A Locus Controlling Leaf Rolling Degree in Wheat under Drought Stress Identified by Bulked Segregant Analysis
Abstract
:1. Introduction
2. Results
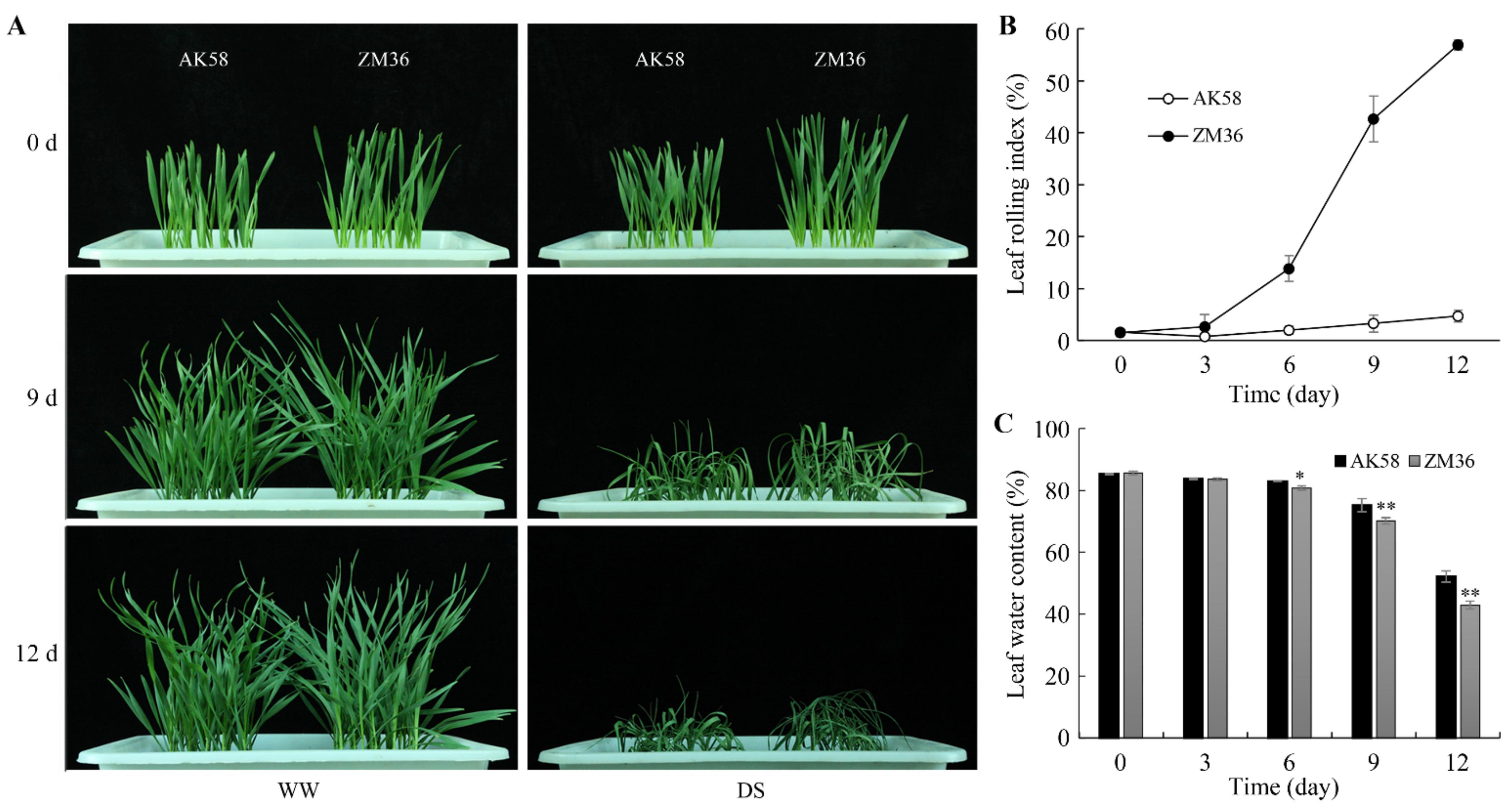
2.1. The Leaf Rolling Degree of AK58 Was Lower than That of ZM36 under Drought Stress
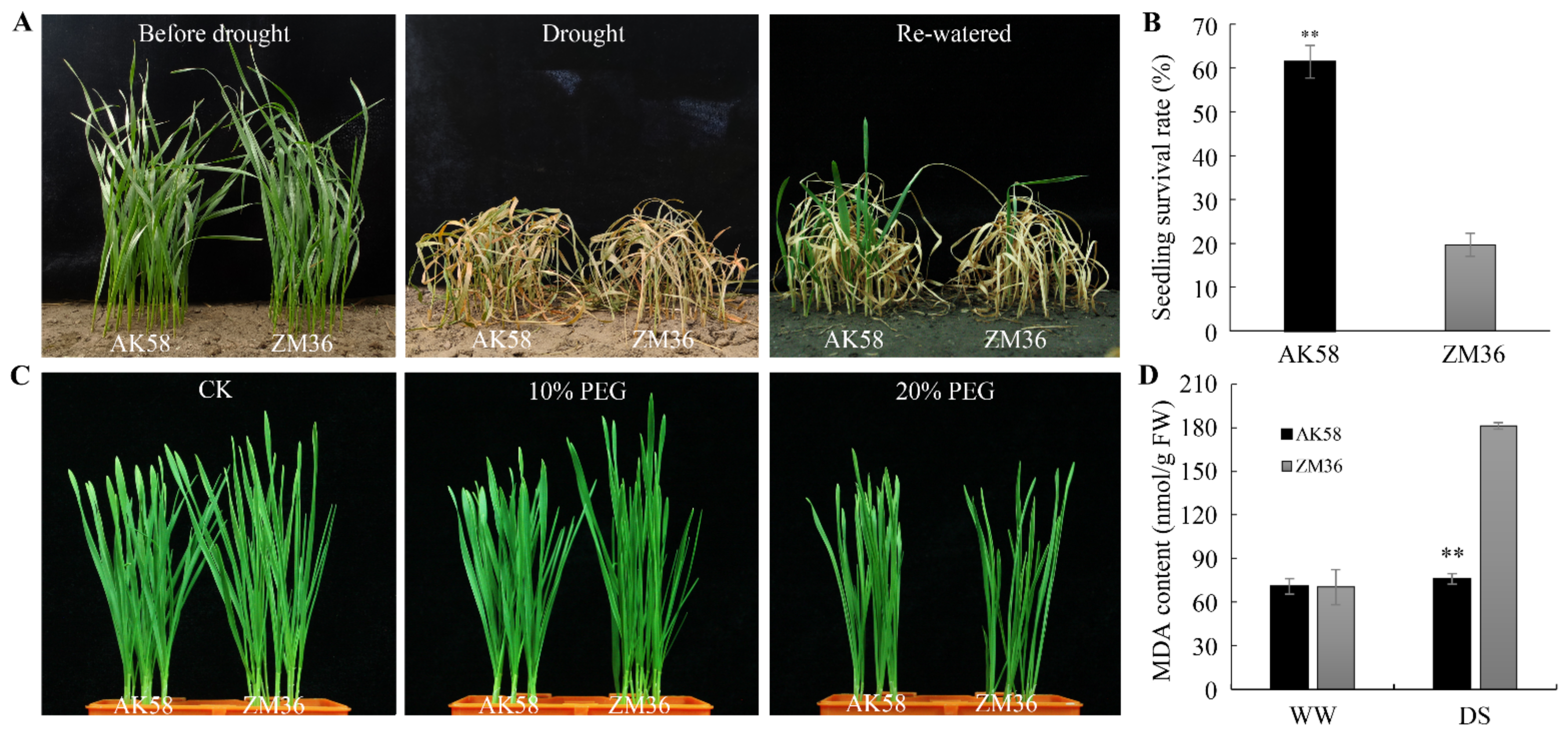
2.2. AK58 Is More Tolerant to Drought than ZM36 at Seedling Stage
2.3. AK58 Leaves Exhibited Lower Stomatal Density than That of ZM36
2.4. Genetic Analysis and Mapping of Leaf Rolling Degree Locus
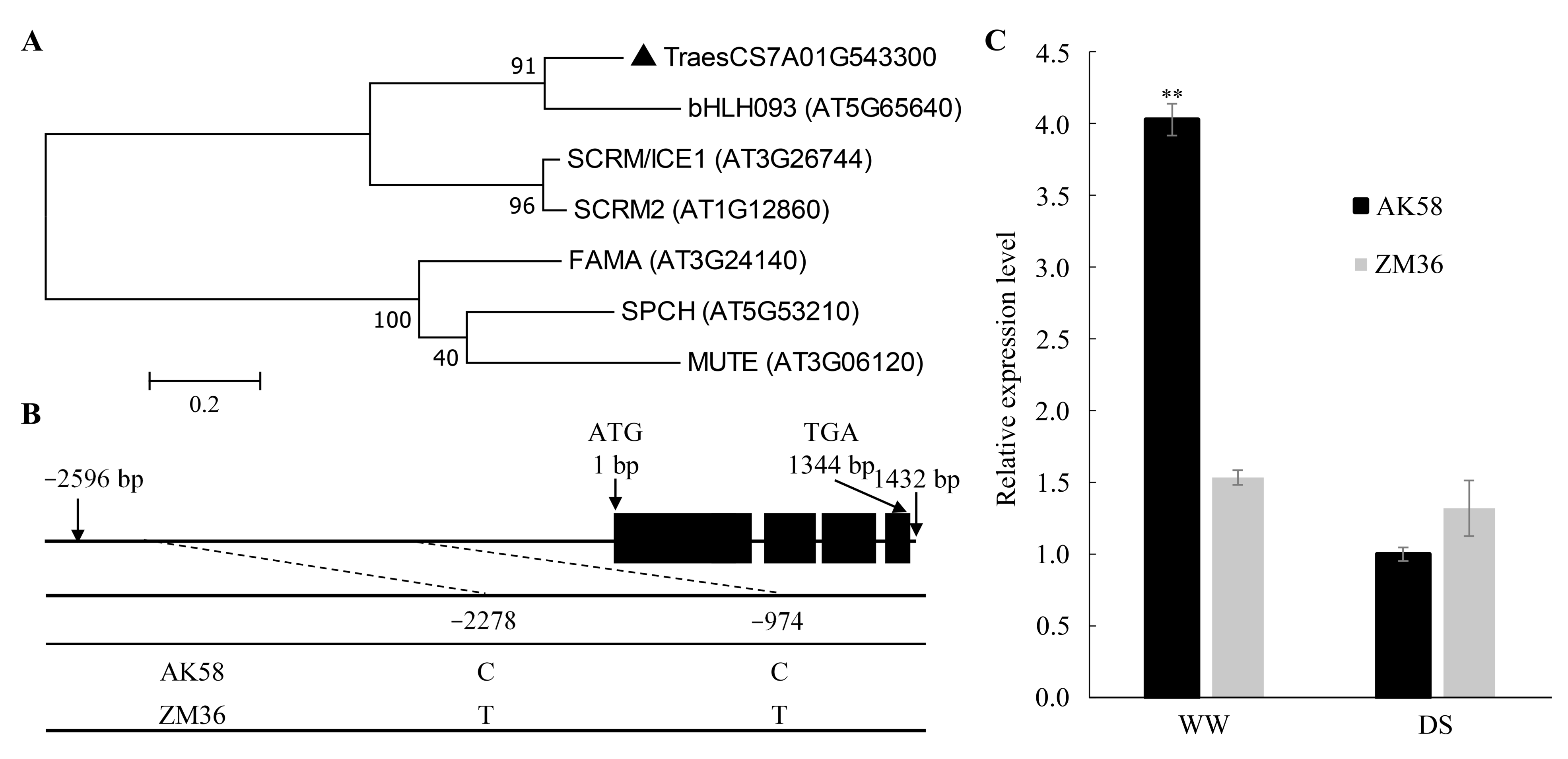
2.5. Candidate Gene Prediction and Bioinformatics Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Condition
4.2. Leaf Rolling Degree Assays
4.3. Drought Tolerance Assays
4.4. Rate of Water Loss (RWL) from Excised-Leaf
4.5. Leaf Structure Assays
4.6. Genetic Analysis of Leaf Rolling
4.7. Fine Mapping
4.8. Bioinformatics Analysis and Expression Analysis of Candidate Gene
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef] [PubMed]
- Juliana, P.; Poland, J.; Huerta-Espino, J.; Shrestha, S.; Crossa, J.; Crespo-Herrera, L.; Toledo, F.H.; Govindan, V.; Mondal, S.; Kumar, U.; et al. Improving grain yield, stress resilience and quality of bread wheat using large-scale genomics. Nat. Genet. 2019, 51, 1530–1539. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.S.; Helander, J.D.M.; Peterson, F.C.; Elzinga, D.; Dejonghe, W.; Kaundal, A.; Park, S.Y.; Xing, Z.; Mega, R.; Takeuchi, J.; et al. Dynamic control of plant water use using designed ABA receptor agonists. Science 2019, 366, 6464. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mao, X.G.; Wang, J.Y.; Chang, X.P.; Reynolds, M.; Jing, R.L. Genetic dissection of drought and heat-responsive agronomic traits in wheat. Plant Cell Environ. 2019, 42, 2540–2553. [Google Scholar] [CrossRef] [Green Version]
- Mao, H.D.; Li, S.M.; Wang, Z.X.; Cheng, X.X.; Li, F.F.; Mei, F.M.; Chen, N.; Kang, Z.S. Regulatory changes in TaSNAC8-6A are associated with drought tolerance in wheat seedlings. Plant Biotechnol. J. 2020, 18, 1078–1092. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Rouphael, Y.; Cardarelli, M.; Colla, G. Vegetable grafting as a tool to improve drought resistance and water use efficiency. Front. Plant Sci. 2017, 8, 1130. [Google Scholar] [CrossRef] [Green Version]
- Myskow, B.; Goralska, M.; Lenarczyk, N.; Czyczylo-Mysza, I.; Stojalowski, S. Putative candidate genes responsible for leaf rolling in rye (Secale cereale L.). BMC Genet. 2018, 19, 57. [Google Scholar] [CrossRef]
- Merrium, S.; Ali, Z.; Tahir, M.H.N.; Habib-Ur-Rahman, M.; Hakeem, S. Leaf rolling dynamics for atmospheric moisture harvesting in wheat plant as an adaptation to arid environments. Environ. Sci. Pollut. Res. Int. 2022, 29, 48995–49006. [Google Scholar] [CrossRef]
- Zhang, G.H.; Hou, X.; Wang, L.; Xu, J.; Chen, J.; Fu, X.; Shen, N.W.; Nian, J.Q.; Jiang, Z.Z.; Hu, J.; et al. PHOTO-SENSITIVE LEAF ROLLING 1 encodes a polygalacturonase that modifies cell wall structure and drought tolerance in rice. New Phytol. 2021, 229, 890–901. [Google Scholar] [CrossRef]
- Li, W.Q.; Zhang, M.J.; Gan, P.F.; Qiao, L.; Yang, S.Q.; Miao, H.; Wang, G.F.; Zhang, M.M.; Liu, W.T.; Li, H.F.; et al. CLD1/SRL1 modulates leaf rolling by affecting cell wall formation, epidermis integrity and water homeostasis in rice. Plant J. 2017, 92, 904–923. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.F.; Li, M.; Liu, K.; Tang, D.; Sun, M.F.; Li, Y.F.; Shen, Y.; Du, G.J.; Cheng, Z.K. Semi-Rolled Leaf2 modulates rice leaf rolling by regulating abaxial side cell differentiation. J. Exp. Bot. 2016, 67, 2139–2150. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Zheng, T.Q.; Hoang, L.; Wang, C.C.; Nafisah; Joseph, C.; Zhang, W.; Xu, J.; Li, Z. Joint mapping and allele mining of the rolled leaf trait in rice (Oryza sativa L.). PLoS ONE 2016, 11, e0158246. [Google Scholar] [CrossRef]
- Zhu, Q.Q.; Yu, S.G.; Chen, G.S.; Ke, L.L.; Pan, D.R. Analysis of the differential gene and protein expression profile of the rolled leaf mutant of transgenic rice (Oryza sativa L.). PLoS ONE 2017, 12, e0181378. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.H.; Teng, S.Z.; Liu, D.; Chang, Y.; Zhang, L.Y.; Cui, X.A.; Wu, J.X.; Ai, P.F.; Sun, X.H.; Lu, T.G.; et al. RLM1, encoding an R2R3 MYB transcription factor, regulates the development of secondary cell wall in rice. Front. Plant Sci. 2022, 13, 905111. [Google Scholar] [CrossRef]
- Yu, N.; Liang, Y.P.; Wang, Q.P.; Peng, X.X.; He, Z.H.; Hou, X.W. Transcriptomic analysis of OsRUS1 overexpression rice lines with rapid and dynamic leaf rolling morphology. Sci. Rep. 2022, 12, 6736. [Google Scholar] [CrossRef]
- Liu, X.; Deng, X.J.; Li, C.Y.; Xiao, Y.K.; Zhao, K.; Guo, J.; Yang, X.R.; Zhang, H.S.; Chen, C.P.; Luo, Y.T.; et al. Mutation of protoporphyrinogen IX oxidase gene causes spotted and rolled leaf and its overexpression generates herbicide resistance in rice. Int. J. Mol. Sci. 2022, 23, 5781. [Google Scholar] [CrossRef]
- Gao, L.L.; Yang, G.H.; Li, Y.F.; Fan, N.N.; Li, H.J.; Zhang, M.; Xu, R.B.; Zhang, M.Y.; Zhao, A.J.; Ni, Z.F.; et al. Fine mapping and candidate gene analysis of a QTL associated with leaf rolling index on chromosome 4 of maize (Zea mays L.). Theor. Appl. Genet. 2019, 132, 3047–3062. [Google Scholar] [CrossRef]
- Rao, Y.C.; Yang, Y.L.; Xu, J.; Li, X.J.; Leng, Y.J.; Dai, L.P.; Huang, L.C.; Shao, G.S.; Ren, D.Y.; Hu, J.; et al. EARLY SENESCENCE1 encodes a SCAR-LIKE PROTEIN2 that affects water loss in rice. Plant Physiol. 2015, 169, 1225–1239. [Google Scholar] [CrossRef] [Green Version]
- Han, X.S.; Qin, Y.; Yu, F.; Ren, X.M.; Zhang, Z.X.; Qiu, F.Z. A megabase-scale deletion is associated with phenotypic variation of multiple traits in maize. Genetics 2019, 211, 305–316. [Google Scholar] [CrossRef] [Green Version]
- Nir, I.; Shohat, H.; Panizel, I.; Olszewski, N.; Aharoni, A.; Weiss, D. The tomato DELLA protein PROCERA acts in guard cells to promote stomatal closure. Plant Cell 2017, 29, 3186–3197. [Google Scholar] [CrossRef] [Green Version]
- You, J.; Zong, W.; Li, X.K.; Ning, J.; Hu, H.H.; Li, X.H.; Xiao, J.H.; Xiong, L.Z. The SNAC1-targeted gene OsSRO1c modulates stomatal closure and oxidative stress tolerance by regulating hydrogen peroxide in rice. J. Exp. Bot. 2013, 64, 569–583. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.Y.; Chao, D.Y.; Gao, J.P.; Zhu, M.Z.; Shi, M.; Lin, H.X. A previously unknown zinc finger protein, DST, regulates drought and salt tolerance in rice via stomatal aperture control. Gene Dev. 2009, 23, 1805–1817. [Google Scholar] [CrossRef] [Green Version]
- Chater, C.C.C.; Caine, R.S.; Fleming, A.J.; Gray, J.E. Origins and evolution of stomatal development. Plant Physiol. 2017, 174, 624–638. [Google Scholar] [CrossRef] [Green Version]
- MacAlister, C.A.; Ohashi-Ito, K.; Bergmann, D.C. Transcription factor control of asymmetric cell divisions that establish the stomatal lineage. Nature 2007, 445, 537–540. [Google Scholar] [CrossRef]
- Pillitteri, L.J.; Bogenschutz, N.L.; Torii, K.U. The bHLH protein, MUTE, controls differentiation of stomata and the hydathode pore in Arabidopsis. Plant Cell Physiol. 2008, 49, 934–943. [Google Scholar] [CrossRef] [Green Version]
- Ohashi-Ito, K.; Bergmann, D.C. Arabidopsis FAMA controls the final proliferation/differentiation switch during stomatal development. Plant Cell 2006, 18, 2493–2505. [Google Scholar] [CrossRef] [Green Version]
- Kanaoka, M.M.; Pillitteri, L.J.; Fujii, H.; Yoshida, Y.; Bogenschutz, N.L.; Takabayashi, J.; Zhu, J.K.; Torii, K.U. SCREAM/ICE1 and SCREAM2 specify three cell-state transitional steps leading to Arabidopsis stomatal differentiation. Plant Cell 2008, 20, 1775–1785. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.Z.; Chen, X.; Mang, H.; Liu, C.L.; Yu, X.; Gao, X.Q.; Torii, K.U.; He, P.; Shan, L.B. Differential function of Arabidopsis SERK family receptor-like kinases in stomatal patterning. Curr. Biol. 2015, 25, 2361–2372. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.; Han, S.K.; Dang, J.H.; Garrick, J.M.; Ito, M.; Hofstetter, A.K.; Torii, K.U. Autocrine regulation of stomatal differentiation potential by EPF1 and ERECTA-LIKE1 ligand-receptor signaling. eLife 2017, 6, e24102. [Google Scholar] [CrossRef]
- Wang, M.; Yang, K.Z.; Le, J. Organ-specific effects of brassinosteroids on stomatal production coordinate with the action of TOO MANY MOUTHS. J. Integr. Plant Biol. 2015, 57, 247–255. [Google Scholar] [CrossRef]
- Liu, T.; Ohashi-Ito, K.; Bergmann, D.C. Orthologs of Arabidopsis thaliana stomatal bHLH genes and regulation of stomatal development in grasses. Development 2009, 136, 2265–2276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.L.; Chen, L.; Yu, Q.; Zhou, W.Q.; Gou, X.P.; Li, J.; Hou, S.W. Multiple transcriptional factors control stomata development in rice. New Phytol. 2019, 223, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Chater, C.C.; Caine, R.S.; Tomek, M.; Wallace, S.; Kamisugi, Y.; Cuming, A.C.; Lang, D.; MacAlister, C.A.; Casson, S.; Bergmann, D.C.; et al. Origin and function of stomata in the moss Physcomitrella patens. Nat. Plants 2016, 2, 16179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caine, R.S.; Chater, C.C.; Kamisugi, Y.; Cuming, A.C.; Beerling, D.J.; Gray, J.E.; Fleming, A.J. An ancestral stomatal patterning module revealed in the non-vascular land plant Physcomitrella patens. Development 2016, 143, 3306–3314. [Google Scholar] [CrossRef] [Green Version]
- Raissig, M.T.; Abrash, E.; Bettadapur, A.; Vogel, J.P.; Bergmann, D.C. Grasses use an alternatively wired bHLH transcription factor network to establish stomatal identity. Proc. Natl. Acad. Sci. USA 2016, 113, 8326–8331. [Google Scholar] [CrossRef] [Green Version]
- Rudall, P.J.; Hilton, J.; Bateman, R.M. Several developmental and morphogenetic factors govern the evolution of stomatal patterning in land plants. New Phytol. 2013, 200, 598–614. [Google Scholar] [CrossRef]
- Raissig, M.T.; Matos, J.L.; Anleu Gil, M.X.; Kornfeld, A.; Bettadapur, A.; Abrash, E.; Allison, H.R.; Badgley, G.; Vogel, J.P.; Berry, J.A.; et al. Mobile MUTE specifies subsidiary cells to build physiologically improved grass stomata. Science 2017, 355, 1215–1218. [Google Scholar] [CrossRef] [Green Version]
- Fiorin, L.; Brodribb, T.J.; Anfodillo, T. Transport efficiency through uniformity: Organization of veins and stomata in angiosperm leaves. New Phytol. 2016, 209, 216–227. [Google Scholar] [CrossRef]
- Deokar, A.; Sagi, M.; Daba, K.; Tar’an, B. QTL sequencing strategy to map genomic regions associated with resistance to ascochyta blight in chickpea. Plant Biotechnol. J. 2019, 17, 275–288. [Google Scholar] [CrossRef]
- Huo, H.; Henry, I.M.; Coppoolse, E.R.; Verhoef-Post, M.; Schut, J.W.; de Rooij, H.; Vogelaar, A.; Joosen, R.V.; Woudenberg, L.; Comai, L.; et al. Rapid identification of lettuce seed germination mutants by bulked segregant analysis and whole genome sequencing. Plant J. 2016, 88, 345–360. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, K.L.; Grondin, A.; Courtois, B.; Gantet, P. Next-Generation Sequencing accelerates grop gene discovery. Trends Plant Sci. 2019, 24, 263–274. [Google Scholar] [CrossRef]
- Wang, C.S.; Tang, S.C.; Zhan, Q.L.; Hou, Q.Q.; Zhao, Y.; Zhao, Q.; Feng, Q.; Zhou, C.C.; Lyu, D.F.; Cui, L.L.; et al. Dissecting a heterotic gene through GradedPool-Seq mapping informs a rice-improvement strategy. Nat. Commun. 2019, 10, 2982. [Google Scholar] [CrossRef]
- Yu, C.C.; Yan, C.H.; Liu, Y.L.; Liu, Y.L.; Jia, Y.; Lavelle, D.; An, G.H.; Zhang, W.Y.; Zhang, L.; Han, R.K.; et al. Upregulation of a KN1 homolog by transposon insertion promotes leafy head development in lettuce. Proc. Natl. Acad. Sci. USA 2020, 117, 33668–33678. [Google Scholar] [CrossRef]
- Sun, C.W.; Dong, Z.D.; Zhao, L.; Ren, Y.; Zhang, N.; Chen, F. The Wheat 660K SNP array demonstrates great potential for marker-assisted selection in polyploid wheat. Plant Biotechnol. J. 2020, 18, 1354–1360. [Google Scholar] [CrossRef]
- Baret, F.; Madec, S.; Irfan, K.; Lopez, J.; Comar, A.; Hemmerle, M.; Dutartre, D.; Praud, S.; Tixier, M.H. Leaf-rolling in maize crops: From leaf scoring to canopy-level measurements for phenotyping. J. Exp. Bot. 2018, 69, 2705–2716. [Google Scholar] [CrossRef]
- Sussmilch, F.C.; Schultz, J.; Hedrich, R.; Roelfsema, M.R.G. Acquiring control: The evolution of stomatal signalling pathways. Trends Plant Sci. 2019, 24, 342–351. [Google Scholar] [CrossRef] [Green Version]
- Buckley, T.N. The control of stomata by water balance. New Phytol. 2005, 168, 275–292. [Google Scholar] [CrossRef]
- Ehonen, S.; Yarmolinsky, D.; Kollist, H.; Kangasjarvi, J. Reactive oxygen species, photosynthesis, and environment in the regulation of stomata. Antioxid. Redox Signal. 2019, 30, 1220–1237. [Google Scholar] [CrossRef]
- Ahmed, K.; Shabbir, G.; Ahmed, M.; Shah, K.N. Phenotyping for drought resistance in bread wheat using physiological and biochemical traits. Sci. Total Environ. 2020, 729, 139082. [Google Scholar] [CrossRef]
- Ali, Z.; Merrium, S.; Habib-Ur-Rahman, M.; Hakeem, S.; Saddique, M.A.B.; Sher, M.A. Wetting mechanism and morphological adaptation; leaf rolling enhancing atmospheric water acquisition in wheat crop-a review. Environ. Sci. Pollut. Res. Int. 2022, 29, 30967–30985. [Google Scholar] [CrossRef]
- Merrium, S.; Ali, Z.; Habib-Ur-Rahman, M.; Hakeem, S.; Khalid, M.A. Leaf rolling and leaf angle improve fog capturing and transport in wheat; adaptation for drought stress in an arid climate. Bot. Stud. 2022, 63, 13. [Google Scholar] [CrossRef]
- Jia, J.Z.; Xie, Y.L.; Cheng, J.F.; Kong, C.Z.; Wang, M.Y.; Gao, L.F.; Zhao, F.; Guo, J.Y.; Wang, K.; Li, G.W.; et al. Homology-mediated inter-chromosomal interactions in hexaploid wheat lead to specific subgenome territories following polyploidization and introgression. Genome Biol. 2021, 22, 26. [Google Scholar] [CrossRef]
- Fang, L.K.; Zhao, F.M.; Cong, Y.F.; Sang, X.C.; Du, Q.; Wang, D.Z.; Li, Y.F.; Ling, Y.H.; Yang, Z.L.; He, G.H. Rolling-leaf14 is a 2OG-Fe (II) oxygenase family protein that modulates rice leaf rolling by affecting secondary cell wall formation in leaves. Plant Biotechnol. J. 2012, 10, 524–532. [Google Scholar] [CrossRef]
- Wu, J.H.; Wang, Q.L.; Xu, L.S.; Chen, X.M.; Li, B.; Mu, J.M.; Zeng, Q.D.; Huang, L.L.; Han, D.J.; Kang, Z.S. Combining single nucleotide polymorphism genotyping array with bulked segregant analysis to map a gene controlling adult plant resistance to stripe rust in wheat line 03031-1-5 H62. Phytopathology 2018, 108, 103–113. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.Y.; Wang, R.T.; Mao, X.G.; Li, L.; Chang, X.P.; Zhang, X.Y.; Jing, R.L. TaARF4 genes are linked to root growth and plant height in wheat. Ann. Bot. 2019, 124, 903–915. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.Y.; Wang, R.T.; Mao, X.G.; Zhang, J.L.; Liu, Y.N.; Xie, Q.; Yang, X.Y.; Chang, X.P.; Li, C.N.; Zhang, X.Y.; et al. RING finger ubiquitin E3 ligase gene TaSDIR1-4A contributes to determination of grain size in common wheat. J. Exp. Bot. 2020, 71, 5377–5388. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Wang, J.; Mao, X.; Li, C.; Li, L.; Xue, Y.; He, L.; Jing, R. A Locus Controlling Leaf Rolling Degree in Wheat under Drought Stress Identified by Bulked Segregant Analysis. Plants 2022, 11, 2076. https://doi.org/10.3390/plants11162076
Yang X, Wang J, Mao X, Li C, Li L, Xue Y, He L, Jing R. A Locus Controlling Leaf Rolling Degree in Wheat under Drought Stress Identified by Bulked Segregant Analysis. Plants. 2022; 11(16):2076. https://doi.org/10.3390/plants11162076
Chicago/Turabian StyleYang, Xi, Jingyi Wang, Xinguo Mao, Chaonan Li, Long Li, Yinghong Xue, Liheng He, and Ruilian Jing. 2022. "A Locus Controlling Leaf Rolling Degree in Wheat under Drought Stress Identified by Bulked Segregant Analysis" Plants 11, no. 16: 2076. https://doi.org/10.3390/plants11162076






