Chilling Tolerance in Maize: Insights into Advances—Toward Physio-Biochemical Responses’ and QTL/Genes’ Identification
Abstract
:1. Introduction
2. Effect of Low Temperature
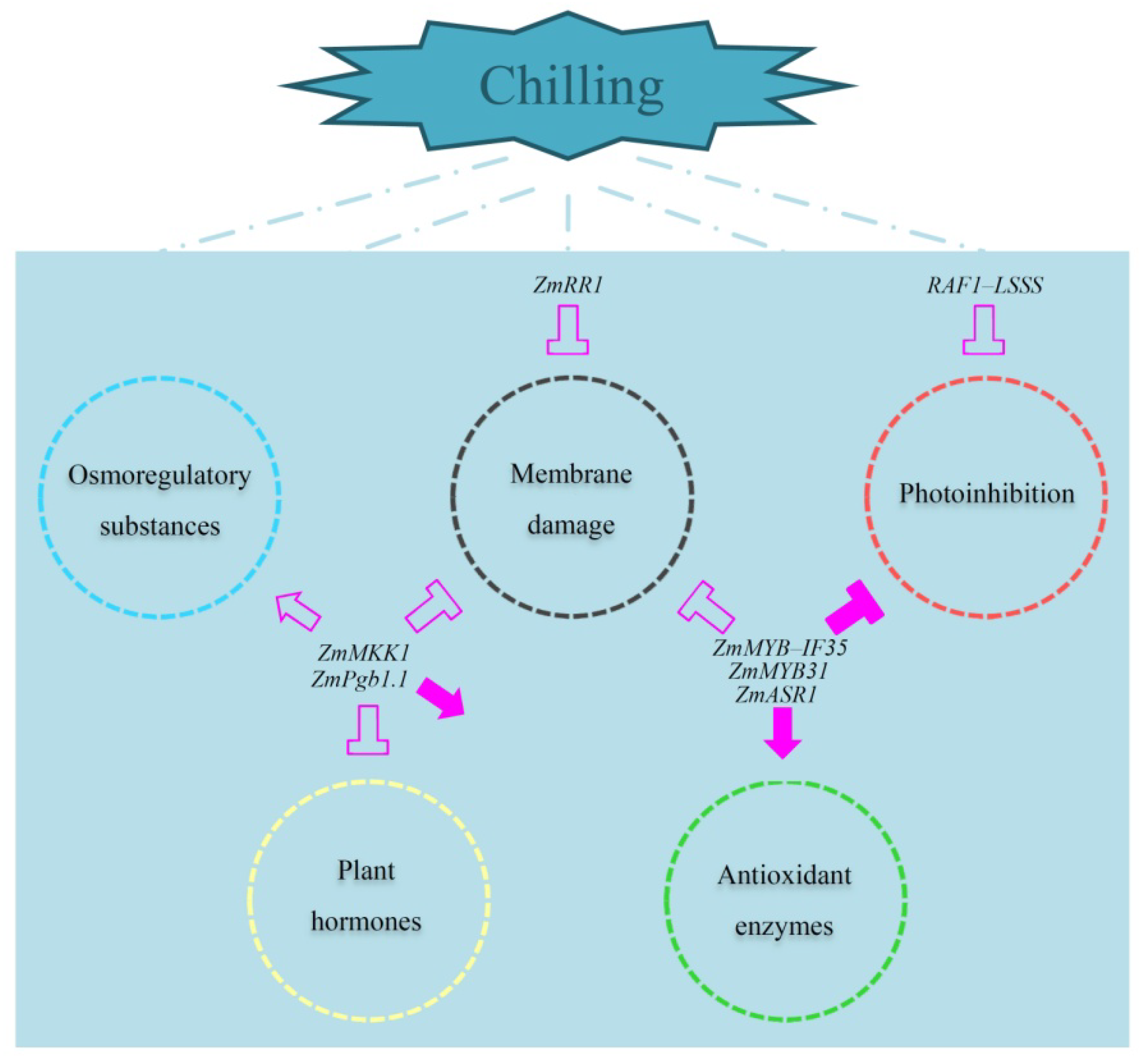
2.1. Effect of Low-Temperature Stress on the Membrane System
2.2. Effect of Low-Temperature Stress on the Antioxidant System
2.3. Effect of Low-Temperature Stress on Photosynthetic Physiology
2.4. Effect of Low-Temperature Stress on Osmoregulatory Substances
2.5. Effect of Low-Temperature Stress on Plant Hormone Levels
3. Identification and Localization of Chilling Tolerance-Related QTL/Genes in Maize
3.1. Identification of Chilling Tolerance-Related QTLs/Genes during Germination
3.2. Identification of Chilling Tolerance-Related QTLs/Genes during the Seedling Stage
4. Trends in Maize Chilling Tolerance-Related Research
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meng, C.; Sui, N. Overexpression of maize myb-if35 increases chilling tolerance in arabidopsis. Plant Physiol. Biochem. 2019, 135, 167–173. [Google Scholar] [CrossRef] [PubMed]
- John, G.A. Improving suboptimal temperature tolerance in maize-the search for variation. J. Exp. Bot. 1996, 47, 307–323. [Google Scholar]
- Hu, G.; Li, Z.; Lu, Y.; Li, C.; Gong, S.; Yan, S.; Li, G.; Wang, M.; Ren, H.; Guan, H.; et al. Genome-wide association study identified multiple genetic loci on chilling resistance during germination in maize. Sci. Rep. 2017, 7, 10840. [Google Scholar] [CrossRef] [PubMed]
- Crevecoeur, M.; Deltour, R.; Bronchart, R. Effects of subminimal temperature on physiology and ultrastructure of zea mays embryo during germination. Can. J. Bot. 1983, 61, 1117–1125. [Google Scholar] [CrossRef]
- Hussain, H.A.; Men, S.; Hussain, S.; Zhang, Q.; Ashraf, U.; Anjum, S.A.; Ali, I.; Wang, L. Maize tolerance against drought and chilling stresses varied with root morphology and antioxidative defense system. Plants 2020, 9, 720. [Google Scholar] [CrossRef]
- Ricardo, A.; Franco, T.; Juan, J.I.; Manuel, S.-D.; Alberto, P. Different root low temperature response of two maize genotypes differing in chilling sensitivity. Plant Physiol. Biochem. 2001, 39, 1067–1073. [Google Scholar]
- Filek, M.; Koscielniak, J. The effect of chilling temperature on the permeability of membranes to K+, Mg2+, Ca2+ ions and on the electric potential of leaves in the seedlings of maize (Zea mays L.). J. Agron. Crop Sci. 1995, 174, 205–212. [Google Scholar] [CrossRef]
- Pinhero, R.G.; Rao, M.V.; Paliyath, G.; Murr, D.P.; Fletcher, R.A. Changes in activities of antioxidant enzymes and their relationship to genetic and paclobutrazol-induced chilling tolerance of maize seedlings. Plant Physiol. 1997, 114, 695–704. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H.; Vanacker, H.; Gomez, L.D.; Harbinson, J. Regulation of photosynthesis and antioxidant metabolism in maize leaves at optimal and chilling temperatures:Review. Plant Physiol. Biochem. 2002, 40, 659–668. [Google Scholar] [CrossRef]
- Frei, O.M. Changes in yield physiology of corn as a result of breeding in northern europe [Zea mays L.]. Maydica 2000, 45, 173–183. [Google Scholar]
- Aroca, R.; Amodeo, G.; Fernandez-Illescas, S.; Herman, E.M.; Chaumont, F.; Chrispeels, M.J. The role of aquaporins and membrane damage in chilling and hydrogen peroxide induced changes in the hydraulic conductance of maize roots. Plant Physiol. 2005, 137, 341–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noblet, A.; Leymarie, J.; Bailly, C. Chilling temperature remodels phospholipidome of zea mays seeds during imbibition. Sci. Rep. 2017, 7, 8886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Santis, A.; Landi, P.; Genchi, G. Changes of mitochondrial properties in maize seedlings associated with selection for germination at low temperature. Fatty acid composition, cytochrome c oxidase, and adenine nucleotide translocase activities. Plant Physiol. 1999, 119, 743–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capell, B.; Dorffling, K. Genotype-specific differences in chilling tolerance of maize in relation to chilling-induced changes in water status and abscisic acid accumulation. Physiol. Plant 1993, 88, 638–646. [Google Scholar] [CrossRef]
- Gu, Y.; He, L.; Zhao, C.; Wang, F.; Yan, B.; Gao, Y.; Li, Z.; Yang, K.; Xu, J. Biochemical and transcriptional regulation of membrane lipid metabolism in maize leaves under low temperature. Front. Plant Sci. 2017, 8, 2053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Sui, N.; Lin, L.; Yang, Z.; Zhang, Y. Transcriptomic profiling revealed genes involved in response to cold stress in maize. Funct. Plant Biol. 2019, 46, 830–844. [Google Scholar] [CrossRef]
- Saczynska, V.; Kargul, J.; Kaniuga, Z. Discrimination between chilling-sensitive and chilling-resistant plants based on measurements of free fatty acid accumulation and inactivation of oxygen evolution in aged chloroplasts. Acta Biochim. Pol. 1993, 40, 507–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilska-Kos, A.; Panek, P.; Szulc-Glaz, A.; Ochodzki, P.; Cislo, A.; Zebrowski, J. Chilling-induced physiological, anatomical and biochemical responses in the leaves of miscanthus x giganteus and maize (Zea mays L.). J. Plant Physiol. 2018, 228, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Prasad, T.K.; Anderson, M.D.; Martin, B.A.; Stewart, C.R. Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell 1994, 6, 65–74. [Google Scholar] [CrossRef]
- Kocsy, G.; Szalai, G.; Galiba, G. Induction of glutathione synthesis and glutathione reductase activity by abiotic stresses in maize and wheat. Sci. World J. 2002, 2, 1699–1705. [Google Scholar] [CrossRef] [Green Version]
- Neta, I.C.S.; de Resende Von Pinho, E.V.; de Abreu, V.M.; Rezende Vilela, D.; Santos, M.C.; Santos, H.O.D.; Diniz Cabral Ferreira, R.A.; Von Pinho, R.G.; de Castro Vasconcellos, R.C. Gene expression and genetic control to cold tolerance during maize seed germination. BMC Plant Biol. 2020, 20, 188. [Google Scholar] [CrossRef]
- Cao, Q.; Li, G.; Cui, Z.; Yang, F.; Jiang, X.; Diallo, L.; Kong, F. Seed priming with melatonin improves the seed germination of waxy maize under chilling stress via promoting the antioxidant system and starch metabolism. Sci. Rep. 2019, 9, 15044. [Google Scholar] [CrossRef] [Green Version]
- Guan, Y.J.; Hu, J.; Wang, X.J.; Shao, C.X. Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J. Zhejiang Univ. Sci. B 2009, 10, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Guan, Y.; Li, Z.; He, F.; Huang, Y.; Song, W.; Hu, J. “On-off” thermoresponsive coating agent containing salicylic acid applied to maize seeds for chilling tolerance. PLoS ONE 2015, 10, e0120695. [Google Scholar] [CrossRef] [Green Version]
- Moradtalab, N.; Weinmann, M.; Walker, F.; Hoglinger, B.; Ludewig, U.; Neumann, G. Silicon improves chilling tolerance during early growth of maize by effects on micronutrient homeostasis and hormonal balances. Front. Plant Sci. 2018, 9, 420. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Cai, Q.; Wang, Z.; Cao, J.; Yu, T.; Xie, T. Exogenous diethyl aminoethyl hexanoate ameliorates low temperature stress by improving nitrogen metabolism in maize seedlings. PLoS ONE 2020, 15, e0232294. [Google Scholar] [CrossRef] [PubMed]
- Prasad, T.K. Role of catalase in inducing chilling tolerance in pre-emergent maize seedlings. Plant Physiol. 1997, 114, 1369–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Li, L.; Zuo, S.; Li, J.; Wei, S. Differentially expressed zmasr genes associated with chilling tolerance in maize (Zea mays) varieties. Funct. Plant Biol. 2018, 45, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lin, L.; Zhang, Y.; Sui, N. Zmmyb31, a r2r3-myb transcription factor in maize, positively regulates the expression of cbf genes and enhances resistance to chilling and oxidative stress. Mol. Biol. Rep. 2019, 46, 3937–3944. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Wang, G.; Wang, L.; Pan, J.; Liu, Y.; Li, D. Zmmkk1, a novel group a mitogen-activated protein kinase kinase gene in maize, conferred chilling stress tolerance and was involved in pathogen defense in transgenic tobacco. Plant Sci. 2014, 214, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Kingston-Smith, A.H.; Foyer, C.H. Bundle sheath proteins are more sensitive to oxidative damage than those of the mesophyll in maize leaves exposed to paraquat or low temperatures. J. Exp. Bot. 2000, 51, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Govindachary, S.; Bukhov, N.G.; Joly, D.; Carpentier, R. Photosystem ii inhibition by moderate light under low temperature in intact leaves of chilling-sensitive and -tolerant plants. Physiol. Plant 2004, 121, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Lopez, A.; Nie, G.Y.; Ort, D.R.; Baker, N.R. The involvement of the photoinhibition of photosystem ii and impaired membrane energization in the reduced quantum yield of carbon assimilation in chilled maize. Planta 1990, 181, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Riva-Roveda, L.; Escale, B.; Giauffret, C.; Perilleux, C. Maize plants can enter a standby mode to cope with chilling stress. BMC Plant Biol. 2016, 16, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szalai, G.; Majlath, I.; Pal, M.; Gondor, O.K.; Rudnoy, S.; Olah, C.; Vankova, R.; Kalapos, B.; Janda, T. Janus-faced nature of light in the cold acclimation processes of maize. Front. Plant Sci. 2018, 9, 850. [Google Scholar] [CrossRef] [Green Version]
- Sowinski, P.; Rudzinska-Langwald, A.; Adamczyk, J.; Kubica, I.; Fronk, J. Recovery of maize seedling growth, development and photosynthetic efficiency after initial growth at low temperature. J. Plant Physiol. 2005, 162, 67–80. [Google Scholar] [CrossRef]
- Bilska, A.; Sowinski, P. Closure of plasmodesmata in maize (Zea mays) at low temperature: A new mechanism for inhibition of photosynthesis. Ann. Bot. 2010, 106, 675–686. [Google Scholar] [CrossRef] [Green Version]
- Meng, A.; Wen, D.; Zhang, C. Maize seed germination under low-temperature stress impacts seedling growth under normal temperature by modulating photosynthesis and antioxidant metabolism. Front. Plant Sci. 2022, 13, 843033. [Google Scholar] [CrossRef] [PubMed]
- Sobkowiak, A.; Jonczyk, M.; Adamczyk, J.; Szczepanik, J.; Solecka, D.; Kuciara, I.; Hetmanczyk, K.; Trzcinska-Danielewicz, J.; Grzybowski, M.; Skoneczny, M.; et al. Molecular foundations of chilling-tolerance of modern maize. BMC Genom. 2016, 17, 125. [Google Scholar] [CrossRef] [Green Version]
- Salesse-Smith, C.E.; Sharwood, R.E.; Busch, F.A.; Stern, D.B. Increased rubisco content in maize mitigates chilling stress and speeds recovery. Plant Biotechnol. J. 2020, 18, 1409–1420. [Google Scholar] [CrossRef] [Green Version]
- Bilska-Kos, A.; Solecka, D.; Dziewulska, A.; Ochodzki, P.; Jonczyk, M.; Bilski, H.; Sowinski, P. Low temperature caused modifications in the arrangement of cell wall pectins due to changes of osmotic potential of cells of maize leaves (Zea mays L.). Protoplasma 2017, 254, 713–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramazan, S.; Qazi, H.A.; Dar, Z.A.; John, R. Low temperature elicits differential biochemical and antioxidant responses in maize (Zea mays) genotypes with different susceptibility to low temperature stress. Physiol. Mol. Biol. Plants 2021, 27, 1395–1412. [Google Scholar] [CrossRef] [PubMed]
- Duran Garzon, C.; Lequart, M.; Rautengarten, C.; Bassard, S.; Sellier-Richard, H.; Baldet, P.; Heazlewood, J.L.; Gibon, Y.; Domon, J.M.; Giauffret, C.; et al. Regulation of carbon metabolism in two maize sister lines contrasted for chilling tolerance. J. Exp. Bot. 2020, 71, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Qi, J.; Hao, G.; Zhang, C.; Wang, C.; Dirk, L.M.A.; Downie, A.B.; Zhao, T. Zmdreb1a regulates raffinose synthase controlling raffinose accumulation and plant chilling stress tolerance in maize. Plant Cell Physiol. 2020, 61, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Janda, T.; Majlath, I.; Szalai, G. Involvement of salicylic acid and other phenolic compounds in light-dependent cold acclimation in maize. Int. J. Mol. Sci. 2020, 21, 1942. [Google Scholar] [CrossRef] [Green Version]
- Xin, Z.; Li, P.H. Alteration of gene expression associated with abscisic acid-induced chilling tolerance in maize suspension-cultured cells. Plant Physiol. 1993, 101, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Prasad, T.K.; Anderson, M.D.; Stewart, C.R. Acclimation, hydrogen peroxide, and abscisic acid protect mitochondria against irreversible chilling injury in maize seedlings. Plant Physiol. 1994, 105, 619–627. [Google Scholar] [CrossRef] [Green Version]
- Xue, C.; Jiang, Y.; Wang, Z.; Shan, X.; Yuan, Y.; Hua, J. Tissue-level transcriptomic responses to local and distal chilling reveal potential chilling survival mechanisms in maize. J. Exp. Bot. 2021, 72, 7610–7625. [Google Scholar] [CrossRef]
- Kang, H.M.; Saltveit, M.E. Chilling tolerance of maize, cucumber and rice seedling leaves and roots are differentially affected by salicylic acid. Physiol. Plant 2002, 115, 571–576. [Google Scholar] [CrossRef] [Green Version]
- Waqas, M.A.; Khan, I.; Akhter, M.J.; Noor, M.A.; Ashraf, U. Exogenous application of plant growth regulators (pgrs) induces chilling tolerance in short-duration hybrid maize. Environ. Sci. Pollut. Res. Int. 2017, 24, 11459–11471. [Google Scholar] [CrossRef]
- Li, Z.; Xu, J.; Gao, Y.; Wang, C.; Guo, G.; Luo, Y.; Huang, Y.; Hu, W.; Sheteiwy, M.S.; Guan, Y.; et al. The synergistic priming effect of exogenous salicylic acid and h2o2 on chilling tolerance enhancement during maize (Zea mays L.) seed germination. Front. Plant Sci. 2017, 8, 1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, I.; Kumar, U.; Singh, S.K.; Gupta, C.; Singh, M.; Kushwaha, S.R. Physiological and biochemical effect of 24-epibrassinoslide on cold tolerance in maize seedlings. Physiol. Mol. Biol. Plants 2012, 18, 229–236. [Google Scholar] [CrossRef] [Green Version]
- Mira, M.M.; Ibrahim, S.; Hill, R.D.; Stasolla, C. Cold stress in maize (Zea mays) is alleviated by the over-expression of phytoglobin 1 (zmpgb1.1). Plant Physiol. Biochem. 2021, 167, 901–910. [Google Scholar] [CrossRef]
- Jompuk, C.; Fracheboud, Y.; Stamp, P.; Leipner, J. Mapping of quantitative trait loci associated with chilling tolerance in maize (Zea mays L.) seedlings grown under field conditions. J. Exp. Bot. 2005, 56, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Strigens, A.; Freitag, N.M.; Gilbert, X.; Grieder, C.; Riedelsheimer, C.; Schrag, T.A.; Messmer, R.; Melchinger, A.E. Association mapping for chilling tolerance in elite flint and dent maize inbred lines evaluated in growth chamber and field experiments. Plant Cell Environ. 2013, 36, 1871–1887. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, G.; Tian, Z.; Wang, Z.; Wang, X.; Zhu, Y.; Chen, Y.; Guo, S.; Qi, J.; Zhang, X.; et al. Genetic dissection of seed vigour traits in maize (Zea mays L.) under low-temperature conditions. J. Genet. 2016, 95, 1017–1022. [Google Scholar] [CrossRef]
- Revilla, P.; Rodriguez, V.M.; Ordas, A.; Rincent, R.; Charcosset, A.; Giauffret, C.; Melchinger, A.E.; Schon, C.C.; Bauer, E.; Altmann, T.; et al. Association mapping for cold tolerance in two large maize inbred panels. BMC Plant Biol. 2016, 16, 127. [Google Scholar] [CrossRef] [Green Version]
- Di Fenza, M.; Hogg, B.; Grant, J.; Barth, S. Transcriptomic response of maize primary roots to low temperatures at seedling emergence. PeerJ 2017, 5, e2839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Wu, Y.; Li, W.; Qin, X.; Wang, Y.; Yue, B. Genetic mapping with testcrossing associations and f2:3 populations reveals the importance of heterosis in chilling tolerance at maize seedling stage. Sci. Rep. 2017, 7, 3232. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhang, J.; Xu, Q.; Wang, D.; Di, H.; Huang, J.; Yang, X.; Wang, Z.; Zhang, L.; Dong, L.; et al. Identification of candidate tolerance genes to low-temperature during maize germination by gwas and rna-seqapproaches. BMC Plant Biol. 2020, 20, 333. [Google Scholar] [CrossRef]
- Aydinoglu, F. Elucidating the regulatory roles of micrornas in maize (Zea mays L.) leaf growth response to chilling stress. Planta 2020, 251, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, P.; Wang, C.; Zhang, N.; Zhu, Y.; Zou, C.; Yuan, G.; Yang, C.; Gao, S.; Pan, G.; et al. Genome-wide association study uncovers new genetic loci and candidate genes underlying seed chilling-germination in maize. PeerJ 2021, 9, e11707. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.; Alvarez-Iglesias, L.; Malvar, R.A.; Romay, M.C.; Revilla, P. A worldwide maize panel revealed new genetic variation for cold tolerance. Theor. Appl. Genet. 2021, 134, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Goering, R.; Larsen, S.; Tan, J.; Whelan, J.; Makarevitch, I. Qtl mapping of seedling tolerance to exposure to low temperature in the maize ibm ril population. PLoS ONE 2021, 16, e0254437. [Google Scholar] [CrossRef]
- Han, Q.; Zhu, Q.; Shen, Y.; Lee, M.; Lubberstedt, T.; Zhao, G. Qtl mapping low-temperature germination ability in the maize ibm syn10 dh population. Plants 2022, 11, 214. [Google Scholar] [CrossRef]
- Zeng, R.; Li, Z.; Shi, Y.; Fu, D.; Yin, P.; Cheng, J.; Jiang, C.; Yang, S. Natural variation in a type-a response regulator confers maize chilling tolerance. Nat. Commun. 2021, 12, 4713. [Google Scholar] [CrossRef]
- Thornsberry, J.M.; Goodman, M.M.; Doebley, J.; Kresovich, S.; Nielsen, D.; Buckler, E.S.t. Dwarf8 polymorphisms associate with variation in flowering time. Nat. Genet. 2001, 28, 286–289. [Google Scholar] [CrossRef]
- Pesev, N.V. Genetic factors affecting maize tolerance to low temperatures at emergence and germination. Theor. Appl. Genet. 1970, 40, 351–356. [Google Scholar] [CrossRef]
- Liu, J.; Shi, Y.; Yang, S. Insights into the regulation of c-repeat binding factors in plant cold signaling. J. Integr. Plant Biol. 2018, 60, 780–795. [Google Scholar] [CrossRef]
- Shi, Y.; Ding, Y.; Yang, S. Molecular regulation of cbf signaling in cold acclimation. Trends Plant Sci. 2018, 23, 623–637. [Google Scholar] [CrossRef]
- Ma, Y.; Dai, X.; Xu, Y.; Luo, W.; Zheng, X.; Zeng, D.; Pan, Y.; Lin, X.; Liu, H.; Zhang, D.; et al. Cold1 confers chilling tolerance in rice. Cell 2015, 160, 1209–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Liu, D.; Chong, K. Cold signaling in plants: Insights into mechanisms and regulation. J. Integr. Plant Biol. 2018, 60, 745–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zhang, Z.; Chong, K.; Xu, Y. Chilling tolerance in rice: Past and present. J. Plant Physiol. 2022, 268, 153576. [Google Scholar] [CrossRef] [PubMed]

| Year | Low-Temperature Treated | Indices | Ref. | |||||
|---|---|---|---|---|---|---|---|---|
| Material (Germplasm) | Condition | Membrane | Antioxidants | Photosynthesis | Osmoprotectants | Hormones | ||
| 1990 | 10-day-old seedlings (Zea mays L. cv. LG11) | 5 °C for 6 h | Fv/Fm | [33] | ||||
| 1993 | 2-week-old seedlings (four inbred lines: Z 7, Mo 17, KW1074 and Penjalinan) | 14/12 °C day/night for 4 days and then chilled for 5 days at 5/3 °C | ion efflux | abscisic acid | [14] | |||
| 1993 | 2- to 3-week-old seedlings (F7-Rp III ) | none | free fatty acids, oxygen evolution | [17] | ||||
| 1993 | maize suspension-cultured cells(Zea mays L. cv Black Mexican Sweet) | 4 °C for 12 h, 24 h, 2 days and 4 days | abscisic acid | [46] | ||||
| 1994 | 3-day-old seedlings (Pioneer inbred G50) | 14 °C for 3 days, then 4 °C for 7 days | catalase 3,hydrogen peroxide | [19] | ||||
| 1994 | 3-day-old seedlings (Pioneer inbred G50) | 14 °C for 3 days, then 4 °C for 7 days, or just 4 °C for 7 days | catalase 3, peroxidase | abscisic acid | [47] | |||
| 1997 | 11-day-old seedlings (chilling-susceptible CO 316 and chilling-tolerant CO 328 inbred lines) | 6/2 °C day/night for 24 and 48 h | superoxide dismutase, glutathione reductase, ascorbate peroxidase | [8] | ||||
| 1997 | 3-day-old seedlings (Pioneer inbred G50) | 14 °C for 1 day or 4 °C for 1 day followed by recovery at 27 °C for 1 day, then 4 °C for 7 days | catalase | [27] | ||||
| 1999 | seed to 21- to 23-day-old seedlings (three F2 from the cross B73×IABO78, as well as the two parental lines, chilling-sensitive germination B73 and chilling-tolerant germination IABO78) | 14 °C | mitochondrial inner membranes, 18-carbon unsaturated fatty acids, fluidity | cytochrome c oxidase mitochondrial peroxidase | [13] | |||
| 2000 | 3-leaf-stage seedlings (Zea mays L. cv. H99) | seed was germinated for 5 days, then transferred at 20, 18, 15 or 10 °C until the third leaf was expanded | oxidative damage | [31] | ||||
| 2002 | 2-leaf-stage seedlings (chilling-sensitive Penjalinan and chilling-tolerant Z7 inbred lines) | 5 °C for 7 days | glutathione, glutathione reductase | [20] | ||||
| 2002 | 2-week-old seedlings (Zea mays L. cv. Golden Jubilee) | 2.5 °C for 1–4 days | electrolyte leakage | glutathione reductase, guaiacol peroxidase | salicylic acid | [49] | ||
| 2004 | 10-to 15-day-old seedlings (variety of Zea mays L.) | 4 °C for 1 to 5 h, sampled at 1-hr intervals | PSII activity | [32] | ||||
| 2005 | root cortex protoplasts (chilling-sensitive Penjalinan and chilling-tolerant Z7 inbred lines) | 5 °C for 3 days | hydraulic conductance | hydrogen peroxide | [11] | |||
| 2005 | seed to 3-leaf-stage seedlings (chilling-tolerant KW 1074 and chilling-sensitive CM 109 inbred lines) | 14/12 °C day/night | photosynthetic efficiency | [36] | ||||
| 2009 | seed (chilling-tolerant HuangC and chilling-sensitive Mo17 inbred lines) | 5 °C for 3 days | relative permeability of the plasma membrane | peroxidase, catalase, malondialdehyde | soluble sugars, proline | [23] | ||
| 2010 | 3-leaf seedlings (chilling-tolerant KW 1074 and chilling-sensitive CM 109 inbred lines) | 14/12 °C day/night for either 4 or 28 h | photosynthesis | [37] | ||||
| 2012 | 10-day-old seedlings (LM-17 inbred lines) | maximum and minimum temperature in net house ranged between 17.6 and 24.5 °C and 2.8 and 7.4 °C; treated for 7, 14 and 21 days | tissue water content, membrane injury index | total chlorophyll | soluble sugar, protein content | 24-epibrassinoslide | [52] | |
| 2014 | 8-week-old transgenic tobacco | 12 °C for 2 and 4 days | antioxidant enzyme activities, ROS-related genes | osmoregulatory substances | [30] | |||
| 2015 | seed (chilling-tolerant HuangC and chilling-sensitive Mo17 inbred lines) | 5 °C for 3 days | protective enzyme activities, malondialdehyde content | [24] | ||||
| 2016 | third-leaf seedlings (three inbred lines: S68911, S50676 and S160) | 14/12 °C day/night for 4 days, followed by 4 days at 8/6 °C day/night | photosynthetic apparatus | [39] | ||||
| 2016 | 5-leaf-stage seedlings (three flint lines: F2, F283, F03802; three dent lines: F353, B73, Mo17; two hybrids: F03802xF353, F2xF353) | 10/7 °C day/night (inbred lines) or 10/4 °C day/night (hybrids) for one week | chlorophyll biosynthesis, CO2 assimilation | [34] | ||||
| 2017 | seed (hybrids A and B provided by Limagrain Europe) | 5, 10, 15 and 18 °C for germination assay; 10 and 18 °C for 24 h for electrolyte leakage measurements, total lipid extraction and phospholipid analysis | saturated or poorly unsaturated fatty acids, electrolyte leakage | [12] | ||||
| 2017 | 2-week-old seedlings (He 344) | 5 °C for 3 days | membrane lipid adjustment | [15] | ||||
| 2017 | 3-leaf-stage seedlings (chilling-tolerant KW 1074 and chilling-sensitive CM 109) | 14/12 °C for either 1, 4, 28, or 168 h | sucrose | [41] | ||||
| 2017 | six-leaf seedlings (hybrid Dekalb-6789) | average chilling temperature was 13–8 °C from sowing to harvesting | electrolyte leakage | [50] | ||||
| 2017 | seed (Meiyuno.3) | 13 °C for 7 days | reactive oxygen species | abscisic acid | [51] | |||
| 2018 | 3-leaf-stage seedlings (chilling-tolerant S68911 and chilling-sensitive B73) | 12–14 °C day/night for 28 h and 3 days | net CO2 assimilation, F’v/F’m, Fv/Fm, ΦPSII | [18] | ||||
| 2018 | 2-week-old seedlings (Zea mays L. cv. Colisee) | 12–14 °C for 2 weeks | superoxide dismutase activity, antioxidants, H2O2 | proline | [25] | |||
| 2018 | 3-week-old seedlings (Zea mays L. cv. Jidan 198 and Jinyu 5) | 5 °C for 2 days | superoxide dismutase, peroxidase activity, malondialdehyde content | Fv/Fm | [28] | |||
| 2018 | 13-day-old-seedlings (Zea mays L. hybrid Norma) | 15/13 °C day/night for 3 days under three different light conditions, then 5 °C for 3 days, then back to 22/20 °C day/night for a 1-day recovery period | photoinhibition | soluble sugars | [35] | |||
| 2019 | 4-leaf-stage seedlings (chilling-tolerant M54 and chilling-sensitive753F inbred lines) | 4 °C for 0, 4 and 24 h | unsaturated fatty acid | PSII, secondary metabolites | [16] | |||
| 2019 | seed (Zea mays L. cv. Jinongnuo 112) | 13 °C for 12 and 24 h | hydrogen peroxide, superoxide dismutase, peroxidase, catalase, ascorbate peroxidase, malondialdehyde concentrations | [22] | ||||
| 2019 | 2-week-old transgenic Arabidopsis | 4 °C for 3, 6, 9 and 12 h | ion leakage | antioxidant enzyme activity, reactive oxygen species | Fv/Fm | [1] | ||
| 2019 | 2-week-old transgenic Arabidopsis | 4 °C for 3, 6, 9 and 12 h | superoxide dismutase, ascorbate peroxidase, reactive oxygen species | Fv/Fm | [29] | |||
| 2020 | 3-leaf-stage seedlings (B73 and W22 background) | 10/8 °C day/night for 4 days | raffinose biosynthesis | [44] | ||||
| 2020 | seeds (three chilling-tolerant germination lines: 91, 64, 63, and three chilling-sensitive germination lines: 44, 54, 57), as well as their hybrid combination by reciprocal crosses | 10 °C for 4 and 7 days | catalase, esterase enzymes | [21] | ||||
| 2020 | 3-leaf-stage seedlings (Q319 and DA-6 inbred lines) | 11±1 °C for 0, 1, 3, 5 and 7 days | oxygen metabolism | photosynthesis | [26] | |||
| 2020 | 11-day-old seedlings (two maize hybrids; Xida889 and Xida319, and two maize inbred; Yu13 and Yu37) | 15/12 °C day/night for 12 days | total antioxidant capability, superoxide dismutase, peroxidase, catalase and glutathione reductase activities | [5] | ||||
| 2020 | 3-week-old seedlings (transgenic maize) | 14 °C/12 °C day/night for 2 weeks | photochemical quenching | [40] | ||||
| 2020 | 4-day-old seedlings (chilling-tolerant CFD04_349 and chilling-sensitive CFD04_332; two double-haploid (DH) population derived from the F1 cross between F353 and D09) | 15 °C/11 °C day/night for about 8 weeks | chlorophyll content, glucose-6-phosphate dehydrogenase activity | sucrose-to-starch ratio | [43] | |||
| 2020 | 11-day-old seedlings | 15/13 °C day/night for 3 days, followed by 5 °C for 3 days | salicylic acid | [45] | ||||
| 2021 | 2-week-old seedlings (chilling-tolerant Gurez local and chilling-sensitive Gujarat-Maize-6) | 6 °C for 2, 4, 6, 8, 10 and 12 h | hydrogen peroxide, malondialdehyde | free proline, total protein, total soluble sugars, trehalose, total phenolics, glycine betaine | [42] | |||
| 2021 | 3-leaf-stage seedlings (B73, B104, CM7, CM37, CML77, CML333, M37W, Mt42, NC300, R177, and Tzi9 inbred lines) | 4 °C for 3–7 days | abscisic acid | [48] | ||||
| 2021 | 2-leaf-stage seedlings (transgenic maize) | 10 °C/4 °C day/night for 72 h | brassinosteroid biosynthetic and response genes | [53] | ||||
| 2022 | seed (chilling-tolerant 04Qun0522-1-1 and chilling-sensitive B283-1 inbred lines) | 13 °C for 4 days | antioxidant metabolism-related pathways | photosynthetic system | [38] | |||
| Year | Low-Temperature Treated | Type of Marker | Number of Markers | Trait | Method | Number of QTL/Loci | Candidate Genes | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Material (Germplasm) | Condition | ||||||||
| 2005 | from seed to 2-leaf-stage seedlings (226 F2:3 population, chilling-tolerant ETH-DH7 and chilling-sensitive ETH-DL3 as parents) | field experiments; plants sown early were exposed to low temperature | SSR | ΦPSII, Fv/Fm, SPAD, Fo, Fm, SDW, LAT, N%, C%, C:N | linkage analysis | 6, 5, 4, 1, 2, 4, 2, 2 and 3 QTLs related to SPAD, ΦPSII, Fv/Fm, Fo, Fm, LAT, SDW, N% and C:N, respectively | none | [54] | |
| 2013 | from seed to 3-leaf-stage seedlings (375 maize inbreeds) | growth chamber (16/13 °C, day/night) and field experiments(mean temperature after sowing for 30 days about 15 °C) | SNP | 56,110 | ΦPSII, Fv/Fm, SPAD, SDW, LDW, LAS, SLA, RGR | association analysis | 16 QTLs | 24 candidate genes: GRMZM2G371167, GRMZM2G049609, GRMZM2G151811, GRMZM2G059165, GRMZM2G103773, GRMZM2G103843, GRMZM2G035584, GRMZM2G099850, GRMZM2G123790, GRMZM2G328742, GRMZM2G394827, GRMZM2G094892, GRMZM2G093346, GRMZM2G381059, GRMZM2G167856, GRMZM2G050649, GRMZM2G130442, GRMZM2G349709, GRMZM2G057386, GRMZM2G057231, GRMZM2G358161, GRMZM2G057709, GRMZM2G021388 and GRMZM2G021277 | [55] |
| 2016 | from seed to 8-day-old seedlings (208 and 212 F10 RILs derived from two crosses, Yu82×Shen137 and Yu537A×Shen137 | 18 ± 1 °C | SNP | 1172 SNPs for RIL from Yu82×Shen137 and 1139 SNPs for Yu537A×Shen137parents, respectively | GP, GI, SL, SVI, MGT | linkage analysis | 5 mQTLs | none | [56] |
| 2016 | from seeds to 2-leaf-stage seedlings (two panels of 306 dent and 292 flint maize inbred lines) | 14/8 °C day/night | SNP | 49,585 | DTE, SPAD in the second leaf,ΦPSII, EV | association analysis | 9 QTLs | 36 candidate genes: GRMZM2G061206, GRMZM2G061127, GRMZM2G174274, GRMZM2G174249, GRMZM2G174221, GRMZM2G174196, GRMZM2G174137, GRMZM2G074241, GRMZM2G375807, GRMZM2G419024, GRMZM5G899800, GRMZM2G416069, GRMZM2G115730, GRMZM2G115750, GRMZM2G130043, GRMZM2G130002, GRMZM2G129979, GRMZM2G178398, GRMZM2G172244, GRMZM2G171420, GRMZM2G171394, GRMZM2G078143, GRMZM2G084825, GRMZM2G154216, GRMZM2G341036, GRMZM2G102862, GRMZM2G405090, GRMZM2G127510, GRMZM2G127499, GRMZM2G429396, GRMZM2G124794, GRMZM2G423478, GRMZM2G180027, GRMZM2G480480, GRMZM2G180080 and GRMZM2G180082 | [57] |
| 2017 | seed (282 inbred lines) | 8 °C (chilling) and 25 °C (normal) | SNP | 2 × 106 | GR, DT50, GI, RGR, RDT50, RGI | association analysis | 17 genetic loci | 18 candidate genes: GRMZM2G704005, GRMZM2G113158, GRMZM2G318156, GRMZM2G012148, GRMZM2G300994, GRMZM5G871707, GRMZM2G462797, GRMZM2G178486, GRMZM5G806387, GRMZM2G148793, GRMZM2G389768, GRMZM2G073535, GRMZM5G802338, GRMZM2G057186, GRMZM2G081928, GRMZM2G019746, GRMZM2G033884 and GRMZM2G170890 | [3] |
| 2017 | root of post-germination (four cultivars: chilling-tolerant Picker and PR39B29, chilling-sensitive Fergus and Codisco) | germination for 1, 2, 3, 4 and5 days | oligo array | 46,000 | growth ratio | microarray analysis | MZ00004486 | [58] | |
| 2017 | 3-leaf-stage seedlings (338 testcrosses and an F2:3 population) | 5.5–6.5 °C for 7 days | SNP and SSR | 556,809 SNP markers for association analysis, 152 SSR markers for linkage map construction | LRD, WCS, RRS, SSC | association analysis and linkage analysis | 32 significant loci for association analysis,7 QTL for linkage analysis | 36 stress tolerance-related candidate genes: GRMZM2G460383, GRMZM2G363229, GRMZM2G082097, GRMZM2G032209, GRMZM2G110242, GRMZM2G159756, GRMZM2G470984, GRMZM2G053384, GRMZM2G000936, GRMZM2G035807, GRMZM2G102927, GRMZM2G102811, GRMZM2G457267, GRMZM2G332258, GRMZM2G110085, GRMZM2G058518, GRMZM2G437460, GRMZM2G580389, GRMZM2G463462, GRMZM2G403609, GRMZM2G132882, GRMZM2G111696, GRMZM2G411288, GRMZM2G019986, GRMZM2G407825, GRMZM2G107481, GRMZM2G051917, GRMZM2G053206, GRMZM2G092327, GRMZM2G138161, GRMZM2G348512, GRMZM2G121878, GRMZM2G027098, GRMZM2G012479, GRMZM2G000404 and GRMZM2G395535 | [59] |
| 2020 | seed (222 diverse inbred lines) | 10 °C for 31 days, at 3-day intervals | SNP | 40,697 | RGR, RGL, RRL, RRSA, RRV, RGI, RVI, RSVI, XYRGR, XYRGL, XYRSVI, KSRGR, KSRGL, KSRSVI | association analysis and RNA-seq | 30 significant SNPs | 82 candidate genes associated with significant SNPs; Zm00001d039219 and Zm00001d034319 were further identified by RNA-seq | [60] |
| 2020 | 4-leaf-stage seedlings (Zea mays L. hybrid ADA313) | 25/4 °C day/night for 2 days | miRNAs | 321 | LL, LA4, LER, Clma, CLme, Length of the cell at the end of meristem, P, D, Tc, Lmer, Lgr, Lel, Nmer, Ngz, Nel, Tel, Rel | microarray analysis | miR408, miR528 and target genes of miR319 and miR396 | [61] | |
| 2021 | seed (300 inbred lines) | 10 °C for 10 days | SNP | 43,943 | FG, TG, RL, SL, RRS | association analysis, candidate gene association study and expression pattern analysis | 15 significant SNPs | Zm00001d010454, Zm00001d010458, Zm00001d010459 and Zm00001d050021 | [62] |
| 2021 | from seed to 2-leaf-stage seedlings (836 maize inbreeds) | 14/10 °C day/night | SNP | 156,164 | DTE, DTSL, EV, SPAD, Fv/Fm, DW | association analysis | 159 QTLs | 226 candidate genes | [63] |
| 2021 | 14-day-old seedlings (97 RILs of IBM Syn4 derived from B73 × Mo17) | 4 °C for 8 h | RFLP and SSR | over 1850 | chlorophyll concentration, leaf color, tissue damage | linkage analysis and RNA-seq | 2 QTLs | 27 candidate genes: GRMZM2G003506, GRMZM2G331652, GRMZM2G331638, GRMZM2G155242, GRMZM2G107774, GRMZM2G103079, GRMZM2G395121, GRMZM2G094444, GRMZM2G098714, GRMZM2G173067, GRMZM2G163043, GRMZM2G095382, GRMZM2G014560, GRMZM2G096753, GRMZM2G170692, GRMZM2G153488, GRMZM2G153359, GRMZM2G153263, GRMZM2G098474, GRMZM2G165290, GRMZM2G175177, GRMZM2G159904, GRMZM5G841914, GRMZM2G178603, GRMZM2G178509, GRMZM2G178497 and GRMZM2G139837 | [64] |
| 2022 | seed (176 B73 × Mo17 (IBM) Syn10 doubled haploid (DH) population) | 10 °C for 21 days | SNP | 6618 | LTPL, LTSL, LTRL, LTGR, LTGI, LTVI, LTSVI, LTAGD | linkage analysis and RNA-seq | 7 QTLs | 6 candidate genes: Zm00001d043166, Zm00001d007315, Zm00001d027974, Zm00001d027976, Zm00001d007311 and Zm00001d053703 | [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Tan, R.; Zhao, J. Chilling Tolerance in Maize: Insights into Advances—Toward Physio-Biochemical Responses’ and QTL/Genes’ Identification. Plants 2022, 11, 2082. https://doi.org/10.3390/plants11162082
Ma Y, Tan R, Zhao J. Chilling Tolerance in Maize: Insights into Advances—Toward Physio-Biochemical Responses’ and QTL/Genes’ Identification. Plants. 2022; 11(16):2082. https://doi.org/10.3390/plants11162082
Chicago/Turabian StyleMa, Yun, Renxiang Tan, and Jiuran Zhao. 2022. "Chilling Tolerance in Maize: Insights into Advances—Toward Physio-Biochemical Responses’ and QTL/Genes’ Identification" Plants 11, no. 16: 2082. https://doi.org/10.3390/plants11162082






