Assessing Seedbank Longevity and Seed Persistence of the Invasive Tussock Grass Nassella trichotoma Using in-Field Burial and Laboratory-Controlled Ageing
Abstract
1. Introduction
2. Methods
2.1. Seed Collection and Preparation
2.2. Seed Longevity
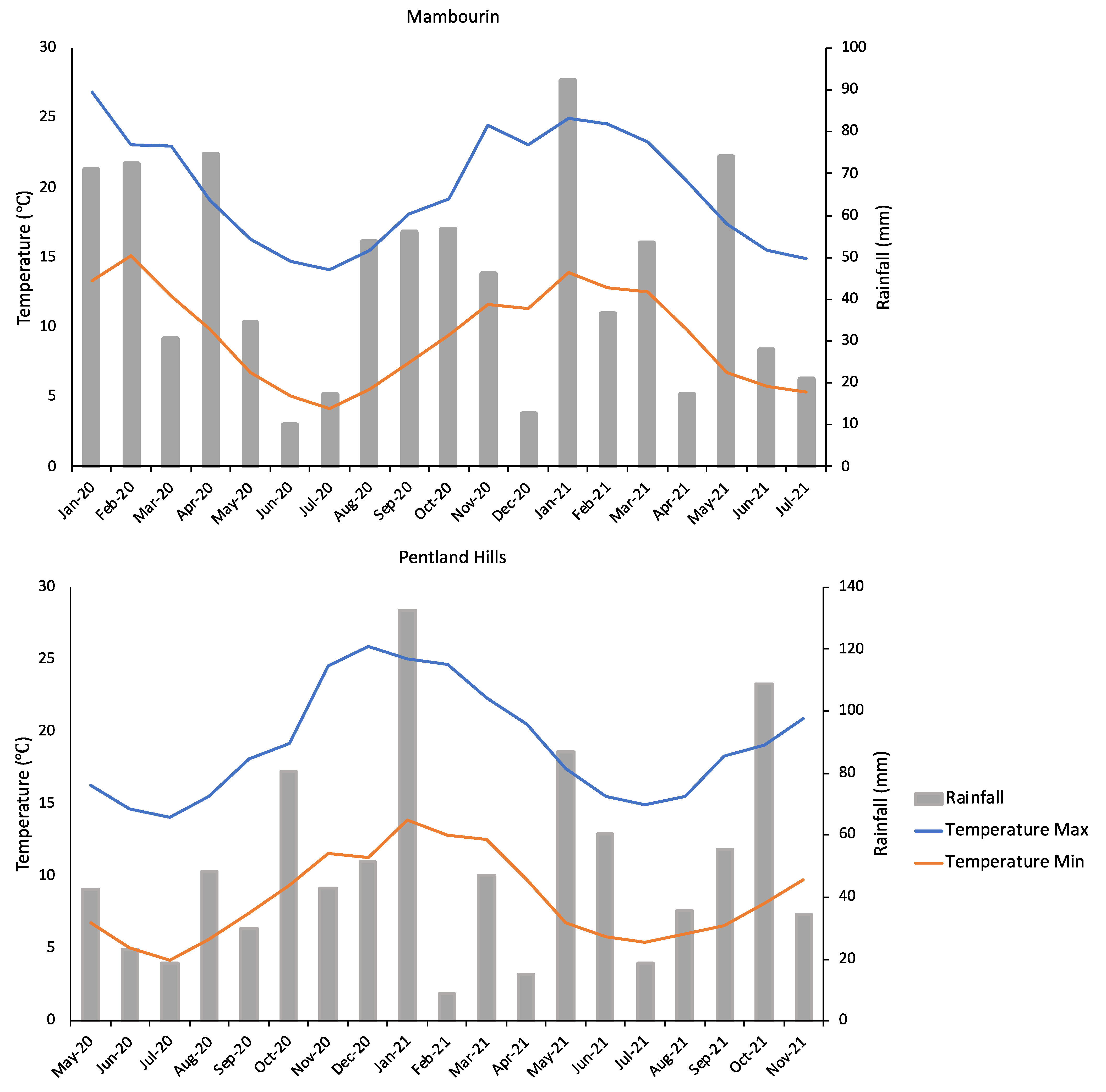
2.3. Seedbank Persistence
2.4. Data Analysis
3. Results
3.1. Seed Longevity
3.2. Seedbank Persistence
4. Discussion
4.1. Seedbank Longevity
4.2. Seedbank Persistence
4.3. Implications for Management
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bardgett, R.D.; Bullock, J.M.; Lavorel, S.; Manning, P.; Schaffner, U.; Ostle, N.; Chomel, M.; Durigan, G.; Fly, E.L.; Shi, H. Combatting global grassland degradation. Nat. Rev. Earth Environ. 2021, 10, 720–735. [Google Scholar] [CrossRef]
- Stuwe, J. An Assessment of the Conservation Status of Native Grasslands on the Western Plains, Victoria and Sites of Botanical Significance; Technical Report, No. 48; Arthur Rylah Institute for Environmental Research: Heidelberg, VIC, Australia, 1986. [Google Scholar]
- Marshall, G.R.; Coleman, M.J.; Sindel, B.M.; Reeve, I.J.; Berney, P.J. Collective action in invasive species control, and prospects for community-based governance: The case study of serrated tussock (Nassella trichotoma) in New South Wales, Australia. Land Use Policy 2016, 56, 100–111. [Google Scholar] [CrossRef]
- Department of the Environment, Water, Heritage and the Arts. Natural Temperate Grassland of the Victorian Volcanic Plain. 2008. Available online: https://websites.mygameday.app/get_file.cgi?id=683727 (accessed on 2 August 2022).
- Bai, X.; Zhao, W.; Wang, J.; Ferreira, C.S.S. Reducing plant community variability and improving resilience for sustainable restoration of temperate grassland. Environ. Res. 2022, 207, 112149. [Google Scholar] [CrossRef] [PubMed]
- Gioria, M.; Carta, A.; Baskin, C.C.; Dawson, W.; Essl, F.; Kreft, H.; Pergl, J.; van Kleunen, M.; Weigelt, P.; Winter, M.; et al. Persistent soil seed banks promote naturalisation and invasiveness in flowering plants. Ecol. Lett. 2021, 24, 1655–1667. [Google Scholar] [CrossRef]
- Presotto, A.; Hernandez, F.; Casquero, M.; Vercellino, R.; Pandolfo, C.; Poverene, M.; Cantamutto, M. Seed bank dynamics of an invasive alien species, Helianthus annuus L. Plant Ecol. 2020, 13, 313–322. [Google Scholar] [CrossRef]
- Dairel, M.; Fidelis, A. The presence of invasive grasses affects the soil seed bank composition and dynamics of both invaded and non-invaded areas of open savannas. J. Environ. Manag. 2020, 276, 111291. [Google Scholar] [CrossRef]
- Abbas, A.M.; Pickart, A.J.; Goldsmith, L.M.; Davenport, D.N.; Newby, B.; Munoz-Rodriguez, A.F.; Grewell, B.J.; Castillo, J.M. Seed bank persistence of a South American cordgrass in invaded northern Atlantic and Pacific Coast estuaries. AoB Plants 2021, 13, plab014. [Google Scholar] [CrossRef]
- Fenellosa, E.; Jené, L.; Munné-Bosch, S.A. Rapid and sensitive method to assess seed longevity through accelerated aging in an invasive plant species. Plant Methods 2020, 16, 64. [Google Scholar] [CrossRef]
- Buhler, D.; Hartzler, R.G.; Forcella, F. Implications of weed seedbank dynamics to weed management. Weed Sci. 1997, 45, 329–336. Available online: http://www.jstor.org/stable/4046027 (accessed on 4 August 2022). [CrossRef]
- Travlos, I.; Gazoulis, I.; Kanatas, P.; Tsekoura, A.; Zannopoulos, S.; Papastylianou, P. Key factors affecting weed seeds’ germination, weed emergence, and their possible role for the efficacy of false seedbed technique as weed management practice. Front. Agron. 2020, 2, 1. [Google Scholar] [CrossRef]
- Wang, N.; Yuan, M.; Chen, H. Effects of artificial aging on seed vigour and physiological characteristics of the invasive alien plant Aegilops tauschii. Acta Soc. Bot. Pol. 2021, 90, 9010. [Google Scholar] [CrossRef]
- Wijayratne, U.C.; Pyke, D.A. Burial increases seed longevity of two Artemisia tridentata (Asteraceae) subspecies. Am. J. Bot. 2012, 99, 428–447. [Google Scholar] [CrossRef] [PubMed]
- Rajjou, L.; Debeaujon, I. Seed longevity: Survival and maintenance of high germination ability of dry seeds. Comptes Rendus Biol. 2008, 331, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Long, R.L.; Gorecki, M.J.; Renton, M.; Scott, J.K.; Colville, L.; Goggin, D.E.; Commander, L.E.; Westcott, D.A.; Cherry, H.; Finch-Savage, W.E. The ecophysiology of seed persistence: A mechanistic view of the journey to germination or demise. Biol. Rev. 2015, 90, 31–59. [Google Scholar] [CrossRef]
- Long, R.L.; Panettta, D.; Steadman, K.J.; Probert, R.; Bekker, R.M.; Brooks, S.; Adkins, S.W. Seed persistence in the field may be predicted by laboratory-controlled aging. Weed Sci. 2008, 56, 523–528. [Google Scholar] [CrossRef]
- Newton, R.; Hay, F.R.; Probert, R.J. Protocol for Comparative Seed Longevity Testing. Millennial Seed Bank Project, Kew. 2009. Available online: www.kew.org/msbp (accessed on 10 January 2020).
- Probert, R.J.; Daws, M.I.; Hay, F.R. Ecological correlates of ex situ seed longevity: A comparative study on 195 species. Ann. Bot. 2009, 104, 57–69. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Manalil, S. Seedbank persistence of four summer grass weed species in the northeast cropping region of Australia. PLoS ONE 2022, 17, e0262288. [Google Scholar] [CrossRef]
- Maskova, T.; Poschlod, P. Soil seed bank persistence across time and burial depth in calcareous grassland habitats. Front. Plant Sci. 2022, 4, 790867. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Johnson, D.E. Seed germination ecology of junglerice (Echinochloa colona): A major weed of rice. Weed Sci. 2009, 57, 235–240. [Google Scholar] [CrossRef]
- Amini, R.; Gholami, F.; Ghsnepour, S. Effects of environmental factors and burial depth on seed germination and emergence of two populations of Caucalis platycarpos. Weed Res. 2017, 57, 247–256. [Google Scholar] [CrossRef]
- Garcia, A.; Loydi, A.; Distel, R.A. Temporal and spatial variation in the soil seed bank of Nassella trichotoma (serrated tussock) in its native range. Aust. J. Bot. 2021, 69, 45–51. [Google Scholar] [CrossRef]
- Campbell, M.H.; Vere, D.T. Nassella trichotoma (Nees) Arech. In The Biology of Australian Weeds; Groves, R.H., Shepherd, R.H., Richardson, R.G., Eds.; R.G. and F.J. Richardson: Merideth, VIC, Australia, 1995; Volume 1, pp. 189–202. [Google Scholar]
- Ruttledge, A.; Whalley, R.D.B.; Falzon, G.; Backhouse, D.; Sindel, B.M. The role of soil temperature and seed dormancy in the creation and maintenance of persistent seed banks of Nassella trichotoma (serrated tussock) on the Northern Tablelands of New South Wales. Rangel 2020, 42, 85–95. [Google Scholar] [CrossRef]
- Sano, N.; Rajju, L.; North, H.M.; Debeaujon, I.; Marion-Poll, A.; Seo, M. Staying alive: Molecular aspects of seed longevity. Plant Cell Physiol. 2015, 57, 660–674. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.; Bakker, J.P.; Bekker, R.M. The Soil Seed Banks of North West Europe: Methodology, Density and Longevity; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Bebawi, F.F.; Campbell, S.D.; Mayer, R. Gamba grass (Andropogon gayanus Kunth.) seed persistence and germination temperature tolerance. Rangeland J. 2018, 40, 463–472. [Google Scholar] [CrossRef]
- Reeves, T.G.; Code, G.R.; Piggin, C.M. Seed production and longevity, seasonal emergence and phenology of wild radish (Raphanus raphanistrum L.). Aust. J. Exp. Agric. 1981, 21, 524–530. [Google Scholar] [CrossRef]
- Vargas, A.A.M.; Agostinetto, D.; Zandna, R.R.; Fraga, D.S.; Neto, R.C.A. Longevity of horseweed seed bank depending on the burial depth. Planta Daninha 2018, 36, e0182073. [Google Scholar] [CrossRef]
- Sarabi, V. Factors that influence the level of weed seed predation: A review. Weed Biol. Manag. 2019, 19, 61–74. [Google Scholar] [CrossRef]
- Chung, N.J.; Paek, N.C. Photoblastism and ecophysiology of seed germination in weedy rice. J. Agron. 2003, 95, 184–190. [Google Scholar] [CrossRef]
- Pearson, T.R.H.; Burslem, D.F.R.P.; Mullins, C.E.; Dalling, J.W. Germination ecology of neotropical pioneers: Interacting effects of environmental conditions and seed size. Ecology 2002, 83, 2798–2807. [Google Scholar] [CrossRef]
- Campbell, M.H.; Nicol, H.I. Seed dormancy in serrated tussock (Nassella trichotoma (Nees) Arech.) in New South Wales. Plant Prot. Q. 1999, 14, 82–85. Available online: https://caws.org.nz/PPQ131415/PPQ%2014-3%20pp082-85%20Campbell.pdf (accessed on 29 June 2020).
- Watt, M.S.; Kriticos, D.J.; Lamoueaux, S.L.; Bourdot, G.W. Climate change and the potential global distribution of serrated tussock (Nassella trichotoma). Weed Sci. 2011, 59, 538–545. [Google Scholar] [CrossRef]
- Kriticos, D.J.; Lamoureaux, S.; Bourdot, G.W.; Pettit, W. Nassella tussock: Current and potential distribution in New Zealand. N. Z. Plant Prot. 2004, 57, 81–88. [Google Scholar] [CrossRef]
- Joubert, D.C. The soil seed bank under nassella tussock infestations at Boschberg. S. Afr. J. 1984, 1, 1–3. [Google Scholar] [CrossRef][Green Version]
- Vere, D.T.; Campbell, M.H. Economics of Controlling serrated tussock in the south-eastern Australian Rangelands. J. Range Manag. 1984, 37, 87–93. [Google Scholar] [CrossRef]
- Badgery, W.B.; Kemp, D.R.; Michalk, D.L.; King, W.M. Rethinking the management of serrated tussock, our worst perennial grass weed. In Proceedings of the 11th Australian Agronomy Conference: Solutions for a Better Environment, Geelong, VIC, Australia, 2–6 February 2003; Volume 1–4. [Google Scholar]
- Osmond, R.; Veebeek, M.; McLaren, D.A.; Michelmore, M.; Wicks, B.; Grech, C.J.; Fullerton, P. Serrated Tussock-National Best Practice Manual; Victorian Department of Primary Industries: Melbourne, VIC, Australia, 2008; Available online: http://serratedtussock.com/wp-content/uploads/files/Serrated-Tussock-National-Best-Practice-Management-Manual.pdf (accessed on 14 April 2022).
- Humphries, T.; Chauhan, B.S.; Florentine, S.K. Environmental factors effecting the germination and seedling emergence of two populations of an aggressive agricultural weed; Nassella trichotoma. PLoS ONE 2018, 13, e0199491. [Google Scholar] [CrossRef] [PubMed]
- Serrated tussock (Nassella trichotoma). Weed Management Guide. 2013. Available online: http://serratedtussock.com/wp-content/uploads/files/CRC-Weed-Management-Guide-Serrated-Tussock.pdf (accessed on 6 June 2022).
- Taylor, N.J. Ecological Aspects of Nassella Tussock (Stipa trichotoma); Botany Division, Department of Scientific and Industrial Research: Lincoln, New Zeland, 1987. [Google Scholar]
- Victorian Serrated Tussock Working Party. 2022. Available online: http://serratedtussock.com/ (accessed on 8 August 2022).
- Borza, J.K.; Westerman, P.R.; Leibman, M. Comparing estimates of seed viability in three foxtail (Setaria) species using the imbibed seed crush test with and without additional tetrazolium testing. Weed Technol. 2007, 21, 518–522. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing; International Seed Testing Association (ISTA): Bassersdorf, Switzerland, 1999; Volume 27 Supplement. Available online: https://www.seedtest.org/en/publications/international-rules-seed-testing-1168.html (accessed on 6 June 2022).
- Sadeghi, H.; Khazaei, L.Y.; Sheidaei, S. Effect of seed osmopriming on seed germination behaviour and vigor of soybean (Glycine max L.). J. Agric. Bio. Sci. 2011, 6, 39–43. Available online: https://www.semanticscholar.org/paper/EFFECT-OF-SEED-OSMOPRIMING-ON-SEED-GERMINATION-AND-Sadeghi-Khazaei/58fe72010ac3119fa402c84d02fe22c7220be040 (accessed on 6 September 2021).
- Association of Official Seed Analysis. 1983. Available online: https://analyzeseeds.com/?v=dfd44cc06c1b (accessed on 8 August 2022).
- Bureau of Meteorology. Climate Data Online; Australian Government: Melbourne, VIC, Australia, 2021. Available online: http://www.bom.gov.au/climate/data/ (accessed on 10 June 2022).
- Dowsett, C.; James, T.; Trivedi, P. Adaption of a technique for the accelerated aging of weed seeds to evaluate their longevity. N. Z. Plant Prot. Soc. 2012, 65, 69–73. [Google Scholar] [CrossRef]
- Willan, R.L. A Guide to Forest Seed Handling: With Special Reference to the Tropics; Food and Agriculture Organization of the United Nations: Washington, DC, USA, 1987; Available online: https://www.fao.org/3/ad232e/ad232e00.htm (accessed on 5 June 2022).
- Willan, R.L. A Guide to Forest Seed Handling; FAO Forestry Paper no 20/2; FAO: Rome, Italy, 1993; Available online: www.fao.org/../AF23E02.htm (accessed on 5 June 2022).
- Mrda, J.; Crnobarac, J.; Dusanic, N.; Jocic, S.; Miklic, V. Germination energy as a parameter of seed quality in different sunflower genotypes. Genetika 2011, 43, 427–436. [Google Scholar] [CrossRef]
- Mavi, K.; Demir, I.; Matthews, S. Mean germination time estimates the relative emergence of seed lots of three cucurbit crops under stress conditions. SST 2010, 38, 14–25. [Google Scholar] [CrossRef]
- Zeng, Y.J.; Wang, Y.R. Methods of topographical tetrazolium testing for seed viability of Nitraria tangutorum Bobr. and N. sibirica Pall. SST 2009, 37, 691–698. [Google Scholar] [CrossRef]
- Hilhorst, H.W.M. Seed Dormancy, Development of Dormancy. In Encyclopedia of Applied Plant Sciences; Thomas, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 1317–1323. [Google Scholar] [CrossRef]
- Buijs, G. A perspective on secondary seed dormancy in Arabidopsis thaliana. Plants 2020, 9, 749. [Google Scholar] [CrossRef] [PubMed]
- Banovetz, S.J.; Scheiner, S.M. Secondary seed dormancy in Coreopsis lanceolata. Am. Midi. Nat. 1994, 131, 75–83. [Google Scholar] [CrossRef]
- Salter, W. The Importance of Soil Seed Banks to Invasive Plant Species; Botany One: Exeter, UK, 2021; Available online: https://botany.one/2021/06/the-importance-of-soil-seed-banks-to-invasive-plant-species/#:~:text=Can%20soil%20seed%20banks%20contribute,the%20keys%20to%20invasion%20success (accessed on 7 August 2022).
- Thompson, K.; Bakker, J.P.; Bekker, R.M.; Hodgson, J.G. Ecological correlates of seed persistence in soil in the north-west European flora. J. Ecol. 1998, 86, 163–169. [Google Scholar] [CrossRef]
- Plue, J.; Vandepitte, K.; Honnay, O.; O’Cousins, S.A. Does the seed bank contribute to the build-up of a genetic extinction debt in the grassland perennial Campanula rotundifolia? Ann. Bot. 2017, 120, 373–385. [Google Scholar] [CrossRef][Green Version]
- Tikka, P.M.; Heikkila, T.; Heiskanen, M.; Kuitunen, M. The role of competition and rarity in the restoration of a dry grassland in Finland. Appl. Veg. Sci. 2001, 4, 139–146. [Google Scholar] [CrossRef]
- Trotter, T.F. The Ecology and Management of Nassella trichotoma on the Northern Tablelands of New South Wales. Ph.D. Thesis, University of New England, Armidale, NSW, Australia, 2006; p. 201. [Google Scholar]
- Sethi, R.; Kaur, N.; Singh, M. Morphological and physiological characterization of seed heteromorphism in Medicago denticulate Willd. Plant Physiol. 2020, 25, 107–119. [Google Scholar] [CrossRef]
- Agriculture Victoria. Serrated Tussock. Weeds Information. 2020. Available online: https://agriculture.vic.gov.au/biosecurity/weeds/weeds-information/serrated-tussock#:~:text=Seedbank%20propagule%20persistence,15%20years%20and%20sometimes%20longer (accessed on 9 August 2022).
- Badgery, W.B.; Kemp, D.R.; Michalk, D.L.; King, W.M.G. Studies of competition between Nassella trichotoma (Nees) Hack. ex Arechav. (serrated tussock) and native pastures. 2. Seedling responses. Aust. J. Agric. Res. 2008, 59, 237–246. [Google Scholar] [CrossRef]
- Healey, A.J. Nassella Tussock: Field Studies and Their Agricultural Significance; Bulletin No. 91; Department of Scientific and Industrial Research: Wellington, New Zealand, 1945. [Google Scholar]

| Artificial Ageing Time (Days) | Germination % | Germination Energy % | Germination Rate Index | Mean Germination Time |
|---|---|---|---|---|
| 1 | ||||
| 2 | ||||
| 5 | ||||
| 9 | ||||
| 20 | ||||
| 30 | ||||
| 50 |
| Time of Burial | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 6 Months | 9 Months | 12 Months | 15 Months | 18 Months | ||||||
| Burial depth (cm) | Germination (%) | |||||||||
| Jan | May | Jan | May | Jan | May | Jan | May | Jan | May | |
| 0 | 0 | 11.2 | 0 | 10 | 0 | 1.6 | 2 | 0 | 2 | 3.20 |
| 1 | 0 | 12 | 3.2 | 15.2 | 0 | 14.8 | 0.4 | 5.2 | 0.4 | 0.40 |
| 2 | 0 | 14 | 0.8 | 5.2 | 0 | 11.2 | 0.4 | 9.2 | 0.4 | 0.40 |
| 4 | 0.4 | 12.8 | 1.6 | 12 | 0.8 | 7.6 | 6.4 | 6.8 | 6.4 | 0.40 |
| Mean | 0.1 | 12.5 | 1.4 | 10.6 | 0.2 | 8.8 | 2.3 | 5.3 | 1.2 | 1.2 |
| SEm± | 1.08 | 1.26 | 1.46 | 1.51 | 2.15 | 2.07 | 2.53 | 2.24 | 3.12 | 2.92 |
| Burial depth (cm) | Seed decay (%) | |||||||||
| Jan | May | Jan | May | Jan | May | Jan | May | Jan | May | |
| 0 | 80.4 | 74.8 | 87.2 | 76.8 | 88.8 | 72.4 | 92 | 70.8 | 66.4 | 78.4 |
| 1 | 80.8 | 78.4 | 88.8 | 77.2 | 57.6 | 63.2 | 77.6 | 57.6 | 81.2 | 79.2 |
| 2 | 77.6 | 74 | 90.4 | 82.4 | 86.4 | 76.4 | 84.4 | 66.8 | 82.8 | 82.4 |
| 4 | 94.8 | 72 | 93.2 | 83.2 | 96.8 | 69.6 | 80.4 | 74 | 78.8 | 86.8 |
| Mean | 83.4 | 74.8 | 89.9 | 79.9 | 82.4 | 70.4 | 83.6 | 67.3 | 77.3 | 81.7 |
| SEm± | 14.39 | 12.60 | 14.80 | 13.00 | 14.21 | 10.84 | 12.72 | 9.87 | 11.14 | 11.71 |
| Burial depth (cm) | Field germination (%) | |||||||||
| Jan | May | Jan | May | Jan | May | Jan | May | Jan | May | |
| 0 | 19.6 | 14 | 12.8 | 13.2 | 11.2 | 26 | 6 | 29.2 | 30.4 | 20.4 |
| 1 | 19.2 | 9.6 | 8 | 7.6 | 42.4 | 22 | 22 | 37.2 | 18.4 | 19.2 |
| 2 | 22.4 | 12 | 8.8 | 12.4 | 13.6 | 12.4 | 15.2 | 24 | 16.8 | 15.2 |
| 4 | 4.8 | 15.2 | 5.2 | 4.8 | 2.4 | 22.8 | 13.2 | 19.2 | 20.8 | 10 |
| Mean | 16.5 | 12.7 | 8.7 | 9.5 | 17.4 | 20.8 | 14.1 | 27.4 | 21.6 | 16.2 |
| SEm± | 3.4 | 1.5 | 1.11 | 1.41 | 6.21 | 2.62 | 2.33 | 3.54 | 2.25 | 1.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Humphries, T.; Florentine, S. Assessing Seedbank Longevity and Seed Persistence of the Invasive Tussock Grass Nassella trichotoma Using in-Field Burial and Laboratory-Controlled Ageing. Plants 2022, 11, 2377. https://doi.org/10.3390/plants11182377
Humphries T, Florentine S. Assessing Seedbank Longevity and Seed Persistence of the Invasive Tussock Grass Nassella trichotoma Using in-Field Burial and Laboratory-Controlled Ageing. Plants. 2022; 11(18):2377. https://doi.org/10.3390/plants11182377
Chicago/Turabian StyleHumphries, Talia, and Singarayer Florentine. 2022. "Assessing Seedbank Longevity and Seed Persistence of the Invasive Tussock Grass Nassella trichotoma Using in-Field Burial and Laboratory-Controlled Ageing" Plants 11, no. 18: 2377. https://doi.org/10.3390/plants11182377
APA StyleHumphries, T., & Florentine, S. (2022). Assessing Seedbank Longevity and Seed Persistence of the Invasive Tussock Grass Nassella trichotoma Using in-Field Burial and Laboratory-Controlled Ageing. Plants, 11(18), 2377. https://doi.org/10.3390/plants11182377






