NADES Compounds Identified in Hypoxis hemerocallidea Corms during Dormancy
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material and Leachate Preparation
4.2. Reagents
4.3. Leachate Analysis and Compound Identification
4.4. Compound Quantification
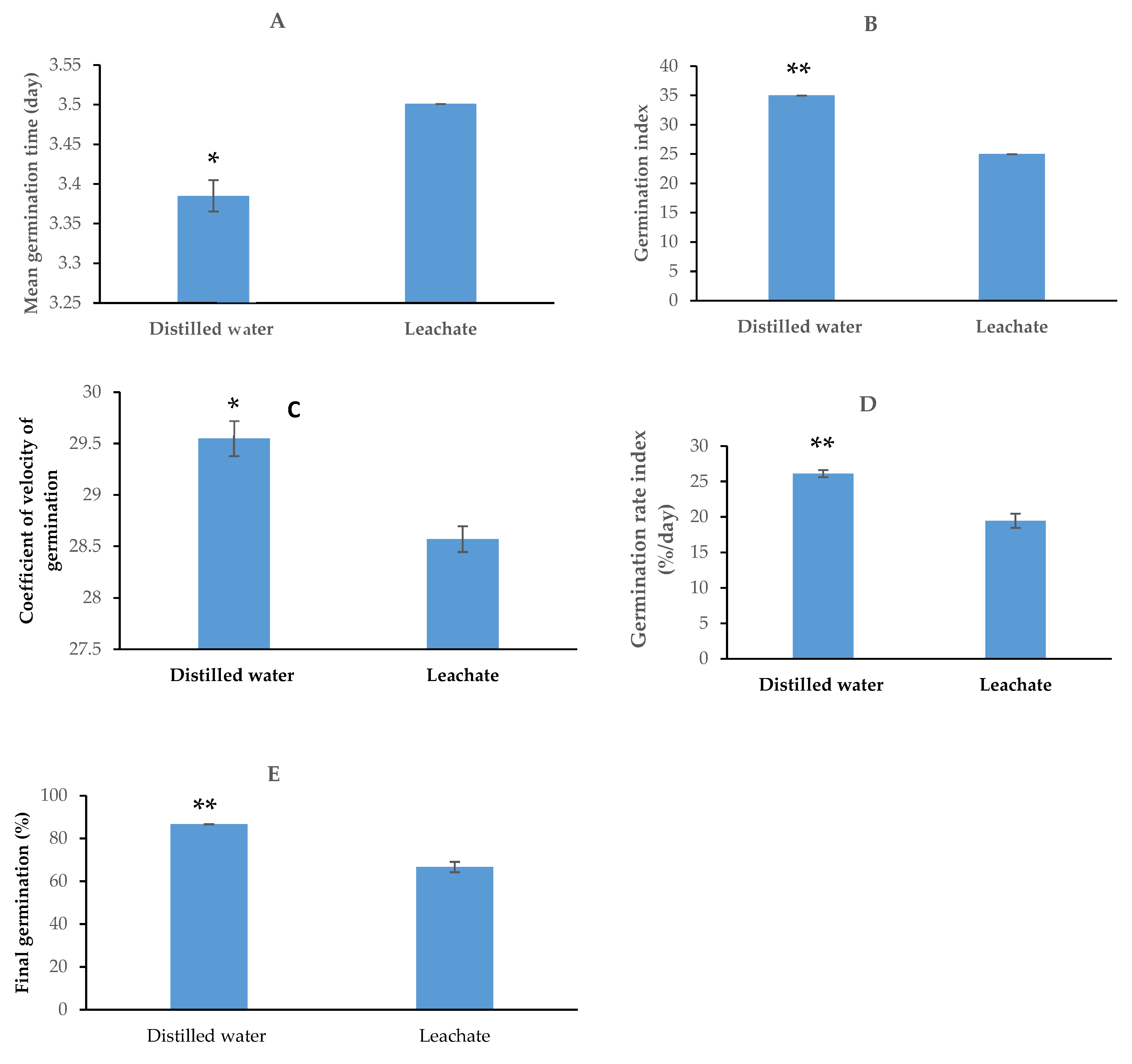
4.5. Effect of Hypoxis Hemerocallidea Leachate on Tomato Seed Germination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaspar, T.; Kevers, C.; Penel, C.; Greppin, H.; Reid, D.M.; Thorpe, T.A. Plant hormones and growth regulators in plant tissues culture. Vitro Cell Dev. Biol. Plant 1996, 32, 272–289. [Google Scholar] [CrossRef]
- Gillespie, L.M.; Volaire, F.A. Are winter and summer dormancy symmetrical seasonal adaptive strategies? The case of temperate herbaceous perennials. Ann. Bot. 2017, 119, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Salvi, N.D.; George, L.; Eapen, S. Micropropagation and field evaluation of micropropagated plants of turmeric. Plant Cell Tissue Organ Cult. 2002, 68, 143–151. [Google Scholar] [CrossRef]
- Shane, M.W.; McCully, M.E.; Canny, M.J.; Pate, J.S.; Ngo, H.; Mathesius, U.; Cawthray, G.R.; Lambers, H. Summer dormancy and winter growth: Root survival strategy in a perennial monocotyledon. New Phytol. 2009, 183, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Murzello, C.; Sun, Y.; Kim, M.-S.; Xie, X.; Jeter, R.M.; Zak, J.C.; Dowd, S.E.; Paré, P.W. Choline and osmotic-stress tolerance induced in Arabidopsis by the soil microbe Bacillus subtilis (GB03). Mol. Plant Microbe Interact. 2010, 23, 1097–1104. [Google Scholar] [CrossRef]
- Tretyn, A.; Kendrick, R.E. Acetylcholine in plants: Presence, metabolism and mechanical action. Bot. Rev. 1991, 57, 33–73. [Google Scholar] [CrossRef]
- Mohammed, A.R.; Tarpley, L. Effects of high night temperature on crop physiology and productivity: Plant growth regulators provide a management option. In Global Warming Impacts—Case Studies on the Economy, Human Health, and on Urban and Natural Environments; Casalegno, S., Ed.; InTech Open Limited: Rijeka, Slovakia, 2011; pp. 153–172. [Google Scholar]
- Karimi, M.; Ahmadi, A.; Hashemi, J.; Abbasi, A.; Tavarini, S.; Pompeiano, A.; Guglielminetti, L.; Angelini, L.G. Plant growth retardants (PGRs) affect growth and secondary metabolite biosynthesis in Stevia rebaudiana Bertoni under drought stress. S. Afr. J. Bot. 2019, 121, 394–401. [Google Scholar] [CrossRef]
- Dastogeer, K.M.G.; Li, H.; Sivasithamparam, K.; Jones, M.G.K.; Du, X.; Ren, Y.; Wylie, S.J. Metabolomic responses of endophytic Nicotiana benthamiana plants experiencing water stress. Environ. Exp. Bot. 2017, 143, 59–71. [Google Scholar] [CrossRef]
- Choi, Y.H.; Van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.-J.; Verpoorte, R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef]
- Liu, Y.; Garzon, J.; Friesen, J.B.; Zhang, Y.; McAlpine, J.B.; Lankin, D.C.; Chen, S.N.; Pauli, G.F. Counter current assisted quantitative recovery of metabolites from plant-associated natural deep eutectic solvents. Fitoterapia 2016, 112, 30–37. [Google Scholar] [CrossRef]
- Możdżeń, K.; Barabasz-Krasny, B.; Stachurska-Swakoń, A.; Zandi, P.; Puła, J. Effect of aqueous extracts of perppermint (Mentha × piperita L.) on the germination and the growth of selected vegetable and cereal seeds. Not. Bot. Horti. Agrobot. Cluj-Napoca 2019, 47, 412–417. [Google Scholar] [CrossRef]
- Palacios, S.M.; Del Corral, S.; Carpinella, M.C.; Ruiz, G. Screening for natural inhibitors of germination and seedling growth in native plants from Central Argentina. Ind. Crops Prod. 2010, 32, 674–677. [Google Scholar] [CrossRef]
- Van Wyk, B.E. A broad review of commercially important southern African medicinal plants. J. Ethnopharmacol. 2008, 119, 342–355. [Google Scholar] [CrossRef]
- Mofokeng, M.M.; Araya, H.T.; Amoo, S.O.; du Plooy, C.P.; Mashela, P.W. Hypoxis hemerocallidea cormlet production in response to corm cutting and exogenous application of plant growth regulators. Hortic. Environ. Biotechnol. 2020, 61, 939–948. [Google Scholar] [CrossRef]
- Goboza, M.; Aboua, Y.G.; Meyer, S.; Oguntibeju, O.O. Diabetes mellitus: Economic and health burden, treatment and the therapeutical effects of Hypoxis hemerrocallidea plant. Med. Technol. SA 2016, 40, 39–46. [Google Scholar]
- Fasinu, P.S.; Gutmann, H.; Schiller, H.; Bouic, P.; Rosenkranz, B. The potential of Hypoxis hemerocallidea for herb-drug interaction. Pharm Biol. 2013, 51, 1499–1507. [Google Scholar] [CrossRef]
- Mills, E.; Cooper, C.; Seely, D.; Kanfer, I. African herbal medicines in the treatment of HIV: Hypoxis and Sutherlandia. An overview of evidence and pharmacology. Nutr. J. 2005, 4, 19–24. [Google Scholar] [CrossRef]
- Mogatle, S.; Skinner, M.; Mills, E.; Kanfer., I. Effect of African potato (Hypoxis hemerocallidea) on the pharmacokinetics of efavirenz. S. Afr. Med. J. 2008, 98, 945–949. [Google Scholar]
- Ojewole, J.A.O.; Olayiwola, G.; Nyinawumuntu, A. Bronchorelaxant property of ‘African potato’ (Hypoxis hemerocallidea corm) aqueous extract in vitro. J. Smooth Muscle Res. 2009, 45, 241–248. [Google Scholar] [CrossRef][Green Version]
- Boukes, G.J.; Van de Venter, M.; Oosthuizen, V. Quantitative and qualitative analysis of sterols/sterolins and hypoxoside contents of three Hypoxis (African potato) spp. Afr. J. Biotechnol. 2008, 7, 1624–1629. [Google Scholar]
- Koduru, S.; Grierson, D.S.; Afolayan, A.J. Ethnobotanical information of medicinal plants used for treatment of cancer in the Eastern Cape Province, South Africa. Curr. Sci. 2007, 92, 906–908. [Google Scholar]
- De Wet, H.; Nzama, V.N.; Van Vuuren, S.F. Medicinal plants used for the treatment of sexually transmitted infections by lay people in northern Maputaland, KwaZulu–Natal Province, South Africa. S. Afr. J. Bot. 2012, 78, 12–20. [Google Scholar] [CrossRef]
- Semenya, S.S.; Potgieter, M.J.; Erasmus, L.J.C. Indigenous plant species used by Bapedi healers to treat sexually transmitted infections: Their distribution, harvesting, conservation and threats. S. Afr. J. Bot. 2013, 87, 66–75. [Google Scholar] [CrossRef]
- Aksenova, N.P.; Sergeeva, L.I.; Konstantinova, T.N.; Golyanovskaya, S.A.; Kolachevskaya, O.O.; Romanov, G.A. Regulation of potato tuber dormancy and sprouting. Russ. J. Plant Physiol. 2013, 60, 301–312. [Google Scholar] [CrossRef]
- Mofokeng, M.M.; Kleynhans, R.; Sediane, L.M.; Morey, L.; Araya, H.T. Propagation of Hypoxis hemerocallidea by inducing corm buds. S. Afr. J. Plant Soil. 2018, 35, 359–365. [Google Scholar] [CrossRef]
- Klein, M.S.; Almstetter, M.F.; Schlamberger, G.; Nürnberger, N.; Dettmer, K.; Oefner, P.J.; Meyer, H.H.D.; Wiedemann, S.; Gronwald, W. Nuclear magnetic resonance and mass spectrometry-based milk metabolomics in dairy cows during early and late lactation. J. Dairy Sci. 2010, 93, 1539–1550. [Google Scholar] [CrossRef]
- Ramachandran, G.K.; Yeow, C.H. Proton NMR characterization of intact primary and metastatic melanoma cells in 2D and 3D cultures. Biol. Res. 2017, 50, 12–22. [Google Scholar] [CrossRef]
- Govindaraju, V.; Young, K.; Maudsley, A.A. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000, 13, 129–153. [Google Scholar] [CrossRef]
- Amini, S.; Ziaratnia, S.M. Effect of plant growth regulators on control of saffron (Crocus sativus L.) corm dormancy. J. Hortic. Postharvest Res. 2019, 2, 167–176. [Google Scholar]
- Jones, R.L.; Phillips, I.D.J. Effect of CCC on the gibberellin content of excised sunflower organs. Planta 1967, 72, 53–59. [Google Scholar] [CrossRef]
- Dennis, D.T.; Upper, C.D.; West, C.A. An enzymatic site of inhibition of gibberellin biosynthesis by Amo 1618 and other plant growth retardants. Plant Physiol. 1965, 40, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Ecklund, P.R.; Moore, T.C. Correlations of growth rate and de-etiolation with rate of ent-kaurene biosynthesis in pea (Pisum sativum L.). Plant Physiol. 1974, 53, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Mehraj, H.; Alam, M.M.; Habiba, S.U.; Mehbub, H. LEDs combined with CHO cources and CCC priming PLB regeration of Phalaenopsis. Horticulturae 2019, 5, 34. [Google Scholar] [CrossRef]
- Jaffe, M.J. Evidence for the regulation of phytochrome-mediated processes in bean roots by the neurohumor, acetylcholine. Plant Physiol. 1970, 46, 768–777. [Google Scholar] [CrossRef]
- Sustr, M.; Soukup, A.; Tylova, E. Potassium in root growth and development. Plants 2019, 8, 435. [Google Scholar] [CrossRef]
- Powar, D.B.; Ambad, S.N.; Katwate, S.M. Effect of different chemicals on breaking dormancy in gladiolus (Gladiolus tristis L.). Indo-Am. J. Agric. Vet. Sci. 2015, 3, 12–17. [Google Scholar]
- Bamel, K.; Gupta, R. Acetylcholine as a regulator of differentiation and development in tomato. In Neurotransmitters in Plants. Perspective and Applications; Ramakrishna, A., Roshchina, V.V., Eds.; CRC Press: London, UK, 2019. [Google Scholar]
- Lindlöf, A. Interplay between low-temperature pathways and light reduction. Plant Signal. Behav. 2010, 5, 820–825. [Google Scholar] [CrossRef]
- Ruttink, T.; Arend, M.; Morreel, K.; Storme, V.; Rombauts, S.; Fromm, J.; Bhalerao, R.P.; Boerjan, W.; Rohde, A. A molecular timetable for apical bud formation and dormancy induction in poplar. Plant Cell 2007, 19, 2370–2390. [Google Scholar] [CrossRef]
- Shim, D.; Ko, J.-H.; Kim, W.-C.; Wang, Q.; Keathley, D.E.; Han, K.H. A molecular framework for seasonal growth-dormancy regulation in perennial plants. Hortic. Res. 2014, 1, 14059. [Google Scholar] [CrossRef]
- Huang, S.; Braun, H.-P.; Gawryluk, R.M.R.; Millar, H. Mitochondrial complex II of plants: Subunit composition, assembly and function in respiration and signalling. Plant J. 2019, 98, 405–417. [Google Scholar] [CrossRef]
- Murphy, M.P.; O’Neill, L.A.J. Krebs cycle reimagined: The emerging roles of succinate and itaconate as signal transducers. Cell 2018, 174, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Soppe, W.J.J.; Bentsink, L. Dormancy in plants. In eLS.; John Wiley and Sons, LTD.: Chichester, UK, 2016. [Google Scholar] [CrossRef]
- Gai, S.; Zhang, Y.; Liu, C.; Zhang, Y.; Zheng, G. Transcript profiling of Paoenia ostia during artificial chilling induced dormancy release identifies activation of GA pathway and carbohydrate metabolism. PLoS ONE 2013, 8, e55297. [Google Scholar] [CrossRef] [PubMed]
- Novelli, S.; Gismondi, A.; Di Marco, G.; Canuti, L.; Nanni, V.; Canini, A. Plant defense factors involved in Olea europaea resistance against Xylella fastidiosa infection. J. Plant Res. 2019, 132, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Airaki, M.; Leterrier, M.; Mateos, R.M.; Valderrama, R.; Chaki, M.; Barroso, J.B.; Del Río, L.A.; Palma, J.M.; Corpas, F.J. Metabolism of reactive oxygen species and reactive nitrogen species in pepper (Capsicum annuum L.) plants under low temperature stress. Plant Cell Environ. 2012, 35, 281–295. [Google Scholar] [CrossRef] [PubMed]
- De Rossi, S.; Di Marco, G.; Bruno, L.; Gismondi, A.; Canini, A. Investigating the Drought and Salinity Effect on the Redox Components of Sulla coronaria (L.) Medik. Antioxidants 2021, 10, 1048. [Google Scholar] [CrossRef]
- Solórzano, E.; Corpas, F.J.; González-Gordo, S.; Palma, J.M. Reactive Oxygen Species (ROS) Metabolism and Nitric Oxide (NO) Content in Roots and Shoots of Rice (Oryza sativa L.) Plants under Arsenic-Induced Stress. Agronomy 2020, 10, 1014. [Google Scholar] [CrossRef]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Saifullah, F.M.; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring control: The evolution of ROS-Induced oxidative stress and redox signalling pathways in plant stress responses. Plant Physiol Biochem. 2019, 141, 353–369. [Google Scholar] [CrossRef]
- Akoumianakis, K.A.; Alexopoulos, A.A.; Karapanos, I.C.; Kalatzopoulos, K.; Aivalakis, G.; Passam, H.C. Carbohydrate metabolism and tissue differentiation during tuber initiation, growth and dormancy induction. Aust. J. Crop Sci. 2016, 10, 185–192. [Google Scholar]
- Viola, R.; Pelloux, J.; Van der Ploeg, A.; Gillespie, T.; Marquis, N.; Roberts, A.G.; Hancock, R.D. Symplastic connection is required for bud outgrowth following dormancy in potato (Solanum tuberosum L.) tubers. Plant Cell Environ. 2007, 30, 973–983. [Google Scholar] [CrossRef]
- Kondrashova, M.N.; Zakharchenko, M.V.; Khunderyakova, N.I.; Fedotcheva, N.I.; Litvinova, E.G.; Romanova, O.I.; Gulayev, A.A. States of succinate dehydrogenase in the organism: Dormant vs. hyperactive (pushed out of equilibrium). Biophysics 2013, 58, 86–94. [Google Scholar] [CrossRef]
- Popov, V.N.; Eprintsev, A.T.; Fedorin, D.N.; Igamberdiev, A.U. Succinate dehydrogenase in Arabidopsis thaliana is regulated by light via phytochrome A. FEBS Lett. 2007, 584, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Toba, T.; Nagashima, S.; Adachi, S. Is lactose really present in plants? J. Sci. Food Agric. 1991, 54, 305–308. [Google Scholar] [CrossRef]
- Krasavina, M.S.; Burmistrova, N.A.; Raldugina, G.N. The role of carbohydrates in plant resistance to abiotic stresses. In Emerging Technologies and Management of Crop Stress Tolerance; Ahmad, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 1. [Google Scholar]
- Saqib, S.; Akram, A.; Halim, S.A.; Tassaduq, R. Source of ß-galactosidase and its applications in food industry. 3 Biotech 2017, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.E.K.; Eriksson, M.E.; Junttila, O. The dynamic nature of bud dormancy in trees: Environmental control and molecular mechanisms. Plant Cell Environ. 2012, 35, 1707–1728. [Google Scholar] [CrossRef]
- Morandini, P. Control limits for accumulation of plant metabolites: Brute force is no substitute for understanding. Plant Biotechnol. J. 2013, 11, 253–267. [Google Scholar] [CrossRef]
- Juers, D.H.; Matthews, B.W.; Huber, R.E. LacZ β-galactosidase: Structure and function of an enzyme of historical and molecular biological importance. Protein Sci. 2012, 21, 1792–1807. [Google Scholar] [CrossRef]
- Ingram, J.; Bartels, D. The molecular basis of dehydration tolerance in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 377–403. [Google Scholar] [CrossRef]
- Sayad, T.; Poturcu, K.; Moradi, M.; Rahimpour, E.; Zhao, H.; Jouyban, A. Solubility study of carvedilol in the aqueous mixtures of a choline chloride/propylene glycol deep eutectic solvent. J. Mol. Liq. 2021, 342, 117537. [Google Scholar] [CrossRef]
- Shang, X.; Duo, Y.; Zhang, Y.; Tan, J.-N.; Liu, X.; Zhang, Z. Tailor-made natural deep eutectic solvents for green extraction of isoflavones from chickpea (Cicer arietinum L.) sprouts. Ind. Crops Prod. 2019, 140, 111724. [Google Scholar] [CrossRef]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety assessment of propylene glycol, tripropylene glycol, and PPGs as used in cosmetics. Int. J. Toxicol. 2012, 31, 245S–260S. [Google Scholar] [CrossRef]
- Harvanko, A.; Kryscio, R.; Martin, C.; Kelly, T. Stimulus effects of propylene glycol and vegetable glycerine in electronic cigarette liquids. Drug Alcohol Depend. 2019, 194, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.A.; Ku, K.; Sue, G.R. Propylene glycol poisoning from excess whiskey ingestion: A case of high osmolal gap metabolic acidosis. J. Investig. Med. High Impact Case Rep. 2015, 3, 2324709615603722. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.C.; Reardon, C.; Farber, H.W. Propylene glycol toxicity in a patient receiving intravenous diazepam. N. Engl. J. Med. 2000, 343, 815. [Google Scholar] [CrossRef]
- Lau, K.; Swiney, B.S.; Reeves, N.; Noguchi, K.K.; Farber, N.B. Propylene glycol produces excessive apoptosis in the developing mouse brain, alone and in combination with phenobarbital. Pediatr. Res. 2012, 71, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Office of Technology Transitions. Kicking the Oil Habit: Making Propylene Glycol from Plants. 2022. Available online: www.energy.gov (accessed on 29 April 2022).
- Mailloux, R.J. Still at the centre of it all; novel functions of the oxidative krebs cycle. Bioenergetics 2015, 4, 122. [Google Scholar] [CrossRef]
- Bozzo, T.T.; Mpambani, B.; Mbenga, A.; Mhlontlo, S. Comparative studies on yield and quality response of soil and soilless grown tomatoes: The case study of Masiphathisane community project and Bathurst experimental farm. S. Afr. J. Agric. Ext. 2019, 47, 75–85. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.T.; Miller, J.H.; Day, M.M.; Munger, J.C.; Brookes, P.S. Accumulation of succinate in cardiac ischemia primarily occurs via canonical krebs cycle activity. Cell Rep. 2018, 23, 2617–2628. [Google Scholar] [CrossRef]
- Musabayane, C.T.; Xozwa, K.; Ojewole, J.A.O. Effects of Hypoxis hemerocallidea (Fisch. & C.A. Mey.) [Hypoxidaceae] corm (African potato) aqueous extract on renal electrolyte and fluid handling in the rat. Ren. Fail. 2005, 27, 763–770. [Google Scholar]
- Matthews, S.B.; Waud, J.P.; Roberts, A.G.; Campbell, A.K. Systematic lactose intolerance: A new perspective on an old problem. Postgrad. Med. J. 2005, 81, 167–173. [Google Scholar] [CrossRef]
- Blusztajn, J.K.; Slack, E.B.; Mellott, T.J. Neuroprotective actions of dietary choline. Nutrients 2017, 9, 815. [Google Scholar] [CrossRef]
- Mehta, A.K.; Gaur, S.N.; Arora, N.; Singh, B.P. Effect of choline chloride in allergen-induced mouse model of airway inflammation. Eur. Respir. J. 2007, 30, 662–671. [Google Scholar] [CrossRef] [PubMed]
- HMDB (The Human Metabolome Database). The Human Metabolome Database. 2021. Available online: www.hmdb.ca (accessed on 3 September 2021).
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.M.; Ungar, I. Germination of the salt tolerant shrub Suaeda fruticosa from pakistan: Salinity and temperature responses. Seed Sci. Technol. 1998, 26, 657–667. [Google Scholar]
- Moyo, M.; Amoo, S.O.; Van Staden, J. Seed priming with smoke water and karrikin improves germination and seedling vigor of Brassica napus under varying environmental conditions. Plant Growth Regul. 2022, 97, 315–326. [Google Scholar] [CrossRef]
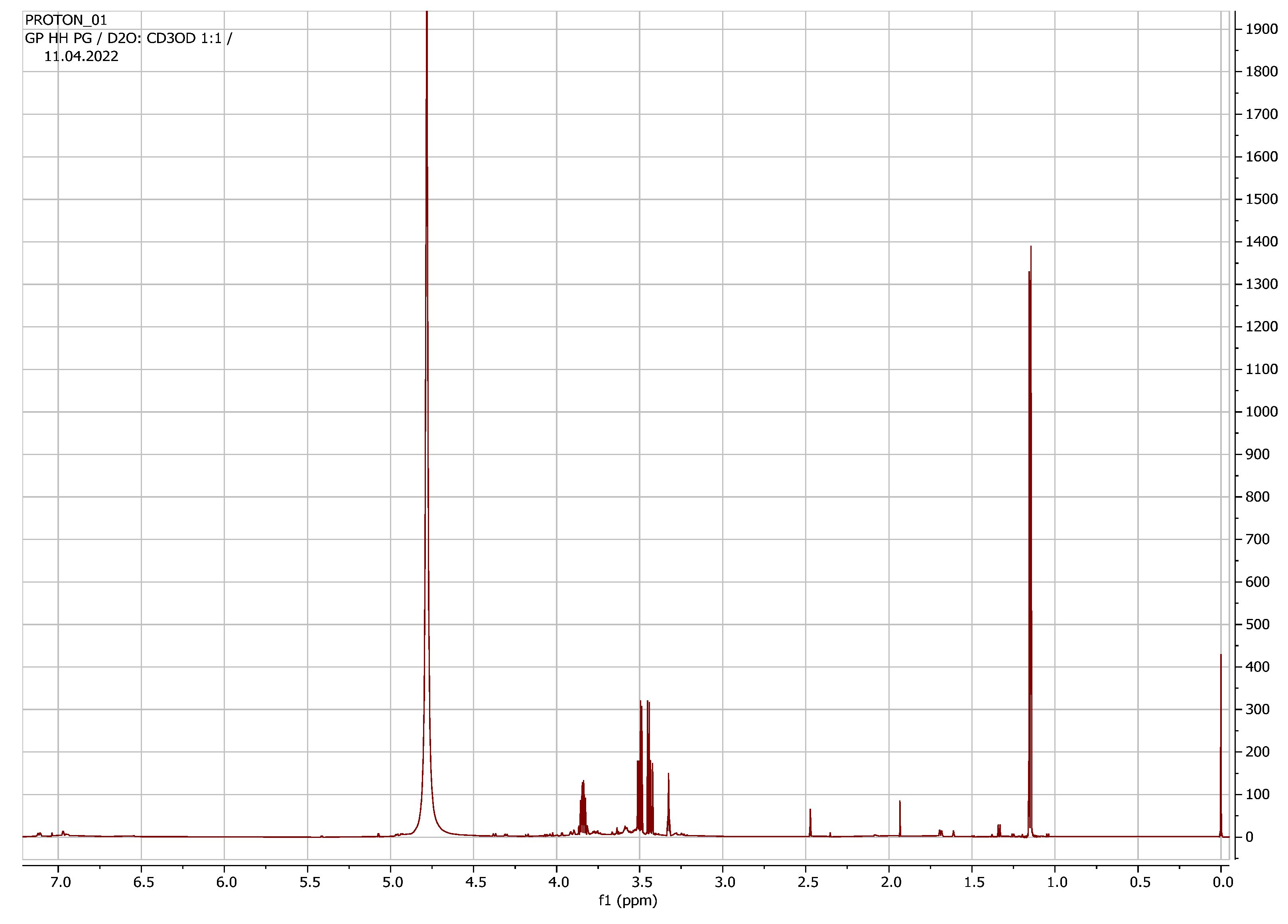
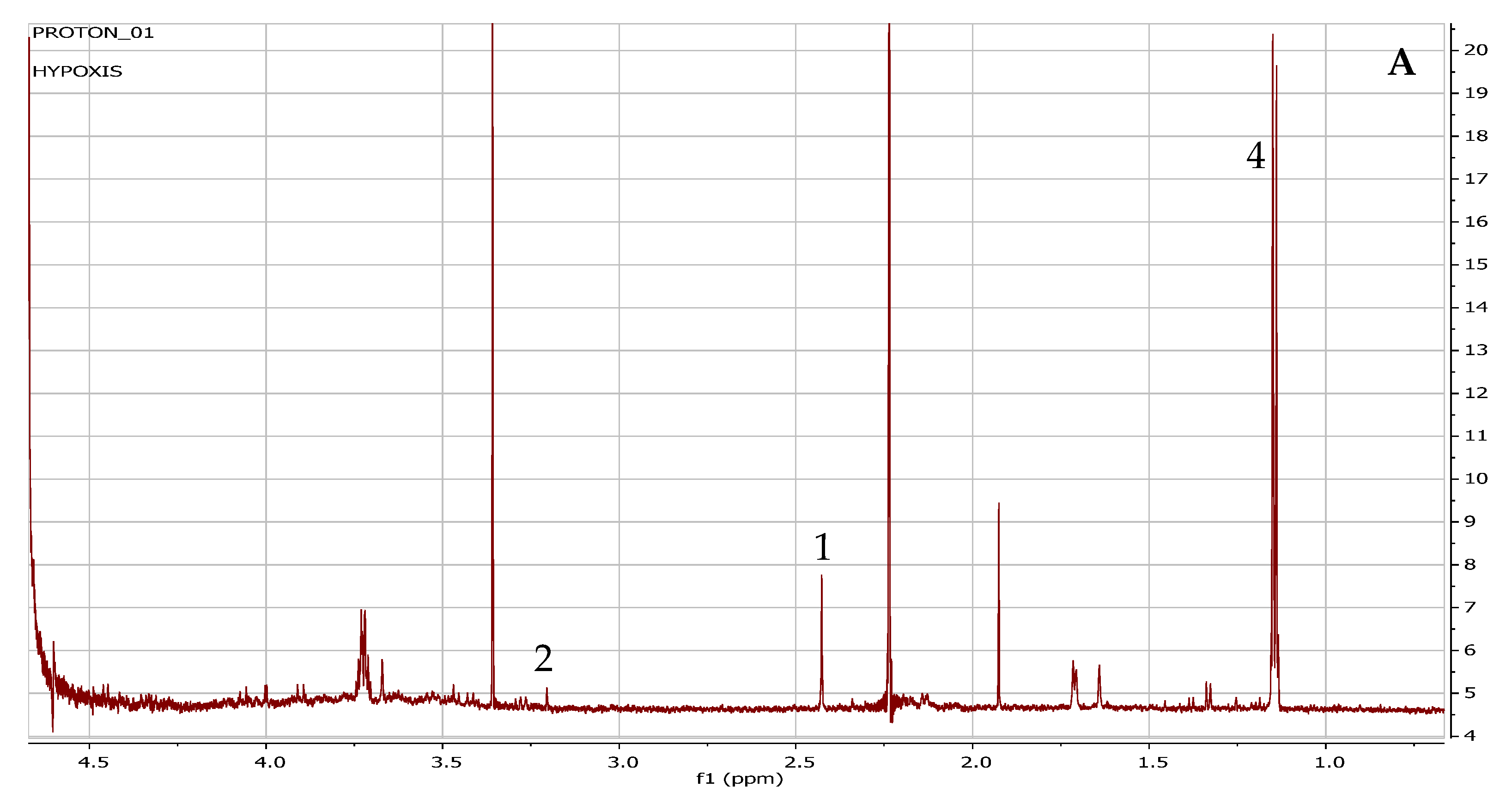


| Compounds | Chemical Group | 1H-NMR Chemical Shifts (ppm) | Reference Chemical Shifts (ppm) | Chenomx (ppm) | References | Human Metabolome Database | Assigned Number |
|---|---|---|---|---|---|---|---|
| Choline (C5H14NO) | Organic acid | 3.192 | 3.18 3.22 | 3.19 | [27] [28] | 3.18 3.50 4.05 | 1 |
| Succinate (C4H6O4) | Organic acid | 2.42 | 2.39 | 2.45 | [29] | 2.39 | 2 |
| Lactose (C12H22O11) | Sugar | 3.25 3.55 3.75 3.85 3.95 5.12 | 3.29 3.67 3.73 4.45 5.23 | 3.23 3.54 3.69 3.85 3.9 4.35 5.17 | [27] | 3.28 3.55 3.60 3.66 3.73 3.79 3.86 3.94 4.45 5.22 | 3 |
| Propylene glycol (C3H8O2) | Alcohol | 1.14 1.16 3.4 3.5 | 1.13 1.15 3.42 3.48 | 1.12 1.13 3.4 3.5 3.9 | 4 |
| Compound | Concentration (mM) | Signature Peak (ppm) |
|---|---|---|
| Propylene glycol | 0.0038 | 1.13, 1.14 (d) |
| Lactose | 0.26 | 3.21, 3.23, 3.25 (t) |
| Choline | 0.0045 | 3.19 (s) |
| Succinate | 0.04 | 2.42 (s) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mofokeng, M.M.; Prinsloo, G.; Araya, H.T.; Amoo, S.O.; du Plooy, C.P.; Mashela, P.W. NADES Compounds Identified in Hypoxis hemerocallidea Corms during Dormancy. Plants 2022, 11, 2387. https://doi.org/10.3390/plants11182387
Mofokeng MM, Prinsloo G, Araya HT, Amoo SO, du Plooy CP, Mashela PW. NADES Compounds Identified in Hypoxis hemerocallidea Corms during Dormancy. Plants. 2022; 11(18):2387. https://doi.org/10.3390/plants11182387
Chicago/Turabian StyleMofokeng, Motiki M., Gerhard Prinsloo, Hintsa T. Araya, Stephen O. Amoo, Christian P. du Plooy, and Phatu W. Mashela. 2022. "NADES Compounds Identified in Hypoxis hemerocallidea Corms during Dormancy" Plants 11, no. 18: 2387. https://doi.org/10.3390/plants11182387
APA StyleMofokeng, M. M., Prinsloo, G., Araya, H. T., Amoo, S. O., du Plooy, C. P., & Mashela, P. W. (2022). NADES Compounds Identified in Hypoxis hemerocallidea Corms during Dormancy. Plants, 11(18), 2387. https://doi.org/10.3390/plants11182387