 ± 0.19 d | 8.03 ± 0.26 c | 6.99 ± 0.20 d |
| Day 5 | 6.00 ± 0.13 e | 6.00 ± 0.14 f | 6.34 ± 0.14 e | 6.64 ± 0.14 e | 8.00 ± 0.28 c | 6.57 ± 0.17 e | |
| Day 10 | 5.34 ± 0.19 g | 5.78 ± 0.18 g | 6.03 ± 0.08 f | 6.03 ± 0.13 g | 7.45 ± 0.20 f | 6.02 ± 0.23 f | |
| Day 15 | 5.22 ± 0.08 g | 5.26 ± 0.13 i | 5.41 ± 0.18 g | 5.73 ± 0.17 h | 7.04 ± 0.18 g | 5.47 ± 0.10 g | |
| Day 20 | 5.11 ± 0.11 h | 5.02 ± 0.12 j | 4.23 ± 0.05 i | 5.35 ± 0.06 i | 6.09 ± 0.12 i | 5.02 ± 0.10 i | |
| Day 25 | 5.01 ± 0.09 h | 4.89 ± 0.11 k | not edible | 4.89 ± 0.08 k | 5.34 ± 0.11 l | 4.65 ± 0.13 j | |
| P. granatum peel-fortified cake 3% | Day 0 | 6.12 ± 0.17 e | 6.67 ± 0.21 c | 7.03 ± 0.12 c | 7.53 ± 0.13 b | 8.92 ± 0.44 a | 7.17 ± 0.14 c |
| Day 5 | 6.02 ± 0.20 e | 6.57 ± 0.17 c | 7.00 ± 0.11 c | 7.34 ± 0.17 c | 8.46 ± 0.42 b | 7.02 ± 0.11 d | |
| Day 10 | 6.00 ± 0.22 e | 6.50 ± 0.11 c | 6.98 ± 0.15 c | 7.01 ± 0.18 d | 7.87 ± 0.11 d | 6.89 ± 0.14 d | |
| Day 15 | 5.93 ± 0.14 e | 6.43 ± 0.10 d | 6.01 ± 0.14 f | 6.45 ± 0.19 f | 7.30 ± 0.14 f | 6.10 ± 0.16 f | |
| Day 20 | 5.78 ± 0.10 f | 6.32 ± 0.08 e | 4.69 ± 0.09 h | 5.69 ± 0.16 h | 6.34 ± 0.19 h | 5.34 ± 0.11 h | |
| Day 25 | 5.74 ± 0.18 f | 6.31 ± 0.09 e | not edible | 5.01 ± 0.14 j | 5.68 ± 0.15 k | 5.03 ± 0.24 i |
| Treatments | Zero Time | 2 Days | 4 Days | 6 Days | 13 Days | 15 Days | 17 Days | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Count | Mold Count | Total Count | Mold Count | Total Count | Mold Count | Total Count | Mold Count | Total Count | Mold Count | Total Count | Mold Count | Total Count | Mold Count | |
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 4 × 102 | 3 × 102 | ND | ND | ND | ND | ND | ND |
| Orange peel | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 × 102 | 2 × 102 | 4 × 102 | 2 × 102 |
| Pomegranate peel | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 × 102 | 2 × 102 | 3 × 102 | 2 × 102 |
| Treatments | Zero Time | 2 Days | 4 Days | 6 Days | 13 Days | 15 Days | 17 Days | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Count | Mold Count | Total Count | Mold Count | Total Count | Mold Count | Total Count | Mold Count | Total Count | Mold Count | Total Count | Mold Count | Total Count | Mold Count | |
| Control | 0 | 0 | 0 | 0 | 6 × 102 | 3 × 102 | 11 × 102 | 7 × 102 | ND | ND | ND | ND | ND | ND |
| Orange peel | ND | ND | ND | ND | ND | ND | ND | ND | 4 × 102 | 2 × 102 | 7 × 102 | 5 × 102 | 11 × 102 | 8 × 102 |
| Pomegranate peel | ND | ND | ND | ND | ND | ND | ND | ND | 3 × 102 | 1 × 102 | 5 × 102 | 3 × 102 | 9 × 102 | 6 × 102 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Beltagi, H.S.; Eshak, N.S.; Mohamed, H.I.; Bendary, E.S.A.; Danial, A.W. Physical Characteristics, Mineral Content, and Antioxidant and Antibacterial Activities of Punica granatum or Citrus sinensis Peel Extracts and Their Applications to Improve Cake Quality. Plants 2022, 11, 1740. https://doi.org/10.3390/plants11131740
El-Beltagi HS, Eshak NS, Mohamed HI, Bendary ESA, Danial AW. Physical Characteristics, Mineral Content, and Antioxidant and Antibacterial Activities of Punica granatum or Citrus sinensis Peel Extracts and Their Applications to Improve Cake Quality. Plants. 2022; 11(13):1740. https://doi.org/10.3390/plants11131740
Chicago/Turabian StyleEl-Beltagi, Hossam S., Nareman S. Eshak, Heba I. Mohamed, Eslam S. A. Bendary, and Amal W. Danial. 2022. "Physical Characteristics, Mineral Content, and Antioxidant and Antibacterial Activities of Punica granatum or Citrus sinensis Peel Extracts and Their Applications to Improve Cake Quality" Plants 11, no. 13: 1740. https://doi.org/10.3390/plants11131740







