Integration of the Connectivity Map and Pathway Analysis to Predict Plant Extract’s Medicinal Properties—The Study Case of Sarcopoterium spinosum L.
Abstract
:1. Introduction
2. Results
2.1. Evaluating the Cytotoxicity Effect of Root Extract from Sarcopoterium spinosum L.
2.2. RNAseq and Pathway Analysis
2.3. Comparative Computational Pathway Analysis of the SSRE Extract Effect on Various Cell Lines
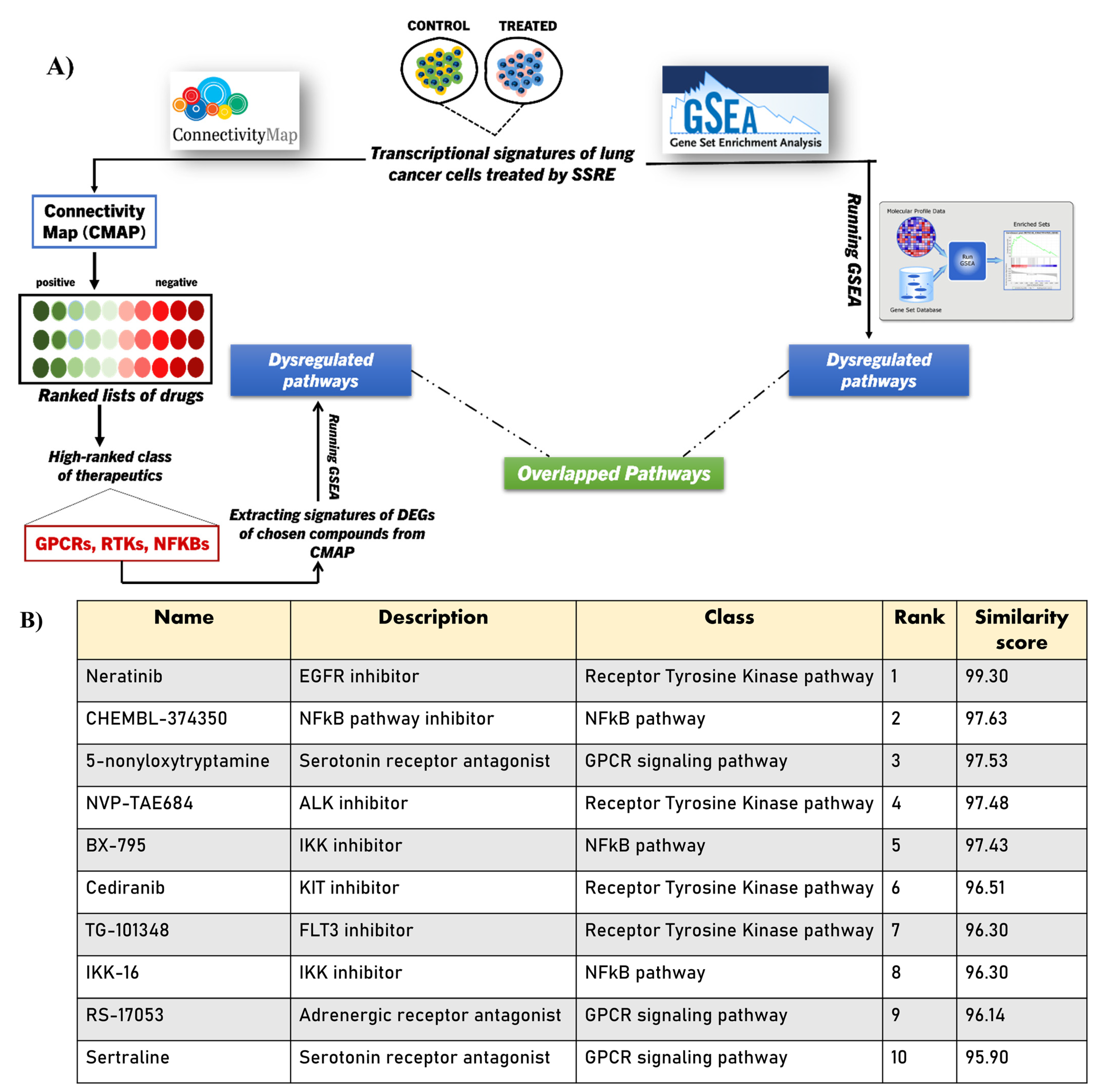
2.4. Integrating CMAP into GSEA Analysis of the SSRE Transcriptome Signature
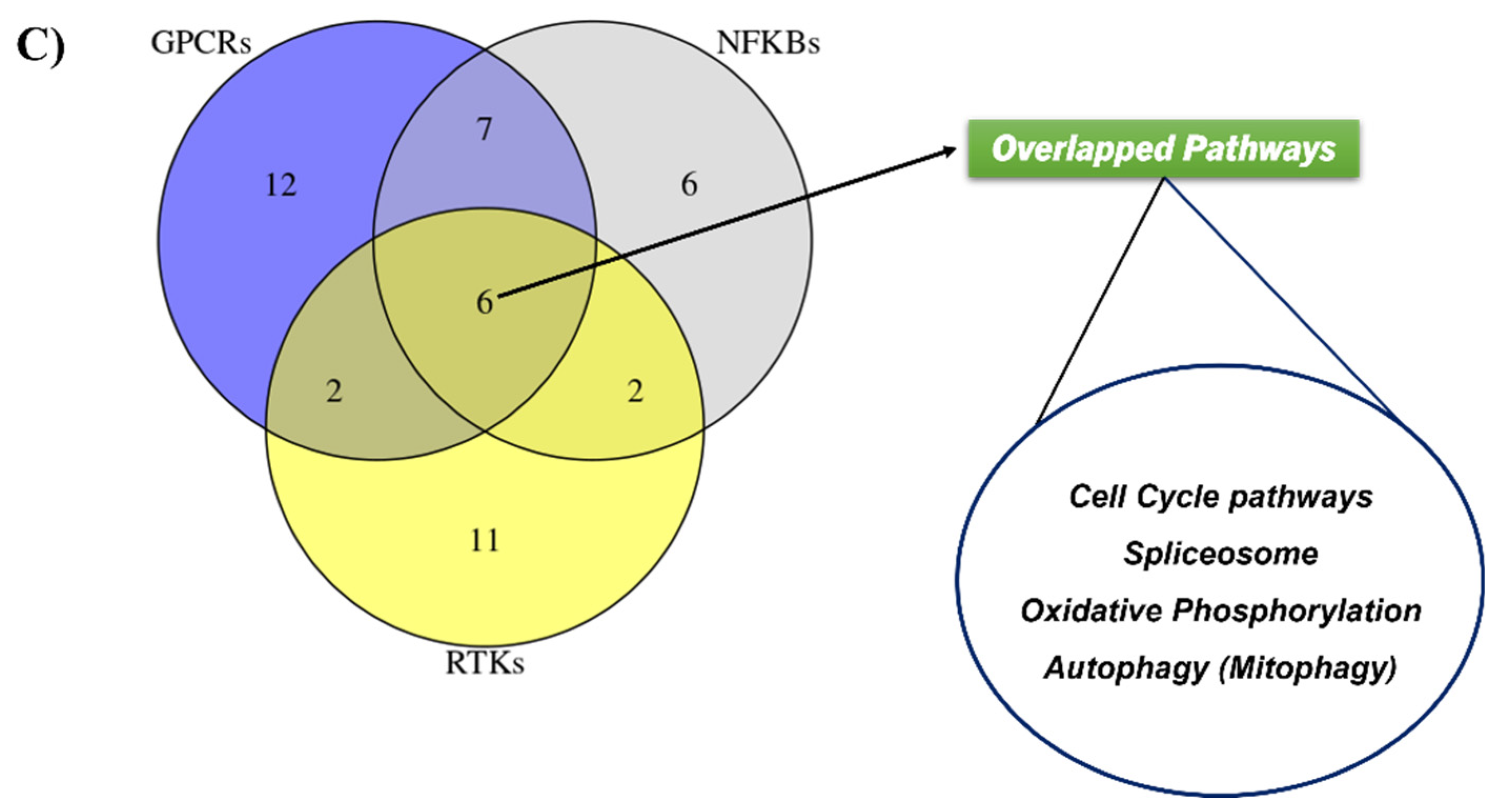
2.5. Validating the Pathways and Predicting Anti-Cancer Effects of SSRE
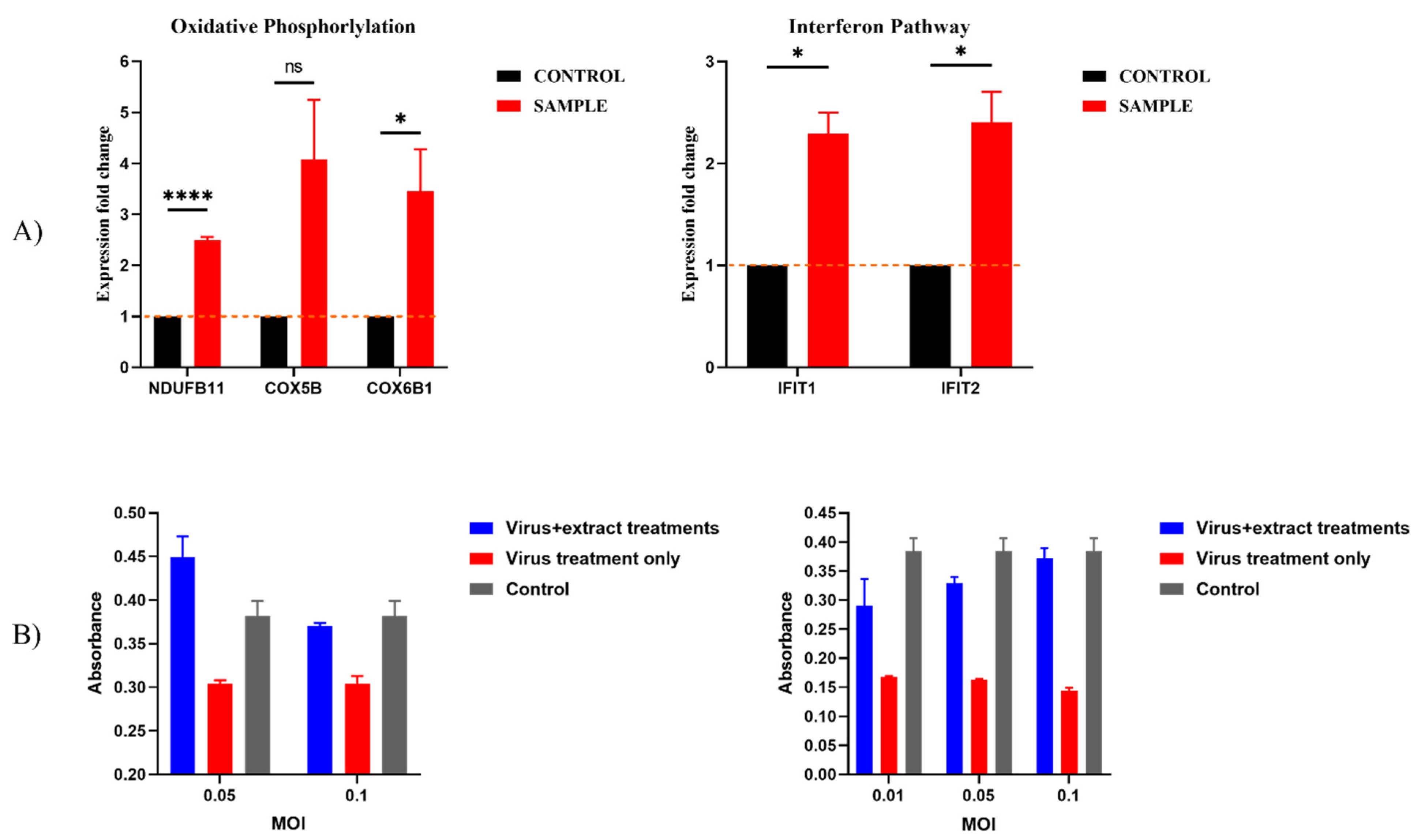
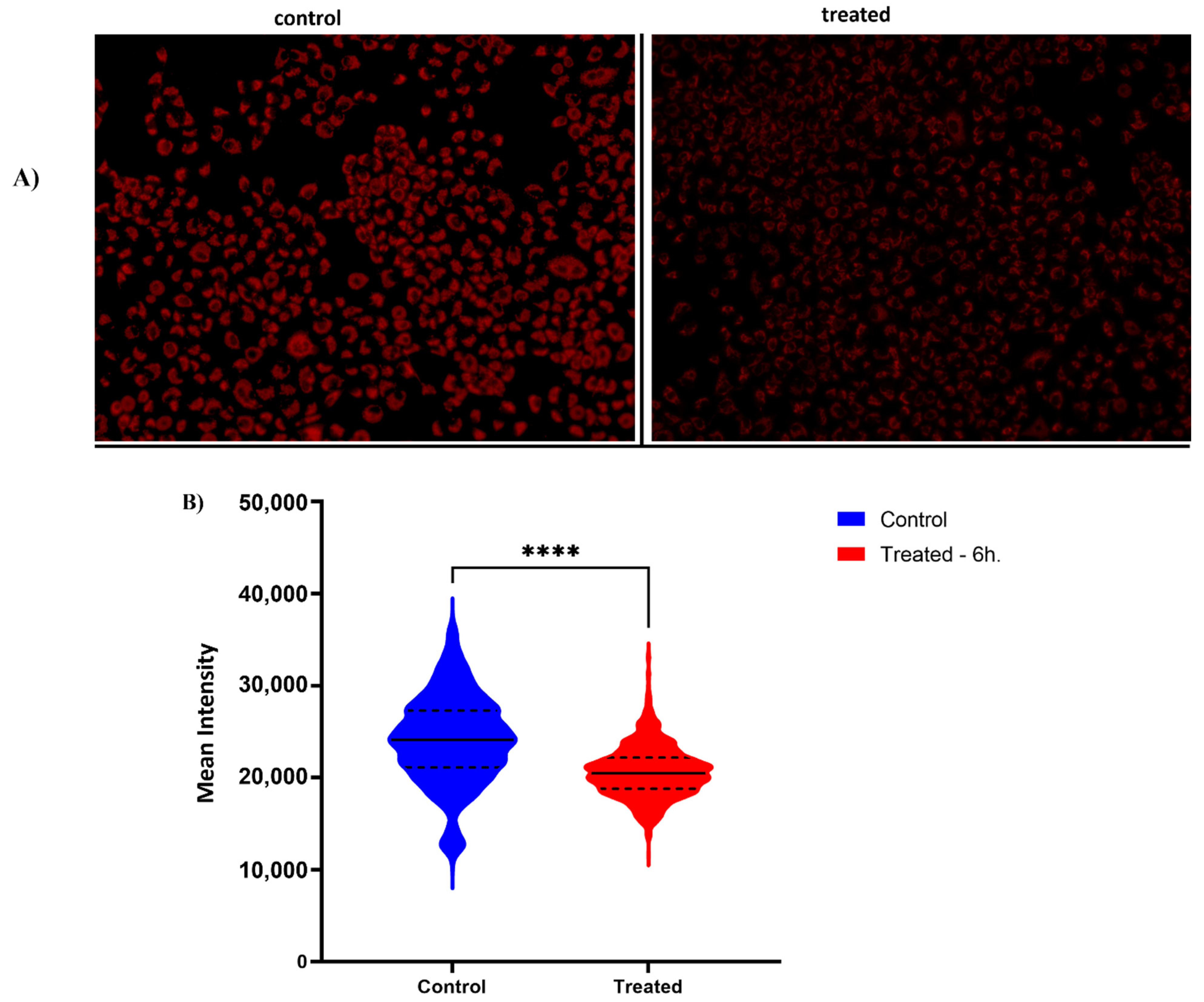
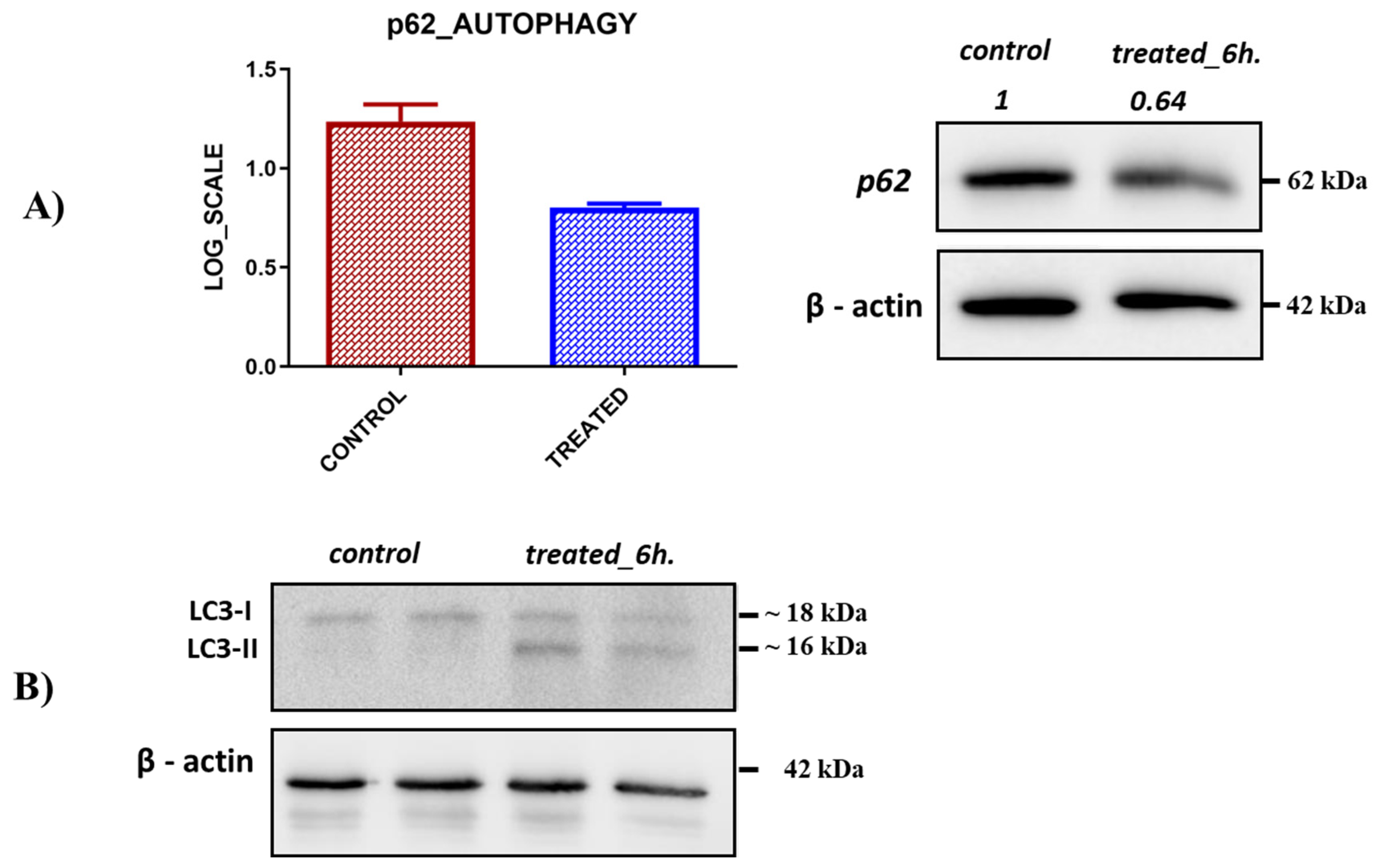
2.6. Previously Unknown Anti-Viral and Autophagy-Stimulating Effects of Sarcopoterium spinosum L. Extract
3. Discussion
4. Materials and Methods
4.1. Plant Collection
4.2. Aqueous Extract
4.3. Cell Lines
4.4. Cytotoxicity Assay
4.5. RNA Extraction and Library Construction
4.6. RNA Sequencing, Quality Control, Sequence Alignment, and Gene Counts
4.7. Normalization and Differential Expression Analysis
4.8. Functional Analysis of RNA-seq (GSEA, STRING, CMAP)
4.9. Cell Cycle Analysis
4.10. cDNA Synthesis
4.11. Real-Time PCR
| Gene Symbols | Forward | Reverse |
| IFIT2 | gacacggttaaagtgtggagg | tccagacggtagcttgctatt |
| IFIT1 | tgagatgtcactttacatggg | tgtattcccacactgtatttgg |
| CCP110 | agacgcagtctgagaggtagt | cagtgtttgcctgtcaactgg |
| CEP152 | ggagtggcagtctaagctgg | tcactggtggttacttggtca |
| NDUFB11 | cgtccgctgggaatctagc | acggggtccttgtcataacca |
| COX5B | tgtgaagaggacaataccagcg | ccagcttgtaatgggctccac |
| COX6B1 | ctacaagaccgccccttttga | tttagcggtcattgccttctg |
| SRSF11 | caggtgactaatgtctccccg | gcagttcgtcgatcttgcct |
| PNN | gaatgacgtgaggcccatcca | ctctgtttggctgggggtcct |
4.12. Analysis of Anti-Viral Activity
4.13. Analysis of Mitochondrial Activity
4.14. Analysis of Mitochondrial Membrane Potential (ΔΨm)
4.15. Autophagy Study
4.16. Western Blot Analysis
4.17. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Fitzgerald, M.; Heinrich, M.; Booker, A. Medicinal Plant Analysis: A Historical and Regional Discussion of Emergent Complex Techniques. Front. Pharmacol. 2020, 10, 1480. [Google Scholar] [CrossRef] [PubMed]
- Britton, E.R.; Kellogg, J.J.; Kvalheim, O.M.; Cech, N.B. Biochemometrics to Identify Synergists and Additives from Botanical Medicines: A Case Study with Hydrastis canadensis (Goldenseal). J. Nat. Prod. 2018, 81, 484–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junio, H.A.; Sy-Cordero, A.A.; Ettefagh, K.A.; Burns, J.T.; Micko, K.T.; Graf, T.N.; Richter, S.J.; Cannon, R.E.; Oberlies, N.H.; Cech, N.B. Synergy-directed fractionation of botanical medicines: A case study with goldenseal (Hydrastis canadensis). J. Nat. Prod. 2011, 74, 1621–1629. [Google Scholar] [CrossRef] [Green Version]
- Suberu, J.O.; Gorka, A.P.; Jacobs, L.; Roepe, P.D.; Sullivan, N.; Barker, G.C.; Lapkin, A.A. Anti-plasmodial polyvalent interactions in Artemisia annua L. aqueous extract--possible synergistic and resistance mechanisms. PLoS ONE 2013, 8, e80790. [Google Scholar] [CrossRef]
- Benbacer, L.; Merghoub, N.; El Btaouri, H.; Gmouh, S.; Attaleb, M.; Morjani, H.; Amzazi, S.; El Mzibri, M. Antiproliferative Effect and Induction of Apoptosis by Inula viscosa L. and Retama monosperma L. Extracts in Human Cervical Cancer Cells. In Topics on Cervical Cancer with an Advocacy for Prevention; InTech: London, UK, 2012; ISBN 978-953-51-0183-3. [Google Scholar]
- Nordin, N.; Salama, S.M.; Golbabapour, S.; Hajrezaie, M.; Hassandarvish, P.; Kamalidehghan, B.; Majid, N.A.; Hashim, N.M.; Omar, H.; Fadaienasab, M.; et al. Anti-ulcerogenic effect of methanolic extracts from Enicosanthellum pulchrum (King) Heusden against ethanol-induced acute gastric lesion in animal models. PLoS ONE 2014, 9, e111925. [Google Scholar] [CrossRef] [Green Version]
- El-Newary, S.A.; Ismail, R.F.; Shaffie, N.M.; Hendawy, S.F.; Omer, E.; Ahmed, M.M.; ELsayed, W.M. Hepatoprotective effects of Tagetes lucida root extract in carbon tetrachloride-induced hepatotoxicity in Wistar albino rats through amelioration of oxidative stress. Pharm. Biol. 2021, 59, 986–997. [Google Scholar] [CrossRef]
- Kuete, V.; Tankeo, S.B.; Saeed, M.E.M.; Wiench, B.; Tane, P.; Efferth, T. Cytotoxicity and modes of action of five Cameroonian medicinal plants against multi-factorial drug resistance of tumor cells. J. Ethnopharmacol. 2014, 153, 207–219. [Google Scholar] [CrossRef]
- Hager, R.; Pitsch, J.; Kerbl-Knapp, J.; Neuhauser, C.; Ollinger, N.; Iken, M.; Ranner, J.; Mittermeier-Kleßinger, V.; Dawid, C.; Lanzerstorfer, P.; et al. A High-Content Screen for the Identification of Plant Extracts with Insulin Secretion-Modulating Activity. Pharmaceuticals 2021, 14, 809. [Google Scholar] [CrossRef]
- Yang, X.; Kui, L.; Tang, M.; Li, D.; Wei, K.; Chen, W.; Miao, J.; Dong, Y. High-Throughput Transcriptome Profiling in Drug and Biomarker Discovery. Front. Genet. 2020, 11, 19. [Google Scholar] [CrossRef]
- Supplitt, S.; Karpinski, P.; Sasiadek, M.; Laczmanska, I. Current Achievements and Applications of Transcriptomics in Personalized Cancer Medicine. Int. J. Mol. Sci. 2021, 22, 1422. [Google Scholar] [CrossRef] [PubMed]
- Casamassimi, A.; Federico, A.; Rienzo, M.; Esposito, S.; Ciccodicola, A. Transcriptome Profiling in Human Diseases: New Advances and Perspectives. Int. J. Mol. Sci. 2017, 18, 1652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, X.; Liu, Y.; Liu, H.; Zhang, Y.; Wang, L.; Zhang, H. Identification of Potential Prognostic Genes for Neuroblastoma. Front. Genet. 2018, 9, 589. [Google Scholar] [CrossRef]
- He, Y.; Zhou, Y.; Ma, W.; Wang, J. An integrated transcriptomic analysis of autism spectrum disorder. Sci. Rep. 2019, 9, 11818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramanian, A.; Narayan, R.; Corsello, S.M.; Peck, D.D.; Natoli, T.E.; Lu, X.; Gould, J.; Davis, J.F.; Tubelli, A.A.; Asiedu, J.K.; et al. A Next Generation Connectivity Map: L1000 platform and the first 1,000,000 profiles. Cell 2017, 171, 1437–1452.e17. [Google Scholar] [CrossRef]
- Asano, T.; Chelvanambi, S.; Decano, J.L.; Whelan, M.C.; Aikawa, E.; Aikawa, M. In silico Drug Screening Approach Using L1000-Based Connectivity Map and Its Application to COVID-19. Front. Cardiovasc. Med. 2022, 9, 842641. [Google Scholar] [CrossRef]
- Lv, C.; Wu, X.; Wang, X.; Su, J.; Zeng, H.; Zhao, J.; Lin, S.; Liu, R.; Li, H.; Li, X.; et al. The gene expression profiles in response to 102 traditional Chinese medicine (TCM) components: A general template for research on TCMs. Sci. Rep. 2017, 7, 352. [Google Scholar] [CrossRef]
- Yoo, M.; Shin, J.; Kim, H.; Kim, J.; Kang, J.; Tan, A.C. Exploring the molecular mechanisms of Traditional Chinese Medicine components using gene expression signatures and connectivity map. Comput. Methods Programs Biomed. 2019, 174, 33–40. [Google Scholar] [CrossRef]
- Wen, Z.; Wang, Z.; Wang, S.; Ravula, R.; Yang, L.; Xu, J.; Wang, C.; Zuo, Z.; Chow, M.S.S.; Shi, L.; et al. Discovery of molecular mechanisms of traditional Chinese medicinal formula Si-Wu-Tang using gene expression microarray and connectivity map. PLoS ONE 2011, 6, e18278. [Google Scholar] [CrossRef] [Green Version]
- Lo, T.-F.; Tsai, W.-C.; Chen, S.-T. MicroRNA-21-3p, a Berberine-Induced miRNA, Directly Down-Regulates Human Methionine Adenosyltransferases 2A and 2B and Inhibits Hepatoma Cell Growth. PLoS ONE 2013, 8, e75628. [Google Scholar]
- Said, O.; Khalil, K.; Fulder, S.; Azaizeh, H. Ethnopharmacological survey of medicinal herbs in Israel, the Golan Heights and the West Bank region. J. Ethnopharmacol. 2003, 83, 251–265. [Google Scholar] [CrossRef]
- Al-Qura’n, S. Ethnopharmacological survey of wild medicinal plants in Showbak, Jordan. J. Ethnopharmacol. 2009, 123, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Elyasiyan, U.; Nudel, A.; Skalka, N.; Rozenberg, K.; Drori, E.; Oppenheimer, R.; Kerem, Z.; Rosenzweig, T. Anti-diabetic activity of aerial parts of Sarcopoterium spinosum. BMC Complement. Altern. Med. 2017, 17, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali-shtayeh, M.S.; Yaniv, Z.; Mahajna, J. Ethnobotanical survey in the Palestinian area: A classification of the healing potential of medicinal plants. J. Ethnopharmacol. 2000, 73, 221–232. [Google Scholar] [CrossRef]
- Yaniv, Z. Ethnobotanical studies of Sarcopoterium spinosum in Israel. Isr. J. Plant Sci. 2007, 55, 111–114. [Google Scholar]
- Hudec, J.; Mojžiš, J.; Habánová, M.; Saraiva, J.; Hradil, P.; Liptaj, T.; Kobida, L.; Haban, M.; Holovičová, M.; Zvercova, D. In Vitro Cytotoxic Effects of Secondary Metabolites Present in Sarcopoterium spinosum L. Appl. Sci. 2021, 11, 5300. [Google Scholar] [CrossRef]
- Durodola, J.I. Antitumour effects against sarcoma 180 ascites of fractions of Annona senegalensis. Planta Med. 1975, 28, 32–36. [Google Scholar] [CrossRef]
- Rozenberg, K.; Wollman, A.; Ben-Shachar, M.; Argaev-Frenkel, L.; Rosenzweig, T. Anti-inflammatory effects of Sarcopoterium spinosum extract. J. Ethnopharmacol. 2020, 249, 112391. [Google Scholar] [CrossRef]
- Aburjai, T.; Hudaib, M.; Tayyem, R.; Yousef, M.; Qishawi, M. Ethnopharmacological survey of medicinal herbs in Jordan, the Ajloun Heights region. J. Ethnopharmacol. 2007, 110, 294–304. [Google Scholar] [CrossRef]
- Reher, G.; Reznicek, G.; Baumann, A. Triterpenoids from Sarcopoterium spinosum and Sanguisorba minor. Planta Med. 1991, 57, 506. [Google Scholar] [CrossRef]
- Smirin, P.; Taler, D.; Abitbol, G.; Brutman-Barazani, T.; Kerem, Z.; Sampson, S.R.; Rosenzweig, T. Sarcopoterium spinosum extract as an antidiabetic agent: In vitro and in vivo study. J. Ethnopharmacol. 2010, 129, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Reher, G.; Slijepcević, M.; Kraus, L. Hypoglycemic Activity of Triterpenes and Tannins from Sarcopoterium spinosum and two Sanguisorba Species. Planta Med. 1991, 57, A57–A58. [Google Scholar] [CrossRef]
- García-Gutiérrez, L.; Delgado, M.D.; León, J. MYC Oncogene Contributions to Release of Cell Cycle Brakes. Genes 2019, 10, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bretones, G.; Delgado, M.D.; León, J. Myc and cell cycle control. Biochim. Biophys. Acta 2015, 1849, 506–516. [Google Scholar] [CrossRef]
- Barnaba, N.; LaRocque, J.R. Targeting cell cycle regulation via the G2-M checkpoint for synthetic lethality in melanoma. Cell Cycle Georget. Tex 2021, 20, 1041–1051. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, J.; Gu, C.; Yang, Y. Alternative splicing and cancer: A systematic review. Signal Transduct. Target. Ther. 2021, 6, 78. [Google Scholar] [CrossRef]
- Anczukow, O.; Krainer, A.R. Splicing-factor alterations in cancers. RNA 2016, 22, 1285–1301. [Google Scholar] [CrossRef] [Green Version]
- Kalev, P.; Hyer, M.L.; Gross, S.; Konteatis, Z.; Chen, C.C.; Fletcher, M.; Lein, M.; Aguado-Fraile, E.; Frank, V.; Barnett, A.; et al. MAT2A Inhibition Blocks the Growth of MTAP-Deleted Cancer Cells by Reducing PRMT5-Dependent mRNA Splicing and Inducing DNA Damage. Cancer Cell 2021, 39, 209–224.e11. [Google Scholar] [CrossRef]
- Park, J.-S.; Davis, R.L.; Sue, C.M. Mitochondrial Dysfunction in Parkinson’s Disease: New Mechanistic Insights and Therapeutic Perspectives. Curr. Neurol. Neurosci. Rep. 2018, 18, 21. [Google Scholar] [CrossRef] [Green Version]
- Prasuhn, J.; Davis, R.L.; Kumar, K.R. Targeting Mitochondrial Impairment in Parkinson’s Disease: Challenges and Opportunities. Front. Cell Dev. Biol. 2021, 8, 1704. [Google Scholar] [CrossRef]
- Lynch, D.R.; Farmer, G. Mitochondrial and metabolic dysfunction in Friedreich ataxia: Update on pathophysiological relevance and clinical interventions. Neuronal Signal. 2021, 5, NS20200093. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, A.; Magrané, J. Mitochondrial dysfunction in neurons in Friedreich’s ataxia. Mol. Cell. Neurosci. 2020, 102, 103419. [Google Scholar] [CrossRef] [PubMed]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef]
- Liu, W.J.; Ye, L.; Huang, W.F.; Guo, L.J.; Xu, Z.G.; Wu, H.L.; Yang, C.; Liu, H.F. p62 links the autophagy pathway and the ubiqutin–proteasome system upon ubiquitinated protein degradation. Cell. Mol. Biol. Lett. 2016, 21, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marinković, M.; Šprung, M.; Buljubašić, M.; Novak, I. Autophagy Modulation in Cancer: Current Knowledge on Action and Therapy. Oxidative Med. Cell. Longev. 2018, 2018, e8023821. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Turnbull, D.M.; Reeve, A.K. Mitochondrial Dysfunction in Parkinson’s Disease—Cause or Consequence? Biology 2019, 8, 38. [Google Scholar] [CrossRef] [Green Version]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [Green Version]
- Masoudi-Sobhanzadeh, Y.; Omidi, Y.; Amanlou, M.; Masoudi-Nejad, A. Drug databases and their contributions to drug repurposing. Genomics 2020, 112, 1087–1095. [Google Scholar] [CrossRef]
- Choi, W.; Lee, H. Inference of Biomedical Relations among Chemicals, Genes, Diseases, and Symptoms Using Knowledge Representation Learning. IEEE Access 2019, 7, 179373–179384. [Google Scholar] [CrossRef]
- Hebels, D.G.; Jetten, M.J.A.; Aerts, H.J.W.; Herwig, R.; Theunissen, D.H.J.; Gaj, S.; Van Delft, J.H.; Kleinjans, J.C.S. Evaluation of database-derived pathway development for enabling biomarker discovery for hepatotoxicity. Biomark. Med. 2014, 8, 185–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rappaport, N.; Twik, M.; Plaschkes, I.; Nudel, R.; Stein, T.I.; Levitt, J.; Gershoni, M.; Morrey, C.P.; Safran, M.; Lancet, D. MalaCards: An amalgamated human disease compendium with diverse clinical and genetic annotation and structured search. Nucleic Acids Res. 2017, 45, D877–D887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Ji, P.; Liu, J.; Broaddus, R.R.; Xue, F.; Zhang, W. Centrosome-associated regulators of the G2/M checkpoint as targets for cancer therapy. Mol. Cancer 2009, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, D.M.; Thomas, S.D.; Islam, A.; Muench, D.; Sedoris, K. c-Myc and cancer metabolism. Clin. Cancer Res. 2012, 18, 5546–5553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabregat, I.; Fernando, J.; Mainez, J.; Sancho, P. TGF-beta Signaling in Cancer Treatment. Curr. Pharm. Des. 2014, 20, 2934–2947. [Google Scholar] [CrossRef]
- Krishnamurthy, N.; Kurzrock, R. Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat. Rev. 2018, 62, 50–60. [Google Scholar] [CrossRef]
- Bai, X.; Yi, M.; Jiao, Y.; Chu, Q.; Wu, K. Blocking TGF-β Signaling To Enhance The Efficacy Of Immune Checkpoint Inhibitor. OncoTargets Ther. 2019, 12, 9527–9538. [Google Scholar] [CrossRef] [Green Version]
- Dang, C.V. MYC on the Path to Cancer. Cell 2012, 149, 22–35. [Google Scholar] [CrossRef] [Green Version]
- Loizzo, M.R.; Bonesi, M.; Passalacqua, N.G.; Saab, A.; Menichini, F.; Tundis, R. Antiproliferative activities on renal, prostate and melanoma cancer cell lines of Sarcopoterium spinosum aerial parts and its major constituent tormentic acid. Anticancer Agents Med. Chem. 2013, 13, 768–776. [Google Scholar] [CrossRef]
- Ramirez, H.; Patel, S.B.; Pastar, I. The Role of TGFβ Signaling in Wound Epithelialization. Adv. Wound Care 2014, 3, 482–491. [Google Scholar] [CrossRef] [Green Version]
- Penn, J.W.; Grobbelaar, A.O.; Rolfe, K.J. The role of the TGF-β family in wound healing, burns and scarring: A review. Int. J. Burns Trauma 2012, 2, 18–28. [Google Scholar] [PubMed]
- Wu, C.; Xue, Y.; Wang, P.; Lin, L.; Liu, Q.; Li, N.; Xu, J.; Cao, X. IFN-γ primes macrophage activation by increasing phosphatase and tensin homolog via downregulation of miR-3473b. J. Immunol. Baltim. Md 1950 2014, 193, 3036–3044. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Chakravarty, S.D.; Ivashkiv, L.B. Regulation of IFN and TLR Signaling During Macrophage Activation by Opposing Feedforward and Feedback Inhibition Mechanisms. Immunol. Rev. 2008, 226, 41–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, L.; Aigner, P.; Stoiber, D. Type I Interferons and Natural Killer Cell Regulation in Cancer. Front. Immunol. 2017, 8, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.J.; Mian, F.; Poznanski, S.M.; Stackaruk, M.; Chan, T.; Chew, M.V.; Ashkar, A.A. Type I Interferon Receptor on NK Cells Negatively Regulates Interferon-γ Production. Front. Immunol. 2019, 10, 1261. [Google Scholar] [CrossRef] [Green Version]
- Paolini, R.; Bernardini, G.; Molfetta, R.; Santoni, A. NK cells and interferons. Cytokine Growth Factor Rev. 2015, 26, 113–120. [Google Scholar] [CrossRef]
- Mantlo, E.; Bukreyeva, N.; Maruyama, J.; Paessler, S.; Huang, C. Antiviral activities of type I interferons to SARS-CoV-2 infection. Antivir. Res. 2020, 179, 104811. [Google Scholar] [CrossRef]
- Sainz, B.; Mossel, E.C.; Peters, C.J.; Garry, R.F. Interferon-beta and interferon-gamma synergistically inhibit the replication of severe acute respiratory syndrome-associated coronavirus (SARS-CoV). Virology 2004, 329, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Zou, C.; Wang, B.; Li, C.; Lin, M.; Mo, L.; Zhang, B.; Huang, N.; Wei, B.; Yang, X.; et al. Network pharmacology and bioinformatics analysis identified essential genes of Jingulian in the treatment of rheumatoid arthritis and COVID-19. Ann. Transl. Med. 2022, 10, 635. [Google Scholar] [CrossRef]
- Shahat, A.A.; Cos, P.; De Bruyne, T.; Apers, S.; Hammouda, F.M.; Ismail, S.I.; Azzam, S.; Claeys, M.; Goovaerts, E.; Pieters, L.; et al. Antiviral and antioxidant activity of flavonoids and proanthocyanidins from Crataegus sinaica. Planta Med. 2002, 68, 539–541. [Google Scholar] [CrossRef]
- Jin, Y.-H.; Lee, J.; Jeon, S.; Kim, S.; Min, J.S.; Kwon, S. Natural Polyphenols, 1,2,3,4,6-O-Pentagalloyglucose and Proanthocyanidins, as Broad-Spectrum Anticoronaviral Inhibitors Targeting Mpro and RdRp of SARS-CoV-2. Biomedicines 2022, 10, 1170. [Google Scholar] [CrossRef] [PubMed]
- Slomski, A. Trials Test Mushrooms and Herbs as Anti–COVID-19 Agents. JAMA 2021, 326, 1997–1999. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, F.; Anderson, D.; Najafzadeh, M. The Antiviral, Anti-Inflammatory Effects of Natural Medicinal Herbs and Mushrooms and SARS-CoV-2 Infection. Nutrients 2020, 12, 2573. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-T.; Hsu, W.-C.; Lin, C.-C. Antiviral Natural Products and Herbal Medicines. J. Tradit. Complement. Med. 2014, 4, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Kong, Q.; Wu, Y.; Gu, Y.; Lv, Q.; Qi, F.; Gong, S.; Chen, X. Analysis of the molecular mechanism of Pudilan (PDL) treatment for COVID-19 by network pharmacology tools. Biomed. Pharmacother. 2020, 128, 110316. [Google Scholar] [CrossRef]
- Rozenberg, K.; Smirin, P.; Sampson, S.R.; Rosenzweig, T. Insulin-sensitizing and insulin-mimetic activities of Sarcopoterium spinosum extract. J. Ethnopharmacol. 2014, 155, 362–372. [Google Scholar] [CrossRef]
- Rozenberg, K.; Rosenzweig, T. Sarcopoterium spinosum extract improved insulin sensitivity in mice models of glucose intolerance and diabetes. PLoS ONE 2018, 13, e0196736. [Google Scholar] [CrossRef] [Green Version]
- Stolz, A.; Ernst, A.; Dikic, I. Cargo recognition and trafficking in selective autophagy. Nat. Cell Biol. 2014, 16, 495–501. [Google Scholar] [CrossRef]
- Bjørkøy, G.; Lamark, T.; Pankiv, S.; Øvervatn, A.; Brech, A.; Johansen, T. Chapter 12 Monitoring Autophagic Degradation of p62/SQSTM1. In Methods in Enzymology; Autophagy in Mammalian Systems, Part B; Academic Press: Cambridge, MA, USA, 2009; Volume 452, pp. 181–197. [Google Scholar]
- Dafni, A.; Yaniv, Z.; Palevitch, D. Ethnobotanical survey of medicinal plants in northern Israel. J. Ethnopharmacol. 1984, 10, 295–310. [Google Scholar] [CrossRef]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a Soluble Tetrazolium/Formazan Assay for Cell Growth and Drug Sensitivity in Culture Using Human and Other Tumor Cell Lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar]
- Ewing, B.; Hillier, L.; Wendl, M.C.; Green, P. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res. 1998, 8, 175–185. [Google Scholar] [CrossRef] [Green Version]
- Babraham Bioinformatics—FastQC A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 10 November 2021).
- HISAT2. Available online: http://DaehwanKimLab.github.io/hisat2/ (accessed on 10 November 2021).
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Yu, D.; Seo, M.; Hersh, C.P.; Weiss, S.T.; Qiu, W. Novel Data Transformations for RNA-seq Differential Expression Analysis. Sci. Rep. 2019, 9, 4820. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [Green Version]
- Connectivity Map (CMAP). Available online: https://www.broadinstitute.org/connectivity-map-cmap (accessed on 20 July 2022).
- Ganger, M.T.; Dietz, G.D.; Ewing, S.J. A common base method for analysis of qPCR data and the application of simple blocking in qPCR experiments. BMC Bioinform. 2017, 18, 534. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gahramanov, V.; Oz, M.; Aouizerat, T.; Rosenzweig, T.; Gorelick, J.; Drori, E.; Salmon-Divon, M.; Sherman, M.Y.; Lubin, B.C.R. Integration of the Connectivity Map and Pathway Analysis to Predict Plant Extract’s Medicinal Properties—The Study Case of Sarcopoterium spinosum L. Plants 2022, 11, 2195. https://doi.org/10.3390/plants11172195
Gahramanov V, Oz M, Aouizerat T, Rosenzweig T, Gorelick J, Drori E, Salmon-Divon M, Sherman MY, Lubin BCR. Integration of the Connectivity Map and Pathway Analysis to Predict Plant Extract’s Medicinal Properties—The Study Case of Sarcopoterium spinosum L. Plants. 2022; 11(17):2195. https://doi.org/10.3390/plants11172195
Chicago/Turabian StyleGahramanov, Valid, Moria Oz, Tzemach Aouizerat, Tovit Rosenzweig, Jonathan Gorelick, Elyashiv Drori, Mali Salmon-Divon, Michael Y. Sherman, and Bat Chen R. Lubin. 2022. "Integration of the Connectivity Map and Pathway Analysis to Predict Plant Extract’s Medicinal Properties—The Study Case of Sarcopoterium spinosum L." Plants 11, no. 17: 2195. https://doi.org/10.3390/plants11172195
APA StyleGahramanov, V., Oz, M., Aouizerat, T., Rosenzweig, T., Gorelick, J., Drori, E., Salmon-Divon, M., Sherman, M. Y., & Lubin, B. C. R. (2022). Integration of the Connectivity Map and Pathway Analysis to Predict Plant Extract’s Medicinal Properties—The Study Case of Sarcopoterium spinosum L. Plants, 11(17), 2195. https://doi.org/10.3390/plants11172195







