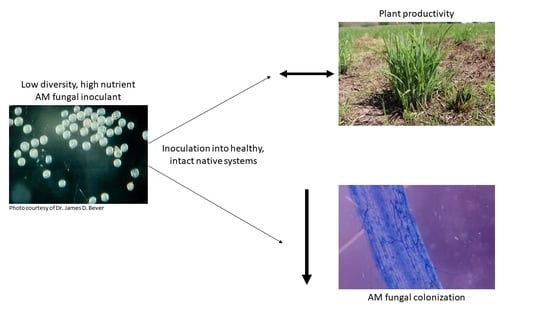
Effects of Commercial Arbuscular Mycorrhizal Inoculants on Plant Productivity and Intra-Radical Colonization in Native Grassland: Unintentional De-Coupling of a Symbiosis?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Preparation
2.2. Seedling Establishment
2.3. Commercial Inoculum Treatments
2.4. Data Collection
2.5. Statistical Analyses
3. Results
3.1. Plant Biomass Production
3.2. AM Fungal Root Colonization
3.3. Inoculum Nutrient Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhardwaj, D.; Ansari, M.W.; Sahoo, R.K.; Tuteja, N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Fact. 2014, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Basiru, S.; Mwanza, H.P.; Hijri, M. Analysis of arbuscular mycorrhizal fungal inoculant benchmarks. Microorganisms 2020, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Stratistics Market Research Consulting. Agricultural Microbials—Global Market Outlook 2017–2026. Available online: https://www.premiummarketinsights.com/reports-smrc/agricultural-microbials-global-market-outlook-2017-2026 (accessed on 22 July 2022).
- Benami, M.; Isack, Y.; Grotsky, D.; Levy, D.; Kofman, Y. The economic potential of arbuscular mycorrhizal fungi in agriculture. In Grand Challenges in Fungal Biotechnology; Nevalainen, H., Ed.; Springer International Publishing: Cham, CH, USA, 2020; pp. 239–279. [Google Scholar]
- Salomon, M.J.; Watts-Williams, S.J.; McLaughlin, M.J.; Bücking, H.; Singh, B.K.; Hutter, I.; Schneider, C.; Martin, F.; Vosatka, M.; Guo, L.D.; et al. Establishing a quality management framework for commercial inoculants containing arbuscular mycorrhizal fungi. iScience 2022, 25, 104636. [Google Scholar] [CrossRef] [PubMed]
- Vosátka, M.; Látr, A.; Gianinazzi, S.; Albrechtová, J. Development of arbuscular mycorrhizal biotechnology and industry: Current achievements and bottlenecks. Symbiosis 2013, 58, 29–37. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, UK, 2010. [Google Scholar]
- Bender, S.F.; Schlaeppi, K.; Held, A.; Van der Heijden, M.G.A. Establishment success and crop growth effects of an arbuscular mycorrhizal fungus inoculated into Swiss corn fields. Agric. Ecosyst. Environ. 2019, 273, 13–24. [Google Scholar] [CrossRef]
- Hijri, M. Analysis of a large dataset of mycorrhiza inoculation field trials on potato shows highly significant increases in yield. Mycorrhiza 2016, 26, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Lekberg, Y.; Koide, R.T. Is plant performance limited by abundance of arbuscular mycorrhizal fungi? A meta-analysis of studies published between 1988 and 2003. New Phytol. 2005, 168, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, E.; Öpik, M.; Bonari, E.; Ercoli, L. Responses of wheat to arbuscular mycorrhizal fungi: A meta-analysis of field studies from 1975 to 2013. Soil Biol. Biochem. 2015, 84, 210–217. [Google Scholar] [CrossRef]
- Zhang, S.; Lehmann, A.; Zheng, W.; You, Z.; Rillig, M.C. Arbuscular mycorrhizal fungi increase grain yields: A meta-analysis. New Phytol. 2019, 222, 543–555. [Google Scholar] [CrossRef]
- Jung, S.C.; Martinez-Medina, A.; Lopez-Raez, J.A.; Pozo, M.J. Mycorrhiza-induced resistance and priming of plant defenses. J. Chem. Ecol. 2012, 38, 651–664. [Google Scholar] [CrossRef]
- Plouznikoff, K.; Declerck, S.; Calonne-Salmon, M. Mitigating abiotic stresses in crop plants by arbuscular mycorrhizal fungi. In Belowground Defence Strategies in Plants; Springer International Publishing: Cham, Switzerland, 2016; pp. 341–400. [Google Scholar]
- Rillig, M.C.; Mummey, D.L. Mycorrhizas and soil structure. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef]
- Wilson, G.W.T.; Rice, C.W.; Rillig, M.C.; Springer, A.; Hartnett, D.C. Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: Results from long-term field studies. Ecol. Lett. 2009, 12, 452–461. [Google Scholar] [CrossRef]
- Cavagnaro, T.R.; Bender, S.F.; Asghari, H.R.; van der Heijden, M.G.A. The role of arbuscular mycorrhizas in reducing soil nutrient loss. Trends Plant Sci. 2015, 20, 283–290. [Google Scholar] [CrossRef]
- Hart, M.M.; Antunes, P.M.; Abbott, L.K. Unknown risks to soil biodiversity from commercial fungal inoculants. Nat. Ecol. Evol. 2017, 1, 1. [Google Scholar] [CrossRef]
- Faye, A.; Dalpé, Y.; Ndung’u-Magiroi, K.; Jewfa, J.; Ndoye, I.; Diouf, M.; Lesueur, D. Evaluation of commercial arbuscular inoculants. Can. J. Plant Sci. 2013, 93, 1201–1208. [Google Scholar] [CrossRef]
- Salomon, M.J.; Demarmels, R.; Watts-Williams, S.J.; McLAughlin, M.J.; Kafle, A.; Ketelsen, C.; Soupir, A.; Bücking, H.; Cavagnaro, T.R.; van der Heijden, M.G.A. Global evaluation of commercial arbuscular mycorrhizal inoculants under greenhouse and field conditions. Appl. Soil Ecol. 2022, 169, 104225. [Google Scholar] [CrossRef]
- Tarbell, T.J.; Koske, R.E. Evaluation of commercial arbuscular mycorrhizal inocula in a sand/peat medium. Mycorrhiza 2007, 18, 51–56. [Google Scholar] [CrossRef]
- Abbott, L.K.; Robson, A.D. The role of vesicular arbuscular mycorrhizal fungi in agriculture and the selection of fungi for inoculation. Aus. J. Agric. Res. 1982, 33, 389–408. [Google Scholar] [CrossRef]
- Allen, M.F.; Allen, E. Effects of mycorrhizal and nontarget organisms on restoration of a seasonal tropical forest in Quintana Roo, Mexico: Factors limiting tree establishment. Rest. Ecol. 2005, 13, 325–333. [Google Scholar] [CrossRef]
- Kachurina, O.M.; Zhang, H.; Raun, W.R.; Krenzer, E.G. Simultaneous determination of soil aluminum, ammonium-and nitrate-nitrogen using 1 M potassium chloride extraction. Comm. Soil Sci. Plan. 2000, 31, 893–903. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Comm. Soil Sci. Plan. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Pittman, J.J.; Zhang, H.; Schroder, J.L.; Payton, M.E. Differences of phosphorus in Mehlich 3 extracts determined by colorimetric and spectroscopic methods. Comm. Soil Sci. Plan. 2005, 36, 1641–1659. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis, Part 2, 2nd ed.; Page, A.L., Ed.; John Wiley & Sons: Madison, WI, USA, 1996; Volume 14, pp. 961–1010. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular—Arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Moora, M.; Öpik, M.; Davison, J.; Jairus, T.; Vasar, M.; Zobel, M.; Eckstein, R.L. AM fungal communities inhabiting the roots of submerged aquatic plant Lobelia dortmanna are diverse and include a high proportion of novel taxa. Mycorrhiza 2016, 26, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Torchiano, M. Package ‘Effsize’. 2020. Available online: https://cran.r-project.org/web/packages/effsize/effsize.pdf (accessed on 28 July 2022).
- R Core Team. 2021. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012. [Google Scholar]
- Wilson, G.W.; Hartnett, D.C. Interspecific variation in plant responses to mycorrhizal colonization in tallgrass prairie. Am. J. Bot. 1998, 85, 1732–1738. [Google Scholar] [CrossRef] [PubMed]
- Gange, A.C.; Ayres, R.L. On the relation between arbuscular mycorrhizal colonization and plant ‘benefit’. Oikos 1999, 87, 615–621. [Google Scholar] [CrossRef]
- Johnson, N.C.; Graham, J.H.; Smith, F.A. Functioning of mycorrhizal associations along the mutualism–parasitism continuum. New Phytol. 1997, 135, 575–585. [Google Scholar] [CrossRef]
- Prado-Tarango, D.E.; Mata-González, R.; Hovland, M.; Schreiner, R.P. Assessing commercial and early-seral arbuscular mycorrhizal fungi inoculation to aid in restoring sagebrush steppe shrubs. Rangel. Ecol. Manag. 2021, 79, 87–90. [Google Scholar] [CrossRef]
- Jastrow, J.D.; Miller, R.M. Soil aggregate stabilization and carbon sequestration: Feedbacks through organomineral associations. In Soil Processes and the Carbon Cycle; Lal, R., Kimble, J.M., Follett, R.F., Stewart, B.A., Eds.; CRC Press: Boca Raton, FL, USA, 1998; pp. 207–223. [Google Scholar]
- Miller, R.M.; Jastrow, J.D. Mycorrhizal fungi influence soil structure. In Arbuscular Mycorrhizae: Physiology and Function; Kapulnik, Y., Douds, D.D., Eds.; Springer Nature: Dordrecht, NL, USA, 2000; pp. 3–18. [Google Scholar]
- Treseder, K.K.; Allen, M.F. Mycorrhizal fungi have a potential role in soil carbon storage under elevated CO2 and nitrogen deposition. New Phytol. 2000, 147, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Dıaz-Zorita, M.; Perfect, E.; Grove, J.H. Disruptive methods for assessing soil structure. Soil Till. Res. 2002, 64, 3–22. [Google Scholar] [CrossRef]
- Johnson, N.C.; Wilson, G.W.T.; Bowker, M.A.; Wilson, J.A.; Miller, R.M. Resource limitation is a driver of local adaptation in mycorrhizal symbioses. Proc. Natl. Acad. Sci. USA 2010, 107, 2093–2098. [Google Scholar] [CrossRef] [PubMed]
- Sylvia, D.M.; Wilson, D.O.; Graham, J.H.; Maddox, J.J.; Millner, P.; Morton, J.B.; Skipper, H.D.; Wright, S.F.; Jarstfer, A.G. Evaluation of vesicular-arbuscular mycorrhizal fungi in diverse plants and soils. Soil Biol. Biochem. 1993, 25, 705–713. [Google Scholar] [CrossRef]
- Anderson, R.C.; Hetrick, B.A.D.; Wilson, G.W.T. Mycorrhizal dependence of Andropogon gerardii and Schizachyrium scoparium in two prairie soils. Am. Midl. Nat. 1994, 132, 366–376. [Google Scholar] [CrossRef]
- Hardesty, J.O. Watch that salt content-excessive high concentration of solublesalts can cause crop injury. for first time available here is a handy guide to salt index values of fertilizer materials. Farm Chem. 1967, 130, 42–47. [Google Scholar]
- Koziol, L.; Schultz, P.A.; House, G.L.; Bauer, J.T.; Middleton, E.L.; Bever, J.D. The plant microbiome and native plant restoration: The example of native mycorrhizal fungi. BioScience 2018, 68, 996–1006. [Google Scholar] [CrossRef]
- Gianinazzi, S.; Vosátka, M. Inoculum of arbuscular mycorrhizal fungi for production systems: Science meets business. Can. J. Bot. 2004, 82, 1264–1271. [Google Scholar] [CrossRef]
- Kokkoris, V.; Hamel, C.; Hart, M.M. Mycorrhizal response in crop versus wild plants. PLoS ONE 2019, 14, e0221037. [Google Scholar] [CrossRef]
- Calvet, C.; Camprubi, A.; Pérez-Hernández, A.; Lovato, P.E. Plant growth stimulation and root colonization potential of in vivo versus in vitro arbuscular mycorrhizal inocula. HortScience 2013, 48, 897–901. [Google Scholar] [CrossRef]
- Jin, H.; Germida, J.J.; Walley, F.L. Impact of arbuscular mycorrhizal fungal inoculants on subsequent arbuscular mycorrhizal fungi colonization in pot-cultured field pea (Pisum sativum L.). Mycorrhiza 2013, 23, 45–59. [Google Scholar] [CrossRef]
- Verbruggen, E.; van der Heijden, M.G.; Rillig, M.C.; Kiers, E.T. Mycorrhizal fungal establishment in agricultural soils: Factors determining inoculation success. New Phytol. 2013, 197, 1104–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farmer, M.J.; Li, X.; Feng, G.; Zhao, B.; Chatagnier, O.; Gianinazzi-Pearson, S.; van Tuinen, D. Molecular monitoring of field- inoculated AMF to evaluate persistence in sweet potato crops in China. Appl. Soil Ecol. 2007, 35, 599–609. [Google Scholar] [CrossRef]
- Martignoni, M.M.; Garnier, J.; Hart, M.M.; Tyson, R.C. Investigating the impact of the mycorrhizal inoculum on the resident fungal community and on plant growth. Ecol. Model. 2020, 438, 109321. [Google Scholar] [CrossRef]
- Thomsen, C.N.; Hart, M.M. Using invasion theory to predict the fate of arbuscular mycorrhizal fungal inoculants. Biol. Invasions 2018, 20, 2695–2706. [Google Scholar] [CrossRef]
- Hart, M.M.; Antunes, P.M.; Chaudhary, V.B.; Abbott, L.K. Fungal inoculants in the field: Is the risk greater than the reward? Funct. Ecol. 2018, 32, 126–135. [Google Scholar] [CrossRef]
- Loján, P.; Senés-Guerrero, C.; Suárez, J.P.; Kromann, P.; Schubler, A.; Declerck, S. Potato field-inoculation in Ecuador with Rhizophagus irregularis: No impact on growth performance and associated arbuscular mycorrhizal fungal communities. Symbiosis 2017, 73, 45–56. [Google Scholar] [CrossRef]
- Koch, A.M.; Antunes, P.M.; Barto, E.K.; Cipollini, D.; Mummey, D.L.; Klironomos, J.N. The effects of arbuscular mycorrhizal (AM) fungal and garlic mustard introductions on native AM fungal diversity. Biol. Invasions 2011, 13, 1627–1639. [Google Scholar] [CrossRef]
- Symanczik, S.; Courty, P.E.; Boller, T.; Wiemken, A.; Al-Yahya’ei, M.N. Impact of water regimes on an experimental community of four desert arbuscular mycorrhizal fungal (AMF) species, as affected by the introduction of a non- native AMF species. Mycorrhiza 2015, 25, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, E.; Turrini, A.; Gamper, H.A.; Cafà, G.; Bonari, E.; Young, J.P.W.; Giovannetti, M. Establishment, persistence and effectiveness of arbuscular mycorrhizal fungal inoculants in the field revealed using molecular genetic tracing and measurement of yield components. New Phytol. 2012, 194, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Antunes, P.M.; Koyama, A. Mycorrhizas as nutrient and energy pumps of soil food webs: Multitrophic interactions and feedbacks. In Mycorrhizal Mediation of Soil; Johnson, N., Gehring, C.C., Jansa, J., Eds.; Elsevier Publishing: Amsterdam, NL, USA, 2017; pp. 149–173. [Google Scholar]
- Schwartz, M.W.; Hoeksema, J.D.; Gehring, C.A.; Johnson, N.C.; Klironomos, J.N.; Abbott, L.K.; Pringle, A. The promise and the potential consequences of the global transport of mycorrhizal fungal inoculum. Ecol. Lett. 2006, 9, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Ohsowski, B.M.; Klironomos, J.N.; Dunfield, K.E.; Hart, M.M. The potential of soil amendments for restoring severely disturbed grasslands. Appl. Soil Ecol. 2012, 60, 77–83. [Google Scholar] [CrossRef]
- Pearse, C.K.; Plummer, A.P.; Savage, D. Restoring the range by reseeding. Yearbook Agric. 1948, 19, 1–7. [Google Scholar]
- Wadley, J.B. The Federal Seed Act: Regulation of seed sales and remedies available to the seed purchaser. SDL Rev. 1981, 27, 453. [Google Scholar]
- Saito, M.; Marumoto, T. Inoculation with arbuscular mycorrhizal fungi: The status quo in Japan and the future prospects. In Diversity and Integration in Mycorrhizas; Smith, F.A., Smith, S.E., Eds.; Springer Publishing: Cham, CH, USA, 2002; pp. 273–279. [Google Scholar]
- Jasper, D.A.; Abbott, L.K.; Robson, A.D. Acacias respond to additions of phosphorus and to inoculation with VA mycorrhizal fungi in soils stockpiled during mineral sand mining. Plant Soil 1989, 115, 99–108. [Google Scholar] [CrossRef]
- Koziol, L.; Bauer, J.T.; Duell, E.B.; Hickman, K.; House, G.L.; Schultz, P.A.; Tipton, A.G.; Wilson, G.W.T.; Bever, J.D. Manipulating plant microbiomes in the field: Native mycorrhizae advance plant succession and improve native plant restoration. J. Appl. Ecol. 2021, 59, 1976–1985. [Google Scholar] [CrossRef]
- Bever, J.; Schultz, P.; Miller, R.; Gades, L.; Jastrow, J. Prairie mycorrhizal fungi inoculant may increase native plant diversity on restored sites (Illinois). Ecol. Rest. 2003, 21, 311–312. [Google Scholar]
- Middleton, E.L.; Bever, J.D. Inoculation with a native soil community advances succession in a grassland restoration. Rest. Ecol. 2012, 20, 218–226. [Google Scholar] [CrossRef]
- Duell, E.B.; O’Hare, A.; Wilson, G.W.T. Inoculation with native soil improves seedling survival and reduces non-native reinvasion in a grassland restoration. Rest. Ecol. 2022, e13685. [Google Scholar] [CrossRef]
- Duell, E.B.; Hickman, K.R.; Wilson, G.W. Inoculation with native grassland soils improves native plant species germination in highly disturbed soil. Grassl. Res. 2022, 1, 75–83. [Google Scholar] [CrossRef]
- Ji, B.; Bentivenga, S.P.; Casper, B.B. Evidence for ecological matching of whole AM fungal communities to the local plant–soil environment. Ecology 2010, 91, 3037–3046. [Google Scholar] [CrossRef]
- Wubs, E.; van der Putten, W.; Bosch, M.; Bezemer, T.B. Soil inoculation steers restoration of terrestrial ecosystems. Nat. Plants 2016, 2, 16107. [Google Scholar] [CrossRef] [PubMed]
- Koziol, L.; Bever, J.D. The missing link in grassland restoration: Arbuscular mycorrhizal fungi inoculation increases plant diversity and accelerates succession. J. Appl. Ecol. 2016, 54, 1301–1309. [Google Scholar] [CrossRef] [Green Version]


| Plant Species | Life Cycle, Provenance |
|---|---|
| C4 Grasses | |
| Andropogon gerardii | Perennial, native |
| Bothriochloa ischaemum | Perennial, non-native, invasive |
| Sorghastrum nutans | perennial, native |
| C3 Grasses | |
| Bromus inermis | Perennial, non-native, invasive |
| Elymus canadensis | perennial, native |
| Forbs | |
| Ratibida columnifera | Perennial, native |
| Salvia azurea | Perennial, native |
| Legumes | |
| Desmanthus illinoensis | Perennial, native |
| Desmodium canadense | Perennial, native |
| Inoculum | Arbuscular Mycorrhizal (AM) Fungal Species (According to Labels) |
|---|---|
| Native field soil | Acaulospra spinosa, Claroideoglomus claroideum, C. etunicatum, Entrophospora infrequens, Funneliformis mosseae, Glomus heterosporum, G. aggregatum, G. macrocarpum, G. constrictum, Scutellospora calospora, |
| A | Rhizophagus irregularis, C. etunicatum, F. mosseae |
| B | R. irregularis, C. etunicatum, F. mosseae |
| C | A. spinosa, Cetraspora pellucida, C. claroideum, C. lamellosum, E. infrequens, F. mosseae, Racocetra fulgida |
| D | R. irregularis |
| E | R. irregularis, C. etunicatum, F. mosseae |
| F | R. irregularis, C. etunicatum, F. mosseae, G. aggregatum |
| Inoculum | pH | Plant-Available N | Plant-Available P |
|---|---|---|---|
| A | 6.0 b | 108.13 ± 9.53 a | 1099.4 ± 36.37 a |
| B | 7.6a | 14.01 ± 0.54 b | 27.72 ± 2.34 c |
| C | 8.1 a | 11.27 ± 1.59 b | 31.03 ± 4.84 c |
| D | 7.7 a | 7.35 ± 0.16 b | 302.69 ± 4.07 b |
| E | 7.5 a | 3.48 ± 0.11b | 58.78 ± 3.04 c |
| F | 7.6 a | 11.14 ± 0.69 b | 36.68 ± 3.22 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duell, E.B.; Cobb, A.B.; Wilson, G.W.T. Effects of Commercial Arbuscular Mycorrhizal Inoculants on Plant Productivity and Intra-Radical Colonization in Native Grassland: Unintentional De-Coupling of a Symbiosis? Plants 2022, 11, 2276. https://doi.org/10.3390/plants11172276
Duell EB, Cobb AB, Wilson GWT. Effects of Commercial Arbuscular Mycorrhizal Inoculants on Plant Productivity and Intra-Radical Colonization in Native Grassland: Unintentional De-Coupling of a Symbiosis? Plants. 2022; 11(17):2276. https://doi.org/10.3390/plants11172276
Chicago/Turabian StyleDuell, Eric B., Adam B. Cobb, and Gail W. T. Wilson. 2022. "Effects of Commercial Arbuscular Mycorrhizal Inoculants on Plant Productivity and Intra-Radical Colonization in Native Grassland: Unintentional De-Coupling of a Symbiosis?" Plants 11, no. 17: 2276. https://doi.org/10.3390/plants11172276
APA StyleDuell, E. B., Cobb, A. B., & Wilson, G. W. T. (2022). Effects of Commercial Arbuscular Mycorrhizal Inoculants on Plant Productivity and Intra-Radical Colonization in Native Grassland: Unintentional De-Coupling of a Symbiosis? Plants, 11(17), 2276. https://doi.org/10.3390/plants11172276