Ferula communis L. (Apiaceae) Root Acetone-Water Extract: Phytochemical Analysis, Cytotoxicity and In Vitro Evaluation of Estrogenic Properties
Abstract
:1. Introduction
2. Results
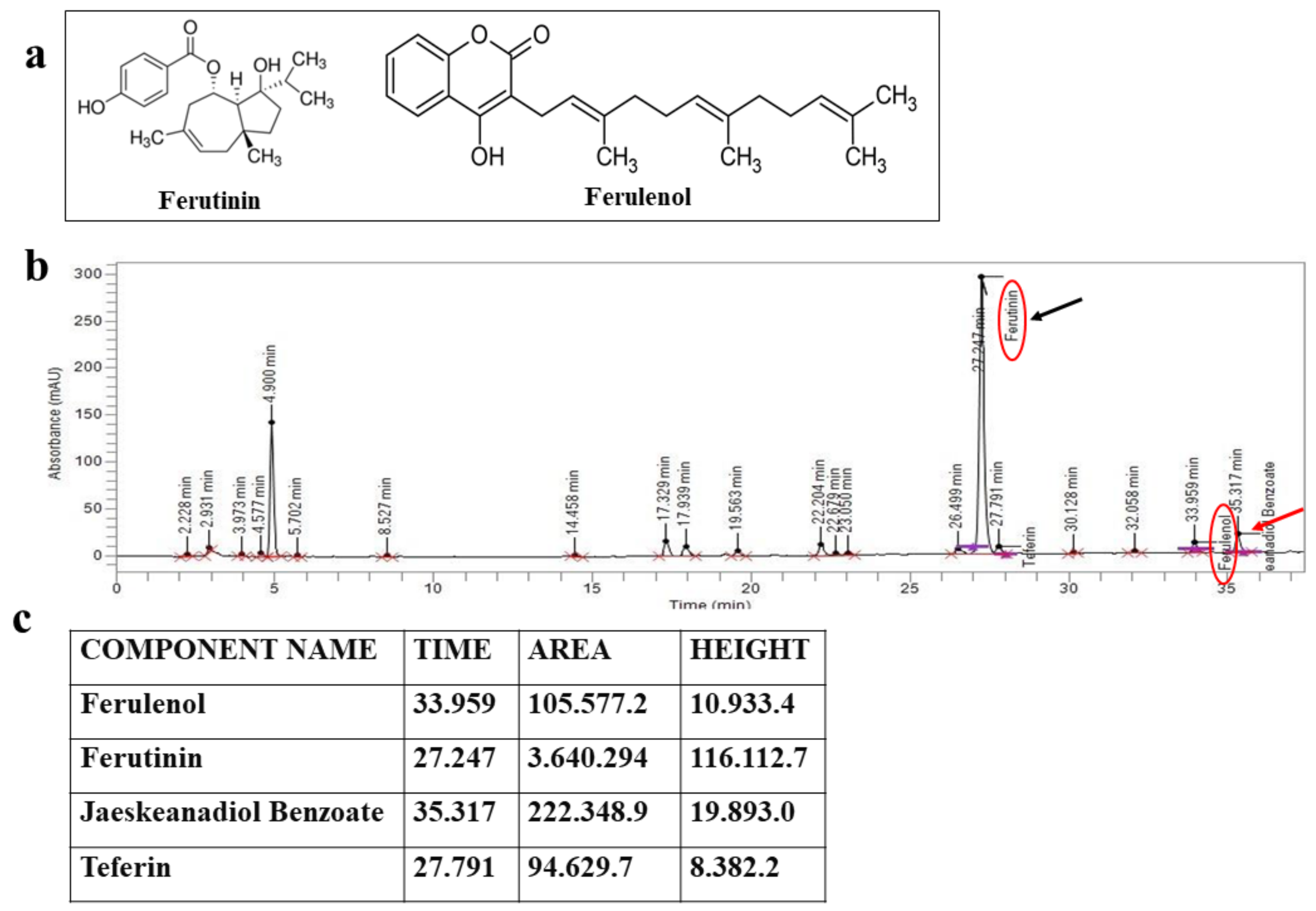
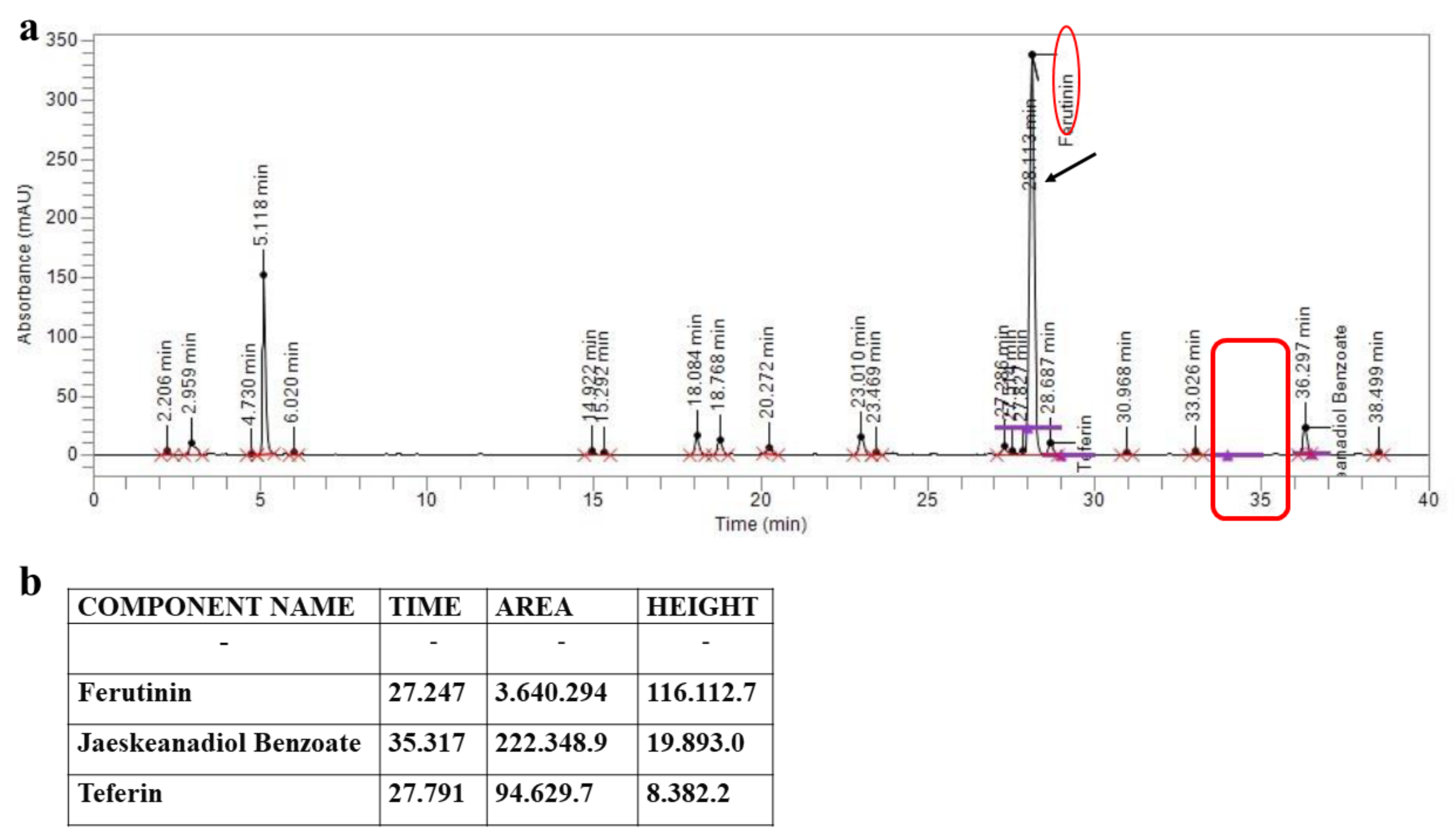
2.1. HPLC Assessment of the Chemical Composition of FER-E
2.2. Treatment with 17-β-Estradiol Increases Cell Proliferation in MCF-7, HeLa and Saos-2 Lines
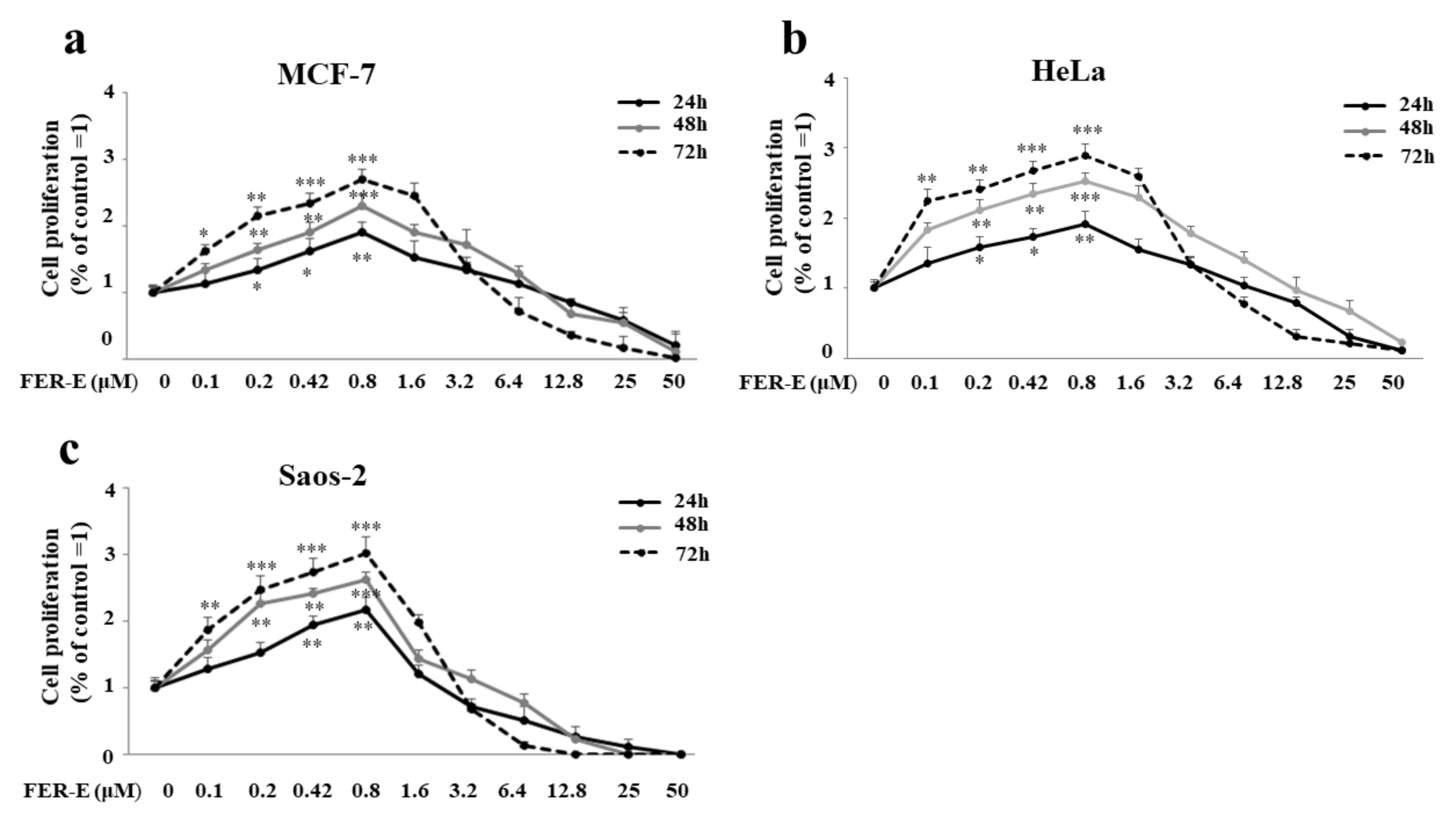
2.3. FER-E Has a Dose-Dependent Effect: Low Concentrations Stimulate Hyperproliferation, While Higher Concentrations Are Toxic
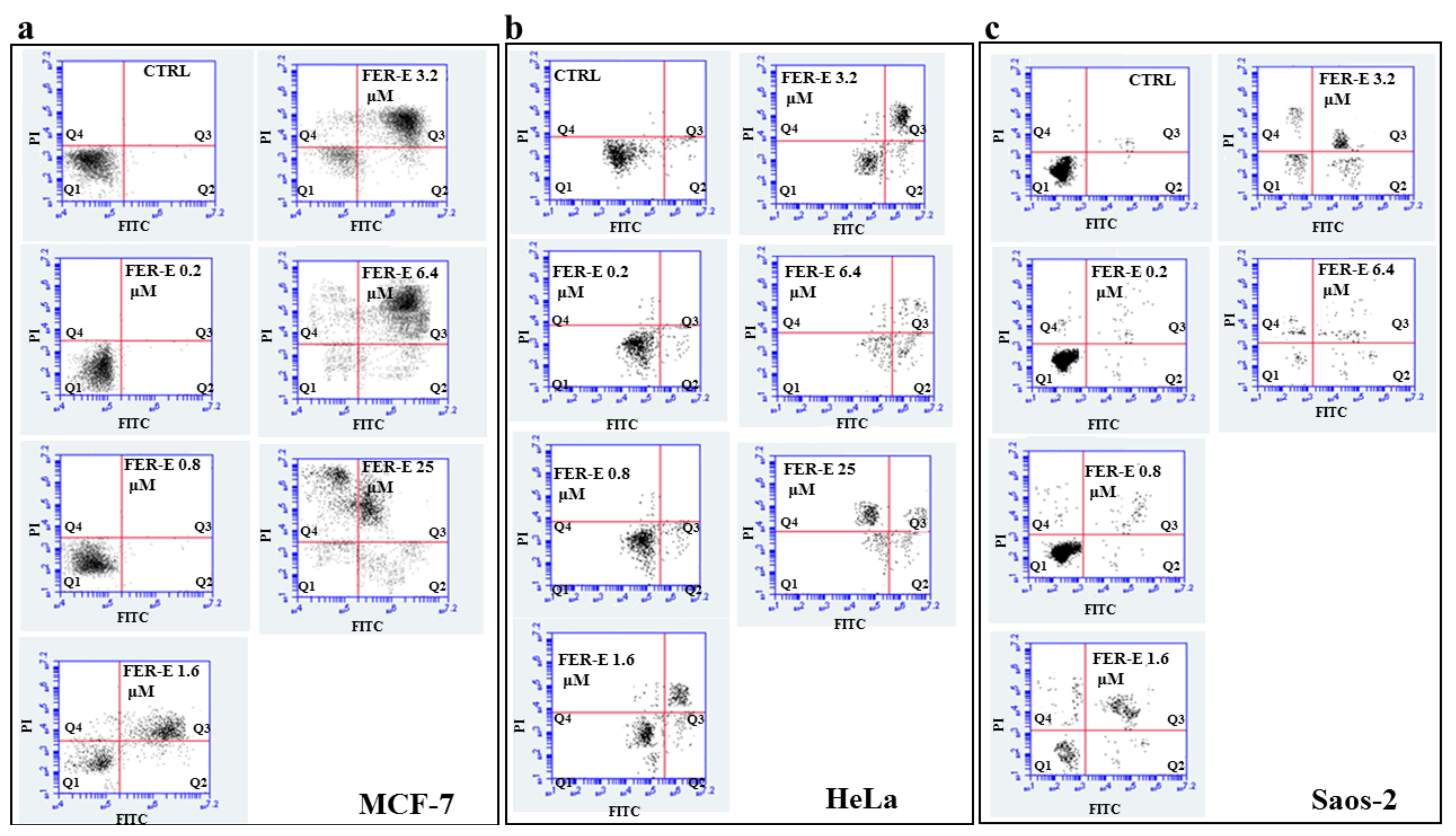
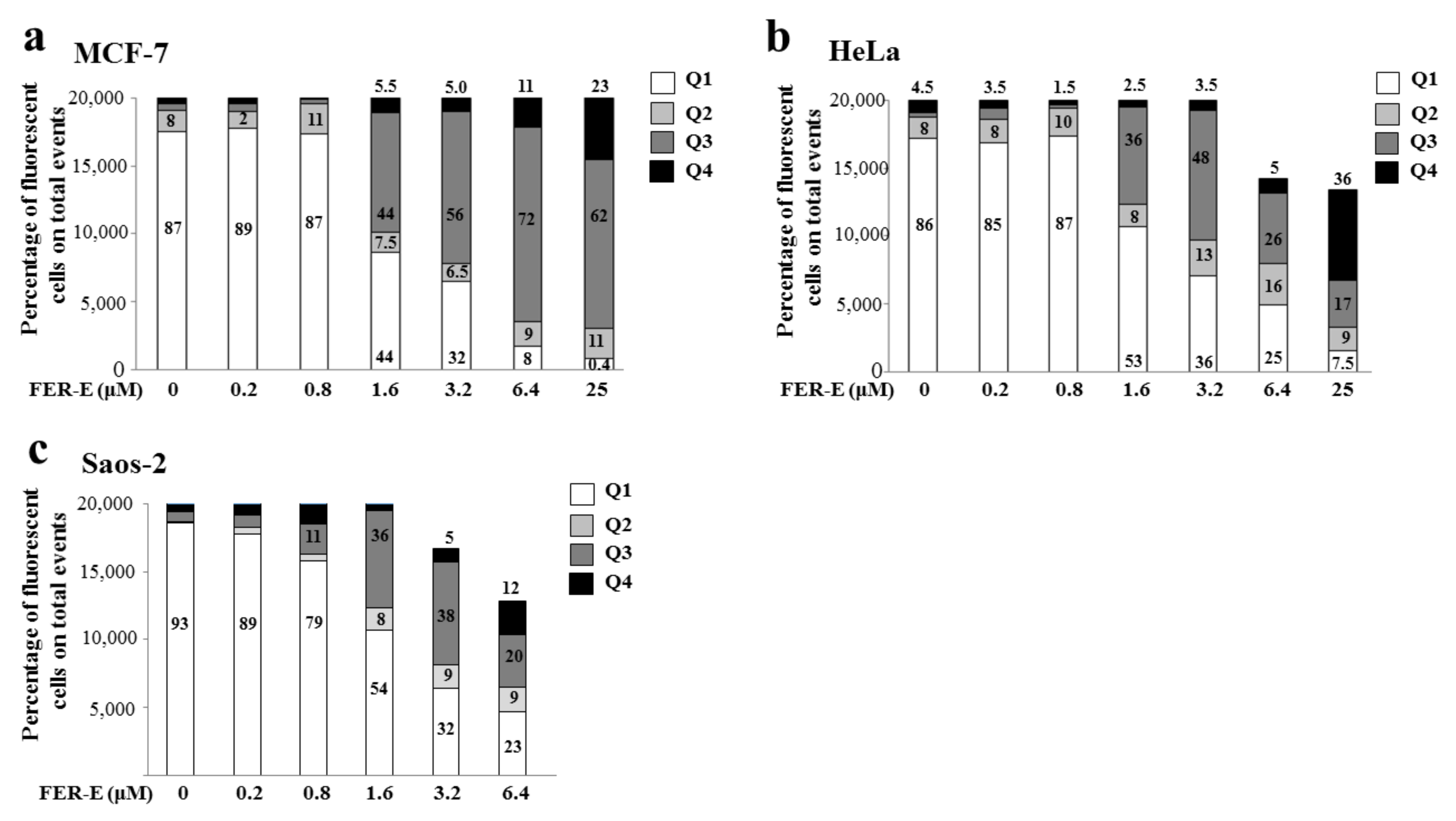
2.4. Assessment of the Type of Cell Death, Induced by FER-E, through the V-PI Annexin Test
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Obtaining and Characterizing of FER-E
5.2. Cell Cultures and Treatments
5.3. Proliferation Assay and Cytotoxicity Study
5.4. Annexin V Staining
5.5. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Sen, T.; Samanta, S.K. Medicinal plants, human health and biodiversity: A broad review. Adv. Biochem. Eng. Biotechnol. 2015, 147, 59–110. [Google Scholar] [PubMed]
- Khan, T.; Ali, M.; Khan, A.; Nisar, P.; Jan, S.A.; Afridi, S.; Shinwari, Z.K. Anticancer Plants: A Review of the Active Phytochemicals, Applications in Animal Models, and Regulatory Aspects. Biomolecules 2019, 10, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonam, S.R.; Wu, Y.S.; Tunki, L.; Chellian, R.; Halmuthur, M.S.K.; Muller, S.; Pandy, V. What Has Come out from Phytomedicines and Herbal Edibles for the Treatment of Cancer? ChemMedChem 2018, 13, 1854–1872. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xin, F.; Zhang, G.; Qu, H.; Yang, D.; Han, X. Recent Advances on Bioactive Constituents in Ferula. Drug Dev. Res. 2017, 78, 321–331. [Google Scholar] [CrossRef]
- Akaberi, M.; Iranshahy, M.; Iranshahi, M. Review of the traditional uses, phytochemistry, pharmacology and toxicology of giant fennel (Ferula communis L. subsp. communis). Iran. J. Basic Med. Sci. 2015, 18, 1050–1062. [Google Scholar]
- Rubiolo, P.; Matteodo, M.; Riccio, G.; Ballero, M.; Christen, P.; Fleury-Souverain, S.; Veuthey, J.L.; Bicchi, C. Analytical discrimination of poisonous and nonpoisonous chemotypes of giant fennel (Ferula communis L.) through their biologically active and volatile fractions. J. Agric. Food Chem. 2006, 54, 7556–7563. [Google Scholar] [CrossRef]
- Sattar, Z.; Iranshahi, M. Phytochemistry and pharmacology of Ferula persica Boiss.: A review. Iran. J. Basic Med. Sci. 2017, 20, 1–8. [Google Scholar]
- Iranshahi, M.; Ghiadi, M.; Sahebkar, A.; Rahimi, A.; Bassarello, C.; Piacente, S. Badrakemonin, a new eremophilane-type sesquiterpene from the roots of Ferula badrakema Kos.-Pol. Iran. J. Pharm. Res. 2009, 8, 275–279. [Google Scholar]
- Iranshahi, M.; Masullo, M.; Asili, A.; Hamedzadeh, A.; Jahanbin, B.; Festa, M.; Capasso, A.; Piacente, S. Sesquiterpene coumarins from Ferula gumosa. J. Nat. Prod. 2010, 73, 1958–1962. [Google Scholar] [CrossRef]
- Kasaian, J.; Iranshahy, M.; Masullo, M.; Piacente, S.; Ebrahimi, F.; Iranshahi, M. Sesquiterpene lactones from Ferula oopoda and their cytotoxic properties. J. Asian Nat. Prod. Res. 2014, 16, 248–253. [Google Scholar] [CrossRef]
- Rubiolo, P.; Matteodo, M.; Riccio, G.; Ballero, M.; Christen, P.; Fleury-Souverain, S.; Veuthey, J.L.; Matin, M.M.; Nakhaeizadeh, H.; Bahrami, A.R.; et al. Ferutinin, an apoptosis inducing terpenoid from Ferula ovina. Asian Pac. J. Cancer Prev. 2014, 15, 2123–2128. [Google Scholar]
- Poli, F.; Appendino, G.; Sacchetti, G.; Ballero, M.; Maggiano, N.; Ranelletti, F.O. Antiproliferative effects of daucane esters from Ferula communis and F. arrigonii on human colon cancer cell lines. Phytother. Res. 2005, 19, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Kiokias, S.; Proestos, C.; Oreopoulou, V. Phenolic Acids of Plant Origin—A Review on Their Antioxidant Activity In Vitro (O/W Emulsion Systems) Along with Their in Vivo Health Biochemical Properties. Foods 2020, 9, 534. [Google Scholar] [CrossRef]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maggi, F.; Papa, F.; Dall’Acqua, S.; Nicoletti, M. Chemical analysis of essential oils from different parts of Ferula communis L. growing in central Italy. Nat. Prod. Res. 2016, 30, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Lamnaouer, D. Anticoagulant activity of coumarins from Ferula communis L. Therapie 1999, 54, 747–751. [Google Scholar] [PubMed]
- Arnoldi, L.; Ballero, M.; Fuzzati, N.; Maxia, A.; Mercalli, E.; Pagni, L. HPLC-DAD-MS identification of bioactive secondary metabolites from Ferula communis roots. Fitoterapia 2004, 75, 342–354. [Google Scholar] [CrossRef]
- Ferretti, M.; Bertoni, L.; Cavani, F.; Benincasa, M.; Sena, P.; Carnevale, G.; Zavatti, M.; Di Viesti, V.; Zanoli, P.; Palumbo, C. Structural and histomorphometric evaluations of ferutinin effects on the uterus of ovariectomized rats during osteoporosis treatment. Life Sci. 2012, 90, 161–168. [Google Scholar] [CrossRef]
- Tiosano, D.; Paris, F.; Grimaldi, M.; Georgescu, V.; Servant, N.; Hochberg, Z.; Balaguer, P.; Sultan, C. Evidence of ERalpha and ERbeta selectivity and partial estrogen agonism in traditional Chinese medicine. Reprod. Biol. Endocrinol. 2014, 12, 97. [Google Scholar] [CrossRef] [Green Version]
- Russo, R.; Navarra, M.; Maiuolo, J.; Rotiroti, D.; Bagetta, G.; Corasaniti, M.T. 17beta-estradiol protects SH-SY5Y Cells against HIV-1 gp120-induced cell death: Evidence for a role of estrogen receptors. Neurotoxicology. 2005, 26, 905–913. [Google Scholar] [CrossRef]
- Safi, R.; El-Sabban, M.; Najjar, F. Ferula hermonis: A Review of Current Use and Pharmacological Studies of its Sesquiterpene Ester Ferutinin. Curr. Drug Targets 2020, 21, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.; Galleguillos, M.; Torres, R.; Tardón, K.; Cáceres, D.D.; Varela, N.M.; Quiñones, L.A. Preliminary Pharmacogenomic-Based Predictive Models of Tamoxifen Response in Hormone-dependent Chilean Breast Cancer Patients. Front. Pharmacol. 2021, 12, 661443. [Google Scholar] [CrossRef] [PubMed]
- Macho, A.; Blanco-Molina, M.; Spagliardi, P.; Appendino, G.; Bremner, P.; Heinrich, M.; Muñoz, E. Calcium ionophoretic and apoptotic effects of ferutinin in the human Jurkat T-cell line. Biochem. Pharmacol. 2004, 68, 875–883. [Google Scholar] [CrossRef]
- Alkhatib, R.; Hennebelle, T.; Joha, S.; Idziorek, T.; Quesnel, B.; Sahpaz, S.; Bailleul, F. Activity of elaeochytrin A from Ferula elaeochytris on leukemia cell lines. Phytochemistry 2008, 69, 2979–2983. [Google Scholar] [CrossRef]
- Kuete, V.; Wiench, B.; Hegazy, M.E.; Mohamed, T.A.; Fankam, A.G.; Shahat, A.A. Antibacterial activity and cytotoxicity of selected Egyptian medicinal plants. Planta Med. 2012, 78, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Arghiani, N.; Matin, M.M.; Iranshahi, M.; Sazgarnia, A.; Rassouli, F.B. Investigating anticancer properties of the sesquiterpene ferutinin on colon carcinoma cells, in vitro and in vivo. Life Sci. 2014, 109, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Spagliardi, P.; Cravotto, G.; Pocock, V.; Milligan, S. Daucane phytoestrogens: A structure-activity study. J. Nat. Prod. 2002, 65, 1612–1615. [Google Scholar] [CrossRef]
- Ikeda, K.; Arao, Y.; Otsuka, S.; Horiguchi, H.; Kato, S. Terpenoids found in the umbelliferae family act as agonists/antagonists for ERalpha and ERbeta: Differential transcription activity between ferutinine-liganded ER(alpha) and ERbeta, Biochem. Biophys. Res. Commun. 2002, 291, 354–360. [Google Scholar] [CrossRef]
- Safi, R.; Hamade, A.; Bteich, N.; El Saghir, J.; Assaf, M.D.; El-Sabban, M.; Najjar, F. A ferutinin analogue with enhanced potency and selectivity against ER-positive breast cancer cells in vitro. Biomed. Pharmacother. 2018, 105, 267–273. [Google Scholar] [CrossRef]
- Dalirfardouei, R.; Mahdipour, E.; Iranshahi, M.; Jamialahmadi, K. Osteogenic induction of menstrual blood mesenchymal stem cell by different Ferula species extracts. Avicenna J. Phytomed. 2021, 11, 281–291. [Google Scholar]
- Jacobson, M.; Mills, K.; Graves, G.; Wolfman, W.; Fortier, M. Guideline No. 422f: Menopause and Breast Cancer. J. Obstet. Gynaecol. Can. 2021, 43, 1450–1456.e1. [Google Scholar] [CrossRef] [PubMed]
- Phillips, N.A.; Bachmann, G.A. Genitourinary syndrome of menopause: Common problem, effective treatments. Cleve. Clin. J. Med. 2018, 85, 390–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira-Baptista, P.; Marchitelli, C.; Haefner, H.K.; Donders, G.; Pérez-López, F. Deconstructing the genitourinary syndrome of menopause. Int. Urogynecol. J. 2017, 28, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Lobo, R.A.; Pickar, J.H.; Stevenson, J.C.; Mack, W.J.; Hodis, H.N. Back to the future: Hormone replacement therapy as part of a prevention strategy for women at the onset of menopause. Atherosclerosis 2016, 254, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Amin, G.; Salehi-Sourmaghi, M.H.; Iranshahi, M. Histone deacetylase inhibitory and cytotoxic activities of the constituents from the roots of three species of Ferula. Iran. J. Basic Med. Sci. 2019, 22, 93–98. [Google Scholar]
- Wang, Y.; Mishra, A.; Brinton, R.D. Transitions in metabolic and immune systems from pre-menopause to post-menopause: Implications for age-associated neurodegenerative diseases. F1000Res. 2020, 9, F1000 Faculty Rev-68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinkerton, J.V. Hormone Therapy for Postmenopausal Women. N. Engl. J. Med. 2020, 382, 446–455. [Google Scholar] [CrossRef]
- Zavatti, M.; Guida, M.; Maraldi, T.; Beretti, F.; Bertoni, L.; de Pol, A. Estrogen receptor signaling in the ferutinin-induced osteoblastic differentiation of human amniotic fluid stem cells. Life Sci. 2016, 164, 15–22. [Google Scholar] [CrossRef]
- Rolph, D.N.; Deb, M.; Kanji, S.; Greene, C.J.; Das, M.; Joseph, M.; Aggarwal, R.; Leblebicioglu, B.; Das, H. Ferutinin directs dental pulp-derived stem cells towards the osteogenic lineage by epigenetically regulating canonical Wnt signaling. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165314. [Google Scholar] [CrossRef]
- Maity, J.; Barthels, D.; Sarkar, J.; Prateeksha, P.; Deb, M.; Rolph, D.; Das, H. Ferutinin induces osteoblast differentiation of DPSCs via induction of KLF2 and autophagy/mitophagy. Cell Death Dis. 2022, 13, 452. [Google Scholar] [CrossRef]
- Li, C.Y.; Chen, M.L. Chemotherapy-Induced Amenorrhea and Menopause Symptoms in Women With Breast Cancer. Hu Li Za Zhi 2016, 63, 19–26. [Google Scholar]
- Heer, E.; Harper, A.; Escandor, N.; Sung, H.; McCormack, V.; Fidler-Benaoudia, M.M. Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. Lancet Glob. Health 2020, 8, e1027–e1037. [Google Scholar] [CrossRef]
- Sydora, B.C.; Graham, B.; Oster, R.T.; Ross, S. Menopause experience in First Nations women and initiatives for menopause symptom awareness; a community-based participatory research approach. BMC Womens Health 2021, 21, 179. [Google Scholar] [CrossRef] [PubMed]
- Dunneram, Y.; Greenwood, D.C.; Cade, J.E. Diet, menopause and the risk of ovarian, endometrial and breast cancer. Proc. Nutr. Soc. 2019, 78, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Arriaga, M.E.; Vajdic, C.M.; Canfell, K.; MacInnis, R.J.; Banks, E.; Velentzis, L.S.; Cumming, R.G.; Hirani, V.; Laaksonen, M.A. The preventable burden of breast cancers for premenopausal and postmenopausal women in Australia: A pooled cohort study. Int. J. Cancer 2019, 145, 2383–2394. [Google Scholar] [CrossRef]
- Lima, S.M.; Kehm, R.D.; Terry, M.B. Global breast cancer incidence and mortality trends by region, age-groups, and fertility patterns. EClinicalMedicine 2021, 38, 100985. [Google Scholar] [CrossRef]
- Fornili, M.; Perduca, V.; Fournier, A.; Jérolon, A.; Boutron-Ruault, M.C.; Maskarinec, G.; Severi, G.; Baglietto, L. Association between menopausal hormone therapy, mammographic density and breast cancer risk: Results from the E3N cohort study. Breast Cancer Res. 2021, 23, 47. [Google Scholar] [CrossRef]
- Macrì, R.; Musolino, V.; Gliozzi, M.; Carresi, C.; Maiuolo, J.; Nucera, S.; Scicchitano, M.; Bosco, F.; Scarano, F.; Ruga, S.; et al. Ferula L. Plant Extracts and Dose-Dependent Activity of Natural Sesquiterpene Ferutinin: From Antioxidant Potential to Cytotoxic Effects. Molecules 2020, 25, 5768. [Google Scholar] [CrossRef]
- Appendino, G.; Cravotto, G.; Sterner, O.; Ballero, M. Oxygenated sesquiterpenoids from a nonpoisonous sardinian chemotype of giant fennel (Ferula communis). J. Nat. Prod. 2001, 64, 393–395. [Google Scholar] [CrossRef]
- Kasaian, J.; Mosaffa, F.; Behravan, J.; Masullo, M.; Piacente, S.; Ghandadi, M.; Iranshah, M. Reversal of P-glycoprotein-mediated multidrug resistance in MCF-7/Adr cancer cells by sesquiterpene coumarins. Fitoterapia 2015, 103, 149–154. [Google Scholar] [CrossRef]
- Hanafi-Bojd, M.Y.; Iranshahi, M.; Mosaffa, F.; Tehrani, S.O.; Kalalinia, F.; Behravan, J. Farnesiferol A from Ferula persica and galbanic acid from Ferula szowitsiana inhibit P-glycoprotein-mediated rhodamine efflux in breast cancer cell lines. Planta Med. 2011, 77, 1590–1593. [Google Scholar] [CrossRef] [PubMed]
- Iranshahi, M.; Amin, G.; Shafiee, A. A new coumarin from Ferula persica. Pharm. Biol. 2004, 42, 440–442. [Google Scholar] [CrossRef]
- Soltani, S.; Amin, G.R.; Salehi-Sourmaghi, M.H.; Schneider, B.; Lorenz, S.; Iranshahi, M. Sulfur-containing compounds from the roots of Ferula latisecta and their cytotoxic activities. Fitoterapia 2018, 124, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Iranshahi, M.; Mojarab, M.; Sadeghian, H.; Hanafi-Bojd, M.Y.; Schneider, B. Polar secondary metabolites of Ferula persica roots. Phytochemistry 2008, 69, 473–478. [Google Scholar] [CrossRef]
- Iranshahi, M. A review of volatile sulfur-containing compounds from terrestrial plants: Biosynthesis, distribution and analytical methods. J. Essent. Oil Res. 2012, 24, 393–434. [Google Scholar] [CrossRef]
- Razavi, B.M.; Arasteh, E.; Imenshahidi, M.; Iranshahi, M. Antihypertensive effect of auraptene, a monoterpene coumarin from the genus Citrus, upon chronic administration. Iran. J. Basic Med. Sci. 2015, 18, 153–158. [Google Scholar]
- Shakeri, A.; Iranshahy, M.; Iranshahi, M. Biological properties and molecular targets of umbelliprenin–a mini-review. J. Asian Nat. Prod. Res. 2014, 16, 884–889. [Google Scholar] [CrossRef]
- Kasaian, J.; Iranshahy, M.; Iranshahi, M. Synthesis, biosynthesis and biological acitivities of galbanic acid-a review. Pharm. Biol. 2014, 52, 524–531. [Google Scholar] [CrossRef] [Green Version]
- Bannoud, N.; Carvelli, F.L.; Troncoso, M.; Sartor, T.; Vargas-Roig, L.M.; Sosa, M. Cation-dependent mannose-6-phosphate receptor expression and distribution are influenced by estradiol in MCF-7 breast cancer cells. PLoS ONE 2018, 13, e0201844. [Google Scholar] [CrossRef]
- Semeikin, A.V.; Kareva, E.N.; Fedotcheva, T.A.; Lunina, A.S.; Levina, I.S.; Rzheznikov, V.M.; Shimanovskii, N.L. Influence of progesterone derivatives on the viability and expression of estrogen receptor-alpha mRNA in hela cells. Eksp. Klin. Farmakol. 2016, 79, 22–24. [Google Scholar]
- Aversa, A.; Fittipaldi, S.; Bimonte, V.M.; Wannenes, F.; Papa, V.; Francomano, D.; Greco, E.A.; Lenzi, A.; Migliaccio, S. Tadalafil modulates aromatase activity and androgen receptor expression in a human osteoblastic cell in vitro model. J. Endocrinol. Invest. 2016, 39, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Bava, I.; Carresi, C.; Gliozzi, M.; Musolino, V.; Scicchitano, M.; Macrì, R.; Oppedisano, F.; Scarano, F.; Zito, M.C.; et al. The Effect of Ferula communis Extract in Escherichia coli Lipopolysaccharide-Induced Neuroinflammation in Cultured Neurons and Oligodendrocytes. Int. J. Mol. Sci. 2021, 22, 7910. [Google Scholar] [CrossRef] [PubMed]
- Hammam, A.M.M.; Elmotayam, A.K.; Elnattat, W.M.; Ali, G.A.; Madbouly, A.E.M.; El Khatteb, R.M.; Abdelhameed, M.F.; Ali, A.H.; Qasim, S.; Ahmed, S.R. Assessment of Ferula hermonis Boiss fertility effects in immature female rats supported by quantification of ferutinin via HPLC and molecular docking. J. Ethnopharmacol. 2022, 289, 115062. [Google Scholar] [CrossRef] [PubMed]
- Alilou, M.; Dibwe, D.F.; Schwaiger, S.; Khodami, M.; Troppmair, J.; Awale, S.; Stuppner, H. Antiausterity Activity of Secondary Metabolites from the Roots of Ferula hezarlalehzarica against the PANC-1 Human Pancreatic Cancer Cell Line. J. Nat. Prod. 2020, 83, 1099–1106. [Google Scholar] [CrossRef] [Green Version]
- Ferretti, M.; Cavani, F.; Manni, P.; Carnevale, G.; Bertoni, L.; Zavatti, M. Ferutinin dose-dependent effects on uterus and mammary gland in ovariectomized rats. Histol. Histopathol. 2014, 29, 1027–1037. [Google Scholar]
- Iranshahi, M.; Rezaee, R.; Najaf, M.; Haghbin, A.; Kasaian, J. Cytotoxic activity of the genus Ferula (Apiaceae) and its bioactive constituents. Avicenna J. Phytomed. 2018, 8, 296–312. [Google Scholar] [PubMed]
- Bagheri, S.M.; Sahebkar, A.; Gohari, A.R.; Saeidnia, S.; Malmir, M.; Iranshahi, M. Evaluation of cytotoxicity and anticonvulsant activity of some Iranian medicinal Ferula species. Pharm. Biol. 2010, 48, 242–246. [Google Scholar] [CrossRef]
- Hajimehdipoor, H.; Esmaeili, S.; Ramezani, R.; Jafari Anaraki, M.; Mosaddegh, M. The cytotoxic effects of Ferula persica var. persica and Ferula hezarlalehzarica against HepG2, A549, HT29, MCF7 and MDBK cell lines. Iran. J. Pharm. Sci. 2012, 8, 113–117. [Google Scholar]
- Zavatti, M.; Resca, E.; Bertoni, L.; Maraldi, T.; Guida, M.; Carnevale, G.; Ferrari, A.; De Pol, A. Ferutinin promotes proliferation and osteoblastic differentiation in human amniotic fluid and dental pulp stem cells. Life Sci. 2013, 92, 993–1003. [Google Scholar] [CrossRef]
- Safi, R.; Rodriguez, F.; Hilal, G.; Diab-Assaf, M.; Diab, Y.; El-Sabban, M.; Najjar, F.; Delfourne, E. Hemisynthesis, Antitumoral Effect, and Molecular Docking Studies of Ferutinin and Its Analogues. Chem. Biol. Drug Des. 2016, 87, 382–897. [Google Scholar] [CrossRef]
- Naji Reyhani Garmroudi, S.; Karimi, E.; Oskoueian, E.; Homayouni-Tabrizi, M.; Iranshahi, M. Ferutinin: A phytoestrogen from ferula and its anticancer, antioxidant, and toxicity properties. J. Biochem. Mol. Toxicol. 2021, 35, e22713. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiuolo, J.; Musolino, V.; Guarnieri, L.; Macrì, R.; Coppoletta, A.R.; Cardamone, A.; Serra, M.; Gliozzi, M.; Bava, I.; Lupia, C.; et al. Ferula communis L. (Apiaceae) Root Acetone-Water Extract: Phytochemical Analysis, Cytotoxicity and In Vitro Evaluation of Estrogenic Properties. Plants 2022, 11, 1905. https://doi.org/10.3390/plants11151905
Maiuolo J, Musolino V, Guarnieri L, Macrì R, Coppoletta AR, Cardamone A, Serra M, Gliozzi M, Bava I, Lupia C, et al. Ferula communis L. (Apiaceae) Root Acetone-Water Extract: Phytochemical Analysis, Cytotoxicity and In Vitro Evaluation of Estrogenic Properties. Plants. 2022; 11(15):1905. https://doi.org/10.3390/plants11151905
Chicago/Turabian StyleMaiuolo, Jessica, Vincenzo Musolino, Lorenza Guarnieri, Roberta Macrì, Anna Rita Coppoletta, Antonio Cardamone, Maria Serra, Micaela Gliozzi, Irene Bava, Carmine Lupia, and et al. 2022. "Ferula communis L. (Apiaceae) Root Acetone-Water Extract: Phytochemical Analysis, Cytotoxicity and In Vitro Evaluation of Estrogenic Properties" Plants 11, no. 15: 1905. https://doi.org/10.3390/plants11151905
APA StyleMaiuolo, J., Musolino, V., Guarnieri, L., Macrì, R., Coppoletta, A. R., Cardamone, A., Serra, M., Gliozzi, M., Bava, I., Lupia, C., Tucci, L., Bombardelli, E., & Mollace, V. (2022). Ferula communis L. (Apiaceae) Root Acetone-Water Extract: Phytochemical Analysis, Cytotoxicity and In Vitro Evaluation of Estrogenic Properties. Plants, 11(15), 1905. https://doi.org/10.3390/plants11151905








