Antioxidants Application Enhances Regeneration and Conversion of Date Palm (Phoenix dactylifera L.) Somatic Embryos
Abstract
:1. Introduction
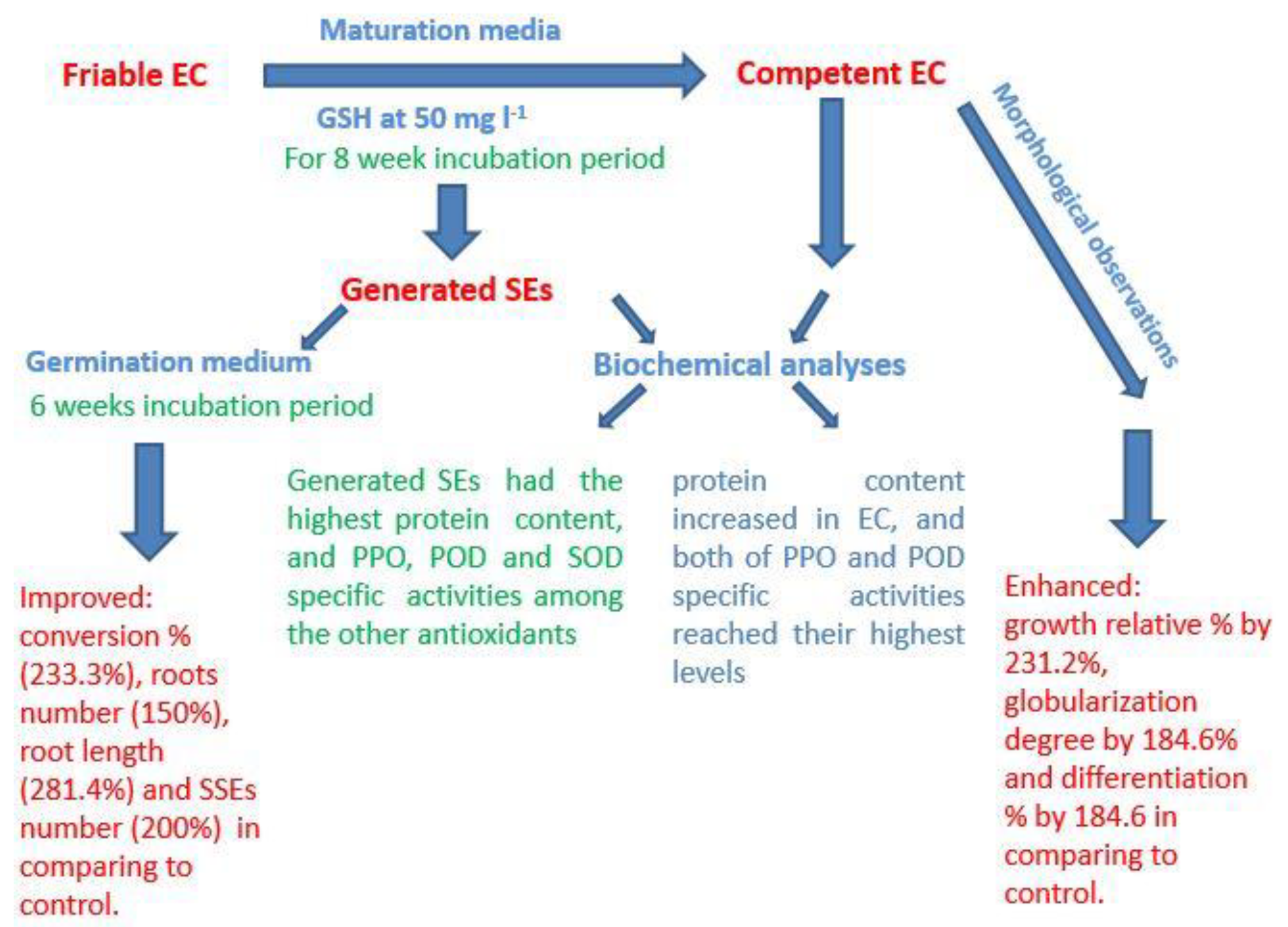
2. Results
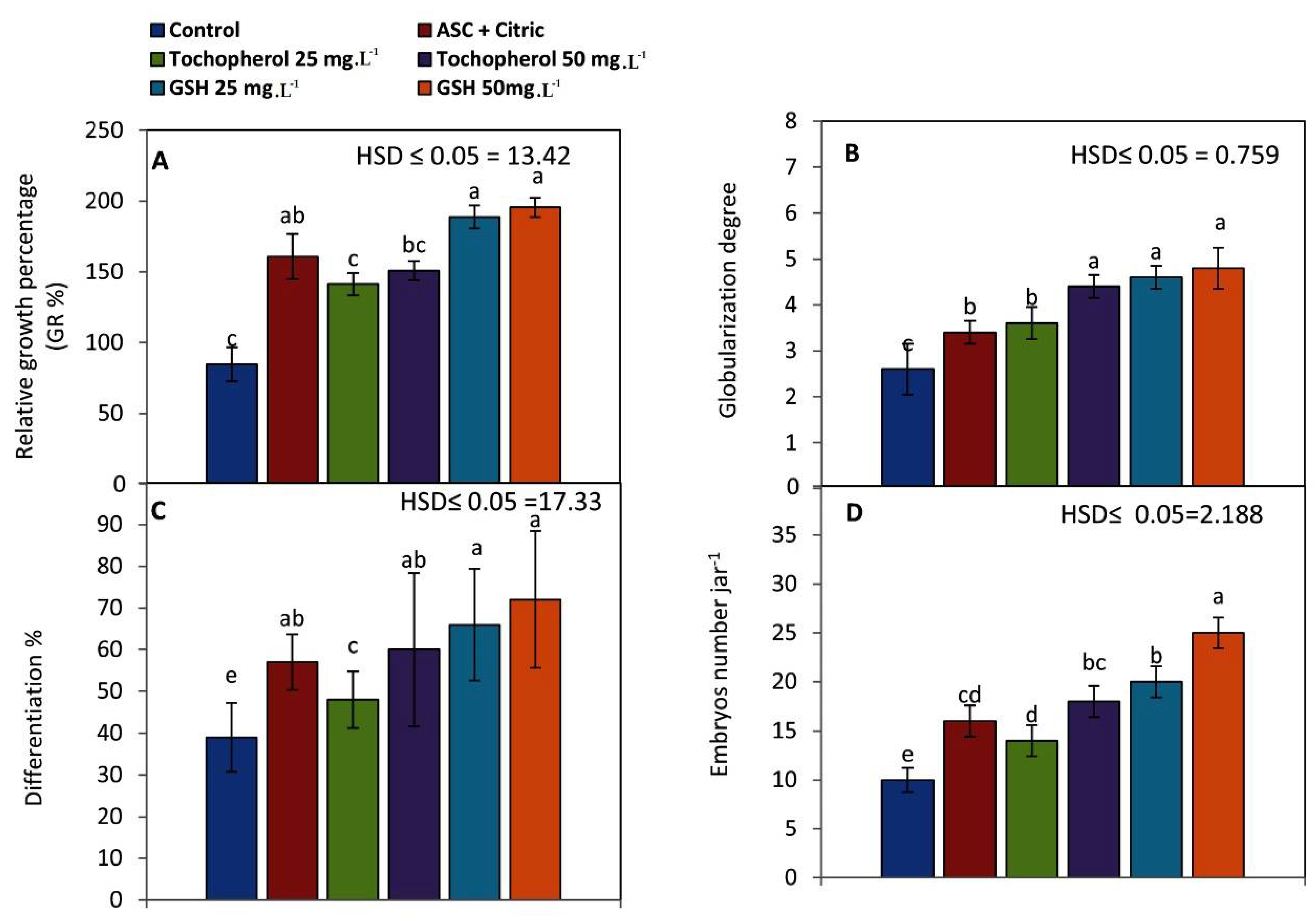
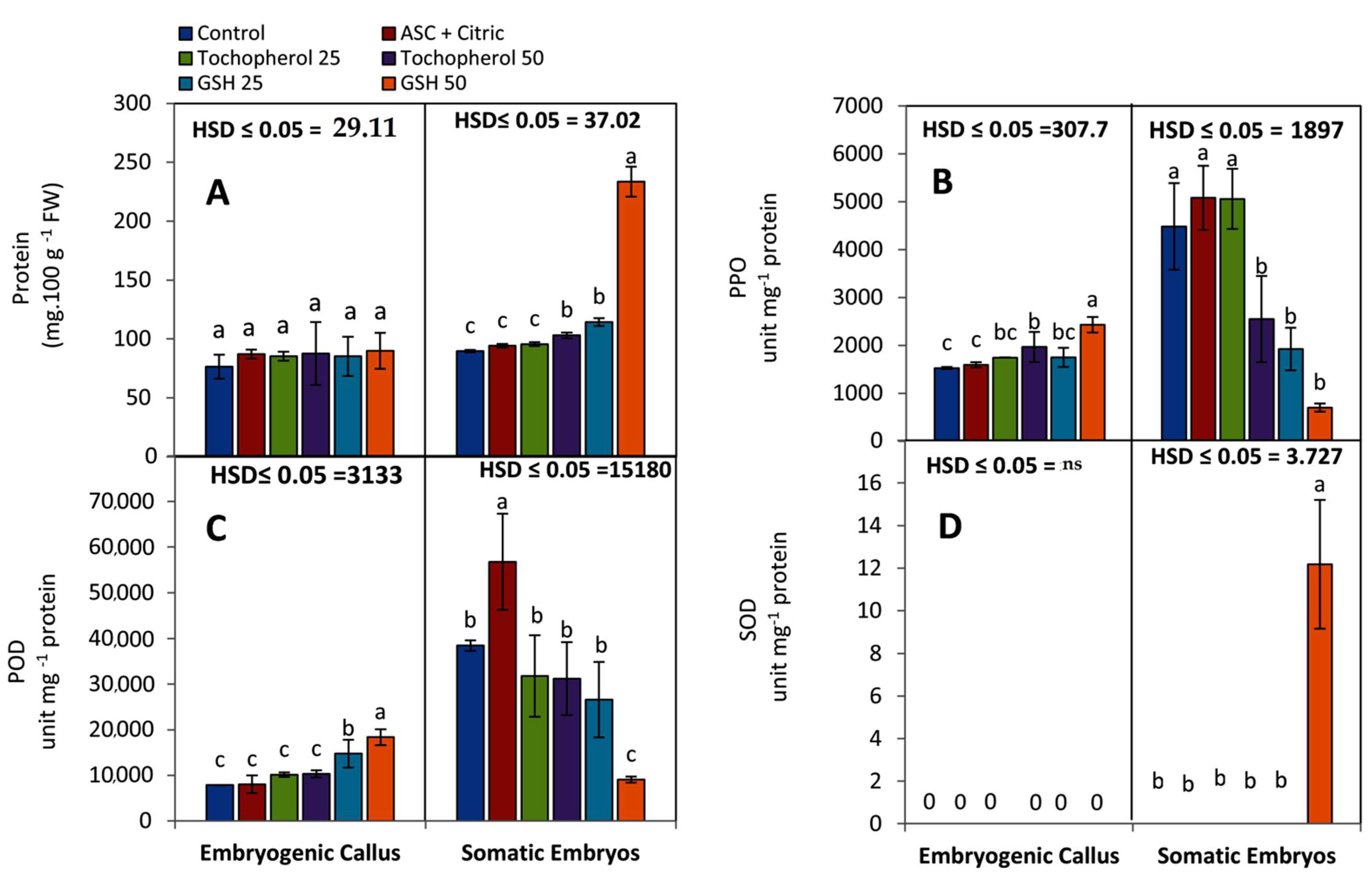
2.1. Maturation of Embryogenic Callus
2.1.1. Morphological Observations
2.1.2. Biochemical Observations
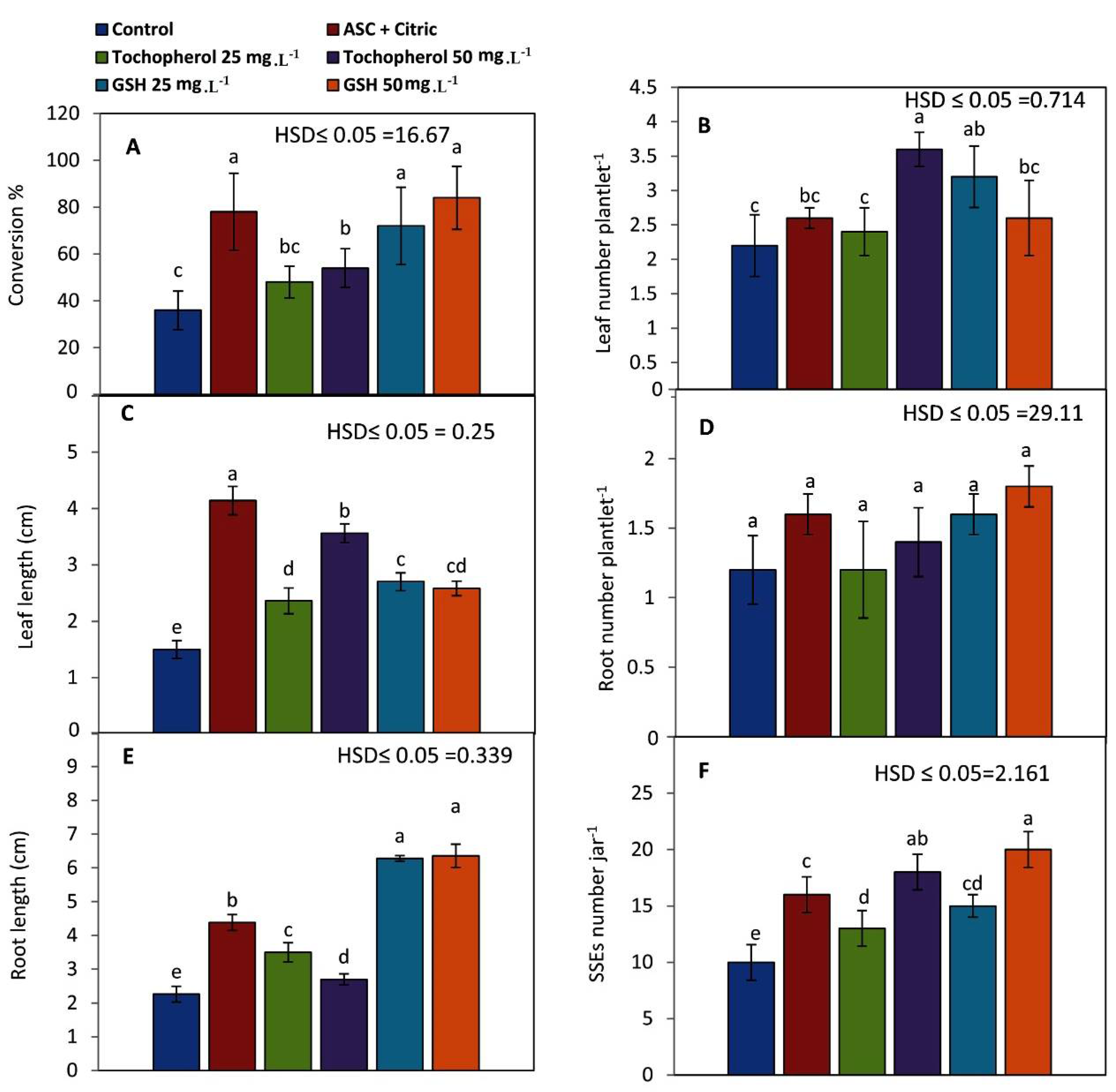
2.2. Conversion of Mature Somatic Embryos into Viable Plantlets
3. Discussion
4. Materials and Methods
4.1. Morphological Observations
4.2. Biochemical Analyses
- Determination of soluble protein;Soluble protein concentration was estimated to calculate specific activity of enzymes. Protein concentration was quantified in the crude extract by the method of Bradford [57] using bovine serum albumin as a standard and expressed as mg 100 g−1 FW.
- Assay of peroxidase activity (POD);POD (E.C 1.11.1.7) activity in enzyme crude extract was determined as described by Hammer Schmidt et al. [58]. The activity was calculated by monitoring the change in absorbance per minute at 470 nm. The enzyme unit (IU) is equivalent to 0.01 POD min−1. The specific activity was expressed as unit mg−1 protein.
- Assay of Polyphenol oxidase (PPO) activity;According to Benjamin and Montgomery [59], the activity of PPO (EC 1.14.18.1) was tested. One unit of PPO activity was determined as the amount of enzyme that induced a 0.001 per minute increase in absorbance at 420 nm. The enzyme activity was measured in unit mg−1 protein.
- Assay of Superoxide dismutase (SOD) activityThe ability of superoxide dismutase (EC 1.15.1.1) to inhibit the photochemical reaction of nitro blue tetrazolium (NBT) at 560 nm was measured using the Beauchamp and Fridovich [60] method. The amount of protein required to inhibit 50% initial decline of NBT under light is one unit of SOD activity. SOD activity was measured in unit mg−1 protein.
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fki, L.; Masmoudi, R.; Kriaa, W.; Mahjoub, A.; Sghaier, B.; Mzid, R.; Mliki, A.; Rival, A.; Drira, N. Date palm micropropagation via somatic embryogenesis. In Date Palm Biotechnology; Jain, S.M., Al-Khayri, J.M., Johnson, D.V., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 47–68. [Google Scholar]
- El Hadrami, I.; Baaziz, M. Somatic embryogenesis and analysis of peroxidases in Phoenix dactylifera L. Biol. Plants 1995, 37, 197–203. [Google Scholar] [CrossRef]
- Pullman, G.S.; Bucalo, K. Pine somatic embryogenesis: Analyses of seed tissue and medium to improve protocol development. New For. 2014, 45, 353–377. [Google Scholar] [CrossRef]
- Pullman, G.S.; Zeng, X.; Copeland-Kamp, B.; Crockett, J.; Lucrezi, J.; May, S.W.; Bucalo, K. Conifer somatic embryogenesis: Improvements by supplementation of medium with oxidation–reduction agents. Tree Physiol. 2015, 35, 209–224. [Google Scholar] [CrossRef]
- El Din, A.F.M.Z.; Elbar, O.H.A.; Turki, S.M.A.; Turki, S.M.; Ramadan, K.M.A.; El-Beltagi, H.S.; Ibrahim, H.A.; Gadalla, E.G.; El-Din, I.M.S.; Ibrahim, I.S.; et al. Morpho-Anatomical and Biochemical Characterization of Embryogenic and Degenerative Embryogenic Calli of Phoenix dactylifera L. Horticulturae 2021, 7, 393. [Google Scholar] [CrossRef]
- Stasolla, C. Changes in the glutathione and ascorbate redox state trigger growth during embryo development and meristem reactivation at germination. In Ascorbate-Glutathione Pathways and Stress Tolerance in Plants; Anjum, N.A., Umar, S., Chan, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 231–249. ISBN 978-90-481-9403-2. [Google Scholar]
- Pullman, G.S.; Copeland, B.; Zeng, X. Analysis of seed redox chemicals in loblolly pine to improve somatic embryo growth and germination. In Proceedings of the 30th Southern Tree Improvement Conference (SFTIC), Blacksburg, VA, USA, 31 May–3 June 2009; pp. 56–65. Available online: http://www.rngr.net/publications/tree-improvement-proceedings/sftic/2009/ (accessed on 28 April 2022).
- Belmonte, M.F.; Stasolla, C.; Loukanina, N.; Yeung, E.C.; Thorpe, T.A. Glutathione modulation of purine metabolism in cultured white spruce embryogenic tissue. Plant. Sci. 2003, 165, 1377–1385. [Google Scholar] [CrossRef]
- Belmonte, M.F.; Macey, J.; Yeung, E.C.; Stasolla, C. The effect of osmoticum on ascorbate and glutathione metabolism during white spruce (Picea glauca) somatic embryo development. Plant Physiol. Biochem. 2005, 43, 337–346. [Google Scholar] [CrossRef]
- Stasolla, C.; Lam, M.S.W.; Yeung, E.C. Exogenous Applications of Ascorbic Acid Enhance Shoot Apical Meristem Growth and Induce Shoot Organogenesis in Germinating White Spruce (Picea glauca) Somatic Embryos. Int. J. Plant Sci. 2006, 167, 429–436. [Google Scholar] [CrossRef]
- Eldin, A.F.M.Z.; Ibrahim, H.A. Some biochemical changes and activities of antioxidant enzymes in developing date palm somatic and zygotic embryos in vitro. Ann. Agric. Sci. 2015, 60, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, A.R.; Hajirezaei, M.; Hofius, D.; Sonnewald, U.; Voll, L.M. Specific roles of a- and g-tocopherol in abiotic stress responses of transgenic tobacco. Plant Physiol. 2007, 143, 1720–1738. [Google Scholar] [CrossRef] [Green Version]
- Sattler, S.E.; Gilliland, L.U.; Magallanes-Lundback, M.; Pollard, M.; DellaPenna, D. Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. Plant Cell 2004, 16, 1419–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangaswamy, N.S. Somatic embryogenesis in angiosperm cell tissue and organ cultures. Proc. Indian Acad. Sci. Plant Sci. 1986, 96, 247–271. [Google Scholar] [CrossRef]
- Orłowska, A.; Kępczyńska, E. Oxidative status in Medicago truncatula Gaertn. non-embryogenic and embryogenic tissues with particular reference to somatic embryogenesis. Plant Cell Tissue Organ Cult. 2020, 140, 35–48. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Han, S.; Yang, W.; Wei, H.; Zhang, M.; Qi, L. Changes in H2O2 content and antioxidant enzyme gene expression during the somatic embryogenesis of Larix leptolepis. Plant Cell Tissue Organ Cult. 2010, 100, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Bagnoli, F.; Giannin, D.; Caparrini, S.; Camussi, A.; Mariotti, D.; Racchi, M.L. Molecular cloning, characterization and expression of a manganese superoxide dismutase gene from peach (Prunus persica [L.] Batsch). Mol. Genet. Genomics. 2002, 267, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants, and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Earnshaw, B.A.; Johnson, M.A. Control of wild carrot somatic embryo development by antioxidants: A probable mode of action of 2,4-dichlorophenoxyacetic acid. Plant Physiol. 1987, 85, 273–276. [Google Scholar] [CrossRef] [Green Version]
- Stern, H. Sulfhydryl groups and cell division. Science 1956, 124, 1292–1293. [Google Scholar] [CrossRef]
- Diaz-Vivancos, P.; Dong, Y.K.; Ziegler, P.; Markovic, J.; Pallard, F.V.; Pellny, T.K.; Verrier, P.; Foyer, C.H. Recruitment of glutathione into the nucleus during cell proliferation adjusts whole cell redox homeostasis in Arabidopsis thaliana and lowers the oxidative defence shield. Plant J. 2010, 64, 825–838. [Google Scholar] [CrossRef]
- Smirnoff, N. The Function and Metabolism of Ascorbic Acid in Plants. Ann. Bot. 1996, 78, 661–669. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.D.; Datta, S. Antioxidant enzyme activities during in vitro morphogenesis of gladiolus and the effect of application of antioxidant on plant regeneration. Biol. Plant 2003, 47, 179–183. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; De Klerk, G.J. Plant Propagation by Tissue Culture, 3rd ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 186–187. [Google Scholar]
- Hoang, M.T.T.; Doan, M.T.A.; Nguyen, T.; Tra, D.P.; Chu, T.N.; Dang, T.P.T.; Quach, P.N.D. Phenotypic characterization of Arabidopsis ascorbate and glutathione deficient mutants under abiotic stresses. Agronomy 2021, 11, 764. [Google Scholar] [CrossRef]
- Considine, M.J.; Foyer, C.H. Oxygen and reactive oxygen species-dependent regulation of plant growth and development. Plant Physiol. 2021, 186, 79–92. [Google Scholar] [CrossRef]
- Rai, M.K.; Shekhawat, N.S.; Gupta, A.K.; Phulwaria, M.; Ram, K.; Jaiswal, U. The role of abscisic acid in plant tissue culture: A review of recent progress. Plant Cell Tissue Organ Cult. 2011, 106, 179–190. [Google Scholar] [CrossRef]
- Ghassemian, M.; Lutes, J.; Chang, H.S.; Lange, I.; Chen, W.; Zhua, T.; Wang, X.; Lange, B.M. Abscisic acid-induced modulation of metabolic and redox control pathways in Arabidopsis thaliana. Phytochemistry 2008, 69, 2899–2911. [Google Scholar] [CrossRef] [PubMed]
- Corredor-Prado, J.P.; De Conti, D.; Guerra, M.P.; Vesco, L.L.D.; Pescador, R. Dynamics of proteins, carbohydrates and global DNA methylation patterns during induction of nodular cluster cultures from seeds of Vriesea reitzii. Acta Scientiarum. 2020, 42, 1807–8621. [Google Scholar] [CrossRef]
- Wu, G.; Wei, X.; Wang, X.; Wei, Y. Changes in biochemistry and histochemical characteristics during somatic embryogenesis in Ormosia henryi Prain. Plant Cell Tissue Organ Cult. 2021, 144, 505–517. [Google Scholar] [CrossRef]
- Von Arnold, S.; Clapham, D.; Abrahamsson, M. Embryology in conifers. Adv. Bot. Res. 2019, 89, 157–184. [Google Scholar] [CrossRef]
- Ammirato, P.V. Patterns of development in culture. In Tissue Culture in Forestry and Agriculture; Plenum Press: New York, NY, USA, 1985; pp. 9–29. [Google Scholar]
- Meister, A.; Anderson, M.E. Glutathione. Ann. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef] [PubMed]
- Vale, E.M.; Heringer, A.S.; Barroso, T.; Da Silva Ferreira, A.T.; Da Costa, M.N.; Perales, J.E.; Santa-Catarina, C.; Silveira, V. Comparative proteomic analysis of somatic embryo maturation in Carica papaya L. Proteome Sci. 2014, 12, 37. [Google Scholar] [CrossRef] [Green Version]
- Eliášová, K.; Konrádová, H.; Dobrev, P.I.; Motyka, V.; Lomenech, A.M.; Fischerová, L.; Lelu-Walter, M.A.; Vondráková, Z.; Teyssier, C. Desiccation as a post-maturation treatment helps complete maturation of Norway spruce somatic embryos: Carbohydrates, phytohormones and proteomic status. Front. Plant Sci. 2022, 13, 823617. [Google Scholar] [CrossRef] [PubMed]
- Arnaldos, T.L.; Muñoz, R.; Ferrer, M.A.; Calderon, A.A. Changes in phenol content during strawberry (Fragaria×ananassa, cv. Chandler) callus culture. Physiol. Plant. 2001, 113, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Leopold, A.C.; Plummer, T.H. Auxin-phenol complexes. Plant Physiol. 1961, 36, 589–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simontacchi, M.; Caro, A.; Fraga, C.G.; Puntarulo, S. Oxidative stress affects [alpha]-tocopherol content in soybean embryonic axes upon imbibition and following germination. Plant Physiol. 1993, 103, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Rasol, M.K. Peroxidase as a developmental marker in plant tissue culture. Int. J. Dev. Biol. 1991, 35, 259–263. [Google Scholar]
- Shohael, A.M.; Ali, M.B.; Hahn, E.J.; Paek, K.Y. Glutathione metabolism and antioxidant responses during Eleutherococcus senticosus somatic embryo development in a bioreactor. Plant Cell Tissue Organ Cult. 2007, 89, 121–129. [Google Scholar] [CrossRef]
- Bido, G.D.; Ferrarese, M.D.L.; Marchiosi, R.; Ferrarese, O. Naringenin inhibits the growth and stimulates the lignification of soybean root. Braz. Arch. Biol. Technol. 2010, 53, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Stonier, T.; Yang, H.M. Studies on Auxin Protectors. Plant Physiol. 1973, 51, 391–395. [Google Scholar] [CrossRef] [Green Version]
- Cordewener, J.; Booij, H.; van der Zandt, H.; Van Engelen, F.; Van Kammen, A.B.; De Vries, S.C. Tunicamycin-inhibited carrot somatic embryogenesis can be restored by secreted cationic peroxidase isoenzymes. Planta 1991, 184, 478–486. [Google Scholar] [CrossRef]
- Cvikrová, M.; Malá, J.; Hrubcová, M.; Eder, J.; Zon, J.; Machácková, I. Effect of inhibition of biosynthesis of phenylpropanoids on sessile oak somatic embryogenesis. Plant Physiol. Biochem. 2003, 41, 251–259. [Google Scholar] [CrossRef]
- Slooten, L.; Capiau, K.; Van Camp, W.; Van Montagu, M.; Sybesma, C.; Inze, D. Factors affecting the enhancement of oxidative stress tolerance in transgenic tobacco overexpressing manganese superoxide dismutase in the chloroplasts. Plant Physiol. 1995, 107, 737–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stasolla, C.; Yeung, E.C. Ascorbic acid improves conversion of white spruce somatic embryos. Vitr. Cell Dev. Biol. Plant. 1999, 35, 316–331. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, N.; Liu, R.; Chen, M.; Zhang, J. H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. J. Exp. Bot. 2010, 61, 2979–2990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vernoux, T.; Wilson, R.C.; Seeley, K.A.; Reichheld, J.P.; Muroy, S.; Brown, S.; Maughan, S.C.; Cobbett, C.S.; Van Montagu, M.; Inzé, D.; et al. The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell 2000, 12, 97–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasternak, T.; Palme, K.; Paponov, I.A. Glutathione enhances auxin sensitivity in Arabidopsis roots. Biomolecules 2020, 10, 1550. [Google Scholar] [CrossRef]
- Cheng, M.C.; Ko, K.; Chang, W.L.; Kuo, W.C.; Chen, G.H.; Lin, T.P. Increased glutathione contributes to stress tolerance and global translational changes in Arabidopsis. Plant J. 2015, 83, 926–939. [Google Scholar] [CrossRef]
- Sanchez-Fernandez, R.; Fricker, M.; Corben, L.B.; White, N.S.; Sheard, N.; Leaver, J.C.; Montagu, M.V.; Inze, D.; May, M.J. Cell proliferation and hair tip growth in the Arabidopsis root are under mechanistically different forms of redox control. Proc. Natl. Acad. Sci. USA 1997, 94, 2745–2750. [Google Scholar] [CrossRef] [Green Version]
- Daigny, G.; Paul, H.; Sangwan, R.S.; Sangwan-Norreel, B.S. Factors influencing secondary somatic embryogenesis in Malus × domestica Borkh. (cv ‘Gloster 69’). Plant Cell Rep. 1996, 16, 153–157. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Santoso, D.; Thornburg, R.W. Isolation and characterization of MF synthase mutant from haploid cell suspension of Nicotiana tabacum. Plant Physiol. 1992, 99, 1216–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pottino, B.G. Methods in Plant Tissue Culture; Department of Horticulture, Agricultural College, Maryland University: College Park, MD, USA, 1981; pp. 8–29. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principal of protein—Dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Schmidt, R.H.; Nuckles, E.M.; Kuc, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant 1982, 20, 73–82. [Google Scholar]
- Benjamin, N.; Montgomery, M.W. Polyphenol oxidase of royal ann cherries: Purification and characterization. J. Food Sci. 1973, 38, 799–806. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assay and an assay applicable to acryl amide gels. Plant Physiol. 1971, 44, 276–287. [Google Scholar]
- CoStat Version 6.311, Copyright (c). CoHort Software 798 Lighthouse Ave. PMB 320, Monterey, CA 93940, USA, (1998–2005). Available online: http://www.Cohort.com (accessed on 29 March 2022).
- Snedocor, G.W.; Cochran, W.C. Statistical Methods, 7th ed.; Iowa State University Press: Ames, IA, USA, 1980; p. 507. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zein El Din, A.F.M.; Darwesh, R.S.S.; Ibrahim, M.F.M.; Salama, G.M.Y.; Shams El-Din, I.M.; Abdelaal, W.B.; Ali, G.A.; Elsayed, M.S.; Ismail, I.A.; Dessoky, E.S.; et al. Antioxidants Application Enhances Regeneration and Conversion of Date Palm (Phoenix dactylifera L.) Somatic Embryos. Plants 2022, 11, 2023. https://doi.org/10.3390/plants11152023
Zein El Din AFM, Darwesh RSS, Ibrahim MFM, Salama GMY, Shams El-Din IM, Abdelaal WB, Ali GA, Elsayed MS, Ismail IA, Dessoky ES, et al. Antioxidants Application Enhances Regeneration and Conversion of Date Palm (Phoenix dactylifera L.) Somatic Embryos. Plants. 2022; 11(15):2023. https://doi.org/10.3390/plants11152023
Chicago/Turabian StyleZein El Din, Amal F. M., Rasmia S. S. Darwesh, Mohamed F. M. Ibrahim, Gehan M. Y. Salama, Ibrahim M. Shams El-Din, Walid B. Abdelaal, Ghada A. Ali, Maha S. Elsayed, Ismail A. Ismail, Eldessoky S. Dessoky, and et al. 2022. "Antioxidants Application Enhances Regeneration and Conversion of Date Palm (Phoenix dactylifera L.) Somatic Embryos" Plants 11, no. 15: 2023. https://doi.org/10.3390/plants11152023






