Characterization of the Water Shortage Effects on Potato Tuber Tissues during Growth Using MRI Relaxometry and Biochemical Parameters
Abstract
1. Introduction
2. Results
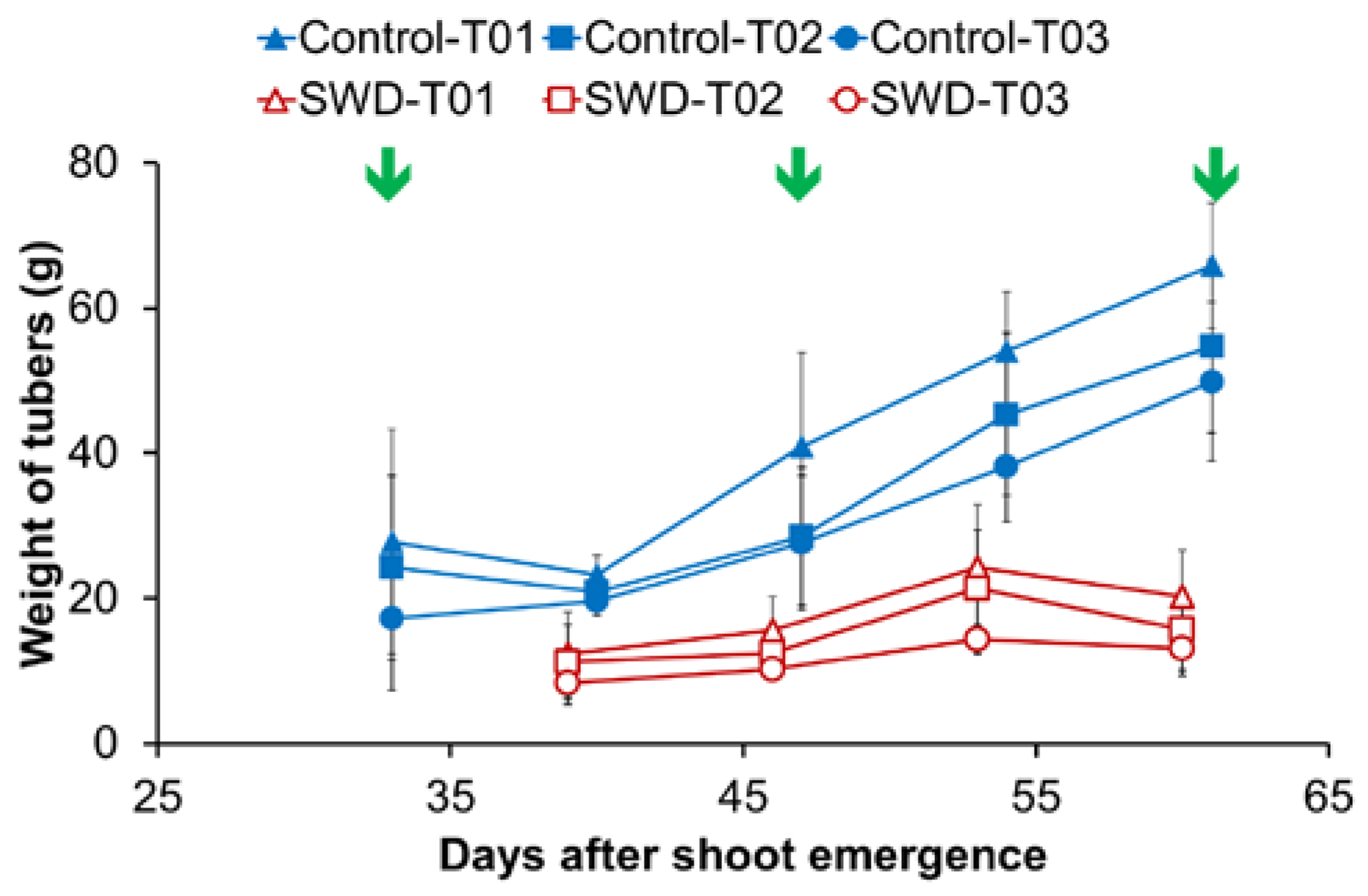
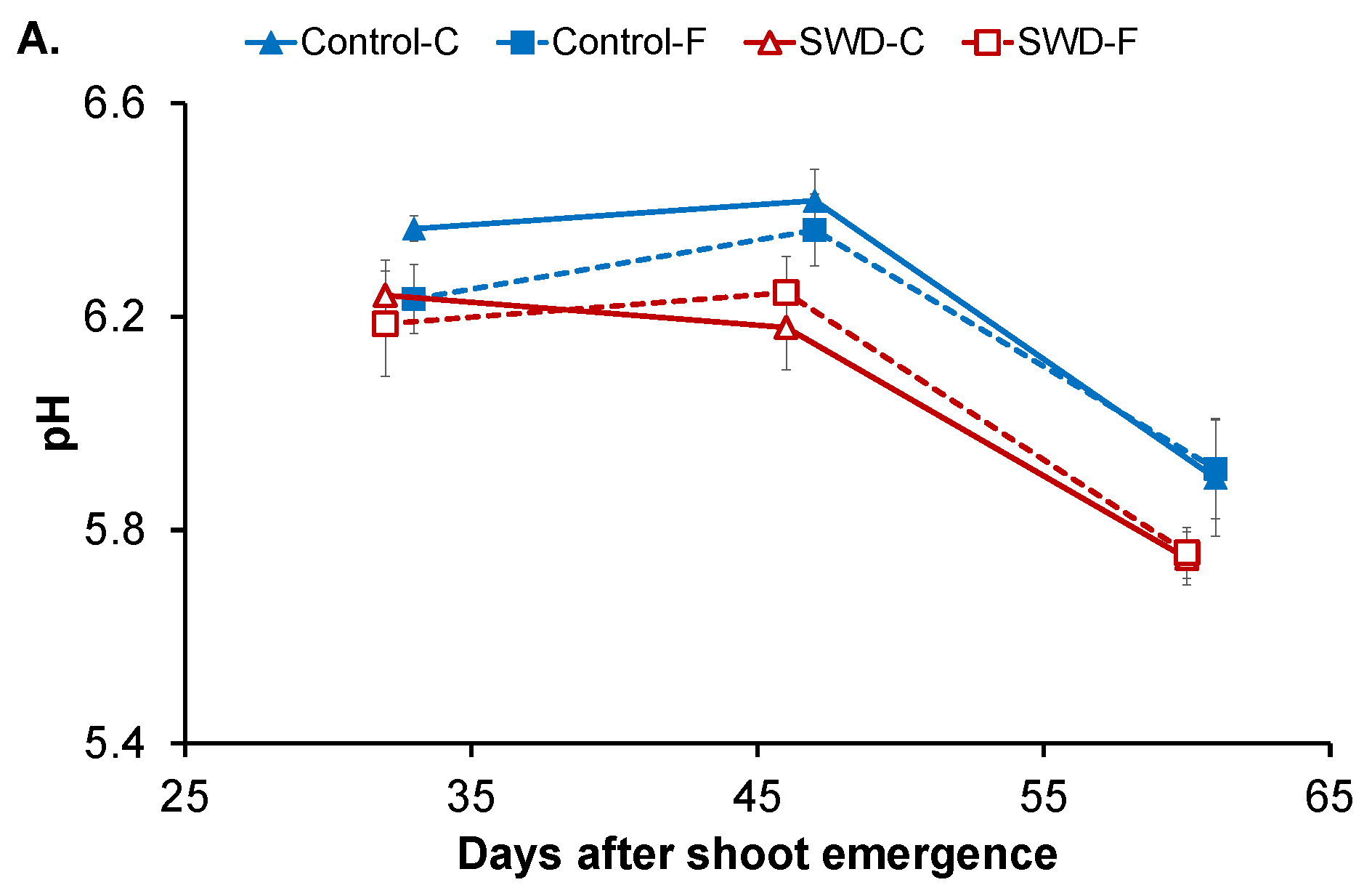
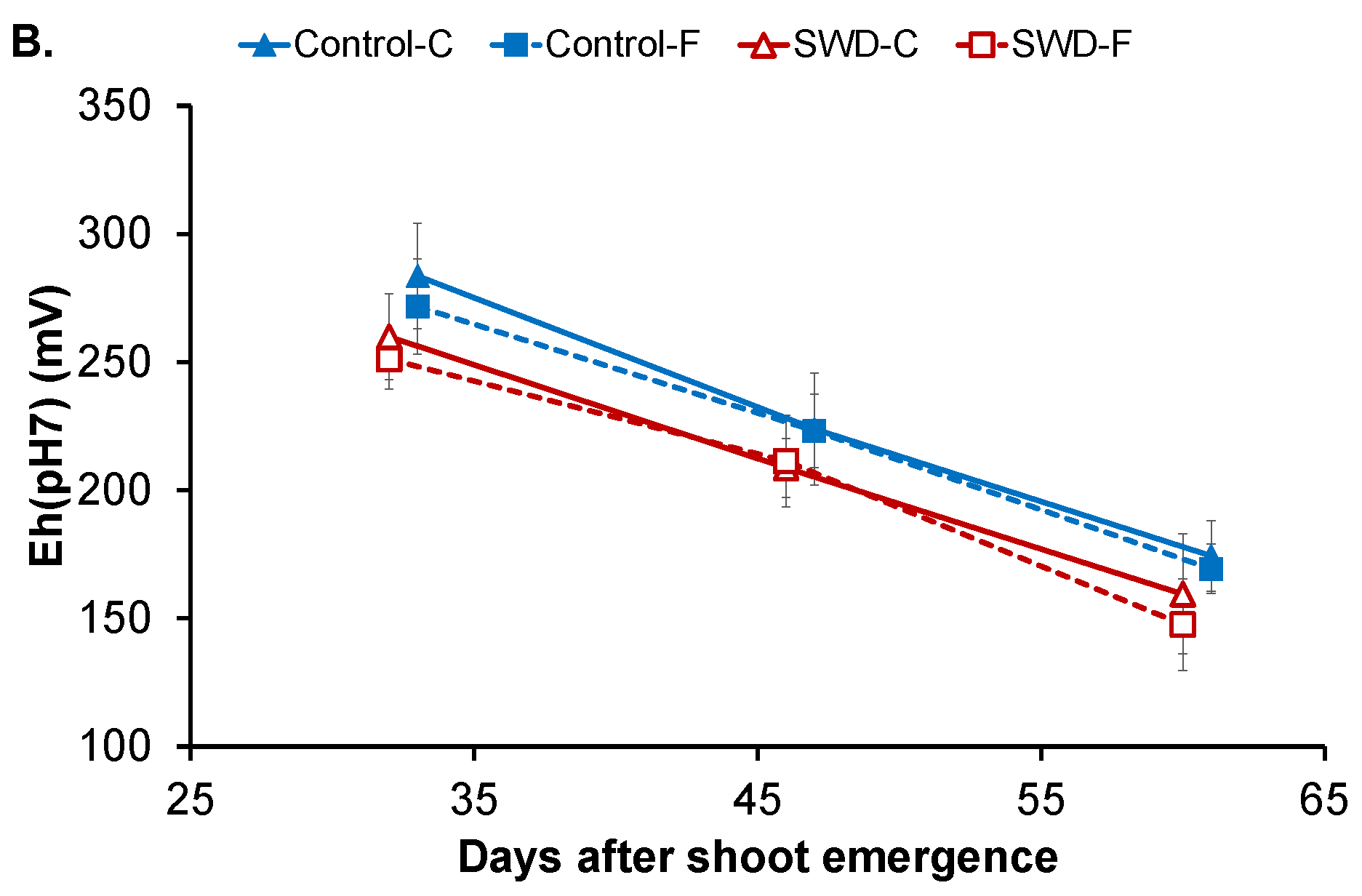
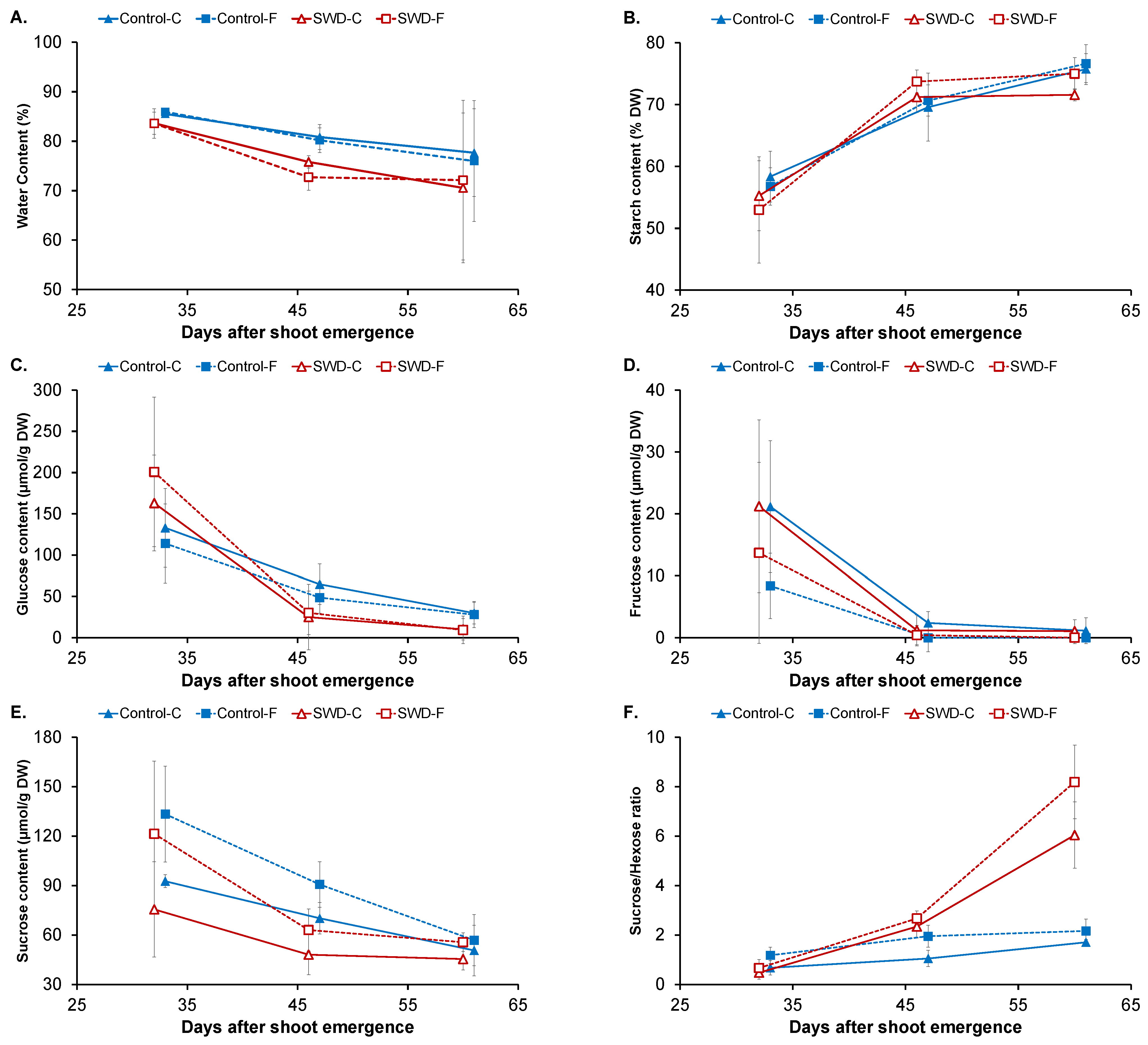
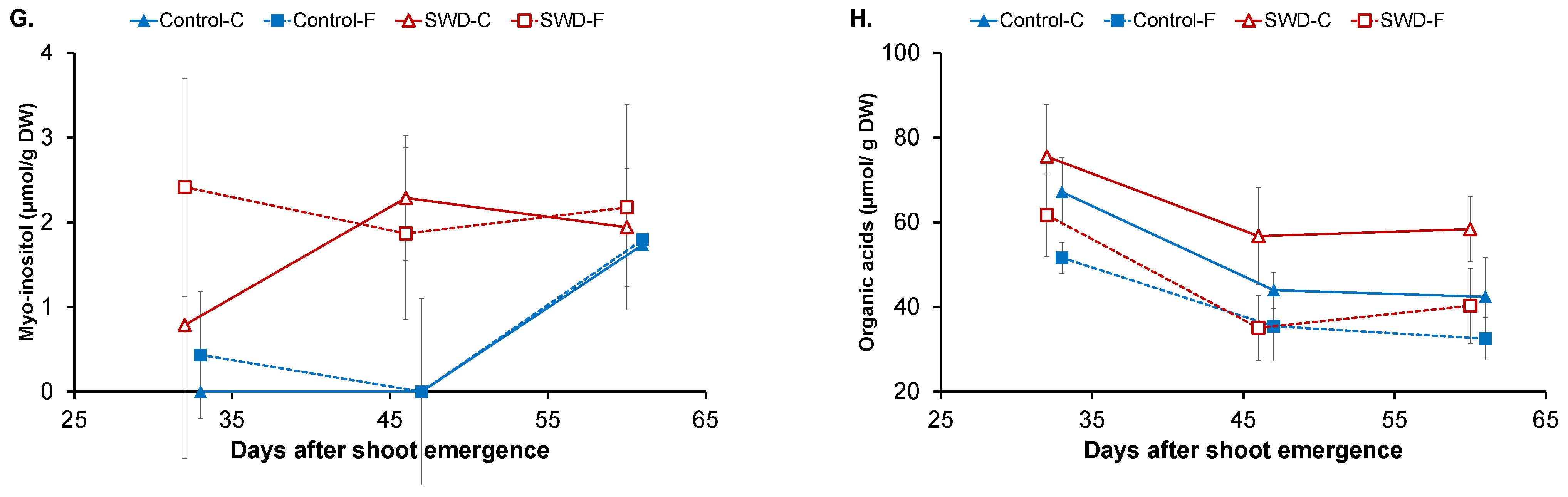
2.1. Changes in Physiological Parameters
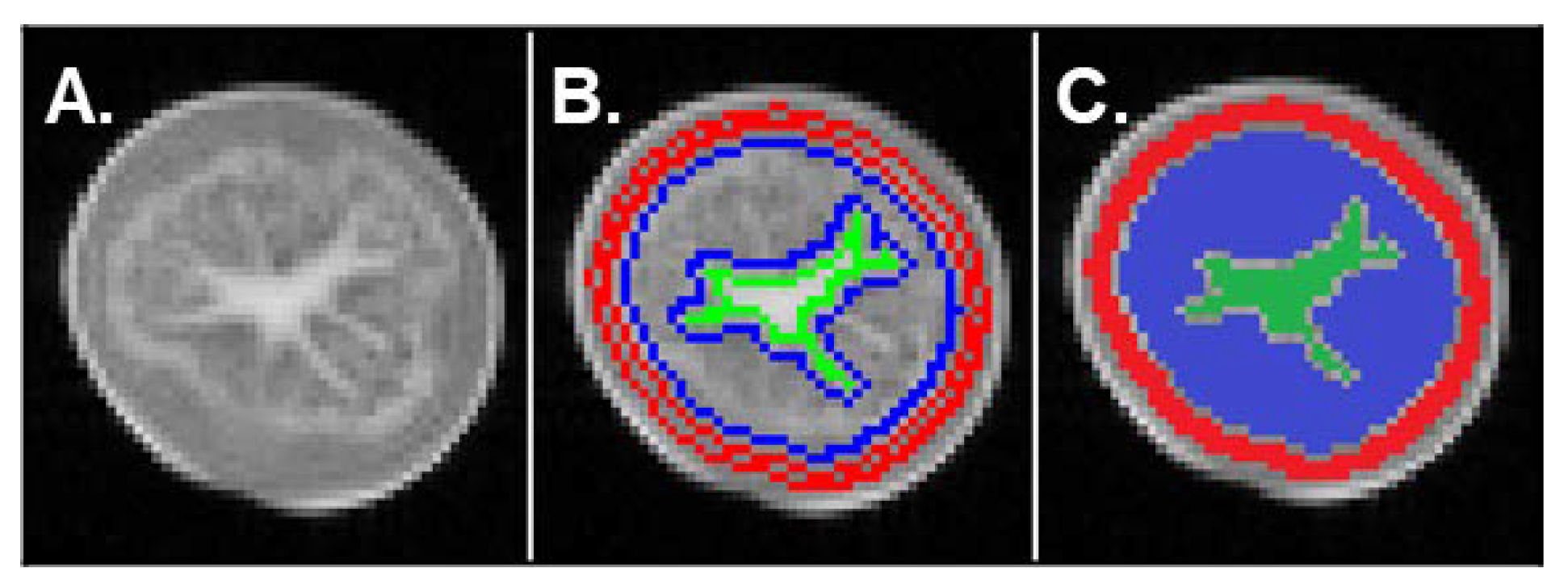
2.2. Evolution of MRI Parameters
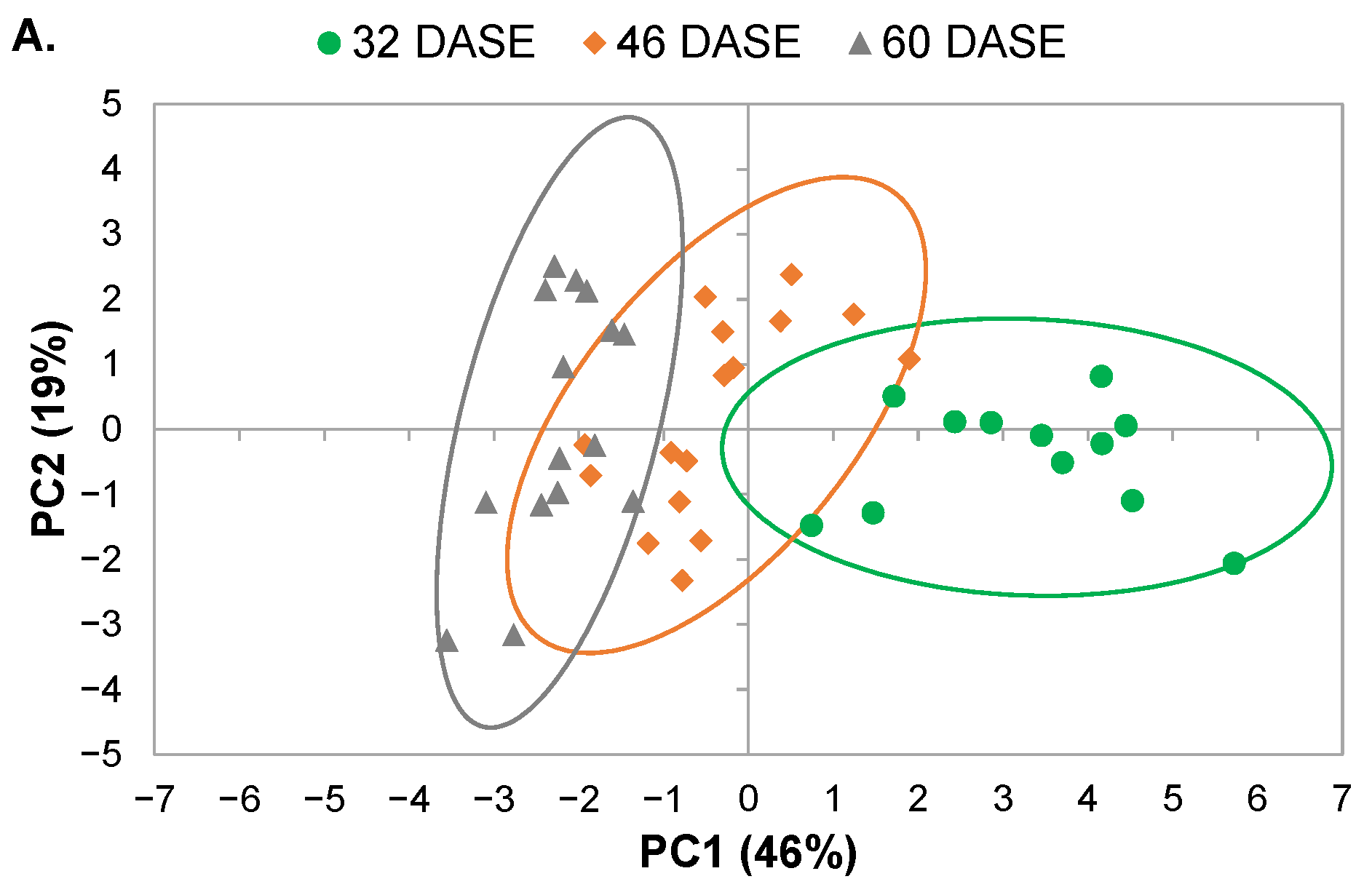
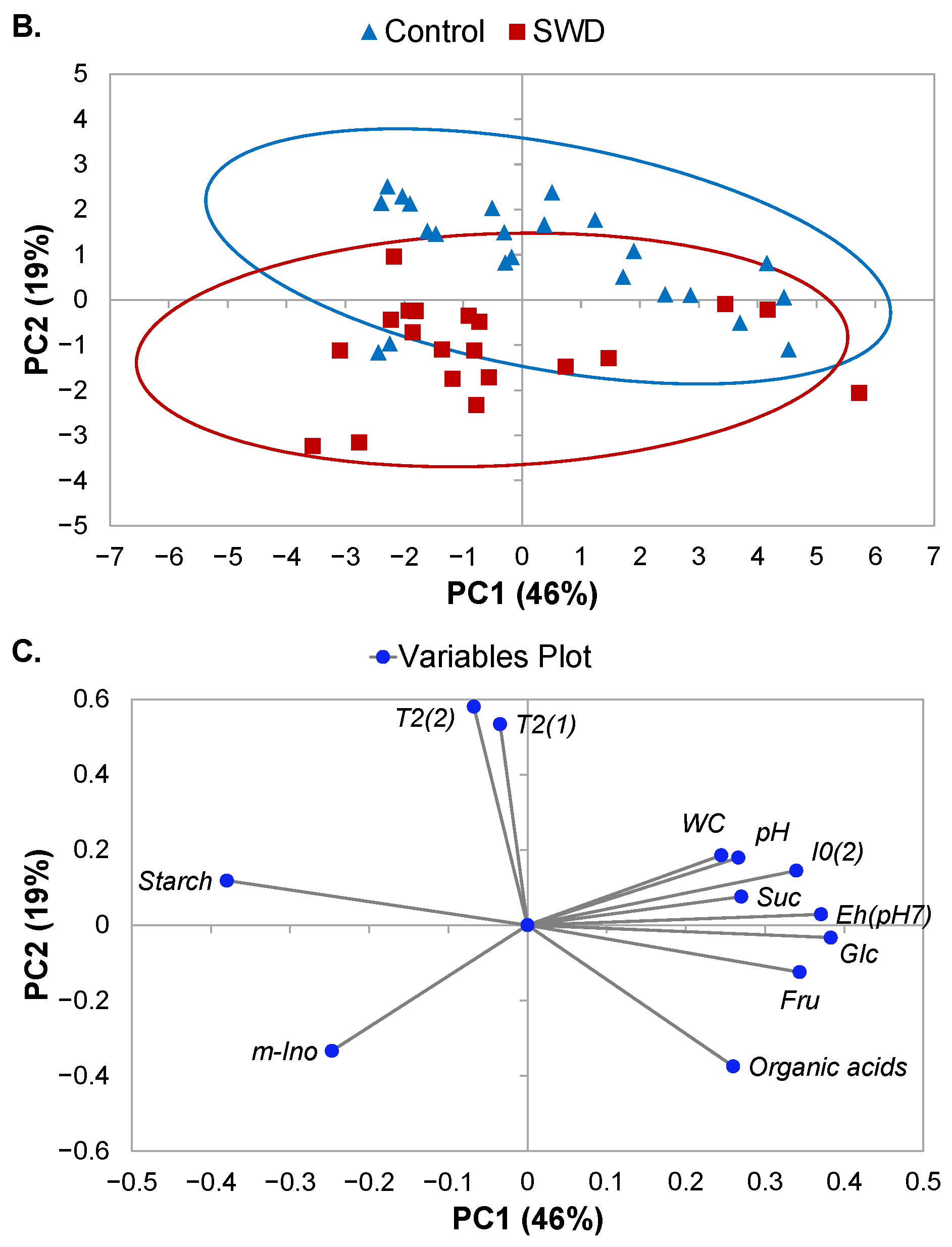
2.3. Global Analysis of Physiological and MRI Parameters
3. Discussion
3.1. Spatial Changes in Composition during Tuber Filling under Well-Irrigated and Stress Conditions
3.2. Relationship between Transverse Relaxation Times and Tissue Features
4. Materials and Methods
4.1. Experimental Design and Sampling
4.2. MRI Acquisition Protocols and Image Processing
4.3. Biochemical Composition
4.4. Redox Potential and pH Measurements
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, L.; Paulo, M.-J.; Strahwald, J.; Lübeck, J.; Hofferbert, H.-R.; Tacke, E.; Junghans, H.; Wunder, J.; Draffehn, A.; van Eeuwijk, F.; et al. Natural DNA variation at candidate loci is associated with potato chip color, tuber starch content, yield and starch yield. Theor. Appl. Genet. 2008, 116, 1167–1181. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, X.; Vreugdenhil, D.; van Lammeren, A.A. Cell division and cell enlargement during potato tuber formation. J. Exp. Bot. 1998, 49, 573–582. [Google Scholar] [CrossRef]
- Reeve, R.M.; Timm, H.; Weaver, M.L. Parenchyma cell growth in potato tubers II. Cell divisions vs. cell enlargement. Am. Potato J. 1973, 50, 71–78. [Google Scholar] [CrossRef]
- Tauberger, E.; Hoffmann-Benning, S.; Fleischer-Notter, H.; Willmitzer, L.; Fisahn, J. Impact of invertase overexpression on cell size, starch granule formation and cell wall properties during tuber development in potatoes with modified carbon allocation patterns. J. Exp. Bot. 1999, 50, 477–486. [Google Scholar] [CrossRef]
- Gancarz, M.; Konstankiewicz, K.; Zgórska, K. Cell orientation in potato tuber parenchyma tissue. Int. Agrophys. 2014, 28, 15–22. [Google Scholar] [CrossRef]
- Viola, R.; Roberts, A.G.; Haupt, S.; Gazzani, S.; Hancock, R.D.; Marmiroli, N.; Machray, G.C.; Oparka, K.J. Tuberization in Potato Involves a Switch from Apoplastic to Symplastic Phloem Unloading. Plant Cell 2001, 13, 385–398. [Google Scholar] [CrossRef]
- Oey, I.; Faridnia, F.; Leong, S.Y.; Burritt, D.J.; Liu, T. Determination of Pulsed Electric Fields Effects on the Structure of Potato Tubers. In Handbook of Electroporation; Miklavčič, D., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1489–1507. [Google Scholar]
- Joshi, M.; Fogelman, E.; Belausov, E.; Ginzberg, I. Potato root system development and factors that determine its architecture. J. Plant Physiol. 2016, 205, 113–123. [Google Scholar] [CrossRef]
- Djaman, K.; Irmak, S.; Koudahe, K.; Allen, S. Irrigation Management in Potato (Solanum tuberosum L.) Production: A Review. Sustainability 2021, 13, 1504. [Google Scholar] [CrossRef]
- Kashyap, P.; Panda, R. Effect of irrigation scheduling on potato crop parameters under water stressed conditions. Agric. Water Manag. 2003, 59, 49–66. [Google Scholar] [CrossRef]
- Hajjar, G.; Quellec, S.; Pépin, J.; Challois, S.; Joly, G.; Deleu, C.; Leport, L.; Musse, M. MRI investigation of internal defects in potato tubers with particular attention to rust spots induced by water stress. Postharvest Biol. Technol. 2021, 180, 111600. [Google Scholar] [CrossRef]
- Herremans, E.; Verboven, P.; Bongaers, E.; Estrade, P.; Verlinden, B.E.; Wevers, M.; Hertog, M.L.A.T.M.; Nicolai, B.M. Characterisation of ‘Braeburn’ browning disorder by means of X-ray micro-CT. Postharvest Biol. Technol. 2013, 75, 114–124. [Google Scholar] [CrossRef]
- Van As, H. Intact plant MRI for the study of cell water relations, membrane permeability, cell-to-cell and long distance water transport. J. Exp. Bot. 2006, 58, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Van der Weerd, L.; Claessens, M.M.; Ruttink, T.; Vergeldt, F.J.; Schaafsma, T.J.; Van As, H. Quantitative NMR microscopy of osmotic stress responses in maize and pearl millet. J. Exp. Bot. 2001, 52, 2333–2343. [Google Scholar] [CrossRef] [PubMed]
- Musse, M.; Bidault, K.; Quellec, S.; Brunel, B.; Collewet, G.; Cambert, M.; Bertin, N. Spatial and temporal evolution of quantitative magnetic resonance imaging parameters of peach and apple fruit—relationship with biophysical and metabolic traits. Plant J. 2020, 105, 62–78. [Google Scholar] [CrossRef]
- Hills, B.P.; Nott, K.P. NMR studies of water compartmentation in carrot parenchyma tissue during drying and freezing. Appl. Magn. Reson. 1999, 17, 521–535. [Google Scholar] [CrossRef]
- Thybo, A.; Andersen, H.; Karlsson, A.; Dønstrup, S.; Stødkilde-Jørgensen, H. Low-field NMR relaxation and NMR-imaging as tools in differentiation between potato sample and determination of dry matter content in potatoes. LWT—Food Sci. Technol. 2003, 36, 315–322. [Google Scholar] [CrossRef]
- Hills, B.; Costa, A.; Marigheto, N.; Wright, K. T 1−T 2 NMR correlation studies of high-pressure-processed starch and potato tissue. Appl. Magn. Reson. 2005, 28, 13–27. [Google Scholar] [CrossRef]
- Borzenkova, R.A.; Borovkova, M.P. Developmental Patterns of Phytohormone Content in the Cortex and Pith of Potato Tubers as Related to Their Growth and Starch Content. Russ. J. Plant Physiol. 2003, 50, 119–124. [Google Scholar] [CrossRef]
- Musse, M.; Hajjar, G.; Ali, N.; Billiot, B.; Joly, G.; Pépin, J.; Quellec, S.; Challois, S.; Mariette, F.; Cambert, M.; et al. A global non-invasive methodology for the phenotyping of potato under water deficit conditions using imaging, physiological and molecular tools. Plant Methods 2021, 17, 81. [Google Scholar] [CrossRef]
- Engels, C.H.; Marschner, H. Allocation of Photosynthate to Individual Tubers of Solanum tuberosum L.: I. relationship between tuber growth rate and enzyme activities of the starch metabolism. J. Exp. Bot. 1986, 37, 1795–1803. [Google Scholar]
- Visser, R.G.F.; Vreugdenhil, D.; Hendriks, T.; Jacobsen, E. Gene expression and carbohydrate content during stolon to tuber transition in potatoes (Solanum tuberosum). Physiol. Plant. 1994, 90, 285–292. [Google Scholar] [CrossRef]
- Roitsch, T. Source-sink regulation by sugar and stress. Curr. Opin. Plant Biol. 1999, 2, 198–206. [Google Scholar] [CrossRef]
- Geigenberger, P.; Reimholz, R.; Geiger, M.; Merlo, L.; Canale, V.; Stitt, M. Regulation of sucrose and starch metabolism in potato tubers in response to short-term water deficit. Planta 1997, 201, 502–518. [Google Scholar] [CrossRef]
- Geigenberger, P.; Stitt, M. Sucrose synthase catalyses a readily reversible reaction in vivo in developing potato tubers and other plant tissues. Planta 1993, 189, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Yildizli, A.; Çevik, S.; Ünyayar, S. Effects of exogenous myo-inositol on leaf water status and oxidative stress of Capsicum annuum under drought stress. Acta Physiol. Plant. 2018, 40, 122. [Google Scholar] [CrossRef]
- Valluru, R.; Van Den Ende, W. Myo-inositol and beyond—Emerging networks under stress. Plant Sci. 2011, 181, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Loewus, F.A.; Murthy, P.P.N. myo-Inositol metabolism in plants. Plant Sci. 2000, 150, 1–19. [Google Scholar] [CrossRef]
- Hockema, B.R.; Etxeberria, E. Metabolic Contributors to Drought-enhanced Accumulation of Sugars and Acids in Oranges. J. Am. Soc. Hortic. Sci. 2001, 126, 599–605. [Google Scholar] [CrossRef]
- Sorin, C.; Musse, M.; Mariette, F.; Bouchereau, A.; Leport, L. Assessment of nutrient remobilization through structural changes of palisade and spongy parenchyma in oilseed rape leaves during senescence. Planta 2014, 241, 333–346. [Google Scholar] [CrossRef]
- Hills, B.; Le Floc’H, G. NMR studies of non-freezing water in cellular plant tissue. Food Chem. 1994, 51, 331–336. [Google Scholar] [CrossRef]
- Leforestier, R.; Mariette, F.; Musse, M. Impact of chemical exchange on transverse relaxation at low and moderate magnetic field strengths for sugar solutions representative of fruit tissues analyzed by simulation and MRI experiments. J. Magn. Reson. 2020, 322, 106872. [Google Scholar] [CrossRef] [PubMed]
- Donker, H.; Van As, H.; Edzes, H.; Jans, A. NMR imaging of white button mushroom (Agaricus bisporis) at various magnetic fields. Magn. Reson. Imaging 1996, 14, 1205–1215. [Google Scholar] [CrossRef]
- Mortensen, M.; Thybo, A.K.; Bertram, H.C.; Andersen, H.J.; Engelsen, S.B. Cooking Effects on Water Distribution in Potatoes Using Nuclear Magnetic Resonance Relaxation. J. Agric. Food Chem. 2005, 53, 5976–5981. [Google Scholar] [CrossRef] [PubMed]
- Winisdorffer, G.; Musse, M.; Quellec, S.; Devaux, M.-F.; Lahaye, M.; Mariette, F. MRI investigation of subcellular water compartmentalization and gas distribution in apples. Magn. Reson. Imaging 2015, 33, 671–680. [Google Scholar] [CrossRef]
- Raffo, A.; Gianferri, R.; Barbieri, R.; Brosio, E. Ripening of banana fruit monitored by water relaxation and diffusion 1H-NMR measurements. Food Chem. 2004, 89, 149–158. [Google Scholar] [CrossRef]
- Geya, Y.; Kimura, T.; Fujisaki, H.; Terada, Y.; Kose, K.; Haishi, T.; Gemma, H.; Sekozawa, Y. Longitudinal NMR parameter measurements of Japanese pear fruit during the growing process using a mobile magnetic resonance imaging system. J. Magn. Reson. 2013, 226, 45–51. [Google Scholar] [CrossRef]
- Earl, H.J. A precise gravimetric method for simulating drought stress in pot experiments. Crop. Sci. 2003, 43, 1868–1873. [Google Scholar] [CrossRef]
- Adriaensen, H.; Musse, M.; Quellec, S.; Vignaud, A.; Cambert, M.; Mariette, F. MSE-MRI sequence optimisation for measurement of bi- and tri-exponential T2 relaxation in a phantom and fruit. Magn. Reson. Imaging 2013, 31, 1677–1689. [Google Scholar] [CrossRef]
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Kapur, J.N.; Sahoo, P.K.; Wong, A.K.C. A new method for gray-level picture thresholding using the entropy of the histogram. Comput. Vis. Graph. Image Process. 1985, 29, 273–285. [Google Scholar] [CrossRef]
- Thouvenot, L.; Deleu, C.; Berardocco, S.; Haury, J.; Thiébaut, G. Characterization of the salt stress vulnerability of three invasive freshwater plant species using a metabolic profiling approach. J. Plant Physiol. 2015, 175, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Gomez, L.; Bancel, D.; Rubio, E.; Vercambre, G. The microplate reader: An efficient tool for the separate enzymatic analysis of sugars in plant tis-sues-validation of a micro-method. J. Sci. Food Agric. 2007, 87, 1893–1905. [Google Scholar] [CrossRef]
- Husson, O.; Husson, B.; Brunet, A.; Babre, D.; Alary, K.; Sarthou, J.-P.; Charpentier, H.; Durand, M.; Benada, J.; Henry, M. Practical improvements in soil redox potential (Eh) measurement for characterisation of soil properties. Application for comparison of conventional and conservation agriculture cropping systems. Anal. Chim. Acta 2016, 906, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Collewet, G.; Musse, M.; El Hajj, C.; Moussaoui, S. Multi-exponential MRI T2 maps: A tool to classify and characterize fruit tissues. Magn. Reson. Imaging 2021, 87, 119–132. [Google Scholar] [CrossRef]

| DASE | 32 | 46 | 60 | |||
|---|---|---|---|---|---|---|
| Control | ||||||
| Variables | Cortex | Flesh | Cortex | Flesh | Cortex | Flesh |
| pH | a | b | a | a | a | a |
| Eh (pH7) | a | a | a | a | a | a |
| WC | a | a | a | a | a | a |
| Starch | a | a | a | a | a | a |
| Glucose | a | a | a | a | a | a |
| Fructose | a | a | a | a | a | a |
| Sucrose | a | b | a | a | a | a |
| S/(G + F) | a | a | a | b | a | a |
| Myo-inositol | - | - | - | - | a | a |
| Organic acids | a | b | a | a | a | b |
| SWD | ||||||
| Variables | Cortex | Flesh | Cortex | Flesh | Cortex | Flesh |
| pH | a | a | a | a | a | a |
| Eh (pH7) | a | a | a | a | a | a |
| WC | a | a | a | a | a | a |
| Starch | a | a | a | a | a | b |
| Glucose | a | a | a | a | a | a |
| Fructose | a | a | a | a | a | a |
| Sucrose | a | a | a | b | a | a |
| S/(G + F) | a | a | a | a | a | a |
| Myo-inositol | a | a | a | a | a | a |
| Organic acids | a | b | a | b | a | b |
| DASE | 32 | 46 | 60 | |||
|---|---|---|---|---|---|---|
| Cortex | ||||||
| Variables | Control | SWD | Control | SWD | Control | SWD |
| pH | a | b | a | b | a | a |
| Eh (pH7) | a | a | a | a | a | a |
| WC | a | a | a | b | a | a |
| Starch | a | a | a | a | a | a |
| Glucose | a | a | a | b | a | b |
| Fructose | a | a | a | a | a | a |
| Sucrose | a | a | a | a | a | a |
| S/(G + F) | a | a | a | b | a | b |
| Myo-inositol | - | - | - | - | a | a |
| Organic acids | a | b | a | b | a | b |
| Flesh | ||||||
| Variables | Control | SWD | Control | SWD | Control | SWD |
| pH | a | a | a | b | a | b |
| Eh (pH7) | a | a | a | a | a | a |
| WC | a | a | a | b | a | a |
| Starch | a | a | a | a | a | a |
| Glucose | a | a | a | a | a | b |
| Fructose | a | a | - | - | - | - |
| Sucrose | a | a | a | a | a | a |
| S/(G + F) | a | a | a | a | a | b |
| Myo-inositol | a | b | - | - | a | a |
| Organic acids | a | a | a | a | a | a |
| DASE | Control | SWD | ||||
|---|---|---|---|---|---|---|
| Cortex | ||||||
| Variables | 32 | 46 | 60 | 32 | 46 | 60 |
| pH | a | a | b | a | a | b |
| Eh (pH7) | a | ab | b | a | b | c |
| WC | a | a | a | a | a | a |
| Starch | a | ab | b | a | b | b |
| Glucose | a | b | b | a | ab | b |
| Fructose | a | ab | b | a | a | a |
| Sucrose | a | a | a | a | a | a |
| S/(G + F) | a | a | b | a | a | b |
| Myo-inositol | - | - | - | a | a | a |
| Organic acids | a | b | b | a | b | b |
| Flesh | ||||||
| Variables | 32 | 46 | 60 | 32 | 46 | 60 |
| pH | a | a | b | a | a | b |
| Eh (pH7) | a | b | c | a | a | b |
| WC | a | a | a | a | a | a |
| Starch | a | b | b | a | b | b |
| Glucose | a | b | b | a | ab | b |
| Fructose | - | - | - | - | - | - |
| Sucrose | a | b | b | a | ab | b |
| S/(G + F) | a | b | b | a | a | b |
| Myo-inositol | a | - | b | a | a | a |
| Organic acids | a | b | b | a | b | b |
| DASE | 32 | 39 | 46 | 53 | 60 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | |||||||||||||||
| Variables | Pith (P) | Cortex (C) | Flesh (F) | P | C | F | P | C | F | P | C | F | P | C | F |
| I0 (comp 2) | a | b | c | a | b | c | a | b | c | a | b | b | a | b | b |
| T2 (comp 1) | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a |
| T2 (comp 2) | a | b | b | a | b | b | a | b | b | a | b | b | a | a | a |
| SWD | |||||||||||||||
| Variables | Pith (P) | Cortex (C) | Flesh (F) | P | C | F | P | C | F | P | C | F | P | C | F |
| I0 (comp 2) | a | a | a | a | ab | b | a | b | c | a | b | b | a | ab | b |
| T2 (comp 1) | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a |
| T2 (comp 2) | a | b | b | a | b | b | a | b | b | a | b | b | a | a | a |
| DASE | 32 | 39 | 46 | 53 | 60 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pith | ||||||||||
| Variables | Control | SWD | Control | SWD | Control | SWD | Control | SWD | Control | SWD |
| I0 (comp 2) | a | a | a | a | a | a | a | a | a | a |
| T2 (comp 1) | a | a | a | a | a | a | a | a | a | a |
| T2 (comp 2) | a | b | a | b | a | b | a | b | a | a |
| Cortex | ||||||||||
| Variables | Control | SWD | Control | SWD | Control | SWD | Control | SWD | Control | SWD |
| I0 (comp 2) | a | a | a | a | a | a | a | a | a | a |
| T2 (comp 1) | a | a | a | b | a | b | a | a | a | a |
| T2 (comp 2) | a | a | a | b | a | b | a | b | a | a |
| Flesh | ||||||||||
| Variables | Control | SWD | Control | SWD | Control | SWD | Control | SWD | Control | SWD |
| I0 (comp 2) | a | a | a | a | a | a | a | a | a | a |
| T2 (comp 1) | a | a | a | a | a | a | a | a | a | a |
| T2 (comp 2) | a | a | a | b | a | b | a | b | a | a |
| DASE | Control | SWD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pith | ||||||||||
| Variables | 32 | 39 | 46 | 53 | 60 | 32 | 39 | 46 | 53 | 60 |
| I0 (comp 2) | a | a | a | a | a | a | ab | bc | abc | c |
| T2 (comp 1) | a | a | a | a | a | a | a | a | a | a |
| T2 (comp 2) | a | a | a | a | a | a | a | a | a | a |
| Cortex | ||||||||||
| Variables | 32 | 39 | 46 | 53 | 60 | 32 | 39 | 46 | 53 | 60 |
| I0 (comp 2) | a | ab | ab | bc | c | a | ab | ab | ab | b |
| T2 (comp 1) | a | a | a | a | a | a | a | a | a | a |
| T2 (comp 2) | a | a | a | a | a | a | a | a | a | a |
| Flesh | ||||||||||
| Variables | 32 | 39 | 46 | 53 | 60 | 32 | 39 | 46 | 53 | 60 |
| I0 (comp 2) | a | a | a | a | a | a | a | a | a | a |
| T2 (comp 1) | a | a | a | a | a | a | a | a | a | a |
| T2 (comp 2) | a | ab | ab | ab | b | a | a | a | a | a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajjar, G.; Quellec, S.; Challois, S.; Bousset-Vaslin, L.; Joly, G.; Langrume, C.; Deleu, C.; Leport, L.; Musse, M. Characterization of the Water Shortage Effects on Potato Tuber Tissues during Growth Using MRI Relaxometry and Biochemical Parameters. Plants 2022, 11, 1918. https://doi.org/10.3390/plants11151918
Hajjar G, Quellec S, Challois S, Bousset-Vaslin L, Joly G, Langrume C, Deleu C, Leport L, Musse M. Characterization of the Water Shortage Effects on Potato Tuber Tissues during Growth Using MRI Relaxometry and Biochemical Parameters. Plants. 2022; 11(15):1918. https://doi.org/10.3390/plants11151918
Chicago/Turabian StyleHajjar, Ghina, Stéphane Quellec, Sylvain Challois, Lydia Bousset-Vaslin, Gisèle Joly, Christophe Langrume, Carole Deleu, Laurent Leport, and Maja Musse. 2022. "Characterization of the Water Shortage Effects on Potato Tuber Tissues during Growth Using MRI Relaxometry and Biochemical Parameters" Plants 11, no. 15: 1918. https://doi.org/10.3390/plants11151918
APA StyleHajjar, G., Quellec, S., Challois, S., Bousset-Vaslin, L., Joly, G., Langrume, C., Deleu, C., Leport, L., & Musse, M. (2022). Characterization of the Water Shortage Effects on Potato Tuber Tissues during Growth Using MRI Relaxometry and Biochemical Parameters. Plants, 11(15), 1918. https://doi.org/10.3390/plants11151918






