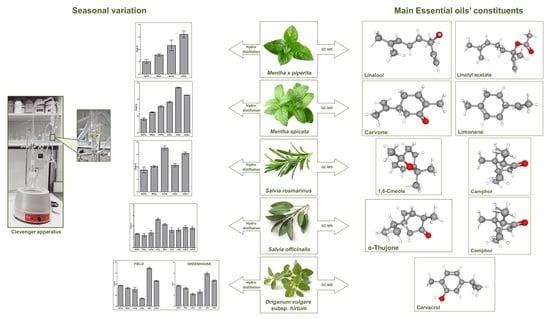
Seasonal Variation of Aromatic Plants under Cultivation Conditions
Abstract
:1. Introduction
2. Results
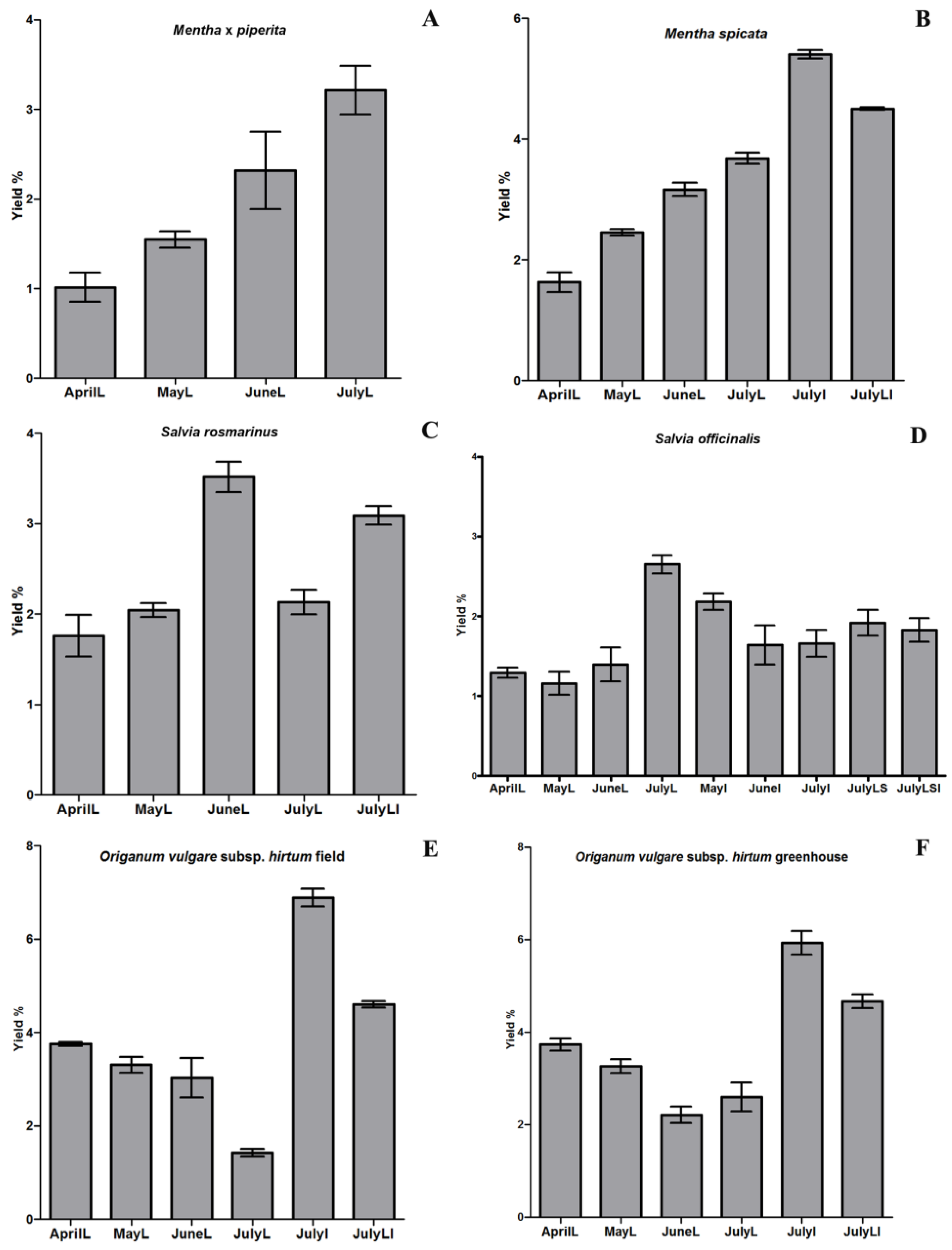
2.1. Yield of Essential Oils
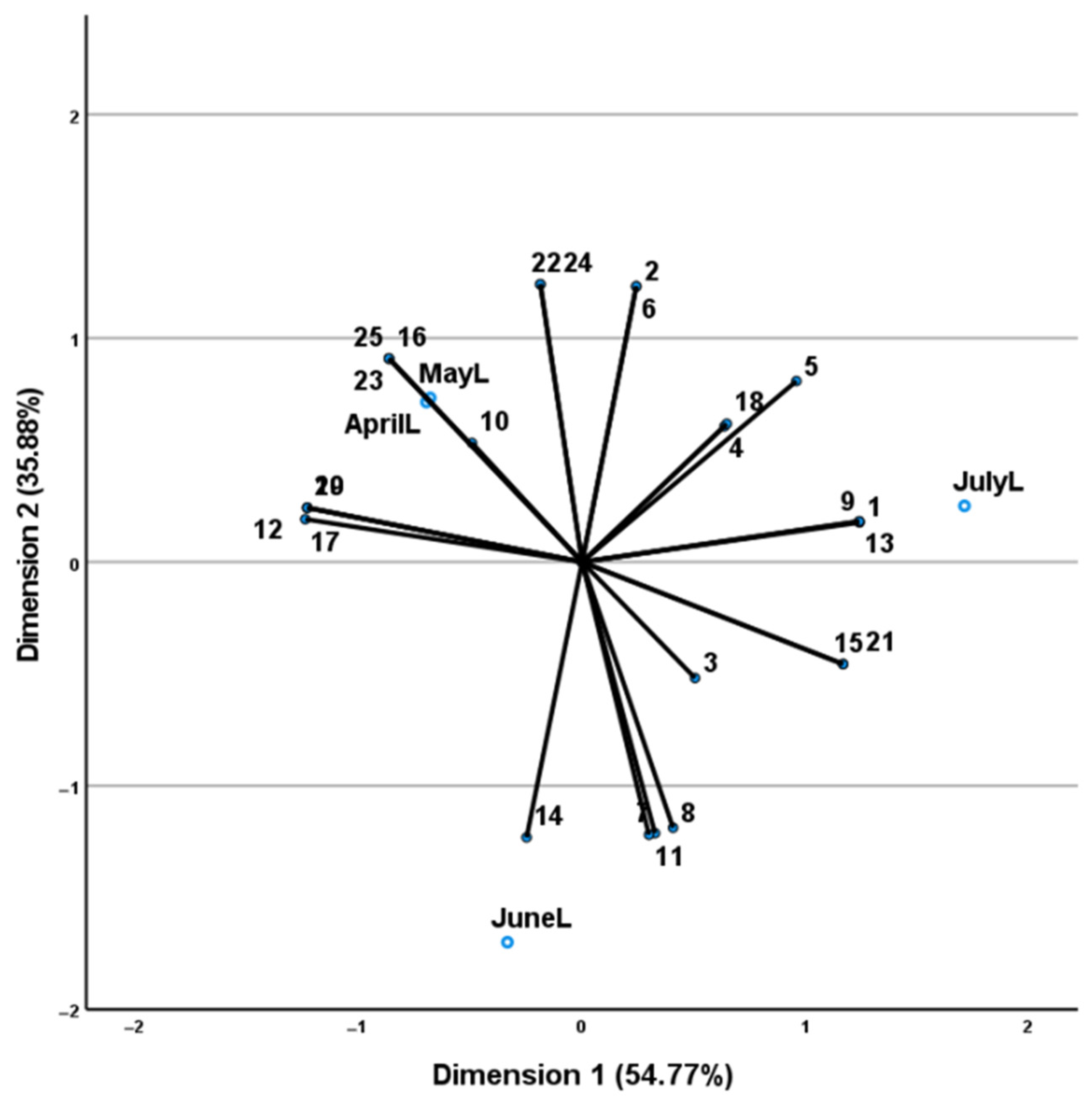
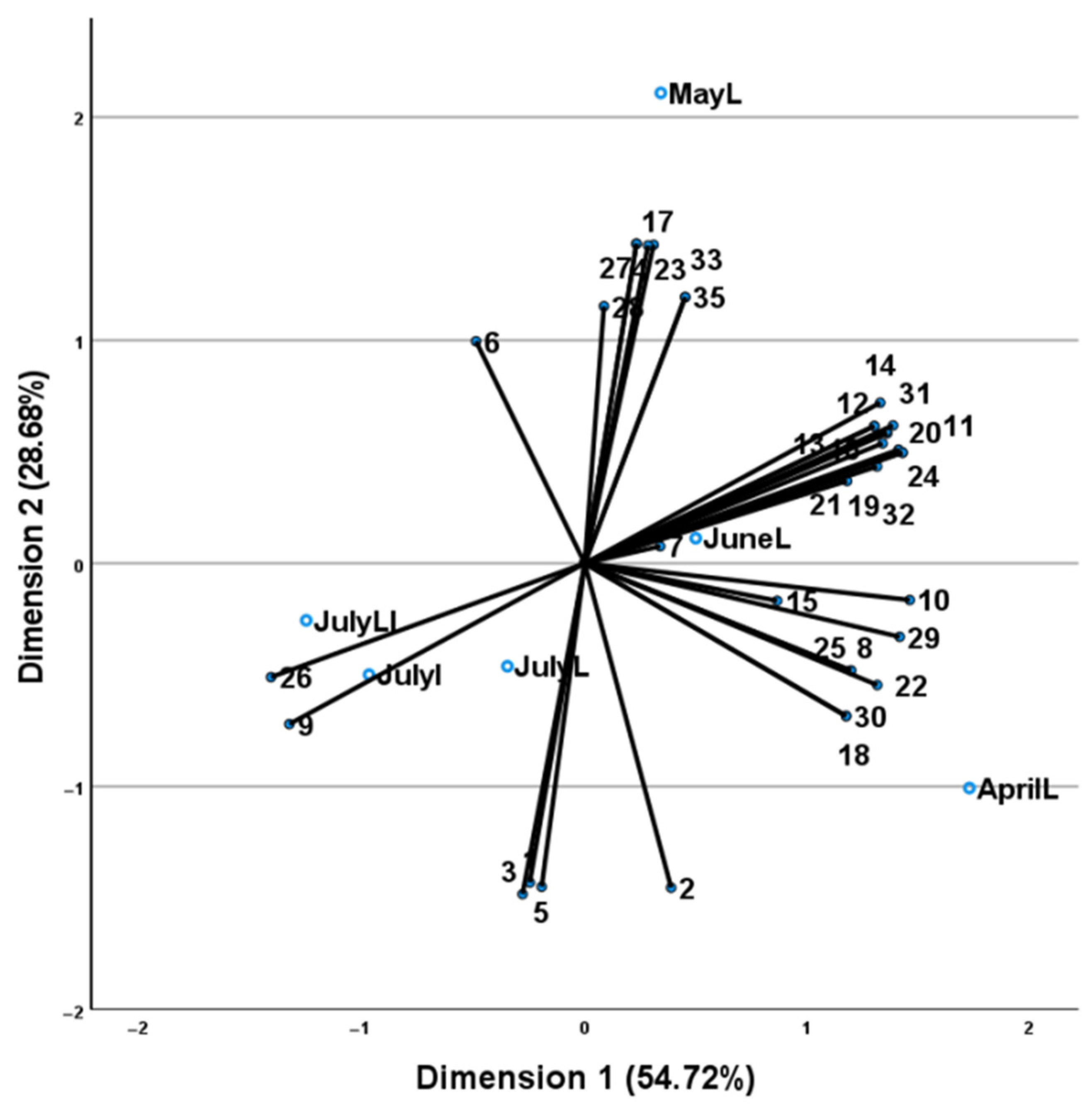
2.2. Volatile Oil Yield and Composition
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Growing Conditions
4.3. Extraction and Isolation
4.4. Analysis of Volatile Components
4.4.1. GC–MS Analysis
4.4.2. Identification of Volatile Components
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanias, G.D.; Souleles, C.; Loukis, A.; Philotheou-Panou, E. Trace elements and essential oil composition in chemotypes of the aromatic plant Origanum vulgare. J. Radioanal. Nucl. Chem. Artic. 1998, 227, 23–31. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Zhiri, A.; Idaomar, M. Cytotoxicity and gene induction by some essential oils in the yeast Saccharomyces cerevisiae. Mutat. Res. Toxicol. Environ. Mutagen. 2005, 585, 13. [Google Scholar] [CrossRef] [PubMed]
- Stefanakis, M.K.; Touloupakis, E.; Anastassopoulos, E.; Ghanotakis, D.; Katerinopoulos, H.E.; Makridis, P. Antibacterial activity of essential oils from plants of the genus Origanum. Food Control 2013, 34, 539–546. [Google Scholar] [CrossRef]
- Morshedloo, M.R.; Salami, S.A.; Nazeri, V.; Maggi, F.; Craker, L. Essential oil profile of oregano (Origanum vulgare L.) populations grown under similar soil and climate conditions. Ind. Crops Prod. 2018, 119, 183–190. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Maarse, H. Volatile oil of Origanum vulgare subsp. vulgare L. III. Changes in composition during maturation. Flavour Ind. 1974, 4, 278–281. [Google Scholar]
- Papageorgiou, V.; Gardeli, C.; Mallouchos, A.; Papaioannou, M.; Komaitis, M. Variation of the Chemical Profile and Antioxidant Behavior of Rosmarinus officinalis L. and Salvia fruticosa Miller Grown in Greece. J. Agric. Food Chem. 2008, 56, 7254–7264. [Google Scholar] [CrossRef]
- Baranauskienė, R.; Venskutonis, P.R.; Dambrauskienė, E.; Viškelis, P. Harvesting time influences the yield and oil composition of Origanum vulgare L. ssp. vulgare and ssp. hirtum. Ind. Crops Prod. 2013, 49, 43–51. [Google Scholar] [CrossRef]
- Ozkan, G.; Baydar, H.; Erbas, S. The influence of harvest time on essential oil composition, phenolic constituents and antioxidant properties of Turkish oregano (Origanum onites L.). J. Sci. Food Agric. 2010, 90, 205–209. [Google Scholar] [CrossRef]
- Russo, A.; Formisano, C.; Rigano, D.; Senatore, F.; Delfine, S.; Cardile, V.; Rosselli, S.; Bruno, M. Chemical composition and anticancer activity of essential oils of Mediterranean sage (Salvia officinalis L.) grown in different environmental conditions. Food Chem. Toxicol. 2013, 55, 42–47. [Google Scholar] [CrossRef]
- Food and Drug Administration. GRAS Notifications. 2005. Available online: http://www.fda.gov (accessed on 20 December 2021).
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Kintzios, S.E. Profile of the multifaced prince of the herbs. In Oregano: The genera Origanum and Lippia, 1st ed.; Kintzios, S.E., Ed.; Taylor & Francis: London, UK, 2002; Volume 25, pp. 3–8. [Google Scholar]
- Marrelli, M.; Statti, G.A.; Conforti, F. Origanum spp.: An update of their chemical and biological profiles. Phytochem. Rev. 2018, 17, 873–888. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Tomic, G.; Nikolić, I.; Nerantzaki, A.A.; Sayyad, N.; Stosic-Grujicic, S.; Stojanović, I.; Gerothanassis, I.P.; Tzakos, A.G. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013, 136, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Lang, G.; Buchbauer, G. A review on recent research results (2008–2010) on essential oils as antimicrobials and antifungals. A review. Flavour Fragr. J. 2011, 27, 13–39. [Google Scholar] [CrossRef]
- Vale-Silva, L.; Silva, M.-J.; Oliveira, D.; Gonçalves, M.-J.; Cavaleiro, C.; Salgueiro, L.; Pinto, E. Correlation of the chemical composition of essential oils from Origanum vulgare subsp. virens with their in vitro activity against pathogenic yeasts and filamentous fungi. J. Med. Microbiol. 2012, 61, 252–260. [Google Scholar] [CrossRef]
- Pilau, M.R.; Alves, S.H.; Weiblen, R.; Arenhart, S.; Cueto, A.P.; Lovato, L.T. Antiviral activity of the Lippia graveolens (Mexican oregano) essential oil and its main compound carvacrol against human and animal viruses. Braz. J. Microbiol. 2011, 42, 1616–1624. [Google Scholar] [CrossRef] [Green Version]
- Skoula, M.; Gotsiou, P.; Naxakis, G.; Johnson, C.B. A chemosystematic investigation on the mono- and sesquiterpenoids in the genus Origanum (Labiatae). Phytochemistry 1999, 52, 649–657. [Google Scholar] [CrossRef]
- Kelley, L.E.; Cadwallader, K.R. Identification and Quantitation of Potent Odorants in Spearmint Oils. J. Agric. Food Chem. 2017, 66, 2414–2421. [Google Scholar] [CrossRef]
- Kokkini, S.; Vokou, D. Carvacrol-rich plants in Greece. Flavour Frag. J. 1989, 4, 1–7. [Google Scholar] [CrossRef]
- Matraka, M.; Ninou, E.; Giannakoula, A.; Lazari, D.; Panou-Filotheou, H.; Bosabalidis, A.M. Effects of soil water content on Mentha spicata L. and Origanum dictamnus L. Isr. J. Plant Sci. 2010, 58, 229–239. [Google Scholar] [CrossRef]
- Kofidis, G.; Bosabalidis, A.; Kokkini, S. Seasonal Variation of Essential Oils in a Linalool-Rich Chemotype of Mentha spicata Grown Wild in Greece. J. Essent. Oil Res. 2004, 16, 469–472. [Google Scholar] [CrossRef]
- Dellacassa, E.; Lorenzo, D.; Moyna, P.; Frizzo, C.D.; Serafini, L.A.; Dugo, P. Rosmarinus officinalis L. (Labiatae) Essential Oils from the South of Brazil and Uruguay. J. Essent. Oil Res. 1999, 11, 27–30. [Google Scholar] [CrossRef]
- Tursun, A.O. Original article Impact of soil types on chemical composition of essential oil of purple basil. Saudi J. Biol. Sci. 2022, 29, 103314. [Google Scholar] [CrossRef]
- Lawrence, B.M. A Study of the Monoterpene Interrelationships in the Genus Mentha with Special Reference to the Origin of Pulegone and Menthofuran; Print Three Inc.: Oakville, ON, Canada, 1978. [Google Scholar]
- Shelepova, O.V.; Dilovarova, T.A.; Gulevich, A.A.; Olekhnovich, L.S.; Shirokova, A.V.; Ushakova, I.T.; Zhuravleva, E.V.; Konovalova, L.N.; Baranova, E.N. Chemical Components and Biological Activities of Essential Oils of Mentha × piperita L. from Field-Grown and Field-Acclimated after In Vitro Propagation Plants. Agronomy 2021, 11, 2314. [Google Scholar] [CrossRef]
- Giménez-Santamarina, S.; Llorens-Molina, J.A.; Sempere-Ferre, F.; Santamarina, C.; Roselló, J.; Santamarina, M.P. Chemical composition of essential oils of three Mentha species and their antifungal activity against selected phytopathogenic and post-harvest fungi. All Life 2022, 15, 64–73. [Google Scholar] [CrossRef]
- Jedrzejczyk, I.; Rewers, M. Genome size and ISSR markers for Mentha L. (Lamiaceae) genetic diversity assessment and species identification. Ind. Crops Prod. 2018, 120, 171–179. [Google Scholar] [CrossRef]
- Gobert, V.; Moja, S.; Colson, M.; Taberlet, P. Hybridization in the section Mentha (Lamiaceae) inferred from AFLP markers. Am. J. Bot. 2002, 98, 2017–2023. [Google Scholar] [CrossRef] [Green Version]
- Djilani, A.; Dicko, A. The Therapeutic Benefits of Essential Oils. In Nutrition, Well-Being and Health; Bouayed, J., Bohn, T., Eds.; IntechOpen: London, UK, 2012. [Google Scholar]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharopov, F.; Antolak, H.; Kręgiel, D.; Sen, S.; Sharifi-Rad, M.; Acharya, K.; Sharifi-Rad, R.; et al. Plants of Genus Mentha: From Farm to Food Factory. Plants 2018, 7, 70. [Google Scholar] [CrossRef] [Green Version]
- Kokkini, S.; Karousou, R.; Hanlidou, E. HERBS|Herbs of the Labiatae, Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 3082–3090. [Google Scholar]
- Goudjil, M.; Segni, L.; Souad, Z.; Hammoya, F.; Messaoud Bachagha, B.; Mehani, M.; Bencheikh, S.E. Bioactivity of Laurus Nobilis and Mentha piperita essential oils on some phytopathogenic fungi (in vitro assay). J. Mater. Environ. Sci. 2016, 7, 4525–4533. [Google Scholar]
- Ludwiczuk, A.; Kiełtyka-Dadasiewicz, A.; Sawicki, R.; Golus, J.; Ginalska, G. Essential Oils of some Mentha Species and Cultivars, their Chemistry and Bacteriostatic Activity. Nat. Prod. Commun. 2016, 11, 1015–1018. [Google Scholar] [CrossRef] [Green Version]
- Satmi, F.R.S.; Hossain, M.A. In vitro antimicrobial potential of crude extracts and chemical compositions of essential oils of leaves of Mentha piperita L native to the Sultanate of Oman. Pac. Sci. Rev. A Nat. Sci. Eng. 2016, 18, 103–106. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Hameed, E.-S.S.; Salman, M.S.; Fadl, M.A.; Elkhateeb, A.; El-Awady, M.A. Chemical Composition of Hydrodistillation and Solvent free Microwave Extraction of Essential Oils from Mentha piperita L. Growing in Taif, Kingdom of Saudi Arabia, and Their Anticancer and Antimicrobial Activity. Orient. J. Chem. 2018, 34, 222–233. [Google Scholar] [CrossRef]
- Sartoratto, A.; Machado, A.L.M.; Delarmelina, C.; Figueira, G.M.; Duarte, M.C.T.; Rehder, V.L.G. Composition and antimicrobial activity of essential oils from aromatic plants used in Brazil Brazilian. J. Microbiol. 2004, 35, 275–280. [Google Scholar]
- Gracindo, L.A.M.B.; Grisi, M.C.M.; Silva, D.B.; Alves, R.B.N.; Bizzo, H.R.; Vieira, R.F. Chemical characterization of mint (Mentha spp.) germplasm at Federal District, Brazil. Rev. Brasil. Plant. Med. 2006, 8, 5–9. [Google Scholar]
- Garlet, T.M.B.; Paulus, D.; Flores, R. Production and chemical composition of Mentha × piperita var. citrata (Ehrh.) Briq. essential oil regarding to different potassium concentrations in the hydroponic solution. J. Biotechnol. Biodivers. 2013, 4, 200–206. [Google Scholar] [CrossRef]
- Deschamps, C.; Castro, L.; Machado, M.; Scheer, A.; Côcco, L. Development, essential oil yield and composition of mint species and chemotypes under different radiation and nitrogen levels. Biosci. J. 2014, 30, 730–736. [Google Scholar]
- El Asbahani, A.; Jilale, A.; Voisin, S.N.; Aït Addi, E.H.; Casabianca, H.; El Mousadik, A.; Hartmann, D.J.; Renaud, F.N. Chemical composition and antimicrobial activity of nine essential oils obtained by steam distillation of plants from the souss-massa region (Morocco). J. Essent. Oil Res. 2015, 27, 34–44. [Google Scholar] [CrossRef]
- Da Silva Ramos, R.; Rodrigues, A.B.L.; Farias, A.L.F.; Simões, R.C.; Pinheiro, M.T.; Dos Anjos Ferreira, M.; Barbosa, L.M.C.; Souto, R.N.P.; Fernandes, J.B.; Santos, L.D.S.; et al. Chemical Composition and In Vitro Antioxidant, Cytotoxic, Antimicrobial, and Larvicidal Activities of the Essential Oil of Mentha piperita L. (Lamiaceae). Sci. World J. 2017, 2017, 4927214. [Google Scholar] [CrossRef] [Green Version]
- Soilhi, Z.; Rhimi, A.; Heuskin, S.; Fauconnier, M.L.; Mekki, M. Essential oil chemical diversity of Tunisian Mentha spp. collection. Ind. Crops Prod. 2019, 131, 330–340. [Google Scholar] [CrossRef] [Green Version]
- Božović, M.; Pirolli, A.; Ragno, R. Mentha suaveolens Ehrh. (Lamiaceae) Essential Oil and Its Main Constituent Piperitenone Oxide: Biological Activities and Chemistry. Molecules 2015, 20, 8605–8633. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi Pirbalouti, A.G.; Mohammadi, M. Phytochemical composition of the essential oil of different populations of Stachys avandulifolia Vahl. Asian Pac. J. Trop. Biomed. 2013, 3, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Ali-Shtayeh, M.S.; Jamous, R.M.; Abu-Zaitoun, S.Y.; Khasati, A.I.; Kalbouneh, S.R. Biological Properties and Bioactive Components of Mentha spicata L. Essential Oil: Focus on Potential Benefits in the Treatment of Obesity, Alzheimer’s Disease, Dermatophytosis, and Drug-Resistant Infections. Evid. Based Complement. Altern. Med. 2019, 2019, 3834265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahboubi, M. Mentha spicata L. essential oil, phytochemistry and its effectiveness in flatulence. J. Tradit. Complement. Med. 2018, 11, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Kokkini, S.; Karousou, R.; Lanaras, T. Essential oil of spearmint (Carvone-rich) plants from the island of Crete (Greece). Biochem. Syst. Ecol. 1995, 23, 425–430. [Google Scholar] [CrossRef]
- Snoussi, M.; Noumi, E.; Trabelsi, N.; Flamini, G.; Papetti, A.; De Feo, V. Mentha spicata Essential Oil: Chemical Composition, Antioxidant and Antibacterial Activities against Planktonic and Biofilm Cultures of Vibrio spp. Strains. Molecules 2015, 20, 14402–14424. [Google Scholar] [CrossRef]
- Hassanzadeh, K.; Aliniaeifard, S.; Farzinia, M.; Ahmadi, M. Effect of Phenological Stages on Essential Oil Content, Composition and Rosmarinic Acid in Rosmarinus officinalis L. Int. J. Hortic. Sci. Technol. 2017, 4, 251–258. [Google Scholar] [CrossRef]
- Melito, S.; Petretto, G.L.; Chahine, S.; Pintore, G.; Chessa, M. Seasonal Variation of Essential Oil in Rosmarinus officinalis Leaves in Sardinia. Nat. Prod. Commun. 2019, 14, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pistelli, L.; Giovanelli, S.; D’Angiolillo, F.; Karkleva, K.; Leonardi, M.; Ambryszewska, K.; Cervelli, C.; Pistelli, L. Antioxidant Activity of Several Essential Oils from Different Rosmarinus officinalis Cultivars Grown in Sanremo (Italy). Nat. Prod. Commun. 2018, 13, 1167–1170. [Google Scholar] [CrossRef] [Green Version]
- Verma, R.S.; Padalia, R.C.; Chauhan, A.; Upadhyay, R.K.; Singh, V.R. Productivity and essential oil composition of rosemary (Rosmarinus officinalis L.) harvested at different growth stages under the subtropical region of north India. J. Essent. Oil Res. 2019, 32, 144–149. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Evangelides, E.; Tzortzakis, N. Seasonal Variation of Antioxidant Capacity, Phenols, Minerals and Essential Oil Components of Sage, Spearmint and Sideritis Plants Grown at Different Altitudes. Agronomy 2021, 11, 1766. [Google Scholar] [CrossRef]
- Zaouali, Y.; Bouzaine, T.; Boussaid, M. Essential oils composition in two Rosmarinus officinalis L. varieties and incidence for antimicrobial and antioxidant activities. Food Chem. Toxicol. 2010, 48, 3144–3152. [Google Scholar] [CrossRef] [PubMed]
- Jordán, M.J.; Lax, V.; Rota, M.C.; Lorán, S.; Sotomayor, J.A. Effect of bioclimatic area on the essential oil composition and antibacterial activity of Rosmarinus officinalis L. Food Control 2013, 30, 463–468. [Google Scholar] [CrossRef]
- Omer, E.; Hendawy, S.; Elgendy, A.N.; Mannu, A.; Petretto, G.L.; Pintore, G. Effect of irrigation systems and soil conditioners on the growth and essential oil composition of Rosmarinus officinalis L. Cultivated in Egypt. Sustainability 2020, 12, 6611. [Google Scholar] [CrossRef]
- Afshar, M.; Najafian, S.; Radi, M. The effect of harvest time on the natural product of Rosmarinus officinalis L. from South Iran (Fars province). Nat. Prod. Res. 2021, 36, 2637–2642. [Google Scholar] [CrossRef] [PubMed]
- Arraiza, M.P.; Arrabal, C.; Lopez, J.V. Seasonal Variation of Essential Oil Yield and Composition of Sage (Salvia officinalis L.) Grown in Castilla—La Mancha (Central Spain). Not. Bot. Horti Agrobot. 2012, 40, 106–108. [Google Scholar] [CrossRef] [Green Version]
- Santos-Gomes, P.C.; Fernandes-Ferreira, M. Organ and season dependent variation in the essential oil composition of Salvia officinalis L. cultivated at two different sites. J. Agric. Food Chem. 2001, 49, 2908–2916. [Google Scholar] [CrossRef]
- Raal, A.; Orav, A.; Arak, E. Composition of the essential oil of Salvia officinalis L. from various European countries. Nat. Prod. Res. 2007, 21, 406–411. [Google Scholar] [CrossRef]
- Verma, R.S.; Padalia, R.C.; Chauhan, A. Harvesting season and plant part dependent variations in the essential oil composition of Salvia officinalis L. grown in northern India. J. Herb. Med. 2015, 5, 165–171. [Google Scholar] [CrossRef]
- Węglarz, Z.; Kosakowska, O.; Przybył, J.L.; Pióro-Jabrucka, E.; Baczek, K. The Quality of Greek Oregano (O. vulgare L. subsp. hirtum (Link) Ietswaart) and Common Oregano (O. vulgare L. subsp. vulgare) Cultivated in the Temperate Climate of Central Europe. Foods 2020, 9, 1671. [Google Scholar] [CrossRef]
- Papaioannou, C.; Stefanakis, M.K.; Batargias, C.; Kilias, G.; Anastasopoulos, E.; Katerinopoulos, H.E.; Papasotiropoulos, V. Genetic Profiling and Volatile Oil Content of Oregano Genotypes from Greece. Rev. Bras. Farmacogn. 2020, 30, 295–300. [Google Scholar] [CrossRef]
- van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology (NIST). 2017. Available online: http://webbook.nist.gov/chemistry/name-ser.html (accessed on 21 December 2021).
- Massada, Y. Analysis of Essential Oils by Gas Chromatography and Spectrometry; Wiley & Sons: New York, NY, USA, 1976. [Google Scholar]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corp.: Carol Stream, IL, USA, 2007. [Google Scholar]
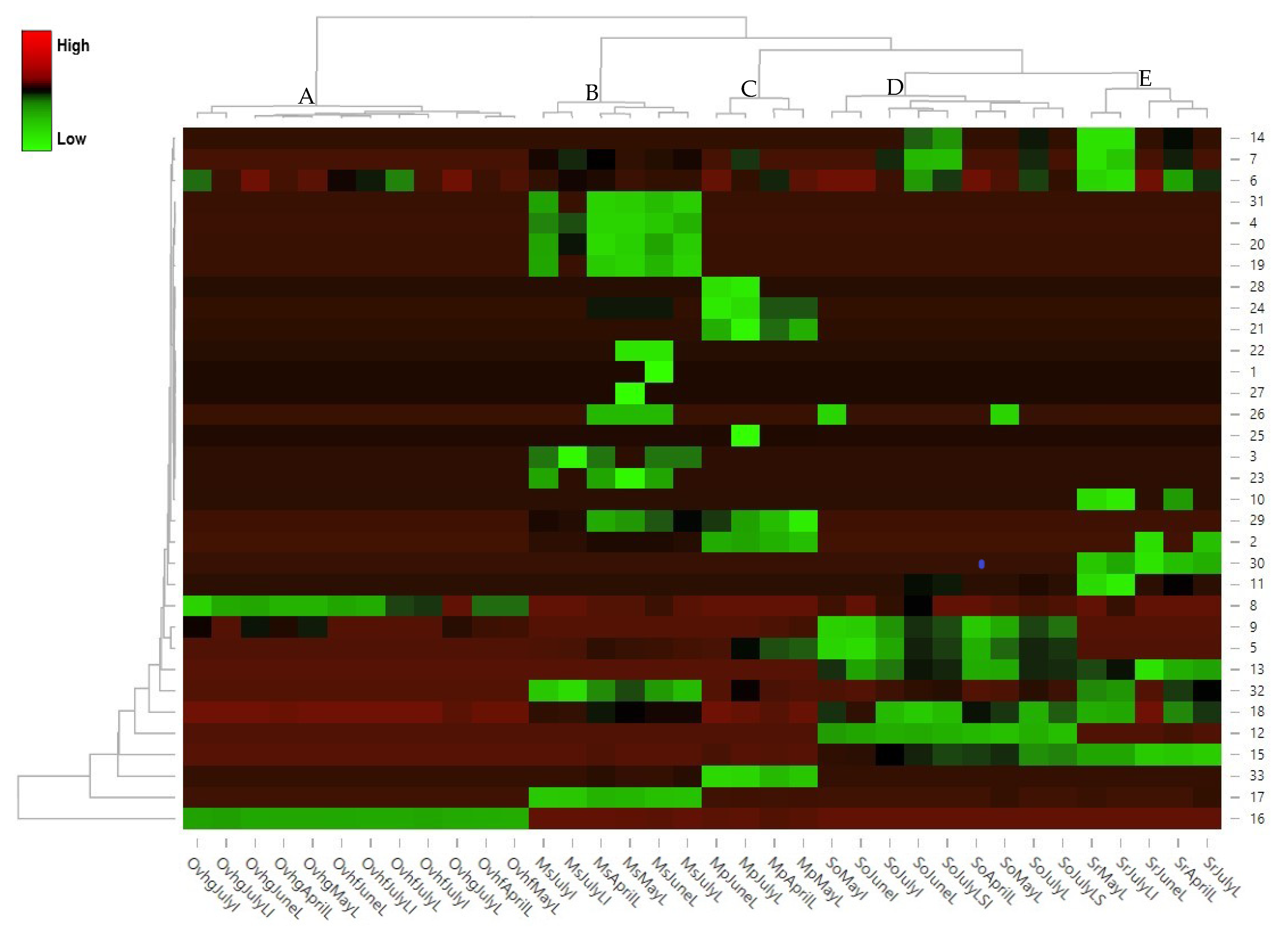
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, 147–153. [Google Scholar] [CrossRef] [PubMed]
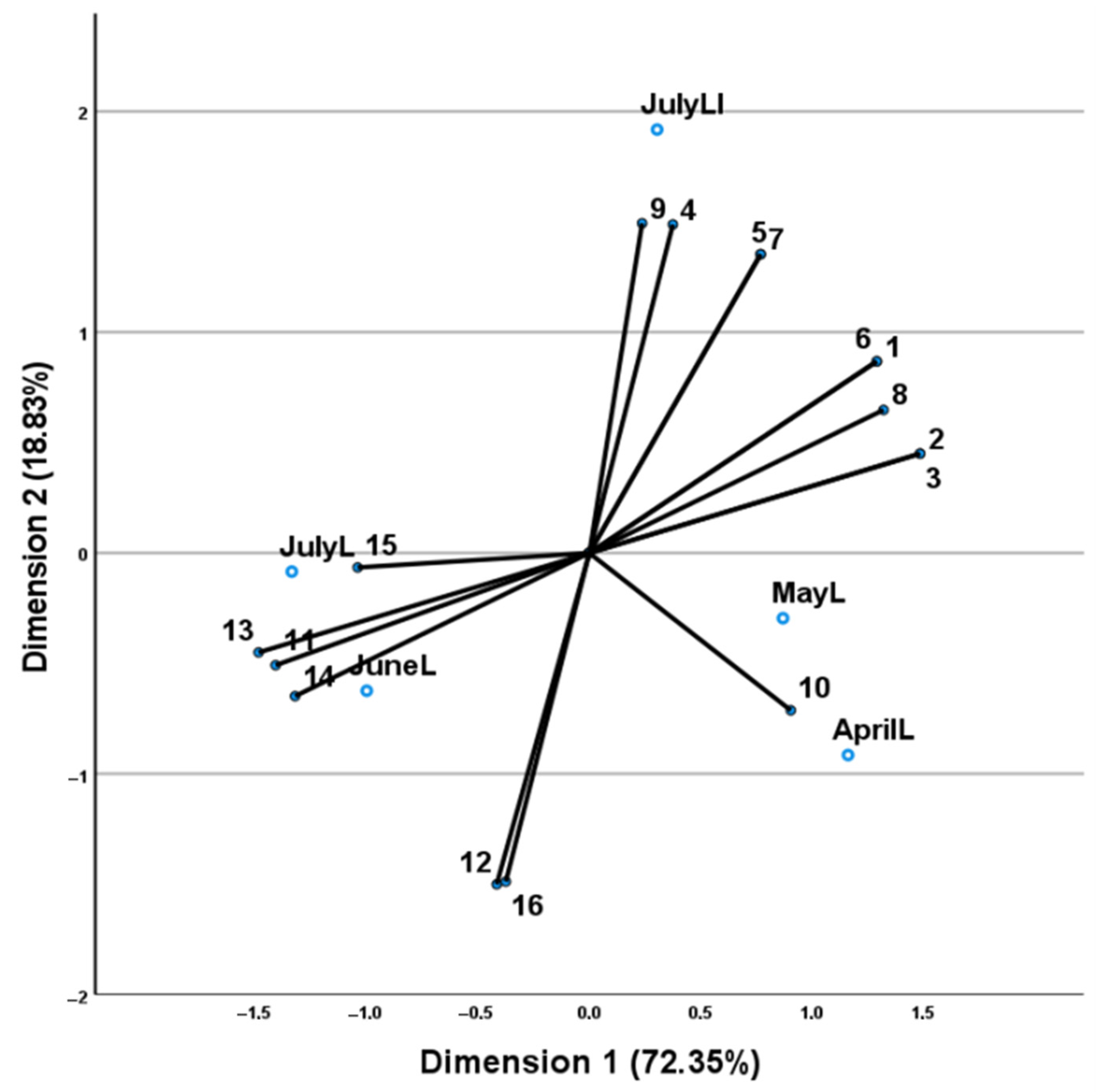

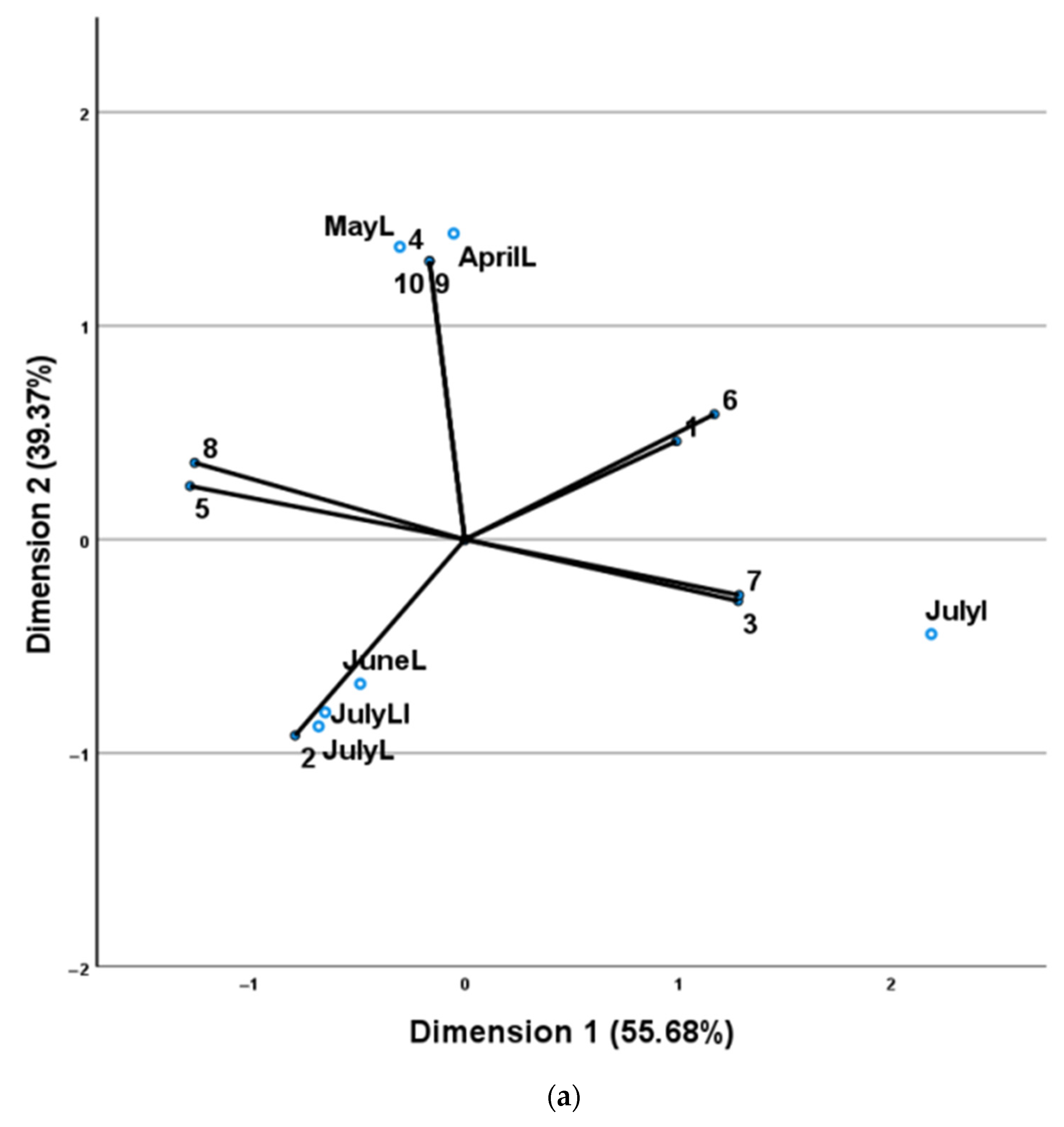
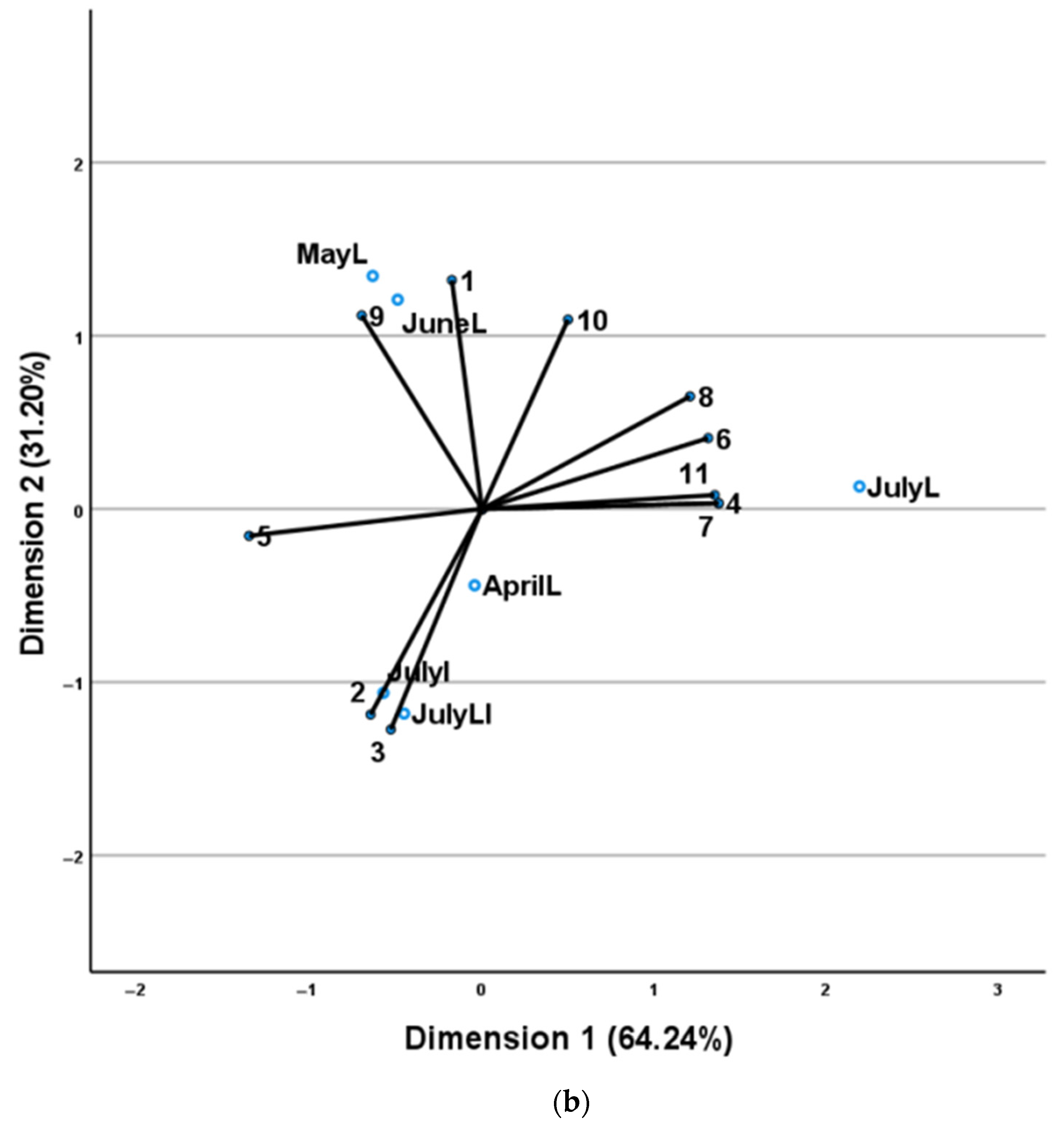
| No | Compounds a | AI b | AI c | AprilL | MayL | JuneL | JulyL |
|---|---|---|---|---|---|---|---|
| 1 | β-Pinene | 973 | 974 | n.d. d | n.d. | n.d. | 0.9 ± 0.12 |
| 2 | β-Myrcene | 990 | 990 | 1.3 ± 0.02 | 0.2 ± 0.00 | 0.1 ± 0.00 | 0.6 ± 0.01 |
| 3 | Octan-3-ol | 995 | 988 | n.d. | 0.1 ± 0.01 | 0.1 ± 0.01 | 0.1 ± 0.01 |
| 4 | p-Cymene | 1022 | 1024 | 0.7 ± 0.10 | 0.1 ± 0.01 | 0.1 ± 0.01 | 0.2 ± 0.02 |
| 5 | Limonene | 1026 | 1029 | 0.3 ± 0.19 | 0.1 ± 0.00 | n.d. | 2.3 ± 0.50 |
| 6 | 1,8-Cineole | 1029 | 1026 | 1.3 ± 0.23 | 0.4 ± 0.15 | n.d. | 0.5 ± 0.30 |
| 7 | (Z)-Ocimene | 1038 | 1037 | 0.6 ± 0.09 | 0.4 ± 0.02 | 1.4 ± 1.25 | 0.7 ± 0.07 |
| 8 | (E)-Ocimene | 1048 | 1044 | 0.2 ± 0.00 | 0.2 ± 0.00 | 1.0 ± 0.04 | 0.8 ± 0.02 |
| 9 | (E)-4-Thujanol | 1065 | 1065 | 0.4 ± 0.15 | 0.7 ± 0.01 | 0.7 ± 0.01 | 2.0 ± 0.98 |
| 10 | Linalool oxide | 1087 | 1084 | n.d. | 0.1 ± 0.00 | n.d. | n.d. |
| 11 | Linalool | 1097 | 1097 | 39.1 ± 2.02 | 46.2 ± 2.13 | 61.1 ± 1.25 | 59.8 ± 1.18 |
| 12 | Octan-3-yl acetate | 1124 | 1120 | 1.9 ± 0.29 | 1.6 ± 0.33 | 1.4 ± 0.43 | 0.8 ± 0.20 |
| 13 | (E)-Pinocarveol | 1135 | 1135 | n.d. | n.d. | n.d. | 0.5 ± 0.00 |
| 14 | Camphor | 1141 | 1141 | 0.4 ± 0.01 | n.d. | 1.6 ± 0.00 | n.d. |
| 15 | (Z)-Pinocamphenone | 1171 | 1172 | 0.3 ± 0.02 | 0.3 ± 0.01 | 0.8 ± 0.03 | 1.7 ± 0.50 |
| 16 | Terpin-4-ol | 1174 | 1174 | 0.2 ± 0.00 | 0.2 ± 0.02 | n.d. | n.d. |
| 17 | α-Terpineol | 1188 | 1186 | 3.5 ± 1.20 | 4.2 ± 1.45 | 3.2 ± 1.00 | 2.8 ± 0.75 |
| 18 | Carvone | 1242 | 1239 | n.d. | 0.4 ± 0.00 | n.d. | 0.4 ± 0.00 |
| 19 | Linalyl acetate | 1255 | 1254 | 32.8 ± 1.20 | 25.0 ± 1.00 | 20.5 ± 1.00 | 15.3 ± 1.35 |
| 20 | Carvacrol | 1299 | 1298 | 5.2 ± 0.08 | 2.9 ± 0.05 | 2.4 ± 0.02 | 1.5 ± 0.30 |
| 21 | Geranyl acetate | 1381 | 1379 | n.d. | n.d. | 1.3 ± 0.25 | 1.5 ± 0.43 |
| 22 | β-Caryophyllene | 1418 | 1417 | 3.4 ± 0.20 | 3.7 ± 1.20 | 0.2 ± 0.00 | 1.8 ± 0.70 |
| 23 | α-Caryophyllene | 1452 | 1454 | 0.2 ± 0.01 | 0.4 ± 0.01 | n.d. | n.d. |
| 24 | Germacrene-D | 1480 | 1484 | 3.3 ± 1.13 | 6.5 ± 2.10 | 1.1 ± 0.07 | 2.1 ± 0.30 |
| 25 | Viridiflorol | 1592 | 1592 | 0.3 ± 0.01 | 0.6 ± 0.02 | n.d. | n.d. |
| Total identified (%) | 95.3 | 94.2 | 97.1 | 96.3 | |||
| Monoterepene hydrocarbons | 3.1 | 1.0 | 2.6 | 5.5 | |||
| Oxygenated monoterpenes | 84.9 | 81.9 | 91.8 | 85.3 | |||
| Sesquiterpene hydrocarbons | 6.9 | 10.6 | 1.3 | 3.9 | |||
| Oxygenated sesquiterpenes | 0.3 | 0.6 | 1.3 | 1.5 | |||
| Others | - | 0.1 | 0.1 | 0.1 | |||
| Essential oil content (mL/100 g dry weight) | 1.01 | 1.55 | 2.32 | 3.22 |
| No | Compounds a | AI b | AI c | AprilL | MayL | JuneL | JulyL | JulyI | JulyLI |
|---|---|---|---|---|---|---|---|---|---|
| 1 | α-Thujene | 931 | 924 | 0.1 ± 0.00 | n.d. d | 0.1 ± 0.00 | 0.1 ± 0.00 | 0.1 ± 0.00 | 0.5 ± 0.01 |
| 2 | Sabinene | 971 | 969 | 0.5 ± 0.01 | 0.2 ± 0.01 | 0.3 ± 0.01 | 0.3 ± 0.01 | 0.3 ± 0.00 | 0.4 ± 0.01 |
| 3 | β-Pinene | 973 | 974 | 0.5 ± 0.01 | 0.2 ± 0.01 | 0.3 ± 0.01 | 0.4 ± 0.01 | 0.4 ± 0.01 | 0.8 ± 0.02 |
| 4 | Oct-1-en-3-ol | 979 | 974 | 0.1 ± 0.00 | 0.2 ± 0.01 | 0.1 ± 0.00 | 0.1 ± 0.00 | n.d. | 0.1 ± 0.00 |
| 5 | β-Myrcene | 990 | 990 | 0.8 ± 0.01 | 0.5 ± 0.02 | 0.6 ± 0.02 | 0.6 ± 0.02 | 0.6 ± 0.02 | 0.9 ± 0.03 |
| 6 | Octan-3-ol | 995 | 988 | 0.5 ± 0.01 | 0.6 ± 0.02 | 0.5 ± 0.02 | 0.4 ± 0.02 | 0.5 ± 0.01 | 0.6 ± 0.02 |
| 7 | α-Terpinene | 1014 | 1014 | n.d. | n.d. | 0.1 ± 0.00 | n.d. | n.d. | n.d. |
| 8 | p-Cymene | 1023 | 1024 | 0.2 ± 0.00 | n.d. | 0.2 ± 0.00 | n.d. | n.d. | n.d. |
| 9 | Limonene | 1026 | 1029 | 6.7 ± 1.25 | 4.7 ± 0.90 | 7.1 ± 1.13 | 10.9 ± 1.00 | 12.8 ± 1.20 | 17.1 ± 1.10 |
| 10 | 1,8-Cineole | 1028 | 1026 | 6.2 ± 1.00 | 5.3 ± 097 | 4.8 ± 0.50 | 4.8 ± 0.82 | 3.4 ± 0.90 | 3.2 ± 0.75 |
| 11 | (Z)-Ocimene | 1038 | 1037 | 0.3 ± 0.01 | 0.3 ± 0.02 | 0.3 ± 0.03 | 0.2 ± 0.00 | 0.1 ± 0.00 | n.d. |
| 12 | (E)-Ocimene | 1048 | 1044 | 0.1 ± 0.00 | 0.1 ± 0.02 | 0.1 ± 0.00 | n.d. | n.d. | n.d. |
| 13 | γ-Terpinene | 1057 | 1054 | 0.3 ± 0.01 | 0.3 ± 0.02 | 0.8 ± 0.05 | 0.2 ± 0.01 | n.d. | n.d. |
| 14 | (Z)-Sabinene hydrate | 1065 | 1065 | 5.1 ± 0.80 | 7.4 ± 0.56 | 4.6 ± 0.20 | 3.1 ± 0.20 | 2.1 ± 0.15 | 0.9 ± 0.20 |
| 15 | Linalool | 1097 | 1097 | 1.6 ± 0.50 | 0.2 ± 0.01 | n.d. | 1.7 ± 0.20 | n.d. | n.d. |
| 16 | (E)-Sabinene hydrate | 1098 | 1098 | 0.2 ± 0.02 | 0.2 ± 0.01 | 0.2 ± 0.02 | n.d. | n.d. | n.d. |
| 17 | Octan-3-yl acetate | 1124 | 1120 | n.d. | 0.1 ± 0.00 | n.d. | n.d. | n.d. | n.d. |
| 18 | Camphor | 1141 | 1141 | 1.0 ± 0.20 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 19 | Terpin-4-ol | 1174 | 1174 | 1.4 ± 0.20 | 1.2 ± 0.20 | 1.0 ± 0.12 | n.d. | 0.4 ± 0.00 | 0.4 ± 0.01 |
| 20 | α-Terpineol | 1188 | 1186 | 0.5 ± 0.03 | 0.5 ± 0.03 | 0.5 ± 0.03 | 0.4 ± 0.02 | 0.3 ± 0.01 | 0.3 ± 0.02 |
| 21 | Dihydrocarveol | 1192 | 1192 | 3.0 ± 0.84 | 3.4 ± 0.55 | 2.2 ± 0.92 | 3.4 ± 0.62 | 1.7 ± 0.98 | n.d. |
| 22 | Dihydrocarvone | 1194 | 1200 | 2.3 ± 0.42 | 2.0 ± 0.38 | 1.0 ± 0.55 | 1.9 ± 0.90 | 1.0 ± 0.36 | 0.3 ± 0.06 |
| 23 | (E)-Carveol | 1216 | 1215 | 0.2 ± 0.01 | 0.7 ± 0.32 | 0.2 ± 0.05 | n.d. | 0.2 ± 0.02 | n.d. |
| 24 | (Z)-Carveol | 1229 | 1226 | 1.3 ± 0.89 | 1.0 ± 0.60 | 0.7 ± 0.22 | 0.5 ± 0.10 | 0.4 ± 0.02 | n.d. |
| 25 | Pulegone | 1237 | 1233 | 0.2 ± 0.01 | n.d. | 0.2 ± 0.01 | n.d. | 0.1 ± 0.00 | n.d. |
| 26 | Carvone | 1242 | 1239 | 54.6 ± 0.00 | 57.1 ± 0.05 | 64.1 ± 0.10 | 65.3 ± 0.22 | 73.0 ± 0.00 | 70.7 ± 0.45 |
| 27 | Geraniol | 1249 | 1249 | n.d. | 0.4 ± 0.02 | n.d. | n.d. | n.d. | n.d. |
| 28 | Carvacrol | 1299 | 1298 | n.d. | 0.8 ± 0.20 | 2.8 ± 1.02 | n.d. | n.d. | 0.1 ± 0.00 |
| 29 | Isodihydrocarveol acetate | 1327 | 1326 | 1.5 ± 0.43 | 1.4 ± 0.92 | 1.1 ± 0.82 | 1.4 ± 0.55 | 0.7 ± 0.12 | n.d. |
| 30 | (Z)-Carveyl acetate | 1361 | 1365 | 1.4 ± 0.30 | 0.7 ± 0.30 | n.d. | n.d. | 0.2 ± 0.05 | n.d. |
| 31 | β-Bourbonene | 1383 | 1387 | 1.8 ± 0.90 | 1.8 ± 0.80 | 1.5 ± 0.82 | 1.1 ± 0.90 | 0.7 ± 0.26 | 0.5 ± 0.10 |
| 32 | β-Caryophyllene | 1418 | 1417 | 0.8 ± 0.10 | 0.6 ± 0.12 | 0.5 ± 0.15 | 0.3 ± 0.12 | 0.1 ± 0.00 | 0.2 ± 0.01 |
| 33 | (E)-β-Farnesene | 1455 | 1454 | n.d. | 0.2 ± 0.00 | 0.2 ± 0.01 | n.d. | n.d. | n.d. |
| 34 | Germacrene-D | 1480 | 1484 | 2.5 ± 0.55 | 2.0 ± 0.48 | 1.4 ± 0.36 | 0.6 ± 0.10 | 0.4 ± 0.08 | 0.3 ± 0.06 |
| 35 | Viridiflorol | 1592 | 1592 | n.d. | 0.5 ± 0.20 | 0.6 ± 0.10 | n.d. | n.d. | n.d. |
| Total identified (%) | 95.7 | 94.6 | 98.1 | 97.7 | 99.5 | 97.3 | |||
| Monoterepene hydrocarbons | 9.4 | 6.2 | 9.8 | 12.8 | 14.3 | 19.7 | |||
| Oxygenated monoterpenes | 80.7 | 82.5 | 83.4 | 82.5 | 83.5 | 76.0 | |||
| Sesquiterpene hydrocarbons | 5.0 | 4.6 | 3.7 | 2.0 | 1.2 | 1.0 | |||
| Oxygenated sesquiterpenes | - | 0.5 | 0.6 | - | - | - | |||
| Others | 0.6 | 0.8 | 0.6 | 0.4 | 0.5 | 0.6 | |||
| Essential oil content (mL/100 g dry weight) | 1.63 | 2.45 | 3.16 | 3.68 | 5.40 | 4.50 |
| No | Compounds a | A.I b | A.I c | AprilL | MayL | JuneL | JulyL | JulyI |
|---|---|---|---|---|---|---|---|---|
| 1 | α-Pinene | 930 | 932 | 0.6 ± 0.00 | 8.7 ± 0.15 | n.d. d | n.d. | 12.0 ± 0.05 |
| 2 | Camphene | 954 | 946 | 0.4 ± 0.01 | 4.5 ± 0.08 | n.d. | n.d. | 4.5 ± 0.02 |
| 3 | β-Pinene | 973 | 974 | 0.7 ± 0.01 | 4.5 ± 0.05 | n.d. | n.d. | 3.0 ± 0.05 |
| 4 | Octan-3-one | 987 | 979 | 3.8 ± 0.80 | 2.4 ± 0.60 | n.d. | 2.9 ± 0.30 | 4.0 ± 0.08 |
| 5 | β-Myrcene | 990 | 990 | 2.5 ± 0.50 | 4.9 ± 0.50 | n.d. | 1.4 ± 0.05 | 5.5 ± 0.20 |
| 6 | α-Phellandrene | 1002 | 1002 | 0.6 ± 0.01 | 1.6 ± 0.05 | n.d. | n.d. | 2.0 ± 0.08 |
| 7 | Limonene | 1026 | 1029 | 4.2 ± 0.95 | 6.6 ± 0.55 | n.d. | 2.5 ± 0.40 | 7.0 ± 0.40 |
| 8 | 1,8-Cineole | 1029 | 1026 | 12.0 ± 1.00 | 15.8 ± 1.10 | n.d. | 7.9 ± 0.15 | 14.6 ± 0.90 |
| 9 | γ-Terpinene | 1059 | 1054 | n.d. | n.d. | n.d. | n.d. | 0.9 ± 0.01 |
| 10 | α-Thujone | 1102 | 1101 | 1.0 ± 0.05 | n.d. | n.d. | n.d. | n.d. |
| 11 | Camphor | 1141 | 1141 | 47.5 ± 1.22 | 29.8 ± 0.98 | 50.4 ± 1.00 | 52.6 ± 0.85 | 30.0 ± 0.28 |
| 12 | Borneol | 1169 | 1165 | 8.6 ± 0.75 | 4.4 ± 0.50 | 17.1 ± 1.02 | 7.1 ± 0.20 | 2.7 ± 0.02 |
| 13 | α-Terpineol | 1188 | 1186 | n.d. | n.d. | 6.6 ± 0.36 | 4.3 ± 0.15 | n.d. |
| 14 | Verbenone | 1205 | 1204 | 3.7 ± 0.24 | 1.7 ± 0.04 | 6.2 ± 0.50 | 5.8 ± 0.40 | 3.0 ± 0.01 |
| 15 | Carvone | 1242 | 1239 | n.d. | 1.6 ± 0.05 | n.d. | 3.1 ± 0.01 | n.d. |
| 16 | Isobornyl acetate | 1288 | 1287 | 5.1 ± 0.60 | 5.8 ± 0.30 | 8.8 ± 0.08 | 4.1 ± 0.08 | 3.6 ± 0.25 |
| Total identified (%) | 90.6 | 92.3 | 89.1 | 91.7 | 92.8 | |||
| Monoterpene hydrocarbons | 9.0 | 30.8 | - | 3.9 | 34.9 | |||
| Oxygenated monoterpenes | 81.6 | 61.5 | 89.1 | 87.8 | 57.9 | |||
| Essential oil content (mL/100 g dry weight) | 1.76 | 2.04 | 3.52 | 2.13 | 3.09 |
| No | Compounds a | A.I. b | A.I. c | AprilL | MayL | JuneL | JulyL | MayI | JuneI | JulyI | JulyLS | JulyLSI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | α-Pinene | 930 | 932 | n.d. d | n.d. | 0.8 ± 0.20 | 0.2 ± 0.01 | n.d. | n.d. | n.d. | n.d. | 1.0 ± 0.00 |
| 2 | Camphene | 954 | 946 | n.d. | n.d. | 1.1 ± 0.02 | 0.5 ± 0.02 | n.d. | n.d. | n.d. | n.d. | 1.5 ± 0.04 |
| 3 | β-Pinene | 973 | 974 | n.d. | n.d. | 2.5 ± 0.92 | 0.8 ± 0.01 | n.d. | n.d. | 0.8 ± 0.02 | n.d. | 2.6 ± 0.80 |
| 4 | β-Myrcene | 990 | 990 | n.d. | 0.3 ± 0.01 | 2.4 ± 0.01 | 1.6 ± 0.36 | n.d. | n.d. | 0.5 ± 0.01 | 0.6 ± 0.01 | 1.5 ± 0.02 |
| 5 | Limonene | 1026 | 1029 | n.d. | 0.2 ± 0.00 | 1.2 ± 0.05 | 1.4 ± 0.10 | 0.2 ± 0.02 | n.d. | 0.7 ± 0.00 | 0.7 ± 0.00 | 1.5 ± 0.04 |
| 6 | 1,8-Cineole | 1029 | 1026 | 6.0 ± 1.10 | 8.0 ± 0.90 | 23.2 ± 0.90 | 16.4 ± 0.65 | 7.7 ± 0.20 | 3.3 ± 1.13 | 18.5 ± 0.74 | 9.9 ± 0.84 | 19.2 ± 0.90 |
| 7 | γ-Terpinene | 1059 | 1054 | n.d. | 0.3 ± 0.01 | 1.9 ± 0.25 | 0.6 ± 0.00 | 0.7 ± 0.00 | n.d. | 1.0 ± 0.05 | 0.4 ± 0.01 | n.d. |
| 8 | (Z)-Sabinene hydrate | 1065 | 1065 | n.d. | 0.5 ± 0.00 | 0.2 ± 0.00 | 0.3 ± 0.01 | n.d. | n.d. | n.d. | 0.3 ± 0.00 | n.d. |
| 9 | (E)-Sabinene hydrate | 1098 | 1098 | n.d. | 0.3 ± 0.01 | n.d. | n.d. | 0.3 ± 0.01 | n.d. | n.d. | n.d. | n.d. |
| 10 | α-Thujone | 1102 | 1101 | 28.4 ± 1.23 | 31.6 ± 0.92 | 23.0 ± 1.56 | 24.8 ± 1.10 | 19.3 ± 1.24 | 21.6 ± 2.02 | 24.5 ± 0.99 | 31.3 ± 2.13 | 25.7 ± 1.00 |
| 11 | β-Thujone | 1112 | 1112 | 4.7 ± 1.00 | 4.9 ± 0.85 | 4.1 ± 0.45 | 4.5 ± 0.30 | 4.6 ± 0.20 | 3.8 ± 0.15 | 4.4 ± 0.08 | 5.9 ± 0.14 | 4.6 ± 0.10 |
| 12 | Camphor | 1141 | 1141 | 16.5 ± 1.20 | 14.3 ± 0.36 | 13.2 ± 0.62 | 25.8 ± 0.84 | 4.9 ± 1.23 | 5.5 ± 0.08 | 9.6 ± 0.33 | 24.5 ± 1.00 | 17.6 ± 0.62 |
| 13 | Borneol | 1169 | 1165 | 8.1 ± 0.05 | 7.8 ± 0.42 | 2.8 ± 0.20 | 3.3 ± 0.05 | 3.6 ± 0.00 | 7.0 ± 0.05 | 5.7 ± 0.45 | 3.5 ± 0.30 | 3.3 ± 0.02 |
| 14 | Carvone | 1242 | 1239 | n.d. | 2.5 ± 0.50 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 15 | Bornyl acetate | 1288 | 1287 | 4.0 ± 0.27 | 4.7 ± 0.50 | 5.0 ± 0.00 | 9.2 ± 0.05 | 22.5 ± 0.02 | 15.5 ± 0.50 | 9.1 ± 0.30 | 10.8 ± 0.55 | 8.2 ± 0.30 |
| 16 | (E)-Cinnamyl alcohol | 1304 | 1303 | n.d. | n.d. | 7.4 ± 0.05 | 1.1 ± 0.02 | n.d. | n.d. | 5.2 ± 0.02 | n.d. | n.d. |
| 17 | β-Caryophyllene | 1418 | 1417 | 6.4 ± 0.20 | 3.9 ± 0.05 | 2.3 ± 0.04 | 2.4 ± 0.01 | 10.7 ± 0.10 | 11.7 ± 0.10 | 5.7 ± 0.02 | 2.8 ± 0.05 | 3.0 ± 0.05 |
| 18 | α-Caryophyllene | 1452 | 1454 | 9.8 ± 0.97 | 6.4 ± 0.55 | 2.9 ± 1.10 | 3.4 ± 0.20 | 10.9 ± 0.09 | 10.3 ± 0.08 | 5.2 ± 0.00 | 4.5 ± 0.05 | 3.6 ± 1.00 |
| 19 | Viridiflorol | 1592 | 1592 | 7.3 ± 0.50 | 9.1 ± 0.30 | 3.5 ± 0.40 | 1.9 ± 0.10 | 8.7 ± 0.10 | 10.8 ± 0.10 | 5.5 ± 0.08 | 2.1 ± 0.10 | 2.5 ± 0.40 |
| Total identified (%) | 91.1 | 94.8 | 97.5 | 98.2 | 94.0 | 89.3 | 96.4 | 97.2 | 95.8 | |||
| Monoterpene hydrocarbons | - | 0.8 | 9.9 | 5.0 | 0.9 | - | 3.0 | 1.7 | 8.1 | |||
| Oxygenated monoterpenes | 67.6 | 74.6 | 78.9 | 85.5 | 62.8 | 56.6 | 77.0 | 86.1 | 78.6 | |||
| Sesquiterpene hydrocarbons | 16.1 | 10.3 | 5.2 | 5.8 | 21.6 | 21.9 | 10.9 | 7.3 | 6.6 | |||
| Oxygenated sesqiterpenes | 7.4 | 9.1 | 3.5 | 1.9 | 8.7 | 10.8 | 5.5 | 2.1 | 2.5 | |||
| Essential oil content (mL/100 g dry weight) | 1.29 | 1.16 | 1.39 | 2.65 | 2.18 | 1.64 | 1.66 | 1.92 | 1.83 |
| No | Compounds a | AI b | AI c | AprilL | MayL | JuneL | JulyL | JulyI | JulyLI |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Octan-3-one | 987 | 979 | 0.6 ± 0.00 | n.d. d | n.d. | n.d. | 0.6 ± 0.00 | n.d. |
| 2 | β-Myrcene | 990 | 990 | 0.5 ± 0.02 | 0.3 ± 0.01 | 0.9 ± 0.20 | 2.2 ± 0.90 | 0.4 ± 0.02 | 1.2 ± 0.50 |
| 3 | p-Cymene | 1022 | 1024 | 1.2 ± 0.05 | 1.6 ± 0.05 | 4.9 ± 1.20 | 2.5 ± 1.40 | 6.1 ± 1.00 | 3.3 ± 1.20 |
| 4 | 1,8-Cineole | 1029 | 1026 | 0.3 ± 0.00 | 0.3 ± 0.00 | n.d. | n.d. | n.d. | n.d. |
| 5 | γ-Terpinene | 1059 | 1054 | 4.0 ± 0.50 | 4.0 ± 0.45 | 5.4 ± 1.00 | 3.2 ± 0.90 | 2.9 ± 0.85 | 5.9 ± 0.55 |
| 6 | Thujol | 1162 | 1168 | 0.7 ± 0.03 | 0.7 ± 0.03 | n.d. | n.d. | 0.8 ± 0.04 | n.d. |
| 7 | Thymol | 1290 | 1289 | 0.1 ± 0.00 | 0.1 ± 0.00 | n.d. | n.d. | 2.6 ± 0.33 | 0.6 ± 0.02 |
| 8 | Carvacrol | 1299 | 1298 | 89.3 ± 1.60 | 90.9 ± 0.80 | 84.0 ± 1.00 | 85.3 ± 1.23 | 82.1 ± 0.90 | 86.1 ± 0.82 |
| 9 | α-Caryophyllene | 1452 | 1454 | 0.6 ± 0.02 | 0.5 ± 0.02 | n.d. | n.d. | n.d. | n.d. |
| 10 | Germacrene A | 1509 | 1508 | 0.2 ± 0.01 | 0.3 ± 0.01 | n.d. | n.d. | n.d. | n.d. |
| Total identified (%) | 97.5 | 98.7 | 95.2 | 93.2 | 95.5 | 97.1 | |||
| Monoterpene hydrocarbons | 5.7 | 5.9 | 11.2 | 7.9 | 9.4 | 10.4 | |||
| Oxygenated monoterpenes | 91.0 | 92.0 | 84.0 | 85.3 | 86.1 | 86.7 | |||
| Sesquiterpene hydrocarbons | 0.8 | 0.8 | - | - | - | - | |||
| Essential oil content (mL/100 g dry weight) | 3.75 | 3.31 | 3.03 | 1.42 | 6.89 | 4.60 |
| No | Compounds a | AI b | AI c | AprilL | MayL | JuneL | JulyL | JulyI | JulyLI |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Octan-3-one | 987 | 979 | 0.7 ± 0.14 | 1.2 ± 0.60 | 1.1 ± 0.02 | 1.0 ± 0.00 | n.d. d | 0.8 ± 0.02 |
| 2 | β-Myrcene | 990 | 990 | 0.4 ± 0.02 | 0.2 ± 0.01 | n.d. | n.d. | 2.0 ± 0.83 | 0.5 ± 0.01 |
| 3 | p-Cymene | 1022 | 1024 | 2.6 ± 0.83 | n.d. | n.d. | n.d. | 5.6 ± 1.20 | 12.4 ± 1.82 |
| 4 | 1,8-Cineole | 1029 | 1026 | 0.3 ± 0.02 | n.d. | n.d. | 1.1 ± 0.02 | n.d. | n.d. |
| 5 | γ-Terpinene | 1059 | 1054 | 6.6 ± 1.00 | 6.6 ± 0.95 | 5.5 ± 0.33 | n.d. | 10.0 ± 1.60 | 5.8 ± 0.83 |
| 6 | Thujol | 1162 | 1168 | 0.5 ± 0.08 | 0.7 ± 0.05 | 0.5 ± 0.01 | 1.6 ± 0.14 | n.d. | n.d. |
| 7 | Thymol | 1290 | 1289 | 0.1 ± 0.00 | n.d. | n.d. | 0.2 ± 0.00 | n.d. | n.d. |
| 8 | Carvacrol | 1299 | 1298 | 83.2 ± 1.50 | 83.4 ± 1.00 | 85.0 ± 0.85 | 87.2 ± 1.00 | 76.3 ± 1.00 | 74.5 ± 1.20 |
| 9 | α-Caryophyllene | 1452 | 1454 | 1.3 ± 0.75 | 2.4 ± 0.84 | 2.2 ± 0.50 | 1.1 ± 0.22 | 1.7 ± 0.05 | n.d. |
| 10 | Germacrene A | 1509 | 1508 | 1.7 ± 0.60 | 1.4 ± 0.50 | 1.4 ± 0.01 | 1.6 ± 0.04 | n.d. | n.d. |
| 11 | Caryophyllene oxide | 1583 | 1582 | n.d. | n.d. | n.d. | 1.1 ± 0.02 | n.d. | n.d. |
| Total identified (%) | 97.4 | 95.9 | 95.7 | 94.9 | 95.6 | 94.0 | |||
| Monoterpene hydrocarbons | 9.6 | 6.8 | 5.5 | - | 17.6 | 18.7 | |||
| Oxygenated monoterpenes | 84.8 | 85.3 | 86.5 | 91.1 | 76.3 | 75.3 | |||
| Sesquiterpene hydrocarbons | 3.0 | 3.8 | 3.6 | 2.7 | 1.7 | - | |||
| Essential oil content (mL/100 g dry weight) | 3.73 | 3.27 | 2.21 | 2.60 | 5.93 | 4.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanakis, M.K.; Papaioannou, C.; Lianopoulou, V.; Philotheou-Panou, E.; Giannakoula, A.E.; Lazari, D.M. Seasonal Variation of Aromatic Plants under Cultivation Conditions. Plants 2022, 11, 2083. https://doi.org/10.3390/plants11162083
Stefanakis MK, Papaioannou C, Lianopoulou V, Philotheou-Panou E, Giannakoula AE, Lazari DM. Seasonal Variation of Aromatic Plants under Cultivation Conditions. Plants. 2022; 11(16):2083. https://doi.org/10.3390/plants11162083
Chicago/Turabian StyleStefanakis, Michalis K., Charikleia Papaioannou, Vaia Lianopoulou, Eleni Philotheou-Panou, Anastasia E. Giannakoula, and Diamanto M. Lazari. 2022. "Seasonal Variation of Aromatic Plants under Cultivation Conditions" Plants 11, no. 16: 2083. https://doi.org/10.3390/plants11162083