The Role of Hemicellulose in Cadmium Tolerance in Ramie (Boehmeria nivea (L.) Gaud.)
Abstract
:1. Introduction
2. Results
2.1. Effects of Cd on Plant Growth
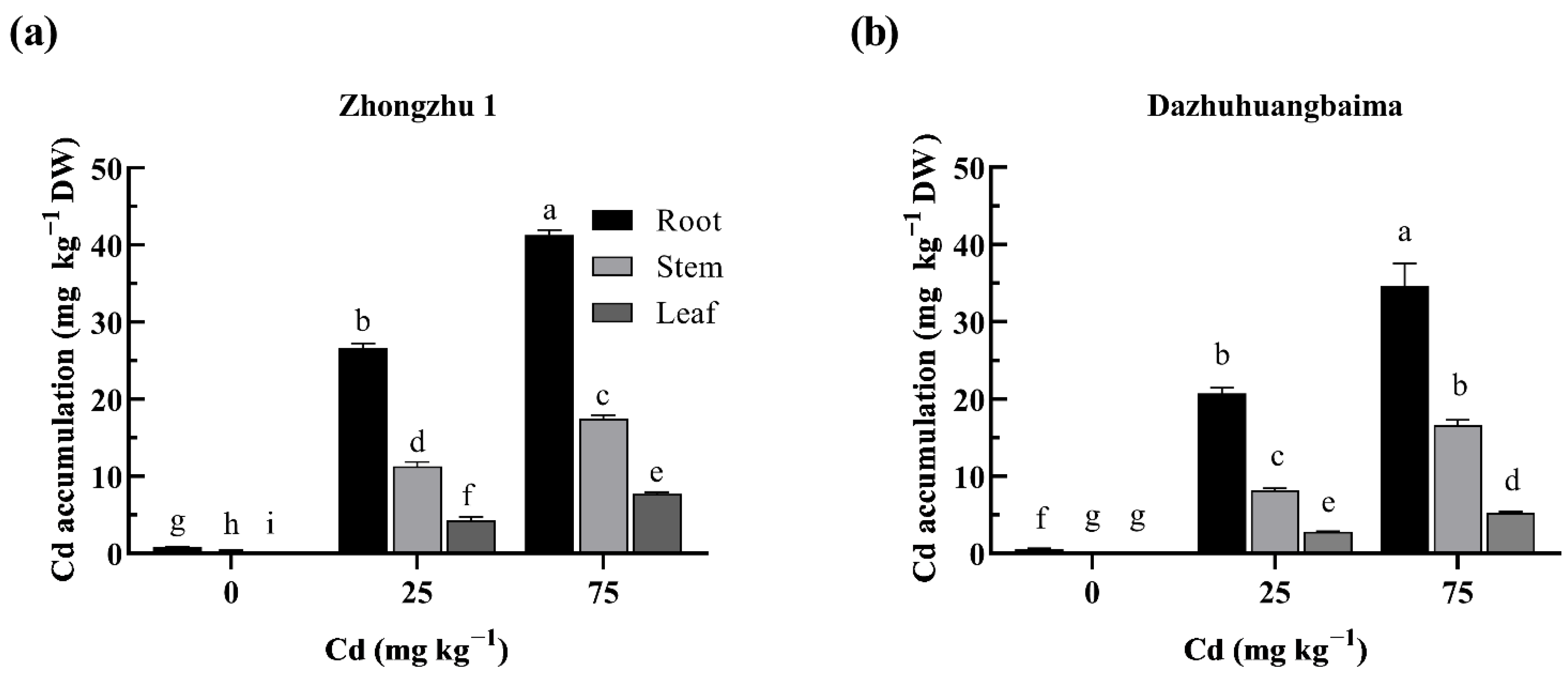
2.2. Cd Accumulation in Ramie under Cd Treatment
2.3. Subcellular Cd Distribution
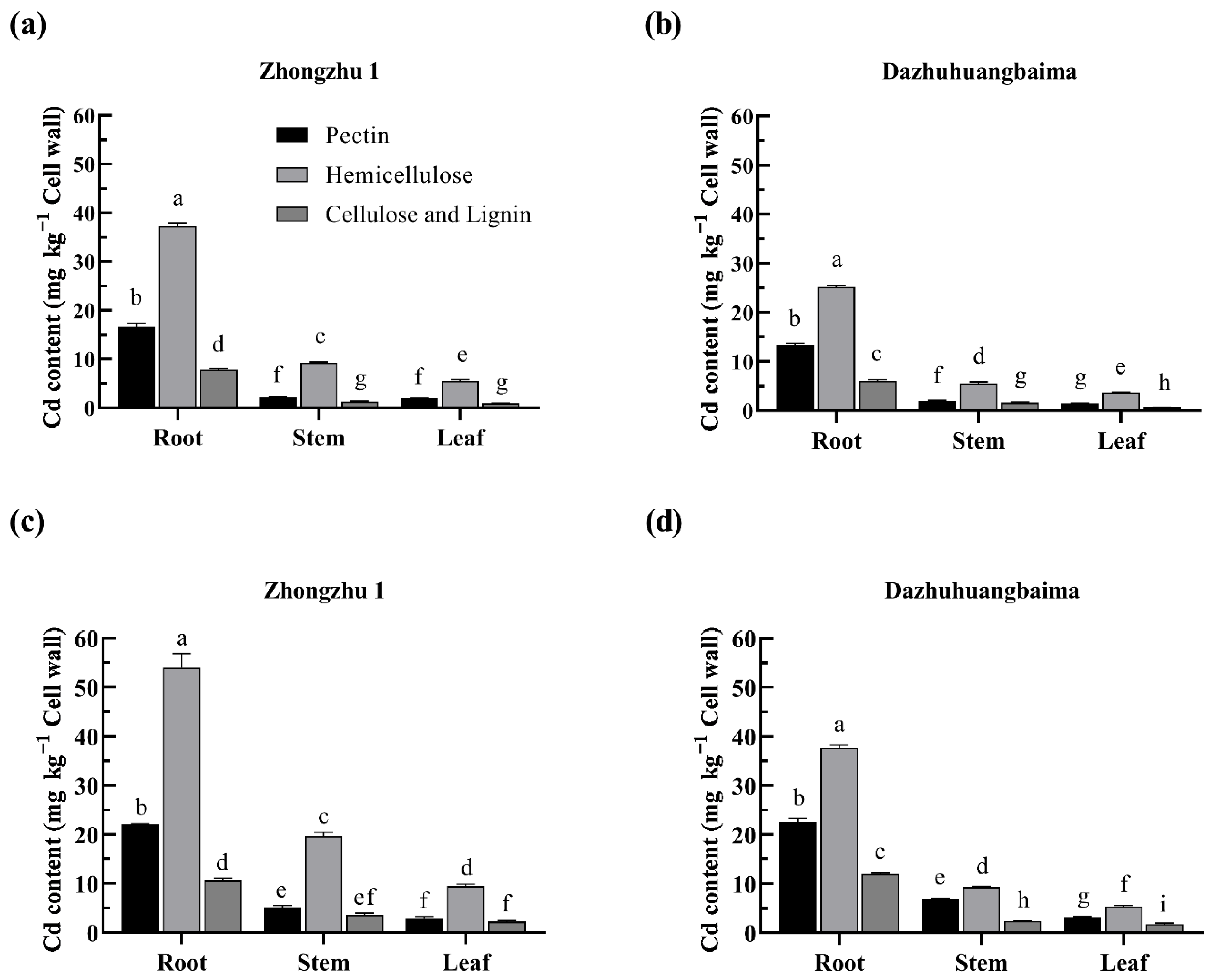
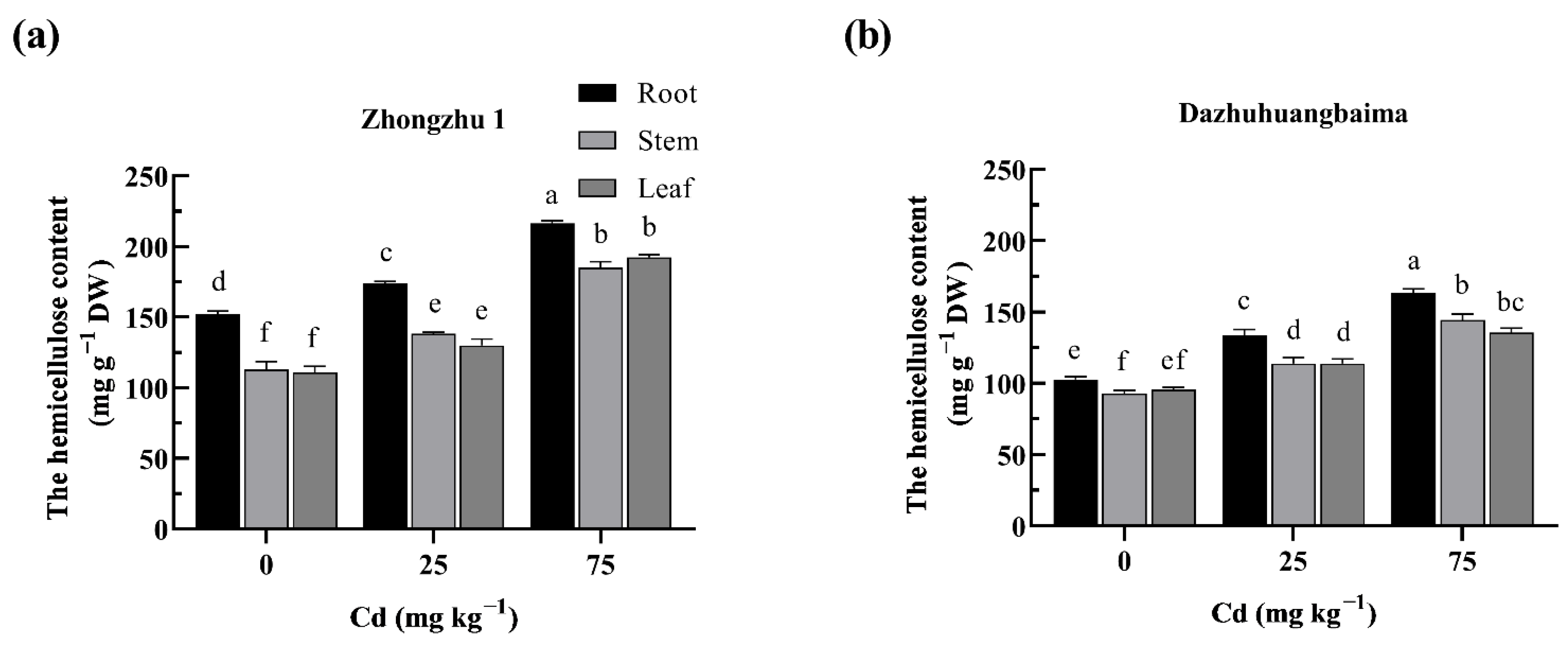
2.4. Hemicellulose Was the Major Target of Cd
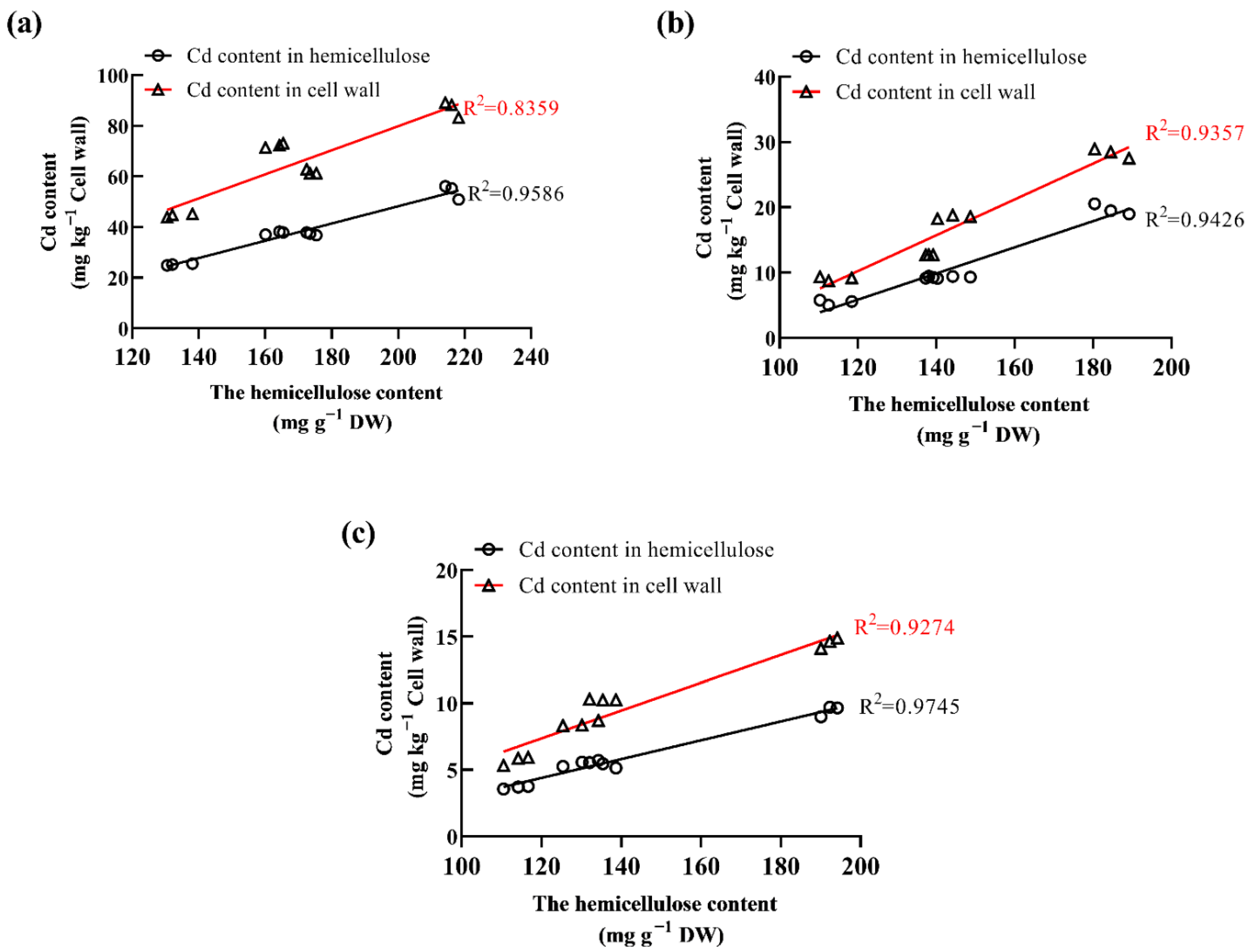
2.5. The Link between Hemicellulose Content and Cd Accumulation
3. Discussion
4. Materials and Methods
4.1. Plant Growth and Cd Treatment
4.2. Separation of Subcellular Fractions
4.3. Cell Wall Extraction and Fractionation
4.4. Extraction of Hemicelluloses, Celluloses, and Pectins from Ramie
4.5. The Contents of Hemicellulose
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Nagpal, A.K. Contamination of vegetables with heavy metals across the globe: Hampering food security goal. J. Food Sci. Technol. 2019, 57, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Gebrekidan, A.; Weldegebriel, Y.; Hadera, A.; Van der Bruggen, B. Toxicological assessment of heavy metals accumulated in vegetables and fruits grown in Ginfel river near Sheba Tannery, Tigray, Northern Ethiopia. Ecotoxicol. Environ. Saf. 2013, 95, 171–178. [Google Scholar] [CrossRef]
- Tiwari, K.; Singh, N.; Patel, M.; Tiwari, M.; Rai, U. Metal contamination of soil and translocation in vegetables growing under industrial wastewater irrigated agricultural field of Vadodara, Gujarat, India. Ecotoxicol. Environ. Saf. 2011, 74, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Proshad, R.; Haque, M.A.; Hoque, F.; Hossin, S.; Sarker, N.I. Assessment of heavy metals in foods around the industrial areas: Health hazard inference in Bangladesh. Geocarto Int. 2018, 35, 280–295. [Google Scholar] [CrossRef]
- Du, Y.; Hu, X.-F.; Wu, X.-H.; Shu, Y.; Jiang, Y.; Yan, X.-J. Affects of mining activities on Cd pollution to the paddy soils and rice grain in Hunan province, Central South China. Environ. Monit. Assess. 2013, 185, 9843–9856. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Chen, Y.; Li, Z.; Hedding, D.W.; Nel, W.; Ji, J.; Chen, J. Geochemical behavior and potential health risk of heavy metals in basalt-derived agricultural soil and crops: A case study from Xuyi County, eastern China. Sci. Total Environ. 2020, 729, 139058. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, W.-T.; Zhou, X.; Liu, L.; Gu, J.-F.; Wang, W.-L.; Zou, J.-L.; Tian, T.; Peng, P.-Q.; Liao, B.-H. Accumulation of Heavy Metals in Vegetable Species Planted in Contaminated Soils and the Health Risk Assessment. Int. J. Environ. Res. Public Health 2016, 13, 289. [Google Scholar] [CrossRef] [Green Version]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Wang, J.; Fang, W.; Yuan, J.; Yang, Z. Cadmium accumulation in different rice cultivars and screening for pollution-safe cultivars of rice. Sci. Total Environ. 2006, 370, 302–309. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, H.; Tao, S. The Challenges and Solutions for Cadmium-contaminated Rice in China: A Critical Review. Environ. Int. 2016, 92–93, 515–532. [Google Scholar] [CrossRef] [PubMed]
- DalCorso, G.; Farinati, S.; Furini, A. Regulatory networks of cadmium stress in plants. Plant Signal. Behav. 2010, 5, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Gogorcena, Y.; Larbi, A.; Andaluz, S.; Carpena, R.O.; Abadía, A.; Abadia, J. Effects of cadmium on cork oak (Quercus suber L.) plants grown in hydroponics. Tree Physiol. 2011, 31, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Sandalio, L.; Dalurzo, H.; Gómez, M.; Romero-Puertas, M.; del Río, L. Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J. Exp. Bot. 2001, 52, 2115–2126. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Weng, B.; Xie, X.; Weiss, D.J.; Liu, J.; Lu, H.; Yan, C. Kandelia obovata (S., L.) Yong tolerance mechanisms to Cadmium: Subcellular distribution, chemical forms and thiol pools. Mar. Pollut. Bull. 2012, 64, 2453–2460. [Google Scholar] [CrossRef] [PubMed]
- Fernández, R.; Fernández-Fuego, D.; Bertrand, A.; González, A. Strategies for Cd accumulation in Dittrichia viscosa (L.) Greuter: Role of the cell wall, non-protein thiols and organic acids. Plant Physiol. Biochem. 2014, 78, 63–70. [Google Scholar] [CrossRef]
- Bora, M.S.; Sarma, K.P. Anatomical and ultrastructural alterations in Ceratopteris pteridoides under cadmium stress: A mechanism of cadmium tolerance. Ecotoxicol. Environ. Saf. 2021, 218, 112285. [Google Scholar] [CrossRef]
- Lan, X.-Y.; Yan, Y.-Y.; Yang, B.; Li, X.-Y.; Xu, F.-L. Subcellular distribution of cadmium in a novel potential aquatic hyperaccumulator—Microsorum pteropus. Environ. Pollut. 2019, 248, 1020–1027. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Zeng, G.; Chai, L.; Song, X.; Min, Z.; Xiao, X. Subcellular distribution and chemical forms of cadmium in Bechmeria nivea (L.) Gaud. Environ. Exp. Bot. 2008, 62, 389–395. [Google Scholar] [CrossRef]
- Gao, M.Y.; Chen, X.W.; Huang, W.X.; Wu, L.; Yu, Z.S.; Xiang, L.; Mo, C.H.; Li, Y.W.; Cai, Q.Y.; Wong, M.H.; et al. Cell wall modification induced by an arbuscular mycorrhizal fungus enhanced cadmium fixation in rice root. J. Hazard. Mater. 2021, 416, 125894. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Yu, S.; Lian, J.; Wang, Q.; He, Z.; Feng, Y.; Yang, X. Physiological and metabolomics responses of two wheat (Triticum aestivum L.) genotypes differing in grain cadmium accumulation. Sci. Total Environ. 2021, 769, 145345. [Google Scholar] [CrossRef] [PubMed]
- Chambat, G.; Barnoud, F.; Joseleau, J.-P. Structure of the Primary Cell Walls of Suspension-Cultured Rosa glauca Cells: I. polysaccharides associated with cellulose. Plant Physiol. 1984, 74, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, A.P.S.; Randhawa, G.S.; Dhugga, K.S. Plant Cell Wall Matrix Polysaccharide Biosynthesis. Mol. Plant 2009, 2, 840–850. [Google Scholar] [CrossRef]
- Tan, L.; Eberhard, S.; Pattathil, S.; Warder, C.; Glushka, J.; Yuan, C.; Hao, Z.; Zhu, X.; Avci, U.; Miller, J.S.; et al. An Arabidopsis Cell Wall Proteoglycan Consists of Pectin and Arabinoxylan Covalently Linked to an Arabinogalactan Protein. Plant Cell 2013, 25, 270–287. [Google Scholar] [CrossRef] [Green Version]
- Haynes, R.J. Ion exchange properties of roots and ionic interactions within the root apoplasm: Their role in ion accumulation by plants. Bot. Rev. 1980, 46, 75–99. [Google Scholar] [CrossRef]
- Davis, T.A.; Volesky, B.; Mucci, A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003, 37, 4311–4330. [Google Scholar] [CrossRef]
- Ren, C.; Qi, Y.; Huang, G.; Yao, S.; You, J.; Hu, H. Contributions of root cell wall polysaccharides to Cu sequestration in castor (Ricinus communis L.) exposed to different Cu stresses. J. Environ. Sci. 2020, 88, 209–216. [Google Scholar] [CrossRef]
- Yang, J.L.; Zhu, X.F.; Peng, Y.X.; Zheng, C.; Li, G.X.; Liu, Y.; Shi, Y.Z.; Zheng, S.J. Cell Wall Hemicellulose Contributes Significantly to Aluminum Adsorption and Root Growth in Arabidopsis. Plant Physiol. 2011, 155, 1885–1892. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.F.; Wang, Z.W.; Dong, F.; Lei, G.J.; Shi, Y.Z.; Li, G.X.; Zheng, S.J. Exogenous auxin alleviates cadmium toxicity in Arabidopsis thaliana by stimulating synthesis of hemicellulose 1 and increasing the cadmium fixation capacity of root cell walls. J. Hazard. Mater. 2013, 263, 398–403. [Google Scholar] [CrossRef]
- Zhu, X.F.; Zhao, X.S.; Wang, B.; Wu, Q.; Shen, R.F. Elevated Carbon Dioxide Alleviates Aluminum Toxicity by Decreasing Cell Wall Hemicellulose in Rice (Oryza sativa). Front. Physiol. 2017, 8, 512. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, R.; Yan, X.; Liang, X.; Sun, Y.; Xu, Y. Pivotal role for root cell wall polysaccharides in cultivar-dependent cadmium accumulation in Brassica chinensis L. Ecotoxicol. Environ. Saf. 2020, 194, 110369. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yang, B.; Wan, H.; Fang, X.; Yang, C. The differences of cell wall in roots between two contrasting soybean cultivars exposed to cadmium at young seedlings. Environ. Sci. Pollut. Res. 2018, 25, 29705–29714. [Google Scholar] [CrossRef]
- Zhu, Q.H.; Huang, D.Y.; Liu, S.L.; Luo, Z.C.; Rao, Z.X.; Cao, X.L.; Ren, X.F. Accumulation and subcellular distribution of cadmium in ramie (Boehmeria nivea L. Gaud.) planted on elevated soil cadmium contents. Plant Soil Environ. 2013, 59, 57–61. [Google Scholar]
- Lan, M.-M.; Liu, C.; Liu, S.-J.; Qiu, R.-L.; Tang, Y.-T. Phytostabilization of Cd and Pb in Highly Polluted Farmland Soils Using Ramie and Amendments. Int. J. Environ. Res. Public Health 2020, 17, 1661. [Google Scholar] [CrossRef] [Green Version]
- Sanità di Toppi, L.; Gabbrielli, R. Response to cadmium in higher plants. Environ. Exp. Bot. 1999, 41, 105–130. [Google Scholar] [CrossRef]
- Ramos, I.; Esteban, E.; Lucena, J.J.; Gárate, A. Cadmium uptake and subcellular distribution in plants of Lactuca sp. Cd–Mn interaction. Plant Sci. 2002, 162, 761–767. [Google Scholar] [CrossRef]
- Mwamba, T.M.; Li, L.; Gill, R.A.; Islam, F.; Nawaz, A.; Ali, B.; Farooq, M.A.; Lwalaba, J.L.; Zhou, W. Differential subcellular distribution and chemical forms of cadmium and copper in Brassica napus. Ecotoxicol. Environ. Saf. 2016, 134, 239–249. [Google Scholar] [CrossRef]
- Fu, X.; Dou, C.; Chen, Y.; Chen, X.; Shi, J.; Yu, M.; Xu, J. Subcellular distribution and chemical forms of cadmium in Phytolacca americana L. J. Hazard. Mater. 2011, 186, 103–107. [Google Scholar] [CrossRef]
- Wu, F.-B.; Dong, J.; Qian, Q.Q.; Zhang, G.-P. Subcellular distribution and chemical form of Cd and Cd–Zn interaction in different barley genotypes. Chemosphere 2005, 60, 1437–1446. [Google Scholar] [CrossRef]
- Dai, M.; Liu, W.; Hong, H.; Lu, H.; Liu, J.; Jia, H.; Yan, C. Exogenous phosphorus enhances cadmium tolerance by affecting cell wall polysaccharides in two mangrove seedlings Avicennia marina (Forsk.) Vierh and Kandelia obovata (S., L.) Yong differing in cadmium accumulation. Mar. Pollut. Bull. 2017, 126, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Liu, Y.K.; Zhang, R.; Luo, J.P.; Li, J.X.; Wu, K.R.; Peng, L.C.; Liu, Y.Y.; Du, Y.L.; Liang, Y.C.; et al. Hemicellulose modification promotes cadmium hyperaccumulation by decreasing its retention on roots in Sedum alfredii. Plant Soil 2020, 447, 241–255. [Google Scholar] [CrossRef]
- Liu, T.; Shen, C.; Wang, Y.; Huang, C.; Shi, J. New Insights into Regulation of Proteome and Polysaccharide in Cell Wall of Elsholtzia splendens in Response to Copper Stress. PLoS ONE 2014, 9, e109573. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, L.; Chen, S.-B.; Li, N.; Zheng, H.; Jin, K.; Pang, H.-C.; Ma, Y.-B. Subcellular Cd accumulation characteristic in root cell wall of rice cultivars with different sensitivities to Cd stress in soil. J. Integr. Agric. 2016, 15, 2114–2122. [Google Scholar] [CrossRef]
- Chen, G.; Liu, Y.; Wang, R.; Zhang, J.; Owens, G. Cadmium adsorption by willow root: The role of cell walls and their subfractions. Environ. Sci. Pollut. Res. 2013, 20, 5665–5672. [Google Scholar] [CrossRef]
- Loix, C.; Hybrechts, M.; Vangronsveld, J.; Gielen, M.; Keunen, E.; Cuypers, A. Reciprocal interactions between cadmium-induced cell wall response and oxidative stress in plants. Front. Plant Sci. 2017, 8, 1867. [Google Scholar] [CrossRef] [Green Version]
- Carpita, N.C.; Gibeaut, D.M. Structural models of primary cell walls in flowering plants: Consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993, 3, 1–30. [Google Scholar] [CrossRef]
- Zhu, C.Q.; Cao, X.C.; Zhu, L.F.; Hu, W.J.; Hu, A.Y.; Bai, Z.G.; Zhong, C.; Sun, L.M.; Liang, Q.D.; Huang, J.; et al. Ammonium mitigates Cd toxicity in rice (Oryza sativa) cvia putrescine dependent alterations of cell wall composition. Plant Physiol. Biochem. 2018, 132, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Rakhshaee, R.; Giahi, M.; Pourahmad, A. Studying effect of cell wall’s carboxyl–carboxylate ratio change of Lemna minor to remove heavy metals from aqueous solution. J. Hazard. Mater. 2009, 163, 165–173. [Google Scholar] [CrossRef]
- Krzesłowska, M. The cell wall in plant cell response to trace metals: Polysaccharide remodeling and its role in defense strategy. Acta Physiol. Plant. 2010, 33, 35–51. [Google Scholar] [CrossRef] [Green Version]
- Sarath, N.G.; Shackira, A.; El-Serehy, H.A.; Hefft, D.I.; Puthur, J.T. Phytostabilization of arsenic and associated physio-anatomical changes in Acanthus ilicifolius L. Environ. Pollut. 2022, 298, 118828. [Google Scholar] [CrossRef]
- Yang, J.L.; Li, Y.Y.; Zhang, Y.J.; Zhang, S.S.; Wu, Y.R.; Wu, P.; Zheng, S.J. Cell Wall Polysaccharides Are Specifically Involved in the Exclusion of Aluminum from the Rice Root Apex. Plant Physiol. 2007, 146, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Tao, Q.; Shohag, M.J.I.; Yang, X.; Sparks, D.L.; Liang, Y. Root cell wall polysaccharides are involved in cadmium hyperaccumulation in Sedum alfredii. Plant Soil 2015, 389, 387–399. [Google Scholar] [CrossRef]
- Wang, X.-H.; Wang, Q.; Nie, Z.-W.; He, L.-Y.; Sheng, X.-F. Ralstonia eutropha Q2-8 reduces wheat plant above-ground tissue cadmium and arsenic uptake and increases the expression of the plant root cell wall organization and biosynthesis-related proteins. Environ. Pollut. 2018, 242, 1488–1499. [Google Scholar] [CrossRef]
- Hossain, A.K.M.Z.; Hossain, M.A.; Asgar, M.A.; Tosaki, T.; Koyama, H.; Hara, T. Changes in Cell Wall Polysaccharides and Hydroxycinnamates in Wheat Roots by Aluminum Stress at Higher Calcium Supply. J. Plant Nutr. 2006, 29, 601–613. [Google Scholar] [CrossRef]
- Wei, L.; Luo, C.; Li, X.; Shen, Z. Copper Accumulation and Tolerance in Chrysanthemum coronarium L. and Sorghum sudanense L. Arch. Environ. Contam. Toxicol. 2008, 55, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Zhang, L.; Li, F.; Zhang, D.; Liu, X.; Wang, H.; Xu, Z.; Chu, C.; Zhou, Y. Control of secondary cell wall patterning involves xylan deacetylation by a GDSL esterase. Nat. Plants 2017, 3, 17017. [Google Scholar] [CrossRef]
- Wan, J.-X.; Zhu, X.-F.; Wang, Y.-Q.; Liu, L.-Y.; Zhang, B.-C.; Li, G.-X.; Zhou, Y.-H.; Zheng, S.-J. Xyloglucan Fucosylation Modulates Arabidopsis Cell Wall Hemicellulose Aluminium binding Capacity. Sci. Rep. 2018, 8, 428. [Google Scholar] [CrossRef] [Green Version]
- Lai, H.-Y. Subcellular distribution and chemical forms of cadmium in Impatiens walleriana in relation to its phytoextraction potential. Chemosphere 2015, 138, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Suska-Malawska, M.; Vyrakhamanova, A.; Ibraeva, M.; Poshanov, M.; Sulwiński, M.; Toderich, K.; Mętrak, M. Spatial and In-Depth Distribution of Soil Salinity and Heavy Metals (Pb, Zn, Cd, Ni, Cu) in Arable Irrigated Soils in Southern Kazakhstan. Agronomy 2022, 12, 1207. [Google Scholar] [CrossRef]

| Populations | Treatments | Organs | Cell Wall | Organelle | Soluble Fraction |
|---|---|---|---|---|---|
| ‘Zhongzhu 1’ | Root | 16.08 ± 0.15a (60.28) | 4.86 ± 0.30c (18.21) | 5.73 ± 0.74b (21.50) | |
| 25 mg kg−1 | Stem | 8.11 ± 0.10a (71.93) | 1.39 ± 0.07c (12.33) | 1.78 ± 0.69b (15.77) | |
| Leaf | 3.03 ± 0.07a (70.72) | 0.44 ± 0.12c (10.35) | 0.81 ± 0.36b (18.88) | ||
| Root | 26.26 ± 0.14a (63.56) | 5.57 ± 0.43c (13.47) | 9.73 ± 0.50b (23.55) | ||
| 75 mg kg−1 | Stem | 11.02 ± 0.14a (62.99) | 1.89 ± 0.10c (10.78) | 4.59 ± 0.45b (26.22) | |
| Leaf | 5.04 ± 0.06a (65.32) | 0.95 ± 0.07c (12.28) | 1.73 ± 0.35b (22.44) | ||
| ‘Dazhuhuangbaima’ | Root | 9.95 ± 1.29a (48.11) | 5.05 ± 0.08c (24.42) | 5.68 ± 0.22b (27.47) | |
| 25 mg kg−1 | Stem | 4.59 ± 0.16a (56.18) | 1.82 ± 0.09b (22.28) | 1.76 ± 0.13b (21.54) | |
| Leaf | 1.66 ± 0.18a (59.19) | 0.42 ± 0.08c (14.95) | 0.73 ± 0.15b (25.88) | ||
| Root | 18.87 ± 0.37a (54.51) | 6.31 ± 0.76c (18.23) | 9.44 ± 0.28b (27.27) | ||
| 75 mg kg−1 | Stem | 8.88 ± 0.13a (53.46) | 2.06 ± 0.05c (12.40) | 5.67 ± 0.39b (34.14) | |
| Leaf | 3.31 ± 0.20a (45.66) | 1.86 ± 0.06b (25.66) | 2.08 ± 0.33b (28.69) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Jie, H.; Tang, Y.; Xing, H.; Jie, Y. The Role of Hemicellulose in Cadmium Tolerance in Ramie (Boehmeria nivea (L.) Gaud.). Plants 2022, 11, 1941. https://doi.org/10.3390/plants11151941
Ma Y, Jie H, Tang Y, Xing H, Jie Y. The Role of Hemicellulose in Cadmium Tolerance in Ramie (Boehmeria nivea (L.) Gaud.). Plants. 2022; 11(15):1941. https://doi.org/10.3390/plants11151941
Chicago/Turabian StyleMa, Yushen, Hongdong Jie, Yanyi Tang, Hucheng Xing, and Yucheng Jie. 2022. "The Role of Hemicellulose in Cadmium Tolerance in Ramie (Boehmeria nivea (L.) Gaud.)" Plants 11, no. 15: 1941. https://doi.org/10.3390/plants11151941
APA StyleMa, Y., Jie, H., Tang, Y., Xing, H., & Jie, Y. (2022). The Role of Hemicellulose in Cadmium Tolerance in Ramie (Boehmeria nivea (L.) Gaud.). Plants, 11(15), 1941. https://doi.org/10.3390/plants11151941






