Antioxidant and Anticancer Potential of Bioactive Compounds from Rhinacanthus nasutus Cell Suspension Culture
Abstract
:1. Introduction
2. Results
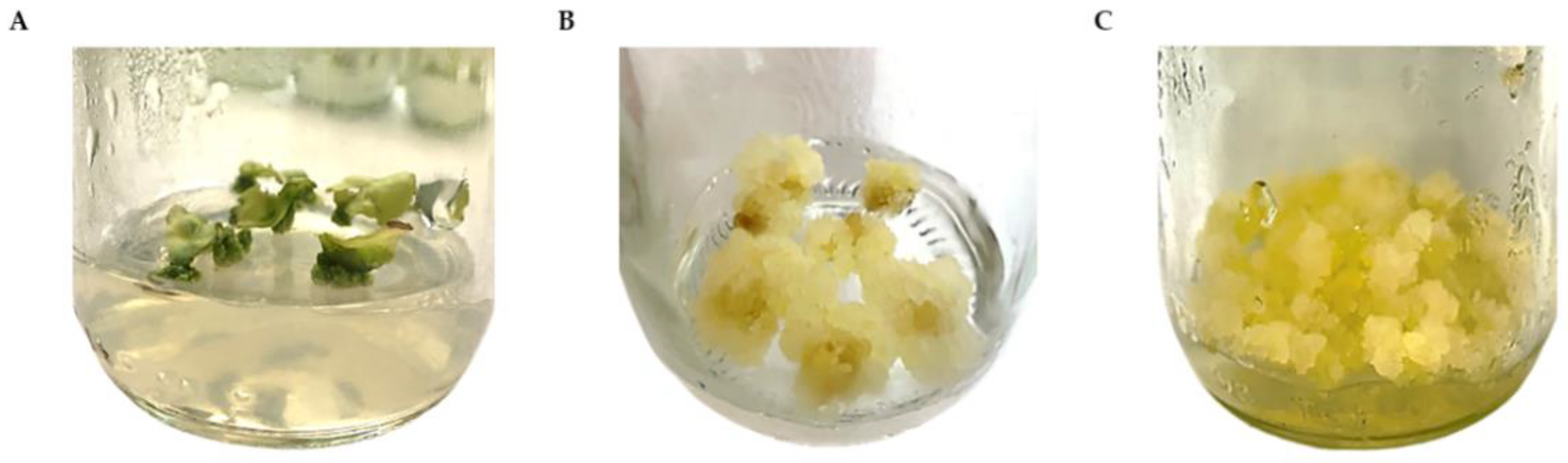
2.1. Callus Induction and Proliferation
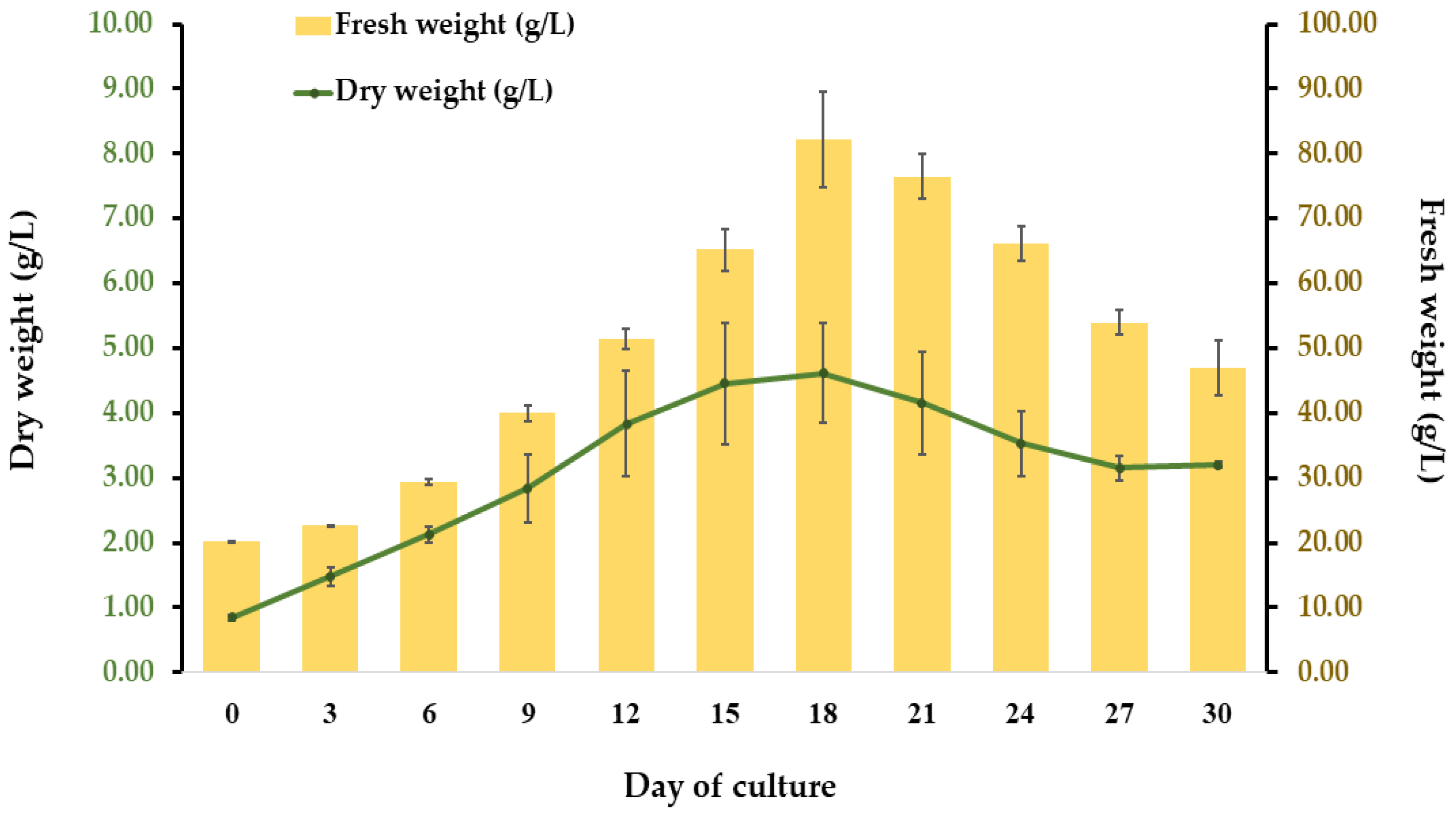
2.2. Establishment of Cell Suspension Culture
2.3. Total Phenolic and Total Flavonoid Contents
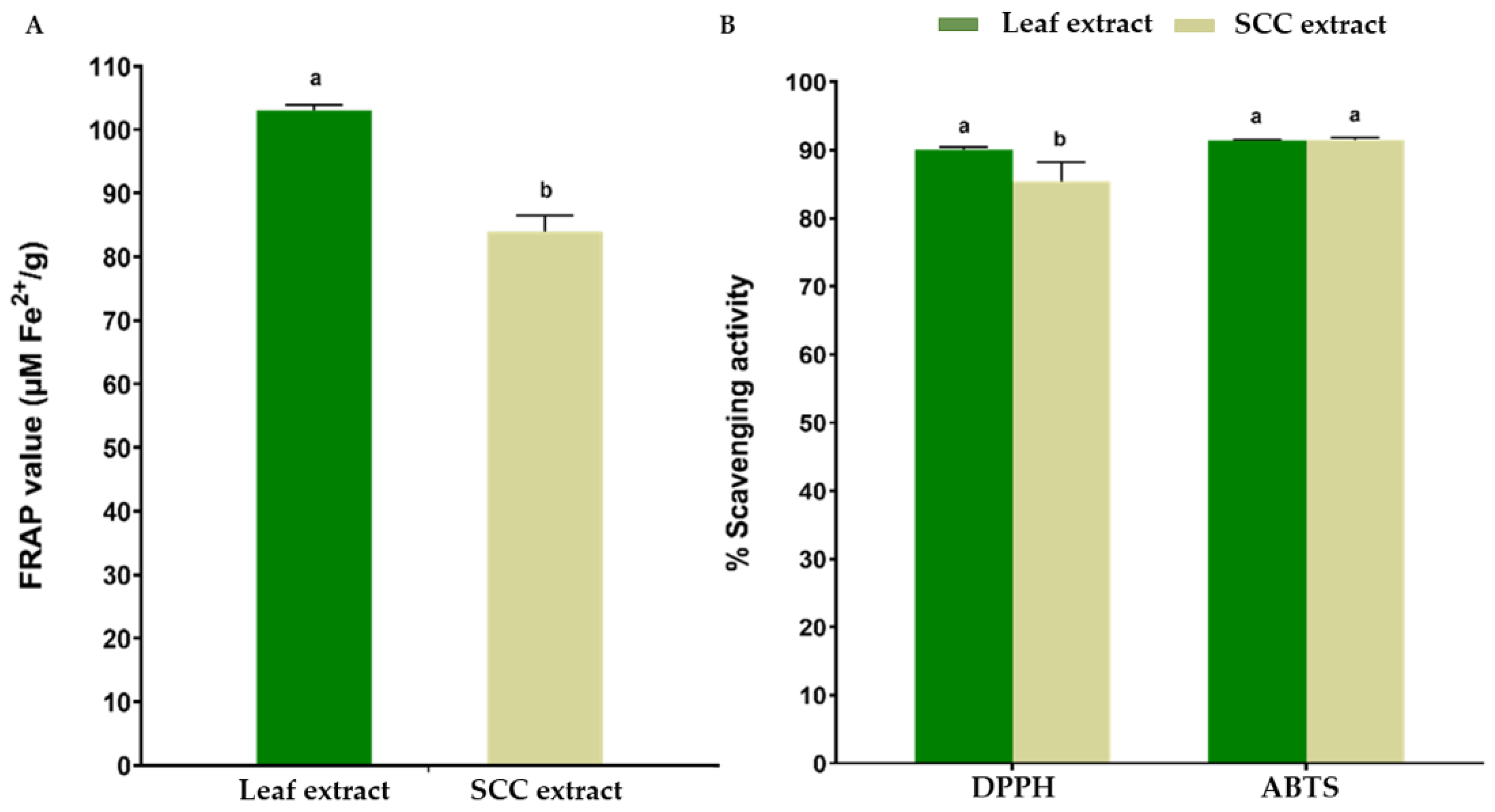
2.4. Antioxidant Properties
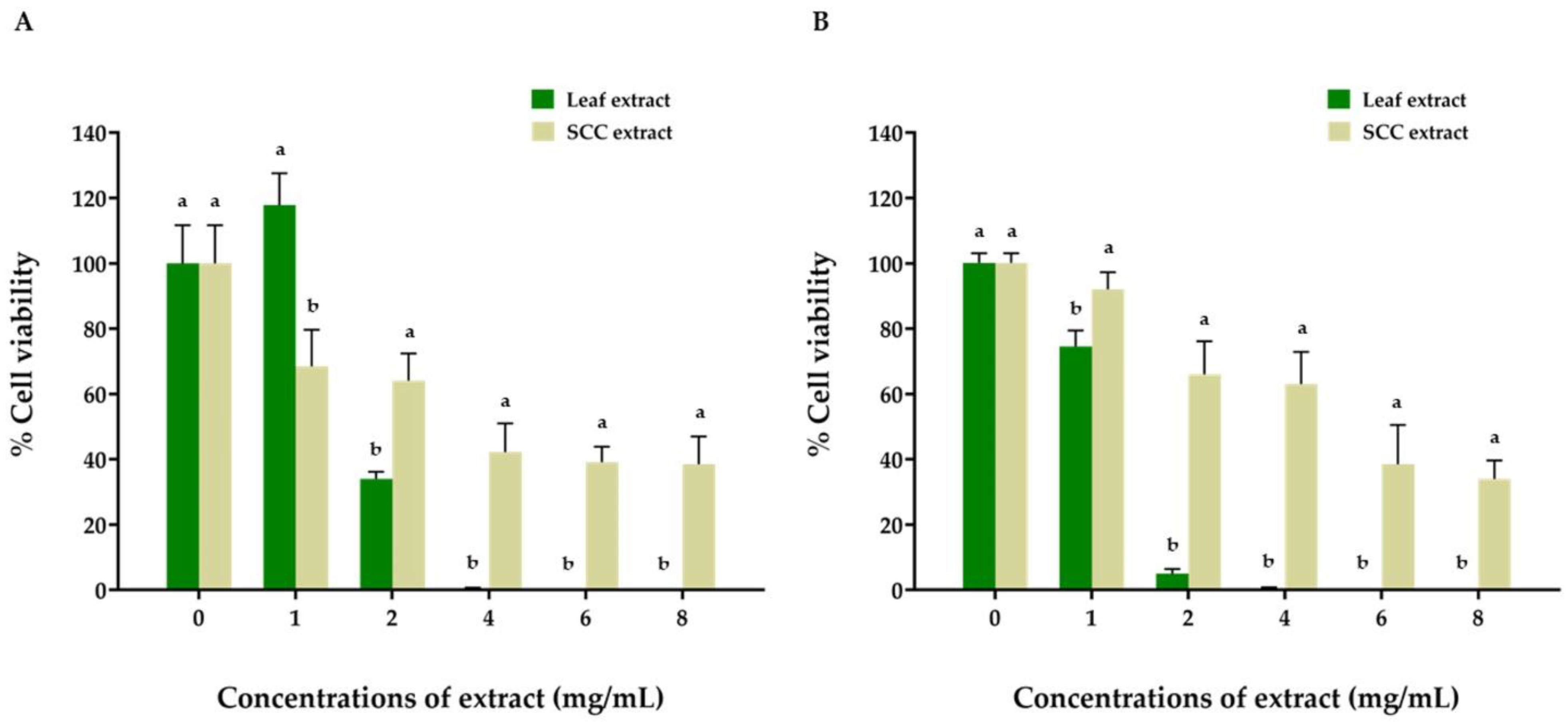
2.5. Anticancer Activity
2.6. Analysis of Metabolites in the Ethanolic Leaf and SCC Extracts by UHPLC–QToF–MS
3. Discussion
| Proposed Compound | Class * | Subclass * | Retention Time (min) | Product Ions (m/z) | Mass | Molecular Formula | Remarks |
|---|---|---|---|---|---|---|---|
| Terpenoids | |||||||
| Austroinulin | Prenol lipids | Diterpenoids | 43.58 | 345.24 | 322.25 | C20 H34 O3 | Found in Stevia/anti-inflammatory [32] |
| Inulicin | Prenol lipids | Terpene lactones | 26.71 | 307.16 | 308.16 | C17 H24 O5 | May suppress angiogenesis and lung cancer cell growth [33] |
| Lucidenic acid | Prenol lipids | Sesquiterpenoids | 41.91 | 481.26 | 458.27 | C27 H38 O6 | Lucidenic acid A, B, N caused cell cycle arrest in G1 phase/inhibited PMA-induced HCC invasion and apoptosis in human leukaemia cells HL-60 [34,35,36] |
| Corosolic acid | Prenol lipids | Triterpenoids | 43.30 | 495.35 | 472.36 | C30 H48 O4 | Inhibits human colorectal cancer cells [37]/inhibits liver cancer cell growth under high-glucose conditions [38] |
| Ganoderic acid | Prenol lipids | Triterpenoids | 40.42 | 555.29 | 532.31 | C30 H44 O8 | Inhibits cell proliferation, viability, and ROS and mRNA expression of TNF [39] |
| Punctaporin B | Prenol lipids | - | 41.32 | 251.17 | 252.17 | C15 H24 O3 | Found in Carolina cucumber and Vernonia cinerea (L.) Less [40] |
| Phenolics | |||||||
| (E)-2-glucosyl-3,4′,5 trihydroxy stilbene | Stilbenes | Stilbene glycosides | 27.74 | 413.12 | 390.13 | C20 H22 O8 | Complementary and alternative medicine for cancer therapy [41] |
| Ferulic acid | Cinnamic acids and derivatives | Hydroxycinnamic acids and derivatives | 16.07 | 193.05 | 194.06 | C10 H10 O4 | Able to suppress tumors in breast cancer, cervical carcinoma cells, prostate cancer cells, and pancreatic cancer cells [42] |
| Trans-cinnamic acid | Cinnamic acids and derivatives | Cinnamic acids | 33.20 | 149.06 | 148.05 | C9 H8 O2 | Some inhibitory activity against enzymes from the human liver and the human cholangiocarcinoma cell line [43] |
| 4-hydroxy coumarin | Coumarins and derivatives | Hydroxycoumarins | 28.54 | 163.04 | 162.03 | C9 H6 O3 | Inhibits cell proliferation in the gastric carcinoma cell line [44] |
| Esculetin | Coumarins and derivatives | Hydroxycoumarins | 13.12 | 177.02 | 178.03 | C9 H6 O4 | Inhibits migration and invasion of laryngeal cancer [45] |
| Quinones | |||||||
| Embelin | Prenol lipids | Quinone and hydroquinone lipids | 27.41 | 293.18 | 294.19 | C17 H26 O4 | Inhibits TNF-α and cancer cell metastasis [46,47,48] |
| Isoplumbagin | Naphthalenes | Naphthoquinones | 23.39 | 187.04 | 188.05 | C11 H8 O3 | Suppresses oral, lung, prostate, and cervical cancer cells [49] |
| 1,3,5,8-tetra hydroxy -6-methoxy-2-methyl anthraquinone | Others | Anthraquinones | 28.85 | 334.09 | 316.06 | C16 H12 O7 | Inhibits cancer progression by kinases, topoisomerases, telomerases, matrix metallo proteinases, and G-quadruplexes [50] |
| Flavonoids | |||||||
| 2′,4′-dihydroxy-7-methoxy-8-prenylflavan | Flavonoids | Flavans | 26.69 | 363.16 | 340.17 | C21 H24 O4 | Also found in Cassia fistula anticancer [51] |
| Rutin | Flavonoids | Flavonoid glycosides | 14.92 | 609.15 | 610.15 | C27 H30 O16 | Anticancer effects on human cervical cancer cells [52] |
| Vitexin 4′-O-galactoside | Flavonoids | Flavonoid glycosides | 15.52 | 593.15 | 594.16 | C27 H30 O15 | Many flavonoids have been isolated from and identified in flowers of the genus Iris [53] |
| Quercetin 3-(2′′-p-hydroxybenzoyl-4′′-p-coumaryl rhamnoside) | Flavonoids | Flavones | 43.83 | 715.17 | 714.16 | C37 H30 O15 | Quercetin suppresses cyclooxygenase-2 (COX-2) expression in human breast cancer cells [54] |
| Proposed Compound | Class * | Subclass * | Retention Time (min) | Product Ions (m/z) | Mass | Molecular Formula | Remarks |
|---|---|---|---|---|---|---|---|
| Terpenoids | |||||||
| Austroinulin | Prenol lipids | Diterpenoids | 43.10 | 345.24 | 322.25 | C20 H34 O3 | Found in Stevia/anti-inflammatory [32] |
| Lucidenic acid | Prenol lipids | Sesquiterpenoids | 38.46 | 463.31 | 462.30 | C27 H42 O6 | Lucidenic acid A, B, N caused cell cycle arrest in G1 phase/inhibited PMA-induced HCC invasion and apoptosis in human leukaemia cells HL-60 [34,35,36] |
| Camelledionol | Prenol lipids | Triterpenoids | 41.48 | 441.34 | 440.33 | C29 H44 O3 | Inhibits the A549, LLC, HL-60, and MCF-7 cancer cell lines [55] |
| Phenolics | |||||||
| Pterostilbene glycinate | Stilbenes | - | 18.38 | 336.12 | 313.13 | C18 H19 N O4 | Effective in treat ment of melanoma and has anticancer activity [56] |
| Esculetin | Coumarins and derivatives | Hydroxycoumarins | 13.08 | 177.02 | 178.03 | C9 H6 O4 | Inhibits migration and invasion of laryngeal cancer [45] |
| N-feruloyltyramine | Cinnamic acids and derivatives | Hydroxycinnamic acids and derivatives | 19.03 | 312.12 | 313.13 | C18 H19 N O4 | Significantly fights against the oxidative damage induced by H2O2 and inhibits HepG2 cells [57] |
| Quinones | |||||||
| Embelin | Prenol lipids | Quinone and hydroquinone lipids | 27.50 | 293.18 | 294.19 | C17 H26 O4 | Inhibits TNF-α and cancer cell metastasis [46,47,48] |
| 1,4-naphtho quinone | Naphthalenes | Naphthoquinones | 14.04 | 159.04 | 158.04 | C10 H6 O2 | Its derivatives demonstrate good anticancer, antioxidant, antimicrobial, and anti-inflammatory effects [58,59] |
| Flavonoids | |||||||
| Hesperetin | Flavonoids | o-methylated flavonoids | 20.359 | 301.07 | 302.08 | C16 H14 O6 | A drug used for lowering cholesterol and treating multiple cancers [60] |
| Quercetin 3-(2″-p-hydroxybenzoyl -4″-p-coumaryl rhamnoside) | Flavonoids | Flavones | 43.83 | 715.17 | 714.16 | C37 H30 O15 | Quercetin suppresses cyclooxygenase-2 (COX-2) expression in human breast cancer cells [54] |
4. Materials and Methods
4.1. Plant Material
4.2. Explant Preparation
4.3. Callus Induction, Cell Line Selection and Proliferation
4.4. Establishment of Cell Suspension Cultures and Growth Measurements
4.5. Preparation of Extracts
4.6. Determination of the TPC and TFC
4.7. Determination of Antioxidant Capacity
4.8. Determination of Anticancer Activity
4.9. UHPLC–QToF–MS
4.9.1. Data Acquisition
4.9.2. Data Processing
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bukke, S.; Raghu, P.S.; Sailaja, G.; Kedam, T.R. The study on morphological, phytochemical and pharmacological aspects of Rhinacanthus nasutus. (L.) Kurz (A Review). J. Appl. Pharm. Sci. 2011, 1, 26–32. [Google Scholar]
- Shah, M.A.; Keach, J.E.; Panichayupakaranant, P. Antidiabetic naphthoquinones and their plant resources in Thailand. Chem. Pharm. Bull. 2018, 66, 483–492. [Google Scholar] [CrossRef] [Green Version]
- Bhusal, N.; Panichayupakaranant, P.; Reanmongkol, W. In vivo analgesic and anti-inflammatory activities of a standardized Rhinacanthus nasutus leaf extract in comparison with its major active constituent rhinacanthin-C. Songklanakarin J. Sci. Technol. 2014, 36, 325–331. [Google Scholar]
- Brimson, J.M.; Prasanth, M.I.; Malar, D.S.; Brimson, S.; Tencomnao, T. Rhinacanthus nasutus “Tea” infusions and the medicinal benefits of the constituent phytochemicals. Nutrients 2020, 12, 3776. [Google Scholar] [CrossRef]
- Huang, R.T.; Lu, Y.F.; Inbaraj, B.S.; Chen, B.H. Determination of phenolic acids and flavonoids in Rhinacanthus nasutus (L.) Kurz by high-performance-liquid-chromatography with photodiode-array detection and tandem mass spectrometry. J. Funct. Foods 2015, 12, 498–508. [Google Scholar] [CrossRef]
- Horii, H.; Suzuki, R.; Sakagami, H.; Tomomura, M.; Tomomura, A.; Shirataki, Y. New biological activities of rhinacanthins from the root of Rhinacanthus nasutus. Anticancer Res. 2013, 33, 453–460. [Google Scholar] [PubMed]
- Kwak, H.J.; Park, S.; Kim, N.; Yoo, G.; Park, J.; OH, Y.; Nhiem, N.X.; Kim, S. Neuraminidase inhibitory activity by compounds isolated from aerial parts of Rhinacanthus nasutus. Nat. Prod. Res. 2018, 32, 2111–2115. [Google Scholar] [CrossRef]
- Siripong, P.; Kanokmedakul, K.; Piyaviriyagul, S.; Yahuafai, J.; Chanpai, R.; Ruchirawat, S.; Oku, N. Antiproliferative naphthoquinone esters from Rhinacanthus nasutus Kurz. roots on various cancer cells. J. Tradit. Med. 2006, 23, 166–172. [Google Scholar] [CrossRef]
- Suzuki, R.; Sakagami, H.; Shirataki, Y. New anti-oxidative compounds from Rhinacanthus nasutus. Heterocycles 2015, 91, 1036–1041. [Google Scholar] [CrossRef] [Green Version]
- Puttarak, P.; Charoonratana, T.; Panichayupakaranant, P. Antimicrobial activity and stability of rhinacanthins-rich Rhinacanthus nasutus extract. Phytomedicine 2010, 17, 323–327. [Google Scholar] [CrossRef]
- Panichayupakaranant, P.; Charoonratana, T.; Sirikatitham, A. RP-HPLC analysis of rhinacanthins in Rhinacanthus nasutus: Validation and application for the preparation of rhinacanthin high-yielding extract. J. Chromatogr. Sci. 2009, 47, 705–708. [Google Scholar] [CrossRef] [Green Version]
- Ngoc, T.M.; Phuong, N.T.T.; Khoi, N.M.; Park, S.; Kwak, H.J.; Nhiem, N.X.; Trang, B.T.T.; Tai, B.H.; Song, J.H.; Ko, H.J.; et al. A new naphthoquinone analogue and antiviral constituents from the root of Rhinacanthus nasutus. Nat. Prod. Res. 2019, 33, 360–366. [Google Scholar] [CrossRef]
- Efferth, T. Biotechnology applications of plant callus cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Phillips, G.C. Plant Cell, Tissue and Organ Culture, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 1–359. [Google Scholar]
- Phillips, G.C.; Hubstenberger, J.F.; Hansen, E.E. Plant regeneration by organogenesis from callus and cell suspension cultures. In Plant Cell, Tissue and Organ Culture, 1st ed.; Gamborg, O.L., Phillips, G.C., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 67–79. [Google Scholar]
- Ali, M.; Abbasi, B.H.; Ihsan-ul-haq. Production of commercially important secondary metabolites and antioxidant activity in cell suspension cultures of Artemisia absinthium L. Ind. Crops Prod. 2013, 49, 400–406. [Google Scholar] [CrossRef]
- Rahimi, S.; Kim, Y.-J.; Sukweenadhi, J.; Zhang, D.; Yang, D.-C. PgLOX6 encoding a lipoxygenase contributes to jasmonic acid biosynthesis and ginsenoside production in Panax ginseng. J. Exp. Bot. 2016, 67, 6007–6019. [Google Scholar] [CrossRef] [Green Version]
- Guadarrama-Flores, B.; Rodríguez-Monroy, M.; Cruz-Sosa, F.; García-Carmona, F.; Gandía-Herrero, F. Production of dihydro xylated betalains and dopamine in cell suspension cultures of Celosia argentea var. Plumosa. J. Agric. Food Chem. 2015, 63, 2741–2749. [Google Scholar] [CrossRef] [PubMed]
- Cheruvathur, M.; Thomas, D. High frequency multiple shoot induction from nodal segments and rhinacanthin production in the medicinal shrub Rhinacanthus nasutus (L.) Kurz. Plant Growth Regul. 2014, 74, 47–54. [Google Scholar] [CrossRef]
- Elangomathavan, R.; Hariharan, S.; Kalaivanan, P.; Beaulah, S.N. Propagation of Rhinacanthus nasutus (L.) Kurz., through encapsulated shoot tips and nodal segments for germplasm exchange and distribution. Int. J. Pharma Bio Sci. 2017, 8, 98–105. [Google Scholar] [CrossRef]
- Reshi, N.A.; Sudarshana, M.S.; Girish, H.V. In vitro micropropagation of Rhinacanthus nasutus (L.) Kurz. Int. J. Biodivers. Conserv. 2018, 10, 357–364. [Google Scholar] [CrossRef]
- Sundar, S.; Jayarami, R.A.; Saravana, K.A.; Justin, K.Y. Effect of plant growth regulators on in vitro propagation of Rhinacanthus nasutus (Acanthaceae). Int. J. Pharm. Ind. Res. 2012, 2, 474–478. [Google Scholar]
- Cheruvathur, M.K.; Sivu, A.R.; Pradeep, N.S.; Thomas, T.D. Shoot organogenesis from leaf callus and ISSR assessment for their identification of clonal fidelity in Rhinacanthus nasutus (L.) Kurz., a potent anticancerous ethnomedicinal plant. Ind. Crops Prod. 2012, 40, 122–128. [Google Scholar] [CrossRef]
- Kumar, R.N.; Ganesan, C.M.; Paulsamy, S. Micropropagation of Rhinacanthus nasutus (L.) Kurz.—An important medicinal plant. Res. Rev. J. Bot. Sci. 2012, 1, 5–10. [Google Scholar]
- Wu, T.; Kerbler, S.M.; Fernie, A.R.; Zhang, Y. Plant cell cultures as heterologous bio-factories for secondary metabolite production. Plant Commun. 2021, 2, 100235. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Ahmad, N.; Anis, M.; Faisal, M.; Alatar, A.A.; Abdel-Salam, E.M.; Meena, R.P.; Sivanesan, I. Biotechnological advances in pharmacognosy and in vitro manipulation of Pterocarpus marsupium Roxb. Plants 2022, 11, 247. [Google Scholar] [CrossRef] [PubMed]
- Haida, Z.; Nakasha, J.J.; Hakiman, M. In vitro responses of plant growth factors on growth, yield, phenolics content and antioxidant activities of Clinacanthus nutans (Sabah Snake Grass). Plants 2020, 9, 1030. [Google Scholar] [CrossRef] [PubMed]
- Cheruvathur, M.K.; Thomas, T.D. Effect of plant growth regulators and elicitors on rhinacanthin accumulation in hairy root cultures of Rhinacanthus nasutus (L.) Kurz. Plant Cell Tissue Organ Cult. (PCTOC) 2014, 118, 169–177. [Google Scholar] [CrossRef]
- Kumar, P.P.; Loh, C.S. Plant tissue culture for biotechnology. In Plant Biotechnology and Agriculture; Elsevier: Amsterdam, The Netherlands, 2012; pp. 131–138. [Google Scholar]
- Ikeuchi, M.; Sugimoto, K.; Iwase, A. Plant callus: Mechanisms of induction and repression. Plant Cell 2013, 25, 3159–3173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjusha, S.; Gangaprasad, A. Callus culture and in vitro production of anthraquinone in Gynochthodes umbellata (L.) Razafim. & B. Bremer (Rubiaceae). Ind. Crops Prod. 2017, 95, 608–614. [Google Scholar] [CrossRef]
- Cho, B.O.; Ryu, H.W.; So, Y.; Cho, J.K.; Woo, H.S.; Jin, C.H.; Seo, K.I.; Park, J.C.; Jeong, I.Y. Anti-inflammatory effect of austroinulin and 6-O-acetyl-austroinulin from Stevia rebaudiana in lipopolysaccharide-stimulated RAW264.7 macrophages. Food Chem. Toxicol. 2013, 62, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Zhengfu, H.; Hu, Z.; Huiwen, M.; Zhijun, L.; Jiaojie, Z.; Xiaoyi, Y.; Xiujun, C. 1-o-acetylbritannilactone (ABL) inhibits angiogenesis and lung cancer cell growth through regulating VEGF-Src-FAK signaling. Biochem. Biophys. Res. Commun. 2015, 464, 422–427. [Google Scholar] [CrossRef]
- Weng, C.J.; Chau, C.F.; Hsieh, Y.S.; Yang, S.F.; Yen, G.C. Lucidenic acid inhibits PMA-induced invasion of human hepatoma cells through inactivating MAPK/ERK signal transduction pathway and reducing binding activities of NF-ΚB and AP-1. Carcinogenesis 2008, 29, 147–156. [Google Scholar] [CrossRef]
- Akihisa, T.; Nakamura, Y.; Tagata, M.; Tokuda, H.; Yasukawa, K.; Uchiyama, E.; Suzuki, T.; Kimura, Y. Anti-inflammatory and anti-tumor-promoting effects of triterpene acids and sterols from the fungus Ganoderma lucidum. Chem. Biodivers. 2007, 4, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Yu, Y.S.; Yen, G.C. Lucidenic acid B induces apoptosis in human leukemia cells via a mitochondria-mediated pathway. J. Agric. Food Chem. 2008, 56, 3973–3980. [Google Scholar] [CrossRef] [PubMed]
- Sung, B.; Kang, Y.J.; Kim, D.H.; Hwang, S.Y.; Lee, Y.; Kim, M.; Yoon, J.-H.; Kim, C.M.; Chung, H.Y.; Kim, N.D. Corosolic acid induces apoptotic cell death in HCT116 human colon cancer cells through a caspase-dependent pathway. Int. J. Mol. Med. 2014, 33, 943–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Niu, Y.; Wang, Z.; Xu, X.; Li, Y.; Ma, L.; Wang, J.; Yu, Y. Corosolic acid inhibits cancer progression by decreasing the level of CDK19-mediated O-GlcNAcylation in liver cancer cells. Cell Death Dis. 2021, 12, 889. [Google Scholar] [CrossRef] [PubMed]
- Gill, B.S.; Navgeet; Kumar, S. Ganoderic acid targeting multiple receptors in cancer: In silico and in vitro study. Tumour Biol. 2016, 37, 14271–14290. [Google Scholar] [CrossRef]
- Javir, G.; Joshi, K.; Rojatkar, S. Anticancer activity, phytochemical analysis of pet-ether extract by UPLC-ESI-QTOF/MS/MS and quantitative analysis of an active major constituent sesquiterpene lactone from Cyathocline purpurea [Buch-Ham Ex D. Don.]. J. Pharmacogn. Phytochem. 2019, 8, 2219–2227. [Google Scholar]
- Berretta, M.; Bignucolo, A.; Di Francia, R.; Comello, F.; Facchini, G.; Ceccarelli, M.; Iaffaioli, R.V.; Quagliariello, V.; Maurea, N. Resveratrol in cancer patients: From bench to bedside. Int. J. Mol. Sci. 2020, 21, 2945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alazzouni, A.S.; Dkhil, M.A.; Gadelmawla, M.H.A.; Gabri, M.S.; Farag, A.H.; Hassan, B.N. Ferulic acid as anticarcinogenic agent against 1,2-dimethylhydrazine induced colon cancer in rats. J. King Saud Univ.-Sci. 2021, 33, 101354. [Google Scholar] [CrossRef]
- Kukongviriyapan, V.; Phromsopha, N.; Tassaneeyakul, W.; Kukongviriyapan, U.; Sripa, B.; Hahnvajanawong, V.; Bhudhisawasdi, V. Inhibitory effects of polyphenolic compounds on human arylamine N-acetyltransferase 1 and 2. Xenobiotica 2006, 36, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Küpeli Akkol, E.; Genç, Y.; Karpuz, B.; Sobarzo-Sánchez, E.; Capasso, R. Coumarins and coumarin-related compounds in pharmacotherapy of cancer. Cancers 2020, 12, 1959. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; Liu, Y.; Zeng, Y.; Wu, G. A review on anti-tumor mechanisms of coumarins. Front. Oncol. 2020, 10, 592853. [Google Scholar] [CrossRef] [PubMed]
- Dhanjal, J.K.; Nigam, N.; Sharma, S.; Chaudhary, A.; Kaul, S.C.; Grover, A.; Wadhwa, R. Embelin inhibits TNF-α converting enzyme and cancer cell metastasis: Molecular dynamics and experimental evidence. BMC Cancer 2014, 14, 775. [Google Scholar] [CrossRef] [Green Version]
- Nigam, N.; Grover, A.; Goyal, S.; Katiyar, S.P.; Bhargava, P.; Wang, P.C.; Sundar, D.; Kaul, S.C.; Wadhwa, R. Targeting mortalin by embelin causes activation of tumor suppressor P53 and deactivation of metastatic signaling in human breast cancer cells. PLoS ONE 2015, 10, e0138192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, J.H.; Lee, S.G.; Yang, W.M.; Um, J.Y.; Sethi, G.; Mishra, S.; Shanmugam, M.K.; Ahn, K.S. The application of embelin for cancer prevention and therapy. Molecules 2018, 23, 621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsao, Y.C.; Chang, Y.J.; Wang, C.H.; Chen, L. Discovery of isoplumbagin as a novel NQO1 substrate and anti-cancer quinone. Int. J. Mol. Sci. 2020, 21, 4378. [Google Scholar] [CrossRef]
- Malik, M.S.; Alsantali, R.I.; Jassas, R.S.; Alsimaree, A.A.; Syed, R.; Alsharif, M.A.; Kalpana, K.; Morad, M.; Althagafi, I.I.; Ahmed, S.A. Journey of anthraquinones as anticancer agents—A systematic review of recent literature. RSC Adv. 2021, 11, 35806–35827. [Google Scholar] [CrossRef] [PubMed]
- Khurm, M.; Wang, X.; Zhang, H.; Hussain, S.N.; Qaisar, M.N.; Hayat, K.; Saqib, F.; Zhang, X.; Zhan, G.; Guo, Z. The genus Cassia L.: Ethnopharmacological and phytochemical overview. Phytother. Res. 2021, 35, 2336–2385. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Pandey, P.; Jha, N.K.; Khalid, M.; Ojha, S. Rutin mediated apoptotic cell death in caski cervical cancer cells via Notch-1 and Hes-1 downregulation. Life 2021, 11, 761. [Google Scholar] [CrossRef] [PubMed]
- Iwashina, T.; Mizuno, T. Flavonoids and xanthones from the genus Iris: Phytochemistry, relationships with flower colors and taxonomy, and activities and function. Nat. Prod. Commun. 2020, 15, 1934578X20937151. [Google Scholar] [CrossRef]
- Smeriglio, A.; Denaro, M.; D’Angelo, V.; Germanò, M.P.; Trombetta, D. Antioxidant, anti-inflammatory and anti-angiogenic properties of Citrus lumia Juice. Front. Pharmacol. 2020, 11, 593506. [Google Scholar] [CrossRef] [PubMed]
- Thao, N.T.P.; Hung, T.M.; Lee, M.K.; Kim, J.C.; Min, B.S.; Bae, K. Triterpenoids from Camellia japonica and their cytotoxic activity. Chem. Pharm. Bull. 2010, 58, 121–124. [Google Scholar] [CrossRef] [Green Version]
- Obrador, E.; Salvador-Palmer, R.; Jihad-Jebbar, A.; López-Blanch, R.; Dellinger, T.H.; Dellinger, R.W.; Estrela, J.M. Pterostilbene in cancer therapy. Antioxidants 2021, 10, 492. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, C.; Chen, Z.; Chen, Y.; Santhanam, R.K.; Xue, Z.; Ma, Q.; Guo, Q.; Liu, W.; Zhang, M.; et al. Effects of N-trans-feruloyltyramine isolated from laba garlic on antioxidant, cytotoxic activities and H2O2-induced oxidative damage in HepG2 and L02 cells. Food Chem. Toxicol. 2019, 130, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.C.; Afraj, S.N.; Hung, C.C.; Barve, B.D.; Kuo, L.M.Y.; Lin, Z.H.; Ho, H.O.; Kuo, Y.H. Synthesis, biological evaluation, and correlation of cytotoxicity versus redox potential of 1,4-naphthoquinone derivatives. Bioorg. Med. Chem. Lett. 2021, 41, 127976. [Google Scholar] [CrossRef] [PubMed]
- Kavaliauskas, P.; Opazo, F.S.; Acevedo, W.; Petraitiene, R.; Grybaitė, B.; Anusevičius, K.; Mickevičius, V.; Belyakov, S.; Petraitis, V. Synthesis, biological activity, and molecular modelling studies of naphthoquinone derivatives as promising anticancer candidates targeting COX-2. Pharmaceuticals 2022, 15, 541. [Google Scholar] [CrossRef] [PubMed]
- Sohel, M.; Sultana, H.; Sultana, T.; Al Amin, M.; Aktar, S.; Ali, M.C.; Rahim, Z.B.; Hossain, M.A.; Al Mamun, A.; Amin, M.N.; et al. Chemotherapeutic potential of hesperetin for cancer treatment, with mechanistic insights: A comprehensive review. Heliyon 2022, 8, e08815. [Google Scholar] [CrossRef] [PubMed]
- Sutton-Jones, B.; Street, H.E. Studies on the growth in culture of plant cells. J. Exp. Bot. 1968, 19, 114–118. [Google Scholar] [CrossRef]
- Shewry, P.R.; Pinfield, N.J.; Stobart, A.K. The effect of 2,4-dichlorophenoxyacetic acid and (2-chloroethyl)-trimethylammonium chloride on chlorophyll synthesis in barley leaves. Planta 1971, 101, 352–359. [Google Scholar] [CrossRef]
- Petsangkrit, N.; Kittipongpatana, N. Establishment of Pseuderanthemum palatiferum (Nees) Radlk callus culture and screening of secondary metabolite production. Int. J. Pharm. Pharm. Sci. 2016, 8, 275–280. [Google Scholar]
- Kumar, M.S.; Nandi, S.C. High frequency plant regeneration with histological analysis of organogenic callus from internode explants of Asteracantha longifolia Nees. J. Genet. Eng. Biotechnol. 2015, 13, 31–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satdive, R.; Shinde, A.N.; Singh, S.; Kamble, S.; Singh, S.; Malpathak, N.; Fulzele, D.P. Aggregate cell suspension cultures of Psoralea corylifolia improved phytoestrogens production. Biotechnol. Bioprocess Eng. 2015, 20, 373–379. [Google Scholar] [CrossRef]
- Seldimirova, O.A.; Bezrukova, M.V.; Galin, I.R.; Lubyanova, A.R.; Shakirova, F.M.; Kruglova, N.N. 24-epibrassinolide effects on in vitro callus tissue formation, growth, and regeneration in wheat varieties with contrasting drought resistance. Russ. J. Plant Physiol. 2017, 64, 919–929. [Google Scholar] [CrossRef]
- Vijayaraghavareddy, P.; Adhinarayanreddy, V.; Vemanna, R.S.; Sreeman, S.; Makarla, U. Quantification of membrane damage/cell death using Evan’s blue staining technique. Bio-Protocol 2017, 7, e2519. [Google Scholar] [CrossRef]
- Maheshu, V.; Sasikumar, J.M.; Darsini, D.T.P.; Aseervatham, G.S.B. In vitro antioxidant activity and polyphenolic contents of Rauvolfia tetraphylla L., Rhinacanthus nasutus Kurz. and Solena amplexicaulis (Lam.). Int. J. Biomed. Pharm. Sci. 2010, 4, 81–86. [Google Scholar]
- Chatatikun, M.; Chiabchalard, A. Thai plants with high antioxidant levels, free radical scavenging activity, anti-tyrosinase and anti-collagenase activity. BMC Complement. Altern. Med. 2017, 17, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brimson, J.M.; Brimson, S.J.; Brimson, C.A.; Rakkhitawatthana, V.; Tencomnao, T. Rhinacanthus nasutus extracts prevent glutamate and amyloid-β neurotoxicity in HT-22 mouse hippocampal cells: Possible active compounds include lupeol, stigmasterol and β-sitosterol. Int. J. Mol. Sci. 2012, 13, 5074–5097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anusha, T.S.; Joseph, M.V.; Elyas, K.K. Callus induction and elicitation of total phenolics in callus cell suspension culture of Celastrus paniculatus—Willd, an endangered medicinal plant in India. Pharmacogn. J. 2016, 8, 471–475. [Google Scholar] [CrossRef] [Green Version]
- Suja, S.R.; Latha, P.G.; Pushpangadan, P.; Rajasekharan, S. Assessment of hepatoprotective and free radical scavenging effects of Rhinacanthus nasutus (Linn.) Kurz in wistar rats. J. Nat. Remedies 2004, 4, 66–72. [Google Scholar]
- Visweswara, R.P.; Sujana, P.; Vijayakanth, T.; Dhananjaya, N.M. Rhinacanthus nasutus—Its protective role in oxidative stress and antioxidant status in streptozotocin induced diabetic rats. Asian Pac. J. Trop. Dis. 2012, 2, 327–330. [Google Scholar] [CrossRef]
- Ho, N.H.; Inbaraj, B.S.; Chen, B.H. Utilization of microemulsions from Rhinacanthus nasutus (L.) Kurz to improve carotenoid bioavailability. Sci. Rep. 2016, 6, 25426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef]
- Martysiak-Żurowska, D.; Wenta, W.A. Comparison of ABTS and DPPH methods for assessing the total antioxidant capacity of human milk. Acta Sci. Pol. Technol. Aliment. 2012, 11, 83–89. [Google Scholar] [PubMed]
- De Oliveira, A.S.; Brighente, I.M.C.; Lund, R.G.; Llanes, L.C.; Nunes, R.J.; Bretanha, L.C.; Yunes, R.A.; Carvalho, P.H.A.; Ribeiro, J.S. Antioxidant and antifungal activity of naphthoquinones dimeric derived from lawsone. J. Biosci. Med. 2017, 5, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Siriwatanametanon, N.; Fiebich, B.L.; Efferth, T.; Prieto, J.M.; Heinrich, M. Traditionally used Thai medicinal plants: In vitro anti-inflammatory, anticancer and antioxidant activities. J. Ethnopharmacol. 2010, 130, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Siripong, P.; Hahnvajanawong, C.; Yahuafai, J.; Piyaviriyakul, S.; Kanokmedhakul, K.; Kongkathip, N.; Ruchirawat, S.; Oku, N. Induction of apoptosis by rhinacanthone isolated from Rhinacanthus nasutus roots in human cervical carcinoma cells. Biol. Pharm. Bull. 2009, 32, 1251–1260. [Google Scholar] [CrossRef] [Green Version]
- Kongkathip, N.; Luangkamin, S.; Kongkathip, B.; Sangma, C.; Grigg, R.; Kongsaeree, P.; Prabpai, S.; Pradidphol, N.; Piyaviriyagul, S.; Siripong, P. Synthesis of novel rhinacanthins and related anticancer naphthoquinone esters. J. Med. Chem. 2004, 47, 4427–4438. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, A.; Sakaeda, T.; Kimura, T.; Shirakawa, T.; Wada, Y.; Wada, A.; Kimachi, T.; Takemoto, Y.; Iida, A.; Iwakawa, S.; et al. Antiproliferative activity of Rhinacanthus nasutus (L.) Kurz extracts and the active moiety, rhinacanthin C. Biol. Pharm. Bull. 2004, 27, 1070–1074. [Google Scholar] [CrossRef] [Green Version]
- Boonyaketgoson, S.; Rukachaisirikul, V.; Phongpaichit, S.; Trisuwan, K. Naphthoquinones from the leaves of Rhinacanthus nasutus having acetylcholinesterase inhibitory and cytotoxic activities. Fitoterapia 2018, 124, 206–210. [Google Scholar] [CrossRef]
- Pai, S.A.; Munshi, R.P.; Panchal, F.H.; Gaur, I.S.; Mestry, S.N.; Gursahani, M.S.; Juvekar, A.R. Plumbagin reduces obesity and nonalcoholic fatty liver disease induced by fructose in rats through regulation of lipid metabolism, inflammation and oxidative stress. Biomed. Pharmacother. 2019, 111, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Trung, N.Q.; Thong, N.M.; Cuong, D.H.; Manh, T.D.; Hoang, L.P.; Hien, N.K.; Nam, P.C.; Quang, D.T.; Mechler, A.; Vo, Q.V. Radical scavenging activity of natural anthraquinones: A theoretical insight. ACS Omega 2021, 6, 13391–13397. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Mehta, A.; Bajpai, V.K.; Shukla, S. In vitro antioxidant activity and total phenolic content of ethanolic leaf extract of Stevia rebaudiana Bert. Food Chem. Toxicol. 2009, 47, 2338–2343. [Google Scholar] [CrossRef]
- Cör, D.; Knez, Ž.; Hrnčič, M.K. Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase effect of Ganoderma lucidum terpenoids and polysaccharides: A review. Molecules 2018, 23, 649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.; Chang, Q.; Wong, L.K.; Chong, F.S.; Li, R.C. Triterpene antioxidants from Ganoderma lucidum. Phytother Res 1999, 13, 529–531. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalteu, V. On tyrosine and tryptophane determinations in proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Zongo, C.; Savadogo, A.; Ouattara, L.; Bassolé, I.H.N.; Ouattara, C.A.T.; Ouattara, A.S.; Barro, N.; Koudou, J.; Traore, A.S. Polyphenols content, antioxidant and antimicrobial activities of Ampelocissus grantii (Baker) Planch. (Vitaceae): A medicinal plant from burkina faso. Int. J. Pharmacol. 2010, 6, 880–887. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Smeriglio, A.; Denaro, M.; Barreca, D.; Calderaro, A.; Bisignano, C.; Ginestra, G.; Bellocco, E.; Trombetta, D. In vitro evaluation of the antioxidant, cytoprotective, and antimicrobial properties of essential oil from Pistacia vera L. variety bronte hull. Int. J. Mol. Sci. 2017, 18, 1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shalaby, E.A.; Shanab, S.M.M. Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanol extracts of Spirulina platensis. Indian J. Geo-Mar. Sci. 2013, 42, 556–564. [Google Scholar]
- Hamid, S.; Lim, K.P.; Zain, R.B.; Ismail, S.M.; Lau, S.H.; Mustafa, W.M.; Abraham, M.T.; Nam, N.A.; Teo, S.H.; Cheong, S.C. Establishment and characterization of Asian oral cancer cell lines as in vitro models to study a disease prevalent in Asia. Int. J. Mol. Med. 2007, 19, 453–460. [Google Scholar] [CrossRef] [Green Version]


| 2,4-D (mg/L) | NAA (mg/L) | % Callus Induction * | Callus Response |
|---|---|---|---|
| 0 | 0 | 0 c | no growth response |
| 0.50 | 1.00 | 100.00 ± 0.00 a | friable, yellow |
| 1.00 | 1.00 | 100.00 ± 0.00 a | friable, yellow |
| 1.50 | 1.00 | 100.00 ± 0.00 a | friable, yellow |
| 2.00 | 1.00 | 100.00 ± 0.00 a | friable, yellow |
| 0.00 | 1.00 | 26.67 ± 0.58 b | compact, yellow |
| 0.00 | 2.00 | 0 c | no growth response |
| 0.50 | 2.00 | 33.33 ± 0.58 b | compact, yellow |
| 1.00 | 2.00 | 33.33 ± 0.58 b | compact, yellow |
| 1.50 | 2.00 | 26.67 ± 0.58 b | compact, yellow |
| 2.00 | 2.00 | 26.67 ± 0.58 b | compact, yellow |
| 1.00 | 0.00 | 0 c | no growth response |
| 2.00 | 0.00 | 0 c | no growth response |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Songserm, P.; Klanrit, P.; Klanrit, P.; Phetcharaburanin, J.; Thanonkeo, P.; Apiraksakorn, J.; Phomphrai, K.; Klanrit, P. Antioxidant and Anticancer Potential of Bioactive Compounds from Rhinacanthus nasutus Cell Suspension Culture. Plants 2022, 11, 1994. https://doi.org/10.3390/plants11151994
Songserm P, Klanrit P, Klanrit P, Phetcharaburanin J, Thanonkeo P, Apiraksakorn J, Phomphrai K, Klanrit P. Antioxidant and Anticancer Potential of Bioactive Compounds from Rhinacanthus nasutus Cell Suspension Culture. Plants. 2022; 11(15):1994. https://doi.org/10.3390/plants11151994
Chicago/Turabian StyleSongserm, Pattralak, Poramaporn Klanrit, Poramate Klanrit, Jutarop Phetcharaburanin, Pornthap Thanonkeo, Jirawan Apiraksakorn, Khamphee Phomphrai, and Preekamol Klanrit. 2022. "Antioxidant and Anticancer Potential of Bioactive Compounds from Rhinacanthus nasutus Cell Suspension Culture" Plants 11, no. 15: 1994. https://doi.org/10.3390/plants11151994







