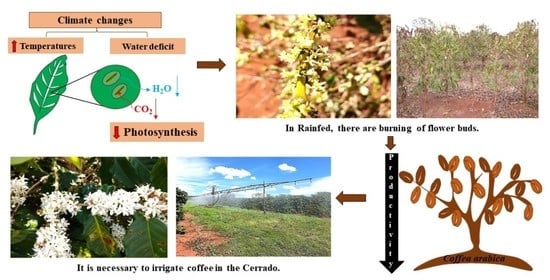
Physiological Changes of Arabica Coffee under Different Intensities and Durations of Water Stress in the Brazilian Cerrado
Abstract
:1. Introduction
2. Results
2.1. Photosynthetic Analysis
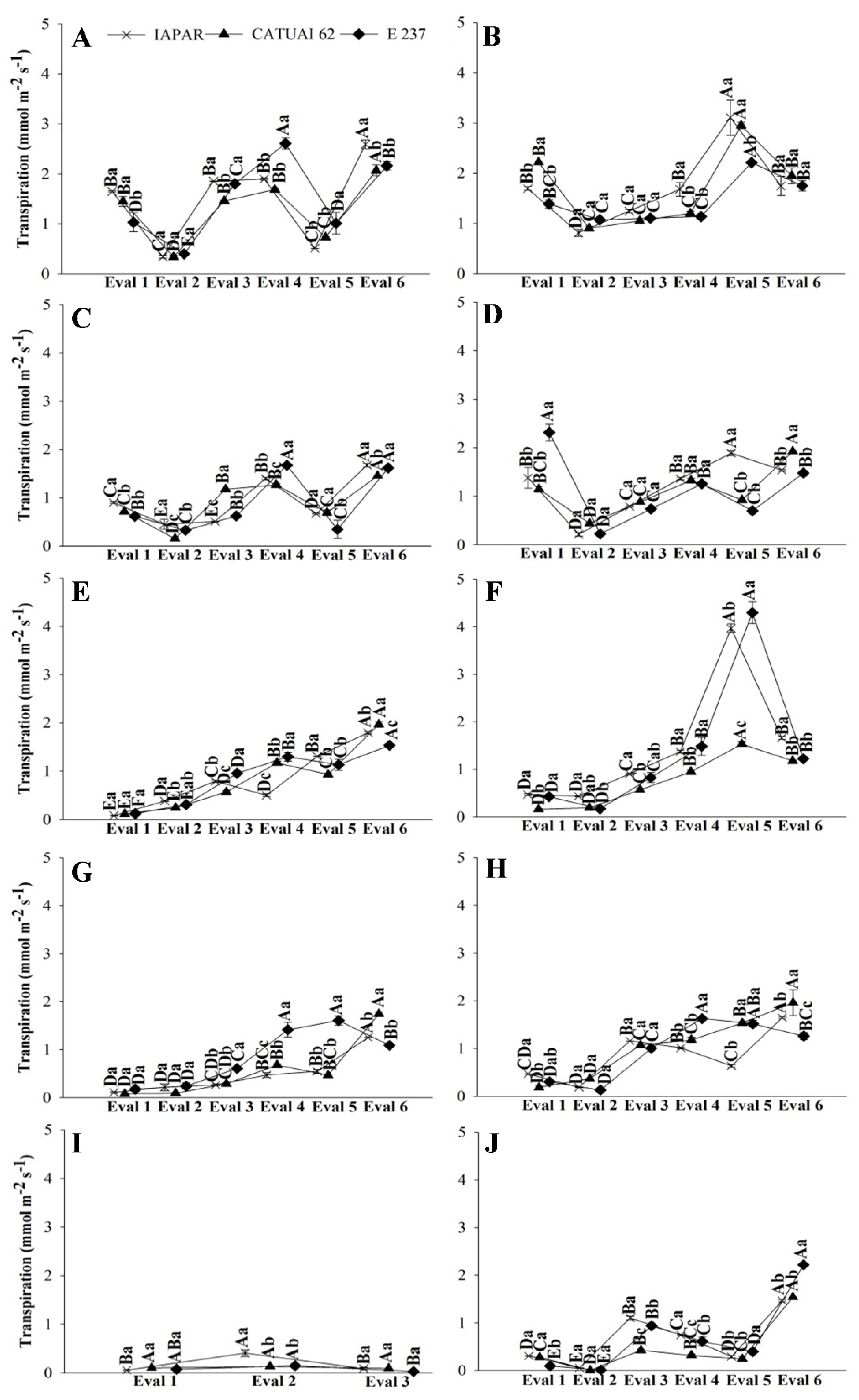
2.1.1. Daily Curve
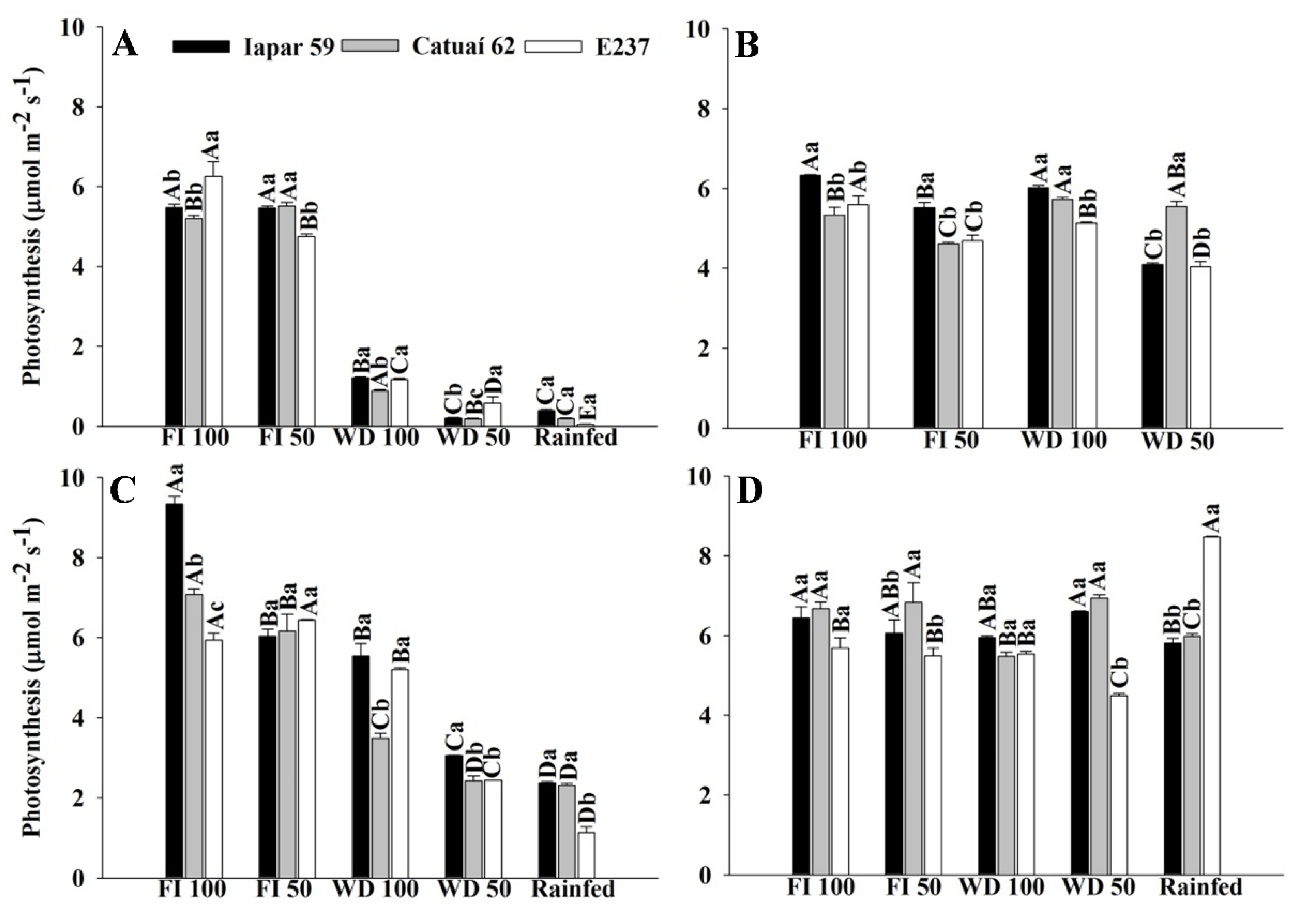
2.1.2. Gas Exchange—Genotypes x Recovery
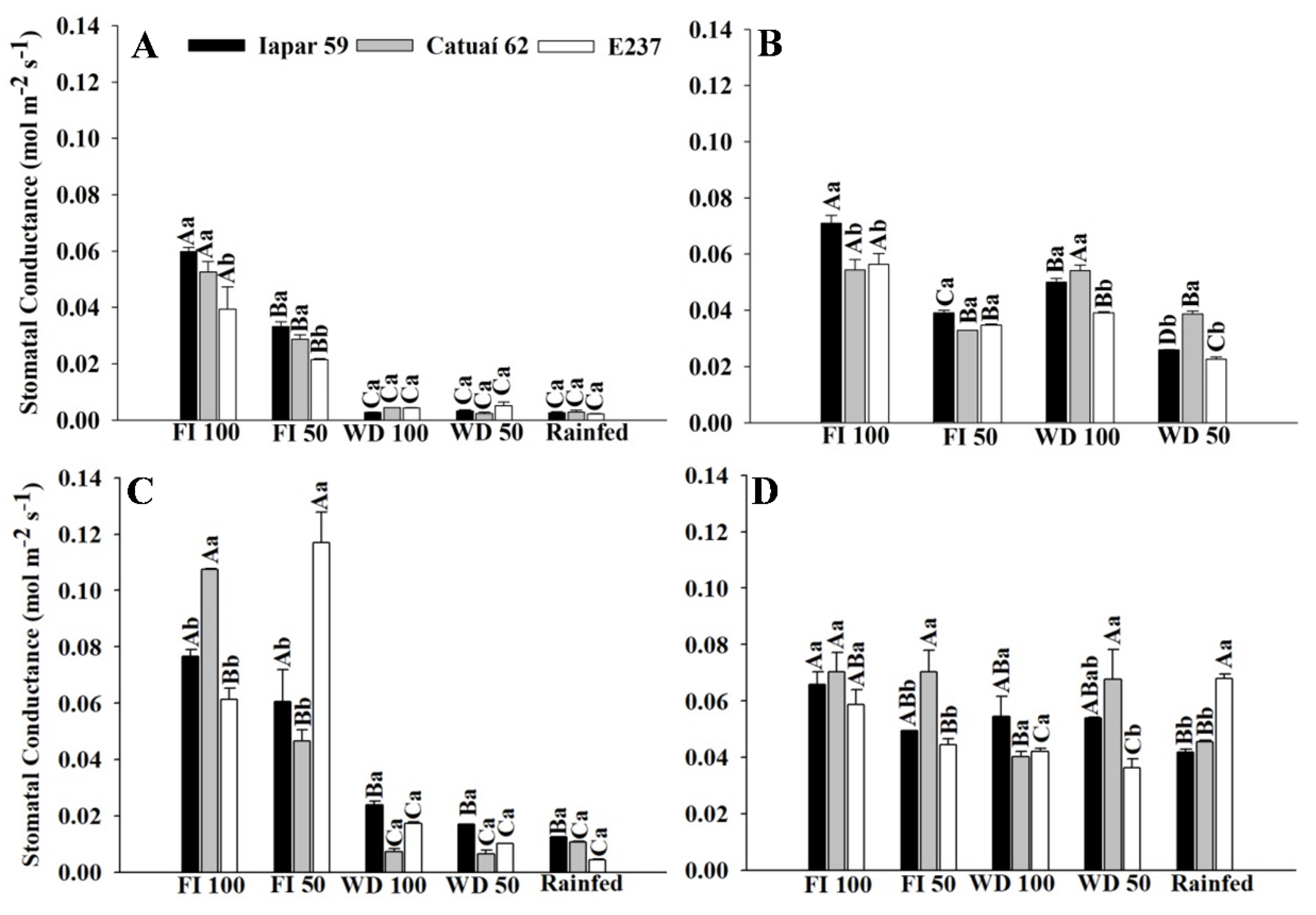
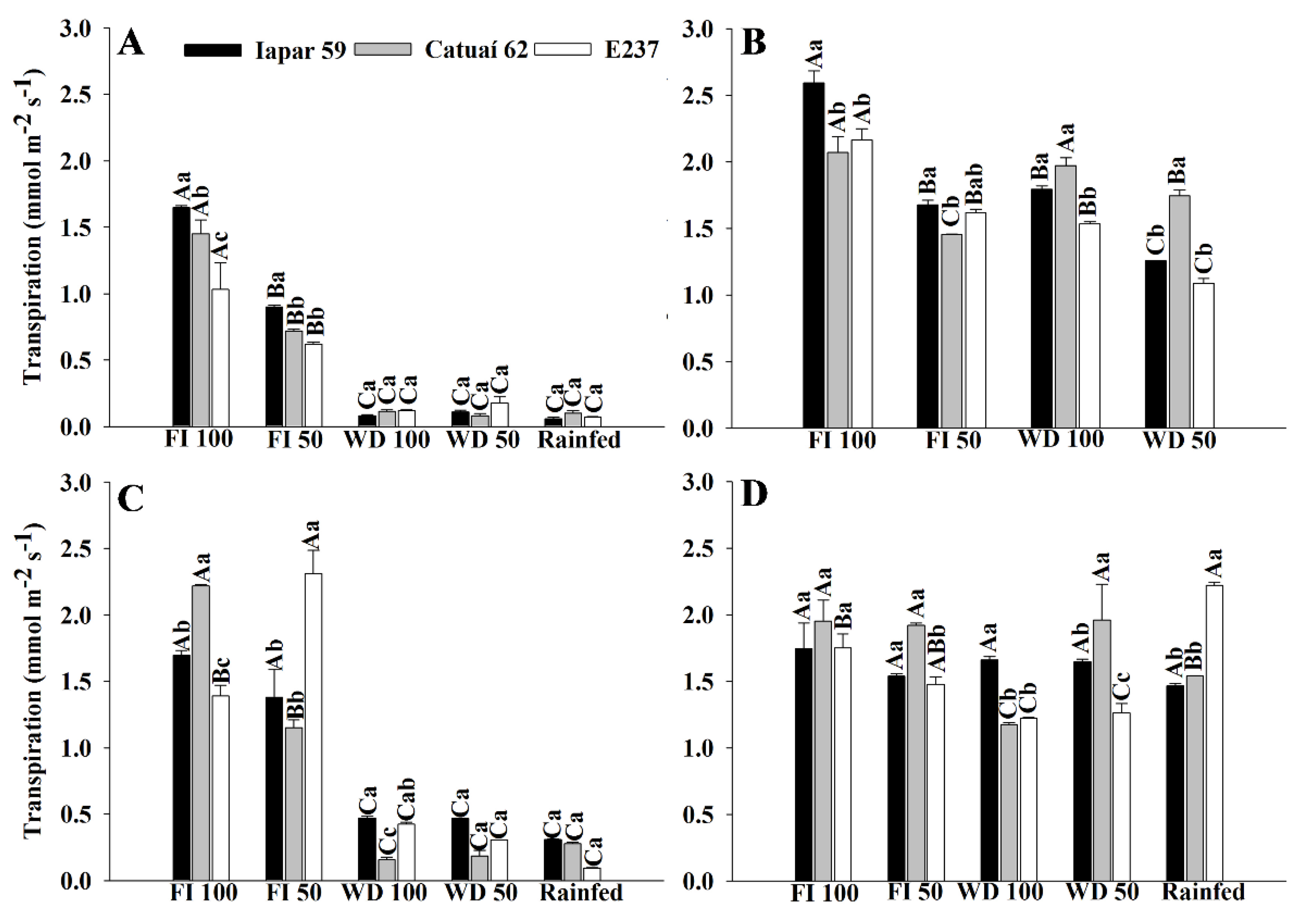
2.1.3. Gas Exchange—Genotypes x Water Regimes
2.1.4. Relative Water Content
2.2. Productivity
3. Discussion
3.1. Gas Exchange
3.2. Relative Water Content
3.3. Productivity
4. Materials and Methods
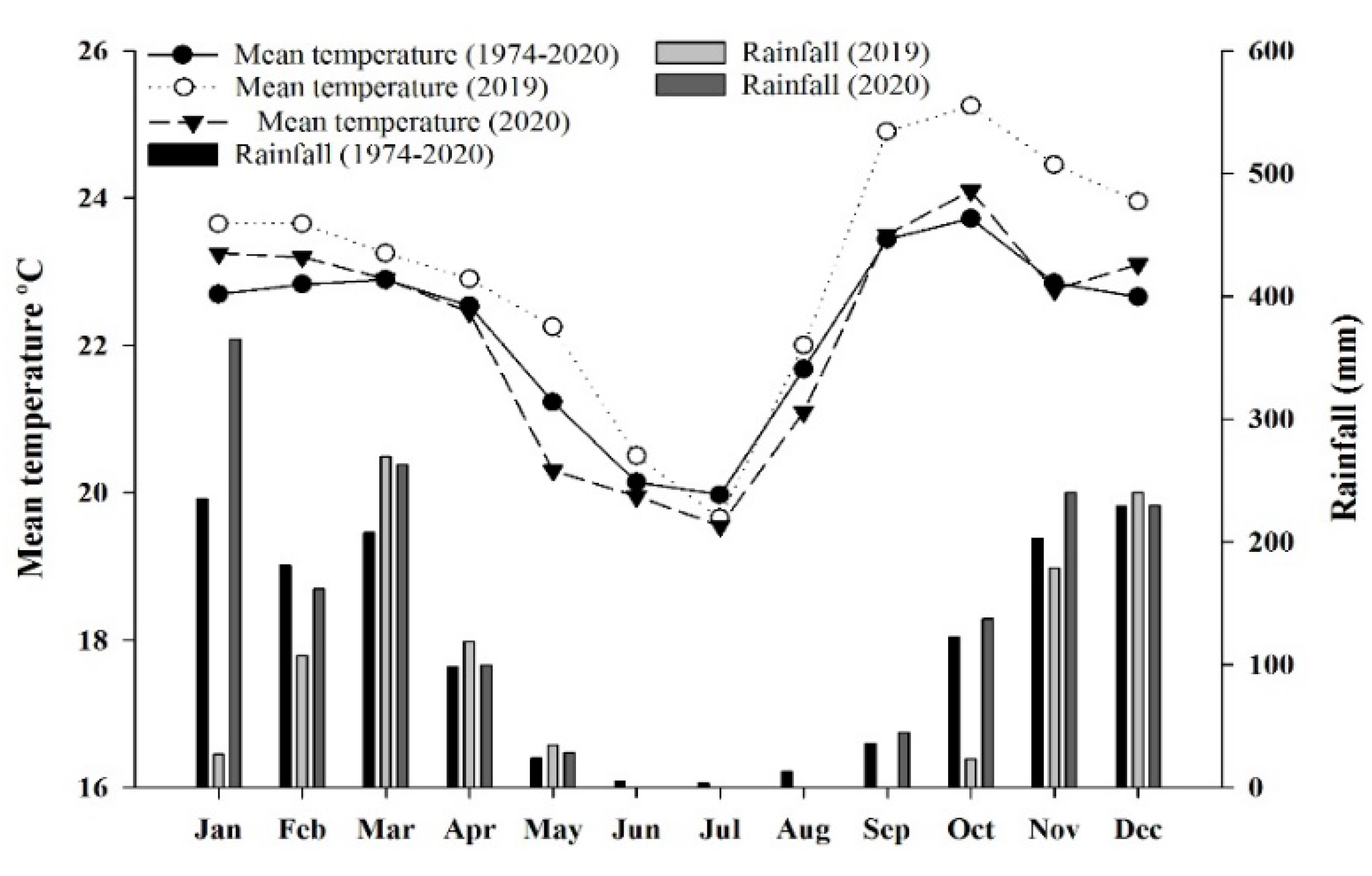
4.1. Characterization of the Experimental Area
4.2. Experimental Setup
4.3. Variables Analyzed
4.3.1. Physiological Variables
4.3.2. Soil Water Content and Relative Water Content in Leaves
4.3.3. Productivity
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DaMatta, F.M.; Avila, R.T.; Cardoso, A.A.; Martins, S.C.; Ramalho, J.C. Physiological and agronomic performance of the coffee crop in the context of climate change and global warming: A review. J. Agric Food Chem. 2018, 66, 5264–5274. [Google Scholar] [CrossRef] [PubMed]
- Wintgens, J.N. Coffee: Growing, Processing, Sustainable Production: A Guidebook for Growers, Processors, Traders, and Researchers, 1st ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004; pp. 1–24. [Google Scholar]
- Veiga, A.D.; Rodrigues, G.C.; Rocha, O.C.; Bartholo, G.F.; Guerra, A.F.; Silva, T.P.D. Arabica coffee cultivars in different water regimes in the central cerrado region. Coffee Sci. 2019, 14, 349–358. [Google Scholar] [CrossRef]
- Zullo, J.; Pinto, H.S.; Assad, E.D. Impact assessment study of climate change on agricultural zoning. Meteorol. Appl. 2006, 13, 69–80. [Google Scholar]
- Magrach, A.; Ghazoul, J. Climate and pest-driven geographic shifts in global coffee production: Implications for forest cover, biodiversity and carbon storage. PLoS ONE 2015, 10, e0133071. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Ronchi, C.P.; Maestri, M.; Barros, R.S. Coffee: Environment and Crop Physiology. In Ecophysiology of Tropical Tree Crops; DaMatta, F.M., Ed.; Nova Science Publishers: New York, NY, USA, 2010; pp. 181–216. [Google Scholar]
- Silva, E.A.D.; Brunini, O.; Sakai, E.; Arruda, F.B.; Pires, R.C.M. Influência de déficits hídricos controlados na uniformização do florescimento e produção do cafeeiro em três diferentes condições edafoclimáticas do Estado de São Paulo. Bragantia 2009, 68, 1678–4499. [Google Scholar] [CrossRef]
- Bhusal, N.; Han, S.G.; Yoon, T.M. Impact of drought stress on photosynthetic response, leaf water potential, and stem sap flow in two cultivars of bi-leader apple trees (Malus× domestica Borkh.). Sci. Hortic. 2019, 246, 535–543. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Han, A.R.; Han, A.; Kim, H.S. Responses to drought stress in Prunus sargentii and Larix kaempferi seedlings using morphological and physiological parameters. For. Ecol. Manag. 2020, 465, 118099. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.-T.; Moutinho-Pereira, J.; Correia, C.M. Drought stress effects and olive tree acclimation under a changing climate. Plants 2019, 8, 232. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Ramalho, J.D.C. Impacts of drought and temperature stress on coffee physiology and production: A review. Braz. J. Plant Physiol. 2006, 18, 55–81. [Google Scholar] [CrossRef]
- Jones, P.; Jones, J.W.; Allen, L.H. Seasonal carbon and water balances of soybeans grown under stress treatments in sunlit chambers. Trans. ASAE 1985, 28, 2021–2028. [Google Scholar] [CrossRef]
- Mielke, M.S.; Schaffer, B.; Schilling, A.C. Evaluation of reflectance spectroscopy indices for estimation of chlorophyll content in leaves of a tropical tree species. Photosynthetica 2012, 50, 343–352. [Google Scholar] [CrossRef]
- Martins, S.C.; Galmes, J.; Cavatte, P.C.; Pereira, L.F.; Ventrella, M.C.; DaMatta, F.M. Understanding the low photosynthetic rates of sun and shade coffee leaves: Bridging the gap on the relative roles of hydraulic, diffusive and biochemical constraints to photosynthesis. PLoS ONE 2014, 9, 95571. [Google Scholar] [CrossRef] [PubMed]
- Franck, N.; Vaast, P.; Génard, M.; Dauzat, J. Soluble sugars mediate sink feedback down-regulation of leaf photosynthesis in field-grown Coffea arabica. Tree Physiol. 2006, 26, 517–525. [Google Scholar] [CrossRef]
- Silva, E.A.; DaMatta, F.M.; Ducatti, C.; Regazzi, A.J.; Barros, R.S. Seasonal changes in vegetative growth and photosynthesis of arabica coffee trees. Field Crops Res. 2004, 89, 349–357. [Google Scholar] [CrossRef]
- Silva, P.C.; Ribeiro Junior, W.Q.; Ramos, M.L.G.; Celestino, S.M.C.; Silva, A.D.N.; Casari, R.A.D.C.N.; Vinson, C.C. Quinoa for the Brazilian Cerrado: Agronomic characteristics of elite genotypes under different water regimes. Plants 2021, 10, 1591. [Google Scholar] [CrossRef] [PubMed]
- Bhusal, N.; Lee, M.; Lee, H.; Adhikari, A.; Han, A.R.; Han, A.; Kim, H.S. Evaluation of morphological, physiological, and biochemical traits for assessing drought resistance in eleven tree species. Sci. Total Environ. 2021, 779, 146466. [Google Scholar] [CrossRef]
- Tombesi, S.; Nardini, A.; Frioni, T.; Soccolini, M.; Zadra, C.; Farinelli, D.; Poni, S.; Palliotti, A. Stomatal closure is induced by hydraulic signals and maintained by ABA in drought-stressed grapevine. Sci. Rep. 2015, 5, 12449. [Google Scholar] [CrossRef]
- Flexas, J.; Ribas-Carbo, M.; Diaz-Espejo, A.; Galmes, J.; Medrano, H. Mesophyll conductance to CO2: Current knowledge and future prospects. Plant Cell Environ. 2008, 31, 602–621. [Google Scholar] [CrossRef]
- Zait, Y.; Shtein, I.; Schwartz, A. Long-term acclimation to drought, salinity and temperature in the thermophilic tree Ziziphus spina-christi: Revealing different tradeoffs between mesophyll and stomatal conductance. Tree Physiol. 2019, 39, 701–716. [Google Scholar] [CrossRef]
- Melo, E.F.; Fernandes-Brum, C.N.; Pereira, F.J.; Castro, E.M.D.; Chalfun-Júnior, A. Anatomic and physiological modifications in seedlings of Coffea arabica cultivar Siriema under drought conditions. Ciênc. Agrotec. 2014, 38, 25–33. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; Cochard, H. Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiol. 2009, 149, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.G.; Sera, G.H.; Andreazi, E.; Sera, T.; Fonseca, I.C.D.B.; Carducci, F.C.; Costa, K.C. Tolerância ao déficit hídrico em mudas de genótipos de café portadores de genes de diferentes espécies. Coffee Sci. 2017, 12, 156–163. [Google Scholar] [CrossRef]
- Queiroz-Voltan, R.B. Caracterização da anatomia foliar de cafeeiros arábica em diferentes períodos sazonais. Biotemas 2014, 27, 1–10. [Google Scholar] [CrossRef]
- Freire, L.P.; Marraccini, P.; Rodrigues, G.C.; Andrade, A.C. Analysis of the mannose 6 phosphate reductase gene expression in coffee trees submitted to water deficit. Coffee Sci. 2013, 8, 17–23. [Google Scholar]
- DaMatta, F.M.; Chaves, A.R.M.; Pinheiro, H.A.; Ducatti, C.; Loureiro, M.E. Drought tolerance of two field-grown clones of Coffea canephora. Plant Sci. 2003, 164, 111–117. [Google Scholar] [CrossRef]
- Pinheiro, H.A.; DaMatta, F.M.; Chaves, A.R.M.; Loureiro, M.E.; Ducatti, C. Drought tolerance is associated with rooting depth and stomatal control of water use in clones of Coffea canephora. Ann. Bot. 2005, 96, 101–108. [Google Scholar] [CrossRef]
- Lugojan, C.; Ciulca, S. Evaluation of relative water content in winter wheat. J. Hortic. Fores. Biotechnol. 2011, 15, 173–177. [Google Scholar]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Da Matta, F.M.; Maestri, M.; Barros, R.S.; Regazzi, A.J. Water relations of coffee leaves (Coffea arabica and C. canephora) in response to drought. J. Hortic. Sci. 1993, 68, 741–746. [Google Scholar] [CrossRef]
- Guerra, A.F.; Rocha, O.C.; Rodrigues, G.C. Manejo do cafeeiro irrigado no Cerrado com estresse hídrico controlado. Irrigação Tecnol. Mod. 2005, 65, 42–45. [Google Scholar]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; De Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Soil Survey Staff. Soil Survey Laboratory Methods Manual. Soil Survey Investigations Report no. 42, Version 5.0; R. Burt and Soil Survey Staff, Ed.; Natural Resources Conservation Service, US Department of Agriculture: Washington, DC, USA, 2014.
- Embrapa—Empresa Brasileira de Pesquisa Agropecuária. Monitoramento de Irrigação no Cerrado. 2017. Available online: http://hidro.cpac.embrapa.br (accessed on 27 April 2020).
- SAS-Statistical Analyses System. Statistical Analysis System User’s Guide; Institute, Statistical Analyses System: Cary, NC, USA, 2008. [Google Scholar]




| Water Regime | Genotype | |||||
|---|---|---|---|---|---|---|
| E237 | Iapar 59 | Catuaí 62 | E237 | Iapar 59 | Catuaí 62 | |
| 2019 | 2020 | |||||
| FI 100 | 78.92 Aa | 76.90 Aa | 74.95 Aa | 61.72 ABb | 77.78 Aa | 77.53 Aa |
| FI 50 | 73.07 Ba | 73.75 Aa | 73.24 Aa | 73.94 Aa | 71.84 Aa | 72.97 Aa |
| WD 100 | 36.04 CDb | 40.15 Bab | 43.04 Ba | 42.42 Cb | 54.36 Ba | 52.95 Ba |
| WD 50 | 31.83 Da | 33.42 Ca | 31.34 Ca | 45.65 Ca | 49.05 Ba | 49.77 Ba |
| Rainfed | 38.02 Ca | 38.09B Ca | 31.98 Cb | 50.11B Ca | 49.72 Ba | 43.18 Ba |
| Water Regime | RWC (%) | |
|---|---|---|
| 2019 | 2020 | |
| FI 100 | 70.82 b | 78.43 a |
| FI 50 | 72.34 ab | 78.54 a |
| WD 100 | 76.13 a | 76.36 ab |
| WD 50 | 71.22 b | 76.30 ab |
| Rainfed | 32.95 c | 74.35 b |
| Genotype | RWC (%) | |
|---|---|---|
| 2019 | 2020 | |
| E237 | 65.07 ab | 80.07 a |
| Iapar 59 | 66.09 a | 75.17 b |
| Catuaí 62 | 62.92 b | 75.15 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, P.C.d.; Junior, W.Q.R.; Ramos, M.L.G.; Rocha, O.C.; Veiga, A.D.; Silva, N.H.; Brasileiro, L.d.O.; Santana, C.C.; Soares, G.F.; Malaquias, J.V.; et al. Physiological Changes of Arabica Coffee under Different Intensities and Durations of Water Stress in the Brazilian Cerrado. Plants 2022, 11, 2198. https://doi.org/10.3390/plants11172198
Silva PCd, Junior WQR, Ramos MLG, Rocha OC, Veiga AD, Silva NH, Brasileiro LdO, Santana CC, Soares GF, Malaquias JV, et al. Physiological Changes of Arabica Coffee under Different Intensities and Durations of Water Stress in the Brazilian Cerrado. Plants. 2022; 11(17):2198. https://doi.org/10.3390/plants11172198
Chicago/Turabian StyleSilva, Patrícia Carvalho da, Walter Quadros Ribeiro Junior, Maria Lucrecia Gerosa Ramos, Omar Cruz Rocha, Adriano Delly Veiga, Nathalia Henriques Silva, Lemerson de Oliveira Brasileiro, Charles Cardoso Santana, Guilherme Filgueiras Soares, Juaci Vitória Malaquias, and et al. 2022. "Physiological Changes of Arabica Coffee under Different Intensities and Durations of Water Stress in the Brazilian Cerrado" Plants 11, no. 17: 2198. https://doi.org/10.3390/plants11172198
APA StyleSilva, P. C. d., Junior, W. Q. R., Ramos, M. L. G., Rocha, O. C., Veiga, A. D., Silva, N. H., Brasileiro, L. d. O., Santana, C. C., Soares, G. F., Malaquias, J. V., & Vinson, C. C. (2022). Physiological Changes of Arabica Coffee under Different Intensities and Durations of Water Stress in the Brazilian Cerrado. Plants, 11(17), 2198. https://doi.org/10.3390/plants11172198