Physiological and Antioxidant Response to Different Water Deficit Regimes of Flag Leaves and Ears of Wheat Grown under Combined Elevated CO2 and High Temperature
Abstract
:1. Introduction
2. Results
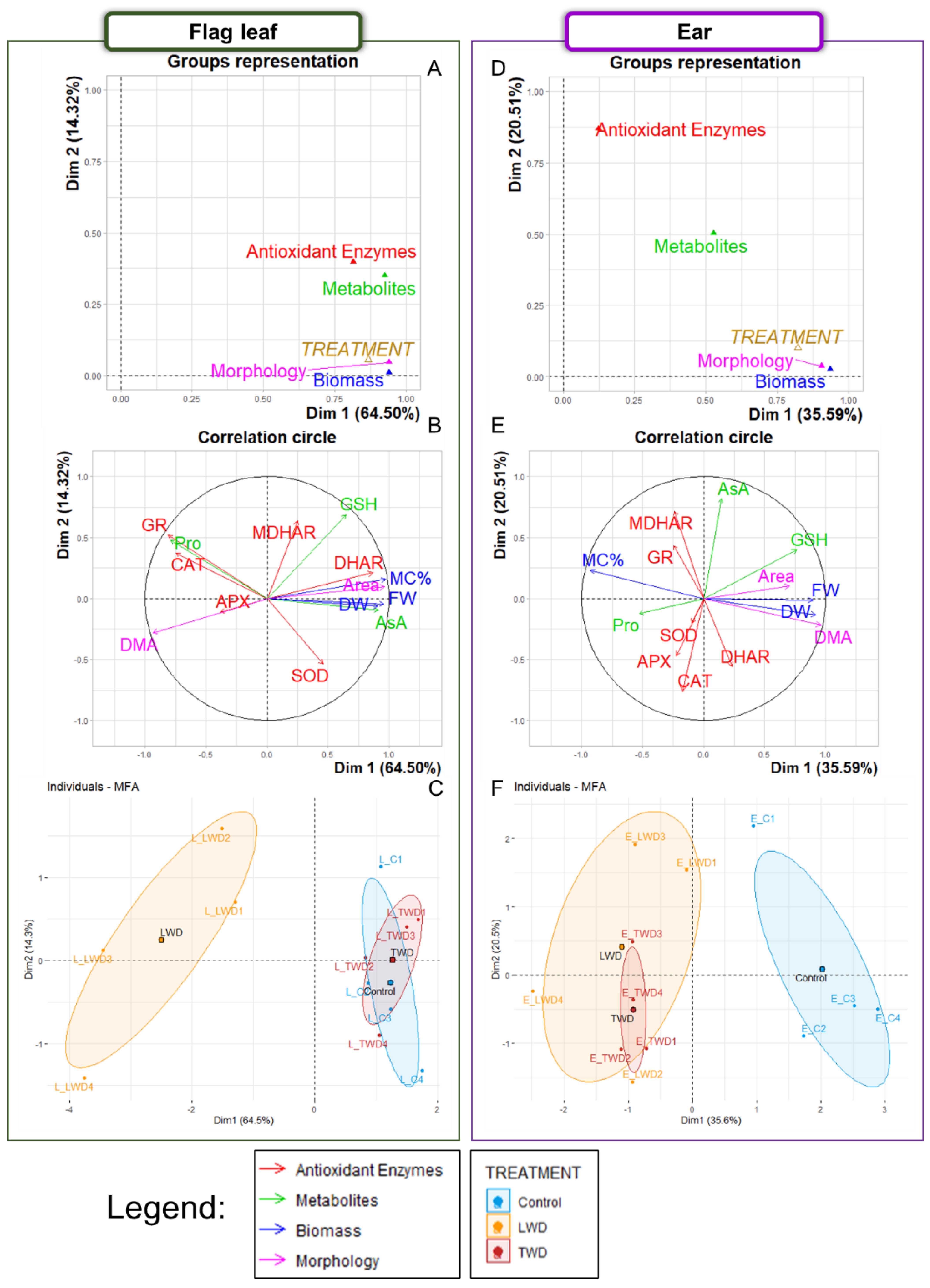
2.1. Multiple Factor Analysis
2.2. Biomass and Morphology
2.3. Chlorophyll and Photosynthesis Status
2.4. Accumulation of Metabolites with Osmoprotectant and Antioxidant Roles
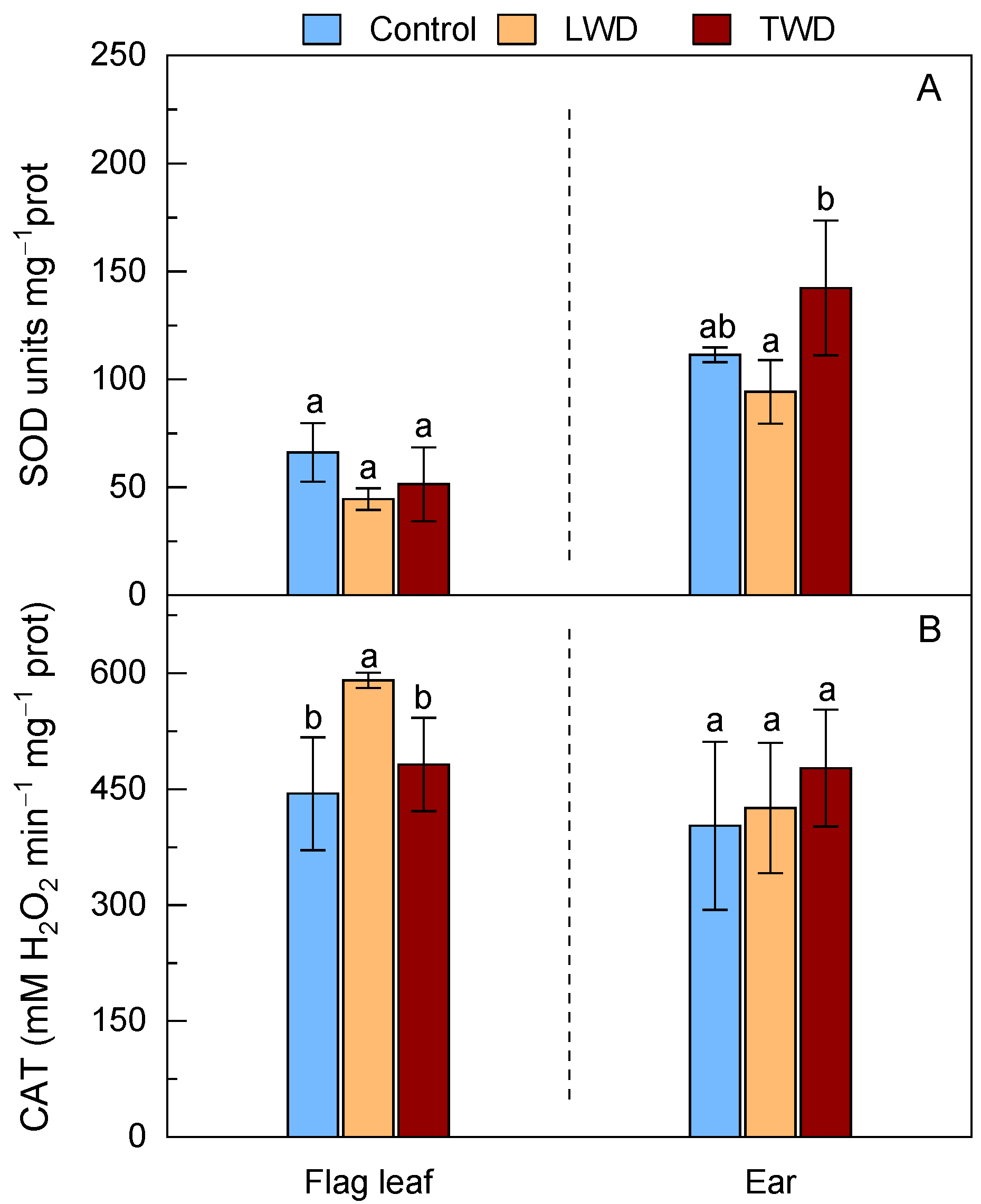
2.5. Superoxide Dismutase and Catalase
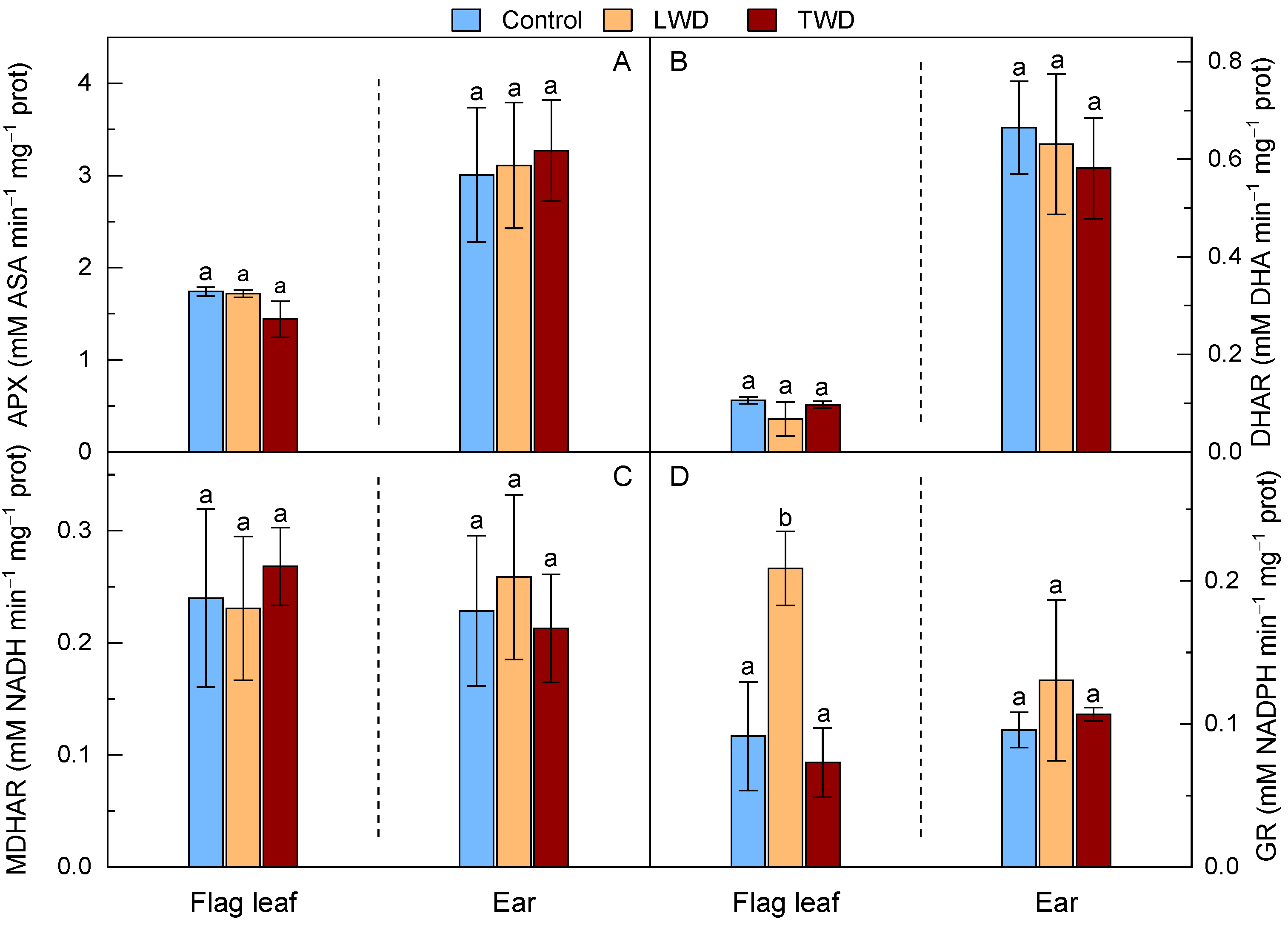
2.6. Ascorbate Glutathione Cycle
3. Discussion
4. Materials and Methods
4.1. Plant Material and Experimental Growth Conditions
4.2. Photosynthetic Parameter Determination
4.3. Biomass Parameters
4.4. Sampling Procedure
4.5. Proline Content Quantification
4.6. Ascorbate and Glutathione Content Quantification
4.7. Enzymatic Antioxidant Activity Assays
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; IPCC: Cambrigde, UK; New York, NY, USA, 2021; pp. 2339–2391. [Google Scholar] [CrossRef]
- Guntukula, R.; Goyari, P. Climate change effects on the crop yield and its variability in telangana, India. Stud. Microecon. 2020, 8, 119–148. [Google Scholar] [CrossRef]
- Hussain, S.; Hussain, S.; Qadir, T.; Khaliq, A.; Ashraf, U.; Parveen, A.; Saqib, M.; Rafiq, M. Drought stress in plants: An overview on implications, tolerance mechanisms and agronomic mitigation strategies. Plant Sci. Today 2019, 6, 389–402. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Plants drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2020. Transforming Food Systems for Affordable Healthy Diets. Available online: https://doi.org/10.4060/ca9692en (accessed on 17 March 2022).
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of climate change on agriculture and its mitigation strategies: A review. Sustainability 2021, 13, 1318. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef]
- Grote, U.; Fasse, A.; Nguyen, T.T.; Erenstein, O. Food security and the dynamics of wheat and maize value chains in Africa and Asia. Front. Sustain. Food Syst. 2021, 4, 207–212. [Google Scholar] [CrossRef]
- Pandey, M.; Shrestha, J.; Subedi, S.; Shah, K.K. Role of nutrients in wheat: A review. Trop. Agrobiodiversity 2020, 1, 18–23. [Google Scholar] [CrossRef]
- Shiferaw, B.; Smale, M.; Braun, H.-J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur. 2013, 5, 291–317. [Google Scholar] [CrossRef]
- Wieser, H.; Koehler, P.; Scherf, K.A. The two faces of wheat. Front. Nutr. 2020, 7, 517313. [Google Scholar] [CrossRef]
- FAO. The Impact of Disasters and Crises on Agriculture and Food Security. 2021. Available online: https://doi.org/10.4060/cb3673en (accessed on 17 March 2022).
- FAO. Special Report—2021 FAO Crop and Food Supply Assessment Mission to the Sudan. Available online: http://www.fao.org/documents/card/en/c/cb9122en (accessed on 8 April 2022).
- Thompson, M.; Gamage, D.; Hirotsu, N.; Martin, A.; Seneweera, S. Effects of elevated carbon dioxide on photosynthesis and carbon partitioning: A Perspective on root sugar sensing and hormonal crosstalk. Front. Physiol. 2017, 8, 578. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, Y.; Zhou, G. Response and adaptation of photosynthesis, respiration, and antioxidant systems to elevated CO2 with environmental stress in plants. Front. Plant Sci. 2015, 6, 701. [Google Scholar] [CrossRef] [PubMed]
- Kadam, N.N.; Xiao, G.; Melgar, R.J.; Bahuguna, R.N.; Quinones, C.; Tamilselvan, A.; Prasad, P.V.V.; Jagadish, K.S.V. Agronomic and physiological responses to high temperature, drought, and elevated CO2 interactions in cereals. In Advances in Agronomy; Sparks, D., Ed.; Academic Press: Cambrigde, MA, USA, 2014; Volume 127, pp. 111–156. [Google Scholar]
- Vicente, R.; Bolger, A.M.; Martínez-Carrasco, R.; Pérez, P.; Gutiérrez, E.; Usadel, B.; Morcuende, R. De novo transcriptome analysis of durum wheat flag leaves provides new insights into the regulatory response to elevated CO2 and high temperature. Front. Plant Sci. 2019, 10, 1605. [Google Scholar] [CrossRef] [PubMed]
- Long, S.P.; Ainsworth, E.A.; Rogers, A.; Ort, D.R. Rising atmospheric carbon dioxide: Plants FACE the future. Annu. Rev. Plant Biol. 2004, 55, 591–628. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, F.; Hao, L.; Yu, J.; Guo, L.; Zhou, H.; Ma, C.; Zhang, X.; Xu, M. Elevated CO2 concentration induces photosynthetic down-regulation with changes in leaf structure, non-structural carbohydrates and nitrogen content of soybean. BMC Plant Biol. 2019, 19, 255. [Google Scholar] [CrossRef]
- Stitt, M.; Krapp, A. The interaction between elevated carbon dioxide and nitrogen nutrition: The physiological and molecular background. Plant Cell Environ. 1999, 22, 583–621. [Google Scholar] [CrossRef]
- Aranjuelo, I.; Cabrera-Bosquet, L.; Morcuende, R.; Avice, J.C.; Nogués, S.; Araus, J.L.; Martínez-Carrasco, R.; Pérez, P. Does ear C sink strength contribute to overcoming photosynthetic acclimation of wheat plants exposed to elevated CO2? J. Exp. Bot. 2011, 62, 3957–3969. [Google Scholar] [CrossRef]
- Vicente, R.; Pérez, P.; Martínez-Carrasco, R.; Usadel, B.; Kostadinova, S.; Morcuende, R. Quantitative RT–PCR platform to measure transcript levels of C and N metabolism-related genes in durum wheat: Transcript profiles in elevated [CO2] and high temperature at different levels of N supply. Plant Cell Physiol. 2015, 56, 1556–1573. [Google Scholar] [CrossRef]
- Pérez, P.; Morcuende, R.; Martín del Molino, I.; Martínez-Carrasco, R. Diurnal changes of Rubisco in response to elevated CO2, temperature and nitrogen in wheat grown under temperature gradient. Environ. Exp. Bot. 2005, 53, 13–27. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef]
- Taub, D.R.; Wang, X. Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. J. Integr. Plant Biol. 2008, 50, 1365–1374. [Google Scholar] [CrossRef]
- Bloom, A.J.; Burger, M.; Kimball, B.A.; Pinter, P.J. Nitrate assimilation is inhibited by elevated CO2 in field-grown wheat. Nat. Clim. Change 2014, 4, 477–480. [Google Scholar] [CrossRef]
- Medina, S.; Vicente, R.; Amador, A.; Araus, J.L. Interactive effects of elevated [CO2] and water stress on physiological traits and gene expression during vegetative growth in four durum wheat genotypes. Front. Plant Sci. 2016, 7, 1738. [Google Scholar] [CrossRef]
- Rehman, H.U.; Tariq, A.; Ashraf, I.; Ahmed, M.; Muscolo, A.; Basra, S.M.A.; Reynolds, M. Evaluation of physio-logical and morphological traits for improving spring wheat adaptation to terminal heat stress. Plants 2021, 10, 455. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, F. Effects of elevated CO2 and heat on wheat grain quality. Plants 2021, 10, 1027. [Google Scholar] [CrossRef] [PubMed]
- Salvucci, M.E.; Crafts-Brandner, S.J. Inhibition of photosynthesis by heat stress: The activation state of Rubisco as a limiting factor in photosynthesis. Physiol. Plant. 2004, 120, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Crafts-Brandner, S.J.; Salvucci, M.E. Rubisco activase constrains the photosynthetic potential of leaves at high temperature and CO2. Proc. Natl. Acad. Sci. USA 2000, 97, 13420–13435. [Google Scholar] [CrossRef]
- Sarkar, S.; Islam, A.K.M.A.; Barma, N.C.D.; Ahmed, J.U. Tolerance mechanisms for breeding wheat against heat stress: A review. South Afr. J. Bot. 2021, 138, 262–277. [Google Scholar] [CrossRef]
- Lal, M.K.; Tiwari, R.K.; Gahlaut, V.; Mangal, V.; Kumar, A.; Singh, M.P.; Paul, V.; Kumar, S.; Singh, B.; Zinta, G. Physiological and molecular insights on wheat responses to heat stress. Plant Cell Rep. 2021, 41, 501–5018. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Xylia, P.; Antoniou, O.; Tzortzakis, N. Climate change due to heat and drought stress can alter the physiology of maratheftiko local Cyprian grapevine variety. J. Water Clim. Change 2018, 9, 715–727. [Google Scholar] [CrossRef]
- Dhakal, A. Effect of drought stress and management in wheat—A review. Food Agribus. Manag. 2021, 2, 62–66. [Google Scholar] [CrossRef]
- Fathi, A.; Tari, D.B. Effect of drought stress and its mechanism in plants. Int. J. Life Sci. 2016, 10, 1–6. [Google Scholar] [CrossRef]
- Araus, J.L.; Slafer, G.A.; Reynolds, M.P.; Royo, C. Plant breeding and drought in C3 cereals: What should we breed for? Ann. Bot. 2002, 89, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Athar, H.-U.-R.; Ashraf, M. Photosynthesis under drought stress. In Handbook of Photosynthesis; Pessaralki, M., Ed.; Taylor and Francis, Inc.: New York, NY, USA, 2005; pp. 793–809. [Google Scholar] [CrossRef]
- Agurla, S.; Gahir, S.; Munemasa, S.; Murata, Y.; Raghavendra, A.S. Mechanism of stomatal closure in plants exposed to drought and cold stress. In Survival Strategies in Extreme Cold and Desiccation. Advances in Experimental Medicine and Biology; Iwaya-Inoue, M., Skurai, M., Uemura, M., Eds.; Springer: Singapore, 2018; Volume 1081, pp. 215–232. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.; Chaitanya, K.V. Photosynthesis and antioxidative defense mechanisms in deciphering drought stress tolerance of crop plants. Biol. Plant. 2016, 60, 201–218. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef]
- Huseynova, I.M. Photosynthetic characteristics and enzymatic antioxidant capacity of leaves from wheat cultivars exposed to drought. Biochim. Et Biophys. Acta Bioenerg. 2012, 1817, 1516–1523. [Google Scholar] [CrossRef] [Green Version]
- Nasirzadeh, L.; Sorkhilaleloo, B.; Majidi Hervan, E.; Fatehi, F. Changes in antioxidant enzyme activities and gene expression profiles under drought stress in tolerant, intermediate, and susceptible wheat genotypes. Cereal Res. Commun. 2021, 49, 83–89. [Google Scholar] [CrossRef]
- Aliyeva, D.R.; Aydinli, L.M.; Zulfugarov, I.S.; Huseynova, I.M. Diurnal changes of the ascorbate-glutathione cycle components in wheat genotypes exposed to drought. Funct. Plant Biol. 2020, 47, 998. [Google Scholar] [CrossRef]
- Lou, L.; Li, X.; Chen, J.; Li, Y.; Tang, Y.; Lv, J. Photosynthetic and ascorbate-glutathione metabolism in the flag leaves as compared to spikes under drought stress of winter wheat (Triticum aestivum L.). PLoS ONE 2018, 13, e0194625. [Google Scholar] [CrossRef]
- Robredo, A.; Pérez-López, U.; de la Maza, H.S.; González-Moro, B.; Lacuesta, M.; Mena-Petite, A.; Muñoz-Rueda, A. Elevated CO2 alleviates the impact of drought on barley improving water status by lowering stomatal conductance and delaying its effects on photosynthesis. Environ. Exp. Bot. 2007, 59, 252–263. [Google Scholar] [CrossRef]
- Zinta, G.; AbdElgawad, H.; Domagalska, M.A.; Vergauwen, L.; Knapen, D.; Nijs, I.; Janssens, I.A.; Beemster, G.T.S.; Asard, H. Physiological, biochemical, and genome-wide transcriptional analysis reveals that elevated CO2 mitigates the impact of combined heat wave and drought stress in Arabidopsis thaliana at multiple organizational levels. Glob. Change Biol. 2014, 20, 3670–3685. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chen, L.; Xu, M.; Huang, B. Effects of elevated CO2 on physiological responses of tall fescue to elevated temperature, drought stress, and the combined stresses. Crop Sci. 2012, 52, 1848–1858. [Google Scholar] [CrossRef]
- Varga, B.; Vida, G.; Varga-László, E.; Hoffmann, B.; Veisz, O. Combined effect of drought stress and elevated atmospheric CO2 concentration on the yield parameters and water use properties of winter wheat (Triticum aestivum L.) genotypes. J. Agron. Crop Sci. 2017, 203, 192–205. [Google Scholar] [CrossRef]
- Li, B.; Feng, Y.; Zong, Y.; Zhang, D.; Hao, X.; Li, P. Elevated CO2-induced changes in photosynthesis, antioxidant enzymes and signal transduction enzyme of soybean under drought stress. Plant Physiol. Biochem. 2020, 154, 105–114. [Google Scholar] [CrossRef]
- Ulfat, A.; Mehmood, A.; Ahmad, K.S.; Ul-Allah, S. Elevated carbon dioxide offers promise for wheat adaptation to heat stress by adjusting carbohydrate metabolism. Physiol. Mol. Biol. Plants 2021, 27, 2345–2355. [Google Scholar] [CrossRef]
- Urban, O.; Hlaváčová, M.; Klem, K.; Novotná, K.; Rapantová, B.; Smutná, P.; Horáková, V.; Hlavinka, P.; Škarpa, P.; Trnka, M. Combined effects of drought and high temperature on photosynthetic characteristics in four winter wheat genotypes. Field Crops Res. 2018, 223, 137–149. [Google Scholar] [CrossRef]
- Li, X.; Kristiansen, K.; Rosenqvist, E.; Liu, F. Elevated CO2 modulates the effects of drought and heat stress on plant water relations and grain yield in wheat. J. Agron. Crop Sci. 2019, 205, 362–371. [Google Scholar] [CrossRef]
- Ben Mariem, S.; Soba, D.; Zhou, B.; Loladze, I.; Morales, F.; Aranjuelo, I. Climate change, crop yields, and grain quality of C3 cereals: A meta-analysis of [CO2], temperature, and drought effects. Plants 2021, 10, 1052. [Google Scholar] [CrossRef]
- Sekhar, K.M.; Kota, V.R.; Reddy, T.P.; Rao, K.V.; Reddy, A.R. Amelioration of plant responses to drought under elevated CO2 by rejuvenating photosynthesis and nitrogen use efficiency: Implications for future climate-resilient crops. Photosynth. Res. 2021, 150, 21–40. [Google Scholar] [CrossRef]
- Gutíérrez, D.; Gutíérrez, E.; Pérez, P.; Morcuende, R.; Verdejo, A.L.; Martínez-Carrasco, R. Acclimation to future atmospheric CO2 levels increases photochemical efficiency and mitigates photochemistry inhibition by warm temperatures in wheat under field chambers. Physiol. Plant. 2009, 137, 86–100. [Google Scholar] [CrossRef]
- Marcos-Barbero, E.L.; Pérez, P.; Martínez-Carrasco, R.; Arellano, J.B.; Morcuende, R. Screening for higher grain yield and biomass among sixty bread wheat genotypes grown under elevated CO2 and high-temperature conditions. Plants 2021, 10, 1596. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Barbero, E.L.; Pérez, P.; Martínez-Carrasco, R.; Arellano, J.B.; Morcuende, R. Genotypic variability on grain yield and grain nutritional quality characteristics of wheat grown under elevated CO2 and high temperature. Plants 2021, 10, 1043. [Google Scholar] [CrossRef] [PubMed]
- Royo, C.; Briceño-Felix, G.A. Spanish wheat pool. In The World Wheat Book: A History of Wheat Breeding; Bojean, A.P., Angus, W.J., van Ginkel, M., Eds.; Lavoisier Publishing Inc.: Paris, France, 2011; Volume 2, pp. 121–154. [Google Scholar]
- Marín-Sanz, M.; Giménez, M.J.; Barro, F.; Savin, R. Prolamin content and grain weight in RNAi silenced wheat lines under different conditions of temperature and nitrogen availability. Front. Plant Sci. 2020, 11, 314. [Google Scholar] [CrossRef] [PubMed]
- White, J.; Edwards, J. Wheat Growth and Development; White, J., Edwards, J., Eds.; NSW Department of Primary Industry: Orange, Australia, 2008; ISBN 978 0 7347 1894 5. [Google Scholar]
- Dowdle, J.; Ishikawa, T.; Gatzek, S.; Rolinski, S.; Smirnoff, N. Two genes in Arabidopsis thaliana encoding GDP-L-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J. 2007, 52, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef]
- Ali, Z.; Merrium, S.; Habib-ur-Rahman, M.; Hakeem, S.; Saddique, M.A.B.; Sher, M.A. Wetting mechanism and morphological adaptation; leaf rolling enhancing atmospheric water acquisition in wheat crop—A review. Environ. Sci. Pollut. Res. 2022, 29, 30967–30985. [Google Scholar] [CrossRef]
- Nezhadahmadi, A.; Prodhan, Z.H.; Faruq, G. Drought tolerance in wheat. Sci. World J. 2013, 2013, 610721. [Google Scholar] [CrossRef]
- Luo, W.; Gong, Y.; Tang, Y.; Pu, P.; Yang, X.; Zhou, C.; Lv, J.; Yan, X. Glutathione and ethylene biosynthesis reveal that the glume and lemma have better tolerance to water deficit in wheat. Plant Physiol. Biochem. 2021, 160, 120–129. [Google Scholar] [CrossRef]
- Vicente, R.; Vergara-Díaz, O.; Medina, S.; Chairi, F.; Kefauver, S.C.; Bort, J.; Serret, M.D.; Aparicio, N.; Araus, J.L. Durum wheat ears perform better than the flag leaves under water stress: Gene expression and physiological evidence. Environ. Exp. Bot. 2018, 153, 271–285. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Merrium, S.; Ali, Z.; Tahir, M.H.N.; Habib-ur-Rahman, M.; Hakeem, S. Leaf rolling dynamics for atmospheric moisture harvesting in wheat plant as an adaptation to arid environments. Environ. Sci. Pollut. Res. 2022, 29, 48995–49006. [Google Scholar] [CrossRef] [PubMed]
- Wada, S.; Takagi, D.; Miyake, C.; Makino, A.; Suzuki, Y. Responses of the photosynthetic electron transport reactions stimulate the oxidation of the reaction center chlorophyll of photosystem I, P700, under drought and high temperatures in rice. Int. J. Mol. Sci. 2019, 20, 2068. [Google Scholar] [CrossRef] [PubMed]
- Lata, C.; Muthamilarasan, M.; Prasad, M. Drought stress responses and signal transduction in plants. In Elucidation of Abiotic Stress Signaling in Plants; Springer: New York, NY, USA, 2015; Volume 2, pp. 195–225. ISBN 9781493925407. [Google Scholar]
- Parkash, V.; Singh, S. A review on potential plant-based water stress indicators for vegetable crops. Sustainability 2020, 12, 3945. [Google Scholar] [CrossRef]
- Onyemaobi, O.; Sangma, H.; Garg, G.; Wallace, X.; Kleven, S.; Suwanchaikasem, P.; Roessner, U.; Dolferus, R. Reproductive stage drought tolerance in wheat: Importance of stomatal conductance and plant growth regulators. Genes 2021, 12, 1742. [Google Scholar] [CrossRef]
- Khan, F.; Feng, Y.; Palta, J.A.; Chen, Y.; Sadras, V.O.; Siddique, K.H.M. Selection for yield over five decades favored anisohydric and phenological adaptations to early-season drought in Australian wheat. Plant Soil 2022, 476, 511–526. [Google Scholar] [CrossRef]
- Gallé, Á.; Csiszár, J.; Benyó, D.; Laskay, G.; Leviczky, T.; Erdei, L.; Tari, I. Isohydric and anisohydric strategies of wheat genotypes under osmotic stress: Biosynthesis and function of ABA in stress responses. J. Plant Physiol. 2013, 170, 1389–1399. [Google Scholar] [CrossRef]
- Abdelhakim, L.O.A.; Mendanha, T.; Palma, C.F.F.; Vrobel, O.; Štefelová, N.; Ćavar Zeljković, S.; Tarkowski, P.; de Diego, N.; Wollenweber, B.; Rosenqvist, E.; et al. Elevated CO2 improves the physiology but not the final yield in spring wheat genotypes subjected to heat and drought stress during anthesis. Front. Plant Sci. 2022, 13, 824476. [Google Scholar] [CrossRef]
- Iqbal, M.J. Role of osmolytes and antioxidant enzymes for drought tolerance in wheat. In Global Wheat Production; Fadh, S., Basir, A., Adnnan, M., Eds.; IntechOpen: London, UK, 2018; pp. 51–65. [Google Scholar] [CrossRef]
- Bueno-Ramos, N.; González-Hernández, A.I.; Marcos-Barbero, E.L.; Miranda-Apodaca, J.; Bendou, O.; Gutiérrez-Fernández, I.; Arellano, J.B.; Morcuende, R. Impact of water deficit on primary metabolism at the whole plant level in bread wheat grown under elevated CO2, and high temperature at different developmental stages. Chem. Proc. 2022, 10, 6. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a multifaceted signalling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant Biol. 2021, 24, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, S. The fermentation analogy: A point of view for understanding the intriguing role of proline accumulation in stressed plants. Front. Plant Sci. 2016, 7, 1339. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Anjum, N.A.; Hasanuzzaman, M.; Gill, R.; Trivedi, D.K.; Ahmad, I.; Pereira, E.; Tuteja, N. Glutathione and glutathione reductase: A boon in disguise for plant abiotic stress defense operations. Plant Physiol. Biochem. 2013, 70, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Hager, J.; Chaouch, S.; Queval, G.; Han, Y.; Taconnat, L.; Saindrenan, P.; Gouia, H.; Issakidis-Bourguet, E.; Renou, J.-P.; et al. Arabidopsis GLUTATHIONE REDUCTASE1 plays a crucial role in leaf responses to intracellular hydrogen peroxide and in ensuring appropriate gene expression through both salicylic acid and jasmonic acid signaling. Plant Physiol. 2010, 153, 1144–1160. [Google Scholar] [CrossRef] [PubMed]
- Abo Gamar, M.I.; Kisiala, A.; Emery, R.J.N.; Yeung, E.C.; Stone, S.L.; Qaderi, M.M. Elevated carbon dioxide decreases the adverse effects of higher temperature and drought stress by mitigating oxidative stress and improving water status in Arabidopsis thaliana. Planta 2019, 250, 1191–1214. [Google Scholar] [CrossRef]
- Jia, S.; Lv, J.; Jiang, S.; Liang, T.; Liu, C.; Jing, Z. Response of wheat ear photosynthesis and photosynthate carbon distribution to water deficit. Photosynthetica 2015, 53, 95–109. [Google Scholar] [CrossRef] [Green Version]
- Tambussi, E.A.; Maydup, M.L.; Carrión, C.A.; Guiamet, J.J.; Araus, J.L. Ear photosynthesis in C3 cereals and its contribution to grain yield: Methodologies, controversies, and perspectives. J. Exp. Bot. 2021, 72, 3956–3970. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Bragado, R.; Vicente, R.; Molero, G.; Serret, M.D.; Maydup, M.L.; Araus, J.L. New avenues for increasing yield and stability in C3 cereals: Exploring ear photosynthesis. Curr. Opin. Plant Biol. 2020, 56, 223–234. [Google Scholar] [CrossRef]
- Gámez, A.L.; Vicente, R.; Sanchez-Bragado, R.; Jauregui, I.; Morcuende, R.; Goicoechea, N.; Aranjuelo, I. Differential flag leaf and ear photosynthetic performance under elevated (CO2) conditions during grain filling period in durum wheat. Front. Plant Sci. 2020, 11, 587958. [Google Scholar] [CrossRef]
- Molero, G.; Reynolds, M.P. Spike photosynthesis measured at high throughput indicates genetic variation independent of flag leaf photosynthesis. Field Crops Res. 2020, 255, 107866. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- LeafWeb. Available online: https://www.leafweb.org/ (accessed on 2 August 2022).
- Goulas, Y.; Cerovic, Z.G.; Cartelat, A.; Moya, I. Dualex: A new instrument for field measurements of epidermal ultraviolet absorbance by chlorophyll fluorescence. Appl. Opt. 2004, 43, 4488–4496. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Foyer, C.H.; Souriau, N.; Perret, S.; Lelandais, M.; Kunert, K.-J.; Pruvost, C.; Jouanin, L. Overexpression of glutathione reductase but not glutathione synthetase leads to increases in antioxidant capacity and resistance to photoinhibition in poplar trees. Plant Physiol. 1995, 109, 1047–1048. [Google Scholar] [CrossRef]
- Pérez-López, U.; Miranda-Apodaca, J.; Muñoz-Rueda, A.; Mena-Petite, A. Interacting effects of high light and el-evated CO2 on the nutraceutical quality of two differently pigmented Lactuca sativa cultivars (Blonde of Paris Ba-tavia and Oak Leaf). Sci. Hortic. 2015, 191, 38–48. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Pérez-López, U.; Robredo, A.; Lacuesta, M.; Sgherri, C.; Muñoz-Rueda, A.; Navari-Izzo, F.; Mena-Petite, A. The oxidative stress caused by salinity in two barley cultivars is mitigated by elevated CO2. Physiol. Plant. 2009, 135, 29–42. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Bendou, O.; Gutiérrez-fernández, I.; Marcos-barbero, E.L.; Bueno-ramos, N.; González-hernández, A.I.; Morcuende, R.; Arellano, J.B. Theoretical and experimental considerations for a rapid and high throughput measurement of catalase in vitro. Antioxidants 2022, 11, 21. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Edwards, E.A.; Rawsthorne, S.; Mullineaux, P.M. Subcellular distribution of multiple forms of glutathione reductase in leaves of pea (Pisum sativum L.). Planta 1990, 180, 278–284. [Google Scholar] [CrossRef]
- Hossain, M.A.; Asada, K. Inactivation of ascorbate peroxidase in spinach chloroplasts on dark addition of hydrogen peroxide: Its protection by ascorbate. Plant Cell Physiol. 1984, 25, 1285–1295. [Google Scholar] [CrossRef]
- Gillham, D.J.; Dodge, A.D. Hydrogen-peroxide-scavenging systems within pea chloroplasts—A quantitative study. Planta 1986, 167, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Arrigoni, O.; de Gara, L.; Tommasi, F.; Liso, R. Changes in the ascorbate system during seed development of Vicia faba L. Plant Physiol. 1992, 99, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ernst, O.; Zor, T. Linearization of the Bradford protein assay. J. Vis. Exp. 2010, 38, 1918. [Google Scholar] [CrossRef]
- R CoreTeam R: A Language and Environment for Statistical Computing. Available online: http://www.r-project.org/ (accessed on 2 August 2022).
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 2.1.3. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 31 August 2022).
- Lê, S.; Josse, J.; Husson, F. {FactoMineR}: A Package for Multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Wickham, H. Reshaping data with the reshape package. J. Stat. Softw. 2007, 21, 1–20. [Google Scholar] [CrossRef]
- Revelle, W. Psych: Procedures for Psychological, Psychometric, and Personality Research. R Package Version 2.1.3. Available online: https://CRAN.R-project.org/package=psych (accessed on 31 August 2022).



| Treatment | Vcmax * (µmol CO2 m−2s−1) | Jmax (µmol e− m−2s−1) | Rd (µmol CO2 m−2s−1) | gs900 (mol H2O m−2s−1) | E900 (mmol H2O m−2s−1) | Ci900 (ppm CO2) |
|---|---|---|---|---|---|---|
| Control | 56.85 ± 9.14b | 128.95 ± 16.61b | 2.22 ± 0.49a | 0.35 ± 0.18a | 5.31 ± 20a | 709.75 ± 61.95a |
| LWD | 78.73 ± 9.82a | 181.78 ± 27.73a | 1.3 ± 0.27b | 0.58 ± 0.13a | 7.35 ± 0.76a | 714.38 ± 23.94a |
| TWD | 81.66 ± 8.22a | 176.55 ± 0.62a | 1.24 ± 0.15b | 0.6 ± 0.06a | 7.35 ± 0.45a | 747.63± 16.38a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bendou, O.; Gutiérrez-Fernández, I.; Marcos-Barbero, E.L.; Bueno-Ramos, N.; Miranda-Apodaca, J.; González-Hernández, A.I.; Morcuende, R.; Arellano, J.B. Physiological and Antioxidant Response to Different Water Deficit Regimes of Flag Leaves and Ears of Wheat Grown under Combined Elevated CO2 and High Temperature. Plants 2022, 11, 2384. https://doi.org/10.3390/plants11182384
Bendou O, Gutiérrez-Fernández I, Marcos-Barbero EL, Bueno-Ramos N, Miranda-Apodaca J, González-Hernández AI, Morcuende R, Arellano JB. Physiological and Antioxidant Response to Different Water Deficit Regimes of Flag Leaves and Ears of Wheat Grown under Combined Elevated CO2 and High Temperature. Plants. 2022; 11(18):2384. https://doi.org/10.3390/plants11182384
Chicago/Turabian StyleBendou, Ouardia, Ismael Gutiérrez-Fernández, Emilio L. Marcos-Barbero, Nara Bueno-Ramos, Jon Miranda-Apodaca, Ana I. González-Hernández, Rosa Morcuende, and Juan B. Arellano. 2022. "Physiological and Antioxidant Response to Different Water Deficit Regimes of Flag Leaves and Ears of Wheat Grown under Combined Elevated CO2 and High Temperature" Plants 11, no. 18: 2384. https://doi.org/10.3390/plants11182384









