Rhizoboxes as Rapid Tools for the Study of Root Systems of Prunus Seedlings
Abstract
:1. Introduction
2. Results
2.1. Whole-Root System Depth: Width Ratio
2.2. Root-Growth Parameters
2.3. Total-Root-Length Distribution by Diameter Classes
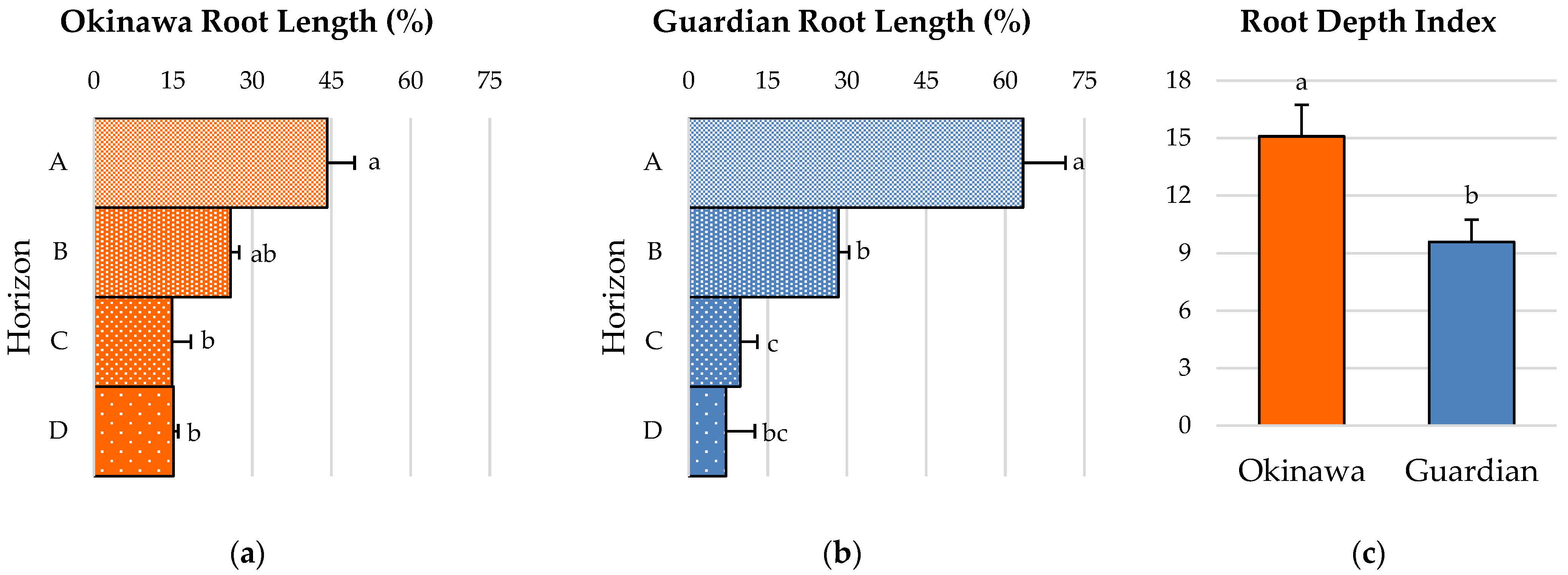
2.4. Root-Depth Pattern and Root-Depth Index
2.5. Root Spreading Angle
3. Discussion
4. Materials and Methods
4.1. Plant Material, Seeds Stratification and Germination
4.2. Seedling Growing Conditions
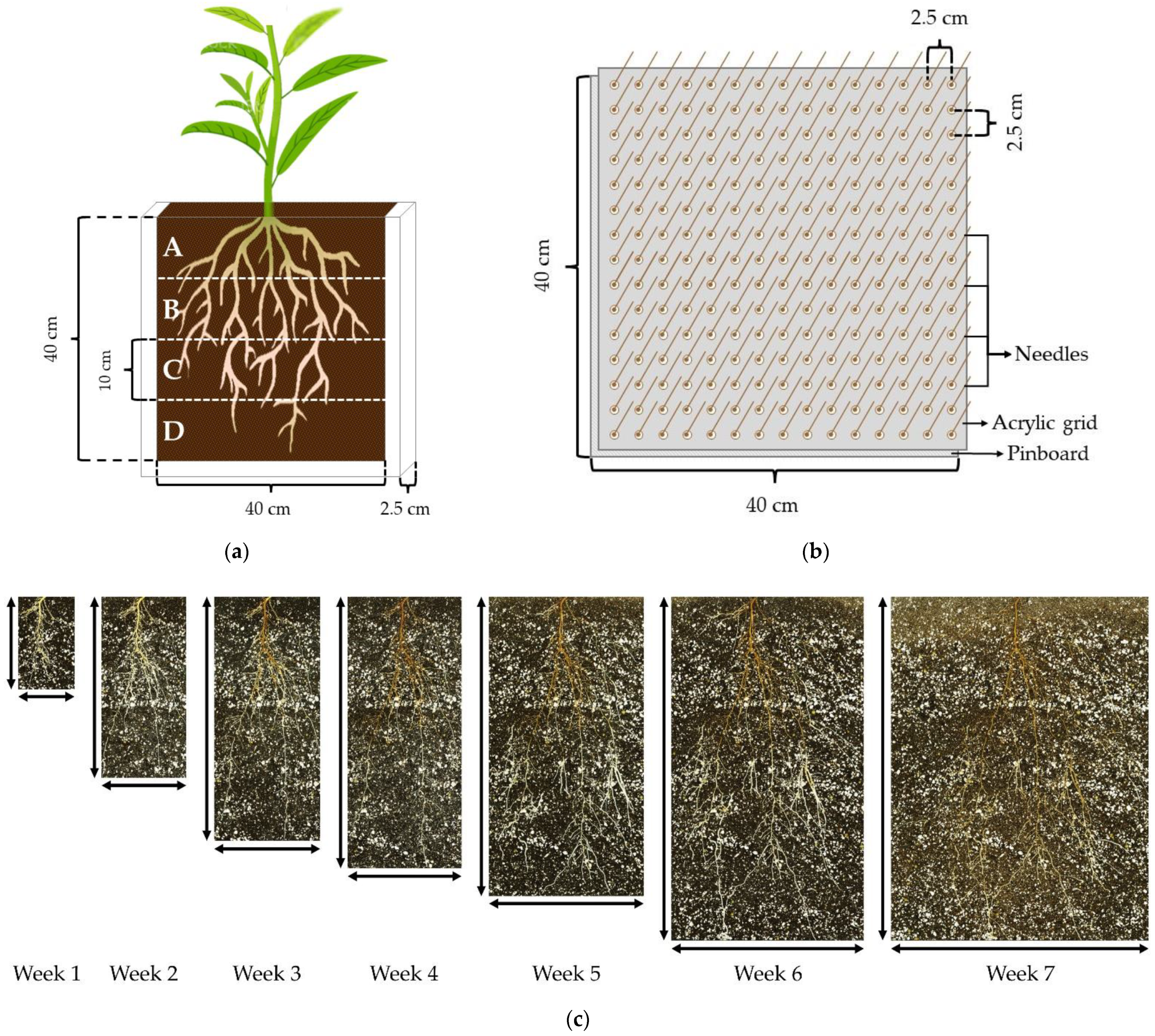
4.3. Root Systems Scanning
4.4. Image-Analysis Software and Measurements
4.5. Experimental Design and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Danjon, F.; Stokes, A.; Bakker, M.R. Root Systems of Woody Plants. In Plant Roots: The Hidden Half, 4th ed.; Eshel, A., Beeckman, T., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 435–460. ISBN 978-1-4398-4649-0. [Google Scholar]
- Moore, J.; Tombleson, J.; Turner, J.; van der Colff, M. Wind Effects on Juvenile Trees: A Review with Special Reference to Toppling of Radiata Pine Growing in New Zealand. For. Int. J. For. Res. 2008, 81, 377–387. [Google Scholar] [CrossRef]
- Basile, B.; Bryla, D.R.; Salsman, M.L.; Marsal, J.; Cirillo, C.; Johnson, R.S.; DeJong, T.M. Growth Patterns and Morphology of Fine Roots of Size-Controlling and Invigorating Peach Rootstocks. Tree Physiol. 2007, 27, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Shahkoomahally, S.; Chang, Y.; Brecht, J.K.; Chaparro, J.X.; Sarkhosh, A. Influence of Rootstocks on Fruit Physical and Chemical Properties of Peach Cv. UFSun. Food Sci. Nutr. 2021, 9, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Chatzissavvidis, C.A.; Therios, I.N.; Molassiotis, A.N. Seasonal Variation of Nutritional Status of Olive Plants as Affected by Boron Concentration in Nutrient Solution. J. Plant Nutr. 2005, 28, 309–321. [Google Scholar] [CrossRef]
- Beckman, T.G.; Chaparro, J.X. Peach Rootstock Development for the Southeastern United States. In Proceedings of the VIII International Peach Symposium, Matera, Italy, 20 May 2015; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2015; pp. 171–178. [Google Scholar]
- Okie, W.R.; Beckman, T.G.; Nyczepir, A.P.; Reighard, G.L.; Newall, W.C.; Zehr, E.I. BY520-9, a Peach Rootstock for the Southeastern United States That Increases Scion Longevity. HortScience 1994, 29, 705–706. [Google Scholar] [CrossRef]
- Fisk, C.L.; Tu, C.; Ritchie, D.F.; Parker, M.L.; Reighard, G.L. Rootstock Effect on Soil Ecology in a Young Peach Orchard. In Proceedings of the XI International Symposium on Integrating Canopy, Rootstock and Environmental Physiology in Orchard Systems, Bologna, Italy, 5 December 2018; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2018; pp. 265–272. [Google Scholar]
- McGee, T.; Shahid, M.A.; Beckman, T.G.; Chaparro, J.X.; Schaffer, B.; Sarkhosh, A. Physiological and Biochemical Characterization of Six Prunus Rootstocks in Response to Flooding. Environ. Exp. Bot. 2021, 183, 104368. [Google Scholar] [CrossRef]
- San, B.; Li, Z.; Hu, Q.; Reighard, G.L.; Luo, H. Adventitious Shoot Regeneration from in Vitro Cultured Leaf Explants of Peach Rootstock Guardian® Is Significantly Enhanced by Silver Thiosulfate. Plant Cell Tissue Organ Cult. PCTOC 2015, 120, 757–765. [Google Scholar] [CrossRef]
- Wilkins, B.S.; Ebel, R.C.; Dozier, W.A.; Pitts, J.; Eakes, D.J.; Himelrick, D.G.; Beckman, T.G.; Nyczepir, A.P. Field Performance of ’Guardian’™ Peach Rootstock Selections. HortScience 2002, 37, 1049–1052. [Google Scholar] [CrossRef]
- Maquilan, M.A.; Sarkhosh, A.; Dickson, D.W. Peach Root-Knot Nematode. EDIS 2018, 2018, HS1320. [Google Scholar] [CrossRef]
- Sharpe, R.H. Okinawa Peach Shows Promising Resistance to Root-Knot Nematodes; University of Florida, Agricultural Experiment Stations Bulletin: Gainesville, FL, USA, 1957; pp. 320–322. [Google Scholar]
- Nyczepir, A.P.; Beckman, T.G. Host Status of “Guardian” Peach Rootstock to Meloidogyne Sp. and M. Javanica. HortScience 2000, 35, 772. [Google Scholar] [CrossRef]
- Sarkhosh, A.; Olmstead, M.A.; Miller, E.P.; Andersen, P.C.; Williamson, J.G. Growing Plums in Florida. EDIS 2018, HS895. [Google Scholar]
- Souza, A.; Spinelli, V.; de Souza, R.; Smiderle, O.; Bianchi, V. Optimization of Germination and Initial Quality of Seedlings of Prunus Persica Tree Rootstocks. J. Seed Sci. 2017, 39, 166–173. [Google Scholar] [CrossRef]
- Ahmed, E.A.; El-Habashy, S.; Maklad, M.F. Trend of Vegetative Growth and Fruiting of Some Peach Cultivars Budded on “Okinawa” and “Nemaguard” Rootstocks. Middle East J. Agric. Res. 2017, 6, 1346–1358. [Google Scholar]
- Lobet, G.; Hachez, C.; Chaumont, F.; Javaux, M.; Draye, X. Root Water Uptake and Water Flow in the Soil–Root Domain. In Plant Roots: The Hidden Half, 4th ed.; Eshel, A., Beeckman, T., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 355–372. ISBN 978-1-4398-4649-0. [Google Scholar]
- Manschadi, A.M.; Hammer, G.L.; Christopher, J.T.; deVoil, P. Genotypic Variation in Seedling Root Architectural Traits and Implications for Drought Adaptation in Wheat (Triticum Aestivum L.). Plant Soil 2008, 303, 115–129. [Google Scholar] [CrossRef]
- King, J.; Gay, A.; Sylvester-Bradley, R.; Bingham, I.; Foulkes, J.; Gregory, P.; Robinson, D. Modelling Cereal Root Systems for Water and Nitrogen Capture: Towards an Economic Optimum. Ann. Bot. 2003, 91, 383–390. [Google Scholar] [CrossRef]
- Pinheiro, H.A.; DaMatta, F.M.; Chaves, A.R.; Loureiro, M.E.; Dicatti, C. Drought Tolerance Is Associated with Rooting Depth and Stomatal Control of Water Use in Clones of Coffea Canephora. Ann. Bot. 2005, 96, 101–108. [Google Scholar] [CrossRef]
- Atger, C.; Edelin, C. Preliminary Data on the Comparative Architecture of Roots and Crowns. Can. J. Bot. 1994, 72, 963–975. [Google Scholar] [CrossRef]
- Pandey, R.; Chinnusamy, V.; Rathod, G.; Paul, V.; Jain, N. Evaluation of Root Growth and Architecture. In Proceedings of the Manual of ICAR Sponsored Training Programme on Physiological Techniques to Analyze the Impact of Climate Change on Crop Plants, New Delhi, India, 16–25 January 2017; ICAR Indian Agricultural Research Institute (IARI): New Delhi, India, 2017; pp. 16–25. [Google Scholar]
- Hammer, G.L.; Dong, Z.; McLean, G.; Doherty, A.; Messina, C.; Schussler, J.; Zinselmeier, C.; Paszkiewicz, S.; Cooper, M. Can Changes in Canopy and/or Root System Architecture Explain Historical Maize Yield Trends in the U.S. Corn Belt? Crop Sci. 2009, 49, 299–312. [Google Scholar] [CrossRef]
- Manschadi, A.M.; Manske, G.; Vlek, P. Root Architecture and Resource Acquisition: Wheat as a Model Plant. In Plant Roots: The Hidden Half, 4th ed.; Eshel, A., Beeckman, T., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 319–336. ISBN 978-1-4398-4649-0. [Google Scholar]
- Bonser, A.M.; Lynch, J.P.; Snapp, S. Effect of Phosphorus Deficiency on Growth Angle of Basal Roots in Phaseolus Vulgaris. New Phytol. 1996, 132, 281–288. [Google Scholar] [CrossRef]
- Liao, H.; Rubio, G.; Yan, X.; Cao, A.; Brown, K.M.; Lynch, J.P. Effect of Phosphorus Availability on Basal Root Shallowness in Common Bean. Plant Soil 2001, 232, 69–79. [Google Scholar] [CrossRef]
- Kato, Y.; Abe, J.; Kamoshita, A.; Yamagishi, J. Genotypic Variation in Root Growth Angle in Rice (Oryza sativa L.) and Its Association with Deep Root Development in Upland Fields with Different Water Regimes. Plant Soil 2006, 287, 117–129. [Google Scholar] [CrossRef]
- Nakamoto, T.; Oyanagi, A. The Direction of Growth of Seminal Roots of Triticum Aestivum L. and Experimental Modification. Ann. Bot. 1994, 73, 363–367. [Google Scholar] [CrossRef]
- Barthélémy, D.; Caraglio, Y. Plant Architecture: A Dynamic, Multilevel and Comprehensive Approach to Plant Form, Structure and Ontogeny. Ann. Bot. 2007, 99, 375–407. [Google Scholar] [CrossRef] [PubMed]
- Thangthong, N.; Jogloy, S.; Pensuk, V.; Kesmala, T.; Vorasoot, N. Distribution Patterns of Peanut Roots under Different Durations of Early Season Drought Stress. Field Crops Res. 2016, 198, 40–49. [Google Scholar] [CrossRef]
- Manschadi, A.M.; Christopher, J.; deVoil, P.; Hammer, G. The Role of Root Architectural Traits in Adaptation of Wheat to Water-Limited Environments. Funct. Plant Biol. 2006, 33, 823–837. [Google Scholar] [CrossRef]
- Oyanagi, A. Gravitropic Response Growth Angle and Vertical Distribution of Roots of Wheat (Triticum aestivum L.). Plant Soil 1994, 165, 323–326. [Google Scholar] [CrossRef]
- Rewald, B.; Ephrath, J.E. Minirhizotron Techniques. In Plant Roots: The Hidden Half, 4th ed.; Eshel, A., Beeckman, T., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 728–743. ISBN 978-1-4398-4649-0. [Google Scholar]
- Mašková, T.; Klimeš, A. The Effect of Rhizoboxes on Plant Growth and Root: Shoot Biomass Partitioning. Front. Plant Sci. 2020, 10, 1693. [Google Scholar] [CrossRef]
- Polomski, J.; Kuhn, N. Root Research Methods. In Plant Roots: The Hidden Half, 3rd ed.; Waisel, Y., Eshel, A., Kafkafi, U., Eds.; CRC Press: New York, NY, USA, 2002; pp. 295–321. ISBN 978-0-8247-0631-9. [Google Scholar]
- Ephrath, J.E.; Klein, T.; Sharp, R.E.; Lazarovitch, N. Exposing the Hidden Half: Root Research at the Forefront of Science. Plant Soil 2020, 447, 1–5. [Google Scholar] [CrossRef]
- Blaauw, B.; Brannen, P.; Lockwood, D.; Schhnabel, G.; Ritchie, D. Southeastern Peach, Nectarine, and Plum Pest Management and Culture Guide; University of Georgia, Cooperative Extension Services: Athens, GA, USA, 2020. [Google Scholar]
- Reighard, G.L.; Beckman, T.G.; Belding, R.; Black, B.; Byers, P.; Cline, J.; Cowgill, W.; Godin, R.; Johnson, R.; Kamas, J.; et al. Six-Year Performance of 14 Prunus Rootstocks at 11 Sites in the 2001 NC-140 Peach Trial. J. Am. Pomol. Soc. 2011, 65, 26–41. [Google Scholar]
- Wongtanet, D.; Boonprakob, U. Effect of Rootstocks on Growth of Peaches in the Highland of Northern Thailand. In Proceedings of the VIII International Symposium on Temperate Zone Fruits in the Tropics and Subtropics, Florianopolis, Brazil, 31 August 2010; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2010; pp. 327–332. [Google Scholar]
- Kucukyumuk, Z.; Erdal, I. Rootstock and Cultivar Effect on Mineral Nutrition, Seasonal Nutrient Variation and Correlations among Leaf, Flower and Fruit Nutrient Concentrations in Apple Trees. Bulg. J. Agric. Sci. 2011, 17, 633–641. [Google Scholar]
- Psarras, G.; Merwin, I.A. Water Stress Affects Rhizosphere Respiration Rates and Root Morphology of Young ‘Mutsu’ Apple Trees on M.9 and MM.111 Rootstocks. J. Am. Soc. Hortic. Sci. Jashs 2000, 125, 588–595. [Google Scholar] [CrossRef]
- An, H.; Luo, F.; Wu, T.; Wang, Y.; Xu, X.; Zhang, X.; Han, Z. Dwarfing Effect of Apple Rootstocks Is Intimately Associated with Low Number of Fine Roots. HortScience 2017, 52, 503–512. [Google Scholar] [CrossRef]
- Eissenstat, D.M. Costs and Benefits of Constructing Roots of Small Diameter. J. Plant Nutr. 1992, 15, 763–782. [Google Scholar] [CrossRef]
- Böhm, W. Methods of Studying Root Systems, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 1979; Volume 16. [Google Scholar]
- Solari, L.I.; Pernice, F.; DeJong, T.M. The Relationship of Hydraulic Conductance to Root System Characteristics of Peach (Prunus Persica) Rootstocks. Physiol. Plant. 2006, 128, 324–333. [Google Scholar] [CrossRef]
- Mayer, N.; Ueno, B.; Silva, V. Leaf Nutrient Content of Peach on Five Rootstocks. Rev. Bras. Frutic. 2015, 37, 1045–1052. [Google Scholar] [CrossRef]
- Mestre, L.; Reig, G.; Betrán, J.A.; Pinochet, J.; Moreno, M.Á. Influence of Peach–Almond Hybrids and Plum-Based Rootstocks on Mineral Nutrition and Yield Characteristics of ‘Big Top’ Nectarine in Replant and Heavy-Calcareous Soil Conditions. Sci. Hortic. 2015, 192, 475–481. [Google Scholar] [CrossRef]
- Kumar, S.; Awasthi, O.P.; Dubey, A.K.; Pandey, R.; Sharma, V.K.; Mishra, A.K.; Sharma, R.M. Root Morphology and the Effect of Rootstocks on Leaf Nutrient Acquisition of Kinnow Mandarin (Citrus Nobilis Loureiro × Citrus Reticulata Blanco). J. Hortic. Sci. Biotechnol. 2018, 93, 100–106. [Google Scholar] [CrossRef]
- Barreto, C.; Moreno, M.; Pricila, S.; Andrade, S.; Cesar, V.; Marcelo, B.; José, C. Growth, Yield and Fruit Quality of “Chimarrita” Peach Trees Grafted on Different Rootstocks. Afr. J. Agric. Res. 2017, 12, 2933–2939. [Google Scholar] [CrossRef]
- Almeida, C.; Souza, A.; Argenta, J.; Fachinello, J.; João, B. The Effect of Rootstocks on the Vigor, Yield, and Fruit Quality of “Maciel” Peach Trees. Rev. Ciênc. Agrar. Amaz. J. Agric. Environ. Sci. 2015, 58, 301–307. [Google Scholar] [CrossRef]
- Jourdan, C.; Rey, H. Architecture and Development of the Oil-Palm (Elaeis Guineensis Jacq.) Root System. Plant Soil 1997, 189, 33–48. [Google Scholar] [CrossRef]
- Vercambre, G.; Pagès, L.; Doussan, C.; Habib, R. Architectural Analysis and Synthesis of the Plum Tree Root System in an Orchard Using a Quantitative Modelling Approach. Plant Soil 2003, 251, 1–11. [Google Scholar] [CrossRef]
- Caruso, T.; Mafrica, R.; Bruno, M.; Vescio, R.; Sorgonà, A. Root Architectural Traits of Rooted Cuttings of Two Fig Cultivars: Treatments with Arbuscular Mycorrhizal Fungi Formulation. Sci. Hortic. 2021, 283, 110083. [Google Scholar] [CrossRef]
- Oyanagi, A.; Nakamoto, T.; Wada, M. Relationship between Root Growth Angle of Seedlings and Vertical Distribution of Roots in the Field in Wheat Cultivars. Jpn. J. Crop Sci. 1993, 62, 565–570. [Google Scholar] [CrossRef]
- Ramalingam, P.; Kamoshita, A.; Deshmukh, V.; Yaginuma, S.; Uga, Y. Association between Root Growth Angle and Root Length Density of a Near-Isogenic Line of IR64 Rice with DEEPER ROOTING 1 under Different Levels of Soil Compaction. Plant Prod. Sci. 2017, 20, 162–175. [Google Scholar] [CrossRef]



| Root Length (cm) Distribution by Diameter Classes a | ||||
|---|---|---|---|---|
| Diameter Class | Cultivar | Response | SE | Group |
| Very Fine (≤0.5 mm) | ‘Okinawa’ | 1955.5 | 264.50 | a |
| ‘Guardian’™ | 611.1 | 74.69 | b | |
| Fine (>0.5 to ≤1.0 mm) | ‘Okinawa’ | 366.5 | 76.10 | bc |
| ‘Guardian’™ | 154.7 | 27.15 | c | |
| Large (>1.0 mm) | ‘Okinawa’ | 53.7 | 7.01 | c |
| ‘Guardian’™ | 23.1 | 4.88 | c | |
| Total Root Length (cm) by Spreading Angle from the Cultivar–Angle Interaction a | ||||
|---|---|---|---|---|
| Angle | Cultivar | Response | SE | Group |
| Shallower (0–25°) | ‘Okinawa’ | 17.89 | 4.51 | a |
| ‘Guardian’™ | 7.15 | 3.88 | a | |
| Shallow (25–45°) | ‘Okinawa’ | 103.57 | 16.84 | ab |
| ‘Guardian’™ | 36.49 | 12.30 | a | |
| Deep (45–65°) | ‘Okinawa’ | 303.35 | 42.92 | c |
| ‘Guardian’™ | 168.08 | 22.31 | b | |
| Deeper (65–90°) | ‘Okinawa’ | 460.17 | 29.89 | d |
| ‘Guardian’™ | 382.70 | 22.72 | cd | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lesmes-Vesga, R.A.; Cano, L.M.; Ritenour, M.A.; Sarkhosh, A.; Chaparro, J.X.; Rossi, L. Rhizoboxes as Rapid Tools for the Study of Root Systems of Prunus Seedlings. Plants 2022, 11, 2081. https://doi.org/10.3390/plants11162081
Lesmes-Vesga RA, Cano LM, Ritenour MA, Sarkhosh A, Chaparro JX, Rossi L. Rhizoboxes as Rapid Tools for the Study of Root Systems of Prunus Seedlings. Plants. 2022; 11(16):2081. https://doi.org/10.3390/plants11162081
Chicago/Turabian StyleLesmes-Vesga, Ricardo A., Liliana M. Cano, Mark A. Ritenour, Ali Sarkhosh, José X. Chaparro, and Lorenzo Rossi. 2022. "Rhizoboxes as Rapid Tools for the Study of Root Systems of Prunus Seedlings" Plants 11, no. 16: 2081. https://doi.org/10.3390/plants11162081
APA StyleLesmes-Vesga, R. A., Cano, L. M., Ritenour, M. A., Sarkhosh, A., Chaparro, J. X., & Rossi, L. (2022). Rhizoboxes as Rapid Tools for the Study of Root Systems of Prunus Seedlings. Plants, 11(16), 2081. https://doi.org/10.3390/plants11162081








