Botryosphaeria Dieback (Lasiodiplodia viticola): An Imminent Emerging Threat to the Moroccan Vineyards
Abstract
:1. Introduction
2. Results
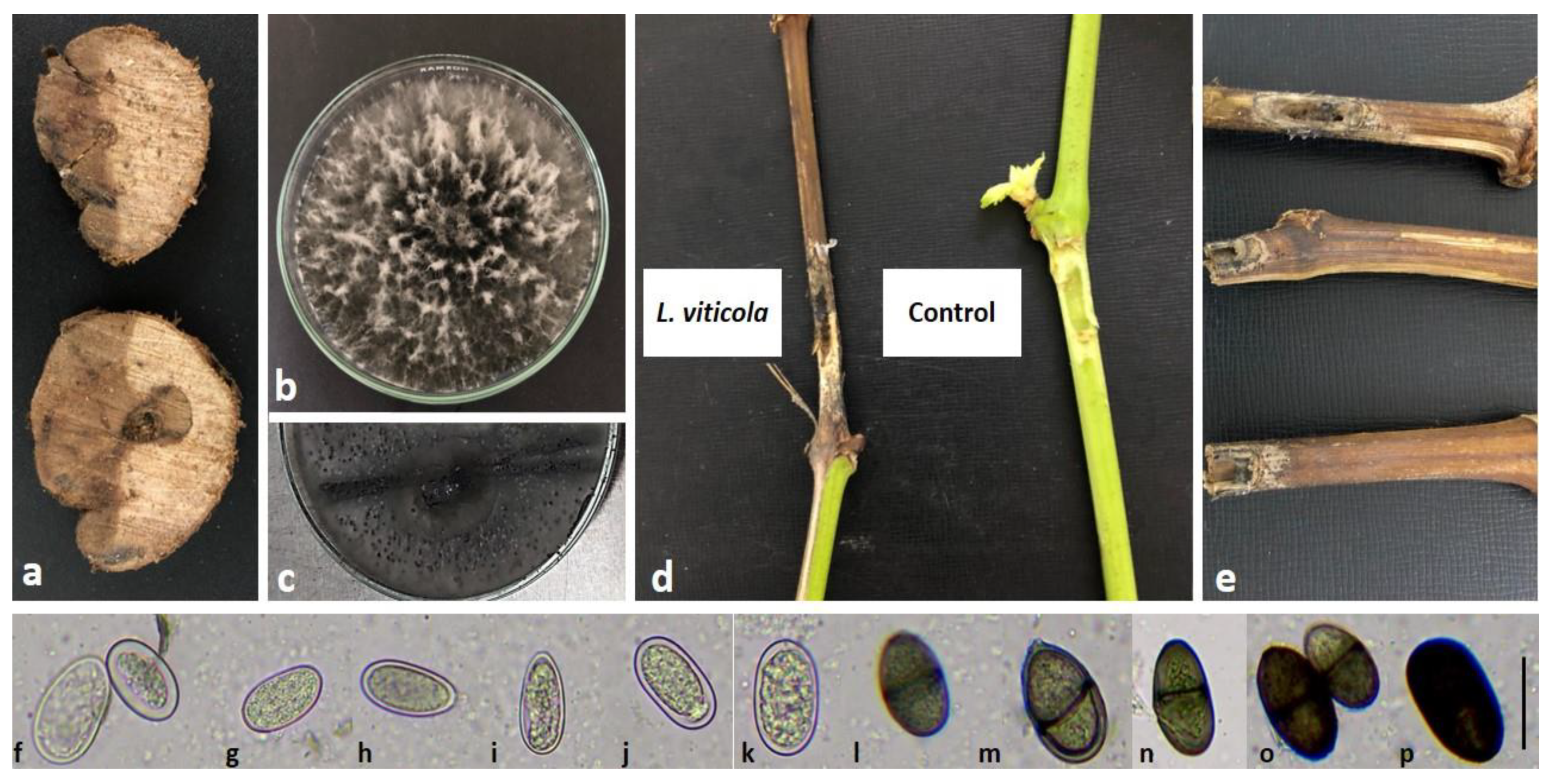
2.1. Species Identification and Morphological Features of Isolates
2.2. Pathogenicity Test
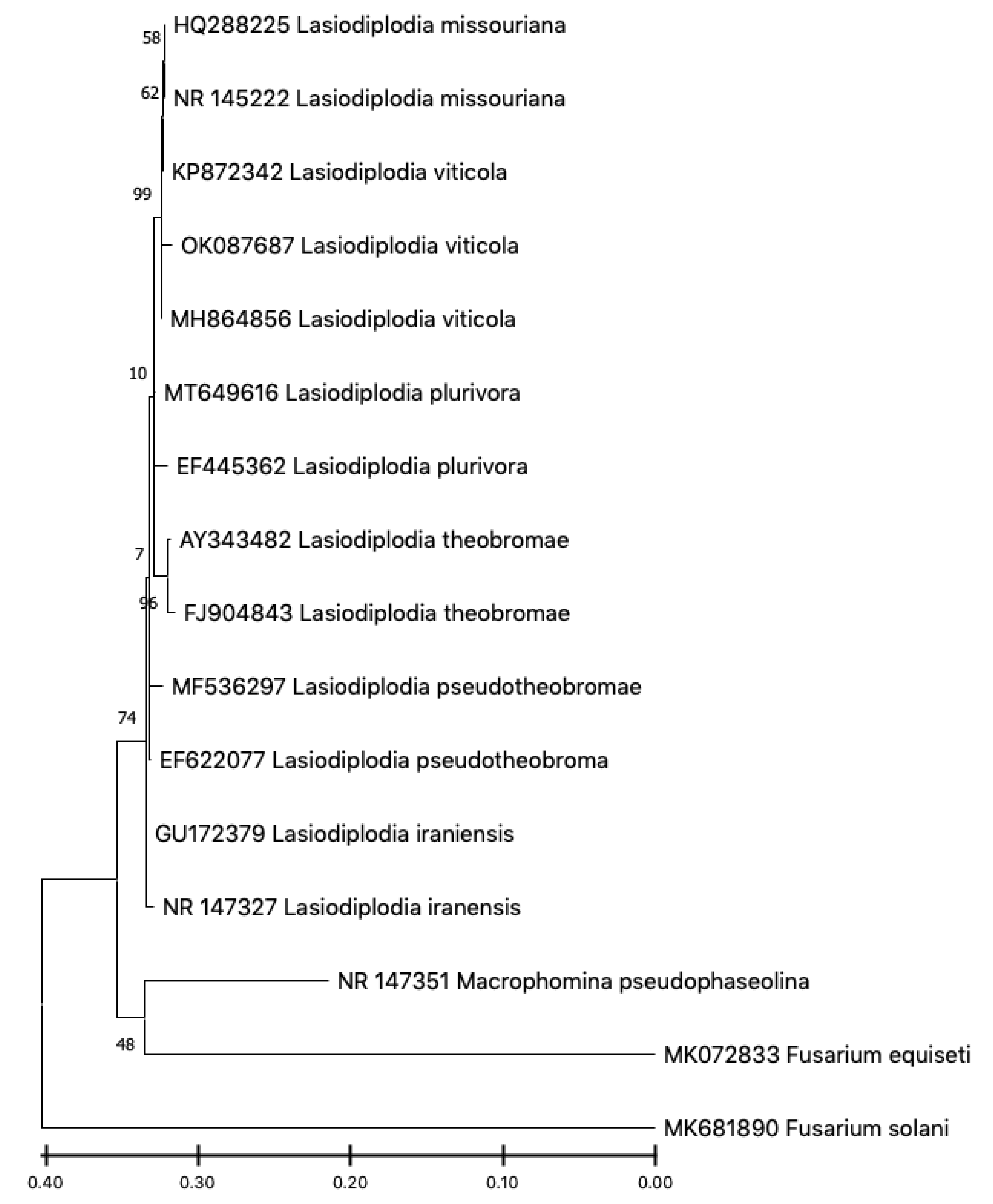
2.3. Molecular Identification
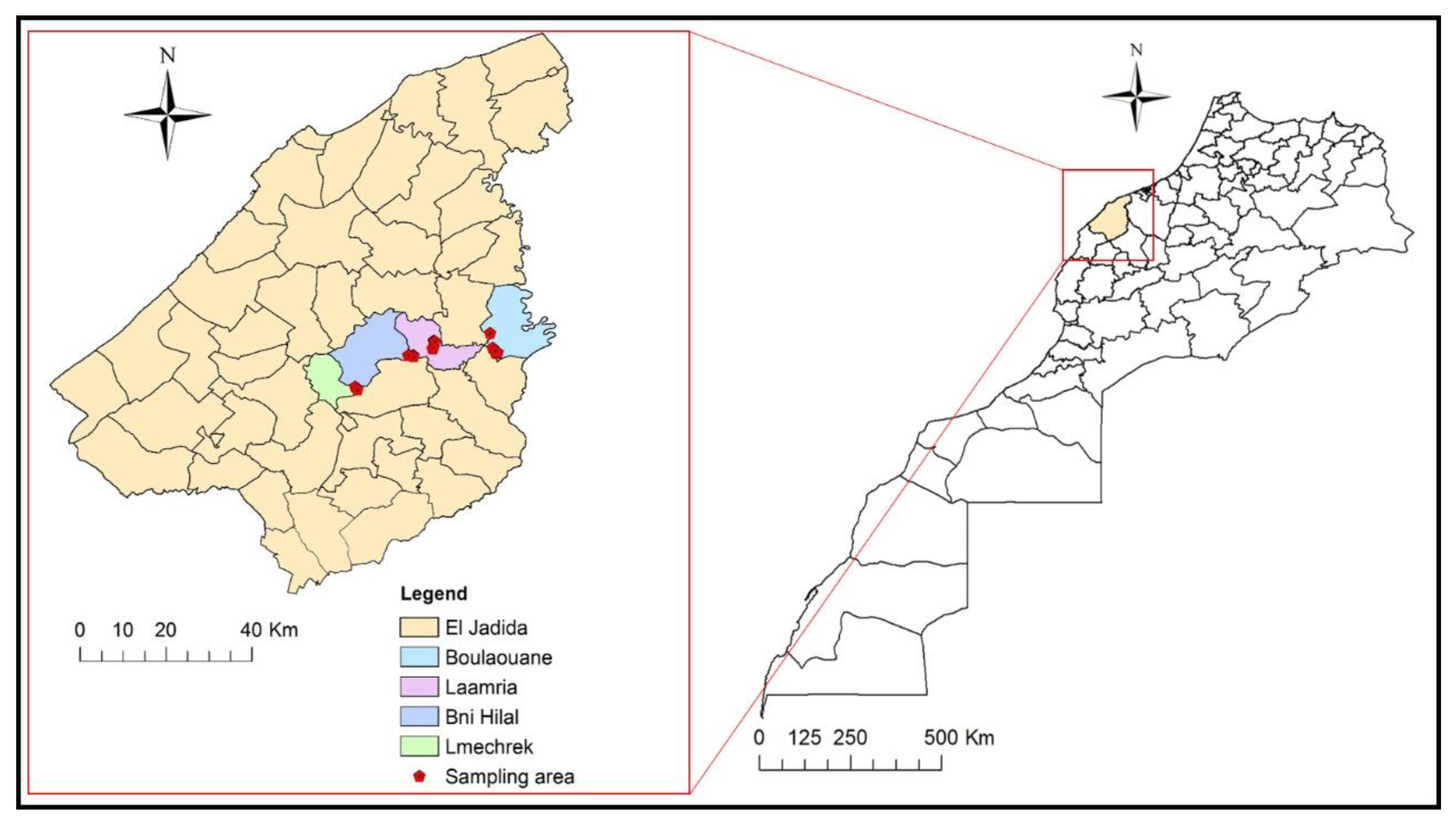
2.4. Distribution of the Pathogenic Fungus in the Surveyed Vineyards
2.5. Physiological Traits
2.5.1. Effect of Temperature on Radial Growth Rate
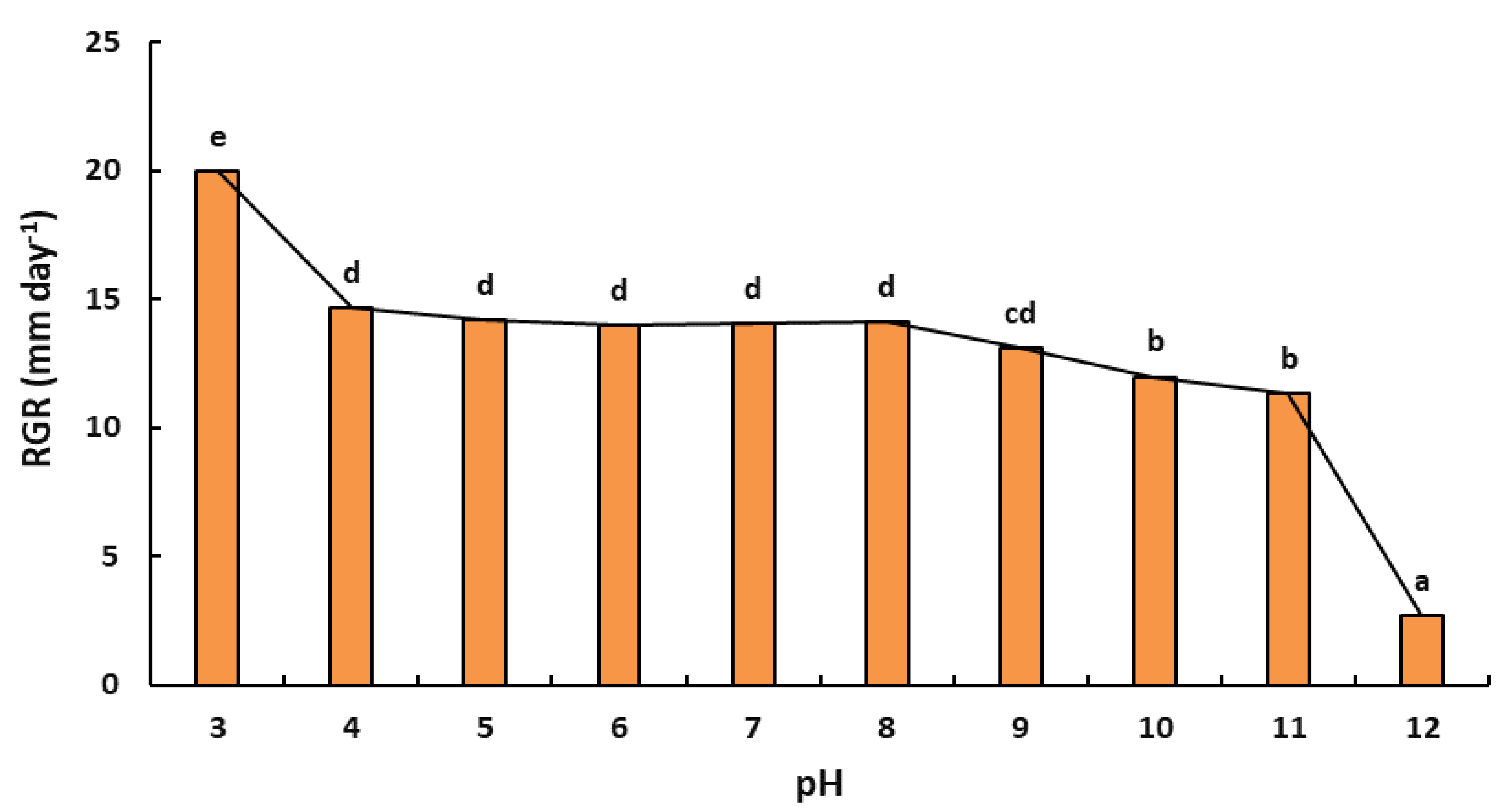
2.5.2. Effect of pH on Radial Growth Rate
3. Discussion
4. Material and Methods
4.1. Survey and Sampling Sites
4.2. Fungal Isolation
4.3. Pathogenicity Test
4.4. Molecular Identification of Isolates
4.5. Morphological Identification of the Pathogen
4.6. Physiological Traits
4.6.1. Effect of Temperature on Radial Growth Rate
4.6.2. Effect of pH on Radial Growth Rate
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pouzoulet, J. Développement d’une Méthodologie PCR en temps réel pour la Détection et la Quantification in planta des Principaux Champignons Pathogènes Associés aux Maladies du bois de la vigne. 2012. Available online: https://oatao.univ-toulouse.fr/8359/ (accessed on 4 January 2022).
- Oiv. State of the World Vitivinicultural Sector In 2020. 2021, pp. 1–19. Available online: https://www.oiv.int/public/medias/7880/oiv-state-of-the-vitivinicultural-world-2020-ppt.pdf (accessed on 4 January 2022).
- DRA. La Culture de la Vigne au Maroc; La Direction Régionale de l’Agriculture Méknes: Meknès, Morocco, 2019.
- Travadon, R.; Lecomte, P.; Diarra, B.; Lawrence, D.P.; Renault, D.; Ojeda, H.; Rey, P.; Baumgartner, K. Grapevine pruning systems and cultivars influence the diversity of wood-colonizing fungi. Fungal Ecol. 2016, 24, 82–93. [Google Scholar] [CrossRef] [Green Version]
- Kenfaoui, J.; Radouane, N.; Mennani, M.; Tahiri, A.; Ghadraoui, L.E.; Belabess, Z.; Fontaine, F.; Hamss, H.E.; Amiri, S.; Lahlali, R.; et al. A Panoramic View on Grapevine Trunk Diseases Threats: Case of Eutypa Dieback, Botryosphaeria Dieback, and Esca Disease. J. Fungi 2022, 8, 595. [Google Scholar] [CrossRef] [PubMed]
- Gramaje, D.; Urbez-Torres, J.R.; Sosnowski, M.R. Managing grapevine trunk diseases with respect to etiology and epidemiology: Current strategies and future prospects. Plant Dis. 2018, 102, 12–39. [Google Scholar] [CrossRef] [Green Version]
- Bertsch, C.; Ramírez-Suero, M.; Magnin-Robert, M.; Larignon, P.; Chong, J.; Abou-Mansour, E.; Spagnolo, A.; Clément, C.; Fontaine, F. Grapevine trunk diseases: Complex and still poorly understood. Plant Pathol. 2013, 62, 243–265. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, D.P.; Travadon, R.; Nita, M.; Baumgartner, K. TrunkDiseaseID.org: A molecular database for fast and accurate identification of fungi commonly isolated from grapevine wood. Crop Prot. 2017, 102, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Larignon, P.; Fontaine, F.; Farine, S.; Clément, C.; Bertsch, C. Esca et Black Dead Arm: Deux acteurs majeurs des maladies du bois chez la Vigne. C. R. Biol. 2009, 332, 765–783. [Google Scholar] [CrossRef]
- Mugnai, L.; Graniti, A.; Surico, G. Esca (Black measles) and brown wood-streaking: Two old and elusive diseases of grapevines. Am. Phytopathol. Soc. 1999, 83, 404–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Frari, G.; Cabral, A.; Nascimento, T.; Ferreira, R.B.; Oliveira, H. Epicoccum layuense a potential biological control agent of esca-associated fungi in grapevine. PLoS ONE 2019, 14, e0213273. [Google Scholar] [CrossRef] [Green Version]
- Rolshausen, P.E.; Baumgartner, K.; Travadon, R.; Fujiyoshi, P.; Pouzoulet, J.; Wilcox, W.F. Identification of Eutypa spp. Causing Eutypa Dieback of Grapevine in Eastern North America. Plant Dis. 2014, 98, 483–491. [Google Scholar] [CrossRef] [Green Version]
- Glawe, D.A.; Dilley, M.A.; Moller, W.J. Isolation and identification of Eutypa armeniacae from Malus domestica in Washington State. Mycotaxon 1983, 18, 315–318. [Google Scholar]
- Eichmeier, A.; Pečenka, J.; Peňázová, E.; Baránek, M.; Català-García, S.; León, M.; Armengol, J.; Gramaje, D. High-throughput amplicon sequencing-based analysis of active fungal communities inhabiting grapevine after hot-water treatments reveals unexpectedly high fungal diversity. Fungal Ecol. 2018, 36, 26–38. [Google Scholar] [CrossRef]
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clément, C.; Mugnai, L.; Fontaine, F. Grapevine trunk diseases: A review of fifteen years of trials for their control with chemicals and biocontrol agents. Plant Dis. 2018, 102, 1189–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larignon, P.; Spagnolo, A.; Bertsch, C.; Fontaine, F. First report of young grapevine decline caused by Neofusicoccum parvum in France. Plant Dis. 2015, 99, 1859. [Google Scholar] [CrossRef]
- Hofstetter, V.; Buyck, B.; Croll, D.; Viret, O.; Couloux, A.; Gindro, K. What if esca disease of grapevine were not a fungal disease? Fungal Divers. 2012, 54, 51–67. [Google Scholar] [CrossRef] [Green Version]
- Romanazzi, G.; Murolo, S.; Pizzichini, L.; Nardi, S. Esca in young and mature vineyards, and molecular diagnosis of the associated fungi. Eur. J. Plant Pathol. 2009, 125, 277–290. [Google Scholar] [CrossRef]
- Slippers, B.; Wingfield, M.J. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: Diversity, ecology and impact. Fungal Biol. Rev. 2007, 21, 90–106. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R.; Gubler, W.D. Pathogenicity of Botryosphaeriaceae species isolated from grapevine cankers in California. Plant Dis. 2009, 93, 584–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbez-Torres, J.R.; Peduto, F.; Striegler, R.K.; Urrea-Romero, K.E.; Rupe, J.C.; Cartwright, R.D.; Gubler, W.D. Characterization of fungal pathogens associated with grapevine trunk diseases in Arkansas and Missouri. Fungal Divers. 2012, 52, 169–189. [Google Scholar] [CrossRef]
- Cruywagen, E.M.; Slippers, B.; Roux, J.; Wingfield, M.J. Phylogenetic species recognition and hybridisation in Lasiodiplodia: A case study on species from baobabs. Fungal Biol. 2017, 121, 420–436. [Google Scholar] [CrossRef] [Green Version]
- Marques, M.W.; Lima, N.B.; De Morais, M.A.; Barbosa, M.A.G.; Souza, B.O.; Michereff, S.J.; Phillips, A.J.L.; Câmara, M.P.S. Species of Lasiodiplodia associated with mango in Brazil. Fungal Divers. 2013, 61, 181–193. [Google Scholar] [CrossRef]
- Comont, G.; Mayet, V.; Marie-France, C. First Report of Lasiodiplodia viticola, Spencermartinsia viticola and Diplodia intermedia Associated With Vitis vinifera Grapevine Decline in French Vineyards. Plant Dis. 2016, 100, 2328. [Google Scholar] [CrossRef]
- Saha, A.; Mandal, P.; Dasgupta, S.; Saha, D. Influence of culture media and environmental factors on mycelial growth and sporulation of Lasiodiplodia theobromae (Pat.) Griffon and Maubl. J. Environ. Biol. 2008, 29, 407. [Google Scholar] [PubMed]
- Úrbez-Torres, J.R. The status of Botryosphaeriaceae species infecting grapevines. Phytopathol. Mediterr. 2015, 54, 241–252. [Google Scholar] [CrossRef]
- Copes, W.E.; Fruit, S.; Hendrix, F.F. Effect of Temperature on Sporulation of Botryosphaeria dothidea, B. obtusa, and B. rhodina. Plant Dis. 2014, 88, 292–296. [Google Scholar] [CrossRef] [Green Version]
- Michailides, T.J.; Morgan, D.P. Spore release by Botryosphaeria dothidea in pistachio orchards and disease control by altering the trajectory angle of sprinklers. Phytopathology 1993, 83, 145–152. [Google Scholar] [CrossRef]
- Michailides, T.J. Pathogenicity, distribution, sources of inoculum, and infection courts of Botryosphaeria dothidea on pistachio. Phytopathology 1991, 81, 566–573. [Google Scholar] [CrossRef]
- Pusey, P.L.; Bertrand, P.F. Seasonal infection of nonwounded peach bark by Botryosphaeria dothidea. Phytopathology 1993, 83, 825–829. [Google Scholar] [CrossRef]
- Ammad, F.; Benchabane, M.; Toumi, M.; Belkacem, N.; Guesmi, A.; Ameur, C.; Lecomte, P.; Merah, O. Occurrence of Botryosphaeriaceae species associated with grapevine dieback in Algeria. Turk. J. Agric. For. 2014, 38, 865–876. [Google Scholar] [CrossRef]
- Kraus, C.; Voegele, R.T.; Fischer, M. Temporal Development of the Culturable, Endophytic Fungal Community in Healthy Grapevine Branches and Occurrence of GTD-Associated Fungi. Microb. Ecol. 2019, 77, 866–876. [Google Scholar] [CrossRef]
- Bruez, E.; Larignon, P.; Compant, S.; Rey, P. Investigating the durable effect of the hot water treatment used in nurseries on pathogenic fungi inhabiting grapevine wood and involved in Grapevine Trunk Diseases. Crop Prot. 2017, 100, 203–210. [Google Scholar] [CrossRef]
- Mondello, V.; Giambra, S.; Conigliaro, G. Fungal pathogens associated with grapevine trunk diseases in young vineyards in Sicily. Phytopathol. Mediterr. 2020, 59, 453–463. [Google Scholar] [CrossRef]
- León, M.; Berbegal, M.; Rodríguez-Reina, J.M.; Elena, G.; Abad-Campos, P.; Ramón-Albalat, A.; Olmo, D.; Vicent, A.; Luque, J.; Miarnau, X.; et al. Identification and characterization of Diaporthe spp. associated with twig cankers and shoot blight of almonds in Spain. Agronomy 2020, 10, 1062. [Google Scholar] [CrossRef]
- Doyle, J.; Doyle, J. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Ezrari, S.; Radouane, N.; Tahiri, A.; Amiri, S.; Lazraq, A.; Lahlali, R. Environmental Effects of Temperature and Water Potential on Mycelial Growth of Neocosmospora solani and Fusarium spp. Causing Dry Root Rot of Citrus. Curr. Microbiol. 2021, 78, 3092–3103. [Google Scholar] [CrossRef]
- Frans, M.; Aerts, R.; Van Laethem, S.; Ceusters, J. Environmental effects on growth and sporulation of Fusarium spp. causing internal fruit rot in bell pepper. Eur. J. Plant Pathol. 2017, 149, 875–883. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press, Inc.: Cambridge, MA, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Chen, M.Y.; Yan, D.D.; Han, D.K.; Ma, D.C.; Gao, P.Z.; Bao, D.X.; Wang, M.F. Occurrence of necrosis of balloon flower (Platycodon grandiflorus) caused by Nigrospora sphaerica in China. Plant Dis. 2022, 106, 1297. [Google Scholar] [CrossRef]


| Location | No. Vineyards 1 | Positive Orchards | |
|---|---|---|---|
| Lasiodiplodia viticola | Disease Prevalence (%) | ||
| Bni Hilal | 1 | 1 | 71.43 |
| 2 | 1 | ||
| 3 | 1 | ||
| 4 | - | ||
| 5 | 1 | ||
| 6 | 1 | ||
| 7 | - | ||
| Laamria | 8 | 1 | 60 |
| 9 | 1 | ||
| 10 | - | ||
| 11 | 1 | ||
| 12 | - | ||
| Boulaouane | 13 | 1 | 40 |
| 14 | - | ||
| 15 | 1 | ||
| 16 | - | ||
| 17 | - | ||
| Lmechrek | 18 | - | 0 |
| 19 | - | ||
| 20 | - | ||
| Total | 20 | 10 | 50 |
| Location | Vineyard Code | Coordinates | |
|---|---|---|---|
| X | Y | ||
| Laamria | 1D | 32°48′16″ N | 8°14′41″ W |
| 2D | 32°49′15″ N | 8°14′42″ W | |
| 3D | 32°49′14″ N | 8°14′41″ W | |
| 4D | 32°49′14″ N | 8°14′41″ W | |
| 5D | 32°49′12″ N | 8°14′36″ W | |
| Bni Hilal | 6D | 32°47′27″ N | 8°17′16″ W |
| 7D | 32°47′20″ N | 8°17′14″ W | |
| 8D | 32°47′28″ N | 8°17′55″ W | |
| 9D | 32°43′36″ N | 8°24′35″ W | |
| 10D | 32°43′23″ N | 8°24′17″ W | |
| 11D | 32°45′28″ N | 8°18′55″ W | |
| 12D | 32°43′37″ N | 8°23′35″ W | |
| Boulaouane | 13D | 32°48′24″ N | 8°07′16″ W |
| 14D | 32°47′45″ N | 8°06′54″ W | |
| 15D | 32°47′48″ N | 8°06′39″ W | |
| 16D | 32°48′00″ N | 8°06′55″ W | |
| 17D | 32°50′12″ N | 8°07′37″ W | |
| Lmechrek | 18D | 32°46′13″ N | 8°15′38″ W |
| 19D | 32°45′11″ N | 8°15′33″ W | |
| 20D | 32°46′09″ N | 8°15′32″ W | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kenfaoui, J.; Lahlali, R.; Mennani, M.; Radouane, N.; Goura, K.; El Hamss, H.; El Ghadraoui, L.; Fontaine, F.; Tahiri, A.; Barka, E.A.; et al. Botryosphaeria Dieback (Lasiodiplodia viticola): An Imminent Emerging Threat to the Moroccan Vineyards. Plants 2022, 11, 2167. https://doi.org/10.3390/plants11162167
Kenfaoui J, Lahlali R, Mennani M, Radouane N, Goura K, El Hamss H, El Ghadraoui L, Fontaine F, Tahiri A, Barka EA, et al. Botryosphaeria Dieback (Lasiodiplodia viticola): An Imminent Emerging Threat to the Moroccan Vineyards. Plants. 2022; 11(16):2167. https://doi.org/10.3390/plants11162167
Chicago/Turabian StyleKenfaoui, Jihane, Rachid Lahlali, Mohammed Mennani, Nabil Radouane, Khadija Goura, Hajar El Hamss, Lahsen El Ghadraoui, Florence Fontaine, Abdessalem Tahiri, Essaid Ait Barka, and et al. 2022. "Botryosphaeria Dieback (Lasiodiplodia viticola): An Imminent Emerging Threat to the Moroccan Vineyards" Plants 11, no. 16: 2167. https://doi.org/10.3390/plants11162167
APA StyleKenfaoui, J., Lahlali, R., Mennani, M., Radouane, N., Goura, K., El Hamss, H., El Ghadraoui, L., Fontaine, F., Tahiri, A., Barka, E. A., & Amiri, S. (2022). Botryosphaeria Dieback (Lasiodiplodia viticola): An Imminent Emerging Threat to the Moroccan Vineyards. Plants, 11(16), 2167. https://doi.org/10.3390/plants11162167










