Effect of Landscape Composition and Invasive Plants on Pollination Networks of Smallholder Orchards in Northeastern Thailand
Abstract
:1. Introduction
2. Results
2.1. Plant and Pollinator Community
2.2. Pollination Network Structures
2.3. Network Modularity and Pollinator Roles
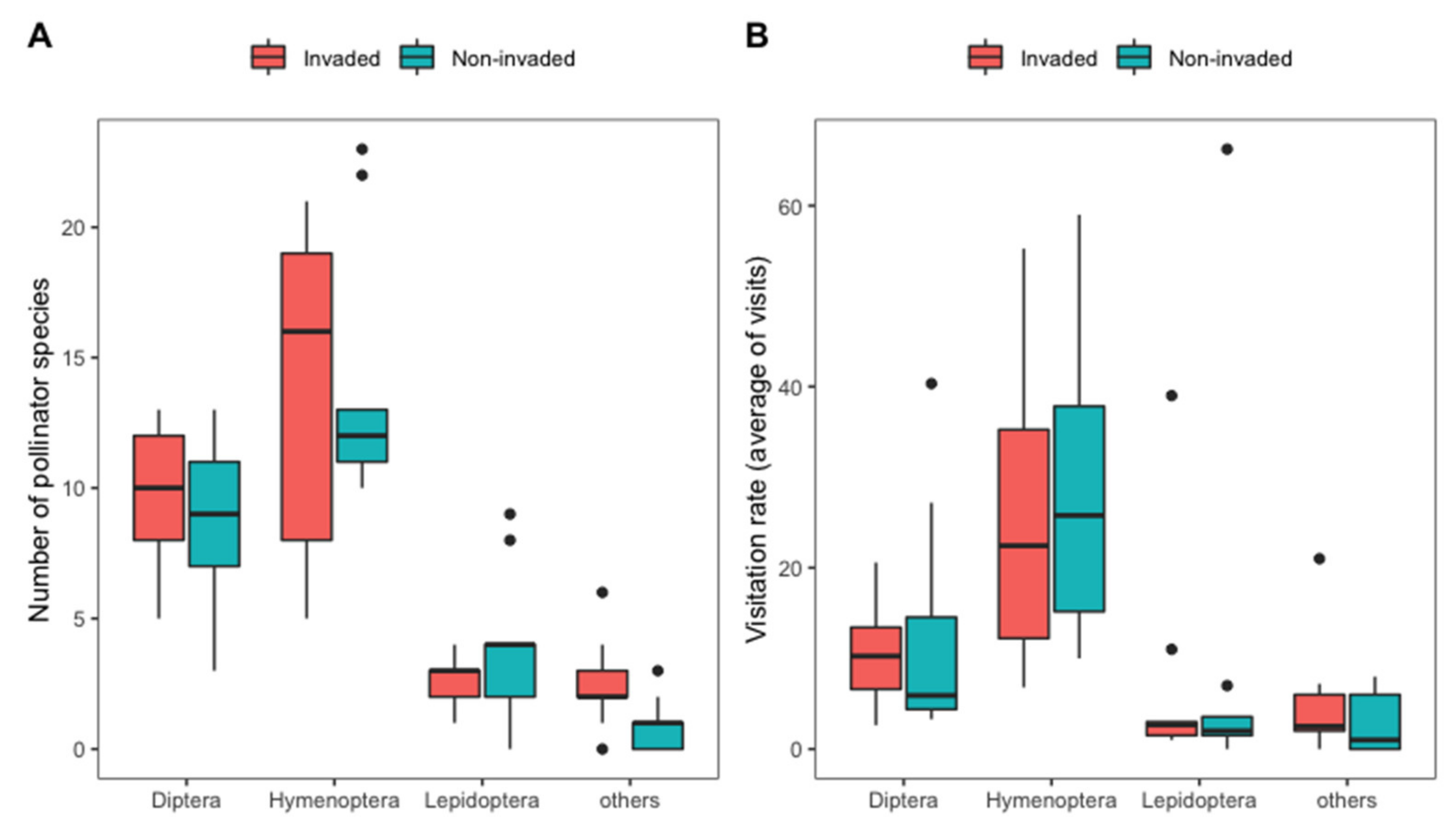
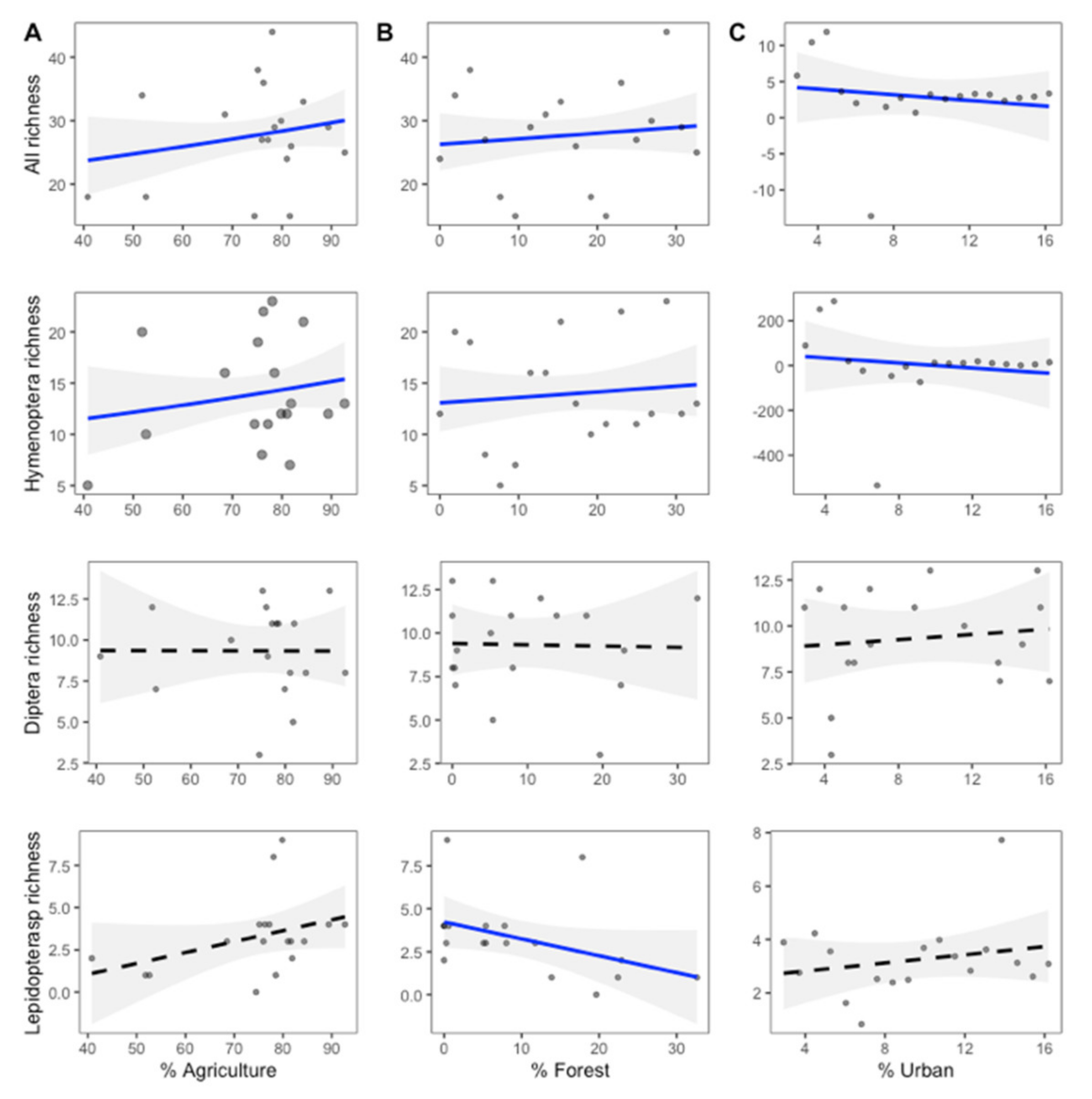
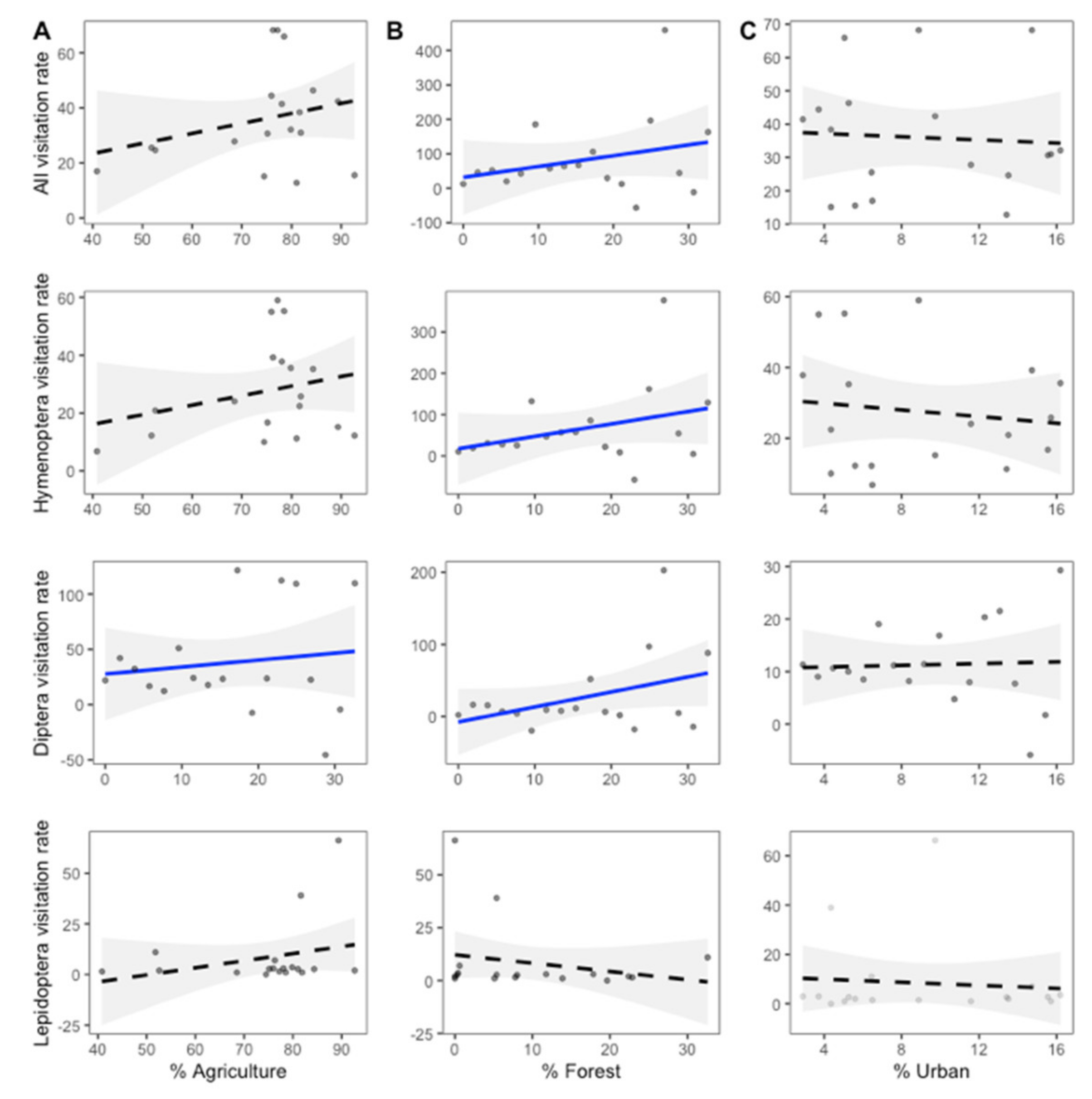
2.4. Effects of Invasion and Landscape Proportions on Pollinator Richness and Visitation Rates
3. Discussion
3.1. Effect of Invasion and Surrounding Landscape on Pollinator Richness and Visitation Rates
3.2. Effect of Invasive Plant and Surrounding Landscape on Pollination Networks
3.3. Implications for Agriculture-Conservation and Future Research
4. Materials and Methods
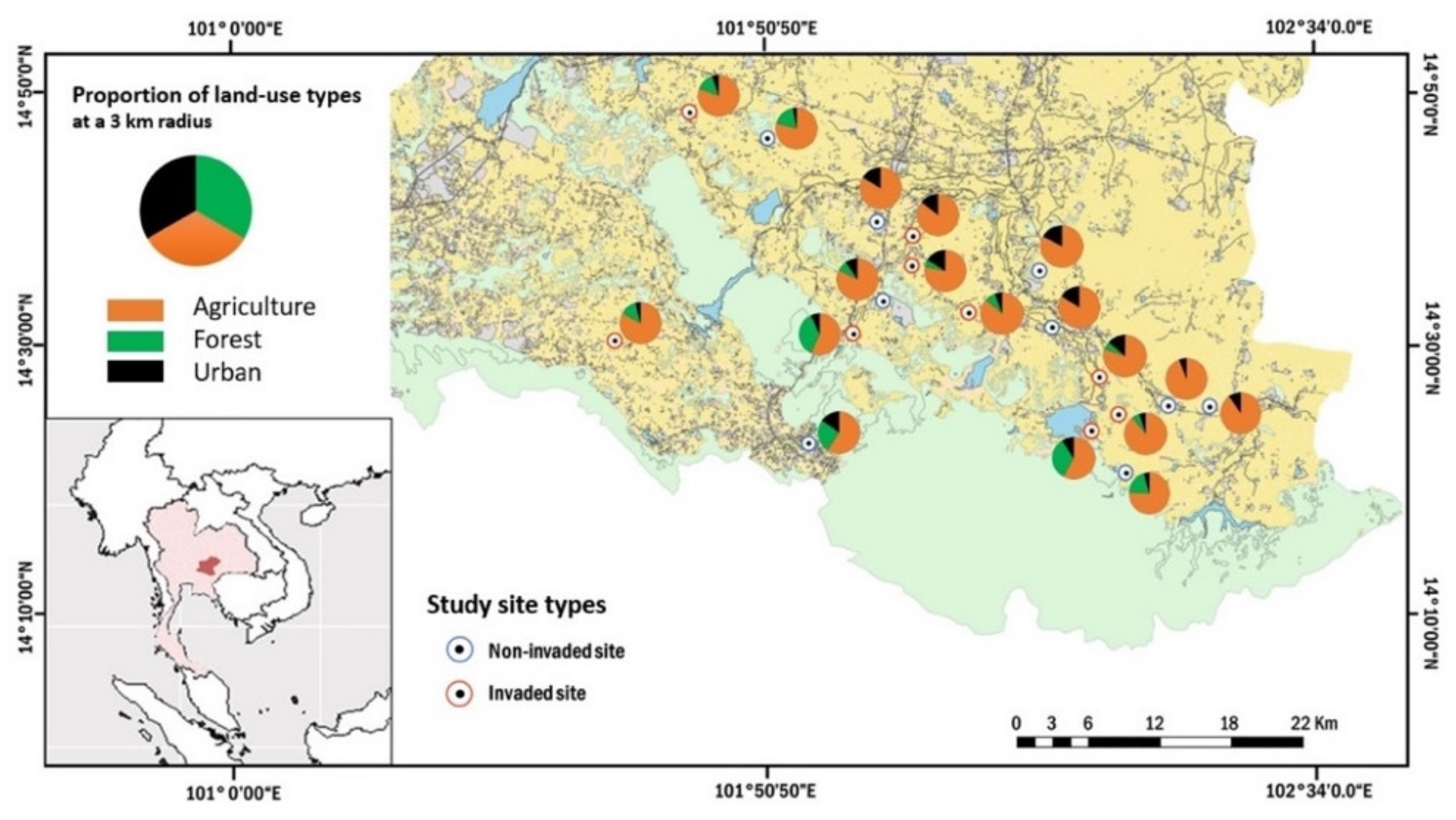
4.1. Study Site and Species
4.2. Sampling Design and Data Collection
4.3. Pollination Network Construction and Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steffan-Dewenter, I.; Westphal, C. The Interplay of Pollinator Diversity, Pollination Services and Landscape Change. J. Appl. Ecol. 2008, 45, 737–741. [Google Scholar] [CrossRef]
- Bartomeus, I.; Vilà, M.; Steffan-Dewenter, I. Combined Effects of Impatiens Glandulifera Invasion and Landscape Structure on Native Plant Pollination. J. Ecol. 2010, 98, 440–450. [Google Scholar] [CrossRef] [Green Version]
- Pyšek, P.; Richardson, D.M. The Biogeography of Naturalization in Alien Plants. J. Biogeogr. 2006, 33, 2040–2050. [Google Scholar] [CrossRef]
- Weber, E.; Sun, S.-G.; Li, B. Invasive Alien Plants in China: Diversity and Ecological Insights. Biol. Invasions 2008, 10, 1411–1429. [Google Scholar] [CrossRef] [Green Version]
- Morales, C.L.; Traveset, A. A Meta-Analysis of Impacts of Alien vs. Native Plants on Pollinator Visitation and Reproductive Success of Co-Flowering Native Plants. Ecol. Lett. 2009, 12, 716–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodell, K.; Parker, I.M. Invasion of a Dominant Floral Resource: Effects on the Floral Community and Pollination of Native Plants. Ecology 2017, 98, 57–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanbergen, A.J.; Espíndola, A.; Aizen, M.A. Risks to Pollinators and Pollination from Invasive Alien Species. Nat. Ecol. Evol. 2018, 2, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Parra-Tabla, V.; Angulo-Pérez, D.; Albor, C.; Campos-Navarrete, M.J.; Juan, T.-G.; Sosenski, P.; Alonso, C.; Ashman, T.-L.; Arceo-Gómez, G. The Effect of Alien Species on Plant-Pollinator Network Structure across a Gradient of Plant Species Invasion. bioRxiv 2019. [Google Scholar] [CrossRef] [Green Version]
- Arroyo-Correa, B.; Burkle, L.A.; Emer, C. Alien Plants and Flower Visitors Disrupt the Seasonal Dynamics of Mutualistic Networks. J. Ecol. 2020, 108, 1475–1486. [Google Scholar] [CrossRef]
- Hansen, S.; Roets, F.; Seymour, C.L.; Thébault, E.; Frank van Veen, J.F.; Pryke, J.S. Alien Plants Have Greater Impact than Habitat Fragmentation on Native Insect Flower Visitation Networks. Divers. Distrib. 2018, 24, 58–68. [Google Scholar] [CrossRef] [Green Version]
- Buchholz, S.; Kowarik, I. Urbanisation Modulates Plant-Pollinator Interactions in Invasive vs. Native Plant Species. Sci. Rep. 2019, 9, 6375. [Google Scholar] [CrossRef]
- Valdovinos, F.S.; Ramos-Jiliberto, R.; Flores, J.D.; Espinoza, C.; López, G. Structure and Dynamics of Pollination Networks: The Role of Alien Plants. Oikos 2009, 118, 1190–1200. [Google Scholar] [CrossRef]
- Parra-Tabla, V.; Arceo-Gómez, G. Impacts of Plant Invasions in Native Plant–Pollinator Networks. New Phytol. 2021, 230, 2117–2128. [Google Scholar] [CrossRef]
- Ferreira, P.A.; Boscolo, D.; Viana, B.F. What Do We Know about the Effects of Landscape Changes on Plant–Pollinator Interaction Networks? Ecol. Indic. 2013, 31, 35–40. [Google Scholar] [CrossRef]
- Harrison, T.; Winfree, R. Urban Drivers of Plant-Pollinator Interactions. Funct. Ecol. 2015, 29, 879–888. [Google Scholar] [CrossRef]
- Redhead, J.W.; Woodcock, B.A.; Pocock, M.J.O.; Pywell, R.F.; Vanbergen, A.J.; Oliver, T.H. Potential Landscape-Scale Pollinator Networks across Great Britain: Structure, Stability and Influence of Agricultural Land Cover. Ecol. Lett. 2018, 21, 1821–1832. [Google Scholar] [CrossRef] [Green Version]
- Viana, B.F.; Boscolo, D.; Neto, E.M.; Lopes, L.E.; Lopes, A.V.; Ferreira, P.A.; Pigozzo, C.M.; Primo, L.M. How Well Do We Understand Landscape Effects on Pollinators and Pollination Services? J. Pollinat. Ecol. 2012, 7, 31–41. [Google Scholar] [CrossRef]
- Montagnana, P.C.; Alves, R.S.C.; Garófalo, C.A. Landscape Heterogeneity and Forest Cover Shape Cavity-Nesting Hymenopteran Communities in a Multi-Scale Perspective. Basic Appl. Ecol. 2021, 56, 239–249. [Google Scholar] [CrossRef]
- Hagen, M.; Kissling, W.D.; Rasmussen, C.; De Aguiar, M.A.M.; Brown, L.E.; Carstensen, D.W.; Alves-Dos-Santos, I.; Dupont, Y.L.; Edwards, F.K.; Genini, J.; et al. Biodiversity, Species Interactions and Ecological Networks in a Fragmented World. Adv. Ecol. Res. 2012, 46, 89–209. [Google Scholar] [CrossRef] [Green Version]
- Moreira, E.F.; Boscolo, D.; Viana, B.F. Spatial Heterogeneity Regulates Plant-Pollinator Networks across Multiple Landscape Scales. PLoS ONE 2015, 10, e0123628. [Google Scholar] [CrossRef] [Green Version]
- Stout, J.C.; Tiedeken, E.J. Direct Interactions between Invasive Plants and Native Pollinators: Evidence, Impacts and Approaches. Funct. Ecol. 2017, 31, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Peh, K.S.-H. Invasive Species in Southeast Asia: The Knowledge so Far. Biodivers. Conserv. 2010, 19, 1083–1099. [Google Scholar] [CrossRef]
- Bjerknes, A.-L.; Totland, Ø.; Hegland, S.J.; Nielsen, A. Do Alien Plant Invasions Really Affect Pollination Success in Native Plant Species? Biol. Conserv. 2007, 138, 1–12. [Google Scholar] [CrossRef]
- Tylianakis, J.M.; Laliberté, E.; Nielsen, A.; Bascompte, J. Conservation of Species Interaction Networks. Biol. Conserv. 2010, 143, 2270–2279. [Google Scholar] [CrossRef]
- Olesen, J.M.; Bascompte, J.; Dupont, Y.L.; Jordano, P. The Modularity of Pollination Networks. Proc. Natl. Acad. Sci. USA 2007, 104, 19891–19896. [Google Scholar] [CrossRef] [Green Version]
- Dormann, C.F.; Strauss, R. A Method for Detecting Modules in Quantitative Bipartite Networks. Methods Ecol. Evol. 2014, 5, 90–98. [Google Scholar] [CrossRef] [Green Version]
- United States Department of Agriculture; Animal and Plant Health Inspection Service. Weed Risk Assessment for Praxelis clematidea R. M. King & H. Rob. (Asteraceae)—Praxelis; United States Department of Agriculture: Washington, DC, USA, 2014.
- Gardner, A.G.; Williges, K.A. Praxelis clematidea (Asteraceae): A New Plant Invader of Florida. Southeast. Nat. 2015, 14, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Shaw, K.A.; Kernot, J.C. Extent of Dense Native Woodland and Exotic Weed Infestation in the Extensive Grazing Lands of the Upper Herbert and Upper Burdekin River Catchments of Far North Queensland: Results of a Producer Survey. Trop. Grassl. 2004, 38, 112–116. [Google Scholar]
- Bartuszevige, A.M.; Gorchov, D.L.; Raab, L. The Relative Importance of Landscape and Community Features in the Invasion of an Exotic Shrub in a Fragmented Landscape. Ecography 2006, 29, 213–222. [Google Scholar] [CrossRef]
- Hall, D.M.; Camilo, G.R.; Tonietto, R.K.; Ollerton, J.; Ahrne, K.; Arduser, M.; Ascher, J.S.; Baldock, K.C.R.; Fowler, R.; Frankie, G.; et al. The City as a Refuge for Insect Pollinators. Conserv. Biol. 2017, 31, 24–29. [Google Scholar] [CrossRef] [Green Version]
- Lavorel, S.; Grigulis, K.; Lamarque, P.; Colace, M.-P.; Garden, D.; Girel, J.; Pellet, G.; Douzet, R. Using Plant Functional Traits to Understand the Landscape Distribution of Multiple Ecosystem Services. J. Ecol. 2011, 99, 135–147. [Google Scholar] [CrossRef]
- González, E.; Salvo, A.; Defagó, M.T.; Valladares, G. A Moveable Feast: Insects Moving at the Forest-Crop Interface Are Affected by Crop Phenology and the Amount of Forest in the Landscape. PLoS ONE 2016, 11, e0158836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willmer, P. Pollination and Floral Ecology; Princeton University Press: Princeton, NJ, USA, 2011; ISBN 978-0-691-12861-0. [Google Scholar]
- Geslin, B.; Gauzens, B.; Thébault, E.; Dajoz, I. Plant Pollinator Networks along a Gradient of Urbanisation. PLoS ONE 2013, 8, e63421. [Google Scholar] [CrossRef] [PubMed]
- Corcos, D.; Cappellari, A.; Mei, M.; Paniccia, D.; Cerretti, P.; Marini, L. Contrasting Effects of Exotic Plant Invasions and Managed Honeybees on Plant-Flower Visitor Interactions. Divers. Distrib. 2020, 26, 1397–1408. [Google Scholar] [CrossRef]
- Hung, K.-L.J.; Kingston, J.M.; Albrecht, M.; Holway, D.A.; Kohn, J.R. The Worldwide Importance of Honey Bees as Pollinators in Natural Habitats. Proc. R. Soc. B 2018, 285, 20172140. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Kunte, K.; Webb, E.L. Flower Specialization of Butterflies and Impacts of Non-Native Flower Use in a Transformed Tropical Landscape. Biol. Conserv. 2016, 201, 184–191. [Google Scholar] [CrossRef]
- Chaianunporn, K.; Chaianunporn, T. Effects of Habitat Types on Butterfly Communities (Lepidoptera, Papilionoidea) in Chulabhorn Dam, Chaiyaphum Province, Thailand. Trop. Nat. Hist. 2019, 19, 70–87. [Google Scholar]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat Management to Conserve Natural Enemies of Arthropod Pests in Agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef]
- Jauker, F.; Jauker, B.; Grass, I.; Steffan-Dewenter, I.; Wolters, V. Partitioning Wild Bee and Hoverfly Contributions to Plant–Pollinator Network Structure in Fragmented Habitats. Ecol. Soc. Am. 2019, 100, e02569. [Google Scholar] [CrossRef]
- Montero-Castaño, A.; Vilà, M. Influence of the Honeybee and Trait Similarity on the Effect of a Non-Native Plant on Pollination and Network Rewiring. Funct. Ecol. 2017, 31, 142–152. [Google Scholar] [CrossRef] [Green Version]
- Papanikolaou, A.D.; Kühn, I.; Frenzel, M.; Schweiger, O. Landscape Heterogeneity Enhances Stability of Wild Bee Abundance under Highly Varying Temperature, but Not under Highly Varying Precipitation. Landsc. Ecol. 2016, 32, 581–593. [Google Scholar] [CrossRef]
- Senapathi, D.; Goddard, M.A.; Kunin, W.E.; Baldock, K.C.R. Landscape Impacts on Pollinator Communities in Temperate Systems: Evidence and Knowledge Gaps. Funct. Ecol. 2017, 31, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Seitz, N.; van Engelsdorp, D.; Leonhardt, S.D. Are Native and Non-Native Pollinator Friendly Plants Equally Valuable for Native Wild Bee Communities? Ecol. Evol. 2020, 10, 12838–12850. [Google Scholar] [CrossRef]
- Stouffer, D.B.; Cirtwill, A.R.; Bascompte, J. How Exotic Plants Integrate into Pollination Networks. J. Ecol. 2014, 102, 1442–1450. [Google Scholar] [CrossRef]
- Bascompte, J.; Jordano, P. Plant-Animal Mutualistic Networks: The Architecture of Biodiversity. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 567–593. [Google Scholar] [CrossRef] [Green Version]
- Dylewski, Ł.; Maćkowiak, Ł.; Banaszak-Cibicka, W. Linking Pollinators and City Flora: How Vegetation Composition and Environmental Features Shapes Pollinators Composition in Urban Environment. Urban For. Urban Green. 2020, 56, 126795. [Google Scholar] [CrossRef]
- Magrach, A.; González-Varo, J.P.; Boiffier, M.; Vilà, M.; Bartomeus, I. Honeybee Spillover Reshuffles Pollinator Diets and Affects Plant Reproductive Success. Nat. Ecol. Evol. 2018, 2, 16–25. [Google Scholar] [CrossRef]
- Valido, A.; Rodríguez-Rodríguez, M.C.; Jordano, P. Honeybees Disrupt the Structure and Functionality of Plant-Pollinator Networks. Sci. Rep. 2019, 9, 4711. [Google Scholar] [CrossRef] [Green Version]
- Santamaría, S.; Sánchez, A.M.; López-Angulo, J.; Ornosa, C.; Mola, I.; Escudero, A. Landscape Effects on Pollination Networks in Mediterranean Gypsum Islands. Plant Biol. 2017, 20, 184–194. [Google Scholar] [CrossRef] [Green Version]
- Sritongchuay, T.; Hughes, A.C.; Memmott, J.; Bumrungsri, S. Forest Proximity and Lowland Mosaic Increase Robustness of Tropical Pollination Networks in Mixed Fruit Orchards. Landsc. Urban Plan. 2019, 192, 103646. [Google Scholar] [CrossRef]
- Klein, A.-M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of Pollinators in Changing Landscapes for World Crops. Proc. R. Soc. B 2007, 274, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Borchardt, K.E.; Morales, C.L.; Aizen, M.A.; Toth, A.L. Plant–Pollinator Conservation from the Perspective of Systems-Ecology. Curr. Opin. Insect Sci. 2021, 47, 154–161. [Google Scholar] [CrossRef]
- Jakobsson, A.; Padrón, B. Does the Invasive Lupinus Polyphyllus Increase Pollinator Visitation to a Native Herb through Effects on Pollinator Population Sizes? Oecologia 2013, 174, 217–226. [Google Scholar] [CrossRef]
- Aizen, M.A.; Aguiar, S.; Biesmeijer, J.C.; Garibaldi, L.A.; Inouye, D.W.; Jung, C.; Martins, D.J.; Medel, R.; Morales, C.L.; Ngo, H.; et al. Global Agricultural Productivity Is Threatened by Increasing Pollinator Dependence without a Parallel Increase in Crop Diversification. Glob. Chang. Biol. 2019, 25, 3516–3527. [Google Scholar] [CrossRef] [Green Version]
- Cunningham-Minnick, M.J.; Peters, V.E.; Crist, T.O. Bee Communities and Pollination Services in Adjacent Crop Fields Following Flower Removal in an Invasive Forest Shrub. Ecol. Appl. 2020, 30, e02078. [Google Scholar] [CrossRef]
- Alvarez, M.E.; Cushman, J.H. Community-Level Consequences of Plant Invasion: Effect on Three Habitats in Coastal California. Ecol. Soc. Am. 2002, 12, 1434–1444. [Google Scholar] [CrossRef]
- Emer, C.; Vaughan, I.P.; Hiscock, S.; Memmott, J. The Impact of the Invasive Alien Plant, Impatiens Glandulifera, on Pollen Transfer Networks. PLoS ONE 2015, 10, e0143532. [Google Scholar] [CrossRef] [Green Version]
- Stewart, A.B.; Sritongchuay, T.; Teartisup, P.; Kaewsomboon, S.; Bumrungsri, S. Habitat and Landscape Factors Influence Pollinators in a Tropical Megacity, Bangkok, Thailand. PeerJ 2018, 6, e5335. [Google Scholar] [CrossRef]
- Memmott, J.; Waser, N.M. Integration of Alien Plants into a Native Flower–Pollinator Visitation Web. Proc. R. Soc. Lond. B 2002, 269, 2395–2399. [Google Scholar] [CrossRef]
- Vizentin-Bugoni, J.; Maruyama, P.K.; Silveira de Souza, C.; Ollerton, J.; Rech, A.R.; Sazima, M. Plant-Pollinator Networks in the Tropics: A Review. In Ecological Networks in the Tropics; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Intanon, S.; Wiengmoon, B.; Mallory-Smith, C.A. Seed Morphology and Allelopathy of Invasive Praxelis clematidea. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Thantawin, W.; Anantanamanee, Y.; Maneechote, C.; Sathuwichan, S.; Shaokongchak, S. Biology and Ecology of Praxelis (Praxelis clematidea); King, R.M., Rob, H., Eds.; University of Florida: Gainesville, FL, USA, 2011. [Google Scholar]
- CRC for Australian Weed Management. Weed Management Guide: Praxelis (Praxelis clematidae); CRC for Australian Weed Management: Sydney, Australia, 2003; ISBN 1-920932-44-5.
- Abbott, J.R.; White, C.L.; Davis, S.B. Praxelis clematidea (Asteraceae), a New Genus and Species New for the Flora of North America. J. Bot. Res. Inst. Tex. 2008, 2, 621–626. [Google Scholar]
- Waterhouse, B.M. Know Your Enemy: Recent Records of Potentially Serious Weeds in Northern Australia, Papua New Guinea and Papua (Indonesia). Telopea 2003, 10, 477–485. [Google Scholar] [CrossRef]
- Land Development Department. GIS Data on Land Use Resources. Available online: https://www.ldd.go.th/WEB_BigData/page_2.htm (accessed on 16 December 2021).
- National Park, Wildlife and Plant Conservation Department National Parks of Thialand. Available online: http://portal.dnp.go.th/ (accessed on 24 May 2021).
- Heumann, B.W.; Walsh, S.J.; Verdery, A.M.; McDaniel, P.M.; Rindfuss, R.R. Land Suitability Modeling Using a Geographic Socio-Environmental Niche-Based Approach: A Case Study from Northeastern Thailand. Ann. Assoc. Am. Geogr. 2013, 103, 764–784. [Google Scholar] [CrossRef]
- Riwthong, S.; Schreinemachers, P.; Grovermann, C.; Berger, T. Land Use Intensification, Commercialization and Changes in Pest Management of Smallholder Upland Agriculture in Thailand. Environ. Sci. Policy 2015, 45, 11–19. [Google Scholar] [CrossRef]
- Smitinand, T. Thai Plant Names; Forest Herbarium, Departure of National Park, Wildlife and Plant Conservation: Bangkok, Thailand, 2014.
- Zurbuchen, A.; Landert, L.; Klaiber, J.; Müller, A.; Hein, S.; Dorn, S. Maximum Foraging Ranges in Solitary Bees: Only Few Individuals Have the Capability to Cover Long Foraging Distances. Biol. Conserv. 2010, 143, 669–676. [Google Scholar] [CrossRef]
- Herron-Sweet, C.R.; Lehnhoff, E.A.; Burkle, L.A.; Littlefield, J.L.; Mangold, J.M. Temporal- and Density-Dependent Impacts of an Invasive Plant on Pollinators and Pollination Services to a Native Plant. Ecosphere 2016, 7, e01233. [Google Scholar] [CrossRef] [Green Version]
- Botanical garden organization of Thailand BGO Plant Databases, the Botanical Garden Organization. Available online: http://www.qsbg.org/ (accessed on 30 October 2020).
- Rader, R.; Edwards, W.; Westcott, D.A.; Cunningham, S.A.; Howlett, B.G. Diurnal Effectiveness of Pollination by Bees and Flies in Agricultural Brassica Rapa: Implications for Ecosystem Resilience. Basic Appl. Ecol. 2013, 14, 20–27. [Google Scholar] [CrossRef]
- Speights, C.J.; Harmon, J.P.; Barton, B.T. Contrasting the Potential Effects of Daytime versus Nighttime Warming on Insects. Curr. Opin. Insect Sci. 2017, 23, 1–6. [Google Scholar] [CrossRef]
- Corbet, S.A.; Fussell, M.; Ake, R.; Fraser, A.; Gunson, C.; Savage, A.; Smith, K. Temperature and the Pollinating Activity of Social Bees. Ecol. Entomol. 1993, 18, 17–30. [Google Scholar] [CrossRef]
- Kremen, C.; Williams, N.M.; Thorp, R.W. Crop Pollination from Native Bees at Risk from Agricultural Intensification. PNAS 2002, 99, 16812–16816. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Jordano, P. Pattern of Mutualistic Interaction and Seed Dispersal: Connectance, Dependence Asymmetries, and Coevolution. Am. Nat. 1987, 129, 657–677. [Google Scholar] [CrossRef]
- Tylianakis, J.M.; Tscharntke, T.; Lewis, O.T. Habitat Modification Alters the Structure of Tropical Host–Parasitoid Food Webs. Nature 2007, 445, 202–205. [Google Scholar] [CrossRef]
- Dormann, C.F. Using Bipartite to Describe and Plot Two-Mode Networks in R. 2021. Available online: https://cran.r-project.org/web/packages/bipartite/vignettes/Intro2bipartite.pdf (accessed on 12 August 2021).
- Fortuna, M.A.; Stouffer, D.B.; Olesen, J.M.; Jordano, P.; Mouillot, D.; Krasnov, B.R.; Poulin, R.; Bascompte, J. Nestedness versus Modularity in Ecological Networks: Two Sides of the Same Coin? J. Anim. Ecol. 2010, 79, 811–817. [Google Scholar] [CrossRef]
- Guimera, R.; Mossa, S.; Turtschi, A.; Amaral, L.A.N. The Worldwide Air Transportation Network: Anomalous Centrality, Community Structure, and Cities’ Global Roles. Proc. Natl. Acad. Sci. USA 2005, 102, 7794–7799. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, M.; Padrón, B.; Bartomeus, I.; Traveset, A. Consequences of Plant Invasions on Compartmentalization and Species’ Roles in Plant–Pollinator Networks. Proc. R. Soc. B 2014, 281, 20140773. [Google Scholar] [CrossRef] [Green Version]
- Saunders, M.E.; Rader, R. Network Modularity Influences Plant Reproduction in a Mosaic Tropical Agroecosystem. Proc. R. Soc. B 2019, 286, 20190296. [Google Scholar] [CrossRef] [PubMed]
- Kishi, S.; Kakutani, T. Male Visitors May Decrease Modularity in Flower–Visitor Networks. Front. Ecol. Evol. 2020, 8, 124. [Google Scholar] [CrossRef]
- Papin, J.A.; Reed, J.L.; Palsson, B.O. Hierarchical Thinking in Network Biology: The Unbiased Modularization of Biochemical Networks. Trends Biochem. Sci. 2004, 29, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Guimera, R.; Amaral, L.A.N. Functional Cartography of Complex Metabolic Networks. Nature 2005, 433, 895–900. [Google Scholar] [CrossRef] [Green Version]
- Poulin, R.; Krasnov, B.R.; Pilosof, S.; Thieltges, D.W. Phylogeny Determines the Role of Helminth Parasites in Intertidal Food Webs. J. Anim. Ecol. 2013, 82, 1265–1275. [Google Scholar] [CrossRef]
- Cirtwill, A.R.; Riva, G.V.D.; Gaiarsa, M.P.; Bimler, M.D.; Cagua, E.F.; Coux, C.; Dehling, D.M. A Review of Species Role Concepts in Food Webs. Food Webs. 2018, 16, e00093. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P. Package “Vegan”. Community Ecology Package. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 20 June 2021).
- Wei, H.; Xu, J.; Quan, G.; Zhang, J.; Qin, Z. Effects of Praxelis clematidea invasion on soil nitrogen fractions and transformation rates in a tropical savanna. Environ. Sci. Pollut. Res. Int. 2017, 24, 3654–3663. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Wang, Q.; Li, X.; Liu, Y.; Huang, Q. Effects of soil nutrients on reproductive traits of invasive and native annual Asteraceae plants. Biodiv. Sci. 2021, 29, 1–9. [Google Scholar]
- Huang, X.R.; Pang, S.L.; Shen, W.H.; Hou, Y.R.; He, F. Herb diversity and its affecting factors of community invaded by Praxelis clematidea in karst mountainous area of Guangxi Province, China. J. Appl. Ecol. 2016, 27, 815–821. [Google Scholar] [CrossRef]


| Community | Variable | Invaded | Non-Invaded | p-Value |
|---|---|---|---|---|
| Plant | Diversity | 3.38 ± 0.20 | 3.38 ± 0.15 | 0.98 NS |
| Richness | 29.89 ± 5.97 | 29.78 ± 4.49 | 0.97 NS | |
| Interacted species | 8.33 ± 2.92 | 8.44 ± 3.05 | 0.94 NS | |
| Flower abundance | 2412.75 ± 1121.46 | 2839.60 ± 1944 | 0.86 NS | |
| Pollinator | Diversity | 3.28 ± 0.31 | 3.28 ± 0.33 | 0.83 NS |
| Richness | 27.67 ± 7.55 | 27.78 ± 8.71 | 0.98 NS | |
| Pollinator abundance | 514.67 ± 282.86 | 590 ± 399.6 | 0.65 NS | |
| Visitation rate | ||||
| All groups | 34.31 ± 16.44 | 37.63 ± 19.83 | 0.70 NS | |
| Hymenoptera | 26.55 ± 18.23 | 28.41 ± 15.94 | 0.82 NS | |
| Lepidoptera | 7.18 ± 12.32 | 9.59 ± 21.34 | 0.93 NS | |
| Diptera | 11.12 ± 6.26 | 12.34 ± 13.04 | 0.60 NS | |
| Other arthropods | 4.86 ± 6.40 | 2.61 ± 3.48 | 0.26 NS |
| Parameters | Invaded Network | Non-Invaded Network | p-Value |
|---|---|---|---|
| Connectance | 0.19 ± 0.06 | 0.19 ± 0.05 | 0.73 NS |
| Interaction evenness | 0.51 ± 0.07 | 0.49 ± 0.06 | 0.44 NS |
| Shannon’s diversity of interactions | 2.76 ± 0.54 | 2.60 ± 0.41 | 0.67 NS |
| Specialization (H′2) | 0.70 ± 0.14 | 0.77 ± 0.12 | 0.27 NS |
| Lower level shared partners (plants) | 0.79 ± 0.53 | 0.71 ± 0.37 | 0.79 NS |
| Higher level shared partners (pollinators) | 0.44 ± 0.12 | 0.47 ± 0.17 | 0.63 NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simla, P.; Chaianunporn, T.; Sankamethawee, W.; Hughes, A.C.; Sritongchuay, T. Effect of Landscape Composition and Invasive Plants on Pollination Networks of Smallholder Orchards in Northeastern Thailand. Plants 2022, 11, 1976. https://doi.org/10.3390/plants11151976
Simla P, Chaianunporn T, Sankamethawee W, Hughes AC, Sritongchuay T. Effect of Landscape Composition and Invasive Plants on Pollination Networks of Smallholder Orchards in Northeastern Thailand. Plants. 2022; 11(15):1976. https://doi.org/10.3390/plants11151976
Chicago/Turabian StyleSimla, Pattraporn, Thotsapol Chaianunporn, Wangworn Sankamethawee, Alice C. Hughes, and Tuanjit Sritongchuay. 2022. "Effect of Landscape Composition and Invasive Plants on Pollination Networks of Smallholder Orchards in Northeastern Thailand" Plants 11, no. 15: 1976. https://doi.org/10.3390/plants11151976
APA StyleSimla, P., Chaianunporn, T., Sankamethawee, W., Hughes, A. C., & Sritongchuay, T. (2022). Effect of Landscape Composition and Invasive Plants on Pollination Networks of Smallholder Orchards in Northeastern Thailand. Plants, 11(15), 1976. https://doi.org/10.3390/plants11151976





