Natural Products in Cardiovascular Diseases: The Potential of Plants from the Allioideae Subfamily (Ex-Alliaceae Family) and Their Sulphur-Containing Compounds
Abstract
:1. Introduction
2. Importance of Allioideae Species in Cardiovascular Diseases
2.1. Traditional Uses of Allioideae
2.2. Pre-Clinical Studies Validating the Cardioprotective Effects of Allioideae
2.2.1. The Effect of Plant Parts or Extracts
2.2.2. The Effect of Isolated Sulphur-Containing Compounds
| Compound | Study Model: Insult or Injury (Route of Administration; Concentration) | Main Findings | Ref. |
|---|---|---|---|
Ajoene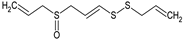 | Smooth muscle cells (1–50 μM) | ↓ Proliferation, cholesterol biosynthesis | [136] |
Allicin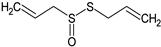 | Mice: ApoE-deficient and LDLR-deficient (p.o.; 9 mg/kg) | ↓ Atherosclerotic plaque, uptake and degradation of oxLDL by macrophages | [137] |
| HUVEC: oxLDL-induced damage (10, 30, 100 μM) | ↓ Apoptosis | [138] | |
| In chemico: Cu2+-induced oxidation of LDL from treated ApoE/LDLR-deficient mice (p.o.; 9 mg/kg) | ↓ LDL oxidation | [137] | |
| In chemico: Cu2+-induced LDL oxidation (0.1, 1 and 10 mM) | ↑ LDL oxidation (at higher doses) | [60] | |
| Phe-contracted PA rings (0.1, 0.3 and 1.0 µg/mL) | Induced relaxation | [95] | |
| Rat: SHR (p.o. for 6 weeks; 80 mg/kg on chow) | ↓ SBP and TG | [139] | |
Alliin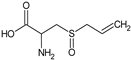 | Rat: High fructose (p.o. for 3 weeks; 0.111 and 0.222 mg/kg) | ↑ Heart function; ↓ SBP | [88] |
| Rat: ISO-induced myocardial infarction (gastric intubation for 35 days; 40 and 80 mg/kg) | ↓ CK, CK-MB, LDH, ALT, AST, TC, LDL, VLDL, TG, FFA, PL, MDA levels, HMGR activity; ↑ HDL levels, LCAT activity | [140] | |
Diallyl disulphide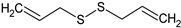 | HEPC: In vitro neovasculogenesis (0.1, 1, and 10 μM) | ↑ Tube formation, c-kit/PI3K/Akt pathway | [141] |
| Rat: Diabetic cardiomyopathy (gavage every other day for 16 days; 40 mg/kg) | ↓ Cardiac apoptosis and apoptotic markers dependent of death receptor and mitochondria; ↑ PI3K/Akt pathway | [68] | |
| HUVEC: Ox-LDL-induced damage (100 and 200 µM) | ↑ eNOS phosphorylation at Ser1177, NO and cGMP levels; stabilised eNOS/Cav-1 interaction; ↓ eNOS degradation, proteosome activity | [142] | |
| HUVEC: Non-stimulated and stimulated (0.2 to 500 µM) | Non-stimulated: ↓ MMP-2 secretion and activity and TIMP-1 secretion Stimulated: ↓ MMP-9 and TIMP-1 secretion | [143] | |
| In chemico: Isolated xanthine-oxidase activity (5 and 10 µM) | Restored activity in the presence of Cu2+ | [144] | |
| In chemico: Cu2+ and amphotericin-induced LDL oxidation (5 and 10 µM) | ↓ MDA | ||
| Rat: ISO-induced myocardial necrosis (p.o. for 14 days; 8.94 mg/kg) | ↓ HW, LDH, CK-MB, cTnC and systemic inflammation; ↑ SOD and cat | [48] | |
Diallyl trisulphide | Rat: Diabetic cardiomyopathy (gavage every other day for 16 days; 40 mg/kg) | ↓ Cardiac apoptosis | [68] |
| HUVEC: Ox-LDL-induced damage (20 and 50 µM) | ↑ eNOS phosphorylation at Ser1177, NO and cGMP levels; stabilised eNOS/Cav-1 interaction; ↓ eNOS degradation, proteosome activity | [142] | |
| Rat: metabolic syndrome (gavage every second day for 3 weeks; 40 mg/kg) | ↓ TG, LDL, homocysteine, BG, insulin, MDA, O22+, NF-κB, IL-17A, Bax, caspase-3 and -9 mRNA; ↑ HDL, H2S, NO2−, cat, GSH, SOD, cardiac function, eNOS, SOD1/2 and Bcl-2 mRNA | [145] | |
| HEK293 cells: Whole cell patch clamp (n/a) | ↓ IKr and hERG channel trafficking | [146] | |
| Cardiomyocytes: HG-induced apoptosis (10 μM) | ↓ Apoptosis | [147,148] | |
| Rat: STZ-induced diabetic (i.p. for 14 days; 500 μg/kg) | ↑ NO, eNOS proteins and phosphorylation levels, blood perfusion and capillary density | [149] | |
| HUVEC (1.3, 2.5, 5, and 10 µM) | ↓ Tube formation, VEGF2 release and VEGF2R expression | [150] | |
| HEPC: In vitro neovasculogenesis (0.1, 1, and 10 μM) | In vitro: ↑ tube formation | [141] | |
| Rat: In vivo neovasculogenesis (gavage for 2 weeks; 10 mg/kg) | In vivo: ↑ new vessels in a xenograft model of neovasculogenesis | ||
Dimethyl disulphide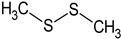 | PA: Phe-induced contractions (cumulative doses from 100 nM to 3 μM) | Induced relaxation; ↑ NOS phosphorylation and Ca2+ influx to ECs | [84] |
S-allylcysteine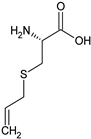 | Rat: Acute myocardial infarction (i.p. for 7 days pre-surgery + 2 days post-surgery; 50 mg/kg) | ↓ Mortality, infarct size; ↑ CTH activity | [151] |
| Cu2+-induced LDL oxidation (0.1, 1 and 10 mM) | ↓ Oxidation | [60,152] | |
| Macrophages and HUVEC: oxLDL stimulated (2.5, 5, 10 and 20 mM) | ↓ H2O2 production | [152] | |
| HUVEC: TNF-α and H2O2 stimulated (2.5, 5, 10 and 20 mM) | ↓ NF-κB activation | ||
| HUVEC and macrophages: LPS- and IFNγ stimulated (20, 40 and 80 µM) | HUVEC: ↑ eNOS activity, cGMP levels Macrophages: ↓ iNOS activity | [127] | |
| Rat: ISO-induced myocardial infarction (p.o. every other day for 3 weeks; 13.1 mg/kg and 32.76 mg/kg) | ↓ LDH, CK-MB; ↑ heart function; SOD and Cat | [42,43,153] |
2.2.3. Clinical Trials
3. Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Townsend, N.; Wilson, L.; Bhatnagar, P.; Wickramasinghe, K.; Rayner, M.; Nichols, M. Cardiovascular Disease in Europe: Epidemiological Update 2016. Eur. Heart J. 2016, 37, 3232–3245. [Google Scholar] [CrossRef] [PubMed]
- WHO Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 15 March 2022).
- North, B.J.; Sinclair, D.A. The Intersection between Aging and Cardiovascular Disease. Circ. Res. 2012, 110, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Buttar, H.S.; Li, T.; Ravi, N. Prevention of Cardiovascular Diseases: Role of Exercise, Dietary Interventions, Obesity and Smoking Cessation. Exp. Clin. Cardiol. 2005, 10, 229–249. [Google Scholar]
- WHO Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases/ (accessed on 20 March 2022).
- Mackay, J.; Mensah, G.A. Risk Factors in the Atlas of Heart Disease and Stroke; World Health Organization: Geneva, Switzerland, 2002; pp. 24–25. [Google Scholar]
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur. Heart J. 2020, 41, 12–85. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of Potentially Modifiable Risk Factors Associated with Myocardial Infarction in 52 Countries (the INTERHEART Study): Case-Control Study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- World Health Organization. Noncommunicable Diseases: Campaign for Action—Meeting the NCD Targets; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Baroletti, S.; Dell’Orfano, H. Medication Adherence in Cardiovascular Disease. Circulation 2010, 121, 1455–1458. [Google Scholar] [CrossRef]
- Jahromi, B.; Pirvulescu, I.; Candido, K.D.; Knezevic, N.N. Herbal Medicine for Pain Management: Efficacy and Drug Interactions. Pharmaceutics 2021, 13, 251. [Google Scholar] [CrossRef]
- Dhara, A.K.; Nayak, A.K. Introduction to Herbal Biomolecules in Herbal Biomolecules in Healthcare Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–19. [Google Scholar]
- Alves-Silva, J.M.; Zuzarte, M.; Marques, C.; Viana, S.; Preguiça, I.; Baptista, R.; Ferreira, C.; Cavaleiro, C.; Domingues, N.; Sardão, V.A.; et al. 1, 8-Cineole Ameliorates Right Ventricle Dysfunction Associated with Pulmonary Arterial Hypertension by Restoring Connexin 43 and Mitochondrial Homeostasis. Pharmacol. Res. 2022, 180, 106151. [Google Scholar] [CrossRef]
- Alves-Silva, J.M.; Zuzarte, M.; Girão, H.; Salgueiro, L. The Role of Essential Oils and Their Main Compounds in the Management of Cardiovascular Disease Risk Factors. Molecules 2021, 26, 3506. [Google Scholar] [CrossRef]
- Alves-Silva, J.M.; Monica, Z.; Carla, M.; Lígia, S.; Henrique, G. Protective Effects of Terpenes on the Cardiovascular System: Current Advances and Future Perspectives. Curr. Med. Chem. 2016, 23, 4559–4600. [Google Scholar] [CrossRef]
- Wachtel-Galor, S.; Benzie, I.F.F. (Eds.) Herbal Medicine: An Introduction to Its History, Usage, Regulation, Current Trends, and Research Needs. In Herbal Medicine: Biomolecular and Clinical Aspects; CRC Press: Boca Raton, FL, USA, 2011; pp. 1–10. ISBN 9781439807132. [Google Scholar]
- Tuso, P.; Stoll, S.R.; Li, W.W. A Plant-Based Diet, Atherogenesis, and Coronary Artery Disease Prevention. Perm. J. 2015, 19, 62–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Dontas, A.S.; Zerefos, N.S.; Panagiotakos, D.B.; Vlachou, C.; Valis, D.A. Mediterranean Diet and Prevention of Coronary Heart Disease in the Elderly. Clin. Interv. Aging 2007, 2, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean Diet, Its Components, and Cardiovascular Disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Temple, N.J.; Guercio, V.; Tavani, A. The Mediterranean Diet and Cardiovascular Disease: Gaps in the Evidence and Research Challenges. Cardiol. Rev. 2019, 27, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Karageorgou, D.; Micha, R.; Zampelas, A. Chapter 9—Mediterranean Diet and Cardiovascular Disease: An Overview of Recent Evidence. In The Mediterranean Diet; Preedy, V.R., Watson, R.R., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 91–104. ISBN 978-0-12-407849-9. [Google Scholar]
- Becerra-Tomás, N.; Mejía, S.B.; Viguiliouk, E.; Khan, T.; Kendall, C.W.C.; Kahleova, H.; Rahelić, D.; Sievenpiper, J.L.; Salas-Salvadó, J. Mediterranean Diet, Cardiovascular Disease and Mortality in Diabetes: A Systematic Review and Meta-Analysis of Prospective Cohort Studies and Randomized Clinical Trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 1207–1227. [Google Scholar] [CrossRef] [PubMed]
- D’Innocenzo, S.; Biagi, C.; Lanari, M. Obesity and the Mediterranean Diet: A Review of Evidence of the Role and Sustainability of the Mediterranean Diet. Nutrients 2019, 11, 1306. [Google Scholar] [CrossRef] [Green Version]
- Jenke-Kodama, H.; Müller, R.; Dittmann, E. Evolutionary Mechanisms Underlying Secondary Metabolite Diversity. Prog. Drug Res. 2008, 65, 121–140. [Google Scholar] [CrossRef]
- Hartmann, T. From Waste Products to Ecochemicals: Fifty Years Research of Plant Secondary Metabolism. Phytochemistry 2007, 68, 2831–2846. [Google Scholar] [CrossRef]
- Bastaki, S.M.A.; Ojha, S.; Kalasz, H.; Adeghate, E. Chemical Constituents and Medicinal Properties of Allium Species. Mol. Cell. Biochem. 2021, 476, 4301–4321. [Google Scholar] [CrossRef]
- Jarić, S.; Mačukanović-Jocić, M.; Djurdjević, L.; Mitrović, M.; Kostić, O.; Karadžić, B.; Pavlović, P. An Ethnobotanical Survey of Traditionally Used Plants on Suva Planina Mountain (South-Eastern Serbia). J. Ethnopharmacol. 2015, 175, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Belda, A.; Zaragozí, B.; Martínez, I.; Seva, E. Traditional Knowledge of Medicinal Plants in the Serra de Mariola Natural Park, South-Eastern Spain. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 299–309. [Google Scholar] [CrossRef] [Green Version]
- Mrabti, H.N.; Jaradat, N.; Kachmar, M.R.; Ed-Dra, A.; Ouahbi, A.; Cherrah, Y.; Faouzi, M.E.A. Integrative Herbal Treatments of Diabetes in Beni Mellal Region of Morocco. J. Integr. Med. 2019, 17, 93–99. [Google Scholar] [CrossRef]
- Bading Taika, B.; Bouckandou, M.; Souza, A.; Bourobou Bourobou, H.P.; MacKenzie, L.S.; Lione, L. An Overview of Anti-Diabetic Plants Used in Gabon: Pharmacology and Toxicology. J. Ethnopharmacol. 2018, 216, 203–228. [Google Scholar] [CrossRef] [Green Version]
- Gbolade, A. Ethnobotanical Study of Plants Used in Treating Hypertension in Edo State of Nigeria. J. Ethnopharmacol. 2012, 144, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Esakkimuthu, S.; Mutheeswaran, S.; Arvinth, S.; Paulraj, M.G.; Pandikumar, P.; Ignacimuthu, S. Quantitative Ethnomedicinal Survey of Medicinal Plants given for Cardiometabolic Diseases by the Non-Institutionally Trained Siddha Practitioners of Tiruvallur District, Tamil Nadu, India. J. Ethnopharmacol. 2016, 186, 329–342. [Google Scholar] [CrossRef]
- Ahmad, L.; Semotiuk, A.; Zafar, M.; Ahmad, M.; Sultana, S.; Liu, Q.-R.; Zada, M.P.; Abidin, S.Z.U.; Yaseen, G. Ethnopharmacological Documentation of Medicinal Plants Used for Hypertension among the Local Communities of DIR Lower, Pakistan. J. Ethnopharmacol. 2015, 175, 138–146. [Google Scholar] [CrossRef]
- Özdemir, E.; Alpınar, K. An Ethnobotanical Survey of Medicinal Plants in Western Part of Central Taurus Mountains: Aladaglar (Nigde-Turkey). J. Ethnopharmacol. 2015, 166, 53–65. [Google Scholar] [CrossRef]
- Odeyemi, S.; Bradley, G. Medicinal Plants Used for the Traditional Management of Diabetes in the Eastern Cape, South Africa: Pharmacology and Toxicology. Molecules 2018, 23, 2759. [Google Scholar] [CrossRef] [Green Version]
- Karou, S.D.; Tchacondo, T.; Djikpo Tchibozo, M.A.; Abdoul-Rahaman, S.; Anani, K.; Koudouvo, K.; Batawila, K.; Agbonon, A.; Simpore, J.; Souza, C.d. Ethnobotanical Study of Medicinal Plants Used in the Management of Diabetes Mellitus and Hypertension in the Central Region of Togo. Pharm. Biol. 2011, 49, 1286–1297. [Google Scholar] [CrossRef]
- Barkaoui, M.; Katiri, A.; Boubaker, H.; Msanda, F. Ethnobotanical Survey of Medicinal Plants Used in the Traditional Treatment of Diabetes in Chtouka Ait Baha and Tiznit (Western Anti-Atlas), Morocco. J. Ethnopharmacol. 2017, 198, 338–350. [Google Scholar] [CrossRef]
- Hyun, S.-W.; Jang, M.; Park, S.W.; Kim, E.J.; Jung, Y.-S. Onion (Allium cepa) Extract Attenuates Brain Edema. Nutrition 2013, 29, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, M.-Y.; Lee, D.H.; Lee, S.H.; Baik, E.J.; Moon, C.-H.; Park, S.W.; Ko, E.Y.; Oh, S.-R.; Jung, Y.-S. Methanolic Extract of Onion (Allium cepa) Attenuates Ischemia/Hypoxia-Induced Apoptosis in Cardiomyocytes via Antioxidant Effect. Eur. J. Nutr. 2009, 48, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Peng, Y.; Li, J.; Shen, H.; Shen, M.; Fang, T. Gualou Xiebai Decoction Prevents Myocardial Fibrosis by Blocking TGF-Beta/Smad Signalling. J. Pharm. Pharmacol. 2013, 65, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Asad, M.; Avula, P.; Asdaq, S. Effect of Aged Garlic Extract and S-Allyl Cysteine and Their Interaction with Atenolol during Isoproterenol Induced Myocardial Toxicity in Rats. Indian J. Pharmacol. 2014, 46, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asdaq, S.M.B.; Challa, O.; Alamri, A.S.; Alsanie, W.F.; Alhomrani, M.; Almutiri, A.H.; Alshammari, M.S. Cytoprotective Potential of Aged Garlic Extract (AGE) and Its Active Constituent, S-Allyl-l-Cysteine, in Presence of Carvedilol during Isoproterenol-Induced Myocardial Disturbance and Metabolic Derangements in Rats. Molecules 2021, 26, 3203. [Google Scholar] [CrossRef]
- Asdaq, S.M.B.; Inamdar, M.N. Pharmacodynamic Interaction of Captopril with Garlic in Isoproterenol-Induced Myocardial Damage in Rat. Phytother. Res. 2010, 24, 720–725. [Google Scholar] [CrossRef]
- Banerjee, S.K.; Sood, S.; Dinda, A.K.; Das, T.K.; Maulik, S.K. Chronic Oral Administration of Raw Garlic Protects against Isoproterenol-Induced Myocardial Necrosis in Rat. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 136, 377–386. [Google Scholar] [CrossRef]
- Banerjee, S.K.; Dinda, A.K.; Manchanda, S.C.; Maulik, S.K. Chronic Garlic Administration Protects Rat Heart against Oxidative Stress Induced by Ischemic Reperfusion Injury. BMC Pharm. 2002, 2, 16. [Google Scholar] [CrossRef] [Green Version]
- Czompa, A.; Szoke, K.; Prokisch, J.; Gyongyosi, A.; Bak, I.; Balla, G.; Tosaki, A.; Lekli, I. Aged (Black) versus Raw Garlic against Ischemia/Reperfusion-Induced Cardiac Complications. Int. J. Mol. Sci. 2018, 19, 1017. [Google Scholar] [CrossRef] [Green Version]
- Asdaq, S.M.B.; Alamri, A.S.; Alsanie, W.F.; Alhomrani, M. Cardioprotective Potential of Garlic Oil and Its Active Constituent, Diallyl Disulphide, in Presence of Carvedilol during Chronic Isoprenaline Injection-Mediated Myocardial Necrosis in Rats. Molecules 2021, 26, 5137. [Google Scholar] [CrossRef] [PubMed]
- Rankovic, M.; Krivokapic, M.; Bradic, J.; Petkovic, A.; Zivkovic, V.; Sretenovic, J.; Jeremic, N.; Bolevich, S.; Kartashova, M.; Jeremic, J.; et al. New Insight Into the Cardioprotective Effects of Allium Ursinum L. Extract Against Myocardial Ischemia-Reperfusion Injury. Front. Physiol. 2021, 12, 690696. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, S.; Pandiyan, A.; Saravanakumar, L.; Moodley, K.; Mackraj, I. Protective Role of Wild Garlic on Isoproterenol-Induced Myocardial Necrosis in Wistar Rats. J. Ethnopharmacol. 2019, 237, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, C.; Mei, X.; Huang, R.; Zhang, S.; Tang, Y.; Dong, Q.; Zhou, C. Effect of the Polyphenol-rich Extract from Allium Cepa on Hyperlipidemic Sprague-dawley Rats. J. Food Biochem. 2021, 45, e13565. [Google Scholar] [CrossRef]
- Ostrowska, E.; Gabler, N.K.; Sterling, S.J.; Tatham, B.G.; Jones, R.B.; Eagling, D.R.; Jois, M.; Dunshea, F.R. Consumption of Brown Onions (Allium Cepa Var. Cavalier and Var. Destiny) Moderately Modulates Blood Lipids, Haematological and Haemostatic Variables in Healthy Pigs. Br. J. Nutr. 2004, 91, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Safaeian, L.; Zolfaghari, B.; Karimi, S.; Talebi, A.; Ghazvini, M. The Effects of Hydroalcoholic Extract of Allium Elburzense Wendelbo Bulb on Dexamethasone-Induced Dyslipidemia, Hyperglycemia, and Oxidative Stress in Rats. Res. Pharm. Sci. 2018, 13, 22. [Google Scholar] [CrossRef]
- Janahmadi, Z.; Nekooeian, A.A.; Mozafari, M. Hydroalcoholic Extract of Allium Eriophyllum Leaves Attenuates Cardiac Impairment in Rats with Simultaneous Type 2 Diabetes and Renal Hypertension. Res. Pharm. Sci. 2015, 10, 125–133. [Google Scholar]
- Khaleghi, S.K. Ethyl Acetate Fraction of Allium Hirtifolium Improves Functional Parameters of Isolated Hearts of Diabetic Rats. Anatol. J. Cardiol. 2017, 17, 452. [Google Scholar] [CrossRef]
- Lee, J. The Hypolipidemic Effect of Allium Hookeri in Rats Fed with a High Fat Diet. Korean J. Community Living Sci. 2016, 27, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Jang, G.-J.; Sung, M.J.; Hur, H.J.; Yoo, M.; Choi, J.H.; Hwang, I.K.; Lee, S. Metabolomics Analysis of the Lipid-Regulating Effect of Allium Hookeri in a Hamster Model of High-Fat Diet-Induced Hyperlipidemia by UPLC/ESI-Q-TOF Mass Spectrometry. Evid.-Based Complement. Altern. Med. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; No, K.; Lee, J. Anti-Obesity Effect of Allium hookeri Leaf Extract in High-Fat Diet-Fed Mice. J. Med. Food 2018, 21, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Torres-Narváez, J.; Pedraza-Chaverri, J.; Rubio-Ruiz, M.; Díaz-Díaz, E.; Valle-Mondragón, L.d.; Martínez-Memije, R.; López, E.V.; Guarner-Lans, V. Effect of the Aged Garlic Extract on Cardiovascular Function in Metabolic Syndrome Rats. Molecules 2016, 21, 1425. [Google Scholar] [CrossRef]
- Lau, B.H.S. Suppression of LDL Oxidation by Garlic Compounds Is a Possible Mechanism of Cardiovascular Health Benefit. J. Nutr. 2006, 136, 765S–768S. [Google Scholar] [CrossRef] [Green Version]
- Morihara, N.; Hino, A.; Yamaguchi, T.; Suzuki, J. Aged Garlic Extract Suppresses the Development of Atherosclerosis in Apolipoprotein E–Knockout Mice. J. Nutr. 2016, 146, 460S–463S. [Google Scholar] [CrossRef] [PubMed]
- El-Sayyad, H.I.; Abou-El-Naga, A.M.; Gadallah, A.A.; Bakr, I.H. Protective Effects of Allium Sativum against Defects of Hypercholesterolemia on Pregnant Rats and Their Offspring. Int. J. Clin. Exp. Med. 2010, 3, 152–163. [Google Scholar] [PubMed]
- Sohn, C.W.; Kim, H.; You, B.R.; Kim, M.J.; Kim, H.J.; Lee, J.Y.; Sok, D.-E.; Kim, J.H.; Lee, K.J.; Kim, M.R. High Temperature- and High Pressure-Processed Garlic Improves Lipid Profiles in Rats Fed High Cholesterol Diets. J. Med. Food 2012, 15, 435–440. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Mageid, A.D.; Abou-Salem, M.E.S.; Salaam, N.M.H.A.; El-Garhy, H.A.S. The Potential Effect of Garlic Extract and Curcumin Nanoparticles against Complication Accompanied with Experimentally Induced Diabetes in Rats. Phytomedicine 2018, 43, 126–134. [Google Scholar] [CrossRef]
- Sultana, M.R.; Bagul, P.K.; Katare, P.B.; Anwar Mohammed, S.; Padiya, R.; Banerjee, S.K. Garlic Activates SIRT-3 to Prevent Cardiac Oxidative Stress and Mitochondrial Dysfunction in Diabetes. Life Sci. 2016, 164, 42–51. [Google Scholar] [CrossRef]
- Amor, S.; González-Hedström, D.; Martín-Carro, B.; Inarejos-García, A.; Almodóvar, P.; Prodanov, M.; García-Villalón, A.; Granado, M. Beneficial Effects of an Aged Black Garlic Extract in the Metabolic and Vascular Alterations Induced by a High Fat/Sucrose Diet in Male Rats. Nutrients 2019, 11, 153. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.-C.; Chang, M.-H.; Tsai, C.-C.; Chen, T.-S.; Fan, C.-C.; Lin, C.-C.; Lai, C.-H.; Tsai, F.-J.; Lin, J.A.; Huang, C.-Y. Garlic Oil Attenuates the Cardiac Apoptosis in Hamster-Fed with Hypercholesterol Diet. Food Chem. 2013, 136, 1296–1302. [Google Scholar] [CrossRef]
- Huang, Y.-T.; Yao, C.-H.; Way, C.-L.; Lee, K.-W.; Tsai, C.-Y.; Ou, H.-C.; Kuo, W.-W. Diallyl Trisulfide and Diallyl Disulfide Ameliorate Cardiac Dysfunction by Suppressing Apoptotic and Enhancing Survival Pathways in Experimental Diabetic Rats. J. Appl. Physiol. 2013, 114, 402–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aziz, H.A.; Tan, Y.T.F.; Peh, K.K.; Yam, M.F. Direct Effect of Khat and Garlic Extracts on Blood Lipids Contents: Preliminary in Vitro Study. Obes. Res. Clin. Pract. 2010, 4, e247–e252. [Google Scholar] [CrossRef]
- Baluchnejadmojarad, T.; Roghani, M. Endothelium-Dependent and -Independent Effect of Aqueous Extract of Garlic on Vascular Reactivity on Diabetic Rats. Fitoterapia 2003, 74, 630–637. [Google Scholar] [CrossRef]
- Baluchnejadmojarad, T.; Roghani, M.; Homayounfar, H.; Hosseini, M. Beneficial Effect of Aqueous Garlic Extract on the Vascular Reactivity of Streptozotocin-Diabetic Rats. J. Ethnopharmacol. 2003, 85, 139–144. [Google Scholar] [CrossRef]
- Baluchnejadmojarad, T.; Roghani, M. Garlic Extract Attenuates Time-Dependent Changes in the Reactivity of Isolated Aorta in Streptozotocin-Diabetic Rats. Life Sci. 2003, 73, 2281–2289. [Google Scholar] [CrossRef]
- Patumraj, S.; Tewit, S.; Amatyakul, S.; Jariyapongskul, A.; Maneesri, S.; Kasantikul, V.; Shepro, D. Comparative Effects of Garlic and Aspirin on Diabetic Cardiovascular Complications. Drug Deliv. 2000, 7, 91–96. [Google Scholar] [CrossRef]
- Supakul, L.; Pintana, H.; Apaijai, N.; Chattipakorn, S.; Shinlapawittayatorn, K.; Chattipakorn, N. Protective Effects of Garlic Extract on Cardiac Function, Heart Rate Variability, and Cardiac Mitochondria in Obese Insulin-Resistant Rats. Eur. J. Nutr. 2014, 53, 919–928. [Google Scholar] [CrossRef]
- Lee, S.; Joo, H.; Kim, C.-T.; Kim, I.-H.; Kim, Y. High Hydrostatic Pressure Extract of Garlic Increases the HDL Cholesterol Level via Up-Regulation of Apolipoprotein A-I Gene Expression in Rats Fed a High-Fat Diet. Lipids Health Dis. 2012, 11, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Madkor, H.R.; Mansour, S.W.; Ramadan, G. Modulatory Effects of Garlic, Ginger, Turmeric and Their Mixture on Hyperglycaemia, Dyslipidaemia and Oxidative Stress in Streptozotocin–Nicotinamide Diabetic Rats. Br. J. Nutr. 2011, 105, 1210–1217. [Google Scholar] [CrossRef] [Green Version]
- Sobenin, I.A.; Andrianova, I.V.; Lakunin, K.Y.; Karagodin, V.P.; Bobryshev, Y.V.; Orekhov, A.N. Anti-Atherosclerotic Effects of Garlic Preparation in Freeze Injury Model of Atherosclerosis in Cholesterol-Fed Rabbits. Phytomedicine 2016, 23, 1235–1239. [Google Scholar] [CrossRef]
- Mukthamba, P.; Srinivasan, K. Protective Effect of Dietary Fenugreek (Trigonella foenum-graecum) Seeds and Garlic (Allium sativum) on Induced Oxidation of Low-Density Lipoprotein in Rats. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Bombicz, M.; Priksz, D.; Varga, B.; Gesztelyi, R.; Kertesz, A.; Lengyel, P.; Balogh, P.; Csupor, D.; Hohmann, J.; Bhattoa, H.; et al. Anti-Atherogenic Properties of Allium Ursinum Liophylisate: Impact on Lipoprotein Homeostasis and Cardiac Biomarkers in Hypercholesterolemic Rabbits. Int. J. Mol. Sci. 2016, 17, 1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olorunnisola, O.S.; Bradley, G.; Afolayan, A.J. Protective Effect of Tulbaghia Violacea Harv. on Aortic Pathology, Tissue Antioxidant Enzymes and Liver Damage in Diet-Induced Atherosclerotic Rats. Int. J. Mol. Sci. 2012, 13, 12747–12760. [Google Scholar] [CrossRef] [Green Version]
- Kawamoto, E.; Sakai, Y.; Okamura, Y.; Yamamoto, Y. Effects of Boiling on the Antihypertensive and Antioxidant Activities of Onion. J. Nutr. Sci. Vitaminol. 2004, 50, 171–176. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-H.; Tsai, S.-J.; Chen, H. Welsh Onion (Allium fistulosum L.) Extracts Alter Vascular Responses in Rat Aortae. J. Cardiovasc. Pharmacol. 1999, 33, 515–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, Y.; Aoyama, S.; Hamaguchi, N.; Rhi, G.-S. Antioxidative and Antihypertensive Effects of Welsh Onion on Rats Fed with a High-Fat High-Sucrose Diet. Biosci. Biotechnol. Biochem. 2005, 69, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Qi, J.; Gao, S.; Li, C.; Ma, Y.; Wang, J.; Bai, Y.; Zheng, X. Vasodilation Effect of Volatile Oil from Allium Macrostemon Bunge Are Mediated by PKA/NO Pathway and Its Constituent Dimethyl Disulfide in Isolated Rat Pulmonary Arterials. Fitoterapia 2017, 120, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Noda, A.; Miyata, S.; Minoshima, M.; Sugiura, M.; Kojima, J.; Otake, M.; Furukawa, M.; Cheng, X.W.; Nagata, K.; et al. Effects of Aged Garlic Extract on Left Ventricular Diastolic Function and Fibrosis in a Rat Hypertension Model. Exp. Anim. 2013, 62, 305–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takashima, M.; Kanamori, Y.; Kodera, Y.; Morihara, N.; Tamura, K. Aged Garlic Extract Exerts Endothelium-Dependent Vasorelaxant Effect on Rat Aorta by Increasing Nitric Oxide Production. Phytomedicine 2017, 24, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Padiya, R.; Chowdhury, D.; Borkar, R.; Srinivas, R.; Pal Bhadra, M.; Banerjee, S.K. Garlic Attenuates Cardiac Oxidative Stress via Activation of PI3K/AKT/Nrf2-Keap1 Pathway in Fructose-Fed Diabetic Rat. PLoS ONE 2014, 9, e94228. [Google Scholar] [CrossRef]
- Asdaq, S.M.; Inamdar, M.N. Potential of Garlic and Its Active Constituent, S-Allyl Cysteine, as Antihypertensive and Cardioprotective in Presence of Captopril. Phytomedicine 2010, 17, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ku, D.D. Allicin in Garlic Protects against Coronary Endothelial Dysfunction and Right Heart Hypertrophy in Pulmonary Hypertensive Rats. Am. J. Physiol.-Heart Circ. Physiol. 2006, 291, H2431–H2438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashraf, M.Z.; Hussain, M.E.; Fahim, M. Endothelium Mediated Vasorelaxant Response of Garlic in Isolated Rat Aorta: Role of Nitric Oxide. J. Ethnopharmacol. 2004, 90, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, A.M.; Darabi, R.; Akbarloo, N. Investigation of Antihypertensive Mechanism of Garlic in 2K1C Hypertensive Rat. J. Ethnopharmacol. 2003, 86, 219–224. [Google Scholar] [CrossRef]
- Ganado, P.; Sanz, M.; Padilla, E.; Tejerina, T. An In Vitro Study of Different Extracts and Fractions of Allium Sativum (Garlic): Vascular Reactivity. J. Pharmacol. Sci. 2004, 94, 434–442. [Google Scholar] [CrossRef] [Green Version]
- Grman, M.; Misak, A.; Cacanyiova, S.; Kristek, F.; Tomaskova, Z.; Bertova, A.; Ondrias, K. The Aqueous Garlic, Onion and Leek Extracts Release Nitric Oxide from S-Nitrosoglutathione and Prolong Relaxation of Aortic Rings. Gen. Physiol. Biophys. 2012, 30, 396–402. [Google Scholar] [CrossRef] [Green Version]
- Kim-Park, S.; Ku, D.D. Garlic Elicits A Nitric Oxide-Dependent Relaxation And Inhibits Hypoxic Pulmonary Vasoconstriction In Rats. Clin. Exp. Pharmacol. Physiol. 2000, 27, 780–786. [Google Scholar] [CrossRef]
- Ku, D.D.; Abdel-Razek, T.T.; Dai, J.; Kim-Park, S.; Fallon, M.B.; Abrams, G.A. Garlic And Its Active Metabolite Allicin Produce Endothelium- And Nitric Oxide-Dependent Relaxation In Rat Pulmonary Arteries. Clin. Exp. Pharmacol. Physiol. 2002, 29, 84–91. [Google Scholar] [CrossRef]
- Radenkovic, M.; Brankovic, S.; Kitic, D.; Veljkovic, S.; Ivetic, V.; Nesić, M.; Miladinovic, B. Inhibitory Effect of Aqueous and Ethanolic Garlic (Allium sativum L., Lilliaceae) Extracts on the Rat Atria. Clin. Exp. Hypertens. 2010, 32, 251–255. [Google Scholar] [CrossRef]
- Bombicz, M.; Priksz, D.; Varga, B.; Kurucz, A.; Kertész, A.; Takacs, A.; Posa, A.; Kiss, R.; Szilvassy, Z.; Juhasz, B. A Novel Therapeutic Approach in the Treatment of Pulmonary Arterial Hypertension: Allium Ursinum Liophylisate Alleviates Symptoms Comparably to Sildenafil. Int. J. Mol. Sci. 2017, 18, 1436. [Google Scholar] [CrossRef] [Green Version]
- Moodley, K.; Mackraj, I.; Naidoo, Y. Cardiovascular Effects of Tulbaghia Violacea Harv. (Alliaceae) Root Methanolic Extract in Dahl Salt-Sensitive (DSS) Rats. J. Ethnopharmacol. 2013, 146, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Alpsoy, S.; Aktas, C.; Uygur, R.; Topcu, B.; Kanter, M.; Erboga, M.; Karakaya, O.; Gedikbasi, A. Antioxidant and Anti-Apoptotic Effects of Onion (Allium cepa) Extract on Doxorubicin-Induced Cardiotoxicity in Rats. J. Appl. Toxicol. 2013, 33, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Alpsoy, S.; Uygur, R.; Aktas, C.; Topcu, B.; Kanter, M.; Erboga, M.; Karakaya, O.; Gedikbasi, A. The Effects of Onion (Allium cepa) Extract on Doxorubicin-Induced Apoptosis in Aortic Endothelial Cells. J. Appl. Toxicol. 2013, 33, 364–369. [Google Scholar] [CrossRef]
- Alpsoy, S.; Kanter, M.; Aktas, C.; Erboga, M.; Akyuz, A.; Akkoyun, D.C.; Oran, M. Protective Effects of Onion Extract on Cadmium-Induced Oxidative Stress, Histological Damage, and Apoptosis in Rat Heart. Biol. Trace Elem. Res. 2014, 159, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Ige, S.F.; Akhigbe, R.E. Common Onion (Allium cepa) Extract Reverses Cadmium-Induced Organ Toxicity and Dyslipidaemia via Redox Alteration in Rats. Pathophysiology 2013, 20, 269–274. [Google Scholar] [CrossRef]
- Alkreathy, H.M.; Damanhouri, Z.A.; Ahmed, N.; Slevin, M.; Osman, A.-M.M. Mechanisms of Cardioprotective Effect of Aged Garlic Extract Against Doxorubicin-Induced Cardiotoxicity. Integr. Cancer Ther. 2012, 11, 364–370. [Google Scholar] [CrossRef]
- Alkreathy, H.; Damanhouri, Z.A.; Ahmed, N.; Slevin, M.; Ali, S.S.; Osman, A.-M.M. Aged Garlic Extract Protects against Doxorubicin-Induced Cardiotoxicity in Rats. Food Chem. Toxicol. 2010, 48, 951–956. [Google Scholar] [CrossRef]
- Mukherjee, S.; Banerjee, S.K.; Maulik, M.; Dinda, A.K.; Talwar, K.K.; Maulik, S.K. Protection against Acute Adriamycin-Induced Cardiotoxicity by Garlic: Role of Endogenous Antioxidants and Inhibition of TNF-Alpha Expression. BMC Pharm. 2003, 3, 16. [Google Scholar] [CrossRef] [Green Version]
- Gomaa, A.M.S.; Abdelhafez, A.T.; Aamer, H.A. Garlic (Allium sativum) Exhibits a Cardioprotective Effect in Experimental Chronic Renal Failure Rat Model by Reducing Oxidative Stress and Controlling Cardiac Na+/K+-ATPase Activity and Ca2+ Levels. Cell Stress Chaperones 2018, 23, 913–920. [Google Scholar] [CrossRef]
- Pop, R.M.; Bocsan, I.C.; Buzoianu, A.D.; Chedea, V.S.; Socaci, S.A.; Pecoraro, M.; Popolo, A. Evaluation of the Antioxidant Activity of Nigella Sativa L. and Allium Ursinum Extracts in a Cellular Model of Doxorubicin-Induced Cardiotoxicity. Molecules 2020, 25, 5259. [Google Scholar] [CrossRef]
- Beretta, H.V.; Bannoud, F.; Insani, M.; Berli, F.; Hirschegger, P.; Galmarini, C.R.; Cavagnaro, P.F. Relationships Between Bioactive Compound Content and the Antiplatelet and Antioxidant Activities of Six Allium Vegetable Species. Food Technol. Biotechnol. 2017, 55, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Cavagnaro, P.F.; Galmarini, C.R. Effect of Processing and Cooking Conditions on Onion (Allium cepa L.) Induced Antiplatelet Activity and Thiosulfinate Content. J. Agric. Food Chem. 2012, 60, 8731–8737. [Google Scholar] [CrossRef] [PubMed]
- Ro, J.-Y.; Ryu, J.-H.; Park, H.-J.; Cho, H.-J. Onion (Allium cepa L.) Peel Extract Has Anti-Platelet Effects in Rat Platelets. Springerplus 2015, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ko, E.Y.; Nile, S.H.; Jung, Y.-S.; Keum, Y.S. Antioxidant and Antiplatelet Potential of Different Methanol Fractions and Flavonols Extracted from Onion (Allium cepa L.). 3 Biotech 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Briggs, W.H.; Folts, J.D.; Osman, H.E.; Goldman, I.L. Administration of Raw Onion Inhibits Platelet-Mediated Thrombosis in Dogs. J. Nutr. 2001, 131, 2619–2622. [Google Scholar] [CrossRef]
- Chen, J.-H.; Chen, H.; Tsai, S.-J.; Jen, C.J. Chronic Consumption of Raw But Not Boiled Welsh Onion Juice Inhibits Rat Platelet Function. J. Nutr. 2000, 130, 34–37. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-H.; Chen, H.; Wang, J.-S.; Tsai, S.-J.; Jen, C.J. Effects of Welsh Onion Extracts on Human Platelet Function in Vitro. Life Sci. 2000, 66, 1571–1579. [Google Scholar] [CrossRef]
- Allison, G.L.; Lowe, G.M.; Rahman, K. Aged Garlic Extract Inhibits Platelet Activation by Increasing Intracellular CAMP and Reducing the Interaction of GPIIb/IIIa Receptor with Fibrinogen. Life Sci. 2012, 91, 1275–1280. [Google Scholar] [CrossRef]
- Allison, G.L.; Lowe, G.M.; Rahman, K. Aged Garlic Extract and Its Constituents Inhibit Platelet Aggregation through Multiple Mechanisms. J. Nutr. 2006, 136, 782S–788S. [Google Scholar] [CrossRef]
- Allison, G.L.; Lowe, G.M.; Rahman, K. Aged Garlic Extract May Inhibit Aggregation in Human Platelets by Suppressing Calcium Mobilization. J. Nutr. 2006, 136, 789S–792S. [Google Scholar] [CrossRef]
- Morihara, N.; Hino, A. Aged Garlic Extract Suppresses Platelet Aggregation by Changing the Functional Property of Platelets. J. Nat. Med. 2017, 71, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.; Billington, D. Dietary Supplementation with Aged Garlic Extract Inhibits ADP-Induced Platelet Aggregation in Humans. J. Nutr. 2000, 130, 2662–2665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, K.; Lowe, G.M.; Smith, S. Aged Garlic Extract Inhibits Human Platelet Aggregation by Altering Intracellular Signaling and Platelet Shape Change. J. Nutr. 2016, 146, 410S–415S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiyasat, B.; Sabha, D.; Grötzinger, K.; Kempfert, J.; Rauwald, J.-W.; Mohr, F.-W.; Dhein, S. Antiplatelet Activity of Allium Ursinum and Allium Sativum. Pharmacology 2009, 83, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Fukao, H.; Yoshida, H.; Tazawa, Y.; Hada, T. Antithrombotic Effects of Odorless Garlic Powder Both in Vitro and in Vivo. Biosci. Biotechnol. Biochem. 2007, 71, 84–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyfi, P.; Mostafaie, A.; Mansouri, K.; Arshadi, D.; Mohammadi-Motlagh, H.-R.; Kiani, A. In Vitro and in Vivo Anti-Angiogenesis Effect of Shallot (Allium ascalonicum): A Heat-Stable and Flavonoid-Rich Fraction of Shallot Extract Potently Inhibits Angiogenesis. Toxicol. In Vitro 2010, 24, 1655–1661. [Google Scholar] [CrossRef]
- Hiramatsu, K.; Tsuneyoshi, T.; Ogawa, T.; Morihara, N. Aged Garlic Extract Enhances Heme Oxygenase-1 and Glutamate-Cysteine Ligase Modifier Subunit Expression via the Nuclear Factor Erythroid 2–Related Factor 2–Antioxidant Response Element Signaling Pathway in Human Endothelial Cells. Nutr. Res. 2016, 36, 143–149. [Google Scholar] [CrossRef]
- Yeh, Y.-Y.; Yeh, S. Homocysteine-Lowering Action Is Another Potential Cardiovascular Protective Factor of Aged Garlic Extract. J. Nutr. 2006, 136, 745S–749S. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.N.; Choi, Y.W.; Kim, H.K.; Park, J.K.; Kim, H.J.; Kim, M.J.; Lee, H.W.; Kim, K.-H.; Bae, S.S.; Kim, B.S.; et al. Chloroform Extract of Aged Black Garlic Attenuates TNF-α-Induced ROS Generation, VCAM-1 Expression, NF-ΚB Activation and Adhesiveness for Monocytes in Human Umbilical Vein Endothelial Cells. Phytother. Res. 2011, 25, 92–100. [Google Scholar] [CrossRef]
- Kim, K.-M.; Chun, S.-B.; Koo, M.-S.; Choi, W.-J.; Kim, T.-W.; Kwon, Y.-G.; Chung, H.-T.; Billiar, T.R.; Kim, Y.-M. Differential Regulation of NO Availability from Macrophages and Endothelial Cells by the Garlic Component S-Allyl Cysteine. Free Radic. Biol. Med. 2001, 30, 747–756. [Google Scholar] [CrossRef]
- Louis, X.L.; Murphy, R.; Thandapilly, S.J.; Yu, L.; Netticadan, T. Garlic Extracts Prevent Oxidative Stress, Hypertrophy and Apoptosis in Cardiomyocytes: A Role for Nitric Oxide and Hydrogen Sulfide. BMC Complement. Altern. Med. 2012, 12, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, P.R.d.S.; Bandeira, F.C.V.; Rolim, J.C.; Nogueira, M.R.S.; Pordeus, M.A.A.; Oliveira, A.F.B.d.; Pitta, G.B.B. Allium Sativum Compared to Cilostazol as an Inhibitor of Myointimal Hyperplasia. Braz. J. Cardiovasc. Surg. 2016, 31, 291–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Recinella, L.; Chiavaroli, A.; Masciulli, F.; Fraschetti, C.; Filippi, A.; Cesa, S.; Cairone, F.; Gorica, E.; Leo, M.D.; Braca, A.; et al. Protective Effects Induced by a Hydroalcoholic Allium Sativum Extract in Isolated Mouse Heart. Nutrients 2021, 13, 2332. [Google Scholar] [CrossRef] [PubMed]
- Rassoul, F.; Salvetter, J.; Reissig, D.; Schneider, W.; Thiery, J.; Richter, V. The Influence of Garlic (Allium sativum) Extract on Interleukin 1α-Induced Expression of Endothelial Intercellular Adhesion Molecule-1 and Vascular Cell Adhesion Molecule-1. Phytomedicine 2006, 13, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.; Whiteman, M.; Moore, P.K.; Zhu, Y.Z. Bioactive S-Alk(En)Yl Cysteine Sulfoxide Metabolites in the Genus Allium: The Chemistry of Potential Therapeutic Agents. Nat. Prod. Rep. 2005, 22, 351–368. [Google Scholar] [CrossRef]
- Fukaya, M.; Nakamura, S.; Hayashida, H.; Noguchi, D.; Nakashima, S.; Yoneda, T.; Matsuda, H. Structures of Cyclic Organosulfur Compounds From Garlic (Allium sativum L.) Leaves. Front. Chem. 2020, 8, 282. [Google Scholar] [CrossRef]
- Golubkina, N.; Caruso, G. Chapter 5—Onion. In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Jaiswal, A.K., Ed.; Academic Press: San Diego, CA, USA, 2020; pp. 73–87. ISBN 978-0-12-812780-3. [Google Scholar]
- Najman, K.; Sadowska, A.; Buczak, K.; Leontowicz, H.; Leontowicz, M. Effect of Heat-Treated Garlic (Allium Sativum L.) on Growth Parameters, Plasma Lipid Profile and Histological Changes in the Ileum of Atherogenic Rats. Nutrients 2022, 14, 336. [Google Scholar] [CrossRef]
- Ferri, N.; Yokoyama, K.; Sadilek, M.; Paoletti, R.; Apitz-Castro, R.; Gelb, M.H.; Corsini, A. Ajoene, a Garlic Compound, Inhibits Protein Prenylation and Arterial Smooth Muscle Cell Proliferation. Br. J. Pharmacol. 2003, 138, 811–818. [Google Scholar] [CrossRef]
- Gonen, A.; Harats, D.; Rabinkov, A.; Miron, T.; Mirelman, D.; Wilchek, M.; Weiner, L.; Ulman, E.; Levkovitz, H.; Ben-Shushan, D.; et al. The Antiatherogenic Effect of Allicin: Possible Mode of Action. Pathobiology 2005, 72, 325–334. [Google Scholar] [CrossRef]
- Chen, X.; Pang, S.; Lin, J.; Xia, J.; Wang, Y. Allicin Prevents Oxidized Low-Density Lipoprotein-Induced Endothelial Cell Injury by Inhibiting Apoptosis and Oxidative Stress Pathway. BMC Complement. Altern. Med. 2016, 16, 133. [Google Scholar] [CrossRef] [Green Version]
- Elkayam, A.; Peleg, E.; Grossman, E.; Shabtay, Z.; Sharabi, Y. Effects of Allicin on Cardiovascular Risk Factors in Spontaneously Hypertensive Rats. Isr. Med. Assoc. J. 2013, 15, 170–173. [Google Scholar] [PubMed]
- Sangeetha, T.; Quine, S.D. Preventive Effect of S-Allyl Cysteine Sulfoxide (Alliin) on Cardiac Marker Enzymes and Lipids in Isoproterenol-Induced Myocardial Injury. J. Pharm. Pharmacol. 2010, 58, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Chiang, E.-P.I.; Chiu, S.-C.; Pai, M.-H.; Wang, Y.-C.; Wang, F.-Y.; Kuo, Y.-H.; Tang, F.-Y. Organosulfur Garlic Compounds Induce Neovasculogenesis in Human Endothelial Progenitor Cells through a Modulation of MicroRNA 221 and the PI3-K/Akt Signaling Pathways. J. Agric. Food Chem. 2013, 61, 4839–4849. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.-P.; Liu, C.-T.; Sheen, L.-Y.; Chen, H.-W.; Lii, C.-K. Diallyl Disulfide and Diallyl Trisulfide Protect Endothelial Nitric Oxide Synthase against Damage by Oxidized Low-Density Lipoprotein. Mol. Nutr. Food Res. 2010, 54, S42–S52. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.; Ueberham, E.; Gebhardt, R. Influence of Organosulphur Compounds from Garlic on the Secretion of Matrix Metalloproteinases and Their Inhibitor TIMP-1 by Cultured HUVEC Cells. Cell Biol. Toxicol. 2004, 20, 253–260. [Google Scholar] [CrossRef]
- Ou, C.; Tsao, S.; Lin, M.; Yin, M. Protective Action on Human LDL against Oxidation and Glycation by Four Organosulfur Compounds Derived from Garlic. Lipids 2003, 38, 219–224. [Google Scholar] [CrossRef]
- Jeremic, J.N.; Jakovljevic, V.L.; Zivkovic, V.I.; Srejovic, I.M.; Bradic, J.V.; Milosavljevic, I.M.; Mitrovic, S.L.; Jovicic, N.U.; Bolevich, S.B.; Svistunov, A.A.; et al. Garlic Derived Diallyl Trisulfide in Experimental Metabolic Syndrome: Metabolic Effects and Cardioprotective Role. Int. J. Mol. Sci. 2020, 21, 9100. [Google Scholar] [CrossRef]
- Li, G.; Cheng, G.; Wu, J.; Ma, S.; Zhang, A.; Han, W.; Sun, C. Allitridin Reduces IKr Current by Disrupting the Trafficking of Human Ether-à-Go-Go-Related Gene Channels. Cardiology 2014, 128, 1–8. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Wang, C.-C.; Lai, T.-Y.; Tsu, H.-N.; Wang, C.-H.; Liang, H.-Y.; Kuo, W.-W. Antioxidant Effects of Diallyl Trisulfide on High Glucose-Induced Apoptosis Are Mediated by the PI3K/Akt-Dependent Activation of Nrf2 in Cardiomyocytes. Int. J. Cardiol. 2013, 168, 1286–1297. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Wen, S.-Y.; Shibu, M.A.; Yang, Y.-C.; Peng, H.; Wang, B.; Wei, Y.-M.; Chang, H.-Y.; Lee, C.-Y.; Huang, C.-Y.; et al. Diallyl Trisulfide Protects against High Glucose-Induced Cardiac Apoptosis by Stimulating the Production of Cystathionine Gamma-Lyase-Derived Hydrogen Sulfide. Int. J. Cardiol. 2015, 195, 300–310. [Google Scholar] [CrossRef]
- Yang, H.-B.; Liu, H.-M.; Yan, J.-C.; Lu, Z.-Y. Effect of Diallyl Trisulfide on Ischemic Tissue Injury and Revascularization in a Diabetic Mouse Model. J. Cardiovasc. Pharmacol. 2018, 71, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Li, M.; Herman-Antosiewicz, A.; Antosiewicz, J.; Xiao, H.; Lew, K.L.; Zeng, Y.; Marynowski, S.W.; Singh, S.V. Diallyl Trisulfide Inhibits Angiogenic Features of Human Umbilical Vein Endothelial Cells by Causing Akt Inactivation and Down-Regulation of VEGF and VEGF-R2. Nutr. Cancer 2006, 55, 94–107. [Google Scholar] [CrossRef]
- Chuah, S.C.; Moore, P.K.; Zhu, Y.Z. S-Allylcysteine Mediates Cardioprotection in an Acute Myocardial Infarction Rat Model via a Hydrogen Sulfide-Mediated Pathway. Am. J. Physiol.-Heart Circ. Physiol. 2007, 293, H2693–H2701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, S.E.; Ide, N.; Lau, B.H.S. S-Allyl Cysteine Reduces Oxidant Load in Cells Involved in the Atherogenic Process. Phytomedicine 2001, 8, 39–46. [Google Scholar] [CrossRef]
- Padmanabhan, M.; Prince, P.S.M. Preventive Effect of S-Allylcysteine on Lipid Peroxides and Antioxidants in Normal and Isoproterenol-Induced Cardiotoxicity in Rats: A Histopathological Study. Toxicology 2006, 224, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Kumari, K.; Augusti, K.T. Lipid Lowering Effect of S-Methyl Cysteine Sulfoxide from Allium Cepa Linn in High Cholesterol Diet Fed Rats. J. Ethnopharmacol. 2007, 109, 367–371. [Google Scholar] [CrossRef]
- Chang, H.S.; Yamato, O.; Sakai, Y.; Yamasaki, M.; Maede, Y. Acceleration of Superoxide Generation in Polymorphonuclear Leukocytes and Inhibition of Platelet Aggregation by Alk(En)Yl Thiosulfates Derived from Onion and Garlic in Dogs and Humans. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 77–83. [Google Scholar] [CrossRef]
- Biró, A.; Markovics, A.; Fazekas, M.É.; Fidler, G.; Szalóki, G.; Paholcsek, M.; Lukács, J.; Stündl, L.; Remenyik, J. Allithiamine Alleviates Hyperglycaemia-Induced Endothelial Dysfunction. Nutrients 2020, 12, 1690. [Google Scholar] [CrossRef]
- Torres-Palazzolo, C.; de Paola, M.; Quesada, I.; Camargo, A.; Castro, C. 2-Vinyl-4H-1,3-Dithiin, a Bioavailable Compound from Garlic, Inhibits Vascular Smooth Muscle Cells Proliferation and Migration by Reducing Oxidative Stress. Plant Foods Hum. Nutr. 2020, 75, 355–361. [Google Scholar] [CrossRef]
- Rai, S.K.; Sharma, M.; Tiwari, M. Inhibitory Effect of Novel Diallyldisulfide Analogs on HMG-CoA Reductase Expression in Hypercholesterolemic Rats: CREB as a Potential Upstream Target. Life Sci. 2009, 85, 211–219. [Google Scholar] [CrossRef]
- Kumar Sharma, D.; Manral, A.; Saini, V.; Singh, A.; Srinivasan, B.P.; Tiwari, M. Novel Diallyldisulfide Analogs Ameliorate Cardiovascular Remodeling in Rats with L-NAME-Induced Hypertension. Eur. J. Pharmacol. 2012, 691, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zou, M.-J.; Zhao, N.; Ren, J.-G.; Zhou, H.; Cheng, G. Effect of Diallyl Trisulfide on the Pharmacokinetics of Nifedipine in Rats. J. Food Sci. 2011, 76, T30–T34. [Google Scholar] [CrossRef] [PubMed]
- Larijani, V.N.; Ahmadi, N.; Zeb, I.; Khan, F.; Flores, F.; Budoff, M. Beneficial Effects of Aged Garlic Extract and Coenzyme Q10 on Vascular Elasticity and Endothelial Function: The FAITH Randomized Clinical Trial. Nutrition 2013, 29, 71–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkin, M.; Laight, D.; Cummings, M.H. The Effects of Garlic Extract upon Endothelial Function, Vascular Inflammation, Oxidative Stress and Insulin Resistance in Adults with Type 2 Diabetes at High Cardiovascular Risk. A Pilot Double Blind Randomized Placebo Controlled Trial. J. Diabetes Its Complicat. 2016, 30, 723–727. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, N.; Nabavi, V.; Hajsadeghi, F.; Zeb, I.; Flores, F.; Ebrahimi, R.; Budoff, M. Aged Garlic Extract with Supplement Is Associated with Increase in Brown Adipose, Decrease in White Adipose Tissue and Predict Lack of Progression in Coronary Atherosclerosis. Int. J. Cardiol. 2013, 168, 2310–2314. [Google Scholar] [CrossRef]
- Wlosinska, M.; Nilsson, A.-C.; Hlebowicz, J.; Hauggaard, A.; Kjellin, M.; Fakhro, M.; Lindstedt, S. The Effect of Aged Garlic Extract on the Atherosclerotic Process—A Randomized Double-Blind Placebo-Controlled Trial. BMC Complement. Med. Ther. 2020, 20, 132. [Google Scholar] [CrossRef]
- Wlosinska, M.; Nilsson, A.; Hlebowicz, J.; Malmsjö, M.; Fakhro, M.; Lindstedt, S. Aged Garlic Extract Preserves Cutaneous Microcirculation in Patients with Increased Risk for Cardiovascular Diseases: A Double-blinded Placebo-controlled Study. Int. Wound J. 2019, 16, 1487–1493. [Google Scholar] [CrossRef]
- Chiu, H.-F.; Shen, Y.-C.; Huang, T.-Y.; Venkatakrishnan, K.; Wang, C.-K. Cardioprotective Efficacy of Red Wine Extract of Onion in Healthy Hypercholesterolemic Subjects. Phytother. Res. 2016, 30, 380–385. [Google Scholar] [CrossRef]
- Asgharpour, M.; Khavandegar, A.; Balaei, P.; Enayati, N.; Mardi, P.; Alirezaei, A.; Bakhtiyari, M. Efficacy of Oral Administration of Allium Sativum Powder “Garlic Extract” on Lipid Profile, Inflammation, and Cardiovascular Indices among Hemodialysis Patients. Evid.-Based Complement. Altern. Med. 2021, 2021, 1–7. [Google Scholar] [CrossRef]
- Brüll, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Naaf, S.; et al. Effects of a Quercetin-Rich Onion Skin Extract on 24 h Ambulatory Blood Pressure and Endothelial Function in Overweight-to-Obese Patients with (Pre-)Hypertension: A Randomised Double-Blinded Placebo-Controlled Cross-over Trial. Br. J. Nutr. 2015, 114, 1263–1277. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.-Y.; Lee, H.; Woo, J.S.; Jang, H.H.; Hwang, S.J.; Kim, H.S.; Kim, W.-S.; Kim, Y.-S.; Choue, R.; Cha, Y.-J.; et al. Effect of Onion Peel Extract on Endothelial Function and Endothelial Progenitor Cells in Overweight and Obese Individuals. Nutrition 2015, 31, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Momenan, A.A.; Azizi, F. Allium Vegetable Intakes and the Incidence of Cardiovascular Disease, Hypertension, Chronic Kidney Disease, and Type 2 Diabetes in Adults. J. Hypertens. 2017, 35, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Boobis, A.R.; Shiga, T.; Edwards, R.J. Genetic Polymorphisms and Cardiovascular Drug Metabolism. In Cardiovascular Pharmacogenetics. Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2004; Volume 160, pp. 39–77. [Google Scholar]
- Liu, Y.; Zhang, L.; Liu, Y.-F.; Yan, F.-F.; Zhao, Y.-X. Effects of Bulbus Allii Macrostemi on Clinical Outcomes and Oxidized Low-Density Lipoprotein and Plasminogen in Unstable Angina/Non-ST-Segment Elevation Myocardial Infarction Patients. Phytother. Res. 2008, 22, 1539–1543. [Google Scholar] [CrossRef]
- Alobaidi, A. Effect of Nigella Sativa and Allium Sativum Coadminstered with Simvastatin in Dyslipidemia Patients: A Prospective, Randomized, Double-Blind Trial. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2014, 13, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Capasso, F.; Gaginella, T.S.; Grandolini, G.; Izzo, A.A. Phytotherapy: A Quick Reference to Herbal Medicine; Springer: Berlin/Heidelberg, Germany, 2003; ISBN 3540000526. [Google Scholar]
- Leite, P.M.; Martins, M.A.P.; Castilho, R.O. Review on Mechanisms and Interactions in Concomitant Use of Herbs and Warfarin Therapy. Biomed. Pharmacother. 2016, 83, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Leite, P.M.; Martins, M.A.P.; Carvalho, M.D.G.; Castilho, R.O. Mechanisms and Interactions in Concomitant Use of Herbs and Warfarin Therapy: An Updated Review. Biomed. Pharmacother. 2021, 143, 112103. [Google Scholar] [CrossRef] [PubMed]
| Scientific Name (Common Name) | Region of Use | Plant Part or Preparation (Mode of Administration; Posology) | Cardiovascular Disease/Risk Factor (Observed Effect) | Ref. |
|---|---|---|---|---|
| Allium ampeloprasum L. (wild leek) | Suva planina, Serbia | Raw aerial parts (oral) | Diabetes | [28] |
| Allium cepa L. (onion) | Serra de Mariola, Spain | Whole plant (oral) | Hypertension | [29] |
| Beni Mellal, Morocco | Raw bulb (oral) | Diabetes | [30] | |
| Gabon | Bulb (oral) | Diabetes, hypertension | [31] | |
| Edo, Nigeria | Mined and blended bulb mixed with honey (oral; one tablespoonful twice a day) | Hypertension | [32] | |
| Bulb maceration—soaked in water with Vernonia amygdalina and Zingiber officinale for 5 days (oral; one cup twice a day) | ||||
| Bulb decoction with snail water and Capsicum frutescens, add small salt and filter (oral; one small cup twice daily) | ||||
| Bulb concoction with Viscum album, Persea americana, Ocimum gratissimum added with Elaeis guineensis kernel oil and boiled for 10 min (oral; drink as a soup) | ||||
| Tamil Nadu, India | Bulb mixed with buttermilk (oral) | Cardiovascular disease | [33] | |
| Bulb boiled in milk, sugar from Borassus flabellifer added (oral; once a day in the evening) | ||||
| Raw bulb, (oral; daily before eating for 45 days) | Dyslipidaemia | |||
| Raw bulb (oral; daily in the morning for 20 days) | ||||
| Juice from bulbs (oral; 25 mL in the morning for two weeks) | Obesity | |||
| Bulb decoction with Macrotyloma uniflorum, Zingiber officinale and honey (oral; daily in the morning for 30 days) | ||||
| Allium jacquemontii Kunth. | Dir, Pakistan | Raw bulb, (oral; for 3 weeks) | Hypertension | [34] |
| Allium rotundum L. | Aladaglar, Turkey | Raw bulb (oral) | Hypertension (regulates blood pressure) | [35] |
| Allium sativum L. (garlic) | Eastern Cape, South Africa | Not referred | Diabetes | [36] |
| Gabon | Bulb maceration | Diabetes, dyslipidaemia | [31] | |
| Suva planina, Serbia | 3 peeled bulbs with 3 chopped lemons in 1 L of hot water for 12 h) (oral; 1 cup a day for 40 days) | Dyslipidaemia (decreases TG), hypertension (improves blood circulation) | [28] | |
| Togo | Bulb decoction with Khaya senegalensis bark (oral) | Diabetes | [37] | |
| Bulb maceration with honey (oral) | Hypertension | |||
| Bulb maceration with Parkia biglobosa (oral) | ||||
| Bulb powder containing Lippia multiflora, Stachytarpheta angustifolia and Persea americana (oral) | ||||
| Edo, Nigeria | Bulb maceration—soaked with guava, Vernonia amygdalina in water for 5 days (oral; half a cup daily) | Hypertension | [32] | |
| Bulb decoction combined with Allium cepa and boiled in water (oral; half a cup twice a day) | ||||
| Bulb decoction combined with Allium cepa and Zingiber officinale and boiled in water (oral; one cup twice a day) | ||||
| Bulb decoction with Cocos nucifera boiled for 3 days (oral; half a cup twice a day) | ||||
| Bulb infusion after pounding with Carica papaya (oral; one small cup 3 times a day) | ||||
| Bulb decoction with Musanga cecropoides, Talinum triangulare, Carica papaya boiled in water (oral; half a cup twice a day) | ||||
| Bulb decoction with Hunteria umbelleta, Sida acuta and potash in cold water (oral; one cup a day) | ||||
| Western Anti-Atlas, Morocco | Raw bulb (oral) | Diabetes | [38] | |
| Tamil Nadu, India | Boiled bulb with Zingiber officinale rhizome and added milk (oral; twice a day) | Cardiovascular disease | [33] | |
| Bulb cooked with Foeniculum vulgare in milk (oral; daily in the morning until cure) | ||||
| Bulb cooked with Trachyspermum ammi in milk (oral; daily in the morning until cure) | ||||
| Bulb boiled in water, and added milk (oral; daily in the evening) | Hypertension | |||
| Bulb boiled in milk (oral; daily in the evening for a month) | Obesity | |||
| Bulb as a food supplement with Moringa oleifera (oral; twice a week at lunch) | Hypertension | |||
| Bulb syrup with Citrus limon juice, Zingiber officinale rhizome, Malus pumila cider vinegar and honey (oral; 10 mL twice a day) | Cardiovascular disease, obesity | |||
| Bulb syrup with Citrus limon juice, Zingiber officinale rhizome, Malus pumila cider vinegar and honey (oral; 15 mL twice a day after meals) | Cardiovascular disease, dyslipidaemia | |||
| Bulb paste with Coriandrum sativum, Solanum torvum and Zingiber officinale, consumed with honey (oral; once a day in the morning) | Cardiovascular disease | |||
| Bulb combined with honey (oral; 5 mL once a day in the morning) | ||||
| Bulb combined with honey (oral; 5 mL twice a day for a month) | Dyslipidaemia | |||
| Bulb combined with honey (oral; 10 mL twice a day) | Hypertension | |||
| Bulb jam with sugar from Borassus flabellifer and oil from Sesamum indicum (oral; 10 g twice a day for 45 days) | Dyslipidaemia | |||
| Tamil Nadu, India | Bulb jam with sugar from Borassus flabellifer (oral; 20 g twice a day till cure) | Obesity | ||
| Bulb jam with sugar from Borassus flabellifer and oil from Sesamum indicum (oral; 10 g twice a day) | Hypertension | |||
| Bulb powder with Cinnamomum verum, Piper cubeba and Vitis vinifera (oral; 2–3 g once a day in the evening) | Dyslipidaemia | |||
| Bulb gravy with Arachis hypogea, Cissus quadrangularis, Murraya koenigii, Tamarindus indica and clarified butter (oral; twice a week until cure) | ||||
| Beni Mellal, Morocco | Raw bulb (oral) | Diabetes | [30] | |
| Allium ursinum L. (wild garlic) | Suva planina, Serbia | Leaf tincture diluted in a small glass of water (oral; 10 drops 3 times a day before meals) | Hypertension, hypercholesterolemia (lowers blood cholesterol) | [28] |
| Tulbaghia alliacea L. | Eastern Cape, South Africa | Not referred | Diabetes | [36] |
| Tulbaghia violaceae Harv. |
| Plant Species | Plant Part or Extract Used (Preparation; Concentration) | Study Model: Insult or Injury | Main Findings | Ref. |
|---|---|---|---|---|
| Ischaemic injury/Myocardial infarction | ||||
| Allium cepa | Aqueous extract (i.v. 30 min before injury; 0.1, 0.3 and 1 g/kg) | Rat: Brain ischaemia | ↓ Brain edema; prevented ZO-1 and occludin disruption; ↑ Cat and GPx; ↓ MDA | [39] |
| Methanolic extract (0.01, 0.05 and 0.1 g/mL) | Cardiomyoblasts (H9c2): Hypoxia | ↓ ROS production, mitochondrial membrane depolarisation, cytochrome c and caspase-3 release | [40] | |
| Methanolic extract (p.o. 14 days before injury; 0.1, 1 and 10 g/Kg) | Rat: Ischaemic injury | ↓ Infarct area, apoptotic cell death and MDA | ||
| Allium macrostemon | Decoction with bulbs and Trichosanthes kirilowii (gavage for 4 weeks; 1.14, 2.27 and 4.53 g/Kg) | Rat: LAD ligation-induced infarction | ↓ HW/BW, LV/BW, systemic inflammation, myocardial fibrosis, and collagen I and III expressions; ↓ TGFβ1, TGFβ2 and Smad 2/3 expression; ↑ Smad7 expression | [41] |
| Allium sativum | Aged garlic extract (p.o. for 3 weeks; 2 and 5 mL/Kg) | Rat: ISO-induced myocardial infarction | ↑ Heart function, SOD and Cat; ↓ LDH, CK-MB and MDA | [42,43] |
| Homogenate (p.o. for 30 days; 125, 250 and 500 mg/kg) | ↑ SOD and cat; ↓ LDH, CK-MB and structural changes | [44] | ||
| Raw homogenate (p.o. 30 days before injury; 125, 250 and 500 mg/kg) | ↓ MDA, LDH and structural changes | [45] | ||
| Rat: I/R | ↓ MDA and structural changes; ↑ SOD, cat, GSH and GPx | [46] | ||
| Black garlic extract (gavage for 4 weeks; 300 mg/kg) | ↑ HO-1 | [47] | ||
| Raw garlic extract (gavage for 4 weeks; 300 mg/kg) | ↑ HO-1 and eNOS | |||
| Garlic oil (intragastric for 14 days, 100 mg/kg) | Rat: ISO-induced myocardial necrosis | ↓ HW, LDH, CK-MB, cTnC and systemic inflammation; ↑ SOD and cat | [48] | |
| Allium ursinum | Methanolic extract (p.o. 28 days before injury in drinking water; 125, 250, and 500 mg/kg) | Rat: I/R | ↑ Cardiac function and antioxidant system | [49] |
| Tulbaghia violacea | Methanolic extract (intragastric for 30 days before injury; 60 mg/kg) | Rat: ISO-induced myocardial infarction | ↓ CK, CK-MB, LDH and MDA; ↑ LV function, SOD and GSH | [50] |
| Dyslipidaemia/Diabetes/Metabolic syndrome | ||||
| Allium cepa | Aqueous extract (p.o. for 4 weeks; 0.5, 1.5 and 4.5 g/kg) | Rat: HFD-induced hyperlipidaemia | ↓ TC, LDL, MDA, lipid droplets in liver, foam cell accumulation and HMG-CoA; ↑ HDL, SOD and LDLR | [51] |
| Allium cepa var. destiny and var. cavalier | Raw onion (p.o. for 6 weeks; 16 and 40 g/kg) | ↓ TC, glucose, LDL, HDL, TG, erythrocyte number and haemoglobin; ↑ white blood cell number | [52] | |
| Allium elburzense | Hydroalcoholic extract (intragastric for 7 days, 100, 200, and 400 mg/kg) | Rat: DEX-induced diabetes | ↓ TG, TC, LDL, MDA and liver steatosis; ↑ HDL | [53] |
| Allium eriophyllum | Hydroalcoholic extract (gavage for 4 weeks; 30 and 100 mg/kg) | Rat: T2DM + Hypertension | ↓ SBP, BG, CK-MB, infarct size and coronary resistance; ↑ SOD, GSR | [54] |
| Allium hirtifolium | Ethyl acetate fraction from hydroalcoholic extract (gavage for 4 weeks; 5 mg/kg) | Rat: STZ-induced diabetes | ↓ BG; ↑ LVDP, HR, RPP and +dp/dt | [55] |
| Allium hookeri | Powder (p.o. for 4 weeks; 3% and 5% in chow) | Rat: HFD-induced obesity | ↓ BW, BW gain, adipose tissue, TG, TC, LDL, AI, cardiac risk factor, LDH, AST and ALP | [56] |
| Powder (p.o. for 13 weeks; 0.2 g/Kg) | Hamster: HFD-induced obesity | ↓ TG, TC and LDL | [57] | |
| Hydroalcoholic extract (p.o. for 4 weeks; 200 and 400 mg/kg in chow) | Mice: HFD-induced obesity | ↓ liver and adipose tissue weight, TG, TC, LDL, AI, AST and ALT; ↑ HDL | [58] | |
| Allium sativum | Aged garlic extract (intra-abdominal injection every 12 h for one month; 125 mg/kg) | Rat: Metabolic syndrome | ↓ TG, insulin, leptin, AGE, SBP and MDA; ↑ GSH, and GPx; restored vascular and cardiac function | [59] |
| Aged garlic extract (1, 2.5 and 5 g/L) | HUVEC: oxLDL | ↓ LDH release and cell damage | [60] | |
| In chemico: Cu2+-induced LDL oxidation | ↓ Cu2+-induced LDL oxidation | |||
| Aged garlic extract (p.o. for 12 or 24 weeks; 3% in chow) | Mice: ApoE−/− | ↓ Atherosclerotic lesions, TC, TG and CD11b+ cells in spleen | [61] | |
| Fresh homogenate (intragastric for 41 days; 100 mg/kg) | Pregnant rat: High cholesterol diet | On mothers: ↓ systemic inflammation, disruption of mitochondrial network, infiltration of foam cells, TC, TG, LDL and CK On offspring: ↓ abnormalities and abortions | [62] | |
| Homogenate and raw garlic (p.o. for 100 weeks; 0.5% in chow) | Rat: High cholesterol diet | Homogenate: ↓ TC, LDL and TG Raw garlic: ↓ TC, LDL and TG; ↑ excretion of TG and TC | [63] | |
| Aged garlic extract (p.o. for 56 days; 500 mg/kg) | Rat: STZ-induced diabetes | ↓ Glucose, CK, LDH and AGER gene expression; ↑ Mn-SOD | [64] | |
| Raw garlic (p.o. for 4 weeks; 250 mg/kg) | ↑ Cat, SOD, SIRT3 activity, TFAM and PGC-1α mRNA; ↓ ROS | [65] | ||
| Black garlic extract (p.o. for one month; 250 mg/kg) | Rat: High fat/sucrose diet | ↓ Calory intake, BW, TG, LDL, insulin, leptin and leptin receptor, pro-inflammatory genes; induced vasorelaxation | [66] | |
| Garlic oil (p.o. for 8 weeks; 1% in chow) | Hamster: High cholesterol | ↓ Cardiac apoptosis and apoptotic markers; ↑ IGFR/PI3K/Akt pathway | [67] | |
| Garlic oil (gavage daily for 16 days; 100 mg/kg) | Rat: Diabetic cardiomyopathy | ↓ Cardiac apoptosis and apoptotic markers dependent of death receptor and mitochondria; ↑ IGFR/PI3K/Akt pathway | [68] | |
| Aqueous extract (5 mg/mL added to the blood collection tube) | Human: Healthy individuals | ↓ TC and TG | [69] | |
| Aqueous extract (i.p. for 8 weeks; 100 mg/kg) | Rat: STZ-induced diabetes | ↓ STZ-induced vasoconstriction | [70,71,72] | |
| Aqueous extract (p.o. for 16 weeks; 100 mg/kg) | ↓ Coronary arterioles thickening and BG; ↑ aortic/coronary blood flow | [73] | ||
| Aqueous extract (gavage for 28 days; 2500 and 500 mg/kg) | Rat: Obese and insulin resistant | ↓ Insulin, BG, and lipid levels; ↑ cardiac function and mitochondrial homeostasis | [74] | |
| High pressure garlic extract (p.o. for 5 weeks; 2% in chow) | Rat: High-fat diet | ↓ Plasma and hepatic LDL and TG; ↑ plasma HDL, hepatic mRNA ApoA1, ABCA1 and LCAT | [75] | |
| Bulb powder (gavage for 28 days; 200 mg/kg) | Rat: STZ/Nicotinamide-induced diabetes | ↓ Hyperglycaemia, dyslipidaemia, AI and MDA; ↑ Insulin production, GSH activity | [76] | |
| Powder (p.o. for 35 days; 300 mg/kg) | Rabbit: HC-induced atherogenesis | ↓ Neointima formation, cholesterol, TG, PL and collagen accumulation; ↓ TG, TC, PL blood levels; ↓ AI | [77] | |
| Powder (n/a) | Rat: In vivo Fe2+-induced LDL oxidation | ↓ LDL oxidation and oxLDL mobility; ↓ MDA hepatic, serum and heart levels | [78] | |
| Allium ursinum | Leaf lyophilizate (p.o. for 8 weeks; 2% in chow) | Rabbit: Hypercholesterolaemic | ↑ Heart function in vivo and ex vivo; ↑ HO-1; ↓ TC, TG, ApoB and atherosclerotic lesions | [79] |
| Tulbaghia violacea | Methanolic extract (p.o. for 2 weeks; 0.25 and 0.50 g/kg) | Rat: Atherogenic diet | ↓ TG, TC, LDL, VLDL, MDA, fibrinogen, LDH, AST, ALT, bilirubin, creatinine, and fatty streak plaques; ↑ HDL, SOD, cat, and NO | [80] |
| Hypertension/Vasorelaxation | ||||
| Allium cepa | Raw onion (p.o. for 3 weeks; 5% in chow) | Rat: L-NAME-induced hypertension | ↓ SBP and TBARS; ↑ NO metabolites excretion | [81] |
| Allium fistulosum | Raw or boiled juice (cumulative doses from 3 × 10−5 to 4 × 10−3 g/mL) | Aortic rings: NE precontracted | Raw juice: Induced relaxation Boiled juice: ↑ EDCF | [82] |
| Raw green part (p.o. for 4 weeks, 5% in chow) | Rat: HFD-induced hypertension | ↓ SBP, O22− and NOX activity; ↑ NO levels | [83] | |
| Allium macrostemon | Volatile extract (cumulative doses from 0.01% to 0.1%) | Pulmonary arteries: Phe contracted | Induced relaxation; ↑ NOS phosphorylation and Ca2+ influx to ECs | [84] |
| Allium sativum | Aged garlic extract (gavage for 12 weeks; 2 g/kg) | Rat: Dahl salt-sensitive hypertensive | ↓ LVEDP, pressure half-time, interstitial fibrosis, LV mass and SBP | [85] |
| Aged garlic extract (cumulative doses from 0.001 to 1%) | Aortic rings: NE-contracted | Induced relaxation in a dose-dependent manner | [86] | |
| Fresh homogenate (p.o. for 8 weeks; 250 mg/kg) | Rat: High fructose | ↓ LVH, NF-κB and oxidative stress; ↑ cat, GSH, GPx, and Nrf2 | [87] | |
| Homogenate (p.o. for 3 weeks; 125 and 250 mg/kg) | ↓ SBP, HR, TC, TG, glucose, LDH, CK-MB; ↑ SOD, cat and heart function | [88] | ||
| Raw garlic (p.o. 1 day or 3 weeks before MCT injection + 3 weeks; 1% in chow) | Rat: MCT-induced PH | ↓ RVSP, RVH, vasoconstriction in CEC; induced relaxation | [89] | |
| Garlic juice (cumulative doses from 1 to 50 μg/mL) | Aortic rings: Phe contracted | Induced relaxation in a dose-dependent manner | [90] | |
| Aqueous extract (p.o. for 4 weeks; 50 mg/kg) | Rat: 2-kindey-1-clip hypertension | ↓ SBP and ACE activity | [91] | |
| 100% methanol fraction from a methanolic extract (cumulative doses from 30 to 750 µg/mL) | Aortic rings | Precontracted with KCl or Phe: Induced relaxation Pre-treatment with the fraction: Prevented contraction evoked by KCl or Phe | [92] | |
| Aqueous extract (0.045 mg/mL) | Aortic rings: NE-contracted | Prolonged relaxation induced by GSNO; Inhibited chloride channels | [93] | |
| Aqueous extract (cumulative doses from 3 to 500 μg/mL) | Pulmonary arteries | Normoxia: Induced dose-dependent relaxation Hypoxia: Inhibited the transient relaxation and sustained contraction elicited by hypoxia ↓ ET-1 induced contractions | [94] | |
| Aqueous and 5% ethanol extracts (cumulative doses from 1 to 500 μg/mL) | ↓ Phe-induced contractions; ↑ ACh-induced relaxation | [95] | ||
| Aqueous and ethanol extract (non-cumulative doses from 0.1 to 3 mg/L) | Atria: Spontaneously or EPI-induced contraction | Negative inotropic and chronotropic effect | [96] | |
| Allium ursinum | Leaf lyophilizate (p.o. for 8 weeks; 2% in chow) | Rat: MCT-induced PH | ↑ RV function and PDE5 activity; ↓ Medial thickness of PA | [97] |
| Tulbaghia violacea | Methanolic extract (i.p. for 7 weeks; 50 mg/kg) | Rat: Dahl salt-sensitive hypertensive | ↓ SBP; ↑ [Na] in urine and AT-1a receptor levels | [98] |
| Protection against cardiotoxic compounds | ||||
| Allium cepa | Raw juice (intragastric intubation for 14 days; 1 mL) | Rat: DOX-induced cardiotoxicity | ↓ Apoptotic cells; ↓ CK, CK-MB, LDH, cTn1 and MDA levels; ↑ SOD, GSH, GPx | [99] |
| Raw juice (intragastric intubation for 14 days; 1 mL) | Rat: DOX-induced endothelial dysfunction | ↓ Apoptotic cells; ↓ MDA levels; ↑ GSH | [100] | |
| Rat: Cd-induced cardiotoxicity | ↓ Apoptotic cells; ↓ CK, CK-MB, LDH, cTnT and MDA levels; ↑ SOD, GSH, GPx | [101] | ||
| Raw juice (intragastric intubation for 8 weeks; 1 mL/100 g BW) | ↓ TC, TG, LDL, albumin and MDA; ↑ HDL and SOD | [102] | ||
| Allium sativum | Aged garlic extract (1000 µg) | Rat: DOX-induced cardiomyocyte apoptosis | ↓ p53 activation, and caspase-3 activity; ↑ 8-isoprostane levels | [103] |
| Aged garlic extract (p.o. for 6 days before DOX; 2860 mg/kg) | Mice: DOX-induced cardiotoxicity | ↑ survivability, and tumour uptake of DOX | [103] | |
| Aged garlic extract (p.o. for 28 days; 250 mg/kg) | ↓ LDH, CK and MDA | [104] | ||
| Homogenate (p.o. days; 250 and 500 mg/kg) | Rat: Adriamycin-induced cardiotoxicity | ↑ SOD, GPx, and cat; ↓ MDA, TNF-α accumulation | [105] | |
| Aqueous extract (p.o. for 3 weeks; 250 mg/kg) | Rat: Gentamycin-induced renal failure | ↑ Renal function, BW, HW/BW, cardiac Na+/K+-ATPase activity, and antioxidant capacity; ↓ BP, LDH, CK-MB, MDA | [106] | |
| Allium ursinum | Water and methanolic extracts (4 h pre-treatment; 50 μg/mL) | Cardiomyoblasts (H9c2): DOX-induced toxicity | Water: ↓ intracellular and mitochondrial ROS and cell death induced by DOX Methanolic: ↓ intracellular and mitochondrial ROS | [107] |
| Antiplatelet aggregation | ||||
| Allium ampeloprasum | Raw juice (n/a) | Human: Platelet aggregation in whole blood | ↓ Platelet aggregation (IC50 = 114.9 and 117.3 mg/mL) | [108] |
| Allium ascalonicum | ↓ Platelet aggregation (IC50 = 6.9 and 30.9 mg/mL) | |||
| Allium cepa | Heated extract (n/a) | Human: Platelet-rich plasma | ↓ Platelet aggregation, which is lost with higher heating times or microwave heating | [109] |
| Peel aqueous extract (50, 100 and 500 μg/mL) | Rat: Collagen-induced platelet aggregation | ↓ Platelet aggregation, [Ca2+]i, TXA2; ↑ cAMP | [110] | |
| Methanolic extract and methanolic fractions (0.5, 1, 3 and 5 mg/mL) | ↓ Platelet aggregation | [111] | ||
| Raw juice (n/a) | Human: Platelet aggregation in whole blood | ↓ Platelet aggregation (IC50 = 46.7 and 116.7 mg/mL) | [108] | |
| Raw juice (i.v. after CFR induction; 0.09 ± 0.01 mL/kg) | Dog: Chronic platelet-mediated thrombosis | ↓ Platelet aggregation | [112] | |
| Raw homogenate (intragastric after CFR induction; 2 g/kg) | ||||
| Raw juice (1, 10 and 100 mL/L) | Human and dog: In vitro platelet aggregation | ↓ Platelet aggregation in both blood type, stronger effect on dog | ||
| Allium fistulosum | Raw juice (n/a) | Human: Platelet aggregation in whole blood | ↓ Platelet aggregation (IC50 = 113.8 and 113.2 mg/mL) | [108] |
| Raw juice (p.o. for 4 weeks; 2 g/Kg) | Rat | ↓ SBP, platelet adhesion to fibrinogen, platelet aggregation and thromboxane release; ↑ bleeding time, cAMP and 6-keto prostacyclin F1α | [113] | |
| Raw or boiled juice (0–4 mg/mL) | Human: ADP-induced aggregation | Raw juice: ↓ [Ca2+]i and thromboxane production; ↑ cAMP levels Boiled juice: ↑ [Ca2+]i and thromboxane production; induced morphological changes | [114] | |
| Allium sativum | Aged garlic extract (3.12 to 12.5%) | Human: Fibrinogen- and ADP-induced platelet aggregation | ↓ Platelet adhesion to fibrinogen; Prevented platelet conformational changes induced by ADP; ↑ cAMP | [115] |
| Aged garlic extract (0.78–25%) | Human: ADP-induced platelet aggregation | ↓ Platelet aggregation and [Ca2+]i | [116,117] | |
| Human: ADP aggregated PRP | Induced platelet disaggregation | [116] | ||
| Aged garlic extract (p.o. for 7 or 14 days; 1, 2 or 5 g/kg) | Rat: Healthy fed AGE | ↓ Platelet aggregation after 14 days without prolonging bleeding time; ↑ extracellular ATP, TXB2 and ↓ phosphorylation of ERK, p38 and JNK after collagen treatment | [118] | |
| Aged garlic extract (p.o. for 13 weeks; 5 mL) | Human: ADP-induced platelet (13 days pre-treatment) | ↓ % of aggregated platelets and the initial rate of aggregation | [119] | |
| Aged garlic extract (0.19–6.25%) | Human: ADP-induced platelet aggregation | ↓ Platelet aggregation; ↑ cGMP and cAMP which were inhibited by ODQ and SQ22536 | [120] | |
| Garlic juice (n/a) | Human: Platelet aggregation in whole blood | ↓ Platelet aggregation (IC50 = 3.2 and 4.0 mg/mL) | [108] | |
| Aqueous and alcoholic extract (n/a) | Human: Platelet-rich plasma | Aqueous: ↓ ADP-induced aggregation Alcoholic: ↓ ADP-, AA-, EPI-induced aggregation | [121] | |
| Odourless powder (p.o. for 2 weeks; 1 g/kg in chow) | Rat: In situ loop | ↓ Thrombus formation | [122] | |
| Allium schoenoprasum | Raw juice (n/a) | Human: Platelet aggregation in whole blood | ↓ Platelet aggregation (IC50 = 45.4 and 50.1 mg/mL) | [108] |
| Allium ursinum | Aqueous extract (n/a) | Rat: Platelet aggregation | ↓ ADP-, collagen-, AA- and EPI-induced aggregation | [121] |
| Other activities | ||||
| Allium ampeloprasum | Aqueous extract (0.045 mg/mL) | NO release from S-nitrosoglutathione | Induced NO release | [93] |
| Allium ascalonicum | Ethyl acetate fraction from a hydroethanolic extract (500 and 800 ng/mL) | HUVEC: Angiogenesis Chorioallantoic membrane assay | Promoted angiogenesis | [123] |
| Allium cepa | Aqueous extract (0.045 mg/mL) | NO release from S-nitrosoglutathione | Induced NO release | [93] |
| Allium sativum | Aged garlic extract (1–4 mg/mL) | HUVEC | ↑ HO-1, GCLM and Nrf2 activation | [124] |
| Aged garlic extract (p.o. for 6 weeks; 4% in chow) | Rat: Folate-deficient diet | ↓ Homocysteine total, protein-bound and free levels | [125] | |
| Chloroform extract of aged black garlic (30 min before treatment; 30 μg/mL) | HUVEC: TNF-α | ↓ ROS, NF-κB activation, VCAM-1 mRNA and protein expression and THP-1 adhesion to HUVEC | [126] | |
| Aqueous extract (0.045 mg/mL) | NO release from S-nitrosoglutathione | Induced NO release | [93] | |
| Aqueous extract (0.2–1.0%) | Macrophages/HUVEC: LPS- and IFNγ stimulated | Macrophages: ↓ iNOS expression HUVEC: ↑ eNOS activity and cGMP levels | [127] | |
| Garlic skin or flesh extract (1, 2.5 and 5 µL/mL) | Cardiomyocyte: NE-induced hypertrophy | ↓ Cell hypertrophy, cell death, apoptosis, and oxidative stress | [128] | |
| Aqueous extract (p.o. for 5 weeks; 800 µg/Kg) | Rabbit: Vascular restenosis | ↓ Myointimal hyperplasia | [129] | |
| Hydroalcoholic extract (1, 10, 50, and 100 μg/mL) | Mice: LPS-stimulated heart | ↓ PGE2 and 8-iso-PGF2α levels; ↓ COX2, IL-6 and NF-κB mRNA | [130] | |
| Aqueous fraction of garlic powder (4 days before treatment; 0.25–4.0 mg/mL) | CAEC: IL-1α | ↓ ICAM-1, VCAM-1 and monocyte adhesion to ECs | [131] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves-Silva, J.M.; Zuzarte, M.; Girão, H.; Salgueiro, L. Natural Products in Cardiovascular Diseases: The Potential of Plants from the Allioideae Subfamily (Ex-Alliaceae Family) and Their Sulphur-Containing Compounds. Plants 2022, 11, 1920. https://doi.org/10.3390/plants11151920
Alves-Silva JM, Zuzarte M, Girão H, Salgueiro L. Natural Products in Cardiovascular Diseases: The Potential of Plants from the Allioideae Subfamily (Ex-Alliaceae Family) and Their Sulphur-Containing Compounds. Plants. 2022; 11(15):1920. https://doi.org/10.3390/plants11151920
Chicago/Turabian StyleAlves-Silva, Jorge M., Mónica Zuzarte, Henrique Girão, and Lígia Salgueiro. 2022. "Natural Products in Cardiovascular Diseases: The Potential of Plants from the Allioideae Subfamily (Ex-Alliaceae Family) and Their Sulphur-Containing Compounds" Plants 11, no. 15: 1920. https://doi.org/10.3390/plants11151920
APA StyleAlves-Silva, J. M., Zuzarte, M., Girão, H., & Salgueiro, L. (2022). Natural Products in Cardiovascular Diseases: The Potential of Plants from the Allioideae Subfamily (Ex-Alliaceae Family) and Their Sulphur-Containing Compounds. Plants, 11(15), 1920. https://doi.org/10.3390/plants11151920








