Abstract
In order to explore the effects of nitrapyrin (N-Serve) application on greenhouse gas emission and nitrogen (N) leaching of a waterlogged maize (Zea mays L.) field, we investigated the effects of applying nitrapyrin on soil ammonium (NH4+-N) and nitrate nitrogen (NO3−-N) content, nitrous oxide (N2O) fluxes, and the warming potential (GWPN2O) in a waterlogged maize field. The design included three treatments: waterlogging treatment with only urea application (V-3WL), waterlogging treatment with urea and nitrapyrin application (V-3WL+N), and no waterlogging treatment applying only urea (CK). Our results revealed that waterlogging led to the increase of nitrate concentrations across the soil profile, thus potentially increasing N leaching and decreasing N use efficiency. The accumulated N2O emissions increased significantly in waterlogged plots compared to control plots, and maximum N2O emission fluxes occurred during the process of soil drying after waterlogging; this resulted in an increase in GWPN2O and N2O greenhouse gas intensity (GHGIN2O) by 299% and 504%, respectively, compared to those of CK. However, nitrapyrin application was able to reduce N2O emissions. Nitrapyrin application was also good for decreasing GWPN2O and GHGIN2O by 34% and 50%, respectively, compared to V-3WL. In addition, nitrapyrin application was conducive to reduce N leaching and improve N use efficiency, resulting in a yield increase by 34%, compared to that of V-3WL. The application of nitrapyrin helped to mitigate agriculture-source greenhouse effects and N leaching induced by waterlogging, and was a high N-efficient fertilizer method for a waterlogged field.
1. Introduction
Agriculture has become a major source of global greenhouse gas emissions (GHGs), which must be substantially reduced to minimize the impacts of climate change [1]. Agriculture accounts for 10%–20% of global GHGs produced by human activities, with nitrous oxide (N2O) accounting for 60% of total agricultural emissions [2,3]. In China, the GHGs produced by the agriculture sector are primarily N2O, comprising 15% of the total GHGs of the country [2]. Nitrous oxide, a long-lived greenhouse gas, contributes to global warming and also serves as an atmospheric tracer of anthropogenic changes to the global N cycle, with a global warming potential 273 times that of CO2 [4,5,6].
In agricultural fields, the N2O emissions are mainly produced by the chemical and organic N inputs [7]. Because of respiration by soil microbes, soil animals, and plant roots after nitrogen (N) fertilization, soil becomes a significant source of N2O emissions [8,9]. The biomass, physiology, and biochemistry of soil microbes are affected by temperature, water content, organic content, pH, redox potential, and the texture of the soil, among other factors; in turn, this affects the rate of soil GHGs [10,11]. Global climate change is predicted to increase the frequency and intensity of extreme precipitation events [12,13,14,15], which could dramatically alter soil GHGs. For example, intensified precipitation regimes would lead to higher incidences of soil waterlogging or flooding and the changes of soil hydrological cycles [16]. N2O emission rates depend on the interaction among soil types, climate, and farm management, which influence soil microbial processes and the diffusion of gaseous N2O to the atmosphere [17]. Humid tropical soils are generally associated with the production of large amounts of gaseous N oxides, including N2O [18].
Excessive rainfall or irrigation can also lead to high rates of nitrate (NO3−) leaching from soils, resulting in the losses of reactive N from maize fields. Waterlogging also restricts nutrient absorption and use by maize roots, leading to a substantial reduction of N efficiency [19]. Nitrification inhibitors can effectively suppress the oxidation of ammonium (NH4+) to NO3−, thus reducing N loss from soils and improving N uptake by crops [20]. The companion of nitrification inhibitors with N fertilizer has been applied as an agronomic practice to reduce N leaching and lessen N2O and NO emissions [20,21]. Nitrapyrin (2-chloro-6-(trichloromethyl) pyridine) is similar to other customarily used nitrification inhibitors, such as dicyandiamide (DCD) and 3,4-dimethylpyrazole phosphate (DMPP), and has been customarily applied to crop fields to inhibit NO3− leaching with great success [22,23,24,25]. The application of nitrapyrin can reduce N leaching by inhibiting the release of soil N2O by 49% and 24%, respectively, compared with urea and urea-ammonium nitrate application during the maize growth period [26,27]. This suppresses the oxidation of NH4+ to NO3− and maintains N in the form of NH4+ in soil for a longer period [28]. The effectiveness of nitrification inhibitors on N2O emissions depends on environmental parameters, such as waterlogging, heat, cold, and so on [21,29,30,31]. Nitrification inhibitors affect N2O emissions more effectively under higher soil moisture levels by regulating the abundance of denitrifying genes (narG, nirK, and nosZ) [32,33,34]. Our previous studies revealed that urea combined with nitrapyrin increased the NH4+ content in the soil, prolonged the fertilizer efficiency, and promoted the absorption and metabolism of N in maize, thereby promoting the recovery of maize and alleviating the reduction of plant dry matter accumulation caused by waterlogging [35]. Moreover, the application of nitrapyrin can lead to higher yields in waterlogged summer maize by optimizing the absorption and relocation of N, which effectively improves N use efficiency (NUE) and the N harvest index [35,36].
However, little research has been conducted on the role of nitrapyrin in mitigating the effects of waterlogging on GHGs and N leaching. In this study, we conducted a field experiment to measure the effects of nitrapyrin application and waterlogging on N2O emissions, and on the content of soil NO3−-N and NH4+-N in a maize field. The results of this study will help in developing a strategy to reduce GHGs and N leaching in waterlogged maize.
2. Materials and Methods
2.1. Plant Materials and Experimental Location
A field experiment was conducted at the experimental farm (36°10′ N, 117°04′ E, 151 m a.s.l.) maintained by the State Key Laboratory of Crop Biology of Shandong Agricultural University, Taian, China in 2016 and 2017. This region was characterized by a temperate continental monsoonal climate with a mean annual temperature of approximately 13 °C, a frost-free period of 195 days, and annual precipitation of 697 mm. The rainfall in two maize growing seasons were mainly concentrated in July, accounting for 46.0% and 54.8%, respectively. The 0–20 cm top-soil of the experimental field consisted of brown loam, which contained 10.7 g kg−1 organic matter, 0.9 g kg−1 total N, 50.7 mg kg−1 available phosphorus (molybdenum-antimony [Mo-Sb] colorimetry), and 86.2 mg kg−1 available potassium (Flame photometry). Denghai605 (DH605), a commonly grown maize (Zea mays L.) hybrid, was used for this experiment. Maize seeds were sown on June 16 at a density of 67,500 plants ha−1.
2.2. Experimental Design
Each plot measured 4 × 4 m2 and was surrounded by four 4 × 2.3 m2 polyvinyl chloride (PVC) boards, which acted as water barriers. Each PVC board was buried 2.0 m below the soil surface, with the remaining 0.3 m above ground. In the waterlogged plots, the water level was maintained at 2~3 cm above the soil surface for 6 days, starting when maize plants were at the third leaf stage (V3). After 6 days, all the water was drained from the soil surface. Two treatments were tested in this experiment: a waterlogging treatment with urea application only (V-3WL); and a waterlogging treatment with urea and nitrapyrin application (V-3WL+N). Control plots (CK) were not waterlogged, but applied only urea. Each treatment had three replicates, and treatments were randomly applied to plots in the field. Fertilizer was applied: 210 kg ha−1 N (urea with 46% N); 84 kg ha−1 phosphorus pentoxide (P2O5; calcium superphosphate with 17% P2O5); and 168 kg ha−1 potassium oxide (K2O; muriate of potash with 60% K2O) at the beginning of the experiment. For nitrapyrin treatment, 2550 mL ha−1 nitrapyrin was mixed uniformly with urea and incorporated into the soil via ploughing. The rate of nitrapyrin was 0.24% of the rate of urea-N application.
2.3. Soil N2O Fluxes Measurements
Soil N2O fluxes were estimated using a static-chamber method [37]. These gas fluxes were measured between 8:00 am and 11:00 am daily from the first day of waterlogging to the last day of soil drying using closed-chamber every other day. The closed chamber (length 0.35 m × width 0.35 m × height 0.2 m) was enclosed by plastic sheets. The exterior of the chamber was insulated with heat-insulating cystosepiment (0.5 cm thick foam board) to prevent temperature changes, and an air vent was installed in the middle of the chamber. A pedestal was placed under the chamber, and the base was sealed using water to ensure that the external environment did not affect the interior of chamber when gas samples were collected. Gas samples (50 mL) were collected using glass syringes from the chamber headspace at 0, 10, 20, and 30 min after placing the chamber on the soil. Concentrations of N2O in the gas samples were detected using an Agilent GC7890 gas chromatograph (Agilent, Santa Clara, CA, USA) equipped with an electron capture detector (ECD). N2O flux was calculated as:
where J is flux (mg m−2 h−1), and dc/dt is the change in gas concentration (c, mg m−3) against time (t, hour). M is the molar mass (mg mol−1) of each gas, P is atmospheric pressure (KPa), T is the absolute temperature (K) during sampling, H is the height (m) of headspace in chamber, and V0, T0, and P0 are the gas molar volume (m3 mol−1), absolute air temperature (K), and atmospheric pressure (KPa), respectively, under standard conditions.
N2O warming potential (GWPN2O, kg CO2-eq m−2) was calculated by multiplying the N2O emission fluxes by radiative forcing potentials. The equation is as follows [4]:
where fN2O is N2O emission flux.
N2O greenhouse gas intensity (GHGIN2O, kg kg−1) represented the comprehensive greenhouse effect of each treatment and was calculated as follows [38,39]:
where Y (kg ha−1) is the grain yield of summer maize for each treatment.
2.4. Soil NH4+-N and NO3-N Content
The soil samples were divided into three layers from 0 to 90 cm, each one with a height of 30 cm. The soil sample of each layer was placed by an earth drill into a Ziploc bag at the sixth leaf stage (V6), tasseling stage (VT), and physiological maturity stage (R6) [37]. Soil NH4+-N and NO3−-N were extracted with 1 M KCl, and filtered through a 0.45-μm membrane filter to remove insoluble particulates. The content of the soil NH4+-N and NO3−-N were measured by the AA3 Continuous Flow Analytical System [40]. Three replicate soil samples were collected in each treatment.
2.5. Nitrogen Efficiency and N Budget
Five representative plant samples were obtained from each plot at the physiological maturity stage (R6). The samples were dried at 80 °C in a force-draft oven (DHG-9420A, Bilon Instruments Co. Ltd., Shanghai, China) to a constant weight and weighed separately. The total N was measured using the Kjedahl method. Nitrogen use efficiency (NUE, kg kg−1), N partial factor productivity (NPFP, kg kg−1), N harvest index (NHI, %), and the apparent N budget (kg ha−1) were calculated to investigate the performance of agricultural management practices, using the following equations:
where NA (kg N ha−1) is N applied, TN (kg ha−1) is the total N uptake by plant, GN (kg ha−1) is the grain N amount, and ∆N (kg ha−1) is the change in the soil inorganic N (including NH4+-N and NO3−-N) before and after maize planting.
2.6. Crop Yield
To determine the maize yield and ear traits, 30 ears were harvested at the physiological maturity stage (R6) from three rows at the center of each plot. All the kernels were air-dried, and the grain yield was measured at 14% moisture, the standard moisture content of maize in storage or for sale in China (GB/T 29890-2013).
2.7. Data Analysis
Analysis of variance (ANOVA) was performed according to the general linear model procedure of SPSS (Ver. 17.0, SPSS, Chicago, IL, USA). The least significant difference (LSD) between the means was estimated at the 95% confidence level. Unless otherwise indicated, significant differences are at p ≤ 0.05. LSD; this was used to compare the adjacent means arranged in order of magnitude.
3. Results
3.1. Grain Yield
Waterlogging significantly decreased grain yield. The grain yield of V-3WL was 31% lower than that of CK across years. However, the application of nitrapyrin was beneficial to increase yields in waterlogged plots, with the grain yield being 34% higher in the V-3WL+N treatment than that in the V-3WL treatment. In addition, the kernel number and 1000-grain weight were significantly increased by 19 and 9% for V-3WL+N, respectively, compared to those of V-3WL across years (Table 1).

Table 1.
Effects of applying nitrapyrin on grain yield and its components under a waterlogged field.
3.2. N2O Emissions
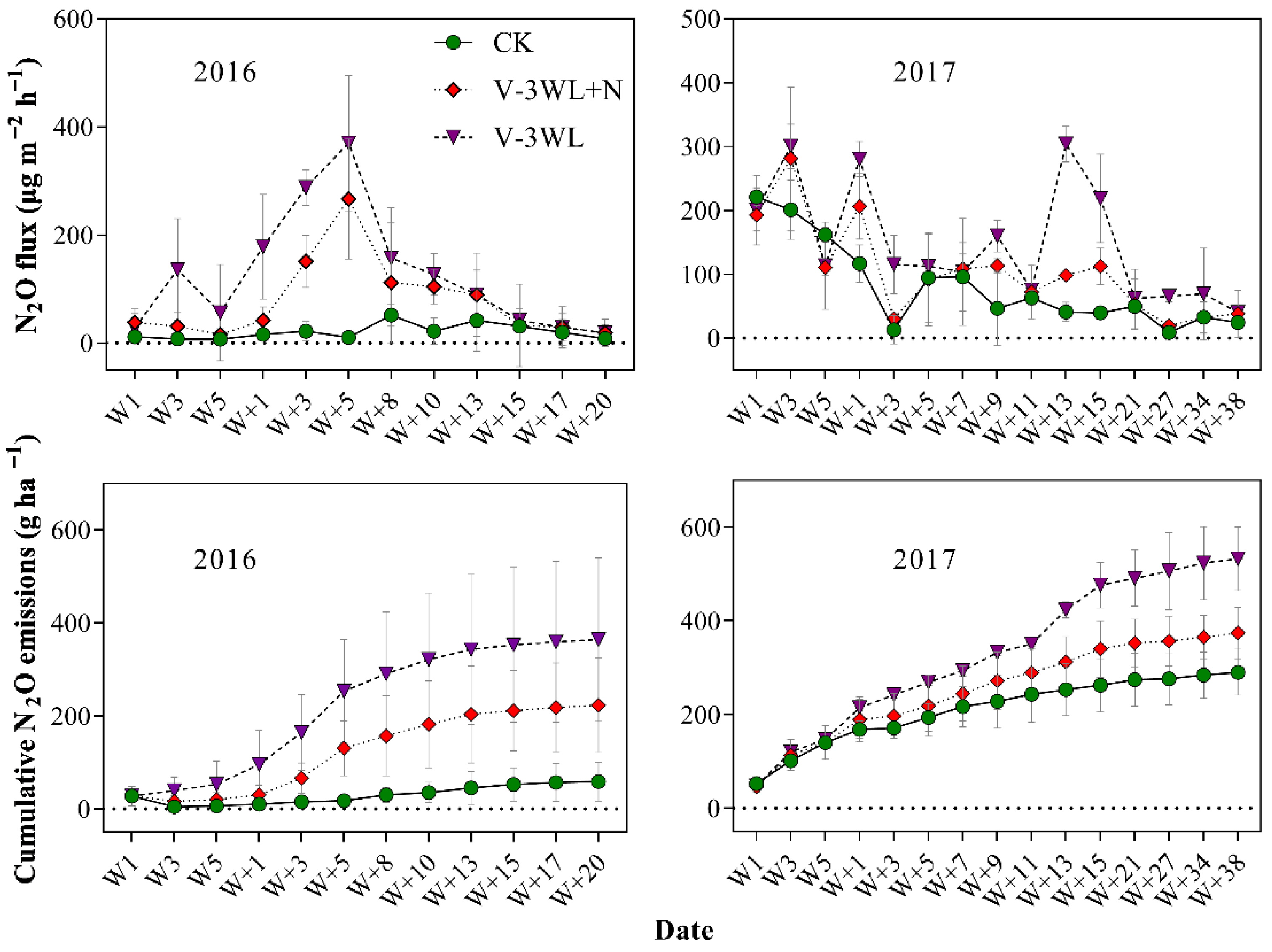
The three treatments during the summer maize season showed significant temporal variation in N2O emissions. N2O emission increased significantly after waterlogging, and the maximum N2O was recorded during the process of soil drying. The N2O emission fluxes in CK, V-3WL+N, and V-3WL ranged from 6.9 to 221.1, 15.8 to 281.0, and 18.6 to 370.0 μg m−2 h−1, respectively. The variation trends of N2O fluxes in the two waterlogging treatments were similar, while the treatment with nitrapyrin decreased significantly. After waterlogging, the cumulative emission flux of N2O increased significantly, showing a trend of V-3WL>V-3WL+N>CK (Figure 1).
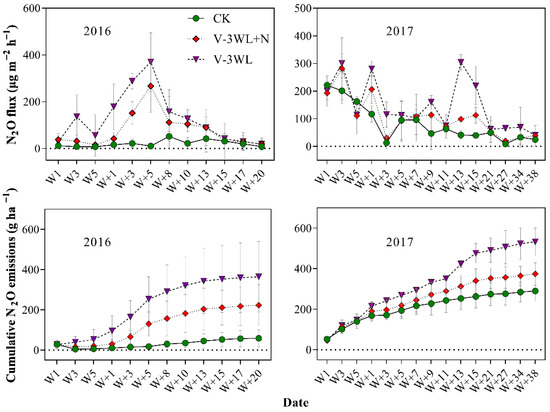
Figure 1.
Effects of applying nitrapyrin on the soil N2O emission flux under a waterlogged field. V-3WL, waterlogging treatment with urea application only; V-3WL+N, waterlogging treatment with urea and nitrapyrin application; CK, not waterlogged, but applied only urea; Wn, waterlogging duration; W+n, the day after waterlogging. The error bars represent 95% confidence intervals.
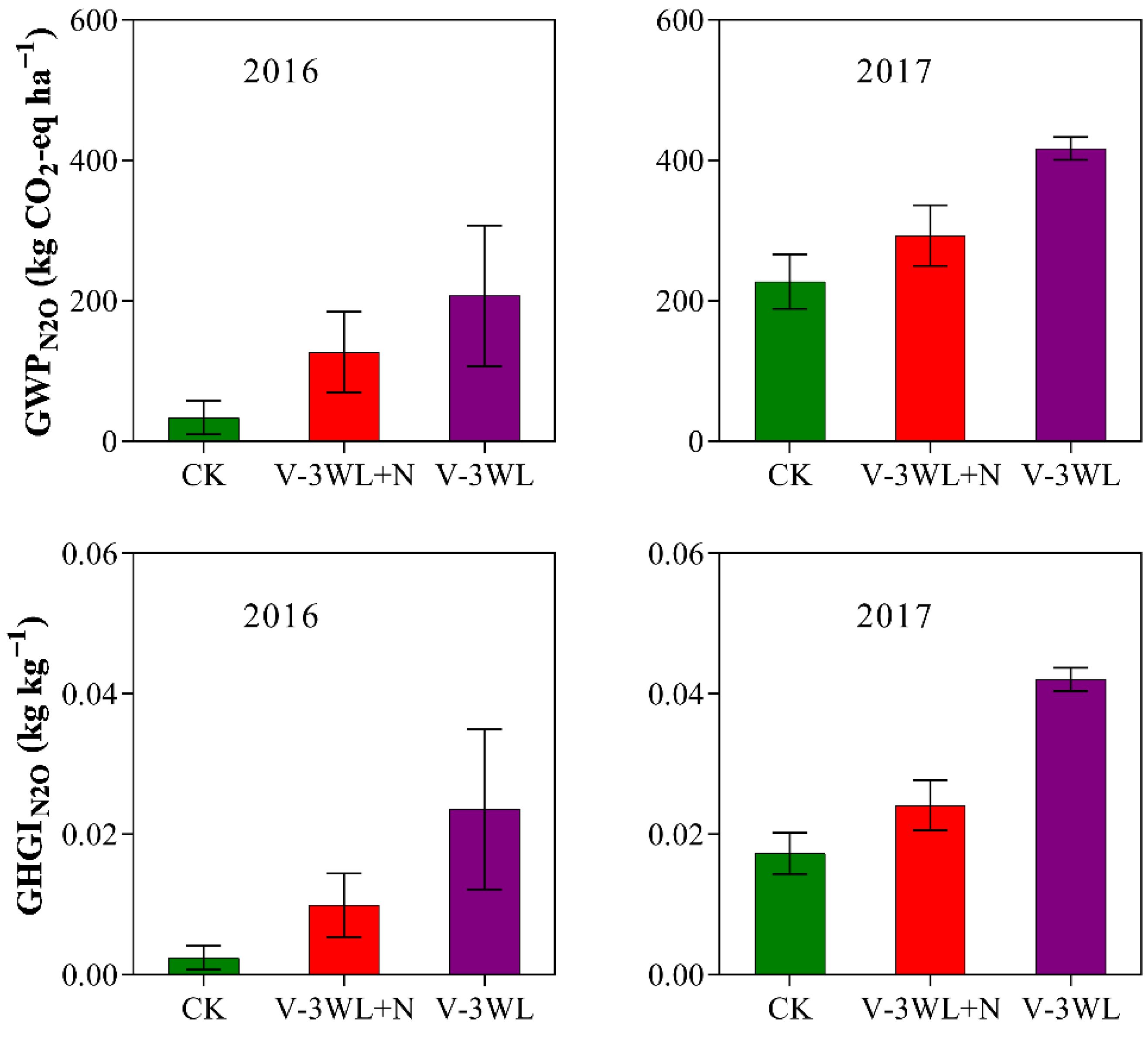
Likewise, waterlogging significantly increased the GWPN2O and GHGIN2O. However, after the addition of nitrapyrin, the GWPN2O and GHGIN2O were significantly decreased, and V-3WL+N decreased by 34% and 50%, respectively, compared with the V-3WL treatment (Figure 2).
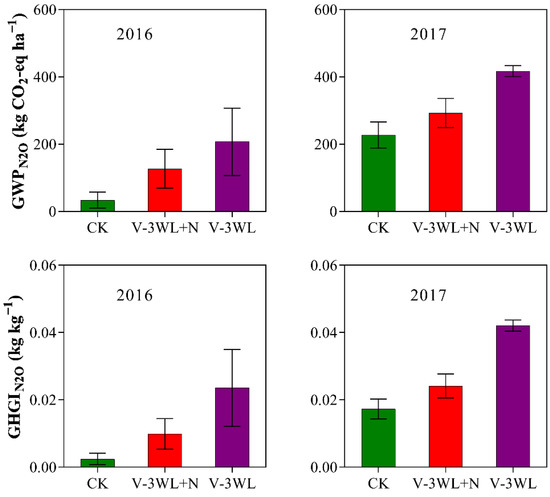
Figure 2.
Effects of applying nitrapyrin on the warming potential under a waterlogged field. V-3WL, waterlogging treatment with urea application only; V-3WL+N, waterlogging treatment with urea and nitrapyrin application; CK, not waterlogged, but applied only urea. The error bars represent 95% confidence intervals.
3.3. Soil NO3−-N and NH4+-N Concentrations
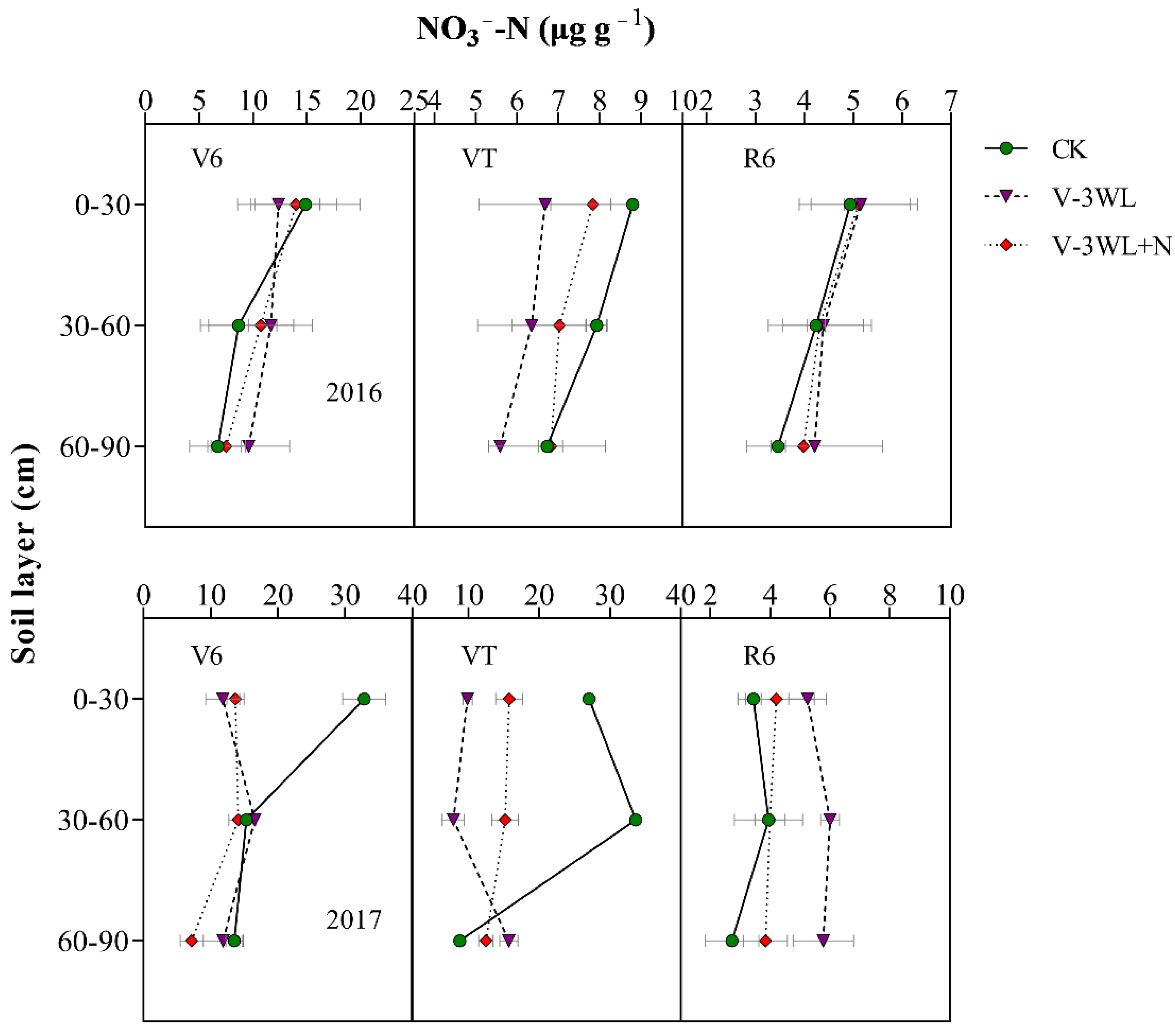
The soil N was rapidly leached in the waterlogged soil due to high levels of soil moisture. The two-year average results showed that the NO3−-N concentration in the top (0–30 cm) soil layer in the V-3WL treatment was 40% lower than that in the CK soil at the V6 stage; however, the NO3−-N concentrations in the mid (30–60 cm) and deep (60–90 cm) soil layers were 22% and 15% higher in the V-3WL treatment, respectively, compared to that in CK. (There was no significant difference between the V-3WL and CK treatments in the 60–90 cm soil layer in 2017.) When nitrapyrin was applied, the transformation of NH4+-N to NO3−-N was inhibited. The NO3−-N concentrations in V-3WL+N treatment increased by 14% in the topsoil layer, and decreased by 12% and 31% in the mid and deep soil layers, respectively, compared to V-3WL. At VT, the NO3−-N concentration of the waterlogging treatment in the 0–30 cm and 30–60 cm soil layers was lower than CK. In 2017, the NO3−-N concentration of the V-3WL treatment in each soil layer at the R6 stage was significantly higher than that of V-3WL+N, while there was no significant difference between the two waterlogging treatments in 2016 (Figure 3).
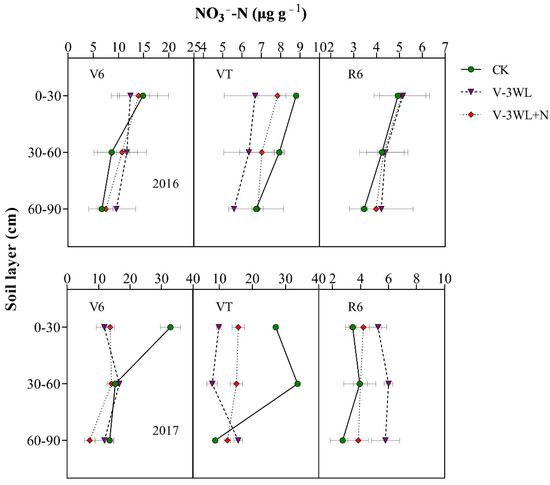
Figure 3.
Effects of applying nitrapyrin on the soil NO3−-N content in a waterlogged maize field. V-3WL, waterlogging treatment with urea application only; V-3WL+N, waterlogging treatment with urea and nitrapyrin application; CK, not waterlogged, but applied only urea. The error bars represent 95% confidence intervals.
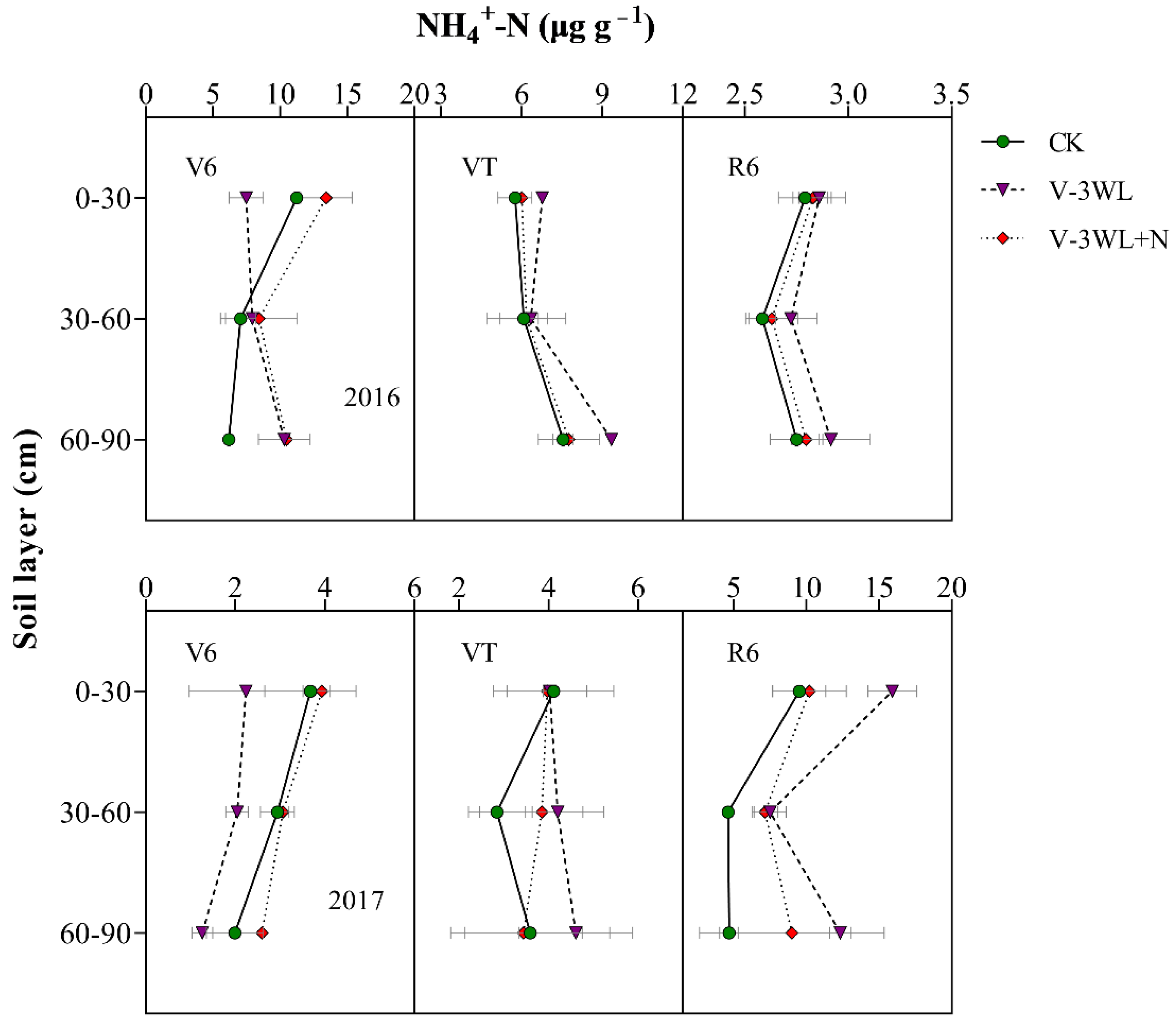
At the V6 stage, the NH4+-N concentration in the 0–30 cm, 30–60 cm, and 60–90 cm soil layers of the V-3WL+N was significantly increased, which was 77%, 28%, and 54% higher than the V-3WL treatment, respectively. However, at VT, the NH4+-N concentration in the deep soil layer of the V-3WL treatment was significantly higher than that of the other treatments, which increased by 26% and 27% compared with CK and V-3WL+N, respectively. Obviously, the NH4+-N concentration of each soil layer at R6 showed a similar trend of V-3WL>V-3WL+N>CK (Figure 4).
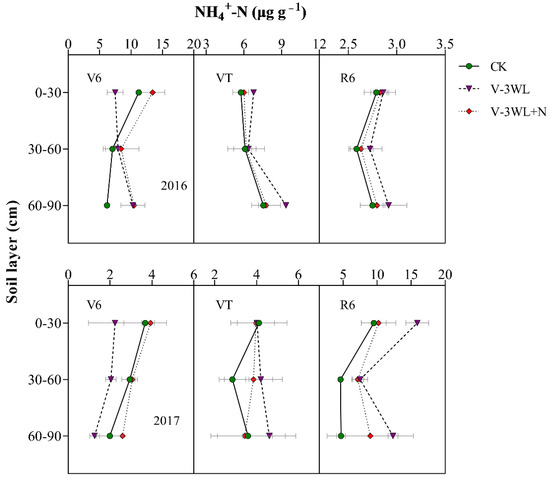
Figure 4.
Effects of applying nitrapyrin on the soil NH4+-N content under a waterlogged field.
V-3WL, waterlogging treatment with urea application only; V-3WL+N, waterlogging treatment with urea and nitrapyrin application; CK, not waterlogged, but applied only urea. The error bars represent 95% confidence intervals.
3.4. Nitrogen Efficiency and N Budget
Nitrogen accumulation was significantly reduced after waterlogging. The total N accumulation was 24% lower than that of CK across years. Moreover, the nitrogen partial factor productivity (NPFP), nitrogen use efficiency (NUE), and nitrogen harvest index (NHI) were 31, 5, and 17% lower than those of CK, respectively. Nitrapyrin application effectively alleviated the reduction of N accumulation and N efficiency induced by waterlogging. The total N accumulation, NPFP, NUE, and NHI of V-3WL+N was increased by 14, 34, 10, and 12% across years, respectively. All the treatments had a surplus of soil N, and the apparent surplus of soil N increased significantly after waterlogging. However, nitrapyrin application reduced the apparent soil N surplus in 2016 (no significant difference in 2017) (Table 2).

Table 2.
Effects of applying nitrapyrin on the nitrogen accumulation and nitrogen efficiency under a waterlogged field.
4. Discussion
The soil N2O was largely produced from microbial nitrification and denitrification, which were affected by environmental conditions such as soil temperature, moisture content, organic matter content, and pH [17,41]. Of these factors, soil moisture content had the greatest effect on N2O emission [42]. An increase in soil moisture, such as from natural (e.g., rainfall) and artificial (e.g., irrigation) processes, resulted in short-term increases in N2O emissions and, thus, lower N acquisition and NUE by crops [35]. Maximum N2O flux was reached at 84–86% water-filled pore space (WFPS), which represented the percentage of soil saturation by water. At less than 70% WFPS, soil N2O flux, mostly produced by nitrification, increased with increasing soil moisture content and the application of N fertilizer [24]. Conversely, when WFPS was more than 70%, N2O was mostly produced by denitrification [43,44,45]. In addition, sufficient mineral N content would promote the release of N2O emissions by changing the nitrification and denitrification rate of microorganisms, and even form “hot spots” of N2O emissions [4].
Our previous results indicated that waterlogging limited plant growth and lowered grain yield by decreasing both NUE and N fertilizer recovery efficiency in summer maize [35,36]. In this present study, we found that soil N2O fluxes were significantly increased after waterlogging (Figure 1). The maximum N2O flux was recorded during the process of soil drying, similar to observations by Jie et al. (1997) [46] for paddy fields. This was probably because the water layer covering the soil surface reduced soil permeability and promoted denitrification. When the soil was waterlogged, gaps among soil particles were completely filled with water. Under this condition, N2O accumulated in the soil and did not revert back to gaseous N (N2). However, N2O was released from the soil as the soil gradually dried. Thus, waterlogging led to increased N2O emissions from our experimental plots and decreased the NUE of the maize crop. The interaction of local temperature, soil, and other environmental factors affect N2O emissions fluxes [47]. Compared with 2016, the peak N2O emission of the waterlogging treatment decreased; however, the duration was prolonged, and the cumulative N2O emission flux increased in 2017. Multi-day rainfall from June to July in 2017 may have contributed to differences in N2O emission fluxes between the two maize seasons. On a broader spatial scale, waterlogging could increase the rate of N2O emissions. This would lead to an increased contribution to the greenhouse effect from agricultural activities with a significant increase of GWPN2O and GHGIN2O by 299% and 504%, respectively, compared to those of CK; this confirms that waterlogging contributed significantly to the greenhouse effect. However, the application of nitrapyrin was shown to improve the absorption and use of N fertilizer and N distribution in grains, which increased the NUE of summer maize grown under waterlogged conditions [35], and enhanced total N accumulation and N fertilizer recovery efficiency (Table 1 and Table 2). Nitrapyrin could reduce N losses through leaching and gas diffusion by inhibiting soil nitrification [32,33]. In this study, the application of nitrapyrin resulted in lowered N2O emissions, decreasing GWPN2O and GHGIN2O by 34% and 50%, respectively. Visibly, nitrapyrin could reduce the effect of waterlogging on soil GHG emissions and help to lower the contribution from agricultural activities to the greenhouse effect.
The leaching of the soil N, which was exacerbated by waterlogging, resulted not only in fertilizer losses, but also in serious environmental problems [48,49]. Soil particles have a negative surface charge (cation exchange capacity); the soil is able to bind positively charged ions (such as NH4+) and prevent such ions from leaching. In contrast, NO3− is a negatively charged ion that does not bind to soil and is easily leached in soil solutions [50]. Our previous study showed that waterlogging limited root growth and development, lowering the ability of the plant to absorb N, and decreased the plant NUE by increasing soil N leaching in the form of NO3− [19]. At the early waterlogging stage, the soil moisture content was very high, and the soil N would be leached at high rates. At the V6 stage, the NH4+-N and NO3−-N concentrations were lower in the topsoil layers, and higher in the mid and deep soil layers in waterlogged soil compared to non-waterlogged soil (Figure 3). By contrast, the NH4+-N concentrations in waterlogged soil with nitrapyrin (V-3WL+N treatment) were higher in all the soil layers compared to the concentrations in waterlogged soil without nitrapyrin (V-3WL treatment). However, the NO3−-N concentrations in the mid and deep soil layers were lower in the V-3WL+N treatment compared to those in the V-3WL treatment (Figure 4). This result might be due to the presence of nitrification inhibitors, which prevented the transformation of NH4+-N into NO3−-N. As the soil dried, the soil moisture content returned to baseline levels, and the process of soil N leaching slowed. However, the disorder of root growth and development caused by waterlogging led to a reduced ability to absorb N and the accumulation of N around the root rhizosphere [19]. Therefore, NH4+-N concentrations in the topsoil layers of waterlogged plots were higher than those of the control plots when the plants were at the VT and R6 stages. These results showed that the N uptake and NUE of summer maize were reduced in waterlogged soils, resulting in an increase in the apparent budget of soil N.
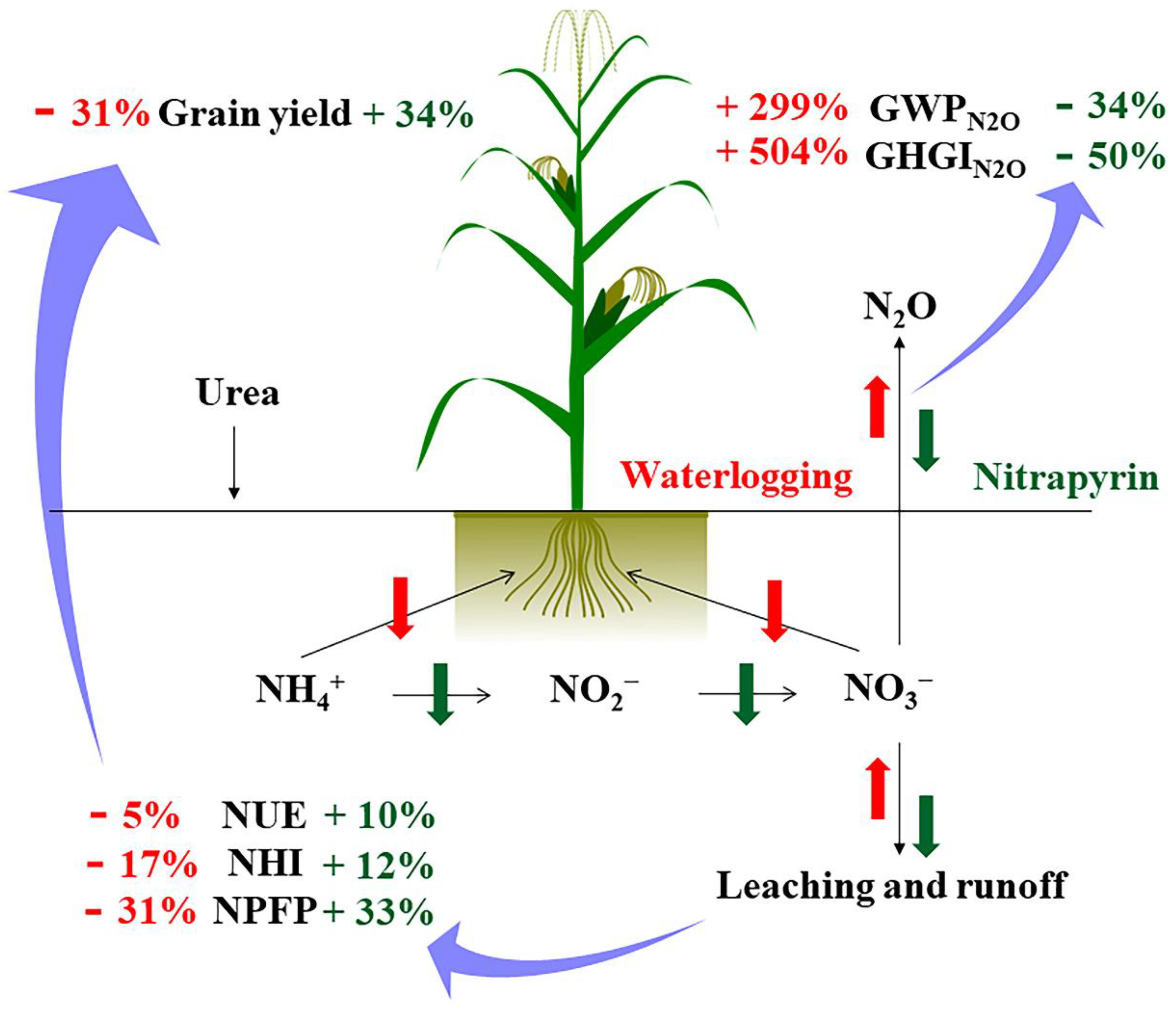
The interannual N2O emission fluxes differences did not have a significant impact on the apparent budget of soil N (Table 2). The interannual differences appeared to be offset by soil inorganic N levels before N application in the summer maize season. In addition, when calculating the apparent budget of the soil N in this study, the mineralized and fixed amount of soil N were not included. The results showed that the N fertilization treatments had a surplus of N in summer maize season, and the waterlogging treatments had a greater risk of N loss. Nitrification inhibitors could reduce N leaching rates, and improve N absorption and use efficiency in plants; thus, this mitigates waterlogging damage on N leaching and N use efficiency. Overall, the application of nitrapyrin to waterlogged fields can help to reduce N loss and GHGN2O flux, and increase grain yield (Figure 5). Nitrapyrin, an eco-friendly and N-efficient fertilizer companion, was useful for waterlogged soil conditions by helping to decrease N leaching and reducing the agricultural contribution to the greenhouse effect.
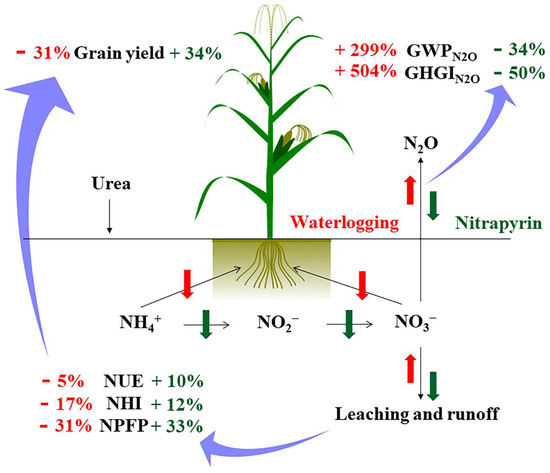
Figure 5.
The influence process of applying nitrapyrin on soil N translocation under a waterlogged field. NUE, nitrogen use efficiency; NHI, nitrogen harvest index; NPFP, nitrogen partial factor productivity; GWPN2O, N2O warming potential; GHGIN2O, N2O greenhouse gas intensity.
5. Conclusions
In this study, waterlogging significantly decreased crop yield and increased the cumulative emission flux of N2O, warming potential and greenhouse gas intensity. Nitrapyrin application not only helped to increase grain yield and N efficiency of maize grown in waterlogged soil, but also reduced the GHGIN2O and GWPN2O of waterlogged soil as well as the contribution to the greenhouse effect from agricultural sources.
Author Contributions
Data curation and writing-original draft, B.R. and Z.M.; conceptualization and methodology, J.Z.; supervision, P.L.; methodology and formal analysis, B.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Nature Science Funds (31801296); National Key Research and Development Program of China (2017YFD0300304; 2018YFD0300603); and National Modern Agricultural Technology & Industry System (CARS-02-21).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author/s.
Acknowledgments
The authors are grateful to the reviewers and editors for their constructive review and suggestions for this paper.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Godfray, H. The challenge of feeding 9–10 billion people equitably and sustainably. J. Agric. Sci. 2014, 152, 2–8. [Google Scholar] [CrossRef]
- Wang, J.; Huang, J.; Rozelle, S. Climate change and China’s agricultural sector: An overview of impacts, adaptation and mitigation. Int. Food Agric. Trade Policy Counc. (IPC) 2010, 5, 1–31. [Google Scholar]
- IPCC. Climate Change 2007: The Physical Science Basis: Contribution of Working Group I to the Fourth Assessment Report of the intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- IPCC. Climate change 2021: The physical science basis In Contribution of Working GROUP I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Denman, K.L. Couplings between changes in the climate system and biogeochemistry. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; pp. 499–587. [Google Scholar]
- Forster, P.; Ramaswamy, V.; Artaxo, P.; Berntsen, T.; Dorland, R.V. The physical science basis of climate change: Changes in atmospheric constituents and in radiative forcing. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; pp. 129–234. [Google Scholar]
- Gil, W.K.; Kim, P.J.; Khan, M.I.; Lee, S.J. Effects of rice planting on nitrous oxide (N2O) emission under different levels of nitrogen fertilization. Agronomy 2021, 11, 217. [Google Scholar]
- Jassal, R.S.; Roy, R.; Ethier, G.; Black, T.A. Effect of nitrogen fertilization on soil CH4 and N2O fluxes, and soil and bole respiration. Geoderma 2011, 162, 182–186. [Google Scholar] [CrossRef]
- Jia, S.; Wang, Z.; Li, X.; Sun, Y.; Zhang, X.; Liang, A. N fertilization affects on soil respiration, microbial biomass and root respiration in Larix gmelinii and Fraxinus mandshurica plantations in China. Plant Soil 2010, 333, 325–336. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, X.; Mclaughlin, N.N.; Liang, A.; Jia, S.; Chen, X.; Chen, X. Effect of soil temperature and soil moisture on CO2 flux from eroded landscape positions on black soil in Northeast China. Soil Tillage Res. 2014, 144, 119–125. [Google Scholar] [CrossRef]
- Weier, K.L. N2O and CH4 emission and CH4 consumption in a sugarcane soil after variation in nitrogen and water application. Soil Biol Biochem. 1999, 31, 1931–1941. [Google Scholar] [CrossRef]
- Cohen, J.; Screen, J.A.; Furtado, J.C. Recent Arctic amplification and extreme mid-latitude weather. Nat. Geosci. 2014, 7, 627–637. [Google Scholar] [CrossRef] [Green Version]
- IPCC. Summary for policymakers. In Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Fischer, E.M.; Knutti, U.B. Robust spatially aggregated projections of climate extremes. Nat. Clim. Change 2013, 3, 1033–1038. [Google Scholar] [CrossRef]
- Min, S.; Zhang, X.; Zwiers, F. Human contribution to more intense precipitation extremes. Nature 2011, 470, 378–381. [Google Scholar] [CrossRef]
- Knapp, A.K.; Claus, B.; Briske, D.D.; Classen, A.T.; Luo, Y.; Markus, R.; Smith, M.D.; Smith, S.D.; Bell, J.E.; Fay, P.A. Consequences of more extreme precipitation regimes for terrestrial ecosystems. Bioscience 2008, 58, 811–821. [Google Scholar] [CrossRef]
- Granli, T.; Bøckman, O.C. Nitrous oxide (N2O) emissions from soils in warm climates. Fertil. Res. 1995, 42, 159–163. [Google Scholar] [CrossRef]
- Weitz, A.M.; Linder, E.; Frolking, S.; Crill, P.M.; Keller, M. N2O emissions from humid tropical agricultural soils: Effects of soil moisture, texture and nitrogen availability. Soil Biol Biochem. 2001, 33, 1077–1093. [Google Scholar] [CrossRef]
- Ren, B.Z.; Zhang, J.W.; Dong, S.T.; Liu, P.; Zhao, B. Root and shoot responses of summer maize to waterlogging at different stages. Agron. J. 2016, 108, 1060–1069. [Google Scholar] [CrossRef]
- Di, H.J.; Cameron, K.C. Nitrous oxide emissions from two dairy pasture soils as affected by different rates of a fine particle suspension nitrification inhibitor, dicyandiamide. Biol. Fertil. Soils 2006, 42, 472–480. [Google Scholar] [CrossRef]
- Wu, D.; Cárdenas, L.M.; Calvet, S.; Brüggemann, N.; Loick, N.; Liu, S.; Bol, R. The effect of nitrification inhibitor on N2O, NO and N2 emissions under different soil moisture levels in a permanent grassland soil. Soil Biol. Biochem. 2017, 113, 153–160. [Google Scholar] [CrossRef]
- Niu, Y.; Luo, J.; Liu, D.; Müller, C.; Mohammad, Z.; Stuart, L.; Ding, W. Effects of biochar and nitrapyrin on nitrous oxide and nitric oxide emissions from a sandy loam soil cropped to maize. Biol. Fertil. Soils 2018, 54, 645–658. [Google Scholar] [CrossRef]
- Chen, D.; Suter, H.C.; Islam, A.; Edis, R. Influence of nitrification inhibitors on nitrification and nitrous oxide (N2O) emission from a clay loam soil fertilized with urea. Soil Biol. Biochem. 2010, 42, 660–664. [Google Scholar] [CrossRef]
- Abbasi, M.K.; Adams, W.A. Estimation of simultaneous nitrification and denitrification in grassland soil associated with urea-N using 15N and nitrification inhibitors. Biol. Fertil. Soils 2000, 31, 38–44. [Google Scholar] [CrossRef]
- McCarty, G.W. Modes of action of nitrification inhibitors. Biol. Fertil. Soils 1999, 29, 1–9. [Google Scholar] [CrossRef]
- Martins, M.R.; Sant’Anna, S.A.C.; Zaman, M.; Santos, R.C.; Monteiro, R.C.; Alves, B.J.R.; Jantalia, C.P.; Boddey, R.M.; Urquiaga, S. Strategies for the use of urease and nitrification inhibitors with urea: Impact on N2O and NH3 emissions, fertilizer-15N recovery and maize yield in a tropical soil. Agric. Ecosyst. Environ. 2017, 247, 54–62. [Google Scholar] [CrossRef]
- Burzaco, J.P.; Smith, D.R.; Tony, V.J. Nitrous oxide emissions in Midwest US maize production vary widely with band-injected N fertilizer rates, timing and nitrapyrin presence. Environ. Res. Lett. 2013, 8, 035031. [Google Scholar] [CrossRef]
- Bronson, K.F.; Mosier, A.R.; Bishnoi, S.R. Nitrous oxide emissions in irrigated corn as affected by nitrification inhibitors. Soil Sci. Soc. Am. J. 1992, 56, 161–165. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Hu, H.; He, J.; Chen, D.; Suter, H. Effects of 3, 4-dimethylpyrazole phosphate (DMPP) on nitrification and the abundance and community composition of soil ammonia oxidizers in three land uses. Biol. Fertil. Soils 2016, 52, 927–939. [Google Scholar] [CrossRef]
- Menéndez, S.; Barrena, I.; Setien, I.; González-Murua, C.; Estavillo, J.M. Efficiency of nitrification inhibitor DMPP to reduce nitrous oxide emissions under different temperature and moisture conditions. Soil Biol. Biochem. 2012, 53, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Parkin, T.B.; Hatfield, J.L. Influence of nitrapyrin on N2O losses from soil receiving fall-applied anhydrous ammonia. Agric. Ecosyst. Environ. 2010, 136, 81–86. [Google Scholar] [CrossRef]
- Borzouei, A.; Mander, U.; Teemusk, A.; Sanz-Cobena, A.; Zaman, M.; Kim, D.G.; Müller, C.; Kelestanie, A.A.; Sayyad, A.P.; Moghiseh, E.; et al. Effects of the nitrification inhibitor nitrapyrin and tillage practices on yield-scaled nitrous oxide emission from a maize field in Iran. Pedosphere 2021, 31, 314–322. [Google Scholar] [CrossRef]
- Dawar, K.; Sardar, K.; Zaman, M.; Müller, C.; Sanz-Cobena, A.; Khan, A.; Borzouei, A.; Pérez-Castillo, A.G. Effects of the nitrification inhibitor nitrapyrin and the plant growth regulator gibberellic acid on yield-scale nitrous oxide emission in maize fields under hot climatic conditions. Pedosphere 2021, 31, 323–331. [Google Scholar] [CrossRef]
- Barrena, I.; Menéndez, S.; Correa-Galeote, D.; Vega-Mas, I.; Bedmar, E.J.; González-Murua, C.; Estavillo, J.M. Soil water content modulates the effect of the nitrification inhibitor 3, 4-dimethylpyrazole phosphate (DMPP) on nitrifying and denitrifying bacteria. Geoderma 2017, 303, 1–8. [Google Scholar] [CrossRef]
- Ren, B.Z.; Zhang, J.W.; Dong, S.T.; Liu, P.; Zhao, B.; Li, H. Nitrapyrin improves grain yield and nitrogen use efficiency of summer maize waterlogged in the field. Agron. J. 2017, 109, 185–192. [Google Scholar] [CrossRef]
- Ren, B.Z.; Dong, S.T.; Zhao, B.; Liu, P.; Zhang, J.W. Responses of nitrogen metabolism, uptake and translocation of maize to waterlogging at different growth stages. Front. Plant Sci. 2017, 8, 01216. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Li, B.; Ren, B.Z.; Zhao, B.; Liu, P.; Zhang, J.W. Effects of residue management strategies on greenhouse gases and yield under double cropping of winter wheat and summer maize. Sci. Total Environ. 2019, 687, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Liu, S.; Guo, Y.; Liu, Q.; Zou, J. Methane and nitrous oxide emissions from organic and conventional rice cropping systems in Southeast China. Biol. Fertil. Soils 2010, 46, 825–834. [Google Scholar] [CrossRef]
- Mosier, A.R.; Halvorson, A.D.; Reule, C.A.; Liu, X.J. Net global warming potential and greenhouse gas intensity in irrigated cropping systems in Northeastern Colorado. J. Environ. Qual. 2006, 35, 1584–1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; He, N.; Wang, Q.; Yuan, G.; Wen, D.; Yu, G.; Jia, Y. The composition, spatial patterns, and influencing factors of atmospheric wet nitrogen deposition in Chinese terrestrial ecosystems. Sci. Total Environ. 2015, 511, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Sahrawat, K.L.; Keeney, D.R. Nitrous oxide emission from soils. Adv. Soil Sci. 1986, 4, 103–148. [Google Scholar]
- Davidson, E.A. Fluxes of nitrous oxide and nitric oxide from terrestrial ecosystems. In Microbial Production and Consumption of Greenhouse Gases: Methane, Nitrogen Oxides, and Halomethanes; Rogers, J.E., Whitman, W.B., Eds.; American Society for Microbiology: Washington, DC, USA, 1991; pp. 219–235. [Google Scholar]
- Ruser, R.; Flessa, H.; Russow, R.; Schmidt, G.; Buegger, F.; Munch, J.C. Emission of N2O, N2 and CO2 from soil fertilized with nitrate: Effect of compaction, soil moisture and rewetting. Soil Biol. Biochem. 2006, 38, 263–274. [Google Scholar] [CrossRef]
- Bateman, E.J.; Baggs, E.M. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol. Fertil. Soils 2005, 41, 379–388. [Google Scholar] [CrossRef]
- Wolf, I.; Russow, R. Different pathways of formation of N2O, N2 and NO in black earth soil. Soil Biol. Biochem. 2000, 32, 229–239. [Google Scholar] [CrossRef]
- Jie, W.U.; Cleemput, O.V.; Oswald, V.C. Relationship between CH4 and N2O emissions from rice field and its microbiological mechanism and impacting factors. Chin. J. Appl. Ecol. 1997, 8, 270–274. [Google Scholar]
- Blagodatskaya, E.; Zheng, X.; Blagodatsky, S.; Wiegl, R.; Dannenmann, M.; Butterbach, B.K. Oxygen and substrate availability interactively control the temperature sensitivity of CO2 and N2O emission from soil. Biol. Fertil. Soils Vol. 2014, 50, 775–783. [Google Scholar] [CrossRef]
- Bryant, J.R.; Snow, V.O.; Cichota, R.; Jolly, B.H. The effect of situational variability in climate and soil, choice of animal type and N fertilisation level on nitrogen leaching from pastoral farming systems around Lake Taupo New Zealand. Agr. Syst. 2011, 104, 271–280. [Google Scholar] [CrossRef]
- Ashraf, M.; Rehman, H. Mineral nutrient status of corn in relation to nitrate and long-term waterlogging. J. Plant Nutr. 1999, 22, 1253–1268. [Google Scholar] [CrossRef]
- Nan, X.; Tan, G.; Wang, H.; Gai, X. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Biol. 2016, 74, 1–8. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).