Abstract
Foeniculum vulgare is used for the treatment of diarrhea in Mexican traditional medicine. Hexane extract showed 94 % inhibition of Giardia duodenalis trophozoites at 300 μg/mL. Therefore, 20 constituents of hexane extract were evaluated to determine their antigiardial activity. Interestingly, six compounds showed good activity toward the parasite. These compounds were (1R,4S) (+)-Camphene (61%), (R)(−)-Carvone (66%), estragole (49%), p-anisaldehyde (67%), 1,3-benzenediol (56%), and trans, trans-2,4-undecadienal (97%). The aldehyde trans, trans-2,4-undecadienal was the most active compound with an IC50 value of 72.11 µg/mL against G. duodenalis trophozoites. This aldehyde was less toxic (IC50 588.8 µg/mL) than positive control metronidazole (IC50 83.5 µg/mL) against Vero cells. The above results could support the use of F. vulgare in Mexican traditional medicine.
1. Introduction
Giardia duodenalis (syn. Giardia lamblia, Giardia intestinalis) is a flagellated enteric protozoan and the etiologic agent of giardiasis, a relevant public health problem. G. duodenalis is the most common protozoal infection in humans and is an important cause of waterborne and foodborne diarrhea, day-care center outbreaks, and traveler’s diarrhea [1]. It contributes to an estimated 280 million human cases of diarrhea every year and has been included as part of the WHO Neglected Disease Initiative associated with poverty [1,2,3]. The prevalence of giardiasis in humans ranges from 2–3% in industrialized countries, up to 30% in low-income and developing countries [4].
The life cycle of G. duodenalis is direct and involves two stages, the trophozoite, which is the replicative stage, and the cyst, which is the infective stage [5]. Giardiasis mainly occurs in children, and a serious manifestation is its contribution to malnutrition and the stunting of growth following early or repeated infections [6]. It is spread among people with poor hygiene habits, and may result in asymptomatic, acute, or chronic disease, depending on multiple factors such as the age of the patient, history of prior exposure, the parasite load, virulence of the parasite, and the immune response of the host [1]. Beyond diarrhea, symptoms include abdominal bloating and cramps, malabsorption and weight loss [2].
Adequate sanitation is not sufficient for protection against giardiasis and there are no available biological prophylactic methods against G. duodenalis infections, meaning that its control is reliant on treatment [3].
There are numerous commercially available drugs for the treatment of giardiasis including nitroimidazole derivatives (metronidazole, tinidazole, secnidazole and ornidazole), benzimidazoles (albendazole, mebendazole), nitazoxanide, furazolidone, quinacrine, chloroquine and paromomycin [6]. Although, there are many antigiardial drugs available, only a few are used in daily practice. In 90% of cases, treatment regimens with a single agent, either metronidazole or albendazole, are effective, but drug resistance has been reported [7,8], leading to combinations of the therapies.
In addition to drug resistance, other factors can also be responsible for treatment failure, such as reinfection in endemic areas with poor sanitation and hygiene, insufficient amounts of drug administered, immunosuppression, or sequestration in the gallbladder or the pancreas [9]. Adverse effects such as nausea, abdominal pain, and diarrhea have also been associated with the use of these drugs, perhaps most controversial is their potential to be genotoxic and carcinogenic in humans [3,10]. Therefore, there is a need to develop new, safer antigiardial agents, and natural products could be an excellent alternative, particularly in communities with limited access to health services.
Foeniculum vulgare Mill. (Apiaceae family), commonly known as fennel, is a widespread perennial plant with an aromatic odor. It is native to Southern Europe and the Mediterranean region and is currently widely cultivated throughout the temperate and tropical regions of the world. The chemical constituents of fennel include fatty acids, phenylpropanoids, terpenes, coumarins, flavonoids, and other types of compounds [11].
The essential oil of fennel fruits is used for flavoring purposes, and in cosmetic and pharmaceutical products. Extracts of F. vulgare and some of its constituents have shown antioxidant, antibacterial, antifungal, estrogenic, and antituberculosis activities [11]. Aerial parts of F. vulgare are used in Mexico for the treatment of bronchitis, coughs, dysmenorrhea, diarrhea, and abdominal pains [12]. The aim of this study was to determine the antigiardial activity of F. vulgare hexane extract and some of its components to support its use in the traditional Mexican medicine.
2. Results
Hexane extract of F. vulgare was prepared previously [13], and tested for antigiardial activity, showing 94% inhibition of G. duodenalis trophozoites at 300 μg/mL. The chemical composition of this extract was determined using gas chromatography-mass spectrometry (GG-MS) by our research group, identifying the compounds by their mass spectra fragmentation patterns using the NIST 1.7 library database [13].
Nineteen compounds identified previously in the hexane extract [13] were tested for antigiardial activity at 300 μg/mL. Results in Table 1 show that six compounds, including the bicyclic monoterpene, (1R,4S)(+)-camphene (61%); the monoterpene, (R)(−)-carvone (66%); the aromatic ether, estragole (49%); the aromatic aldehyde, p-anisaldehyde (67%); the diphenol, 1,3-benzenediol (56%); and the aldehyde, rans, trans-2,4-undecadienal (97%), inhibited G. duodenalis trophozoites in the range of 49 to 97%, with the aldehyde trans, trans-2,4-undecadienal being the most active compound. The above activity of the monoterpenes (1R,4S) (+) camphene was shown by one stereoisomer; however, it is necessary to determine the antigiardial activity of the other stereoisomers to see if there is a structure–activity relationship.

Table 1.
Antigiardial activity of F. vulgare hexane extract and its chemical constituents.
On the other hand, the activity of the monoterpene (R) (−)-carvone was given by one enantiomer, so it will be necessary to examine the other enantiomer. For example, the antigiardial activity of the enantiomers (S) (+)-fenchone and (R)(−)-fenchone showed 16 and 6.5 % of inhibition at 300 µg/mL, respectively. It can be seen that tructure–activity relationship may be reflected in their antigiardial activity because the first terpene was 2.46-times more active than the second one.
Lastly, we have not anticipated if there is any difference in the antigiardial activity of the o-, m, or p-cymenes. Thus, it will be necessary to carry out these experiments in the future.
In addition, half-maximal inhibitory concentrations (IC50) towards G. duodenalis trophozoites and Vero cells were determined for the hexane extract, as well as for the six most active compounds that showed a percentage of inhibition (≥49%) of trophozoites (Table 2). These were all compared to metronidazole, the most common first choice of treatment against G. duodenalis.

Table 2.
Antigiardial and cytotoxic activities of F. vulgare hexane and some of its constituents.
The most active compound against G. duodenalis was the aldehyde trans, trans-2,4-undecadienal (IC50 72.1 μg/mL) as seen in Table 2. However, it was not as active as the positive control metronidazole (IC50 0.5 μg/mL) against the parasite. Interestingly, the mentioned aldehyde was less toxic (IC50 588.8 μg/mL) than metronidazole (IC50 83.5 μg/mL) against Vero cells.
Additionally, we determined the selectivity index (SI) of the most active compounds against G. duodenalis. SI values ≥ 1 are considered promising candidates against parasitic infection. In our study, the six most active compounds of F. vulgare could be considered as potential candidates for the treatment of giardiasis.
3. Discussions
Esquivel-Ferriño et al. identified the aldehyde trans, trans-2,4-undecadienal in the hexane extract of F. vulgare and reported its activity against the sensitive strain H37Rv (MIC 25 μg/mL) and three multidrug resistant (MDR) clinical isolates (MIC 25–50 μg/mL) of Mycobacterium tuberculosis [13]. This aldehyde has also been identified by GC-MS from coriander (Coriandrum sativum L.) and found to be a highly effective deodorant compound against the offensive odor of the porcine large intestine [14].
It is worth mentioning that unsaturated aldehyde trans, trans-2,4-undecadienal is lipophilic in nature (log P 3.8) in contrast to the positive control metronidazole which is hydrophilic in nature (log P 0.1), its structure is seen in Figure 1. Therefore, it is possible that they have different mechanisms of action. It is well known that metronidazole forms covalent adducts with cysteines in proteins. Specifically, metronidazole targets the redox enzyme thioredoxin reductase in Entamoeba histolytica, Trichomonas vaginalis, and G. duodenalis and inhibits the enzyme’s function as a disulfide reductase, resulting in severe oxidative stress [15,16,17].
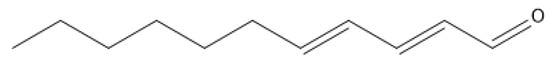
Figure 1.
Structure of trans, trans-2,4-undecadienal.
As constituents of plants, aldehydes play an important role in antimicrobial activity [18]. Aldehydes react with biologically nucleophile groups. In particular, α,β-unsaturated aldehydes are known to react with sulfhydryl, amino and hydroxyl groups under physiological conditions. The mode of action of the aldehyde trans, trans-2,4-undecadienal is likely due to its penetration in the outer layer of the parasite and the alteration of the function of membrane-associated proteins in its cellular surface causing its destabilization and death.
In addition, it has been reported that aldehydes such as trans, trans-2,4-decadienal are metabolites of lipid peroxidation decomposition. These are able to form adducts of type 1, N6-etheno-2’-deoxyadenosine, producing direct damage to DNA, the cell wall and membrane, and generating the activation of antioxidant factors such as GSH, cytochrome C and protein stress to hypoxia [19,20,21].
Brandelli et al. have shown that the aqueous extract of aerial parts of F. vulgare was inactive against the trophozoites of G. duodenalis [22]. In contrast, we were able to show inhibition against G. duodenalis because the hexane extract has lipophilic constituents whereas the aqueous extract has hydrophilic compounds. It is the lipophilic nature of these compounds that seems to be responsible for the antigiardial activity of F. vulgare.
The essential oil of F. vulgare has been tested against the helminth Schistosoma mansoni, exhibiting moderate in vitro antiparasitic activity on its adult worm form [23].
Moreover, F. vulgare aqueous extract was tested against the parasitic protozoa Blastocystis spp. showing a dose- and time-dependent anti-Blastocystis activity at 250 µg/mL [24].
Finally, Karami et al., have tested the efficacy of the extracts, essential oil, and trans-anethole (its main essential oil component) of F. vulgare against the protozoan parasite Trichomonas vaginalis suggesting an in vitro antiprotozoal property [25].
Our results support the use of F. vulgare as an antidiarrheal treatment in Mexican traditional medicine. Additionally, the compound trans, trans-2,4-undecadienal showed a promising toxicity effect toward G. duodenalis. This aldehyde could serve as a basic structure for future modifications in the search of new antiprotozoal drugs.
To our knowledge, this is the first report of the antigiardial activity of F. vulgare and some of its constituents. In addition, in vivo assays of the most active compounds as well as the elucidation of their mechanisms of action should be performed.
4. Materials and Methods
4.1. Plant Material and Extraction
Stems and leaves of F. vulgare var. dulce were collected in Pesquería, Nuevo León (México) in July 2017. Plant material was provided and identified by biologist Mauricio González Ferrara; a sample with a voucher number 024771 was deposited at the herbarium of the Department of Botany, Universidad Autónoma of Nuevo León, Mexico. Powdered air-dried leaves and stems (944 g) were extracted by maceration a at room temperature using 5 L of hexane once. Extract was filtrated and distilled under vacuum yielding 6.2 g of hexane extract [13].
4.2. Chemicals
The compounds identified in the hexane extract by GC-MS were purchased from Sigma-Aldrich (St. Louis, MO, USA). These were: fenchylacetate, α-tujone, (R) (−)-carvone, o-cymene, p-anisaldehyde, (R)(−)-fenchone, (S)(+)-fenchone, terpinolene, methylchavicol, trans-anethol, estragole, eugenol, 1,3-benzenediol, undecanal, (R)-(+)-β-citronellol, trans, trans- 2,4-undecadienal, (1R, 4S)(+)-camphene, and oleic acid. The solvents used for the extractions were purchased from J.T. Baker (USA).
4.3. Parasite Culture
G. duodenalis strain 0989: IMSS was used. G. duodenalis was cultured in TYI-S-33 media supplemented with bile, as previously described [26]. G. duodenalis trophozoites were sub-cultured three times a week. Parasites used in the assays to determine drug susceptibility were harvested when cultures reached the middle of their logarithmic growth phase.
4.4. Cytotoxic Activity on Vero Cell Line
Cytotoxicity assay was performed on Vero cell line (ATCC CCL-81), as previously described [27]. Briefly, Vero cells were cultivated in Roswell Park Memorial Institute (RPMI) medium supplemented with 10% fetal bovine serum (FBS). Only the most active compounds were tested. Each compound was dissolved in dimethyl sulfoxide (DMSO) and sterilized by filtration, and brought to final concentrations of 487, 243.5, 121.75 and 60.875 μg/mL with RPMI medium supplemented with 10% FBS. The cell suspension was adjusted to 1 × 104 cells/mL with RPMI medium supplemented with 10% FBS. Cell suspension (200 μL) was placed into a 96-well plate and incubated at 37 °C in a 5% CO2 atmosphere for 24 h. Each compound solution was added to each well containing the Vero cells and incubated for 24 h. The assay was carried out in triplicate. The number of cells were determined in a Neubauer chamber, while the percentage of viable cells was measured with the Trypan blue staining method. The half-maximum inhibitory concentration (IC50) of each drug was calculated by Probit method.
Additionally, selectivity index (SI) for the compounds was determined to identify the quantity of the compound that is active against the parasite with low toxicity to the host cells. The SI is the ratio of the IC50 concentration in host cells (Vero cells) to that in parasite (G. duodenalis) [28].
4.5. Antigiardial Activity
The concentration of all standard drugs was adjusted to 10 mg/mL. Metronidazole (used as a positive control), and pure compounds were dissolved in (DMSO). All stock solutions were stored at –20 °C until use. Before use in the assays, serial two-fold dilutions of the stock solutions were made in basal TYI medium (without serum). Fifty micro liters of each solution was added into 1 mL glass screw-capped cylindrical vials with a conical interior (Wheaton vial). All vials were filled with 950 μL of a freshly prepared parasite suspension in TYI-S-33 medium plus 10 % bovine serum. G. duodenalis was tested at density of 2 × 105 trophozoites/mL. All vials were incubated at 36 °C for 24 h. The vials were then chilled on iced water for 20 min, and the number of trophozoites per milliliter in each tube were counted using a hemocytometer (Neubauer cell-counter chamber) [29]. The percentage of growth inhibition with respect to untreated controls was then determined.
4.6. Statistical Analysis
All experiments were performed in triplicate and in at least three independent bioassays (n = 9 cultures for each analyzed compound). The data were analyzed using GraphPad Prism software version 5.0 (USA). The half-maximum inhibitory concentration (IC50) of each drug was calculated by Probit analysis and 95% confidence limits were calculated.
5. Conclusions
The results suggest the in vitro antigiardial properties of F. vulgare hexane extract. Antigiardial properties of F. vulgare are mainly due to the aldehyde trans, trans-2,4-undecadienal found in the hexane extract of this plant. In addition, it is possible that the other compounds contribute to the antigiardial activity or act in a synergist way. The results found in this study justify the use of F. vulgare for the treatment of diarrhea associated to giardiasis in Mexican traditional medicine.
Author Contributions
Conceptualization, M.d.R.C.-C. and B.D.M.-C.; methodology, software, validation, formal analysis, I.G.D.-V.; investigation, resources, B.D.M.-C. and M.d.R.C.-C.; methodology and investigation J.V.-V.; data curation and writing—original draft preparation, I.G.D.-V.; writing—review, editing, P.C.E.-F. and F.G.A.-A.; visualization, I.G.D.-V.; supervision, project administration, funding acquisition, M.d.R.C.-C. and B.D.M.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Secretaria de Educación Publica through the project Redes Temáticas de Colaboración Académica PROMEP/103.5/13/6922.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors express their appreciation and thanks to biologist Mauricio González Ferrara for providing the plant material for this study.
Conflicts of Interest
The authors declare no conflict of interest regarding the publication of this paper.
References
- Leung, A.K.C.; Leung, A.A.M.; Wong, A.H.C.; Sergi, C.M.; Kam, J.K.M. Giardiasis: An overview. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Ryan, U.; Hijjawi, N.; Feng, Y.; Xiao, L. Giardia: An under-reported foodborne parasite. Int. J. Parasitol. 2019, 49, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Argüello-García, R.; Leitsch, D.; Skinner-Adams, T.; Ortega-Pierres, M.G. Drug resistance in Giardia: Mechanisms and alternative treatments for Giardiasis. Adv. Parasitol. 2020, 107, 201–282. [Google Scholar]
- CCernikova, L.; Faso, C.; Hehl, A.B. Five facts about Giardia lamblia. PLoS Pathog. 2018, 14, e1007250. [Google Scholar] [CrossRef]
- Ryan, U.; Cacciò, S.M. Zoonotic potential of Giardia. Int. J. Parasitol. 2013, 43, 943–956. [Google Scholar] [CrossRef] [PubMed]
- Mørch, K.; Hanevik, K. Giardiasis treatment: An update with a focus on refractory disease. Curr. Opin. Infect. Dis. 2020, 33, 355–364. [Google Scholar] [CrossRef]
- Tejman-Yarden, N.; Eckmann, L. New approaches to the treatment of giardiasis. Curr. Opin. Infect. Dis. 2011, 24, 451–456. [Google Scholar] [CrossRef]
- Leitsch, D. Drug Resistance in the Microaerophilic Parasite Giardia lamblia. Curr. Trop. Med. Rep. 2015, 2, 128–135. [Google Scholar] [CrossRef]
- Nash, T.E. Treatment of Giardia lamblia Infections. Pediatr. Infect. Dis. J. 2001, 20, 193–195. [Google Scholar] [CrossRef]
- Hernández Ceruelos, A.; Romero-Quezada, L.C.; Ruvalcaba Ledezma, J.C.; López Contreras, L. Therapeutic uses of metronidazole and its side effects: An update. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 397–401. [Google Scholar]
- He, W.; Huang, B. A review of chemistry and bioactivities of a medicinal spice: Foeniculum vulgare. J. Med. Plants Res. 2011, 5, 3595–3600. [Google Scholar]
- Argueta Villamar, A.; Cano Asseleih, L.M. Atlas de las Plantas de la Medicina Tradicional Mexicana, 1st ed.; Instituto Nacional Indigenista: Mexico City, Mexico, 1994; 650p. [Google Scholar]
- Esquivel-Ferriño, P.C.; Favela-Hernández, J.M.J.; Garza-González, E.; Waksman, N.; Ríos, M.Y.; Del Rayo Camacho-Corona, M. Antimycobacterial activity of constituents from Foeniculum vulgare var. Dulce Grown in Mexico. Molecules 2012, 17, 8471–8482. [Google Scholar] [CrossRef] [PubMed]
- Ikeura, H.; Kohara, K.; Li, X.-X.; Kobayashi, F.; Hayata, Y. Identification of (E,E)-2,4-Undecadienal from Coriander (Coriandrum sativum L.) as a Highly Effective Deodorant Compound against the Offensive Odor of Porcine Large Intestine. J. Agric. Food Chem. 2010, 58, 11014–11017. [Google Scholar] [CrossRef] [PubMed]
- Leitsch, D.; Kolarich, D.; Wilson, I.B.H.; Altmann, F.; Duchêne, M. Nitroimidazole action in Entamoeba histolytica: A central role for thioredoxin reductase. PLoS Biol. 2007, 5, 1820–1834. [Google Scholar] [CrossRef] [PubMed]
- Leitsch, D.; Kolarich, D.; Binder, M.; Stadlmann, J.; Altmann, F.; Duchêne, M. Trichomonas vaginalis: Metronidazole and other nitroimidazole drugs are reduced by the flavin enzyme thioredoxin reductase and disrupt the cellular redox system. Implications for nitroimidazole toxicity and resistance. Mol. Microbiol. 2009, 72, 518–536. [Google Scholar] [CrossRef]
- Williams, C.F.; Lloyd, D.; Kolarich, D.; Alagesan, K.; Duchêne, M.; Cable, J.; Williams, D.; Leitsch, D. Disrupted intracellular redox balance of the diplomonad fish parasite Spironucleus vortens by 5-nitroimidazoles and garlic-derived compounds. Vet. Parasitol. 2012, 190, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Bisignano, G.; Laganà, M.G.; Trombetta, D.; Arena, S.; Nostro, A.; Uccella, N.; Mazzanti, G.; Saija, A. In vitro antibacterial activity of some aliphatic aldehydes from Olea europaea L. FEMS Microbiol. Lett. 2001, 198, 9–13. [Google Scholar] [CrossRef]
- Carvalho, V.M.; Di Mascio, P.; de Arruda Campos, I.P.; Douki, T.; Cadet, J.; Medeiros, M.H.G. Formation of 1,N6-Etheno-2‘-deoxyadenosine Adducts by trans, trans-2,4-Decadienal. Chem. Res. Toxicol. 1998, 11, 1042–1047. [Google Scholar] [CrossRef]
- Carvalho, V.M.; Asahara, F.; Di Mascio, P.; De Arruda Campos, I.P.; Cadet, J.; Medeiros, M.H.G. Novel 1,N6-etheno-2′-deoxyadenosine adducts from lipid peroxidation products. Chem. Res. Toxicol. 2000, 13, 397–405. [Google Scholar] [CrossRef]
- Sigolo, C.A.O.; Di Mascio, P.; Medeiros, M.H.G. Covalent modification of cytochrome c exposed to trans,trans-2,4-decadienal. Chem. Res. Toxicol. 2007, 20, 1099–1110. [Google Scholar] [CrossRef]
- Brandelli, C.L.C.; Giordani, R.B.; De Carli, G.A.; Tasca, T. Indigenous traditional medicine: In vitro anti-giardial activity of plants used in the treatment of diarrhea. Parasitol. Res. 2009, 104, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, K.A.L.; De Melo, N.I.; Aguiar, D.P.; De Oliveira, P.F.; Groppo, M.; Da Silva Filho, A.A.; Rodrigues, V.; Cunha, W.R.; Tavares, D.C.; Magalhães, L.G.; et al. Anthelmintic Effects of the Essential Oil of Fennel (Foeniculum vulgare Mill., Apiaceae) against Schistosoma mansoni. Chem. Biodivers. 2015, 12, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Méabed, E.M.; El Sayed, N.M.; Abou-Sreea, A.I.; Roby, M.H. Chemical analysis of aqueous extracts of Origanum majorana and Foeniculum vulgare and their efficacy on Blastocystis spp. cysts. Phytomedicine 2018, 43, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Karami, F.; Dastan, D.; Fallah, M.; Matini, M. In vitro activity of foeniculum vulgare and its main essential oil component trans-anethole on trichomonas vaginalis. Iran. J. Parasitol. 2019, 14, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Keister, D.B. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans. R. Soc. Trop. Med. Hyg. 1983, 77, 487–488. [Google Scholar] [CrossRef]
- Uc-Cachón, A.H.; Borges-Argáez, R.; Said-Fernández, S.; Vargas-Villarreal, J.; González-Salazar, F.; Méndez-González, M.; Cáceres-Farfán, M.; Molina-Salinas, G.M. Naphthoquinones isolated from Diospyros anisandra exhibit potent activity against pan-resistant first-line drugs Mycobacterium tuberculosis strains. Pulm. Pharmacol. Ther. 2014, 27, 114–120. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Atolani, O.; Awakan, O.J.; Olaolu, T.D.; Nwonuma, C.O.; Alejolowo, O.; Otohinoyi, D.A.; Rotimi, D.; Owolabi, A.; Batiha, G.E.-S. In vitro screening to identify anti-toxoplasma compounds and in silico modeling for bioactivities and toxicity. Yale J. Biol. Med. 2019, 92, 369–383. [Google Scholar]
- Mata-Cárdenas, B.D.; Vargas-Villarreal, J.; González-Salazar, F.; Palacios-Corona, R.; Said-Fernández, S. A new vial microassay to screen antiprotozoal drugs. Pharmacologyonline 2008, 1, 529–537. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).