Effects of Insect-Resistant Maize 2A-7 Expressing mCry1Ab and mCry2Ab on the Soil Ecosystem
Abstract
:1. Introduction
2. Results
2.1. Effects of Transgenic Insect-Resistant Maize 2A-7 on the Physical and Chemical Properties of Rhizosphere Soil
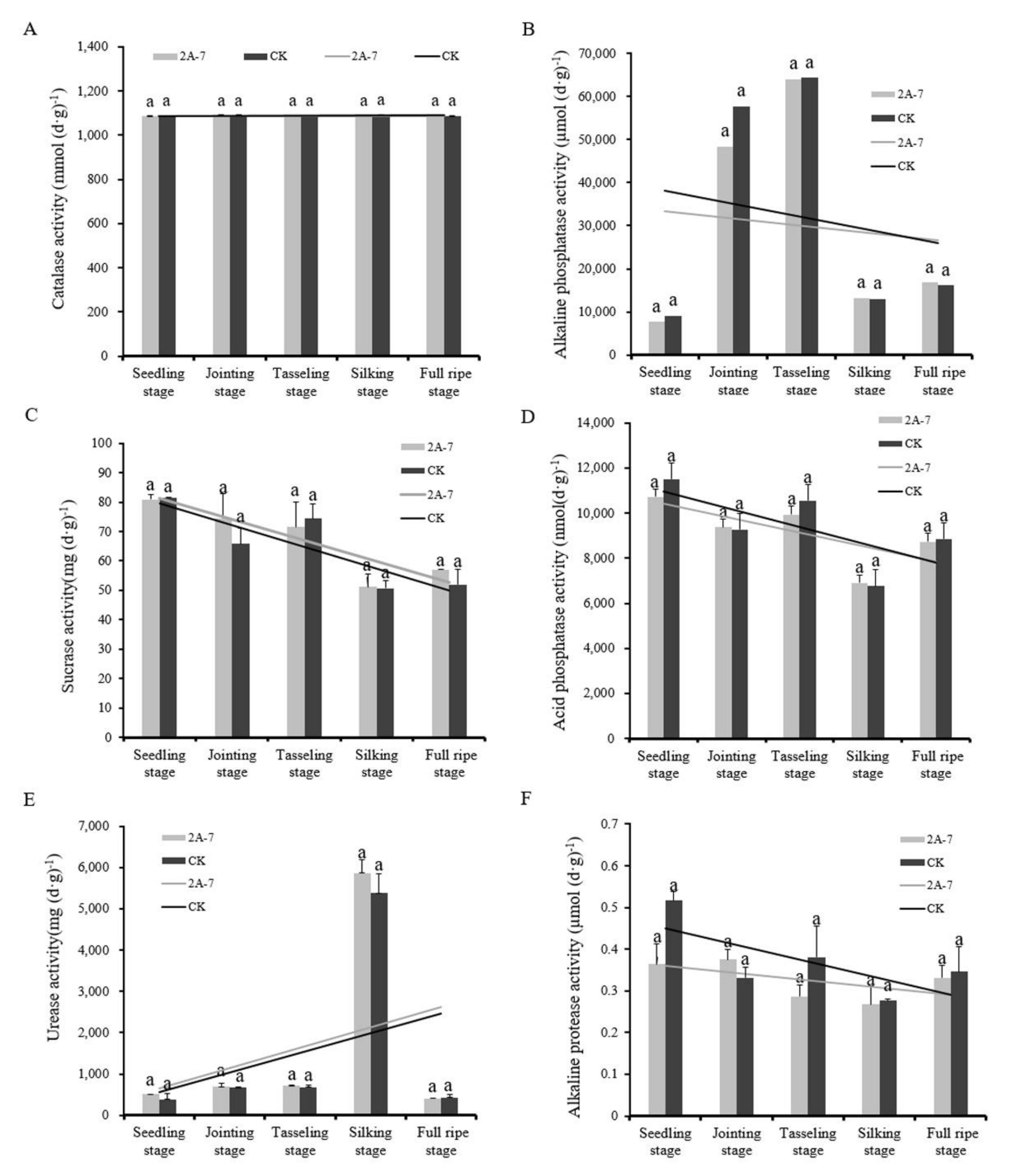
2.2. Effects of Transgenic Insect-Resistant Maize 2A-7 on the Enzyme Activity Levels in Rhizosphere Soil
2.3. Metabolic Functional Diversity of Microorganisms in the Rhizosphere Soils of Transgenic Maize 2A-7 and Its Control
2.3.1. Metabolic Activity Changes in Microorganisms Inhabiting the Rhizosphere Soils of Transgenic Maize 2A-7 and ITS Control
2.3.2. Changes in the Rhizosphere Soil Microorganisms’ Utilization of Different Carbon Sources between the Two Maize Lines
2.3.3. Diversity Index Analysis of the Rhizosphere Soil Microbial Communities of Transgenic Maize 2A-7 and Its Control
3. Discussion
3.1. Effects of Transgenic Maize 2A-7 on Physical and Chemical Properties of Rhizosphere Soil
3.2. Effects of Transgenic Maize 2A-7 on Soil Enzyme Activities in Rhizosphere
3.3. Effects of Transgenic Maize 2A-7 on the Functional Diversity of Rhizosphere Soil Microorganisms
4. Materials and Methods
4.1. Plant Materials
4.2. Test Design
4.3. Determination of Physical and Chemical Properties of the Rhizosphere Soil
4.4. Determination of Enzyme Activities in the Rhizosphere Soil
4.5. Determination of Microbial Functional Diversity in the Rhizosphere Soil
4.6. Data Processing
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, J.A.; Ellsworth, P.C.; Faria, J.C.; Head, G.P.; Owen, M.D.K.; Pilcher, C.D.; Shelton, A.M.; Meissle, M. Genetically engineered crops: Importance of diversified integrated pest management for agricultural sustainability. Front. Bioeng. Biotechnol. 2019, 7, 24. [Google Scholar] [CrossRef]
- Ramankutty, N.; Mehrabi, Z.; Waha, K.; Jarvis, L.; Kremen, C.; Herrero, M.; Rieseberg, L.H. Trends in global agricultural land use: Implications for environmental health and food security. Annu. Rev. Plant Biol. 2018, 69, 789–815. [Google Scholar] [CrossRef] [PubMed]
- ISAAA. Global Status of Commercialized Biotech/GM Crops in 2019 (ISAAA Brief No. 55); ISAAA: Ithaca, NY, USA, 2019. [Google Scholar]
- Brookes, G.; Barfoot, P. Farm income and production impacts of using GM crop technology 1996–2016. GM Crops Food 2018, 9, 59–89. [Google Scholar] [CrossRef] [PubMed]
- Campos, S.O.; Santana, I.V.; Silva, C.; Santos-Amaya, O.F.; Guedes, R.N.C.; Pereira, E.J.G. Bt-induced hormesis in Bt-resistant insects: Theoretical possibility or factual concern? Ecotoxicol. Environ. Saf. 2019, 183, 109577. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, X.; Qi, L.; Chen, F.; Liu, M.; Whalen, J.K. Root and detritus of transgenic Bt crop did not change nematode abundance and community composition but enhanced trophic connections. Sci. Total Environ. 2018, 644, 822–829. [Google Scholar] [CrossRef]
- Cheng, M.M.; Shu, Y.H.; Wang, J.W. Effect of Bt rice straw returning in soil on the growth and reproduction of Eisenia fetida. Ying Yong Sheng Tai Xue Bao 2016, 27, 3667–3674. [Google Scholar]
- Zhang, W.; Cao, Z.; Wang, M.; Chen, X.; Wang, B. Absorption, translocation, and effects of Bt Cry1Ac peptides from transgenic cotton to the intercrops and soil functional bacteria. Sci. Rep. 2020, 10, 17294. [Google Scholar] [CrossRef]
- Song, X.; Chang, L.; Reddy, G.V.P.; Zhang, L.; Fan, C.; Wang, B. Use of taxonomic and trait-based approaches to evaluate the effects of transgenic Cry1Ac corn on the community characteristics of soil collembola. Environ. Entomol. 2019, 48, 263–269. [Google Scholar] [CrossRef]
- Fan, C.; Wu, F.; Dong, J.; Wang, B.; Yin, J.; Song, X. No impact of transgenic cry1Ie maize on the diversity, abundance and composition of soil fauna in a 2-year field trial. Sci. Rep. 2019, 9, 10333. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Carrière, Y. Surge in insect resistance to transgenic crops and prospects for sustainability. Nat. Biotechnol. 2017, 35, 926–935. [Google Scholar] [CrossRef]
- Li, J.; Shu, Y.; Wang, F.; Wang, J. Effects of Cry1Ab-expressing Bt rice straw return on juvenile and adult Eisenia fetida. Ecotoxicol. Environ. Saf. 2019, 169, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wu, K. Recent progress on the interaction between insects and Bacillus thuringiensis crops. Philos. Trans. R Soc. Lond. B Biol. Sci. 2019, 374, 20180316. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Zhang, Y.; Zeng, H.; Zhang, Y.; Wang, J. Effects of Cry1Ab Bt maize straw return on bacterial community of earthworm Eisenia fetida. Chemosphere 2017, 173, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, J.; Luo, Z.; Wang, H.; Liu, F. The fate of fusion Cry1Ab/1Ac proteins from Bt-transgenic rice in soil and water. Ecotoxicol. Environ. Saf. 2016, 124, 455–459. [Google Scholar] [CrossRef]
- Van Wyk, D.A.B.; Adeleke, R.; Rhode, O.H.J.; Bezuidenhout, C.C.; Mienie, C. Ecological guild and enzyme activities of rhizosphere soil microbial communities associated with Bt-maize cultivation under field conditions in North West Province of South Africa. J. Basic Microbiol. 2017, 57, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Rui, Y.K.; Yi, G.X.; Zhao, J.; Wang, B.M.; Li, Z.H.; Zhai, Z.X.; He, Z.P.; Li, Q.X. Changes of Bt toxin in the rhizosphere of transgenic Bt cotton and its influence on soil functional bacteria. World J. Microbiol. Biotechnol. 2005, 21, 1279–1284. [Google Scholar] [CrossRef]
- Valldor, P.; Miethling-Graff, R.; Martens, R.; Tebbe, C.C. Fate of the insecticidal Cry1Ab protein of GM crops in two agricultural soils as revealed by ¹⁴C-tracer studies. Appl. Microbiol. Biotechnol. 2015, 99, 7333–7341. [Google Scholar] [CrossRef]
- Crecchio, C.; Stotzky, G. Insecticidal activity and biodegradation of the toxin from Bacillus thuringiensis subsp. Kustuki bound to humic acids from soil. Soil Biol. Biochem. 1998, 30, 463–470. [Google Scholar]
- Li, F.; Wang, M.; Sun, H.W.; Yang, S.K.; Lu, X.B. Dynamics of Cry1Ab protein content in the rhizosphere soil and straw debris of transgenic Bt corn. Chin. J. Appl. Ecol. 2013, 24, 1907–1913. [Google Scholar]
- Liu, W.; Zhao, H.; Miao, C.; Jin, W. Integrated proteomics and metabolomics analysis of transgenic and gene-stacked maize line seeds. GM Crops Food 2021, 12, 361–375. [Google Scholar] [CrossRef]
- Yin, Y.; Xu, Y.; Cao, K.; Qin, Z.; Zhao, X.; Dong, X.; Shi, W. Impact assessment of Bt maize expressing the Cry1Ab and Cry2Ab protein simultaneously on non-target arthropods. Environ. Sci. Pollut. Res. Int. 2020, 27, 21552–21559. [Google Scholar] [CrossRef]
- Mulder, C.; Wouterse, M.; Rutgers, M.; Posthuma, L. Transgenic maize containing the Cry1Ab protein ephemerally enhances soil microbial communities. Ambio 2007, 36, 359–361. [Google Scholar] [CrossRef]
- Barriuso, J.; Valverde, J.R.; Mellado, R.P. Effect of Cry1Ab protein on rhizobacterial communities of Bt-maize over a four-year cultivation period. PLoS ONE 2012, 7, e35481. [Google Scholar] [CrossRef] [PubMed]
- Baumgarte, S.; Tebbe, C.C. Field studies on the environmental fate of the Cry1Ab Bt-toxin produced by transgenic maize (MON810) and its effect on bacterial communities in the maize rhizosphere. Mol. Ecol. 2005, 14, 2539–2551. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, C.; Ge, L.; Hu, C.; Wu, G.; Sun, Y.; Song, L.; Wu, X.; Pan, A.; Xu, Q.; et al. Environmental behaviors of Bacillus thuringiensis (Bt) insecticidal proteins and their effects on microbial ecology. Plants 2022, 11, 1212. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pan, L.; Ren, M.; Li, J.; Guan, X.; Tao, J. Comparison of genetically modified insect-resistant maize and non-transgenic maize revealed changes in soil metabolomes but not in rhizosphere bacterial community. GM Crops Food 2022, 13, 1–14. [Google Scholar] [CrossRef]
- Liu, N.; Zhu, P.; Peng, C.; Kang, L.; Gao, H.; Clarke, N.J.; Clarke, J.L. Effect on soil chemistry of genetically modified (GM) vs. non-GM maize. GM Crops 2010, 1, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.Y.; Li, Z.B.; Xiao, J.B.; Zhang, L.T.; Ma, B.; Li, J.M.; Cheng, D.B. Research progress on the effects of freeze-thaw on soil physical and chemical properties and wind and water erosion. Ying Yong Sheng Tai Xue Bao 2019, 30, 337–347. [Google Scholar] [PubMed]
- Lei, L.; Guo, Q.S.; Wang, C.L.; Li, X.; An, J. Effect of compound planting on soil physical and chemical properties and soil enzyme activities of Salvia miltiorrhiza. Zhongguo Zhong Yao Za Zhi 2018, 43, 2480–2488. [Google Scholar]
- Chen, Q.; Yang, B.; Liu, X.; Chen, F.; Ge, F. Long-term cultivation of Bt rice expressing the Cry1Ab/1Ac gene reduced phytoparasitic nematode abundance but did not affect other nematode parameters in paddy fields. Sci. Total Environ. 2017, 607–608, 463–474. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Heckmann, L.H.; Caul, S.; Thompson, J.; Scrimgeour, C.; Krogh, P.H. Varietal effects of eight paired lines of transgenic Bt maize and near-isogenic non-Bt maize on soil microbial and nematode community structure. Plant Biotechnol. J. 2007, 5, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Zhong, W.; Tan, F.; Shu, Y.; Feng, Y.; Wang, J. The influence of Bt maize cultivation on communities of arbuscular mycorrhizal fungi revealed by MiSeq sequencing. Front. Microbiol. 2018, 9, 3275. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Ning, D.; Mao, L.; Wang, B.; Fang, Q.; Yao, H.; Wang, F.; Ye, G. Metabolic analysis reveals Cry1C gene transformation does not affect the sensitivity of rice to rice dwarf virus. Metabolites 2021, 11, 209. [Google Scholar] [CrossRef] [PubMed]
- Ahamd, M.; Abbasi, W.M.; Jamil, M.; Iqbal, M.; Hussain, A.; Akhtar, M.F.; Nazli, F. Comparison of rhizosphere properties as affected by different Bt- and non-Bt-cotton (Gossypium hirsutum L.) genotypes and fertilization. Environ. Monit. Assess. 2017, 189, 278. [Google Scholar] [CrossRef]
- Du, J.; Hou, F.; Zhou, Q. Response of soil enzyme activity and soil bacterial community to PCB dissipation across different soils. Chemosphere 2021, 283, 131229. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sun, Y.N.; Lin, L.; Li, Y.K.; Du, Y.G.; Guo, X.W.; Yang, Y.S.; Cao, G.M. Changes of soil enzyme activities and nutrients across different succession stages of grazing alpine Kobresia grassland. Ying Yong Sheng Tai Xue Bao 2019, 30, 2267–2274. [Google Scholar] [PubMed]
- Feng, H.L.; Xu, C.S.; He, H.H.; Zeng, Q.; Chen, N.; Li, X.L.; Ren, T.B.; Ji, X.M.; Liu, G.S. Effect of biochar on soil enzyme activity & the bacterial community and its mechanism. Huan Jing Ke Xue 2021, 42, 422–432. [Google Scholar]
- Liang, J.G.; Xin, L.T.; Luan, Y.; Song, X.Y.; Zhang, Z.G. Effect of Cry1Ie Bt maize on carbon source metabolism of rhizosphere microorganisms. J. Agric. Sci. Technol. Iran 2019, 21, 104–110. [Google Scholar]
- Zeng, X.; Pei, T.; Song, Y.; Guo, P.; Zhang, H.; Li, X.; Li, H.; Di, H.; Wang, Z. A three-year plant study of salt-tolerant transgenic maize showed no effects on soil enzyme activity and nematode community. Life 2022, 12, 412. [Google Scholar] [CrossRef]
- Wu, J.; Yu, M.; Xu, J.; Du, J.; Ji, F.; Dong, F.; Li, X.; Shi, J. Impact of transgenic wheat with wheat yellow mosaic virus resistance on microbial community diversity and enzyme activity in rhizosphere soil. PLoS ONE 2014, 9, e98394. [Google Scholar]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant-microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Shi, W.; Liu, W.; Gao, Z.; Han, L.; Wang, X. Differential impact of Bt-transgenic rice plantings on bacterial community in three niches over consecutive years. Ecotoxicol. Environ. Saf. 2021, 223, 112569. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, Y.; Shi, J.; Yu, Z.; Pan, A.; Tang, X.; Ming, F. Impact of transgenic Cry1Ac + CpTI cotton on diversity and dynamics of rhizosphere bacterial community of different root environments. Sci. Total Environ. 2018, 637–638, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Dai, R.; Ruan, Y.; Rensing, C.; Liu, M.; Guo, S.; Ling, N.; Shen, Q. Probing active microbes involved in Bt-containing rice straw decomposition. Appl. Microbiol. Biotechnol. 2018, 102, 10273–10284. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Liu, R.H.; Wang, L.L.; Li, J.; Li, G.; Yang, D.L.; Zhao, J.N. Effects of continuous planting of phosphorus-efficient transgenic rice on soil microbial functional diversity. J. Tianjin Agric. Univ. 2019, 26, 1–7. [Google Scholar]
- Sun, H.W.; Zhang, Y.X.; Xu, X.H.; Gao, R.; Li, F.; Yang, S.K.; Lu, X.B. Effects of transgenic maize variety (double resistance 12-5) on enzyme activity and microbial diversity in rhizosphere soil. Soils 2019, 51, 61–67. [Google Scholar]
- Jiang, Z.; Zhou, L.; Wang, B.; Wang, D.; Wu, F.; Yin, J.; Song, X. Toxicological and biochemical analyses demonstrate no toxic effect of Bt maize on the Folsomia candida. PLoS ONE 2020, 15, e0232747. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Y.; Hu, J.; Du, N.; Chen, F. Functional diversity of the microbial community in healthy subjects and periodontitis patients based on sole carbon source utilization. PLoS ONE 2014, 9, e91977. [Google Scholar]
- Liu, W.; Pan, N.; Chen, W.; Jiao, W.T.; Wang, M.E. Effect of veterinary oxytetracycline on functional diversity of soil microbial community. Plant Soil Environ. 2012, 58, 295–301. [Google Scholar] [CrossRef]
- Xu, X.H.; Guo, Y.; Sun, H.; Li, F.; Yang, S.; Gao, R.; Lu, X. Effects of phytase transgenic maize on the physiological and biochemical responses and the gut microflora functional diversity of Ostrinia furnacalis. Sci. Rep. 2018, 8, 4413. [Google Scholar] [CrossRef] [Green Version]
- Riley, D.; Barber, S.D. Salt accumulation at the soybean (Glycine max (L.) Merr.) root-soil interface. Soil Sci. Soc. Am. J. 1970, 34, 154–155. [Google Scholar] [CrossRef]
- Hong, S.; Gan, P.; Chen, A. Environmental controls on soil pH in planted forest and its response to nitrogen deposition. Environ. Res. 2019, 172, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Rong, Y.; Su, Y.; Du, Z. Characteristics of soil respiration in farmland of Hexi oasis and its response to long-term fertilization. J. Desert Res. 2022, 42, 178–186. [Google Scholar]
- Hughes, J.B.; Bohannan, B.J.M. Section 7 update: Application of ecological diversity statistics in microbial ecology. Mol. Microb. Ecol. Man. 2004, 7, 1321–1344. [Google Scholar]
- Preston-Mafham, J.; Boddy, L.; Randerson, P.F. Analysis of microbial community functional diversity usingsole-carbon-source utilisation profiles—A critique. FEMS Microbiol. Ecol. 2002, 42, 1–14. [Google Scholar]


| Developmental Stage | Variety | pH | Total Nitrogen (%) | Total Phosphorus (%) | Organic Matter (g/Kg) | Available Phosphorus (mg/Kg) | Alkali-Hydrolyzed Nitrogen (mg/Kg) |
|---|---|---|---|---|---|---|---|
| Seedling stage | 2A-7 | 6.70 ± 0.10 a | 0.15 ± 0.01 a | 0.10 ± 0.01 a | 22.3 ± 2.70 a | 96.10 ± 4.62 a | 75.47 ± 5.67 a |
| CK | 6.80 ± 0.00 a | 0.14 ± 0.01 a | 0.08 ± 0.03 a | 21.63 ± 1.34 a | 98.63 ± 10.73 a | 94.60 ± 16.32 a | |
| Jointing stage | 2A-7 | 7.17 ± 0.06 a | 0.12 ± 0.01 a | 0.10 ± 0.02 a | 21.00 ± 4.66 a | 90.83 ± 3.50 a | 89.13 ± 11.48 a |
| CK | 7.27 ± 0.06 a | 0.13 ± 0.01 a | 0.10 ± 0.01 a | 23.23 ± 0.55 a | 88.70 ± 0.35 a | 82.20 ± 9.00 a | |
| Tasseling stage | 2A-7 | 7.40 ± 0.10 a | 0.14 ± 0.01 a | 0.10 ± 0.01 a | 21.60 ± 0.61 a | 80.00 ± 8.15 a | 85.47 ± 8.59 a |
| CK | 7.50 ± 0.00 a | 0.13 ± 0.01 a | 0.11 ± 0.00 a | 24.93 ± 0.74 a | 80.80 ± 2.05 a | 79.93 ± 11.22 a | |
| Silking stage | 2A-7 | 7.47 ± 0.06 a | 0.14 ± 0.02 a | 0.08 ± 0.03 a | 26.60 ± 6.32 a | 73.47 ± 14.72 a | 91.27 ± 17.07 a |
| CK | 7.60 ± 0.00 a | 0.12 ± 0.02 a | 0.11 ± 0.00 a | 17.57 ± 0.32 a | 57.27 ± 1.80 a | 88.30 ± 16.63 a | |
| Full ripe stage | 2A-7 | 7.73 ± 0.06 a | 0.13 ± 0.00 a | 0.12 ± 0.00 a | 20.60 ± 3.21 a | 59.33 ± 2.40 a | 85.10 ± 1.39 a |
| CK | 7.80 ± 0.00 a | 0.12 ± 0.01 a | 0.12 ± 0.01 a | 17.17 ± 3.27 a | 59.17 ± 4.28 a | 85.63 ± 5.59 a |
| Period | Variety | Shannon Index (H′) | Simpson Index (D) | McIntosh Index (U) |
|---|---|---|---|---|
| Seedling stage | 2A-7 | 3.34 ± 0.00 a | 0.96 ± 0.00 a | 6.40 ± 0.20 a |
| CK | 3.35 ± 0.01 a | 0.96 ± 0.00 a | 6.65 ± 0.21 a | |
| Jointing stage | 2A-7 | 3.34 ± 0.01 a | 0.96 ± 0.00 a | 6.33 ± 0.22 a |
| CK | 3.32 ± 0.02 a | 0.96 ± 0.00 a | 6.20 ± 0.52 a | |
| Tasseling stage | 2A-7 | 3.34 ± 0.03 a | 0.96 ± 0.00 a | 6.57 ± 0.21 a |
| CK | 3.34 ± 0.04 a | 0.96 ± 0.00 a | 6.35 ± 0.34 a | |
| Silking stage | 2A-7 | 3.34 ± 0.00 a | 0.96 ± 0.00 a | 6.71 ± 0.10 a |
| CK | 3.31 ± 0.01 a | 0.96 ± 0.00 a | 6.25 ± 0.27 a | |
| Full ripe stage | 2A-7 | 3.33 ± 0.01 a | 0.96 ± 0.00 a | 5.91 ± 0.14 a |
| CK | 3.33 ± 0.01 a | 0.96 ± 0.00 a | 5.92 ± 0.17 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Liu, X.; Xu, X.; Sun, H.; Li, F.; Hao, C.; Lu, X. Effects of Insect-Resistant Maize 2A-7 Expressing mCry1Ab and mCry2Ab on the Soil Ecosystem. Plants 2022, 11, 2218. https://doi.org/10.3390/plants11172218
Yang S, Liu X, Xu X, Sun H, Li F, Hao C, Lu X. Effects of Insect-Resistant Maize 2A-7 Expressing mCry1Ab and mCry2Ab on the Soil Ecosystem. Plants. 2022; 11(17):2218. https://doi.org/10.3390/plants11172218
Chicago/Turabian StyleYang, Shuke, Xin Liu, Xiaohui Xu, Hongwei Sun, Fan Li, Chaofeng Hao, and Xingbo Lu. 2022. "Effects of Insect-Resistant Maize 2A-7 Expressing mCry1Ab and mCry2Ab on the Soil Ecosystem" Plants 11, no. 17: 2218. https://doi.org/10.3390/plants11172218
APA StyleYang, S., Liu, X., Xu, X., Sun, H., Li, F., Hao, C., & Lu, X. (2022). Effects of Insect-Resistant Maize 2A-7 Expressing mCry1Ab and mCry2Ab on the Soil Ecosystem. Plants, 11(17), 2218. https://doi.org/10.3390/plants11172218





