Enhanced Apiaceous Potyvirus Phylogeny, Novel Viruses, and New Country and Host Records from Sequencing Apiaceae Samples
Abstract
:1. Introduction
2. Results
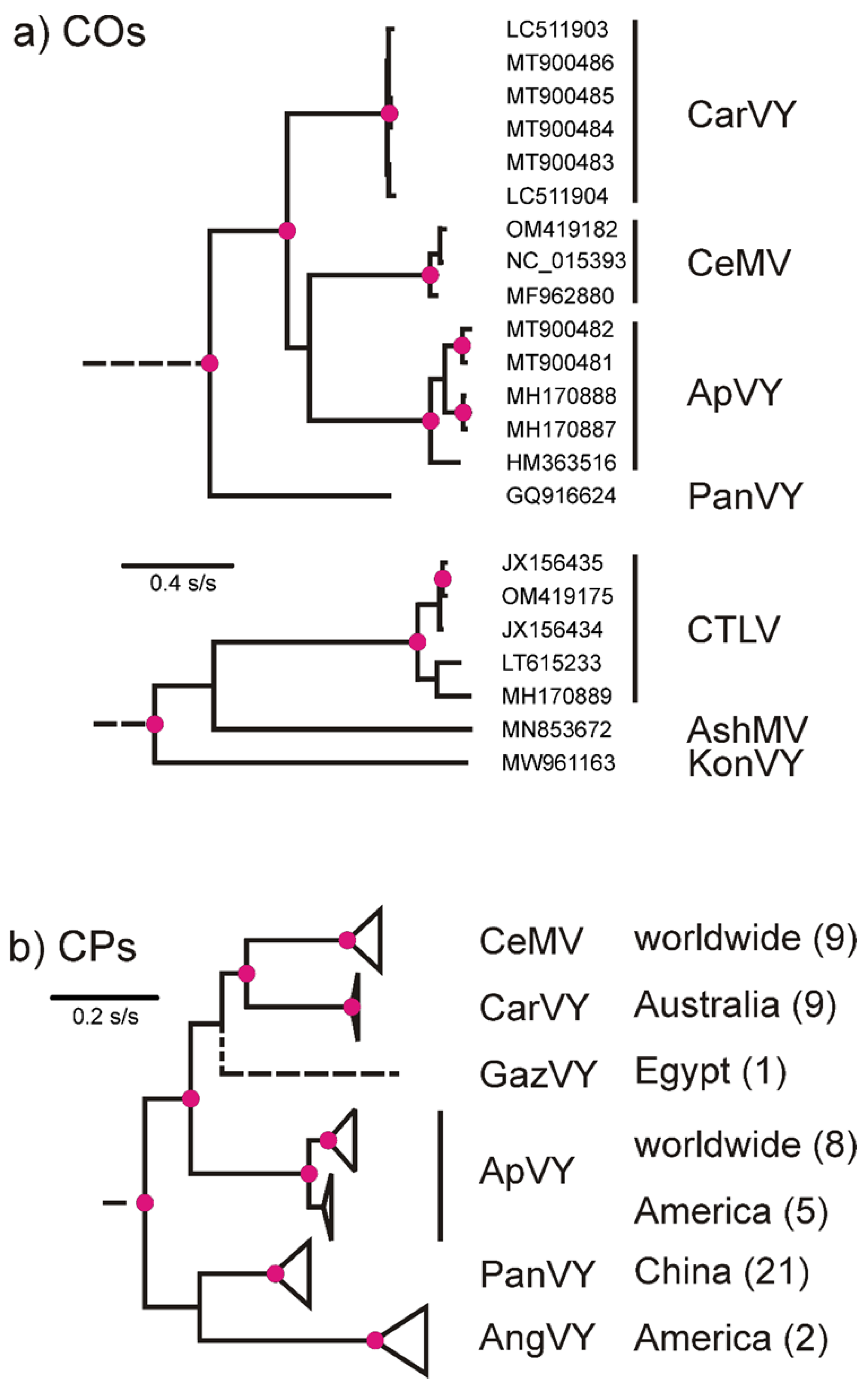
2.1. Potyvirus Detection and Diversity
2.2. Beet Western Yellows Virus Detected from Coriander—A New Host Record in the Apiaceae
2.3. Carrot Torrado Virus 1—A New Country Record for Australia
2.4. A Novel Ophiovirus and an Unclassified RNA Virus from Australian Carrot
2.5. A Novel Umbravirus from Parsley Imported into the UK
3. Discussion
4. Materials and Methods
4.1. Sample Source
4.2. High Throughput Sequencing (HTS) and Bioinformatics
4.3. Phylogenetic Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petruzzello, M. List of Plants in the Family Apiaceae; Encyclopædia Britannica, Inc.: Chicago, IL, USA, 2019. [Google Scholar]
- Brunt, A.; Crabtree, K.; Dallwitz, M.; Gibbs, A.; Watson, L. Viruses of Plants; CAB International: Wallingford, UK, 1996. [Google Scholar]
- Menzel, W.; Vetten, H. Complete nucleotide sequence of an isolate of the Anthriscus strain of Parsnip yellow fleck virus. Arch. Virol. 2008, 153, 2173–2175. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, P.; Bos, L. Survey and biological differentiation of viruses of wild and cultivated Umbelliferae in the Netherlands. Neth. J. Plant Pathol. 1989, 95, 1–34. [Google Scholar] [CrossRef]
- Watson, M.; Tian, T.; Estabrook, E.; Falk, B. A small RNA identified as a component of California carrot motley dwarf resembles the beet western yellows luteovirus ST9-associated RNA. Phytopathology 1998, 88, 164–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbs, M.; Ziegler, A.; Robinson, D.; Waterhouse, P.; Cooper, J. Carrot mottle mimic virus (CMoMV): A second umbravirus associated with carrot motley dwarf disease recognised by nucleic acid hybridisation. Mol. Plant Pathol. Line 1996. [Google Scholar]
- Tang, J.; Quinn, B.; Clover, G. First report of Carrot red leaf virus-associated RNA co-infecting carrot with Carrot red leaf virus and Carrot mottle mimic virus to cause carrot motley dwarf disease in New Zealand. Australas. Plant Dis. Notes 2009, 4, 15–16. [Google Scholar]
- Jones, R.; Smith, L.; Gajda, B.; Latham, L. Patterns of spread of Carrot virus Y in carrot plantings and validation of control measures. Ann. Appl. Biol. 2005, 147, 57–67. [Google Scholar] [CrossRef]
- Jones, R.; Smith, L.; Gajda, B.; Smith, T.; Latham, L. Further studies on Carrot virus Y: Hosts, symptomatology, search for resistance, and tests for seed transmissibility. Aust. J. Agric. Res. 2005, 56, 859–868. [Google Scholar] [CrossRef]
- Latham, L.J.; Jones, R.A. Carrot virus Y: Symptoms, losses, incidence, epidemiology and control. Virus Res. 2004, 100, 89–99. [Google Scholar] [CrossRef]
- Latham, L.; Traicevski, V.; Persley, D.; Wilson, C.; Tesoriero, L.; Coles, R.; Jones, R. Distribution and incidence of Carrot virus Y in Australia. Australas. Plant Pathol. 2004, 33, 83–86. [Google Scholar] [CrossRef] [Green Version]
- Moran, J.; Van Rijswijk, B.; Traicevski, V.; Kitajima, E.W.; Mackenzie, A.; Gibbs, A. Potyviruses, novel and known, in cultivated and wild species of the family Apiaceae in Australia. Arch. Virol. 2002, 147, 1855–1867. [Google Scholar] [CrossRef]
- Latham, L.; Jones, R. Incidence of celery mosaic virus in celery crops in south-west Australia and its management using a ‘celery-free period’. Australas. Plant Pathol. 2003, 32, 527–531. [Google Scholar] [CrossRef]
- Tang, J.; Clover, G.; Alexander, B. First report of apium virus Y in celery in New Zealand. Plant Dis. 2007, 91, 1682. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Liu, H.-Y.; Koike, S. First report of Apium virus Y on cilantro, celery, and parsley in California. Plant Dis. 2008, 92, 1254. [Google Scholar] [CrossRef]
- Mehle, N.; Kutnjak, D.; Tušek Žnidarič, M.; Ravnikar, M. First report of apium virus Y and carrot thin leaf virus in parsley in Slovenia. Plant Dis. 2019, 103, 592. [Google Scholar] [CrossRef]
- Bos, L.; Mandersloot, H.; Vader, F.; Steenbergen, B. An epidemic of celery mosaic potyvirus in celeriac (Apium graveolens var. rapaceum) in the Netherlands. Neth. J. Plant Pathol. 1989, 95, 225–240. [Google Scholar] [CrossRef]
- Fernández, T.; Carballo, O.; Zambrano, K.; Romano, M.; Marys, E. First report of celery mosaic virus infecting celery in Venezuela. Plant Dis. 2006, 90, 1111. [Google Scholar] [CrossRef] [PubMed]
- Sutabutra, T.; Campbell, R. Strains of celery mosaic virus from parsley and poison hemlock in California. Plant Dis. Report. 1971, 55, 328–332. [Google Scholar]
- Howell, W.; Mink, G. Incidence of carrot thin leaf virus and carrot motley dwarf virus diseases in commercial carrots grown in Washington State during 1974 and 1975. Plant Dis. Report. 1976, 60, 1047–1049. [Google Scholar]
- Xu, D.; Liu, H.; Li, F.; Howell, B.; Tian, T.; Li, R. Biological characterization and complete genomic sequence of carrot thin leaf virus. Phytopathology 2011, 101, S195. [Google Scholar]
- Menzel, W.; Barg, E.; Vetten, H. First evidence of the carrot thin leaf virus for Germany and Europe-studies on the variability and distribution. Jul. Kühn-Arch. 2010, 428, 333–334. [Google Scholar]
- Lotos, L.; Olmos, A.; Katis, N.; Maliogka, V.I. First report of carrot torrado virus 1 and carrot thin leaf virus naturally infecting Torilis arvensis ssp. arvensis in Greece. Plant Dis. 2018, 102, 2049. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.; Ahmed, A.A.; Mohamed, M.S.; Hanna, D.H.; Barsoum, B. Gazar virus Y, a new member of the celery mosaic virus group of potyviruses, isolated from carrots in Egypt. Australas. Plant Pathol. 2012, 41, 529–534. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Soliman, A.M.; Waziri, H.M. Occurrence of carrot virus Y potyvirus in Egypt. Egypt. J. Virol. 2012, 9, 60–79. [Google Scholar]
- Robertson, N. Identification and characterization of a new virus in the genus Potyvirus from wild populations of Angelica lucida L. and A. genuflexa Nutt., family Apiaceae. Arch. Virol. 2007, 152, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, R.; Wang, J.; Chang, J.; Zhang, X.; Chen, T.; An, L.; Xu, S. A new potyvirus first isolated and identified from Angelica sinensis. Virus Genes 2009, 39, 120–125. [Google Scholar] [CrossRef]
- Nury, S.; Hosseini, A.; Gibbs, A.J.; Mohammadi, M. Poison hemlock virus Y (PHVY), a novel potyvirus from Iranian Conium maculatum (Apiaceae). Australas. Plant Pathol. 2020, 49, 119–126. [Google Scholar] [CrossRef]
- Bem, F.; Murant, A. Transmission and differentiation of six viruses infecting hogweed (Heracleum sphondylium) in Scotland. Ann. Appl. Biol. 1979, 92, 237–242. [Google Scholar] [CrossRef]
- Howell, W.; Mink, G. Viruses isolated from wild carrot and poison hemlock. Plant Dis. 1981, 65, 277–279. [Google Scholar] [CrossRef]
- Eastwell, K.; Glass, J.; Seymour, L.; Druffel, K. First report of infection of poison hemlock and celery by Apium virus Y in Washington State. Plant Dis. 2008, 92, 1710. [Google Scholar] [CrossRef]
- Adams, I.; Skelton, A.; Macarthur, R.; Hodges, T.; Hinds, H.; Flint, L.; Nath, P.; Boonham, N.; Fox, A. Carrot yellow leaf virus is associated with carrot internal necrosis. PLoS ONE 2014, 9, e109125. [Google Scholar] [CrossRef] [Green Version]
- Rozado-Aguirre, Z.; Marais, A.; Svanella-Dumas, L.; Faure, C.; Latour, F.; Villeneuve, F.; Dickinson, M.; Fox, A.; Boonham, N.; Candresse, T. First report of Carrot torradovirus 1 (CaTV1), a member of the Torradovirus Genus, infecting carrots in France. Plant Dis. 2017, 101, 1333. [Google Scholar] [CrossRef] [Green Version]
- Gaafar, Y.Z.; Ziebell, H. Complete genome sequence of a highly divergent carrot torradovirus 1 strain from Apium graveolens. Arch. Virol. 2019, 164, 1943–1947. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, R.; Nishikawa, M.; Hosoe, N.; Nijo, T.; Iwabuchi, N.; Yoshida, T.; Watanabe, K.; Maejima, K.; Yamaji, Y.; Namba, S. Complete genome sequence of a carrot torradovirus 1 isolate, obtained from Angelica keiskei in Japan. Microbiol. Resour. Announc. 2019, 8, e00110-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verbeek, M.; Dullemans, A.; Van den Heuvel, J.; Maris, P.; Van der Vlugt, R. Identification and characterisation of tomato torrado virus, a new plant picorna-like virus from tomato. Arch. Virol. 2007, 152, 881–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vetten, H.J.; Knierim, D.; Rakoski, M.S.; Menzel, W.; Maiss, E.; Gronenborn, B.; Winter, S.; Krenz, B. Identification of a novel nanovirus in parsley. Arch. Virol. 2019, 164, 1883–1887. [Google Scholar] [CrossRef]
- Alabi, O.J.; Gaytán, B.C.; Al Rwahnih, M.; Villegas, C. A description of the possible etiology of the cilantro yellow blotch disease. Plant Dis. 2020, 104, 630–633. [Google Scholar] [CrossRef]
- Jones, R.A.; Boonham, N.; Adams, I.P.; Fox, A. Historical virus isolate collections: An invaluable resource connecting plant virology’s pre-sequencing and post-sequencing eras. Plant Pathol. 2021, 70, 235–248. [Google Scholar] [CrossRef]
- Malmstrom, C.M.; Martin, M.D.; Gagnevin, L. Exploring the emergence and evolution of plant pathogenic microbes using historical and paleontological sources. Annu. Rev. Phytopathol. 2022, 60. [Google Scholar] [CrossRef]
- Martin, D.P.; Murrell, B.; Golden, M.; Khoosal, A.; Muhire, B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015, 1, vev003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, D.; Liu, H.-Y.; Li, F.; Li, R. Complete genome sequence of Celery mosaic virus and its relationship to other members of the genus Potyvirus. Arch. Virol. 2011, 156, 917–920. [Google Scholar] [CrossRef]
- Rambaut, A.; Lam, T.T.; Max Carvalho, L.; Pybus, O.G. Exploring the temporal structure of heterochronous sequences using TempEst (formerly Path-O-Gen). Virus Evol. 2016, 2, vew007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasaka, R.; Fukagawa, H.; Ikematsu, M.; Soda, H.; Korkmaz, S.; Golnaraghi, A.; Katis, N.; Ho, S.Y.; Gibbs, A.J.; Ohshima, K. The timescale of emergence and spread of turnip mosaic potyvirus. Sci. Rep. 2017, 7, 4240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbs, A.J.; Hajizadeh, M.; Ohshima, K.; Jones, R.A. The potyviruses: An evolutionary synthesis is emerging. Viruses 2020, 12, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, J. Evolution of eastern Asian and eastern North American disjunct distributions in flowering plants. Annu. Rev. Ecol. Syst. 1999, 30, 421–455. [Google Scholar] [CrossRef]
- Flegontov, P.; Altınışık, N.E.; Changmai, P.; Rohland, N.; Mallick, S.; Adamski, N.; Bolnick, D.A.; Broomandkhoshbacht, N.; Candilio, F.; Culleton, B.J. Palaeo-eskimo genetic ancestry and the peopling of Chukotka and North America. Nature 2019, 570, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Wallace-Senft, D. The Plants of St.Paul Island. 2019. Available online: https://stpaulislandtour.com/wp-content/uploads/2019/01/The_Plants_of_St_Paul_Island_Alaska.pdf (accessed on 3 March 2022).
- Veltre, D.W.; Pendleton, C.L.; Schively, S.A.; Hay, J.A.; Tatarenkova, N. Aleut Ethnobotany: An Annotated Bibliography; Conservation of Arctic Flora and Fauna (CAFF): Norðurslóð, Iceland, 2006. [Google Scholar]
- CABI. Beet Western Yellows Virus, Turnip (Mild) Yellows, Datasheet 10259; CABI Crop Protection Compendium: Wallingford, UK, 2021; Available online: https://www.cabi.org/cpc/datasheet/10259 (accessed on 3 March 2022).
- van der Vlugt, R.A.; Verbeek, M.; Dullemans, A.M.; Wintermantel, W.M.; Cuellar, W.J.; Fox, A.; Thompson, J.R. Torradoviruses. Annu. Rev. Phytopathol. 2015, 53, 485–512. [Google Scholar] [CrossRef]
- Rozado, Z.; Adams, I.; Collins, L.; Fox, A.; Dickinson, M.; Boonham, N. Detection and transmission of carrot torrado virus, a novel putative member of the Torradovirus genus. J. Virol. Methods 2016, 235, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, N. Biological and genetic characterization of carrot red leaf virus and its associated virus/RNA isolated from carrots in Hokkaido, Japan. Plant Pathol. 2020, 69, 1379–1389. [Google Scholar] [CrossRef]
- Fox, A. Reconsidering causal association in plant virology. Plant Pathol. 2020, 69, 956–961. [Google Scholar] [CrossRef]
- Sidharthan, V.K.; Chaturvedi, K.K.; Baranwal, V.K. Diverse RNA viruses in a parasitic flowering plant (spruce dwarf mistletoe) revealed through RNA-seq data mining. J. Gen. Plant Pathol. 2022, 88, 138–144. [Google Scholar] [CrossRef]
- Hodgetts, J.; Boonham, N.; Mumford, R.; Dickinson, M. Panel of 23S rRNA gene-based real-time PCR assays for improved universal and group-specific detection of phytoplasmas. Appl. Environ. Microbiol. 2009, 75, 2945–2950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryabov, E.; Taliansky, M.; Robinson, D.; Waterhouse, P.; Murant, A.; de Zoeten, G.; Falk, B.; Vetten, H.; Gibbs, M. Genus Umbravirus. In Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses; Elsevier Science: London, UK, 2012; pp. 1191–1195. [Google Scholar]
- Fowkes, A.R.; McGreig, S.; Pufal, H.; Duffy, S.; Howard, B.; Adams, I.P.; Macarthur, R.; Weekes, R.; Fox, A. Integrating high throughput sequencing into survey design reveals turnip yellows virus and soybean dwarf virus in pea (pisum sativum) in the United Kingdom. Viruses 2021, 13, 2530. [Google Scholar] [CrossRef] [PubMed]
- Gaafar, Y.Z.; Herz, K.; Hartrick, J.; Fletcher, J.; Blouin, A.G.; MacDiarmid, R.; Ziebell, H. Investigating the pea virome in Germany—Old friends and new players in the field (s). Front. Microbiol. 2020, 11, 583242. [Google Scholar] [CrossRef] [PubMed]
- Redila, C.D.; Prakash, V.; Nouri, S. Metagenomics analysis of the wheat virome identifies novel plant and fungal-associated viral sequences. Viruses 2021, 13, 2457. [Google Scholar] [CrossRef] [PubMed]
- Syller, J. Molecular and biological features of umbraviruses, the unusual plant viruses lacking genetic information for a capsid protein. Physiol. Mol. Plant Pathol. 2003, 63, 35–46. [Google Scholar] [CrossRef]
- Falk, B.; Duffus, J.; Morris, T. Transmission, host range, and serological properties of the viruses that cause lettuce speckles disease. Phytopathology 1979, 69, 612–617. [Google Scholar] [CrossRef]
- Murant, A. Carrot Mottle Virus. DPV 137. Available online: https://dpvweb.net/dpv/showdpv/?dpvno=137 (accessed on 10 March 2022).
- Fox, A.; Fowkes, A.; Skelton, A.; Harju, V.; Buxton-Kirk, A.; Kelly, M.; Forde, S.; Pufal, H.; Conyers, C.; Ward, R. Using high-throughput sequencing in support of a plant health outbreak reveals novel viruses in Ullucus tuberosus (Basellaceae). Plant Pathol. 2019, 68, 576–587. [Google Scholar]
- Joshi, N.; Fass, J. Sickle: A sliding-window, adaptive, quality-based trimming tool for FastQ files. Ref. Source 2011, 455–477. [Google Scholar]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644. [Google Scholar] [CrossRef] [Green Version]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [Green Version]
- Huson, D.H.; Beier, S.; Flade, I.; Górska, A.; El-Hadidi, M.; Mitra, S.; Ruscheweyh, H.-J.; Tappu, R. MEGAN community edition-interactive exploration and analysis of large-scale microbiome sequencing data. PLoS Comput. Biol. 2016, 12, e1004957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasimuddin, M.; Misra, S.; Li, H.; Aluru, S. Efficient architecture-aware acceleration of BWA-MEM for multicore systems. In Proceedings of the 2019 IEEE International Parallel and Distributed Processing Symposium (IPDPS), Rio de Janeiro, Brazil, 20–24 May 2019; pp. 314–324. [Google Scholar]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M. Twelve years of SAMtools and BCFtools. Gigascience 2021, 10, giab008. [Google Scholar] [CrossRef] [PubMed]
- Birolo, G.; Telatin, A. BamToCov: An efficient toolkit for sequence coverage calculations. Bioinformatics 2022, 38, 2617–2618. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In Proceedings of the Nucleic Acids Symposium Series; Information Retrieval Ltd.: London, UK, 1999; pp. 95–98. [Google Scholar]
- Abascal, F.; Zardoya, R.; Telford, M.J. TranslatorX: Multiple alignment of nucleotide sequences guided by amino acid translations. Nucleic Acids Res. 2010, 38, W7–W13. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Maynard-Smith, J. Analyzing the mosaic structure of genes. J. Mol. Evol. 1992, 34, 126–129. [Google Scholar]
- Holmes, E.C.; Worobey, M.; Rambaut, A. Phylogenetic evidence for recombination in dengue virus. Mol. Biol. Evol. 1999, 16, 405–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padidam, M.; Sawyer, S.; Fauquet, C.M. Possible emergence of new geminiviruses by frequent recombination. Virology 1999, 265, 218–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbs, M.J.; Armstrong, J.S.; Gibbs, A.J. Sister-scanning: A Monte Carlo procedure for assessing signals in recombinant sequences. Bioinformatics 2000, 16, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Lemey, P.; Lott, M.; Martin, D.P.; Moulton, V. Identifying recombinants in human and primate immunodeficiency virus sequence alignments using quartet scanning. BMC Bioinform. 2009, 10, 126. [Google Scholar] [CrossRef] [Green Version]
- Martin, D.; Posada, D.; Crandall, K.; Williamson, C. A modified bootscan algorithm for automated identification of recombinant sequences and recombination breakpoints. AIDS Res. Hum. Retrovir. 2005, 21, 98–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, D.; Rybicki, E. RDP: Detection of recombination amongst aligned sequences. Bioinformatics 2000, 16, 562–563. [Google Scholar] [CrossRef]
- Boni, M.F.; Posada, D.; Feldman, M.W. An exact nonparametric method for inferring mosaic structure in sequence triplets. Genetics 2007, 176, 1035–1047. [Google Scholar] [CrossRef] [Green Version]
- Posada, D.; Crandall, K.A. Evaluation of methods for detecting recombination from DNA sequences: Computer simulations. Proc. Natl. Acad. Sci. USA 2001, 98, 13757–13762. [Google Scholar] [CrossRef] [Green Version]
- McGuire, G.; Wright, F. TOPAL 2.0: Improved detection of mosaic sequences within multiple alignments. Bioinformatics 2000, 16, 130–134. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [Green Version]
- Miura, S.; Tamura, K.; Tao, Q.; Huuki, L.A.; Kosakovsky Pond, S.L.; Priest, J.; Deng, J.; Kumar, S. A new method for inferring timetrees from temporally sampled molecular sequences. PLoS Comput. Biol. 2020, 16, e1007046. [Google Scholar] [CrossRef] [Green Version]





| Sample ID | Host (Latin/Common Name) | Paired End Reads | Virus Present | Coverage | Accession Code |
|---|---|---|---|---|---|
| 21506527 | Coriandrum sativum Coriander | 745985 | carrot thin leaf virus beet western yellows ** | 11686.84 231.82 | OM419175 OM419176 |
| 21604167 | Petroselinum crispum Parsley | 1092446 | parsley umbravirus 1 * | 428.83 | OM419177 |
| 21625530 | Coriandrum sativum Coriander | 5823 | apium virus Y | 0.52 | OM419183-OM419187 (partial sequence) |
| 21806162 | Coriandrum sativum Coriander | 147561 | apium virus Y | 1532.98 | MT900482 |
| ApVY isolate WA-1 | Petroselinum crispum Parsley | 1083897 | apium virus Y | 649.53 | MT900481 |
| CeMV isolate WA-1 | Apium graveolens Celery | 1629326 | celery mosaic virus | 628.97 | OM419182 |
| CarVY isolate SA-2 | Daucus carota Carrot | 1172179 | carrot virus Y | 7198.74 | MT900484 |
| CarVY isolate SA-3 | Daucus carota Carrot | 918475 | carrot virus Y | 6467.35 | MT900486 |
| CarVY isolate SA-4 | Daucus carota Carrot | 969303 | carrot virus Y | 2725.14 | MT900485 |
| CarVY isolate VIC-1 | Daucus carota Carrot | 1105338 | carrot virus Y carrot torrado virus-1 *** carrot ophiovirus-1 * Carrot associated RNA virus 1 | 2883.78 1080.96, 1534.21 13.29, 87.48, 129.41, 45.37 3.71, 4.67 | MT900483 OM419190-OM419191 OM419178-OM419181 OM419188-OM419188 |
| Sample ID | Host (Latin/Common Name) | Sample Description | Origin | Symptoms |
|---|---|---|---|---|
| 21506527 | Coriandrum sativum Coriander | Frozen RNA extract from fresh leaf | UK import interception Ex. Cyprus, 2015 | Chlorotic spotting See Figure 5a. |
| 21604167 | Petroselinum crispum Parsley | Frozen RNA extract from fresh leaf | UK Import interception Ex. Israel 2016 | General chlorosis and chlorotic mottle See Figure 5b. |
| 21625530 | Coriandrum sativum Coriander | Frozen RNA extract from fresh leaf | UK Import interception Ex. Egypt 2016 | Vein yellowing and chlorotic mottle See Figure 5c. |
| 21806162 | Coriandrum sativum Coriander | Frozen RNA extract from fresh leaf | UK Import interception Ex. Egypt 2018 | Vein yellowing and chlorotic spotting See Figure 5d. |
| ApVY isolate WA-1 | Petroselinum crispum Parsley | Freeze-dried leaf | Survey sample Ex. Perth metropolitan area, south-west Australia, 2002 [10] | Mosaic |
| CeMV isolate WA-1 | Apium gravelolens Celery | Freeze dried leaf | Survey sample Ex. Perth metropolitan area, south-west Australia, 1998 [13] | Mosaic |
| CarVY isolate SA-2 | Daucus carota Carrot | Freeze dried leaf | Survey sample Ex. South Australia, 2000–2002, [9] | Unknown, from random sample |
| CarVY isolate SA-3 | Daucus carota Carrot | Freeze dried leaf | Survey sample Ex. South Australia, 2000–2002 [9] | Unknown, from random sample |
| CarVY isolate SA-4 | Daucus carota Carrot | Freeze dried leaf | Survey sample Ex. South Australia, 2000–2002 [9] | Unknown, random sample |
| CarVY isolate VIC-1 | Daucus carota Carrot | Freeze dried leaf | Survey sample Ex. Victoria, Australia, 2000–2002 [9] | Unknown, from random sample |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fox, A.; Gibbs, A.J.; Fowkes, A.R.; Pufal, H.; McGreig, S.; Jones, R.A.C.; Boonham, N.; Adams, I.P. Enhanced Apiaceous Potyvirus Phylogeny, Novel Viruses, and New Country and Host Records from Sequencing Apiaceae Samples. Plants 2022, 11, 1951. https://doi.org/10.3390/plants11151951
Fox A, Gibbs AJ, Fowkes AR, Pufal H, McGreig S, Jones RAC, Boonham N, Adams IP. Enhanced Apiaceous Potyvirus Phylogeny, Novel Viruses, and New Country and Host Records from Sequencing Apiaceae Samples. Plants. 2022; 11(15):1951. https://doi.org/10.3390/plants11151951
Chicago/Turabian StyleFox, Adrian, Adrian J. Gibbs, Aimee R. Fowkes, Hollie Pufal, Sam McGreig, Roger A. C. Jones, Neil Boonham, and Ian P. Adams. 2022. "Enhanced Apiaceous Potyvirus Phylogeny, Novel Viruses, and New Country and Host Records from Sequencing Apiaceae Samples" Plants 11, no. 15: 1951. https://doi.org/10.3390/plants11151951
APA StyleFox, A., Gibbs, A. J., Fowkes, A. R., Pufal, H., McGreig, S., Jones, R. A. C., Boonham, N., & Adams, I. P. (2022). Enhanced Apiaceous Potyvirus Phylogeny, Novel Viruses, and New Country and Host Records from Sequencing Apiaceae Samples. Plants, 11(15), 1951. https://doi.org/10.3390/plants11151951







