The Role of Medicago lupulina Interaction with Rhizophagus irregularis in the Determination of Root Metabolome at Early Stages of AM Symbiosis
Abstract
:1. Introduction
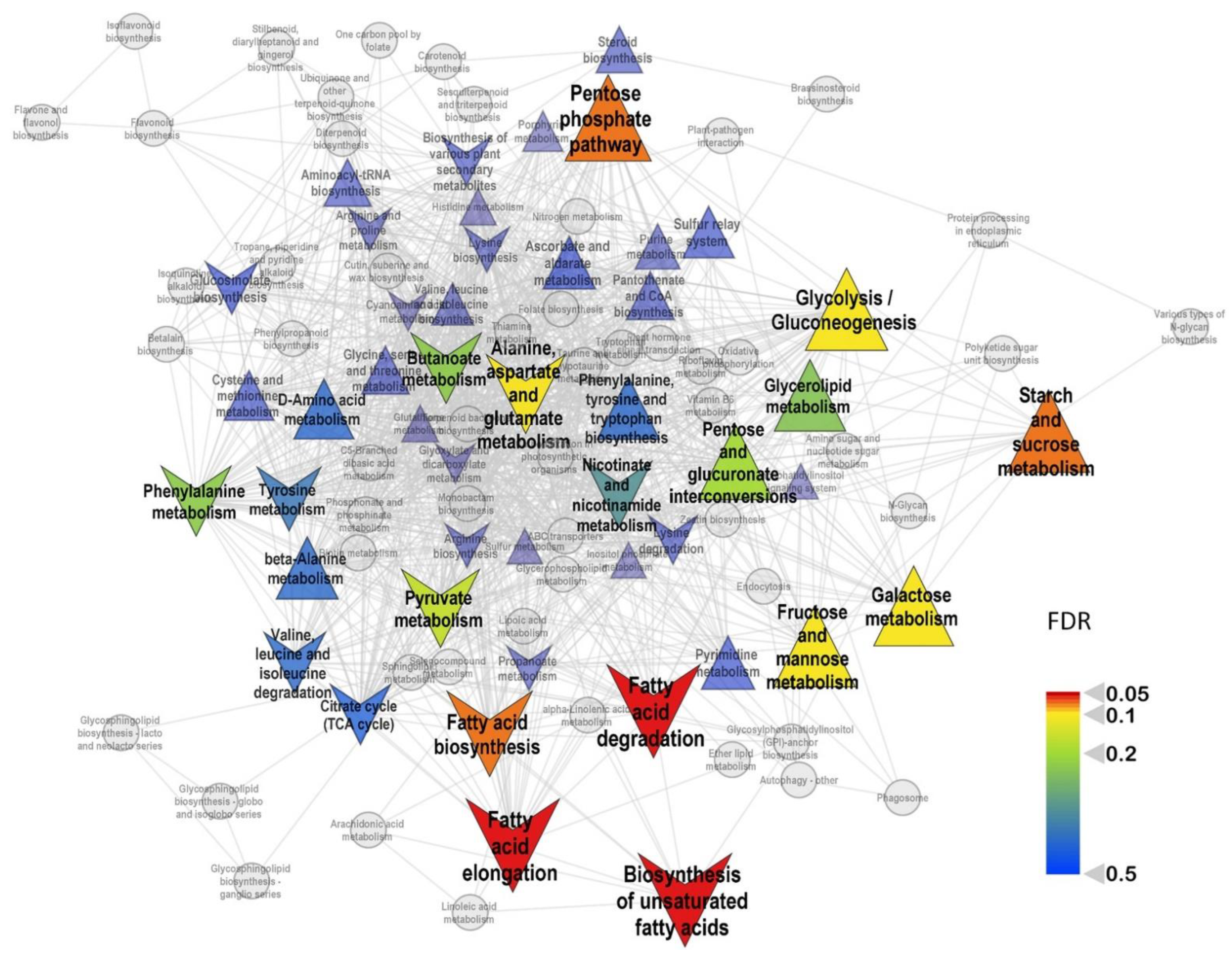
2. Results
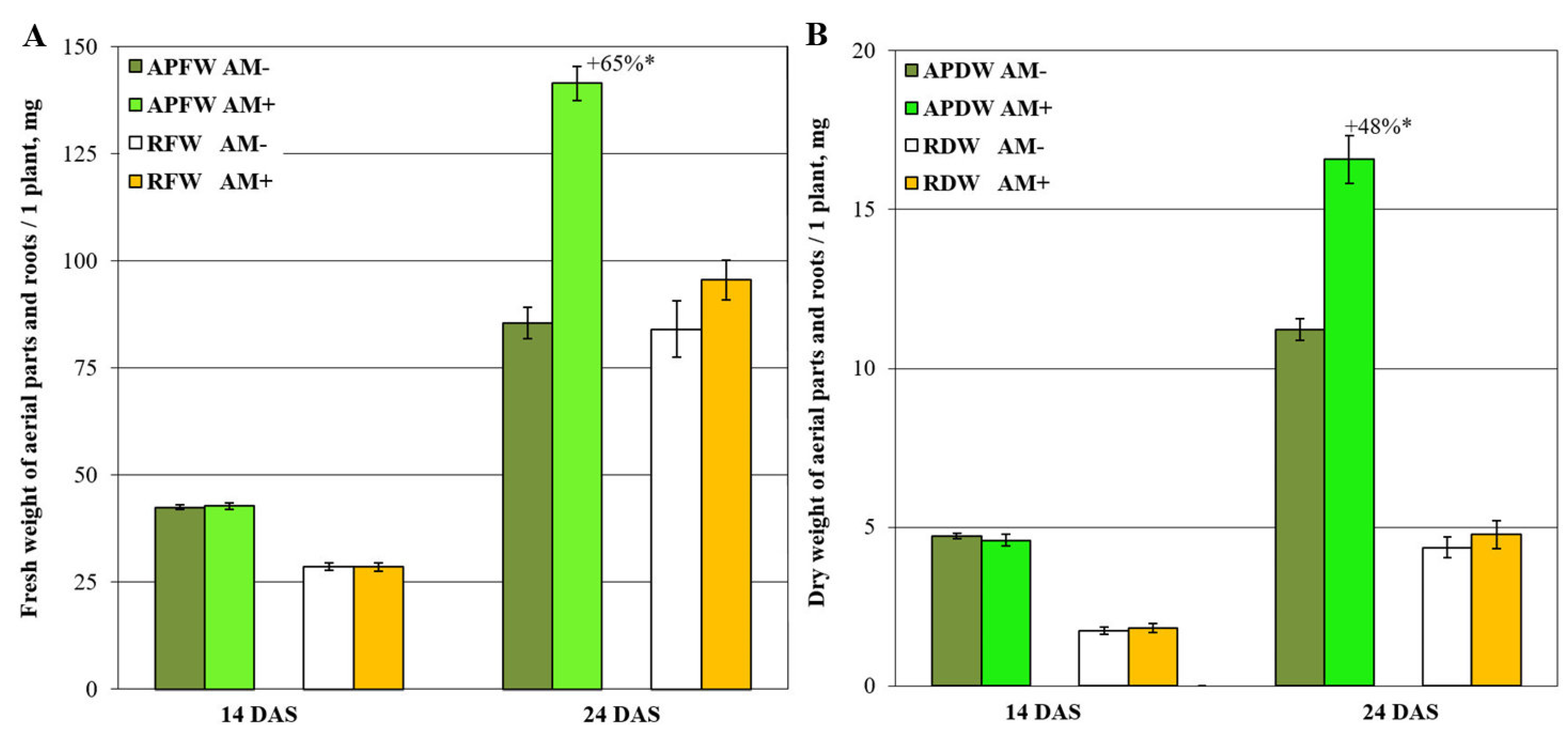
2.1. Medicago lupulina MlS-1 Line Plants Development under Low Phosphorus Level
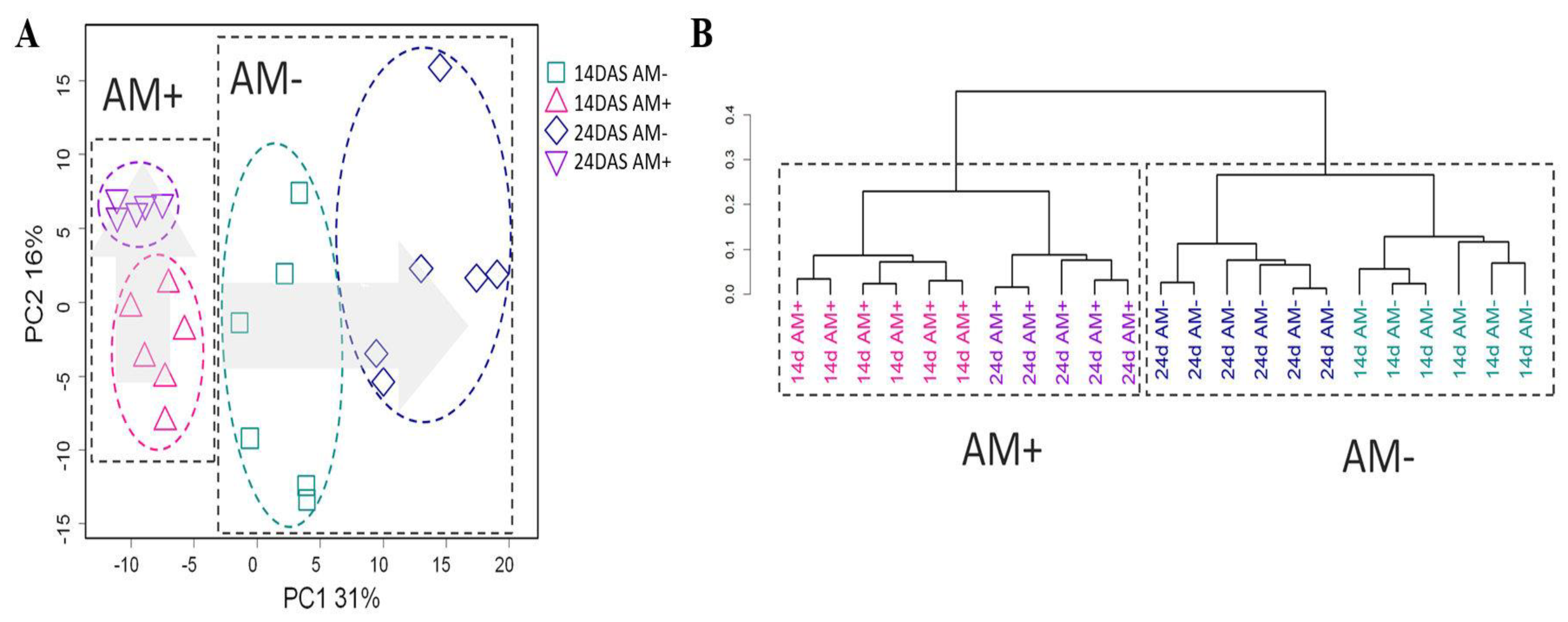
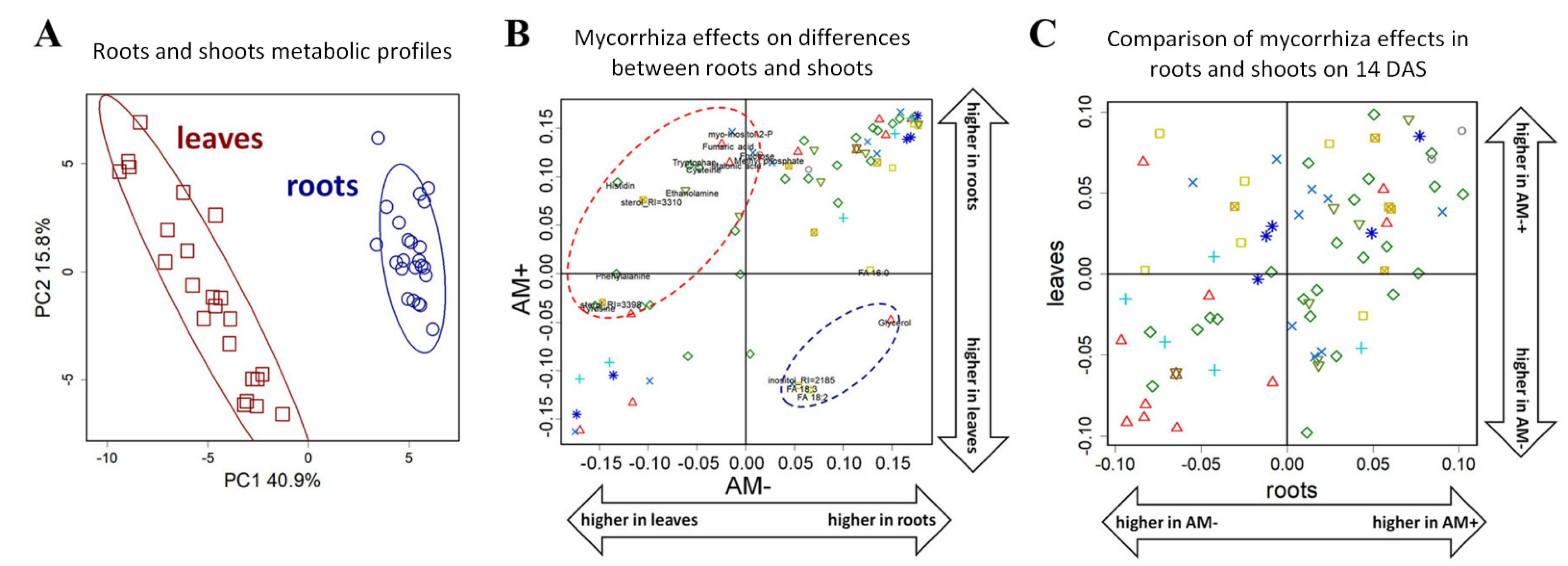
2.2. General Characteristics of Metabolic Profiles
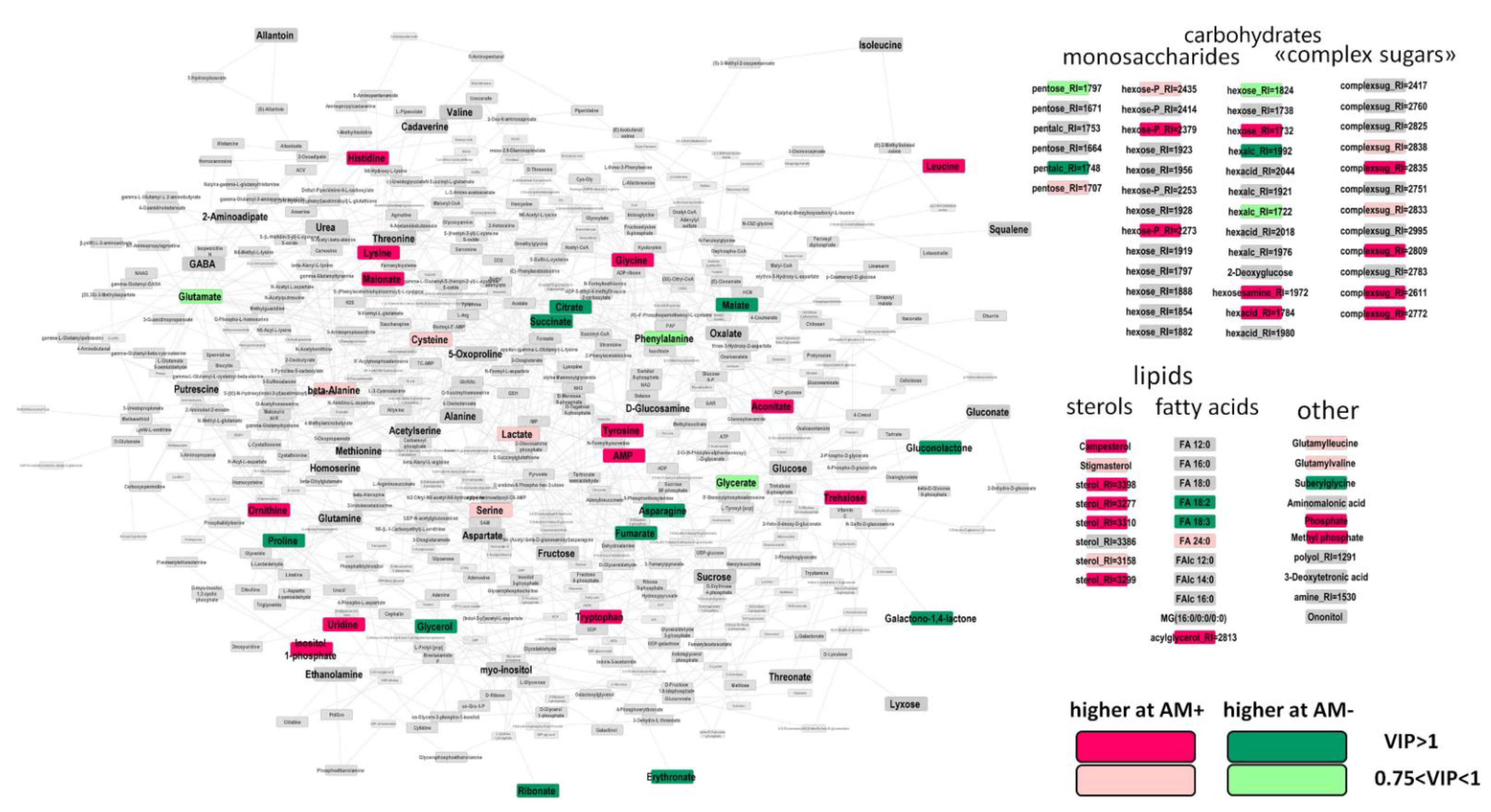
2.3. The Effect of Mycorrhization at 14th DAS
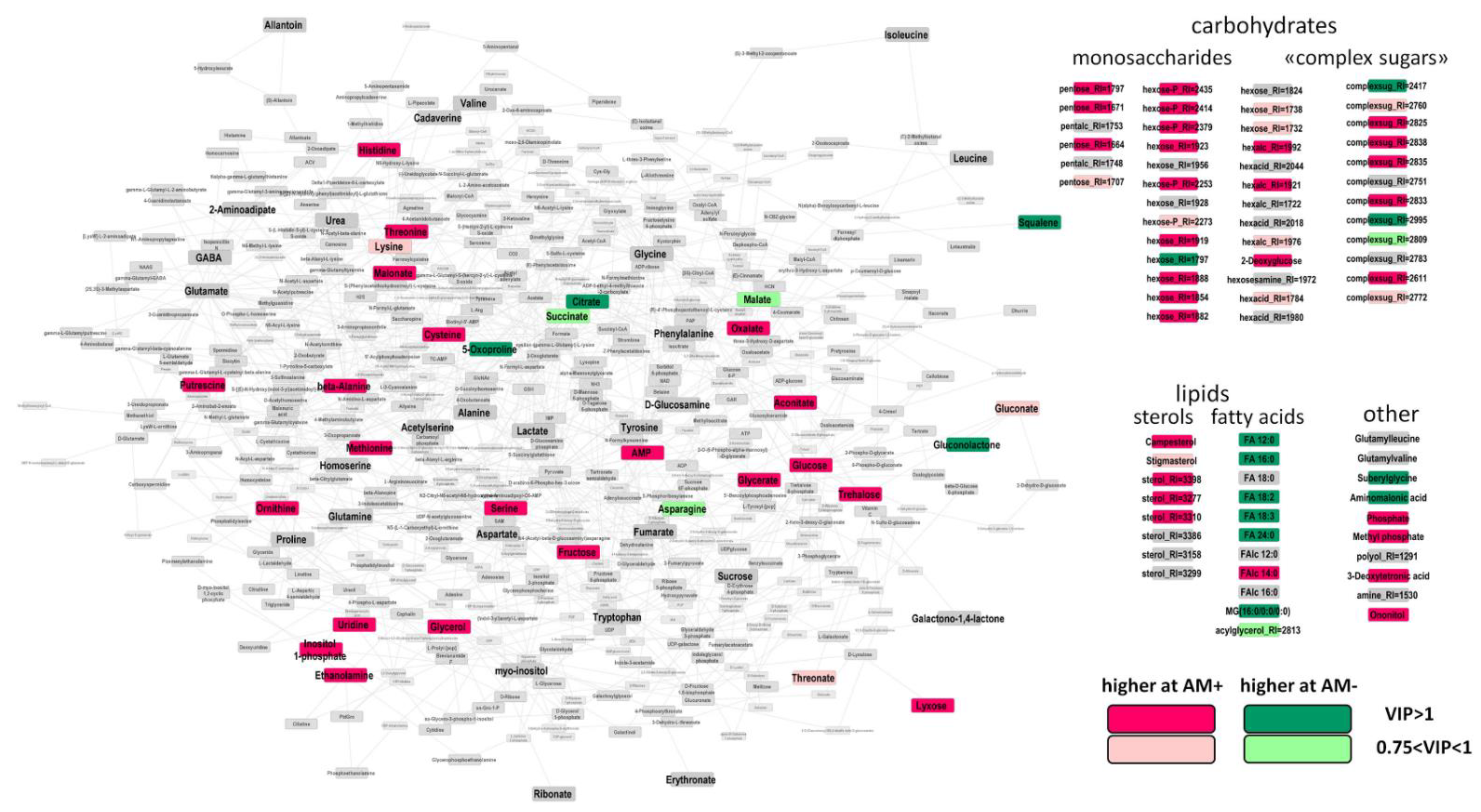
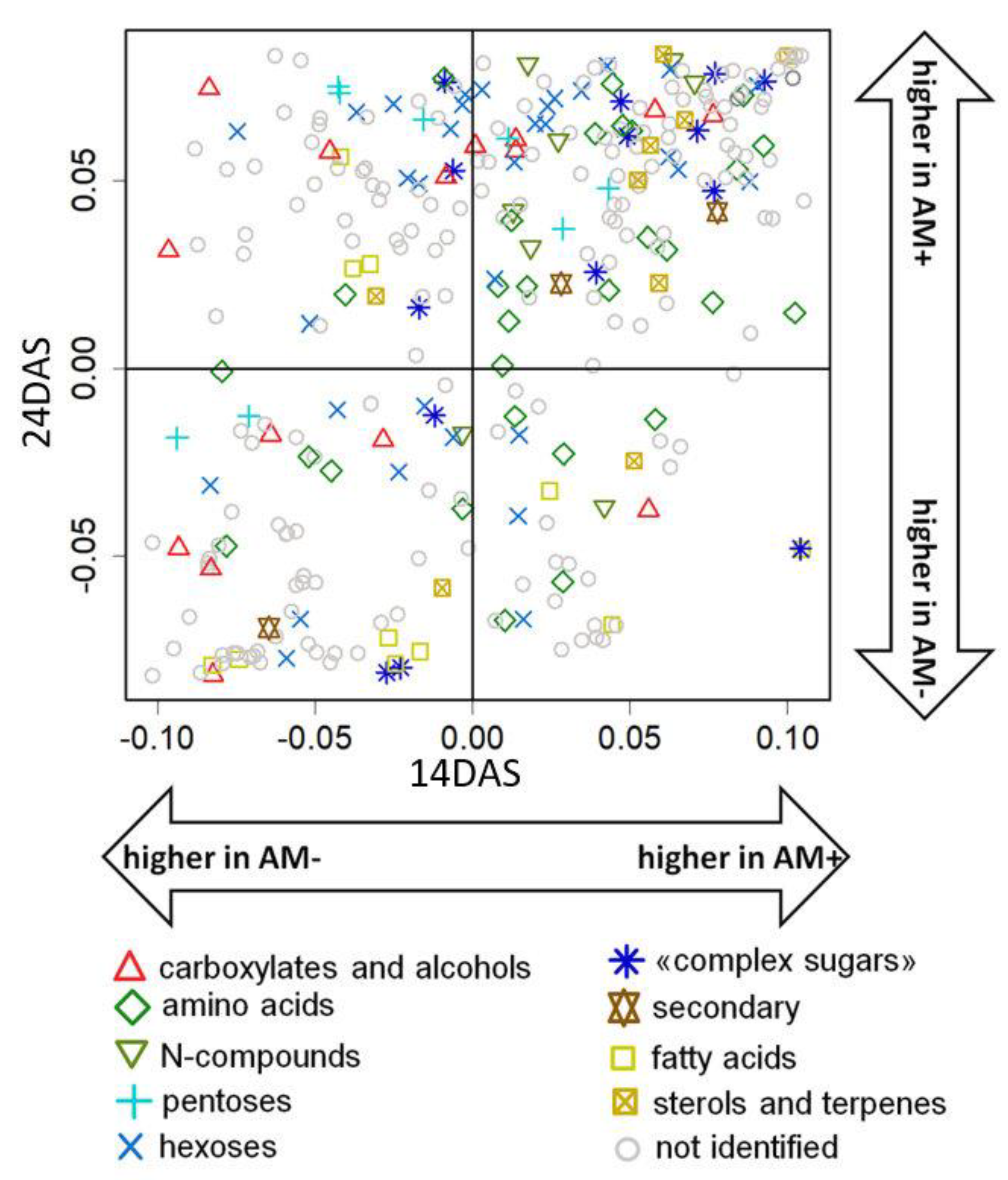
2.4. The Effect of Mycorrhization at 24 DAS
2.5. The Effect of Mycorrhization on Metabolite Allocation between Leaves and Roots
3. Discussion
4. Materials and Methods
4.1. Plant and Fungus Biomaterials
4.2. Experimental Design and Plant Growth Conditions
4.3. Evaluation of Mycorrhization Parameters
4.4. Evaluation of Mycorrhizal Growth Response: AM Symbiotic Efficiency
4.5. GC-MS Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: San Diego, CA, USA, 2008. [Google Scholar]
- Jung, S.C.; Martinez-Medina, A.; Lopez-Raez, J.A.; Pozo, M.J. Mycorrhiza-induced resistance and priming of plant defenses. J. Chem. Ecol. 2012, 38, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Gbongue, L.-R.; Lalaymia, I.; Zeze, A.; Delvaux, B.; Declerck, S. Increased silicon acquisition in bananas colonized by Rhizophagus irregularis MUCL 41833 reduces the incidence of Pseudocercospora fijiensis. Front. Plant Sci. 2019, 9, 1977. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Abiotic stresses and their effects on plant growth, yield and nutritional quality of agricultural produce. J. Sci. Food Agric. 2020, 4, 367–378. [Google Scholar] [CrossRef]
- Shtark, O.Y.; Puzanskiy, R.K.; Avdeeva, G.S.; Yurkov, A.P.; Smolikova, G.N.; Yemelyanov, V.V.; Kliukova, M.S.; Shavarda, A.L.; Kirpichnikova, A.A.; Zhernakov, A.I.; et al. Metabolic alterations in pea leaves during arbuscular mycorrhiza development. PeerJ 2019, 7, e7495. [Google Scholar] [CrossRef]
- Schweiger, R.; Baier, M.C.; Persicke, M.; Müller, C. High specificity in plant leaf metabolic responses to arbuscular mycorrhiza. Nat. Commun. 2014, 5, 3886. [Google Scholar] [CrossRef]
- Goddard, M.-L.; Belval, L.; Martin, I.R.; Roth, L.; Laloue, H.; Deglène-Benbrahim, L.; Valat, L.; Bertsch, C.; Chong, J. Arbuscular mycorrhizal symbiosis triggers major changes in primary metabolism together with modification of defense responses and signaling in both roots and leaves of Vitis vinifera. Front. Plant Sci. 2021, 12, 721614. [Google Scholar] [CrossRef]
- Watts-Williams, S.J.; Emmett, B.D.; Levesque-Tremblay, V.; MacLean, A.M.; Sun, X.; Satterlee, J.W.; Fei, Z.; Harrison, M.J. Diverse Sorghum bicolor accessions show marked variation in growth and transcriptional responses to arbuscular mycorrhizal fungi. Plant Cell Environ. 2019, 42, 1758–1774. [Google Scholar] [CrossRef]
- Grunwald, U.; Guo, W.; Fischer, K.; Isayenkov, S.; Ludwig-Müller, J.; Hause, B.; Yan, X.; Küster, H.; Franken, P. Overlapping expression patterns and differential transcript levels of phosphate transporter genes in arbuscular mycorrhizal, Pi-fertilised and phytohormone-treated Medicago truncatula roots. Planta 2009, 229, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wilde, J.; Baldwin, I.T.; Groten, K. Nicotiana attenuata’s capacity to interact with arbuscular mycorrhiza alters its competitive ability and elicits major changes in the leaf transcriptome. J. Integr. Plant Biol. 2018, 60, 242–261. [Google Scholar] [CrossRef]
- Watson, B.S.; Asirvatham, V.S.; Wang, L.; Sumner, L.W. Mapping of the proteome of barrel medic (Medicago truncatula). Plant Physiol. 2003, 131, 1104–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valot, B.; Negroni, L.; Zivy, M.; Gianinazzi, S.; Dumas-Gaudot, E. A mass spectrometric approach to identify arbuscular mycorrhiza-related proteins in root plasma membrane fractions. Proteomics 2006, 6, S145–S155. [Google Scholar] [CrossRef] [PubMed]
- Aloui, A.; Recorbet, G.; Lemaître-Guillier, C.; Mounier, A.; Balliau, T.; Zivy, M.; Wipf, D.; Dumas-Gaudot, E. The plasma membrane proteome of Medicago truncatula roots as modified by arbuscular mycorrhizal symbiosis. Mycorrhiza 2018, 281, 1–16. [Google Scholar] [CrossRef]
- Olalde-Portugal, V.; Cabrera-Ponce, J.L.; Gastelum-Arellanez, A.; Guerrero-Rangel, A.; Winkler, R.; Valdés-Rodríguez, S. Proteomic analysis and interactions network in leaves of mycorrhizal and nonmycorrhizal sorghum plants under water deficit. PeerJ 2020, 8, e8991. [Google Scholar] [CrossRef] [PubMed]
- Casarrubias-Castillo, K.; Montero-Vargas, J.M.; Dabdoub-González, N.; Winkler, R.; Martínez-Gallardo, N.A.; Avilés-Arnaut, H.; Délano-Frier, J.P. Distinct gene expression and secondary metabolite profiles for suboptimal mycorrhizal colonization in wild-type and the jasmonic acid deficient spr2 tomato mutant. PeerJ 2020, 8, e8888:1–e8888:43. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Suseela, V. Unraveling arbuscular mycorrhiza-induced changes in plant primary and secondary metabolome. Metabolites 2020, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Yurkov, A.P.; Puzanskiy, R.K.; Avdeeva, G.S.; Jacobi, L.M.; Gorbunova, A.O.; Kryukov, A.A.; Kozhemyakov, A.P.; Laktionov, Y.V.; Kosulnikov, Y.V.; Romanyuk, D.A.; et al. Mycorrhiza-induced alterations in metabolome of Medicago lupulina leaves during symbiosis development. Plants 2021, 10, 2506. [Google Scholar] [CrossRef]
- Davey, M.P.; Burrell, M.M.; Woodward, F.I.; Quick, W.P. Population-specific metabolic phenotypes of Arabidopsis lyrata ssp. petraea. New Phytol. 2008, 177, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Bertram, H.C.; Weisbjerg, M.R.; Jensen, C.S.; Pedersen, M.G.; Didion, T.; Petersen, B.O.; Duus, J.Ø.; Larsen, M.K.; Nielsen, J.H. Seasonal changes in the metabolic fingerprint of 21 grass and legume cultivars studied by nuclear magnetic resonance-based metabolomics. J. Agric. Food Chem. 2010, 58, 4336–4341. [Google Scholar] [CrossRef]
- Kim, H.K.; Saifullah; Khan, S.; Wilson, E.G.; Kricun, S.D.; Meissner, A.; Goraler, S.; Deelder, A.M.; Choi, Y.H.; Verpoorte, R. Metabolic classification of South American Ilex species by NMR-based metabolomics. Phytochemistry 2010, 71, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, A.; Takahashi, H.; Takahara, K.; Hirabayashi, T. Principal component and hierarchical clustering analysis of metabolites in destructive weeds; polygonaceous plants. Metabolomics 2010, 6, 146–155. [Google Scholar] [CrossRef]
- Schweiger, R.; Mueller, C. Leaf metabolome in arbuscular mycorrhizal symbiosis. Curr. Opin. Plant Biol. 2015, 26, 120–126. [Google Scholar] [CrossRef]
- Duc, G.; Messager, A. Mutagenesis of pea Pisum sativum L. and the isolation of mutants for nodulation and nitrogen fixation. Plant Sci. 1989, 60, 207–213. [Google Scholar] [CrossRef]
- Borisov, A.Y.; Barmicheva, E.M.; Jacobi, L.M.; Tsyganov, V.E.; Voroshilova, V.A.; Tikhonovich, I.A. Pea Pisum sativum L. mendelian genes controlling development of nitrogen-fixing nodules and arbuscular mycorrhiza. Czech J. Genet. Plant Breed. 2000, 36, 106–110. [Google Scholar]
- Shtark, O.; Puzanskiy, R.; Avdeeva, G.; Yemelyanov, V.; Shavarda, A.; Romanyuk, D.; Kliukova, M.; Kirpichnikova, A.; Tikhonovich, I.; Zhukov, V.; et al. Metabolic alterations in Pisum sativum roots during plant growth and arbuscular mycorrhiza development. Plants 2021, 10, 1033. [Google Scholar] [CrossRef] [PubMed]
- Sagan, M.; Morandi, D.; Tarenghi, E.; Duc, G. Selection of nodulation and mycorrhizal mutants in the model plant Medicago truncatula Gaertn. after γ-ray mutagenesis. Plant Sci. 1995, 111, 63–71. [Google Scholar] [CrossRef]
- Schliemann, W.; Ammer, C.; Strack, D. Metabolite profiling of mycorrhizal roots of Medicago truncatula. Phytochemistry 2008, 69, 112–146. [Google Scholar] [CrossRef]
- Yurkov, A.P.; Jacobi, L.M.; Gapeeva, N.E.; Stepanova, G.V.; Shishova, M.F. Development of arbuscular mycorrhiza in highly responsive and mycotrophic host plant—Black medick (Medicago lupulina L.). Russ. J. Dev. Biol. 2015, 46, 263–275. [Google Scholar] [CrossRef]
- Wegel, E.; Schauser, L.; Sandal, N.; Stougaard, J.; Parniske, M. Mycorrhiza mutants of Lotus japonicus define genetically independent steps during symbiotic infection. MPMI 1998, 11, 933–936. [Google Scholar] [CrossRef]
- Shiztliffe, S.J.; Vessey, J.K. A nodulation (Nod+/Fix−) mutant of Phaseolus vulgaris L. has nodulelike structures lacking peripherial vascular bundles (Pvb−) and is resistant to mycorrhizal infection (Myc−). Plant Sci. 1996, 118, 209–220. [Google Scholar] [CrossRef]
- Klingner, A.; Bothe, H.; Wray, V. Identification of a yellow pigment formed in maize roots upon mycorrhizal colonization. Phytochemistry 1995, 38, 53–55. [Google Scholar] [CrossRef]
- Cordier, C.; Pozo, M.J.; Barea, J.M.; Gianinazzi, S.; Gianinazzi-Pearson, V. Cell defense responses assosiated with localized and systemic resistance to Phitophthora parasitica induced in tomato by an arbuscular mycorrhizal fungus. MPMI 1998, 11, 1017–1028. [Google Scholar] [CrossRef] [Green Version]
- Reddy, D.M.R.S.; Schorderet, M.; Feller, U.; Reinhardt, D. A petunia mutant affected in intracellular accommodation and morphogenesis of arbuscular mycorrhizal fungi. Plant J. 2007, 51, 739–750. [Google Scholar] [CrossRef]
- Luginbuehl, L.H.; Oldroyd, G.E.D. Understanding the arbuscule at the heart of endomycorrhizal symbioses in plants. Curr. Biol. 2017, 27, 952–963. [Google Scholar] [CrossRef]
- Trepanier, M.; Becard, G.; Moutoglis, P.; Willemot, C.; Gagne, S.; Avis, T.J.; Rioux, J.A. Dependence of arbuscular-mycorrhizal fungi on their plant host for palmitic acid synthesis. Appl. Environ. Microbiol. 2005, 71, 5341–5347. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, S.; Harrison, M.J. A set of fluorescent protein-based markers expressed from constitutive and arbuscular mycorrhiza-inducible promoters to label organelles, membranes and cytoskeletal elements in Medicago truncatula. Plant J. 2014, 80, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.M.; Reader, R.J. Host plant benefit from association with arbuscular mycorrhizal fungi: Variation due to differences in size of mycelium. Biol. Fertil. Soils 2002, 36, 357–366. [Google Scholar] [CrossRef]
- Kaur, S.; Campbell, B.J.; Suseela, V. Root metabolome of plant-arbuscular mycorrhizal symbiosis mirrors the mutualistic or parasitic mycorrhizal phenotype. New Phytol. 2022, 234, 672–687. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A.; Jakobsen, I. Functional diversity in arbuscular mycorrhizal (AM) symbioses: The contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. New Phytol. 2004, 162, 511–524. [Google Scholar] [CrossRef]
- Bucher, M. Functional biology of plant phosphate uptake at root and mycorrhiza interfaces. New Phytol. 2007, 173, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Rivero, J.; Gamir, J.; Aroca, R.; Pozo, M.J.; Flors, V. Metabolic transition in mycorrhizal tomato roots. Front. Microbiol. 2015, 6, 598. [Google Scholar] [CrossRef]
- Pedone-Bonfim, M.V.; Lins, M.A.; Coelho, I.R.; Santana, A.S.; Silva, F.S.; Maia, L.C. Mycorrhizal technology and phosphorus in the production of primary and secondary metabolites in cebil (Anadenanthera colubrina (Vell.) Brenan) seedlings. J. Sci. Food Agric. 2013, 93, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Stockinger, H.; Walker, C.; Schüßler, A. “Glomus intraradices DAOM197198”, a model fungus in arbuscular mycorrhiza research, is not Glomus intraradices. New Phytol. 2009, 183, 1176–1187. [Google Scholar] [CrossRef]
- Saia, S.; Rappa, V.; Ruisi, P.; Abenavoli, M.R.; Sunseri, F.; Giambalvo, D.; Frenda, A.S.; Martinelli, F. Soil inoculation with symbiotic microorganisms promotes plant growth and nutrient transporter genes expression in durum wheat. Front. Plant Sci. 2015, 6, 815. [Google Scholar] [CrossRef] [PubMed]
- Schliemann, W.; Schmidt, J.; Nimtz, M.; Wray, V.; Fester, T.; Strack, D. Accumulation of apocarotenoids in mycorrhizal roots of Ornithogalum umbellatum. Phytochemistry 2006, 67, 1196–1205. [Google Scholar] [CrossRef]
- Wang, M.; Schäfer, M.; Li, D.; Halitschke, R.; Dong, C.; McGale, E.; Paetz, C.; Song, Y.; Li, S.; Dong, J.; et al. Blumenols as shoot markers of root symbiosis with arbuscular mycorrhizal fungi. eLife 2018, 7, e37093. [Google Scholar] [CrossRef]
- Hill, E.M.; Robinson, L.A.; Abdul-Sada, A.; Vanbergen, A.J.; Hodge, A.; Hartley, S.E. Arbuscular mycorrhizal fungi and plant chemical defence: Effects of colonisation on above ground and below ground metabolomes. J. Chem. Ecol. 2018, 44, 198–208. [Google Scholar] [CrossRef]
- Adolfsson, L.; Nziengui, H.; Abreu, I.N.; Šimura, J.; Beebo, A.; Herdean, A.; Aboalizadeh, J.; Široká, J.; Moritz, T.; Novák, O.; et al. Enhanced secondary- and hormone metabolism in leaves of arbuscular mycorrhizal Medicago truncatula. Plant Physiol. 2017, 175, 392–411. [Google Scholar] [CrossRef]
- Johny, L.; Cahill, D.M.; Adholeya, A. AMF enhance secondary metabolite production in ashwagandha, licorice, and marigold in a fungi-host specific manner. Rhizosphere 2021, 17, 100314. [Google Scholar] [CrossRef]
- Tavarini, S.; Passera, B.; Martini, A.; Avio, L.; Sbrana, C.; Giovannetti, M.; Angelini, L.G. Plant growth, steviol glycosides and nutrient uptake as affected by arbuscular mycorrhizal fungi and phosphorous fertilization in Stevia rebaudiana Bert. Ind. Crops Prod. 2018, 111, 899–907. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, J.; Yang, F.; Tao, S.; Yan, X.; Zhou, Z.; Zhang, Y. Arbuscular mycorrhizal fungi improve the growth and performance in the seedlings of Leymus chinensis under alkali and drought stresses. PeerJ 2022, 10, e12890. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, W.; Geng, Y.; Liu, K.; Shao, X. Cooperation with arbuscular mycorrhizal fungi increases plant nutrient uptake and improves defenses against insects. Front. Ecol. Evol. 2022, 23, 833389. [Google Scholar] [CrossRef]
- Fester, T.; Fetzer, I.; Buchert, S.; Lucas, R.; Rillig, M.C.; Härtig, C. Towards a systemic metabolic signature of the arbuscular mycorrhizal interaction. Oecologia 2011, 167, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, F.; Amini, T.; Asadi, M.; Hassanpouraghdam, M.B.; Aazami, M.A.; Ercisli, S.; Skrovankova, S.; Mlcek, J. Growth and antioxidant responses of lettuce (Lactuca sativa L.) to arbuscular mycorrhiza inoculation and seaweed extract foliar application. Agronomy 2022, 12, 401. [Google Scholar] [CrossRef]
- Vo, A.T.; Haddidi, I.; Daood, H.; Mayer, Z.; Posta, K. Impact of arbuscular mycorrhizal inoculation and growth substrate on biomass and content of polyphenols in Eclipta prostrata. Hortic. Sci. 2019, 54, 1976–1983. [Google Scholar] [CrossRef]
- Dreher, D.; Baldermann, S.; Schreiner, M.; Hause, B. An arbuscular mycorrhizal fungus and a root pathogen induce different volatiles emitted by Medicago truncatula roots. J. Adv. Res. 2019, 19, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Tsiokanos, E.; Cartabia, A.; Tsafantakis, N.; Lalaymia, I.; Termentzi, A.; Miguel, M.; Declerck, S.; Fokialakis, N. The metabolic profile of Anchusa officinalis L. differs according to its associated arbuscular mycorrhizal fungi. Metabolites 2022, 12, 573. [Google Scholar] [CrossRef]
- Yurkov, A.; Kryukov, A.; Gorbunova, A.; Sherbakov, A.; Dobryakova, K.; Mikhaylova, Y.; Afonin, A.; Shishova, M. AM-induced alteration in the expression of genes, encoding phosphorus transporters and enzymes of carbohydrate metabolism in Medicago lupulina. Plants 2020, 9, 486. [Google Scholar] [CrossRef]
- Peret, B.; Desnos, T.; Jost, R.; Kanno, S.; Berkowitz, O.; Nussaume, L. Root architecture responses: In search of phosphate. Plant Physiol. 2014, 166, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Strock, C.F.; Morrow, L.D.L.R.; Lynch, J. Reduction in root secondarygrowth as a strategy for phosphorus acquisition. Plant Physiol. 2018, 176, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P.; Brown, K.M. Topsoil foraging: An architectural adaptation of plants to low phosphorus availability. Plant Soil 2001, 237, 225–237. [Google Scholar] [CrossRef]
- Ticconi, C.A.; Delatorre, C.A.; Lahner, B.; Salt, D.E.; Abel, S. Arabidopsis pdr2 reveals a phosphate-sensitive checkpoint in root development. Plant J. 2004, 37, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.; Godde, V.; Niehaus, K.; Zorb, C. Metabolic adaptations of white lupin roots and shoots under phosphorus deficiency. Front. Plant Sci. 2015, 6, e0129520. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Liu, Y.; Zhang, H.; Wang, J.; Chen, Z.; Luo, L.; Liu, G.; Liu, P. Metabolic alterations provide insights into Stylosanthes roots responding to phosphorus deficiency. BMC Plant Biol. 2020, 20, 85. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, M.; Zhao, M.; Chen, R.; Tigabu, M.; Wu, P.; Li, M.; Ma, X. Transcriptome analysis provides insights into the root response of Chinese firto phosphorus deficiency. BMC Plant Biol. 2021, 21, 525. [Google Scholar] [CrossRef] [PubMed]
- Dokwal, D.; Cocuron, J.-C.; Alonso, A.P.; Dickstein, R. Metabolite shift in Medicago truncatula occurs in phosphorus deprivation. J. Exp. Bot. 2022, 73, 2093–2111. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; Kumar, A.; Kaur, N.; Kaur, H. Molecular cues of sugar signaling in plants. Physiol. Plant. 2022, 174, e13630. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, A.; Ando, F.; Toyofuku, K.; Kawashima, C. Sucrose metabolism for the development of seminal root in maize seedlings. Plant Prod. Sci. 2009, 12, 9–16. [Google Scholar] [CrossRef]
- Yurkov, A.P.; Veselova, S.V.; Jacobi, L.M.; Stepanova, G.V.; Yemelyanov, V.V.; Kudoyarova, G.R.; Shishova, M.F. The effect of inoculation with arbuscular mycorrhizal fungus Rhizophagus irregularis on cytokinin content in highly mycotrophic Medicago lupulina line under low phosphorus level in soil. Plant Soil Environ. 2017, 63, 519–524. [Google Scholar] [CrossRef]
- Helber, N.; Wippel, K.; Sauer, N.; Schaarschmidt, S.; Hause, B.; Requena, N. A versatile monosaccharide transporter that operates in the arbuscular mycorrhizal fungus Glomus sp. is crucial for the symbiotic relationship with plants. Plant Cell 2011, 23, 3812–3823. [Google Scholar] [CrossRef]
- Douds, D.D., Jr.; Pfeffer, P.E.; Shachar-Hill, Y. Carbon partitioning, cost and metabolism of arbuscular mycorrhizas. In Arbuscular Mycorrhizas: Physiology and Function; Kapulnik, Y., Douds, D.D., Jr., Eds.; Kluwer Academic Press: Dordrecht, The Netherlands, 2000; pp. 107–130. [Google Scholar] [CrossRef]
- Klugh, K.R.; Cumming, J.R. Variations in organic acid exudation and aluminum resistance among arbuscular mycorrhizal species colonizing Liriodendron tulipifera. Tree Physiol. 2007, 27, 1103–1112. [Google Scholar] [CrossRef] [Green Version]
- Kloppholz, S.; Kuhn, H.; Requena, N. A secreted fungal effector of Glomus intraradices promotes symbiotic biotrophy. Curr. Biol. 2011, 21, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, S.; Handa, Y.; Takeda, N.; Kawaguchi, M. Strigolactone induced putative secreted protein 1 is required for the establishment of symbiosis by the arbuscular mycorrhizal fungus Rhizophagus irregularis. Mol. Plant-Microbe Interact. 2016, 29, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Holmer, R.; Hontelez, J.; Lintel-Hekkert, B.T.; Marufu, L.; de Zeeuw, T.; Wu, F.; Schijlen, E.; Bisseling, T.; Limpens, E. Host- and stage-dependent secretome of the arbuscular mycorrhizal fungus Rhizophagus irregularis. Plant J. 2018, 94, 411–425. [Google Scholar] [CrossRef]
- Wewer, V.; Brands, M.; Dormann, P. Fatty acid synthesis and lipid metabolism in the obligate biotrophic fungus Rhizophagus irregularis during mycorrhization of Lotus japonicus. Plant J. 2014, 79, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Bravo, A.; Brands, M.; Wewer, V.; Dormann, P.; Harrison, M.J. Arbuscular mycorrhiza-specific enzymes FatM and RAM2 fine-tune lipid biosynthesis to promote development of arbuscular mycorrhiza. New Phytol. 2017, 214, 1631–1645. [Google Scholar] [CrossRef] [PubMed]
- Olsson, P.A.; Larsson, L.; Bago, B.; Wallander, H.; van Aarle, I. Ergosterol and fatty acids for biomass estimation of mycorrhizal fungi. New Phytol. 2003, 159, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.H.; Tibbett, M.; Edmonds-Tibbett, T.; Suriyagoda, L.D.B.; Lambers, H.; Cawthray, G.R.; Pang, J. Carbon trading for phosphorus gain: The balance between rhizosphere carboxylates and arbuscular mycorrhizal symbiosis in plant phosphorus acquisition. Plant Cell Environ. 2012, 35, 2170–2180. [Google Scholar] [CrossRef]
- Kirpichnikov, N.A.; Volkov, A.A.; Chernyshkova, L.B.; Yurkov, A.P.; Yakobi, L.M.; Kozhemyakov, A.P.; Zavalin, A.A. Effect of phosphorus fertilizers, lime materials, and biopreparations in barley and clover plants in a mixed plantation. Agrohimia 2012, 11, 16–27. [Google Scholar]
- Efimova, I.L.; Yurkov, A.P. New methods of agroecology for improving the quality of apple planting material. Proc. Kuban State Univ. 2015, 4, 73–77. [Google Scholar]
- Yurkov, A.P.; Stepanova, G.V.; Yakobi, L.M.; Kozhemyakov, A.P.; Sergaliev, N.H.; Amenova, R.K.; Djaparov, R.S.; Volodin, M.A.; Tlepov, A.S.; Baymukanov, E.N. Productivity of spring and winter wheat in drought conditions dependent on the application of arbuscular mycorrhizal fungus Glomus intraradices. Fodd. Prod. 2012, 12, 18–24. [Google Scholar]
- Kryukov, A.A.; Yurkov, A.P. Optimization procedures for molecular-genetic identification of arbuscular mycorrhizal fungi in symbiotic phase on the example of two closely kindred strains. Mikol. Fitopatol. 2018, 52, 38–48. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.L.; Gianinazzi-Pearson, V. Mesure du taux de mycorhization VA d’un système radiculaire. Recherche de méthodes ayant une signification fonctionnelle. In Physiological and Genetical Aspects of Mycorrhizae; Gianinazzi-Pearson, V., Gianinazzi, S., Eds.; INRA-Press: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Vorobyev, N.I.; Yurkov, A.P.; Provorov, N.A. Certificate N2010612112 about the Registration of the Computer Program “Program for Calculating the Mycorrhization Indices of Plant Roots” (Dated 2 December 2016); The Federal Service for Intellectual Property: Moscow, Russia, 2016. [Google Scholar]
- Johnsen, L.G.; Skou, P.B.; Khakimov, B.; Bro, R. Gas chromatography—Mass spectrometry data processing made easy. J. Chromatogr. A 2017, 1503, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Hummel, J.; Selbig, J.; Walther, D.; Kopka, J. The Golm Metabolome Database: A database for GC-MS based metabolite profiling. In Topics in Current Genetics; Nielsen, J., Jewett, M.C., Eds.; Metabolomics; Springer: Heidelberg, Germany, 2007; Volume 18, pp. 75–95. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 19 July 2022).
- Komsta, L. Outliers: Tests for Outliers. 2011. Available online: https://CRAN.R-project.org/package=outliers (accessed on 19 July 2022).
- Hastie, T.; Tibshirani, R.; Narasimhan, B.; Chu, G. Impute: Imputation for Microarray Data. R Package Version 1.60.0. 2019. Available online: https://bioconductor.org/packages/release/bioc/html/impute.html (accessed on 19 July 2022).
- Stacklies, W.; Redestig, H.; Scholz, M.; Walther, D.; Selbig, J. pcaMethods-a bioconductor package providing PCA methods for incomplete data. Bioinformatics 2007, 23, 1164–1167. [Google Scholar] [CrossRef] [PubMed]
- Thévenot, E.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the human adult urinary metabolome variations with age, body mass index, and gender by implementing a comprehensive workflow for univariate and OPLS statistical analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef]
- Korotkevich, G.; Sukhov, V.; Sergushichev, A. Fast gene set enrichment analysis. bioRxiv 2021, 060012. [Google Scholar] [CrossRef]
- Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019, 28, 1947–1951. [Google Scholar] [CrossRef] [PubMed]
- Tenenbaum, D.; Maintainer, B. KEGGREST: Client-Side REST Access to the Kyoto Encyclopedia of Genes and Genomes (KEGG). R Package Version 1.36.3. 2022. Available online: https://bioconductor.org/packages/release/bioc/html/KEGGREST.html (accessed on 19 July 2022). [CrossRef]
- Csardi, G.; Nepusz, T. The Igraph Software Package for Complex Network Research. InterJournal, Complex Systems 1695. 2006. Available online: https://igraph.org (accessed on 19 July 2022).
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yurkov, A.P.; Puzanskiy, R.K.; Kryukov, A.A.; Gorbunova, A.O.; Kudriashova, T.R.; Jacobi, L.M.; Kozhemyakov, A.P.; Romanyuk, D.A.; Aronova, E.B.; Avdeeva, G.S.; et al. The Role of Medicago lupulina Interaction with Rhizophagus irregularis in the Determination of Root Metabolome at Early Stages of AM Symbiosis. Plants 2022, 11, 2338. https://doi.org/10.3390/plants11182338
Yurkov AP, Puzanskiy RK, Kryukov AA, Gorbunova AO, Kudriashova TR, Jacobi LM, Kozhemyakov AP, Romanyuk DA, Aronova EB, Avdeeva GS, et al. The Role of Medicago lupulina Interaction with Rhizophagus irregularis in the Determination of Root Metabolome at Early Stages of AM Symbiosis. Plants. 2022; 11(18):2338. https://doi.org/10.3390/plants11182338
Chicago/Turabian StyleYurkov, Andrey P., Roman K. Puzanskiy, Alexey A. Kryukov, Anastasiia O. Gorbunova, Tatyana R. Kudriashova, Lidija M. Jacobi, Andrei P. Kozhemyakov, Daria A. Romanyuk, Ekaterina B. Aronova, Galina S. Avdeeva, and et al. 2022. "The Role of Medicago lupulina Interaction with Rhizophagus irregularis in the Determination of Root Metabolome at Early Stages of AM Symbiosis" Plants 11, no. 18: 2338. https://doi.org/10.3390/plants11182338
APA StyleYurkov, A. P., Puzanskiy, R. K., Kryukov, A. A., Gorbunova, A. O., Kudriashova, T. R., Jacobi, L. M., Kozhemyakov, A. P., Romanyuk, D. A., Aronova, E. B., Avdeeva, G. S., Yemelyanov, V. V., Shavarda, A. L., & Shishova, M. F. (2022). The Role of Medicago lupulina Interaction with Rhizophagus irregularis in the Determination of Root Metabolome at Early Stages of AM Symbiosis. Plants, 11(18), 2338. https://doi.org/10.3390/plants11182338





