Characterization of Four New Compounds from Protea cynaroides Leaves and Their Tyrosinase Inhibitory Potential
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Characterization
Structural Elucidation of the Isolated Compounds
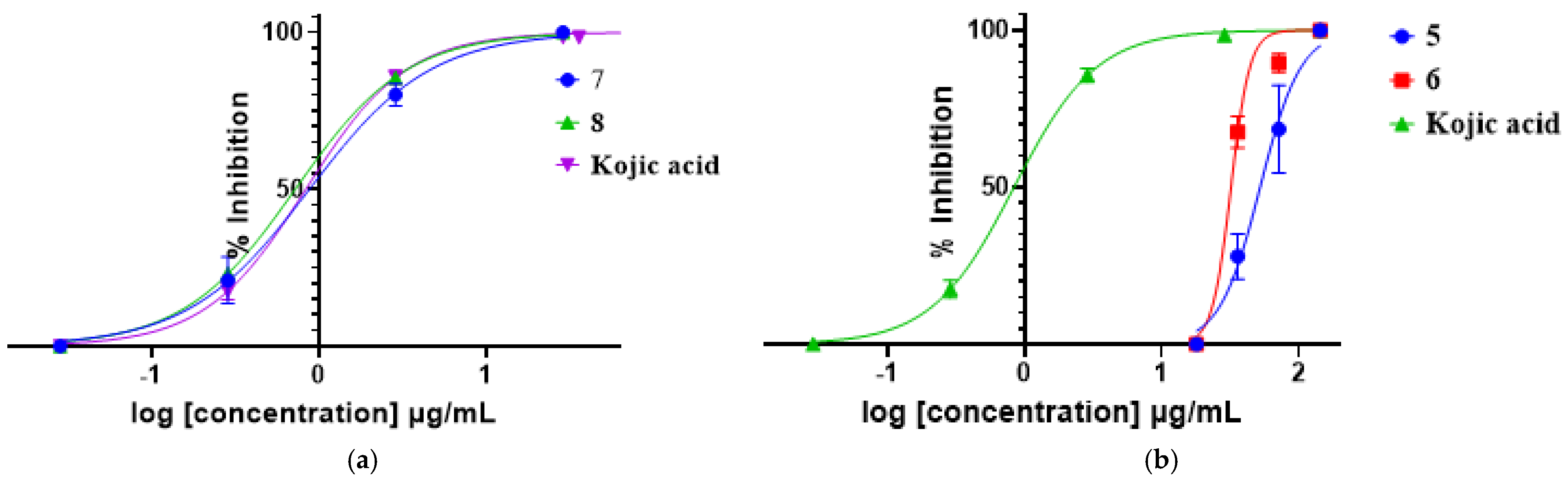
2.2. Inhibitory Activities of Isolated Compounds on Tyrosinase
3. Materials and Methods
3.1. Plant Material
3.2. Equipment and Chemical Reagents
3.3. Extraction and Fractionation of the Plant Material
3.4. Antityrosinase Inhibition Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. CMLS 2005, 62, 1707–1723. [Google Scholar] [CrossRef] [PubMed]
- Bernard, P.; Berthon, J.Y. Resveratrol: An original mechanism on tyrosinase inhibition. Int. J. Cosmet. Sci. 2000, 22, 219–226. [Google Scholar] [CrossRef]
- Malik, A.; Khan, M.T.H.; Khan, S.B.; Ahmad, A.; Choudhary, M.I. Tyrosinase inhibitory lignans from the methanol extract of the roots of Vitex negundo Linn. and their structure–activity relationship. Phytomedicine 2006, 13, 255–260. [Google Scholar]
- Kim, M.; Meng, X.F.; Kim, A.; Wang, M.; Simon, J.E. Developing a long-lasting tyrosinase inhibitor from Morus alba L. Nat. Org. Cosmet. RD Marketpl. 2007, 121, 299–313. [Google Scholar]
- Lee, M.H.; Lin, Y.P.; Hsu, F.L.; Zhan, G.R.; Yen, K.Y. Bioactive constituents of Spatholobus suberectus in regulating tyrosinase-related proteins and mRNA in HEMn cells. Phytochemistry 2006, 67, 1262–1270. [Google Scholar] [CrossRef]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [Green Version]
- MCasanola-Martin, G.; Le-Thi-Thu, H.; Marrero-Ponce, Y.; A Castillo-Garit, J.; Torrens, F.; Rescigno, A.; Abad, C.; Tareq Hassan Khan, M. Tyrosinase enzyme: 1. An overview on a pharmacological target. Curr. Top. Med. Chem. 2014, 14, 1494–1501. [Google Scholar] [CrossRef]
- Stulberg, D.L.; Clark, N.; Tovey, D. Common hyperpigmentation disorders in adults: Part II. Melanoma, seborrheic keratoses, acanthosis nigricans, melasma, diabetic dermopathy, tinea versicolor, and postinflammatory hyperpigmentation. Am. Fam. Physician 2003, 68, 1963–1968. [Google Scholar]
- Akhtar, M.N.; Sakeh, N.M.; Zareen, S.; Gul, S.; Lo, K.M.; Ul-Haq, Z.; Shah, S.A.A.; Ahmad, S. Design and synthesis of chalcone derivatives as potent tyrosinase inhibitors and their structural activity relationship. J. Mol. Struct. 2015, 1085, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, G.; Yan, J.; Gong, D. Inhibitory effect of morin on tyrosinase: Insights from spectroscopic and molecular docking studies. Food Chem. 2014, 163, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Choi, J.U.; Lee, E.A.; Park, H.R. Flaniostatin, a new isoflavonoid glycoside isolated from the leaves of Cudrania tricuspidata as a tyrosinase inhibitor. Food Sci. Biotechnol. 2013, 22, 1–4. [Google Scholar] [CrossRef]
- Kim, J.H.; Yoon, J.Y.; Yang, S.Y.; Choi, S.K.; Kwon, S.J.; Cho, I.S.; Jeong, M.H.; Ho Kim, Y.; Choi, G.S. Tyrosinase inhibitory components from Aloe vera and their antiviral activity. J. Enzym. Inhib. Med. Chem. 2017, 32, 78–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, T.S.; Ding, H.Y.; Lin, H.C. Identifying 6, 7, 4′-trihydroxyisoflavone as a potent tyrosinase inhibitor. Biosci. Biotechnol. Biochem. 2005, 69, 1999–2001. [Google Scholar] [CrossRef] [PubMed]
- Christenhusz, M.J.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef] [Green Version]
- Johnson, L.A.; Briggs, B.G. On the Proteaceae—The evolution and classification of a southern family. Bot. J. Linn. Soc. 1975, 70, 83–182. [Google Scholar] [CrossRef]
- Verotta, L.; Orsini, F.; Pelizzoni, F.; Torri, G.; Rogers, C.B. Polyphenolic glycosides from african proteaceae. J. Nat.Prod. 1999, 62, 1526–1531. [Google Scholar] [CrossRef]
- Bremer, K.; Bremer, B.; Thulin, M. Introduction to Phylogeny and Systematics of Flowering Plants; Department of Systematic Botany, University of Uppsala: Uppsala, Sweden, 2003; Volume 33. [Google Scholar]
- Crous, P.W.; Palm, M.E. Systematics of selected foliicolous fungi associated with leaf spots of Proteaceae. Mycol. Res. 1999, 103, 1299–1304. [Google Scholar] [CrossRef]
- Razafintsalama, V.; Sarter, S.; Mambu, L.; Randrianarivo, R.; Petit, T.; Rajaonarison, J.F.; Mertz, C.; Rakoto, D.; Jeannoda, V. Antimicrobial activities of Dilobeia thouarsii Roemer and Schulte, a traditional medicinal plant from Madagascar. South Afr. J. Bot. 2013, 87, 1–3. [Google Scholar] [CrossRef]
- Rebelo, T. Field guide to the Proteas of the Cape Peninsula; Protea Atlas Project, National Botanical Institute: Cape Town, South Africa, 2000. [Google Scholar]
- Paterson-Jones, C. Protea family in Southern Africa; Struik: Cape Town, South Africa, 2000. [Google Scholar]
- Arendse, M.L. Medicinal Plant Use in the Dwarsrivier Valley, Stellenbosch. Ph.D. Thesis, University of the Western Cape, Cape Town, South Africa, 2013. [Google Scholar]
- Perold, G.W.; Carlton, L. Neriifolin, an ester glucoside of benzene-1, 2, 4-triol. J. Chem.Soc. Perkin Trans. 1989, 1, 1215–1217. [Google Scholar] [CrossRef]
- León, F.; Alfayate, C.; Batista, C.V.; López, A.; Rico, M.; Brouard, I. Phenolic compounds, antioxidant activity and ultrastructural study from Protea hybrid ‘Susara’. Ind. Crops Prod. 2014, 55, 230–237. [Google Scholar] [CrossRef]
- Bieleski, R.L.; Briggs, B.G. Taxonomic patterns in the distribution of polyols within the Proteaceae. Aust. J. Bot. 2005, 53, 205–217. [Google Scholar] [CrossRef]
- Perold, G.W.; Rosenberg, M.E.; Howard, A.S.; Huddle, P.A. Metabolites of proteaceae. Part 9. Eximin (6-O-benzoylarbutin) and the synthesis of aryl glycoside esters. J. Chem. Soc. Perkin Trans. 1 1979, 239–243. [Google Scholar] [CrossRef]
- Wu, H.C.; Du Toit, E.S.; Reinhardt, C.F.; Rimando, A.M.; Van der Kooy, F.; Meyer, J.J.M. The phenolic, 3, 4-dihydroxybenzoic acid, is an endogenous regulator of rooting in Protea cynaroides. Plant Growth Regul. 2007, 52, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Masike, K.; de Villiers, A.; Hoffman, E.W.; Brand, D.J.; Causon, T.; Stander, M.A. Detailed Phenolic Characterization of Protea Pure and Hybrid Cultivars by Liquid Chromatography–Ion Mobility–High Resolution Mass Spectrometry (LC-IM-HR-MS). J. Agric. Food Chem. 2019, 68, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Kamagaju, L.; Bizuru, E.; Minani, V.; Morandini, R.; Stévigny, C.; Ghanem, G.; Duez, P. An ethnobotanical survey of medicinal plants used in Rwanda for voluntary depigmentation. J. Ethnopharmacol. 2013, 150, 708–717. [Google Scholar] [CrossRef]
- Kamori, A.; Kato, A.; Miyawaki, S.; Koyama, J.; Nash, R.J.; Fleet, G.W.; Miura, D.; Ishikawa, F.; Adachi, I. Dual action of acertannins as potential regulators of intracellular ceramide levels. Tetrahedron Asymmetry 2016, 27, 1177–1185. [Google Scholar] [CrossRef]
- Machida, S.; Mukai, S.; Kono, R.; Funato, M.; Saito, H.; Uchiyama, T. Synthesis and comparative structure–activity study of carbohydrate-based phenolic compounds as α-glucosidase inhibitors and antioxidants. Molecules 2019, 24, 4340. [Google Scholar] [CrossRef] [Green Version]
- Miyase, T.; Ueno, A.; Takizawa, N.; Kobayashi, H.; Oguchi, H. Ionone and lignan glycosides from Epimedium diphyllum. Phytochemistry 1989, 28, 3483–3485. [Google Scholar] [CrossRef]
- Ariga, G.; Nandibewoor, S.T.; Chimatadar, S.A. Oxidative degradation of antibacterial drug, methylparaben, by Mn-VII in perchloric acid medium: A kinetic and mechanistic approach. J. Indian Chem. Soc. 2015, 92, 1705–1714. [Google Scholar]
- Syafni, N.; Putra, D.P.; Arbain, D. 3, 4-dihydroxybenzoic acid and 3, 4-dihydroxybenzaldehyde from the fern Trichomanes chinense L.; isolation, antimicrobial and antioxidant properties. Indones. J. Chem. 2012, 12, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Terada, O.; Suzuki, S.; Kinoshita, S. 3-Oxykojic Acid, a New γ-Pyrone formed by Gluconobacter. Agric. Biol. Chem. 1961, 25, 802–803. [Google Scholar] [CrossRef]
- Liu, W.R.; Qiao, W.L.; Liu, Z.Z.; Wang, X.H.; Jiang, R.; Li, S.Y.; Shi, R.B.; She, G.M. Gaultheria: Phytochemical and pharmacological characteristics. Molecules 2013, 18, 12071–12108. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.B.; Hassan Khan, M.T.; Jang, E.S.; Akhtar, K.; Seo, J.; Han, H. Tyrosinase inhibitory effect of benzoic acid derivatives and their structure-activity relationships. J. Enzym. Inhib. Med. Chem. 2010, 25, 812–817. [Google Scholar] [CrossRef]
- Curto, E.V.; Kwong, C.; Hermersdörfer, H.; Glatt, H.; Santis, C.; Virador, V.; Hearing Jr, V.J.; Dooley, T.P. Inhibitors of mammalian melanocyte tyrosinase: In vitro comparisons of alkyl esters of gentisic acid with other putative inhibitors. Biochem. Pharmacol. 1999, 57, 663–672. [Google Scholar] [CrossRef]
- Nerya, O.; Vaya, J.; Musa, R.; Izrael, S.; Ben-Arie, R.; Tamir, S. Glabrene and isoliquiritigenin as tyrosinase inhibitors from licorice roots. J. Agric. Food Chem. 2003, 51, 1201–1207. [Google Scholar] [CrossRef]
- Kim, B.J.; Kim, J.H.; Kim, H.P.; Heo, M.Y. Biological screening of 100 plant extracts for cosmetic use (II): Anti-oxidative activity and free radical scavenging activity. Int. J. Cosmet. Sci. 1997, 19, 299–307. [Google Scholar] [CrossRef]

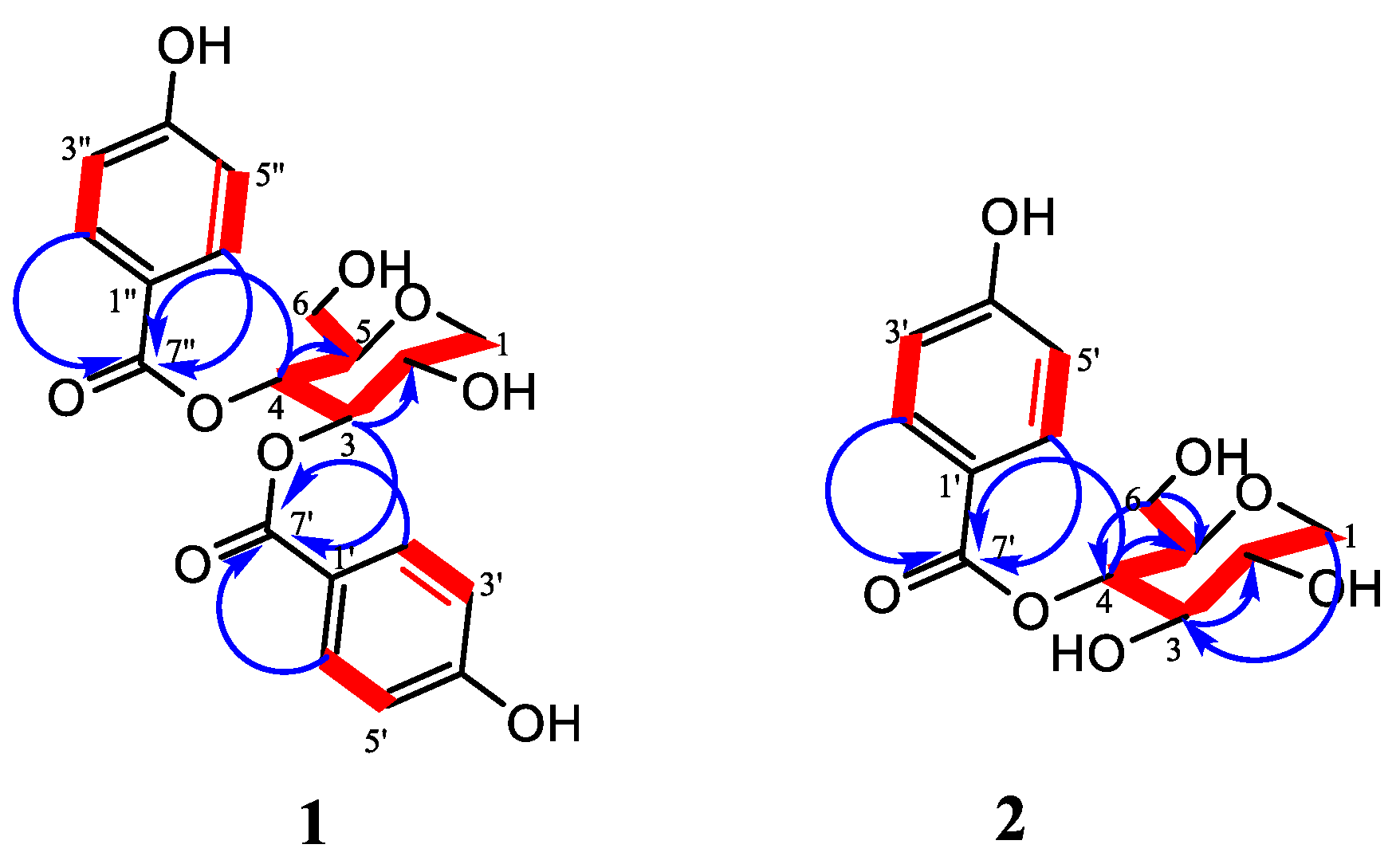

| 1 in DMSO-d6 | 2 in CD3OD | |||
|---|---|---|---|---|
| Position | ẟH (mult, J) | ẟC | ẟH (mult, J) | ẟC |
| 1a | 3.33 (dd, 11.1, 5.5) | 69.9 t | 3.16 (t, 10.6) | 71.1 t |
| 1b | 3.94 (dd, 11.1, 5.5) | 3.89 (dd, 11.2, 4.6) | ||
| 2 | 3.76 (m) | 68.3 d | 3.51 (m) * | 71.7 d |
| 3 | 5.27 (t, 9.3) | 77.5 d | 3.51 (m) * | 77.9 d |
| 4 | 5.05 (t, 9.7) | 69.7 d | 4.81 (t, 9.3) | 73.0 d |
| 5 | 3.59 (ddd, 4.2, 2.3, 2.2) | 79.5 d | 3.38 (m) * | 81.0 d |
| 6a | 3.38 (dd, *) | 61.2 t | 3.40 (m) * | 62.9 t |
| 6b | 3.45 (dd, *) | 3.49 (m) | ||
| 1′ | - | 120.8 s | - | 122.1 s |
| 2′ | 7.71 (d, 8.8) | 132.0 d | 7.83 (d, 8.8) | 133.2 d |
| 3′ | 6.78 (d, 8.8) | 115.6 d | 6.75 (d, 8.8) | 116.3 d |
| 4′ | - | 162.3 s | - | 163.8 s |
| 5′ | 6.78 (d, 8.8) | 115.6 d | 6.75 (d, 8.8) | 116.3 d |
| 6′ | 7.71 (d, 8.8) | 132.0 d | 7.83 (d, 8.8) | 133.2 d |
| 7′ | - | 165.6 s | 167.7 s | |
| 1″ | - | 120.1 s | ||
| 2″ | 7.68 (d, 8.8) | 131.9 d | ||
| 3″ | 6.78 (d, 8.8) | 115.7 d | ||
| 4″ | - | 162.6 s | ||
| 5″ | 6.78 (d, 8.8) | 115.7 d | ||
| 6″ | 7.68 (d, 8.8) | 131.9 d | ||
| 7″ | - | 165.0 s |
| 3 in CD3OD | 4 in DMSO-d6 | ||||
|---|---|---|---|---|---|
| Position | ẟH (mult, J) | ẟC | Position | ẟH (mult, J) | ẟC |
| 2 | - | 163.9 s | 1 | - | 42.2 s |
| 3 | - | 142.3 s | 2 | 3.16 (m) | 76.9 d |
| 4 | - | 177.4 s | 3 | 3.70 (d, 6.7) * | 75.7 d |
| 5 | 6.31 (d, 5.6) | 117.7 d | 4a | 2.00 (dd, 17.2, 7.1) * | 37.9 t |
| 6 | 7.97 (d, 5.6) | 157.5 d | 4b | 2.31 (dd, 17.4, 6.8) | |
| 7a | 4.69 (d, 14.1) | 57.7 t | 5 | - | 123.9 s |
| 7b | 4.47 (d, 14.1) | 6 | - | 136.0 s | |
| 1′ | 4.94 (d, 7.7) | 104.0 d | 7a | 2.09 (m) | 22.2 t |
| 2′ | 3.41 (m) | 75.4 d | 7b | 2.16 (m) | |
| 3′ | 3.45 (m) | 77.8 d | 8 | 2.45 (t, 8.1) | 43.9 (CH2) |
| 4′ | 3.43 (m) | 71.7 d | 9 | - | 208.9 s |
| 5′ | 3.56 (m) | 76.2 d | 10 | 2.07 (s) | 30.1 (CH3) |
| 6′a | 4.58 (dd, 11.8, 2.2) | 64.4 s t | 11 | 1.01 (s) | 25.6 (CH3) |
| 6′b | 4.45 (dd, 11.8, 2.2) | 12 | 0.86 (s) | 22.0 (CH3) | |
| 1″ | - | 122.1 s | 13 | 1.56 (s) | 19.5 (CH3) |
| 2″ | 7.84 (d, 8.8) | 130.0 d | 1′ | 4.28 (d, 7.8) | 101.4 d |
| 3″ | 6.83 (d, 8.8) | 116.4 d | 2′ | 3.00 (m) | 73.7 d |
| 4″ | - | 163.7 s | 3′ | 3.15 (m) | 77.2 d |
| 5″ | 6.83 (d, 8.8) | 116.4 d | 4′ | 3.05 (m) | 70.4 d |
| 6″ | 7.84 (d, 8.8) | 130.0 d | 5′ | 3.16 (m) | 76.8 d |
| 7″ | - | 167.9 s | 6′a | 3.44 (m) | 61.4 t |
| 6′b | 3.65 (m) |
| Extracts/ Compounds | IC50(µg/mL) ± SD |
|---|---|
| TE Bu-F 1 | 85.2 75.5 NA * |
| 2 | NA * |
| 3 | NA * |
| 4 | NA * |
| 5 | 274.5 ± 2.12 |
| 6 | 149.2 ± 1.06 |
| 7 | 0.8776 ± 0.12 |
| 8 | 0.7215 ± 0.09 |
| Kojic acid | 0.8347 ± 0.093 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yalo, M.; Makhaba, M.; Hussein, A.A.; Sharma, R.; Koki, M.; Nako, N.; Mabusela, W.T. Characterization of Four New Compounds from Protea cynaroides Leaves and Their Tyrosinase Inhibitory Potential. Plants 2022, 11, 1751. https://doi.org/10.3390/plants11131751
Yalo M, Makhaba M, Hussein AA, Sharma R, Koki M, Nako N, Mabusela WT. Characterization of Four New Compounds from Protea cynaroides Leaves and Their Tyrosinase Inhibitory Potential. Plants. 2022; 11(13):1751. https://doi.org/10.3390/plants11131751
Chicago/Turabian StyleYalo, Masande, Masixole Makhaba, Ahmed A. Hussein, Rajan Sharma, Mkhuseli Koki, Ndikho Nako, and Wilfred T. Mabusela. 2022. "Characterization of Four New Compounds from Protea cynaroides Leaves and Their Tyrosinase Inhibitory Potential" Plants 11, no. 13: 1751. https://doi.org/10.3390/plants11131751
APA StyleYalo, M., Makhaba, M., Hussein, A. A., Sharma, R., Koki, M., Nako, N., & Mabusela, W. T. (2022). Characterization of Four New Compounds from Protea cynaroides Leaves and Their Tyrosinase Inhibitory Potential. Plants, 11(13), 1751. https://doi.org/10.3390/plants11131751











