Heat Stress Tolerance: A Prerequisite for the Selection of Drought- and Low Phosphorus-Tolerant Common Beans for Equatorial Tropical Regions Such as Ghana
Abstract
:1. Introduction
2. Results
2.1. Ambient Temperature and Humidity Conditions at the Screen-House
2.2. Assessment of Reproductive Growth on Drought-Tolerant Common Bean Accessions in a Phosphorus–Water Factorial Treatment under Terminal Drought, Screen-House Conditions
2.3. Assessment of the Root Characteristics of Drought-Tolerant Common Bean Accessions in a Phosphorus–Water Factorial Treatment under Screen-House Conditions of Terminal Drought
2.4. Soil Moisture Content during Terminal Drought
3. Discussion
4. Materials and Methods
4.1. Screen-House Factorial Experiment of 40 Selected Common Bean Lines with Phosphorus and Water as Treatment Factors
4.2. Soil Sample Collection and Laboratory Analysis
4.3. Temperature Data Logger at the Screen-House
4.4. Moisture-Pro-Check 10HS Soil Moisture Sensor (Decagon Devices, Inc.)
4.5. Imposing Terminal Drought
4.6. Root Characteristics Measurement Using WinRHIZO™
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Calculations for the Amount of TSP Used per Kg Sample Soil
- I.
- TSP = 46% P2O5
- II.
- Mass of soil per hectare at 15 cm depth
References
- Celmeli, T.; Sari, H.; Canci, H.; Sari, D.; Adak, A.; Eker, T.; Toker, C. The nutritional content of common bean (Phaseolus vulgaris L.) landraces in comparison to modern varieties. Agronomy 2018, 8, 166. [Google Scholar] [CrossRef]
- Margaret, N.; Tenywa, J.S.; Otabbong, E.; Mubiru, D.N.; Basamba, T.A. Development of common bean (Phaseolus vulgaris L.) production under low soil phosphorus and drought in Sub-Saharan Africa: A review. J. Sustain. Dev. 2014, 7, 128–139. [Google Scholar] [CrossRef]
- Miklas, P.N.; Kelly, J.D.; Beebe, S.E.; Blair, M.W. Common bean breeding for resistance against biotic and abiotic stresses: From classical to MAS breeding. Euphytica 2006, 147, 105–131. [Google Scholar] [CrossRef]
- Darkwa, K.; Ambachew, D.; Mohammed, H.; Asfaw, A.; Blair, M.W. Evaluation of common bean (Phaseolus vulgaris L.) genotypes for drought stress adaptation in Ethiopia. Crop J. 2016, 4, 367–376. [Google Scholar] [CrossRef]
- Rao, I.M.; Beebe, S.E.; Polania, J.; Grajales, M.; Cajiao, C.; Ricaurte, J.; García, R.; Rivera, M. Evidence for genotypic differences among elite lines of common bean in the ability to remobilize photosynthate to increase yield under drought. J. Agric. Sci. 2017, 155, 857–875. [Google Scholar] [CrossRef]
- Rainey, K.M.; Griffiths, P.D. Differential response of common bean genotypes to high temperature. J. Am. Soc. Hortic. Sci. 2005, 130, 18–23. [Google Scholar] [CrossRef]
- Vargas, Y.; Mayor-Duran, V.M.; Buendia, H.F.; Ruiz-Guzman, H.; Raatz, B. Physiological and genetic characterization of heat stress effects in a common bean RIL population. PLoS ONE 2021, 16, e0249859. [Google Scholar] [CrossRef]
- Deva, C.R.; Urban, M.O.; Challinor, A.J.; Falloon, P.; Svitákova, L. Enhanced Leaf Cooling Is a Pathway to Heat Tolerance in Common Bean. Front. Plant Sci. 2020, 11, 19. [Google Scholar] [CrossRef]
- Lanna, A.C.; Silva, R.A.; Ferraresi, T.M.; Mendonça, J.A.; Coelho, G.R.C.; Moreira, A.S.; Valdisser, P.A.M.R.; Brondani, C.; Vianello, R.P. Physiological characterization of common bean (Phaseolus vulgaris L.) under abiotic stresses for breeding purposes. Environ. Sci. Pollut. Res. 2018, 25, 31149–31164. [Google Scholar] [CrossRef]
- Suárez, J.C.; Polanía, J.A.; Contreras, A.T.; Rodríguez, L.; Machado, L.; Ordoñez, C.; Beebe, S.; Rao, I.M. Adaptation of common bean lines to high temperature conditions: Genotypic differences in phenological and agronomic performance. Euphytica 2020, 216, 28. [Google Scholar] [CrossRef]
- López-Hernández, F.; Cortés, A.J. Last-Generation Genome–Environment Associations Reveal the Genetic Basis of Heat Tolerance in Common Bean (Phaseolus vulgaris L.). Front. Genet. 2019, 10, 954. [Google Scholar] [CrossRef]
- Soltani, A.; Weraduwage, S.M.; Sharkey, T.D.; Lowry, D.B. Elevated temperatures cause loss of seed set in common bean (Phaseolus vulgaris L.) potentially through the disruption of source-sink relationships. BMC Genom. 2019, 20, 312. [Google Scholar] [CrossRef] [PubMed]
- Asamoah, Y.; Ansah-Mensah, K. Temporal Description of Annual Temperature and Rainfall in the Bawku Area of Ghana. Adv. Meteorol. 2020, 2020, 3402178. [Google Scholar] [CrossRef]
- Asante, F.A.; Amuakwa-Mensah, F. Climate change and variability in Ghana: Stocktaking. Climate 2015, 3, 78–99. [Google Scholar] [CrossRef]
- Ramirez-Vallejo, P.; Kelly, J.D. Traits related to drought resistance in common bean. Euphytica 1998, 99, 127–136. [Google Scholar] [CrossRef]
- Julius, P.M.; Susan, N.; Magdalena, N.W.; Jean. Assessment of the effects of water stress on seed yield of common bean genotypes. Afr. J. Agric. Res. 2020, 16, 1069–1077. [Google Scholar] [CrossRef]
- Urrea, C.A.; Yonts, C.D.; Lyon, D.J.; Koehler, A.E. Selection for drought tolerance in dry bean derived from the Mesoamerican gene pool in western Nebraska. Crop Sci. 2009, 49, 2005–2010. [Google Scholar] [CrossRef]
- Smith, M.R.; Dinglasan, E.; Veneklaas, E.; Polania, J.; Rao, I.M.; Beebe, S.E.; Merchant, A. Effect of Drought and Low P on Yield and Nutritional Content in Common Bean. Front. Plant Sci. 2022, 13, 4325. [Google Scholar] [CrossRef]
- Farooq, M.; Gogoi, N.; Barthakur, S.; Baroowa, B.; Bharadwaj, N.; Alghamdi, S.S.; Siddique, K.H.M. Drought Stress in Grain Legumes during Reproduction and Grain Filling. J. Agron. Crop Sci. 2017, 203. [Google Scholar] [CrossRef]
- Dipp, C.C.; Marchese, J.A.; Woyann, L.G.; Bosse, M.A.; Roman, M.H.; Gobatto, D.R.; Paludo, F.; Fedrigo, K.; Kovali, K.K.; Finatto, T. Drought stress tolerance in common bean: What about highly cultivated Brazilian genotypes? Euphytica 2017, 213, 102. [Google Scholar] [CrossRef]
- Abbam, T.; Johnson, F.A.; Dash, J.; Padmadas, S.S. Spatiotemporal Variations in Rainfall and Temperature in Ghana over the Twentieth Century, 1900–2014. Earth Sp. Sci. 2018, 5, 120–132. [Google Scholar] [CrossRef]
- Beebe, S.; Rao, I.M.; Blair, M.W.; Butare, L. Breeding for abiotic stress tolerance in common bean: Present and future challenges. Australas. Plant Breed. SABRAO Conf 2009, 14, 1–12. [Google Scholar]
- Karmalkar, A.V.; Bradley, R.S. Consequences of global warming of 1.5 °C and 2 °C for regional temperature and precipitation changes in the contiguous United States. PLoS ONE 2017, 12, e0168697. [Google Scholar] [CrossRef]
- Thompson, L.G. Climate change: The evidence and our options. Behav. Anal. 2010, 33, 153–170. [Google Scholar] [CrossRef]
- Beebe, S.E.; Rao, I.M.; Blair, M.W.; Acosta-Gallegos, J.A. Phenotyping common beans for adaptation to drought. Front. Physiol. 2013, 4, 35. [Google Scholar] [CrossRef]
- Pornaro, C.; Macolino, S.; Menegon, A.; Richardson, M. WinRHIZO technology for measuring morphological traits of bermudagrass stolons. Agron. J. 2017, 109, 3007–3010. [Google Scholar] [CrossRef]
- Wang, M.B.; Zhang, Q. Issues in using the WinRHIZO system to determine physical characteristics of plant fine roots. Shengtai Xuebao/Acta Ecol. Sin. 2009, 29, 136–138. [Google Scholar] [CrossRef]
- Camilo, S.; Odindo, A.O.; Kondwakwenda, A.; Sibiya, J. Root traits related with drought and phosphorus tolerance in common bean (Phaseolus vulgaris L.). Agronomy 2021, 11, 552. [Google Scholar] [CrossRef]
- Polania, J.; Rao, I.M.; Cajiao, C.; Grajales, M.; Rivera, M.; Velasquez, F.; Raatz, B.; Beebe, S.E. Shoot and root traits contribute to drought resistance in recombinant inbred lines of MD 23–24 × SEA 5 of common bean. Front. Plant Sci. 2017, 8, 296. [Google Scholar] [CrossRef]
- Hernández, G.; Ramírez, M.; Valdés-López, O.; Tesfaye, M.; Graham, M.A.; Czechowski, T.; Schlerethdrey, M.; Erban, A.; Wan, A.; Cheung, F.; et al. Phosphorus stress in common bean: Root transcript and metabolic responses. Plant Physiol. 2007, 144, 752–767. [Google Scholar] [CrossRef]
- Beebe, S.E.; Rao, I.M.; Cajiao, C.; Grajales, M. Selection for drought resistance in common bean also improves yield in phosphorus limited and favorable environments. Crop Sci. 2008, 48, 582–592. [Google Scholar] [CrossRef]
- Ontel, I.; Irimescu, A.; Boldeanu, G.; Mihailescu, D.; Angearu, C.V.; Nertan, A.; Craciunescu, V.; Negreanu, S. Assessment of soil moisture anomaly sensitivity to detect drought spatio-temporal variability in romania. Sensors 2021, 21, 8371. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
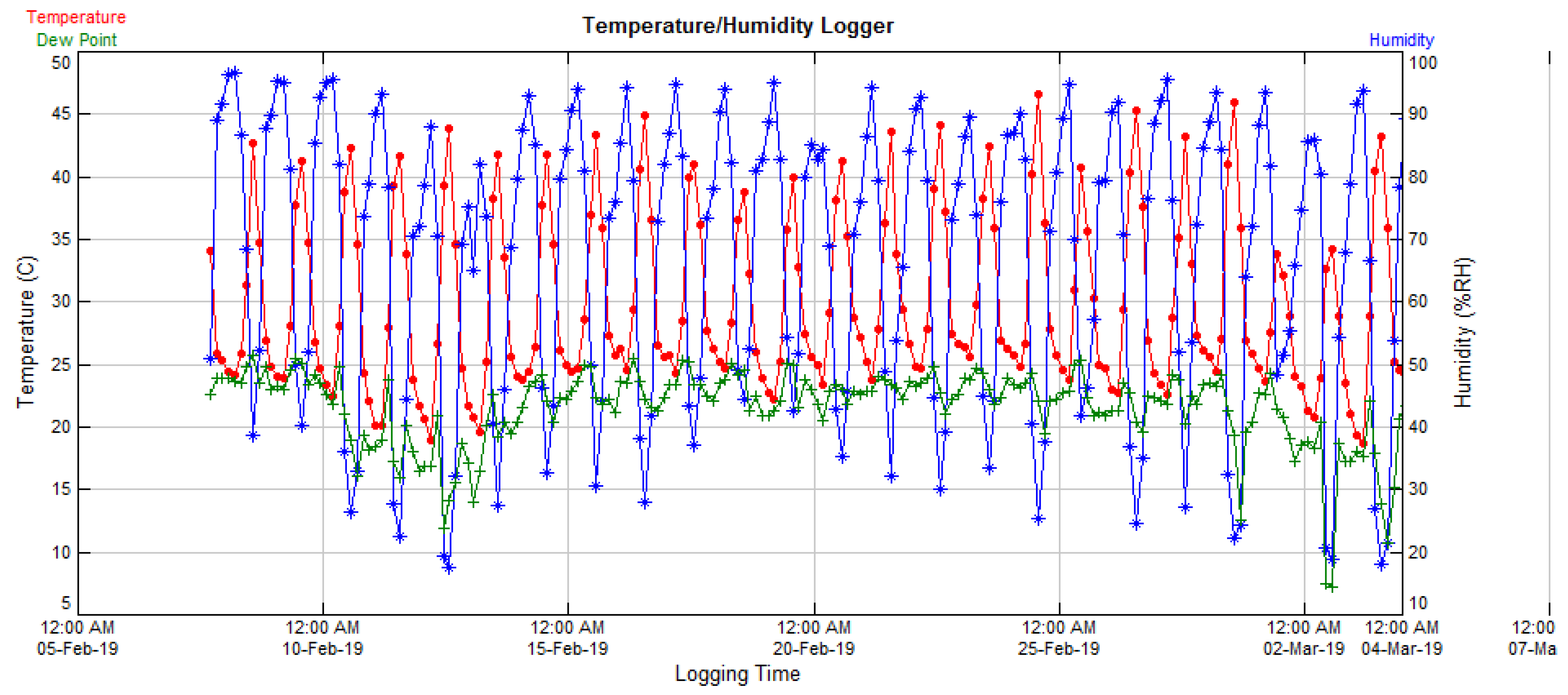
| Parameter | N | Mean | StdErr | StdDev | Min | Max |
|---|---|---|---|---|---|---|
| Temperature (°C) | 391 | 30.18 | 0.35 | 6.83 | 18.78 | 46.72 |
| Rel. Humidity (%) | 391 | 67.86 | 1.14 | 22.55 | 17.75 | 97.9 |
| Dew Point (°C) | 391 | 22.28 | 0.13 | 2.48 | 7.23 | 25.94 |
| Variable | Nighttime Temperature (°C) | Daytime Temperature (°C) |
|---|---|---|
| Avg. Temp | 24.95 | 35.45 |
| Max. Temp | 30.72 | 46.72 |
| Min. Temp | 18.78 | 23.08 |
| Variable | Nighttime % Rel. Humidity | Daytime % Rel. Humidity |
|---|---|---|
| Avg. Rel. Humidity | 84.84 | 50.78 |
| Max. Rel. Humidity | 97.9 | 90.45 |
| Min. Rel. Humidity | 53.95 | 17.75 |
| Temp (°C) | Rel. Humidity (%) | Dew Point (°C) | |
|---|---|---|---|
| Temp (°C) | 1 | ||
| Rel. Humidity (%) | −0.935 *** | 1 | |
| Dew Point (°C) | 0.095 ns | 0.214 *** | 1 |
| Reproductive Character Status on Bean Genotypes | Phosphorus–Water Factorial Treatments | Frequency Occurrence | Percentage Occurrence |
|---|---|---|---|
| No Flower | P1W1 | 20 | 4.18 |
| No Flower | P1W2 | 17 | 3.56 |
| No Flower | P2W1 | 54 | 11.3 |
| No Flower | P2W2 | 50 | 10.46 |
| Total Not Flowered | 141 | 29.5 | |
| Flower Aborted | P1W1 | 15 | 3.14 |
| Flower Aborted | P1W2 | 16 | 3.35 |
| Flower Aborted | P2W1 | 15 | 3.14 |
| Flower Aborted | P2W2 | 24 | 5.02 |
| Total Flower Aborted | 70 | 14.65 | |
| Pod Aborted | P1W1 | 59 | 12.34 |
| Pod Aborted | P1W2 | 54 | 11.3 |
| Pod Aborted | P2W1 | 31 | 6.49 |
| Pod Aborted | P2W2 | 24 | 5.02 |
| Total Pod Aborted | 168 | 35.15 | |
| Pod Matured | P1W1 | 26 | 5.44 |
| Pod Matured | P1W2 | 33 | 6.9 |
| Pod Matured | P2W1 | 20 | 4.18 |
| Pod Matured | P2W2 | 20 | 4.18 |
| Total Pod Matured | 99 | 20.7 |
| Genotype | Frequency Occurrence | Percent Occurrence | Cumulative Frequency | Cumulative Percentage |
|---|---|---|---|---|
| AWASH1 | 7 | 1.46 | 7 | 1.46 |
| BFS30 | 4 | 0.84 | 11 | 2.3 |
| BFS35 | 5 | 1.05 | 16 | 3.35 |
| BFS39 | 1 | 0.21 | 17 | 3.56 |
| BFS55 | 2 | 0.42 | 19 | 3.98 |
| BFS59 | 6 | 1.26 | 25 | 5.24 |
| BFS60 | 1 | 0.21 | 26 | 5.45 |
| BFS62 | 1 | 0.21 | 27 | 5.66 |
| ENNEPA (check) | 9 | 1.88 | 36 | 7.54 |
| SEF17 | 1 | 0.21 | 37 | 7.75 |
| SEF60 | 1 | 0.21 | 38 | 7.96 |
| SEF73 | 2 | 0.42 | 40 | 8.38 |
| SMC146 | 5 | 1.05 | 45 | 9.43 |
| SMC155 | 4 | 0.84 | 49 | 10.27 |
| SMC157 | 7 | 1.46 | 56 | 11.73 |
| SMC158 | 9 | 1.88 | 65 | 13.61 |
| SMC159 | 8 | 1.67 | 73 | 15.28 |
| SMC160 | 4 | 0.84 | 77 | 16.12 |
| SMC161 | 5 | 1.05 | 82 | 17.17 |
| SMN58 | 8 | 1.67 | 90 | 18.84 |
| SMN63 | 8 | 1.67 | 98 | 20.51 |
| SMR101 | 8 | 1.67 | 106 | 22.18 |
| SMR103 | 2 | 0.42 | 108 | 22.6 |
| SMR107 | 3 | 0.63 | 111 | 23.23 |
| SMR113 | 11 | 2.3 | 122 | 25.53 |
| SMR118 | 10 | 2.09 | 132 | 27.62 |
| SMR127 | 5 | 1.05 | 137 | 28.67 |
| SMR128 | 4 | 0.84 | 141 | 29.51 |
| Genotype | Frequency Occurrence | Percent Occurrence | Cumulative Frequency | Cumulative Percentage |
|---|---|---|---|---|
| AWASH1 | 1 | 0.21 | 1 | 0.21 |
| BFS30 | 2 | 0.42 | 3 | 0.63 |
| BFS35 | 4 | 0.84 | 7 | 1.46 |
| BFS39 | 5 | 1.05 | 12 | 2.51 |
| BFS55 | 3 | 0.63 | 15 | 3.14 |
| BFS59 | 1 | 0.21 | 16 | 3.35 |
| BFS60 | 2 | 0.42 | 18 | 3.77 |
| BFS62 | 3 | 0.63 | 21 | 4.39 |
| ENNEPA (check) | 3 | 0.63 | 24 | 5.02 |
| SEF17 | 1 | 0.21 | 25 | 5.23 |
| SEF28 | 2 | 0.42 | 27 | 5.65 |
| SEF44 | 1 | 0.21 | 28 | 5.86 |
| SEF53 | 1 | 0.21 | 29 | 6.07 |
| SEF62 | 1 | 0.21 | 30 | 6.28 |
| SEF73 | 1 | 0.21 | 31 | 6.49 |
| SMC146 | 1 | 0.21 | 32 | 6.69 |
| SMC155 | 3 | 0.63 | 35 | 7.32 |
| SMC157 | 4 | 0.84 | 39 | 8.16 |
| SMC158 | 3 | 0.63 | 42 | 8.79 |
| SMC159 | 3 | 0.63 | 45 | 9.41 |
| SMC160 | 4 | 0.84 | 49 | 10.25 |
| SMC161 | 2 | 0.42 | 51 | 10.67 |
| SMN58 | 4 | 0.84 | 55 | 11.51 |
| SMN63 | 1 | 0.21 | 56 | 11.72 |
| SMR101 | 2 | 0.42 | 58 | 12.13 |
| SMR103 | 2 | 0.42 | 60 | 12.55 |
| SMR107 | 2 | 0.42 | 62 | 12.97 |
| SMR113 | 1 | 0.21 | 63 | 13.18 |
| SMR118 | 2 | 0.42 | 65 | 13.6 |
| SMR127 | 3 | 0.63 | 68 | 14.23 |
| SMR128 | 2 | 0.42 | 70 | 14.64 |
| Genotype | Frequency Occurrence | Percent Occurrence | Cumulative Frequency | Cumulative Percentage |
|---|---|---|---|---|
| AWASH1 | 2 | 0.42 | 2 | 0.42 |
| BFS30 | 6 | 1.26 | 8 | 1.68 |
| BFS35 | 3 | 0.63 | 11 | 2.31 |
| BFS39 | 6 | 1.26 | 17 | 3.57 |
| BFS55 | 7 | 1.46 | 24 | 5.03 |
| BFS59 | 3 | 0.63 | 27 | 5.66 |
| BFS60 | 9 | 1.88 | 36 | 7.54 |
| BFS62 | 5 | 1.05 | 41 | 8.59 |
| BFS67 | 12 | 2.51 | 53 | 11.1 |
| SEF10 | 3 | 0.63 | 56 | 11.73 |
| SEF17 | 5 | 1.05 | 61 | 12.78 |
| SEF28 | 7 | 1.46 | 68 | 14.24 |
| SEF29 | 7 | 1.46 | 75 | 15.7 |
| SEF44 | 6 | 1.26 | 81 | 16.96 |
| SEF47 | 2 | 0.42 | 83 | 17.38 |
| SEF52 | 7 | 1.46 | 90 | 18.84 |
| SEF53 | 11 | 2.3 | 101 | 21.14 |
| SEF55 | 4 | 0.84 | 105 | 21.98 |
| SEF60 | 1 | 0.21 | 106 | 22.19 |
| SEF64 | 3 | 0.63 | 109 | 22.82 |
| SEF73 | 9 | 1.88 | 118 | 24.7 |
| SMC146 | 5 | 1.05 | 123 | 25.75 |
| SMC155 | 5 | 1.05 | 128 | 26.8 |
| SMC157 | 1 | 0.21 | 129 | 27.01 |
| SMC160 | 4 | 0.84 | 133 | 27.85 |
| SMC161 | 5 | 1.05 | 138 | 28.9 |
| SMN63 | 3 | 0.63 | 141 | 29.53 |
| SMR101 | 2 | 0.42 | 143 | 29.95 |
| SMR103 | 8 | 1.67 | 151 | 31.62 |
| SMR107 | 7 | 1.46 | 158 | 33.08 |
| SMR127 | 4 | 0.84 | 162 | 33.92 |
| SMR128 | 6 | 1.26 | 168 | 35.18 |
| Genotype | Frequency Occurrence | Percent Occurrence | Cumulative Frequency | Cumulative Percentage |
|---|---|---|---|---|
| AWASH1 | 2 | 0.42 | 2 | 0.42 |
| BFS59 | 2 | 0.42 | 4 | 0.84 |
| BFS62 | 3 | 0.63 | 7 | 1.47 |
| SEF10 | 9 | 1.88 | 16 | 3.35 |
| SEF15 | 12 | 2.51 | 28 | 5.86 |
| SEF17 | 4 | 0.84 | 32 | 6.7 |
| SEF28 | 2 | 0.42 | 34 | 7.12 |
| SEF29 | 5 | 1.05 | 39 | 8.17 |
| SEF44 | 5 | 1.05 | 44 | 9.22 |
| SEF47 | 10 | 2.09 | 54 | 11.31 |
| SEF52 | 5 | 1.05 | 59 | 12.36 |
| SEF55 | 8 | 1.67 | 67 | 14.03 |
| SEF60 | 10 | 2.09 | 77 | 16.12 |
| SEF62 | 11 | 2.3 | 88 | 18.42 |
| SEF64 | 9 | 1.88 | 97 | 20.3 |
| SMC146 | 1 | 0.21 | 98 | 20.51 |
| SMC159 | 1 | 0.21 | 99 | 20.72 |
| Treatment Levels | N | Mean Total Root Length (cm) | Mean StdErr | Min. Length | Max. Length |
|---|---|---|---|---|---|
| P1W1 | 34 | 5453.82 | 692.33 | 488.41 | 17,209.56 |
| P1W1 | 35 | 7605.21 | 540.57 | 2221.37 | 14,141.84 |
| P2W1 | 32 | 8017.05 | 907.61 | 142.69 | 19,636.44 |
| P2W2 | 34 | 10,882.05 | 578.54 | 5413.16 | 17,714.55 |
| Treatment Levels | N | Mean Root Surface Area (cm2) | Mean StdErr | Min. Surface Area | Max. Surface Area |
|---|---|---|---|---|---|
| P1W1 | 34 | 410.58 | 55.24 | 31.46 | 1299.13 |
| P1W2 | 35 | 600.37 | 44.42 | 147.99 | 1238.19 |
| P2W1 | 32 | 650.18 | 77.74 | 12.98 | 1692.43 |
| P2W2 | 34 | 907.17 | 49.73 | 449.36 | 1468.25 |
| Treatment Levels | N | Mean Root Diameter (mm) | Mean StdErr | Min. Diameter | Max. Diameter |
|---|---|---|---|---|---|
| P1W1 | 34 | 0.82 | 0.1 | 0.19 | 2.07 |
| P1W2 | 35 | 1.19 | 0.08 | 0.43 | 2.5 |
| P2W1 | 32 | 1.19 | 0.13 | 0.21 | 2.76 |
| P2W2 | 34 | 1.64 | 0.1 | 0.75 | 3.08 |
| Treatment Levels | N | Mean Root Volume (cm3) | Mean StdErr | Min. Volume | Max. Volume |
|---|---|---|---|---|---|
| P1W1 | 34 | 2.51 | 0.36 | 0.16 | 7.83 |
| P1W2 | 35 | 3.81 | 0.3 | 0.79 | 8.64 |
| P2W1 | 32 | 4.24 | 0.54 | 0.09 | 11.62 |
| P2W2 | 34 | 6.08 | 0.36 | 2.9 | 10.27 |
| Common Bean Genotype | Mean Root Length (cm) | Mean Surface Area (cm2) | Average Root Diameter (mm) | Mean Root Volume (cm3) |
|---|---|---|---|---|
| AWASH1 | 10,359.04 | 825.64 | 1.34 | 5.27 |
| BFS59 | 9466.82 | 744.26 | 1.41 | 4.72 |
| BFS62 | 7880.31 | 652.21 | 1.26 | 4.39 |
| SEF10 | 9630.07 | 772.49 | 1.41 | 5.00 |
| SEF15 | 5518.09 | 424.08 | 0.83 | 2.62 |
| SEF17 | 8084.45 | 650.05 | 1.32 | 4.22 |
| SEF28 | 10,929.92 | 879.14 | 1.58 | 5.68 |
| SEF29 | 9923.6 | 803.75 | 1.59 | 5.23 |
| SEF44 | 6749.95 | 550.07 | 0.97 | 3.61 |
| SEF47 | 4833.63 | 382.98 | 0.79 | 2.44 |
| SEF52 | 9654.93 | 825.69 | 1.6 | 5.66 |
| SEF55 | 10,203.17 | 813.87 | 1.43 | 5.24 |
| SEF60 | 3013.99 | 224.02 | 0.47 | 1.34 |
| SEF62 | 6468.47 | 489.13 | 0.92 | 2.97 |
| SEF64 | 7983.79 | 610.03 | 1.11 | 3.74 |
| SEF73 | 6272.05 | 489 | 0.97 | 3.08 |
| SMC146 | 5112.89 | 435 | 0.97 | 3.02 |
| SMC159 | 10,307.57 | 872.81 | 1.64 | 5.93 |
| CV % | 39.46 | 42.82 | 43.92 | 47.7 |
| Mean | 7986.28 | 641.64 | 1.21 | 4.16 |
| MSE | 3151.16 | 274.76 | 0.53 | 1.98 |
| R-square | 0.76 | 0.75 | 0.69 | 0.73 |
| Days | P1W1 Soil Moisture (m3/m3) | P2W1 Soil Moisture (m3/m3) |
|---|---|---|
| 1 | 0.25 | 0.24 |
| 2 | 0.17 | 0.13 |
| 3 | 0.1 | 0.07 |
| 4 | 0.02 | 0.01 |
| 5 | −0.01 | −0.01 |
| 6 | −0.03 | −0.06 |
| 7 | −0.03 | −0.06 |
| 8 | −0.04 | −0.07 |
| 9 | −0.06 | −0.08 |
| 10 | −0.06 | −0.09 |
| 11 | −0.07 | −0.09 |
| 12 | −0.07 | −0.09 |
| 13 | −0.07 | −0.09 |
| 14 | −0.09 | −0.09 |
| 1.SMR_113 | 11. SEF_29 | 21. SMC_160 | 31. SEF_62 |
| 2. SMC_158 | 12. BFS_35 | 22. SEF_64 | 32. SMC_161 |
| 3. SEF_17 | 13. BFS_60 | 23. BFS_59 | 33. SMC_146 |
| 4. SMN_63 | 14. SMR_107 | 24. SEF_28 | 34. SEF_47 |
| 5. SMN_58 | 15. BFS_39 | 25. SEF_55 | 35. BFS_67 |
| 6. BFS_30 | 16. SMC_155 | 26. SEF_10 | 36. ENNEPA (non-drought check) |
| 7. SMC_159 | 17. SMR_103 | 27. SEF_52 | 37. SEF_15 |
| 8. SMR_118 | 18. SMR_101 | 28. BFS_62 | 38. SMR_129 |
| 9. SMC_157 | 19. AWASH_1 | 29. SMR_128 | 39. BFS_55 |
| 10. SMR_127 | 20. SEF_60 | 30. SEF_44 | 40. SEF_68 |
| T1 = P1W1 | T2 = P1W2 | T3 = P2W1 | T4 = P2W2 |
| Sample | pH | Available P (mg/kg) | % Total N | Exch. Bases (cmol/kg) | Exch. Acidity (cmol/kg) | % Org. Carbon | % Org. Matter | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| K | Ca | Mg | Na | Al | H | ||||||
| Soil Sample | 6.55 | 5.445 | 0.0824 | 0.135 | 1.51 | 1.04 | 0.042 | 0.508 | 0.345 | 1.077 | 1.857 |
| Sample | % Sand | %Clay | % Silt | Textural Class | Water Holding Capacity |
|---|---|---|---|---|---|
| Soil Sample | 83.20 | 6.40 | 10.40 | Loamy sand | 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Appiah-Kubi, D.; Asibuo, J.Y.; Butare, L.; Yeboah, S.; Appiah-Kubi, Z.; Kena, A.W.; Tuffour, H.O.; Akromah, R. Heat Stress Tolerance: A Prerequisite for the Selection of Drought- and Low Phosphorus-Tolerant Common Beans for Equatorial Tropical Regions Such as Ghana. Plants 2022, 11, 2352. https://doi.org/10.3390/plants11182352
Appiah-Kubi D, Asibuo JY, Butare L, Yeboah S, Appiah-Kubi Z, Kena AW, Tuffour HO, Akromah R. Heat Stress Tolerance: A Prerequisite for the Selection of Drought- and Low Phosphorus-Tolerant Common Beans for Equatorial Tropical Regions Such as Ghana. Plants. 2022; 11(18):2352. https://doi.org/10.3390/plants11182352
Chicago/Turabian StyleAppiah-Kubi, David, James Yaw Asibuo, Louis Butare, Stephen Yeboah, Zippora Appiah-Kubi, Alexander Wireko Kena, Henry Oppong Tuffour, and Richard Akromah. 2022. "Heat Stress Tolerance: A Prerequisite for the Selection of Drought- and Low Phosphorus-Tolerant Common Beans for Equatorial Tropical Regions Such as Ghana" Plants 11, no. 18: 2352. https://doi.org/10.3390/plants11182352





