Crosstalk of Cytokinin with Ethylene and Auxin for Cell Elongation Inhibition and Boron Transport in Arabidopsis Primary Root under Boron Deficiency
Abstract
:1. Introduction
2. Results
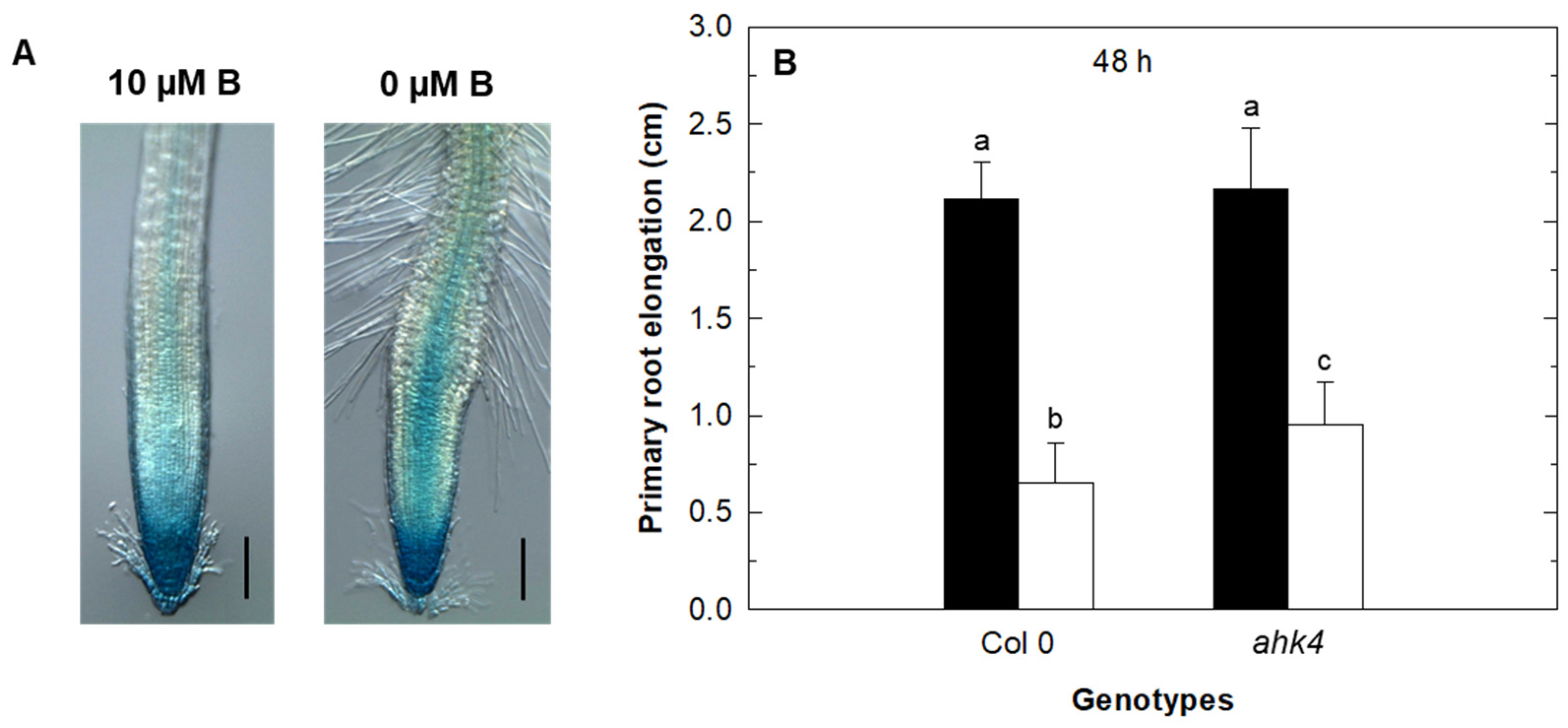
2.1. Effect of Cytokinin Treatment on Primary Root Growth of Auxin and Ethylene Arabidopsis Mutants under B Deficiency
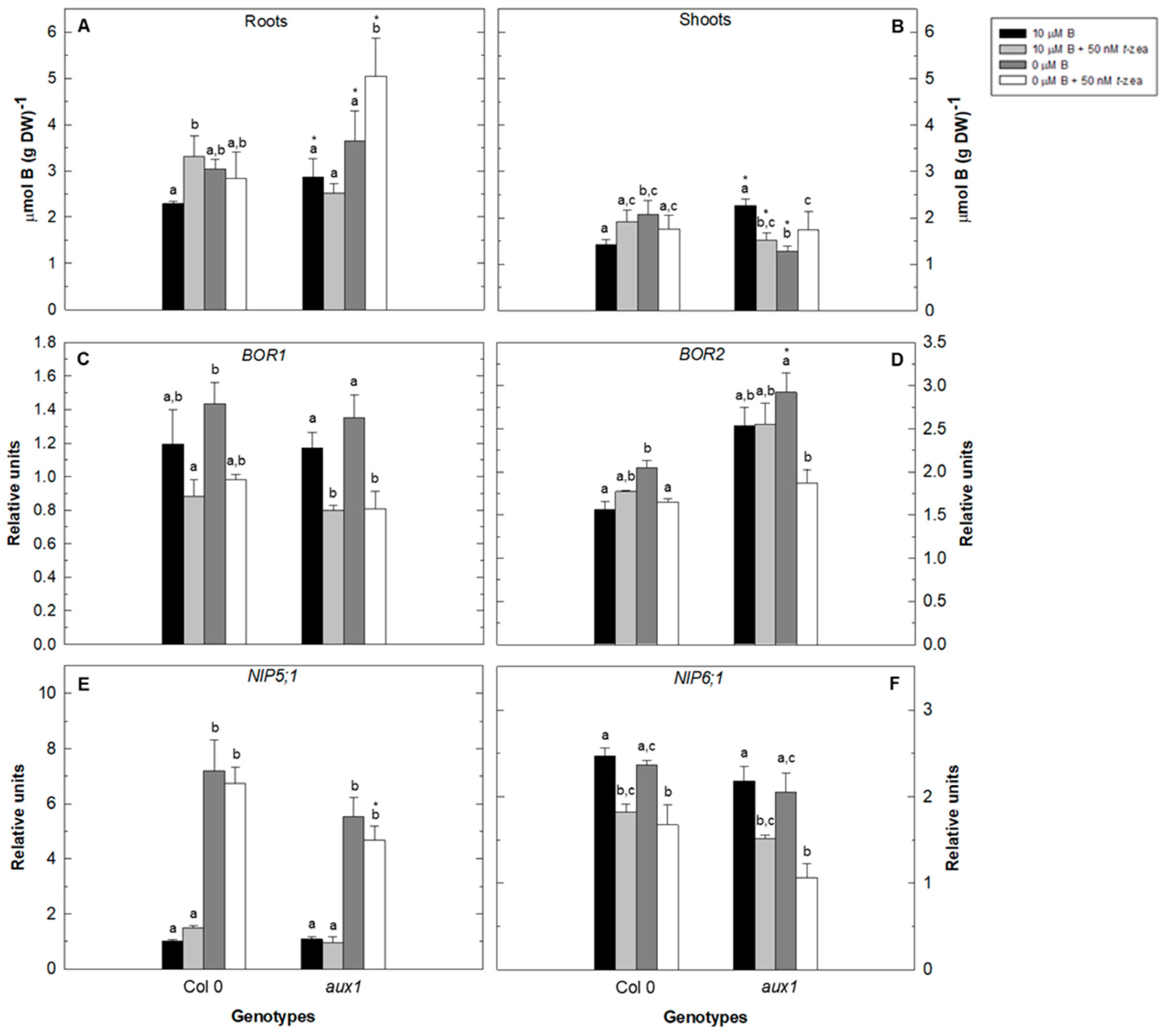
2.2. Effect of Cytokinin Treatment on Total B Content and Gene Expression of B Transporters of Auxin and Ethylene Arabidopsis Mutants under B Deficiency
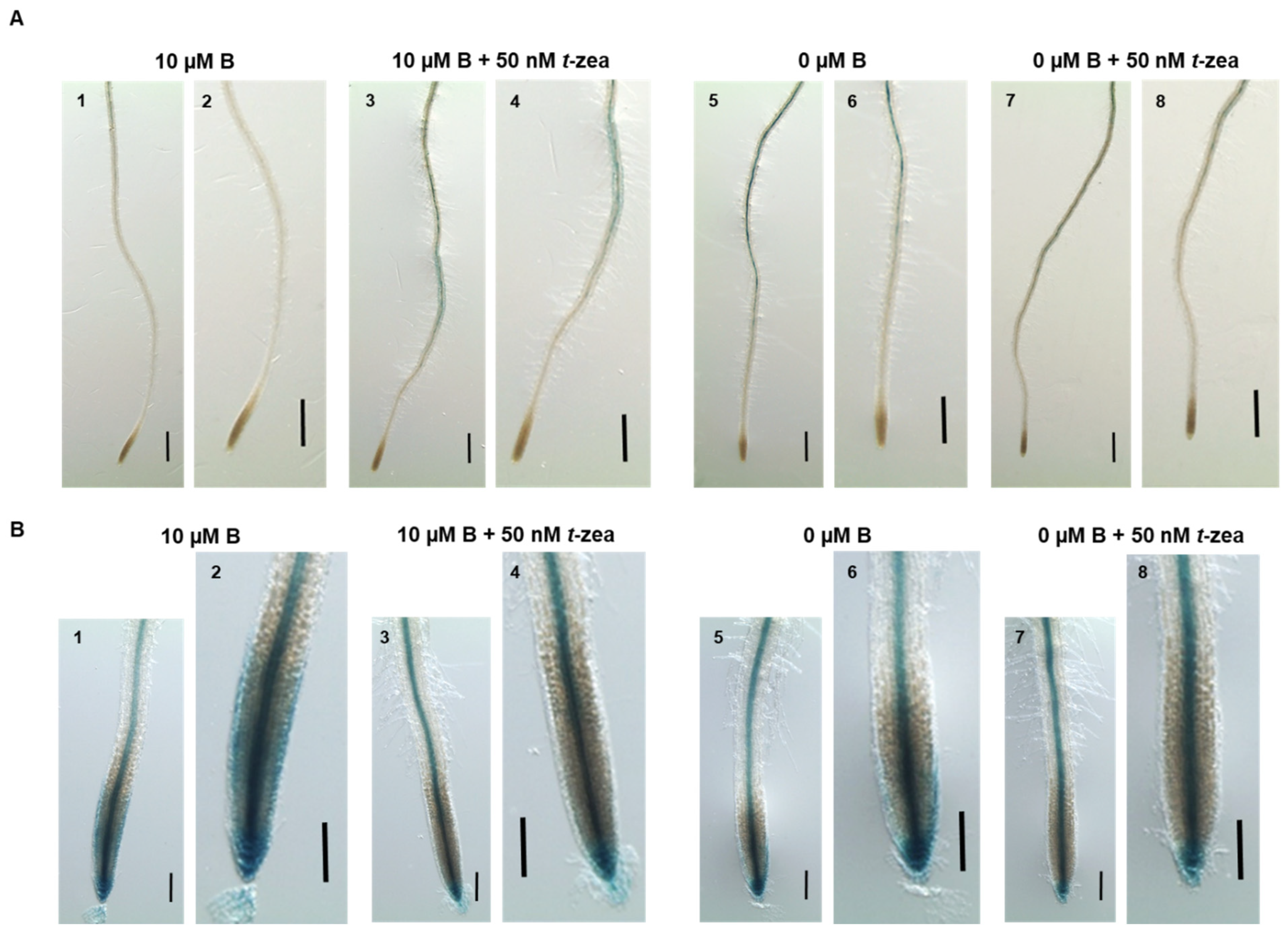
2.3. Interaction among Cytokinin, Auxin, and Ethylene under B Deficiency
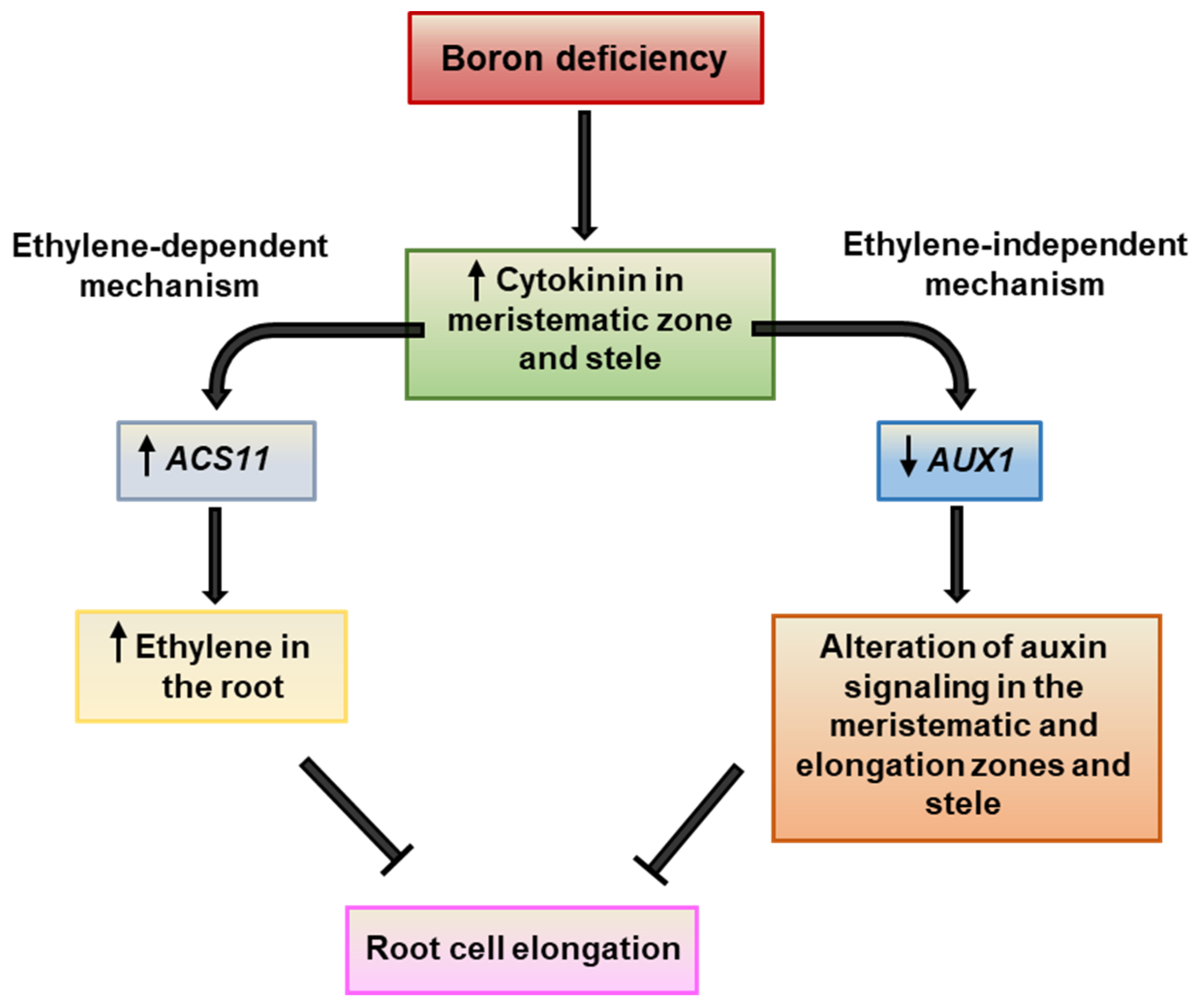
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Root Length, Elongation and LEH Measurements
4.3. Boron Determination
4.4. RNA Isolation, cDNA Synthesis, and Quantitative RT-PCR Analyses
4.5. Histochemical Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shorrocks, V.M. The occurrence and correction of boron deficiency. Plant Soil 1997, 193, 121–148. [Google Scholar] [CrossRef]
- Blevins, D.G.; Lukaszewski, K.M. Boron in plant structure and function. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 481–500. [Google Scholar] [CrossRef]
- Graham, R.D. Micronutrient Deficiencies in Crops and Their Global Significance. In Micronutrient Deficiencies in Global Crop Production; Alloway, B.J., Ed.; Springer: Dordrecht, Netherlands, 2008; pp. 41–61. [Google Scholar]
- Tanaka, M.; Fujiwara, T. Physiological roles and transport mechanisms of boron: Perspectives from plants. Eur. J. Physiol. 2008, 456, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.K.; Kaur, H.; Khanna, K.; Handa, N.; Bhardwaj, R.; Rinklebe, J.; Ahmad, P. Boron in plants: Uptake, deficiency and biological potential. Plant Growth Regul. 2022. [Google Scholar] [CrossRef]
- Ahmad, W.; Zia, M.H.; Malhi, S.S.; Niaz, A.; Saifullah. Boron Deficiency in Soils and Crops: A Review. In Crop Plant; Goyal, A., Ed.; IntechOpen: London, UK, 2012; pp. 77–114. [Google Scholar]
- Cakmak, I.; Römheld, V. Boron deficiency-induced impairments of cellular functions in plants. Plant Soil 1997, 193, 71–83. [Google Scholar] [CrossRef]
- Brown, P.H.; Bellaloui, N.; Wimmer, M.A.; Bassil, E.S.; Ruiz, J.; Hu, H.; Pfeffer, H.; Dannel, F.; Römheld, V. Boron in plant biology. Plant Biol. 2002, 4, 205–223. [Google Scholar] [CrossRef]
- Goldbach, H.E.; Wimmer, M. Boron in plants and animals: Is there a role beyond cell-wall structure? J. Plant Nutr. Soil Sci. 2007, 170, 39–48. [Google Scholar] [CrossRef]
- Camacho-Cristóbal, J.J.; Herrera-Rodríguez, M.B.; Beato, V.M.; Rexach, J.; Navarro-Gochicoa, M.T.; Maldonado, J.M.; González-Fontes, A. The expression of several cell wall-related genes in Arabidopsis roots is down-regulated under boron deficiency. Environ. Exp. Bot. 2008, 63, 351–358. [Google Scholar] [CrossRef]
- Camacho-Cristóbal, J.J.; Rexach, J.; Herrera-Rodríguez, M.B.; Navarro-Gochicoa, M.T.; González-Fontes, A. Boron deficiency and transcript level changes. Plant Sci. 2011, 181, 85–89. [Google Scholar] [CrossRef]
- Herrera-Rodríguez, M.B.; González-Fontes, A.; Rexach, J.; Camacho-Cristóbal, J.J.; Maldonado, J.M.; Navarro-Gochicoa, M.T. Role of boron in vascular plants and response mechanisms to boron stress. Plant Stress 2010, 4, 115–122. [Google Scholar]
- Takano, J.; Miwa, K.; Fujiwara, T. Boron transport mechanisms: Collaboration of channels and transporters. Trends Plant Sci. 2008, 13, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Miwa, K.; Fujiwara, T. Boron transport in plants: Co-ordinated regulation of transporters. Ann. Bot. 2010, 105, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Reid, R. Understanding the boron transport network in plants. Plant Soil 2014, 385, 1–13. [Google Scholar] [CrossRef]
- Ishii, T.; Matsunaga, T. Isolation and characterization of a boron-rhamnogalacturonan-II complex from cell walls of sugar beet pulp. Carbohydr. Res. 1996, 284, 1–9. [Google Scholar] [CrossRef]
- Kobayashi, M.; Matoh, T.; Azuma, J. Two chains of rhamnogalacturonan II are cross-linked by borate-diol ester bonds in higher plant cell walls. Plant Physiol. 1996, 110, 1017–1020. [Google Scholar] [CrossRef]
- O’Neill, M.A.; Warrenfeltz, D.; Kates, K.; Pellerin, P.; Doco, T.; Darvill, A.G.; Albersheim, P. Rhamnogalacturonan-II, a pectic polysaccharide in the walls of growing plant cell, forms a dimer that is covalently cross-linked by a borate ester. J. Biol. Chem. 1996, 271, 22923–22930. [Google Scholar] [CrossRef]
- O’Neill, M.A.; Ishii, T.; Albersheim, P.; Darvill, A.G. Rhamnogalacturonan II: Structure and function of a borate cross-linked cell wall pectic polysaccharide. Annu. Rev. Plant Biol. 2004, 55, 109–139. [Google Scholar] [CrossRef]
- Sechet, J.; Htwe, S.; Urbanowicz, B.; Agyeman, A.; Feng, W.; Ishikawa, T.; Colomes, M.; Kumar, K.S.; Kawai-Yamada, M.; Dinneny, J.R.; et al. Suppression of Arabidopsis GGLT1 affects growth by reducing the L-galactose content and borate cross-linking of rhamnogalacturonan-II. Plant J. 2018, 96, 1036–1050. [Google Scholar] [CrossRef]
- Dell, B.; Huang, L. Physiological response of plants to low boron. Plant Soil 1997, 193, 103–120. [Google Scholar] [CrossRef]
- Rubio, V.; Bustos, R.; Irigoyen, M.L.; Cardona-López, X.; Rojas-Triana, M.; Paz-Ares, J. Plant hormones and nutrient signaling. Plant Mol. Biol. 2009, 69, 361–373. [Google Scholar] [CrossRef]
- Martín, A.C.; del Pozo, J.C.; Iglesias, J.; Rubio, V.; Solano, R.; de La Pena, A.; Leyva, A.; Paz-Ares, J. Influence of cytokinins on the expression of phosphate starvation responsive genes in Arabidopsis. Plant J. 2000, 24, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Franco-Zorrilla, J.M.; Martín, A.C.; Solano, R.; Rubio, V.; Leyva, A.; Paz-Ares, J. Mutations at CRE1 impair cytokinin-induced repression of phosphate starvation responses in Arabidopsis. Plant J. 2002, 32, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Franco-Zorrilla, J.M.; González, E.; Bustos, R.; Linhares, F.; Leyva, A.; Paz-Ares, J. The transcriptional control of plant responses to phosphate limitation. J. Exp. Bot. 2004, 55, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Franco-Zorrilla, J.M.; Martin, A.C.; Leyva, A.; Par-Ares, J.P. Interaction between phosphate-starvation, sugar, and cytokinin signaling in Arabidopsis and the roles of cytokinin receptors CRE1/AHK4 and AHK3. Plant Physiol. 2005, 138, 847–857. [Google Scholar] [CrossRef]
- Takei, K.; Takahashi, T.; Sugiyama, T.; Yamaya, T.; Sakakibara, H. Multiple routes communicating nitrogen availability from roots to shoots: A signal transduction pathway mediated by cytokinin. J. Exp. Bot. 2002, 53, 971–977. [Google Scholar] [CrossRef]
- Sakakibara, H. Nitrate-specific and cytokinin-mediated nitrogen signaling pathways in plants. J. Plant Res. 2003, 116, 253–257. [Google Scholar] [CrossRef]
- Maruyama-Nakashita, A.; Nakamura, Y.; Yamaya, T.; Takahashi, H. A novel regulatory pathway of sulfate uptake in Arabidopsis roots: Implication of CRE1/WOL/AHK4-mediated cytokinin-dependent regulation. Plant J. 2004, 38, 779–789. [Google Scholar] [CrossRef]
- Nam, Y.-J.; Phan Tran, L.-S.; Kojima, M.; Sakakibara, H.; Nishiyama, R.; Shin, R. Regulatory Roles of Cytokinins and Cytokinin Signaling in Response to Potassium Deficiency in Arabidopsis. PLoS ONE 2012, 7, e47797. [Google Scholar] [CrossRef]
- Martín-Rejano, E.M.; Camacho-Cristóbal, J.J.; Herrera-Rodríguez, M.B.; Rexach, J.; Navarro- Gochicoa, M.T.; González-Fontes, A. Auxin and ethylene are involved in the responses of root system architecture to low boron supply in Arabidopsis seedlings. Physiol. Plant. 2011, 142, 170–178. [Google Scholar] [CrossRef]
- Camacho-Cristóbal, J.J.; Martín-Rejano, E.M.; Herrera-Rodríguez, M.B.; Navarro-Gochicoa, M.T.; Rexach, J.; González-Fontes, A. Boron deficiency inhibits root cell elongation via an ethylene/auxin/ROS-dependent pathway in Arabidopsis seedlings. J. Exp. Bot. 2015, 66, 3831–3840. [Google Scholar] [CrossRef]
- González-Fontes, A.; Herrera-Rodríguez, M.B.; Martín-Rejano, E.M.; Navarro-Gochicoa, M.T.; Rexach, J.; Camacho-Cristóbal, J.J. Root responses to boron deficiency mediated by ethylene. Front. Plant Sci. 2016, 6, 1103. [Google Scholar] [CrossRef] [Green Version]
- Abreu, I.; Poza, L.; Bonilla, I.; Bolaños, L. Boron deficiency results in early repression of a cytokinin receptor gene and abnormal cell differentiation in the apical root meristem of Arabidopsis thaliana. Plant Physiol. Biochem. 2014, 77, 117–121. [Google Scholar] [CrossRef]
- Poza-Viejo, L.; Abreu, I.; González-García, M.P.; Allauca, P.; Bonilla, I.; Bolaños, L.; Reguera, M. Boron deficiency inhibits root growth by controlling meristem activity under cytokinin regulation. Plant Sci. 2018, 270, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.S.; Faure, F.; Kieber, J.J. The eto1, eto2, and eto3 mutations and cytokinin treatment increase ethylene biosynthesis in Arabidopsis by increasing the stability of ACS protein. Plant Cell 2003, 15, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Dello Ioio, R.; Nakamura, K.; Moubayidin, L.; Perilli, S.; Taniguchi, M.; Morita, M.T.; Aoyama, T.; Costantino, P.; Sabatini, S. A genetic framework for the control of cell division and differentiation in the root meristem. Science 2008, 322, 1380–1384. [Google Scholar] [CrossRef]
- Hansen, M.; Chae, H.S.; Kieber, J.J. Regulation of ACS protein stability by cytokinin and brassinosteroid. Plant J. 2009, 57, 606–614. [Google Scholar] [CrossRef]
- Schaller, G.E.; Bishopp, A.; Kieber, J.J. The yin-yang of hormones: Cytokinin and auxin interactions in plant development. Plant Cell 2015, 27, 44–63. [Google Scholar] [CrossRef] [PubMed]
- Street, I.H.; Mathews, D.E.; Yamburkenko, M.V.; Sorooshzadeh, A.; John, R.T.; Swarup, R.; Bennett, M.J.; Kieber, J.J.; Schaller, G.E. Cytokinin acts through the auxin influx carrier AUX1 to regulate cell elongation in the root. Development 2016, 143, 3982–3993. [Google Scholar] [CrossRef]
- Le, J.; Vandenbussche, F.; Van Der Straeten, D.; Verbelen, J.-P. In the early response of Arabidopsis roots to ethylene, cell elongation is up- and down regulated and uncoupled from differentiation. Plant Physiol. 2001, 125, 519–522. [Google Scholar] [CrossRef]
- De Cnodder, T.; Vissenberg, K.; Van Der Straeten, D.; Verbelen, J.P. Regulation of cell length in the Arabidopsis thaliana root by the ethylene precursor 1-aminocyclopropane-1-carboxylic acid: A matter of apoplastic reactions. New Phytol. 2005, 168, 541–550. [Google Scholar] [CrossRef]
- Tsang, D.L.; Edmond, C.; Harrington, J.L.; Nüshse, T.S. Cell Wall integrity controls root elongation via a general 1-aminocyclopropane-1-carboxylic acid-dependent, ethylene-independent pathway. Plant Physiol. 2011, 156, 596–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swarup, R.; Friml, J.; Marchant, A.; Ljung, K.; Sandberg, G.; Palme, K.; Bennett, M. Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes Dev. 2001, 15, 2648–2653. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, I.B.; Deruere, J.; Kieber, J.J. Characterization of the response of the Arabidopsis response regulator gene family to cytokinin. Plant Physiol. 2000, 124, 1706–1717. [Google Scholar] [CrossRef] [PubMed]
- Werner, T.; Motyka, V.; Laucou, V.; Smets, R.; Van Onckelen, H.; Schmülling, T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 2003, 15, 2532–2550. [Google Scholar] [CrossRef]
- Aloni, R.; Langhans, M.; Aloni, E.; Dreieicher, E.; Ullrich, C.I. Root-synthesized cytokinin in Arabidopsis is distributed in the shoot by the transpiration stream. J. Exp. Bot. 2005, 56, 1535–1544. [Google Scholar] [CrossRef]
- Shtratnikova, V.Y.; Kudryakova, N.V.; Kudoyarova, G.R.; Korobova, A.V.; Akhiyarova, G.R.; Danilova, M.N.; Kusnetsov, V.V.; Kulaeva, O.N. Effects of Nitrate and Ammonium on Growth of Arabidopsis thaliana Plants Transformed with the ARR5::GUS Construct and a Role for Cytokinins in Suppression of Disturbances Induced by the Presence of Ammonium. Russ. J. Plant Physiol. 2015, 62, 741–752. [Google Scholar] [CrossRef]
- Kieber, J.J.; Schaller, G.E. Cytokinin signaling in plant development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef]
- Dello Ioio, R.; Linhares, F.S.; Scacchi, E.; Casamitjana-Martinez, E.; Heidstra, R.; Costantino, P.; Sabatini, S. Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Curr. Biol. 2007, 17, 678–682. [Google Scholar] [CrossRef]
- Růžička, K.; Šimášková, M.; Duclercq, J.; Petrášek, J.; Zažímalová, E.; Simon, S.; Friml, J.; Van Montagu, M.C.E.; Benková, E. Cytokinin regulates root meristem activity via modulation of the polar auxin transport. Proc. Natl. Acad. Sci. USA 2009, 106, 4284–4299. [Google Scholar] [CrossRef]
- To, J.P.C.; Deruere, J.; Maxwell, B.B.; Morris, V.F.; Hutchison, C.E.; Ferreira, F.J.; Schaller, G.E.; Kieber, J.J. Cytokinin regulates Type-A Arabidopsis response regulator activity and protein stability via two-component phosphorelay. Plant Cell 2007, 19, 3901–3914. [Google Scholar] [CrossRef]
- Kieber, J.J.; Rothenberg, M.; Roman, G.; Feldmann, K.A. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell 1993, 72, 427–441. [Google Scholar] [CrossRef]
- Růžička, K.; Ljung, K.; Vanneste, S.; Podhorská, R.; Beeckman, T.; Friml, J.; Benková, E. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 2007, 19, 2197–2212. [Google Scholar] [CrossRef]
- Swarup, R.; Perry, P.; Hagenbeek, D.; Van Der Straeten, D.; Beemster, G.T.S.; Sandberg, G.; Bhalerao, R.; Ljung, K.; Bennett, M.J. Ethylene regulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell 2007, 19, 2186–2196. [Google Scholar] [CrossRef]
- Muday, G.K.; Rahman, A.; Binder, B.M. Auxin and ethylene: Collaborators or competitors? Trends Plant Sci. 2012, 17, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Tsuchisaka, A.; Theologis, A. Unique and overlapping expression patterns among the Arabidopsis 1-amino-cyclopropane-1-carboxylate synthase gene family members. Plant Physiol. 2004, 136, 2982–3000. [Google Scholar] [CrossRef] [PubMed]
- Argueso, C.T.; Hansen, M.; Kieber, J.J. Regulation of ethylene biosynthesis. J. Plant Growth Regul. 2007, 26, 92–105. [Google Scholar] [CrossRef]
- Gómez-Soto, D.; Galván, S.; Rosales, E.; Bienert, P.; Abreu, I.; Bonilla, I.; Bolaños, L.; Reguera, M. Insights into the role of phytohormones regulating pAtNIP5;1 activity and boron transport in Arabidopsis thaliana. Plant Sci. 2019, 287, 110198. [Google Scholar] [CrossRef]
- Dordas, C.; Chrispeels, M.J.; Brown, P.H. Permeability and channel-mediated transport of boric acid across membrane vesicles isolated from squash roots. Plant Physiol. 2000, 124, 1349–1362. [Google Scholar] [CrossRef]
- Fitzpatrick, K.L.; Reid, R.J. The involvement of aquaglyceroporins in transport of boron in barley roots. Plant Cell Environ. 2009, 32, 1357–1365. [Google Scholar] [CrossRef]
- Takano, J.; Wada, M.; Ludewig, U.; Schaaf, G.; von Wirén, N.; Fujiwara, T. The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell 2006, 18, 1498–1509. [Google Scholar] [CrossRef]
- Miwa, K.; Wakuta, S.; Takada, S.; Ide, K.; Takano, J.; Naito, S.; Omori, H.; Matsunaga, T.; Fujiwara, T. Roles of BOR2, a boron exporter, in cross linking of rhamnogalacturonan II and root elongation under boron limitation in Arabidopsis. Plant Physiol. 2013, 163, 1699–1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Yoshinari, A.; Shimada, T.; Hara-Nishimura, I.; Mitani-Ueno, N.; Ma, J.F.; Naito, S.; Takano, J. Polar localization of the NIP5;1 boric acid channel is maintained by endocytosis and facilitates boron transport in Arabidopsis roots. Plant Cell 2017, 29, 824–842. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, A.; Takano, J. Insights into the Mechanisms Underlying Boron Homeostasis in Plants. Front. Plant Sci. 2017, 8, 1951. [Google Scholar] [CrossRef] [PubMed]
- Takano, J.; Noguchi, K.; Yasumori, M.; Kobayashi, M.; Gajdos, Z.; Miwa, K.; Hayashi, H.; Yoneyama, T.; Fujiwara, T. Arabidopsis boron transporter for xylem loading. Nature 2002, 420, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Kiba, T.; Kudo, T.; Kojima, M.; Sakakibara, H. Hormonal control of nitrogen acquisition: Roles of auxin, abscisic acid, and cytokinin. J. Exp. Bot. 2011, 62, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Beato, V.M.; Rexach, J.; Navarro-Gochicoa, M.T.; Camacho-Cristóbal, J.J.; Herrera Rodríguez, M.B.; Maldonado, J.M.; González-Fontes, A. A tobacco asparagine synthetase gene responds to carbon and nitrogen status and its root expression is affected under boron stress. Plant Sci. 2010, 178, 289–298. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Rodríguez, M.B.; Camacho-Cristóbal, J.J.; Barrero-Rodríguez, R.; Rexach, J.; Navarro-Gochicoa, M.T.; González-Fontes, A. Crosstalk of Cytokinin with Ethylene and Auxin for Cell Elongation Inhibition and Boron Transport in Arabidopsis Primary Root under Boron Deficiency. Plants 2022, 11, 2344. https://doi.org/10.3390/plants11182344
Herrera-Rodríguez MB, Camacho-Cristóbal JJ, Barrero-Rodríguez R, Rexach J, Navarro-Gochicoa MT, González-Fontes A. Crosstalk of Cytokinin with Ethylene and Auxin for Cell Elongation Inhibition and Boron Transport in Arabidopsis Primary Root under Boron Deficiency. Plants. 2022; 11(18):2344. https://doi.org/10.3390/plants11182344
Chicago/Turabian StyleHerrera-Rodríguez, María Begoña, Juan José Camacho-Cristóbal, Rafael Barrero-Rodríguez, Jesús Rexach, María Teresa Navarro-Gochicoa, and Agustín González-Fontes. 2022. "Crosstalk of Cytokinin with Ethylene and Auxin for Cell Elongation Inhibition and Boron Transport in Arabidopsis Primary Root under Boron Deficiency" Plants 11, no. 18: 2344. https://doi.org/10.3390/plants11182344
APA StyleHerrera-Rodríguez, M. B., Camacho-Cristóbal, J. J., Barrero-Rodríguez, R., Rexach, J., Navarro-Gochicoa, M. T., & González-Fontes, A. (2022). Crosstalk of Cytokinin with Ethylene and Auxin for Cell Elongation Inhibition and Boron Transport in Arabidopsis Primary Root under Boron Deficiency. Plants, 11(18), 2344. https://doi.org/10.3390/plants11182344





