Comparison of Leaf Shape between a Photinia Hybrid and One of Its Parents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Data Acquisition
2.3. Methods
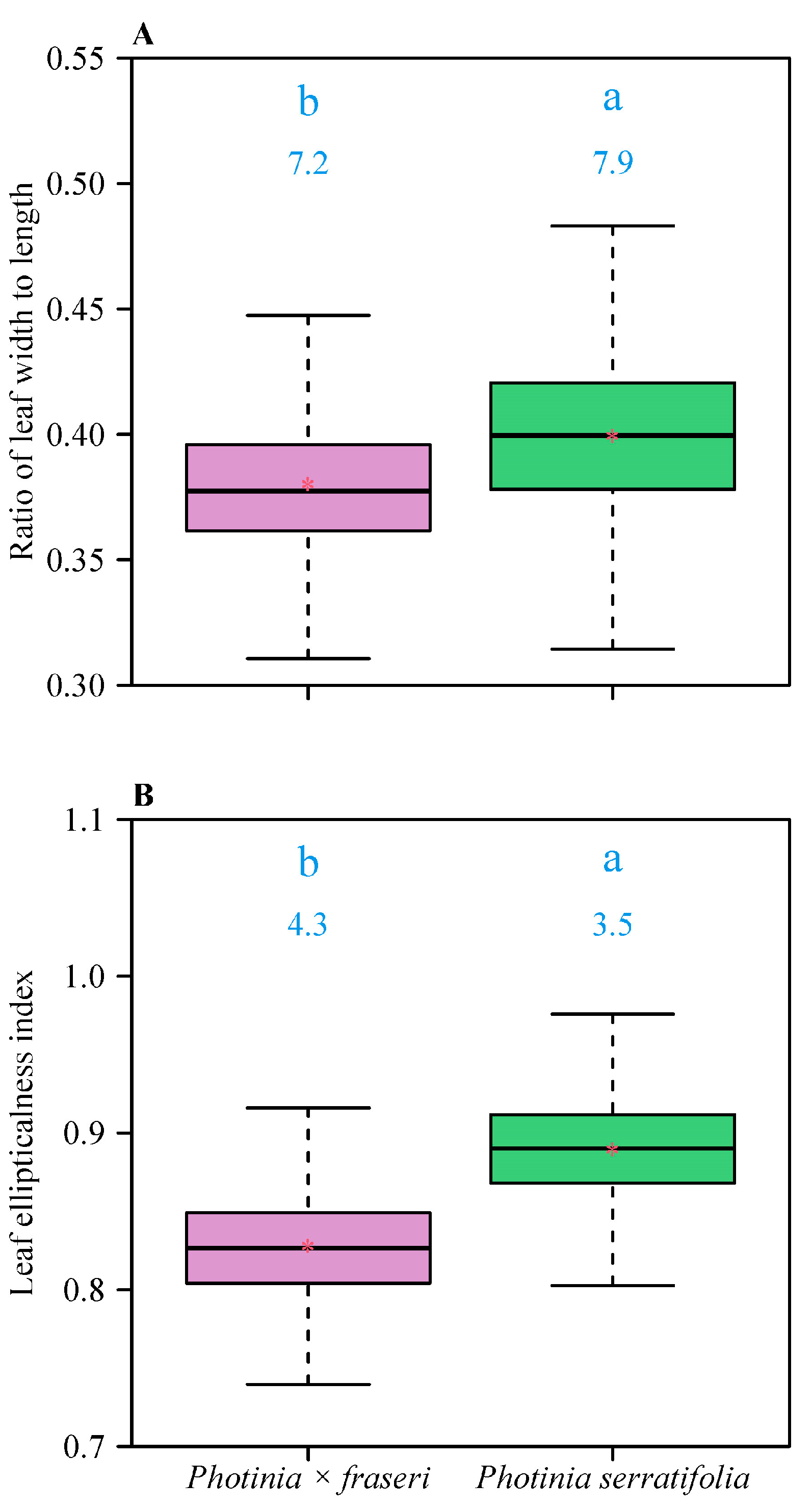
3. Results
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhagsari, A.S.; Brown, R.H. Leaf photosynthesis and its correlation with leaf area. Crop Sci. 1986, 26, 127–132. [Google Scholar] [CrossRef]
- Reich, P.B.; Ellsworth, D.S.; Walters, M.B. Leaf structure (specific leaf area) modulates photosynthesis–nitrogen relations: Evidence from within and across species and functional groups. Funct. Ecol. 2002, 12, 948–958. [Google Scholar] [CrossRef]
- Wu, Y.; Gong, W.; Wang, Y.; Yong, T.; Yang, F.; Liu, W.; Wu, X.; Du, J.; Shu, K.; Liu, J.; et al. Leaf area and photosynthesis of newly emerged trifoliolate leaves are regulated by mature leaves in soybean. J. Plant Res. 2018, 131, 671–680. [Google Scholar] [CrossRef]
- Shi, P.; Miao, Q.; Niinemets, Ü.; Liu, M.; Li, Y.; Yu, K.; Niklas, K.J. Scaling relationships of leaf vein and areole traits versus leaf size for nine Magnoliaceae species differing in venation density. Am. J. Bot. 2022, 109, 899–909. [Google Scholar] [CrossRef]
- Montgomery, E.G. Correlation Studies in Corn, Annual Report No.24; Nebraska Agricultural Experimental Station: Lincoln, NB, USA, 1911; pp. 108–159. [Google Scholar]
- Kemp, C.D. Methods of estimating leaf area of grasses from linear measurements. Ann. Bot. 1960, 24, 491–499. [Google Scholar] [CrossRef]
- Stickler, F.C.; Wearden, S.; Pauli, A.W. Leaf area determination in grain sorghum. Agronony 1961, 53, 187–188. [Google Scholar] [CrossRef]
- Jani, T.C.; Misra, D.K. Leaf area estimation by linear measurements in Ricinus communis. Nat. 1966, 212, 741–742. [Google Scholar] [CrossRef]
- Palaniswamy, K.M.; Gomez, K.A. Length-width method for estimating leaf area of rice. Agron. J. 1974, 66, 430–433. [Google Scholar] [CrossRef]
- Shi, P.; Liu, M.; Ratkowsky, D.A.; Gielis, J.; Su, J.; Yu, X.; Wang, P.; Zhang, L.; Lin, Z.; Schrader, J. Leaf area-length allometry and its implications in leaf-shape evolution. Trees Struct. Funct. 2019, 33, 1073–1085. [Google Scholar] [CrossRef]
- Yu, X.; Shi, P.; Schrader, J.; Niklas, K.J. Nondestructive estimation of leaf area for 15 species of vines with different leaf shapes. Am. J. Bot. 2020, 107, 1481–1490. [Google Scholar] [CrossRef]
- Schrader, J.; Shi, P.; Royer, D.L.; Peppe, D.J.; Gallagher, R.V.; Li, Y.; Wang, R.; Wright, I.J. Leaf size estimation based on leaf length, width and shape. Ann. Bot. 2021, 128, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Niklas, K.J.; Liu, L.; Fang, Z.; Li, Y.; Shi, P. Tree size influences leaf shape but does not affect the proportional relationship between leaf area and the product of length and width. Front. Plant Sci. 2022, 13, 850203. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Niinemets, Ü.; Ma, J.; Schrader, J.; Wang, R.; Shi, P. Plant age has a minor effect on non-destructive leaf area calculations in moso bamboo (Phyllostachys edulis). Symmetry 2021, 13, 369. [Google Scholar] [CrossRef]
- Webb, L.J. Environmental relationships of the structural types of Australian rain forest vegetation. Ecology 1968, 49, 296–311. [Google Scholar] [CrossRef]
- Lewis, M.C. The physiological significance of variation in leaf structure. Sci. Prog. 1972, 60, 25–51. [Google Scholar]
- Givnish, T. On the adaptive significance of leaf form. In Topics in Plant Population Biology; Solbrig, O.T., Jain, S., Johnson, G.B., Raven, P.H., Eds.; Columbia University Press: New York, NY, USA, 1979; pp. 375–407. [Google Scholar]
- Royer, D.L.; Wilf, P. Why do toothed leaves correlate with cold climates? Gas exchange at leaf margins provides new insights into a classic paleotemperature proxy. Int. J. Plant Sci. 2006, 167, 11–18. [Google Scholar] [CrossRef]
- Baxes, G.A. Digital Image Processing: Principles and Applications; John Wiley and Sons, Inc.: New York, NY, USA, 1994. [Google Scholar]
- Niinemets, Ü.; Cescatti, A.; Christian, R. Constraints on light interception efficiency due to shoot architecture in broad-leaved Nothofagus species. Tree Physiol. 2004, 24, 617–630. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Sparrow, A.; Cescatti, A. Light capture efficiency decreases with increasing tree age and size in the southern hemisphere gymnosperm Agathis australis. Trees Struct. Funct. 2005, 19, 177–190. [Google Scholar] [CrossRef]
- Roth-Nebelsick, A.; Konrad, W. Fossil leaf traits as archives for the past—And lessons for the future? Flora 2019, 254, 59–70. [Google Scholar] [CrossRef]
- Li, Y.; Quinn, B.K.; Niinemets, Ü.; Schrader, J.; Gielis, J.; Liu, M.; Shi, P. Ellipticalness index—A simple measure of the complexity of oval leaf shape. Pak. J. Bot. 2022, 54, 2233–2240. [Google Scholar] [CrossRef]
- Shi, P.; Yu, K.; Niinemets, Ü.; Gielis, J. Can leaf shape be represented by the ratio of leaf width to length? Evidence from nine species of Magnolia and Michelia (Magnoliaceae). Forests 2021, 12, 41. [Google Scholar] [CrossRef]
- Shi, P.; Ratkowsky, D.A.; Li, Y.; Zhang, L.; Lin, S.; Gielis, J. A general leaf-area geometric formula exists for plants—Evidence from the simplified Gielis equation. Forests 2018, 9, 714. [Google Scholar] [CrossRef]
- Su, J.; Niklas, K.J.; Huang, W.; Yu, X.; Yang, Y.; Shi, P. Lamina shape does not correlate with lamina surface area: An analysis based on the simplified Gielis equation. Glob. Ecol. Conserv. 2019, 19, e00666. [Google Scholar] [CrossRef]
- Shi, P.; Gielis, J.; Quinn, B.K.; Niklas, K.J.; Ratkowsky, D.A.; Schrader, J.; Ruan, H.; Wang, L.; Niinemets, Ü. ‘biogeom’: An R package for simulating and fitting natural shapes. Ann. N. Y. Acad. Sci. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 1 June 2022).
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman and Hall/CRC: New York, NY, USA, 1993. [Google Scholar]
- Sandhu, H.S.; Shi, P.; Kuang, X.; Xue, F.; Ge, F. Applications of the bootstrap to insect physiology. Fla. Entomol. 2011, 94, 1036–1041. [Google Scholar] [CrossRef]
- Hsu, J.C. Multiple Comparisons: Theory and Methods; Chapman and Hall/CRC: New York, NY, USA, 1996. [Google Scholar]
- Xue, Y.; Chen, L. Statistical Models and R Software; Tsinghua University Press: Beijing, China, 2007. [Google Scholar]
- Shi, P.; Li, Y.; Niinemets, Ü.; Olson, E.; Schrader, J. Influence of leaf shape on the scaling of leaf surface area and length in bamboo plants. Trees Struct. Funct. 2021, 35, 709–715. [Google Scholar] [CrossRef]
- Lamé, G. Examen des Différentes Méthodes Employées Pour Résoudre les Problèmes de Géométrie; V. Courcier: Paris, France, 1818. [Google Scholar]
- Shi, P.; Huang, J.; Hui, C.; Grissino-Mayer, H.D.; Tardif, J.C.; Zhai, L.; Wang, F.; Li, B. Capturing spiral radial growth of conifers using the superellipse to model tree-ring geometric shape. Front. Plant Sci. 2015, 6, 856. [Google Scholar] [CrossRef]
- Huang, W.; Li, Y.; Niklas, K.J.; Gielis, J.; Ding, Y.; Cao, L.; Shi, P. A superellipse with deformation and its application in describing the cross-sectional shapes of a square bamboo. Symmetry 2020, 12, 2073. [Google Scholar] [CrossRef]
- Peppe, D.J.; Royer, D.L.; Gariglino, B.; Oliver, S.Y.; Newman, S.; Leight, E.; Enikolopov, G.; Fernandez-Burgos, M.; Herrera, F.; Adams, J.M.; et al. Sensitivity of leaf size and shape to climate: Global patterns and paleoclimatic applications. New Phytol. 2011, 190, 724–739. [Google Scholar] [CrossRef] [Green Version]



| Taxon/Leaf-Shape Index | Equation † | Parameter | Estimate | Significance | r2 |
|---|---|---|---|---|---|
| Photinia × fraseri Leaf width/length ratio | y = a + bx | a | 0.3920 | <0.001 | 0.0192 |
| b | −0.0022 | <0.001 | |||
| y = a + bx + cx2 | a | 0.3733 | <0.001 | 0.0233 | |
| b | 0.0054 | 0.0365 | |||
| c | −0.0006 | 0.0031 | |||
| Photinia × fraseri Leaf ellipticalness index | y = a + bx | a | 0.8549 | <0.001 | 0.0591 |
| b | −0.0050 | <0.001 | |||
| y = a + bx + cx2 | a | 0.9668 | <0.001 | 0.1452 | |
| b | 0.0504 | <0.001 | |||
| c | 0.0041 | <0.001 | |||
| Photinia serratifolia Leaf width/length ratio | y = a + bx | a | 0.4096 | <0.001 | 0.0099 |
| b | −0.0014 | <0.001 | |||
| y = a + bx + cx2 | a | 0.3974 | <0.001 | 0.0111 | |
| b | 0.0022 | 0.268 | |||
| c | −0.0002 | 0.071 | |||
| Photinia serratifolia Leaf ellipticalness index | y = a + bx | a | 0.8932 | <0.001 | 0.0014 |
| b | −0.0005 | 0.0562 | |||
| y = a + bx + cx2 | a | 0.9010 | <0.001 | 0.0018 | |
| b | −0.0028 | 0.166 | |||
| c | 0.0002 | 0.252 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Niklas, K.J.; Ratkowsky, D.A.; Jiao, Y.; Ding, H.; Shi, P. Comparison of Leaf Shape between a Photinia Hybrid and One of Its Parents. Plants 2022, 11, 2370. https://doi.org/10.3390/plants11182370
Zheng X, Niklas KJ, Ratkowsky DA, Jiao Y, Ding H, Shi P. Comparison of Leaf Shape between a Photinia Hybrid and One of Its Parents. Plants. 2022; 11(18):2370. https://doi.org/10.3390/plants11182370
Chicago/Turabian StyleZheng, Xiao, Karl J. Niklas, David A. Ratkowsky, Yabing Jiao, Hui Ding, and Peijian Shi. 2022. "Comparison of Leaf Shape between a Photinia Hybrid and One of Its Parents" Plants 11, no. 18: 2370. https://doi.org/10.3390/plants11182370






