Determining Reproductive Parameters, which Contribute to Variation in Yield of Olive Trees from Different Cultivars, Irrigation Regimes, Age and Location
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
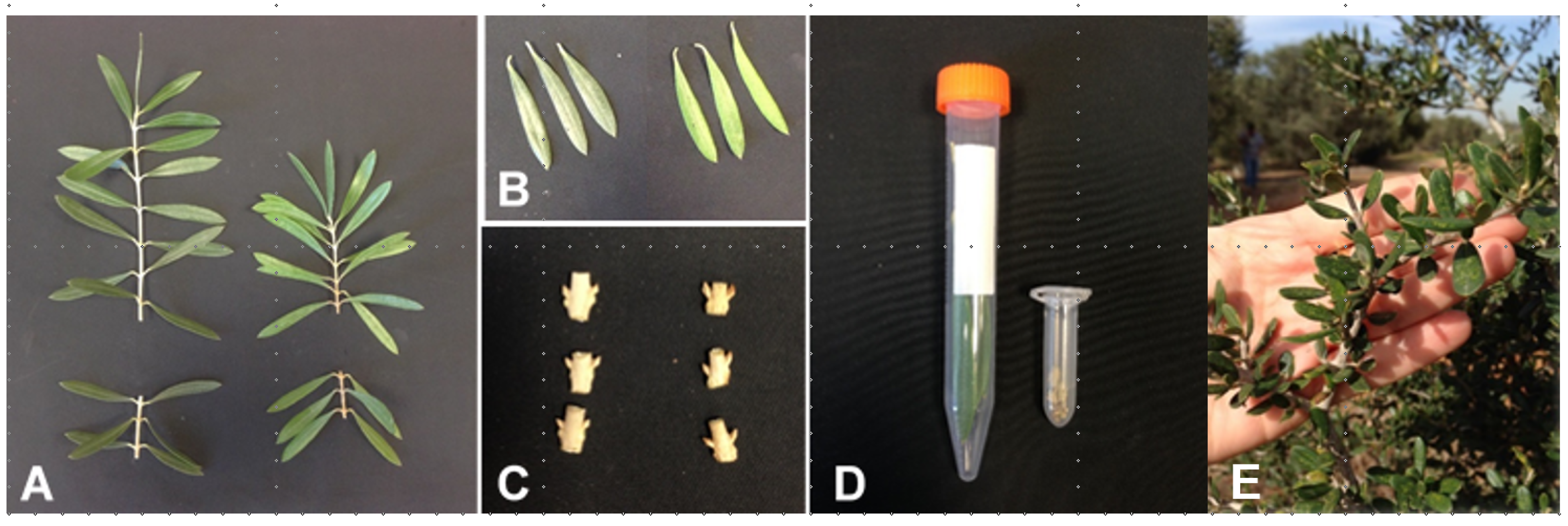
2.2. Trees and Branches Used for Measurements
2.3. RNA Sampling
2.4. RNA Production and cDNA Synthesis
2.5. Expression Analysis by Quantitative Real-Time PCR
2.6. Reproductive Parameters Collected on Selected Branches
2.7. Calculation of Alternate Bearing (AB) Index
2.8. Statistical Analysis
3. Results
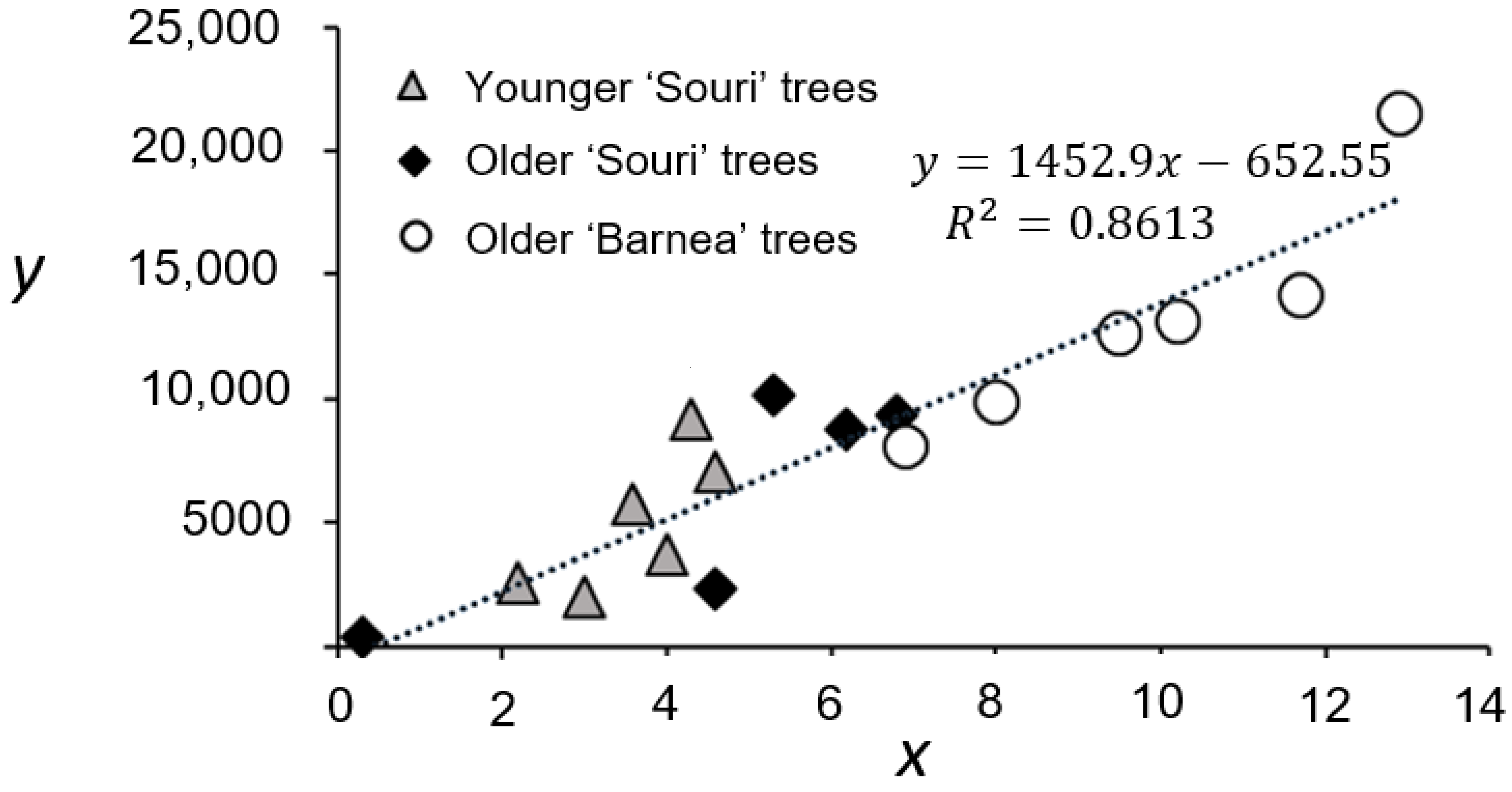
3.1. An Estimate of Total Fruit Yield Based on 25 Pre-Marked Branches
3.2. Older Trees do Not Necessarily have Lower Yields
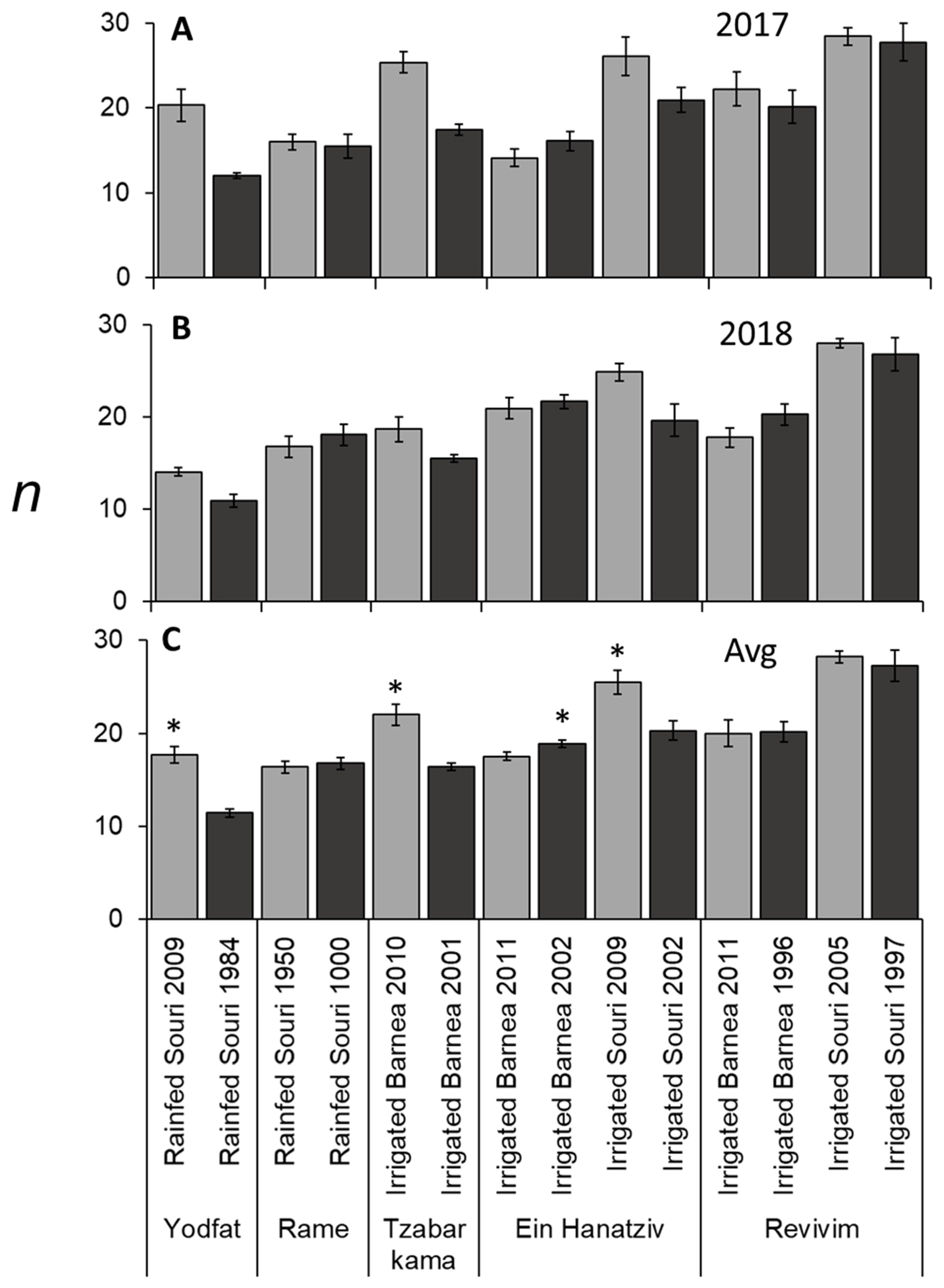
3.3. Rainfed Trees Form Fewer Buds per Branch (n) Compared to Irrigated Trees
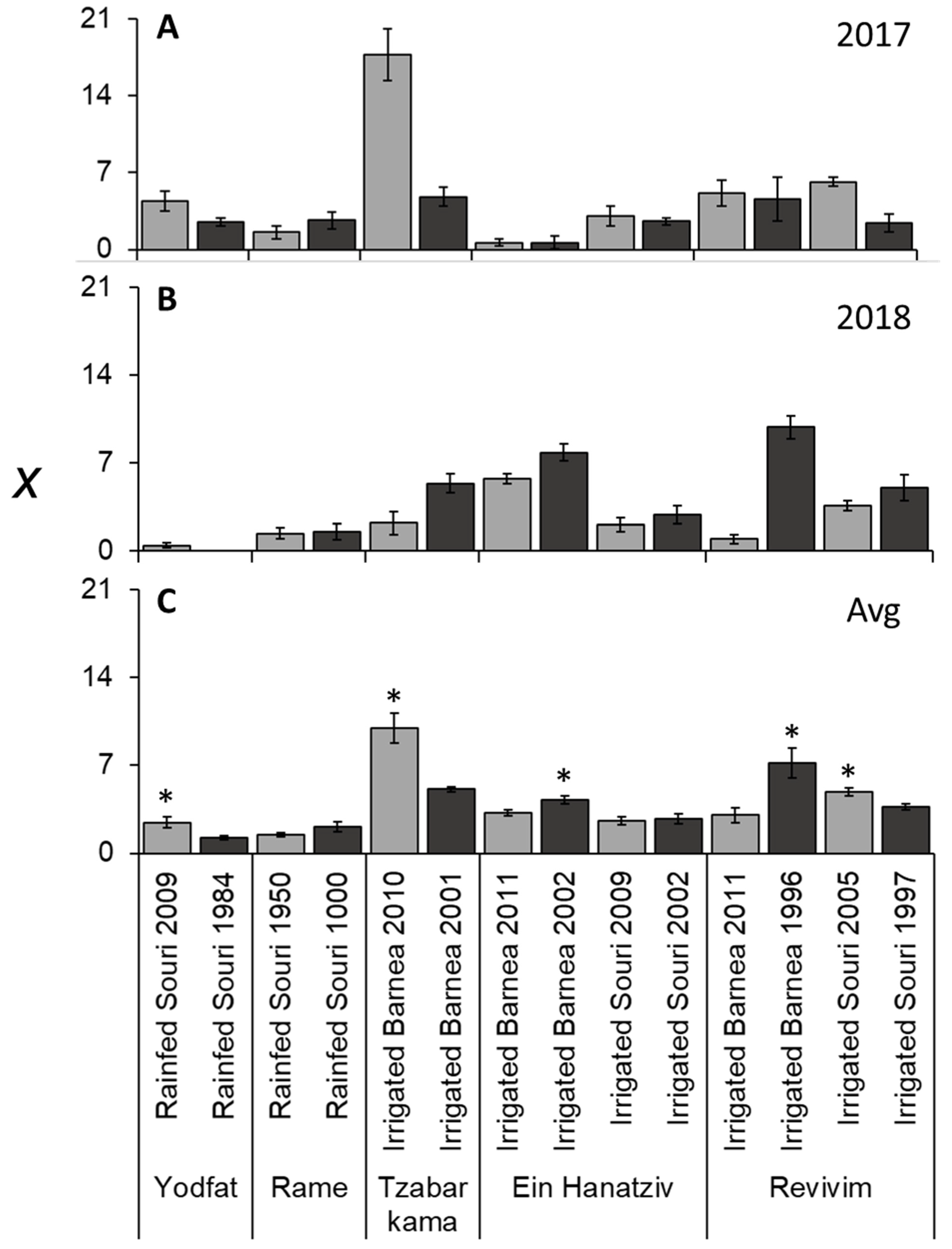
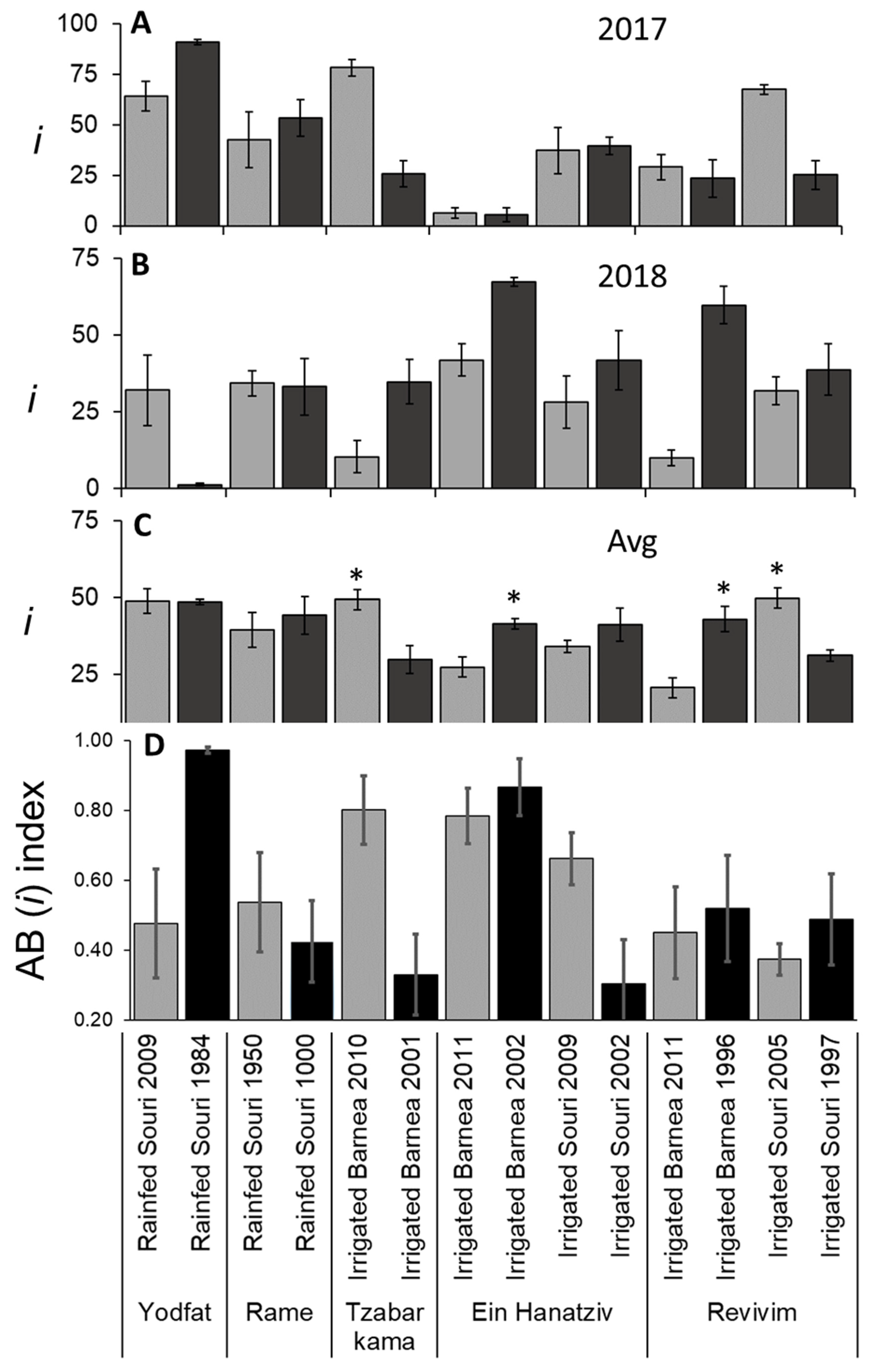
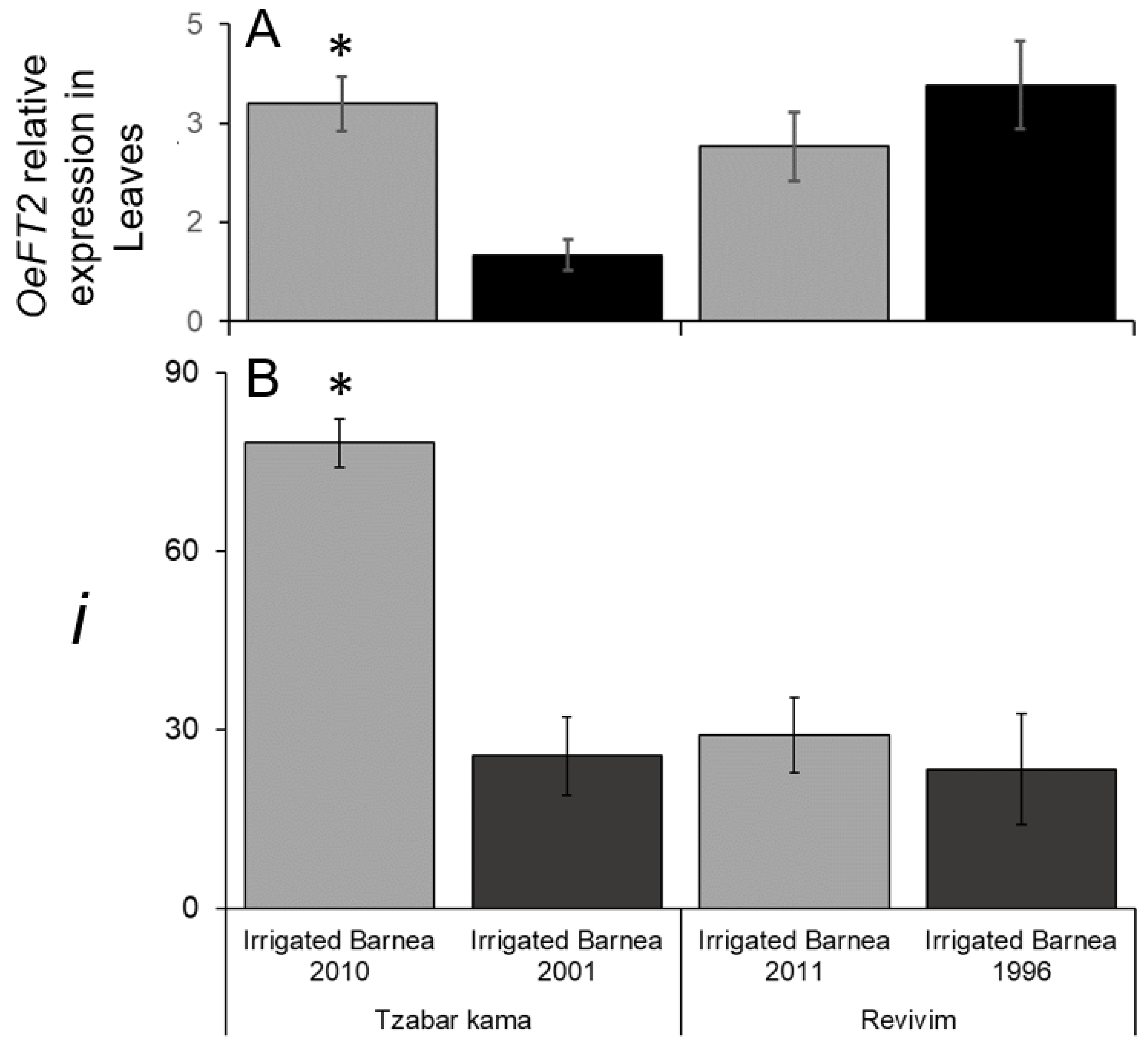
3.4. Variation in I due to Alternate Bearing and OeFT2 Expression in Leaves
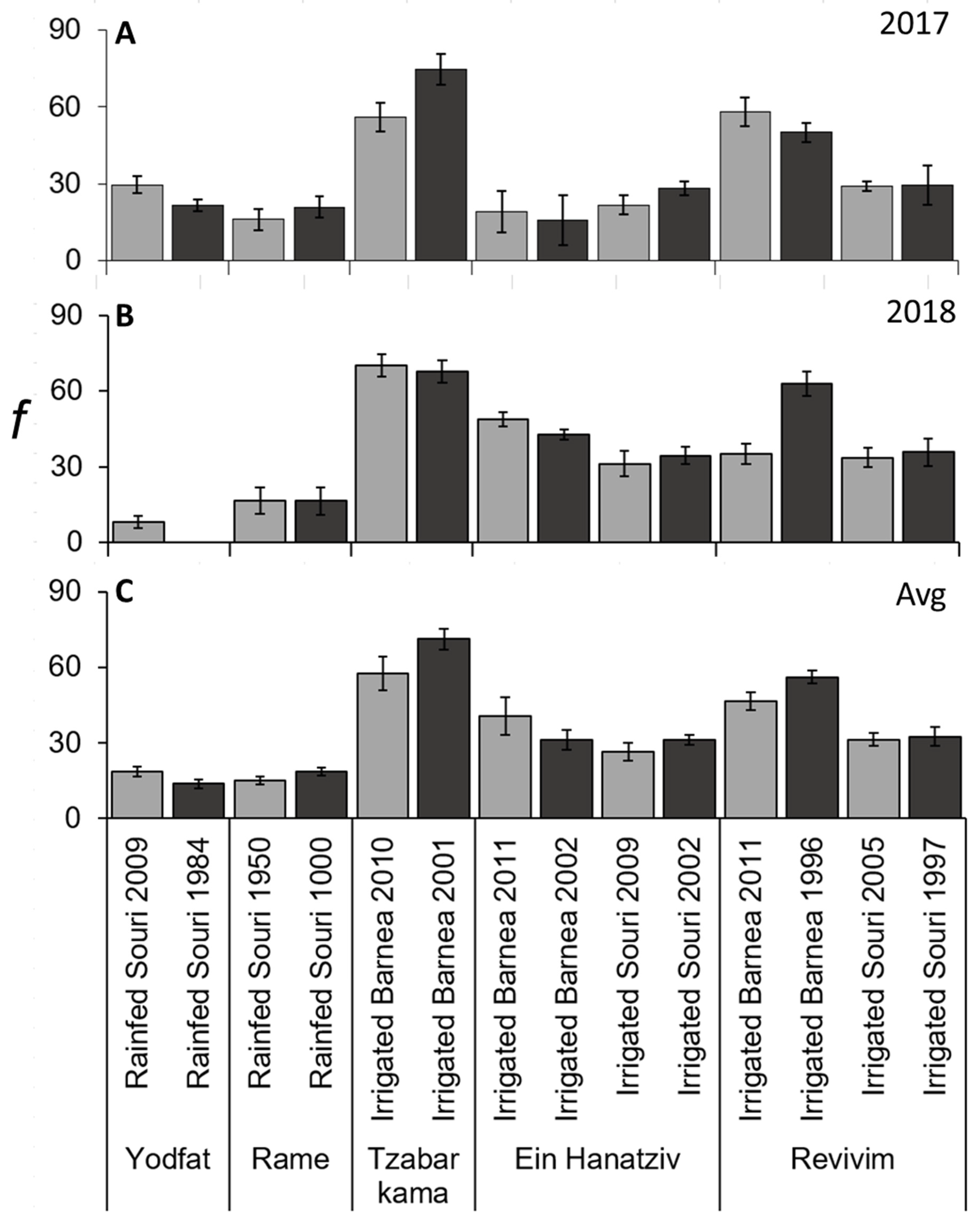
3.5. f and m Values Were Significantly Lower in ‘Souri’ Compared to ‘Barnea’
3.6. i and f Are the Main Contributors to Variation in x
3.7. Juvenile Olive Seedlings Do Not Flower, Likely due to Low Levels of OeFT2 and Higher Levels of Potential Flowering Inhibitors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kaniewski, D.; Van Campo, E.; Boiy, T.; Terral, J.-F.; Khadari, B.; Besnard, G. Primary domestication and early uses of the emblematic olive tree: Palaeobotanical, historical and molecular evidence from the Middle East. Biol. Rev. 2012, 87, 885–899. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Mora, J.J.; García-Vigara, A.; Sánchez-Sánchez, M.L.; García-Pérez, M.-Á.; Tarín, J.; Cano, A. The Mediterranean diet: A historical perspective on food for health. Maturitas 2020, 132, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Morze, J.; Hoffmann, G. Mediterranean diet and health status: Active ingredients and pharmacological mechanisms. Br. J. Pharmacol. 2020, 177, 1241–1257. [Google Scholar] [CrossRef]
- Langgut, D.; Cheddadi, R.; Carrión, J.S.; Cavanagh, M.; Colombaroli, D.; Eastwood, W.J.; Greenberg, R.; Litt, T.; Mercuri, A.M.; Miebach, A.; et al. The origin and spread of olive cultivation in the Mediterranean Basin: The fossil pollen evidence. Holocene 2019, 29, 902–922. [Google Scholar] [CrossRef]
- Arnan, X.; López, B.C.; Martínez-Vilalta, J.; Estorach, M.; Poyatos, R. The age of monumental olive trees (Olea europaea) in northeastern Spain. Dendrochronologia 2012, 30, 11–14. [Google Scholar] [CrossRef]
- Bernabei, M. The age of the olive trees in the Garden of Gethsemane. J. Archaeol. Sci. 2015, 53, 43–48. [Google Scholar] [CrossRef]
- Ehrlich, Y.; Regev, L.; Kerem, Z.; Boaretto, E. Radiocarbon dating of an olive tree cross-section: New insights on growth patterns and implications for age estimation of olive trees. Front. Plant Sci. 2017, 8, 1918. [Google Scholar] [CrossRef]
- Wareing, P.F. Problems of juvenility and flowering in trees. Bot. J. Linn. Soc. 1959, 56, 282–289. [Google Scholar] [CrossRef]
- Bellini, E. Behaviour of some genetic characters in olive seedlings obtained by cross-breeding. Acta Hortic. 1992, 317, 197–208. [Google Scholar] [CrossRef]
- Aukerman, M.J.; Sakai, H. Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 2003, 15, 2730–2741. [Google Scholar] [CrossRef]
- Chen, X. A microRNA as a translational repressor of APETALA2 in arabidopsis flower development. Science 2004, 303, 2022–2025. [Google Scholar] [CrossRef] [PubMed]
- Yant, L.; Mathieu, J.; Dinh, T.T.; Ott, F.; Lanz, C.; Wollmann, H.; Chen, X.; Schmid, M. Orchestration of the Floral Transition and Floral Development in Arabidopsis by the Bifunctional Transcription Factor APETALA2. Plant Cell 2010, 22, 2156–2170. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F.; Julca, I.; Gómez-Garrido, J.; Loska, D.; Marcet-Houben, M.; Cano, E.; Galán, B.; Frias, L.; Ribeca, P.; Derdak, S.; et al. Genome sequence of the olive tree, Olea europaea. GigaScience 2016, 5, 29. [Google Scholar] [CrossRef]
- Julca, I.; Marcet-Houben, M.; Cruz, F.; Gómez-Garrido, J.; Gaut, B.S.; Díez, C.M.; Gut, I.G.; Alioto, T.S.; Vargas, P.; Gabaldón, T. Genomic evidence for recurrent genetic admixture during the domestication of Mediterranean olive trees (Olea europaea L.). BMC Biol. 2020, 18, 148. [Google Scholar] [CrossRef] [PubMed]
- Donaire, L.; Pedrola, L.; de la Rosa, R.; Llave, C. High-throughput sequencing of RNA silencing-associated small RNAs in olive (Olea europaea L.). PLoS ONE 2011, 6, e27916. [Google Scholar] [CrossRef]
- García-López, M.C.; Vidoy, I.; Jiménez-Ruiz, J.; Muñoz-Mérida, A.; Fernández-Ocaña, A.; de la Rosa, R.; Barroso, J.B.; Navarro, F.; Trelles, O.; Beuzón, C.R.; et al. Genetic changes involved in the juvenile-to-adult transition in the shoot apex of Olea europaea L. occur years before the first flowering. Tree Genet. Genomes 2014, 10, 585–603. [Google Scholar] [CrossRef]
- Dag, A.; Bustan, A.; Avni, A.; Tzipori, I.; Lavee, S.; Riov, J. Timing of fruit removal affects concurrent vegetative growth and subsequent return bloom and yield in olive (Olea europaea L.). Sci. Hortic. 2010, 123, 469–472. [Google Scholar] [CrossRef]
- Lavee, S. Biology and physiology of the olive. In World Olive Encyclopaedia; International Olive Oil Council: Madrid, Spain, 1996; pp. 61–110. [Google Scholar]
- Haberman, A.; Bakhshian, O.; Cerezo-Medina, S.; Paltiel, J.; Adler, C.; Ben Ari, G.; Mercado, J.A.; Pliego-Alfaro, F.; Lavee, S.; Samach, A. A possible role for FT-encoding genes in interpreting environmental and internal cues affecting olive (Olea europaea L.) flower induction. Plant Cell Environ. 2017, 40, 1263–1280. [Google Scholar] [CrossRef]
- Samach, A.; Smith, H.M. Constraints to obtaining consistent annual yields in perennials II: Environment and fruit load affect flowering induction. Plant Sci. 2013, 207, 168–176. [Google Scholar] [CrossRef]
- Lavee, S. Alternate bearing in olive initiated by abiotic induction leading to biotic responses. Adv. Hortic. Sci. 2015, 29, 213–219. [Google Scholar]
- Saumitou-Laprade, P.; Vernet, P.; Vekemans, X.; Billiard, S.; Gallina, S.; Essalouh, L.; Mhaïs, A.; Moukhli, A.; El Bakkali, A.; Barcaccia, G.; et al. Elucidation of the genetic architecture of self-incompatibility in olive: Evolutionary consequences and perspectives for orchard management. Evol. Appl. 2017, 10, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, H.F.; Rallo, L. Fruit Set and Enlargement in Fertilized and Unfertilized Olive Ovaries. HortScience 1991, 26, 896–898. [Google Scholar] [CrossRef]
- Lavee, S. Biennial bearing in olive (Olea europea). Ann. Ser. Hist. Nat. 2007, 17, 101–112. [Google Scholar]
- Rallo, L.; Barranco, D.; Castro-García, S.; Connor, D.J.; Gómez del Campo, M.; Rallo, P. High-Density Olive Plantations. Hortic. Rev. 2013, 41, 303–384. [Google Scholar]
- Sibbett, G.S. (Ed.) Pruning Mature Bearing Olive Trees, 1st ed.; University of California, Agriculture and Natural Resources: Oakland, CA, USA, 1994; pp. 57–60. [Google Scholar]
- Ben-Rouina, B.; Omri, A.; Trigui, A. Effect of a Hard Pruning on Trees Vigor and Yields of Old Olive Orchards. Acta Hortic. 2002, 586, 321–323. [Google Scholar] [CrossRef]
- Metzidakis, I. Effect of regeneration pruning for the recovery of olive productivity and fruit characteristics in ten olive cultivars. Acta Hortic. 2002, 586, 333–336. [Google Scholar] [CrossRef]
- Dias, A.B.; Pinheiro, A.; Peca, J.O. Fifteen-year evaluation of the influence of mechanical pruning on olive yield. Acta Hortic. 2014, 1057, 335–340. [Google Scholar] [CrossRef]
- Fatal, V. Description and Analysis of the Olive Sector in Israel; The Knesset, Center for Research and Information: Jerusalem, Israel, 2014. (In Hebrew) [Google Scholar]
- Connor, D.J.; Gómez-del-Campo, M.; Rousseaux, M.C.; Searles, P.S. Structure, management and productivity of hedgerow olive orchards: A review. Sci. Hortic. 2014, 169, 71–93. [Google Scholar] [CrossRef]
- Samish, R.M.; Speigel, P. The use of irrigation in growing olives for oil production. Isr. J. Agric. Res. 1961, 11, 87–95. [Google Scholar]
- Lavee, S. The revolutionary impact of introducing irrigation-intensification to the olive oil industry. Acta Hortic. 2011, 888, 21–30. [Google Scholar] [CrossRef]
- Logemann, J.; Schell, J.; Willmitzer, L. Improved method for the isolation of RNA from plant tissues. Anal. Biochem. 1987, 163, 16–20. [Google Scholar] [CrossRef]
- Teper-Bamnolker, P.; Samach, A. The flowering integrator FT regulates SEPALLATA3 and FRUITFULL accumulation in Arabidopsis leaves. Plant Cell 2005, 17, 2661–2675. [Google Scholar] [CrossRef] [PubMed]
- El-Motaium, R.A.; Hashim, M.E. Boron efficiency in increasing olive (cv. Frantoio) fruit productivity and oil yield and quality. J. Plant Nutr. 2020, 43, 2981–2989. [Google Scholar] [CrossRef]
- Erel, R.; Dag, A.; Ben-Gal, A.; Schwartz, A.; Yermiyahu, U. Flowering and Fruit Set of Olive Trees in Response to Nitrogen, Phosphorus, and Potassium. J. Am. Soc. Hortic. Sci. 2008, 133, 639–647. [Google Scholar] [CrossRef]
- Rallo, L.; Martin, G.C. The role of chilling in releasing olive floral buds from dormancy. J. Am. Soc. Hortic. Sci. 1991, 116, 1058–1062. [Google Scholar] [CrossRef]
- Ben-Ari, G.; Biton, I.; Many, Y.; Namdar, D.; Samach, A. Elevated temperatures negatively affect olive productive cycle and oil quality. Agronomy 2021, 11, 1492. [Google Scholar] [CrossRef]
- Tsamir-Rimon, M.; Ben-Dor, S.; Feldmesser, E.; Oppenhimer-Shaanan, Y.; David-Schwartz, R.; Samach, A.; Klein, T. Rapid starch degradation in the wood of olive trees under heat and drought is permitted by three stress-specific beta amylases. New Phytol. 2021, 229, 1398–1414. [Google Scholar] [CrossRef]
- Rosecrance, R.C.; Krueger, W.H.; Milliron, L.; Bloese, J.; Garcia, C.; Mori, B. Moderate regulated deficit irrigation can increase olive oil yields and decrease tree growth in super high density ‘arbequina’ olive orchards. Sci. Hortic.-Amst. 2015, 190, 75–82. [Google Scholar] [CrossRef]
- Goldental-Cohen, S.; Biton, I.; Many, Y.; Tavrizov, K.; Dourou, A.M.; Zemach, H.; Tonutti, P.; Kerem, Z.; Avidan, B.; Sperling, O.; et al. Removal of flowers or inflorescences affects ‘Barnea’ olive fruitlet post-anthesis abscission. J. Hortic. Sci. Biotechnol. 2019, 94, 488–498. [Google Scholar] [CrossRef]
- Moreno-Alias, I.; Rapoport, H.F.; Leon, L.; de la Rosa, R. Olive seedling first-flowering position and management. Sci. Hortic.-Amst. 2010, 124, 74–77. [Google Scholar] [CrossRef]
- Nakagawa, M.; Honsho, C.; Kanzaki, S.; Shimizu, K.; Utsunomiya, N. Isolation and expression analysis of FLOWERING LOCUS T-like and gibberellin metabolism genes in biennial-bearing mango trees. Sci. Hortic. 2012, 139, 108–117. [Google Scholar] [CrossRef]
- Jiménez-Ruiz, J.; de la Leyva-Pérez, M.O.; Vidoy-Mercado, I.; Barceló, A.; Luque, F. Transcriptomic time-series analysis of early development in olive from germinated embryos to juvenile tree. BMC Genom. 2018, 19, 824. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, K.E.; Pullen, N.; Lamzin, S.; Morris, R.J.; Wigge, P.A. Interlocking feedback loops govern the dynamic behavior of the floral transition in arabidopsis. Plant Cell 2013, 25, 820–833. [Google Scholar] [CrossRef] [PubMed]
- Bradley, D.; Ratcliffe, O.; Vincent, C.; Carpenter, R.; Coen, E. Inflorescence commitment and architecture in arabidopsis. Science 1997, 275, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]


| Irrigation Regime | Location | Cultivar | Grove Planting Year | |
|---|---|---|---|---|
| Older | Younger | |||
| Rain fed | Rame | Souri | 1000 (a) | 1950 |
| Yodfat | Souri | 1984 | 2009 | |
| Irrigated | Revivim | Barnea | 1996 | 2011 |
| Souri | 1997 | 2005 | ||
| Ein Hanatiziv (b) | Barnea | 2002 | 2011 | |
| Souri | 2002 | 2009 | ||
| Tzabar Kama | Barnea | 2001 | 2010 | |
| Olea europaea | Arabidopsis Reference Protein (from Tair) | Primers | |||||
|---|---|---|---|---|---|---|---|
| Name | Accession (Transcripts) | Genomic Location | Protein Name | Arabidopsis Accession | E-Value (Blastp) | Primer Name | Primer Sequence |
| OeACT7 | OE6A117728T1 | Oe6_s00163 | ACTIN 7 (ACT7) | AT5G09810.1 | 5.6 × 10−199 | OeACT7RTfor | 5′-AAGATCAAAGTTGTTGCACCACC-3′ |
| OeACT7RTrev | 5′-CTTAGAAATCCACATCTGCTGGAAT-3′ | ||||||
| OeFT2 | OE6A103537T1 | Oe6_s04126 | FLOWERING LOCUS T (FT) | AT1G65480.1 | 3.8 × 10−74 | OeFT2RTfor | 5′-CCTTCGTACTTTCTACACGCTCATT-3′ |
| OeFT2RTrev | 5′-TCAGTCACCAACCAGTGCAAA-3′ | ||||||
| OeTFL1-1 | Oe6A037908T1 | Oe6_s02173 | TERMINAL FLOWER 1 (TFL1) | AT5G03840.1 | 3.2 × 10−64 | OeTFL1.1RTfor | 5′-CGTGAGTTCTGTCCGTCTGC-3′ |
| OeTFL1.1RTrev | 5′-TCAGGATCAATCATCACCAGTGTA-3′ | ||||||
| OeTFL1.1Probe | 5′-FAM-CCAGACCAAGGGTTGAGATTCAAGGAGGT-BHQ-1-3′ | ||||||
| OeAP2_1 Group | OE6A031451T1 | Oe6_s10134 | TARGET OF EARLY ACTIVATION TAGGED (EAT) 1 | AT2G28550.3 | 1.00 × 10−101 | OeAP2_1_RTfor | 5′-AACTTGGGCATTGCTCC-3′ |
| OE6A037406T2 | Oe6_s07880 | AT2G28550.3 | 9.00 × 10−98 | ||||
| OE6A055418T1 | Oe6_s02174 | AT2G28550.3 | 5.00 × 10−105 | OeAP2_1_RTrev | 5′-TTGAATTAGCGAATCCTGATG-3′ | ||
| OE6A105872T1 | Oe6_s02170 | AT2G28550.3 | 2.00 × 10−103 | ||||
| OeAP2_2 Group | OE6A061030T2 | Oe6_s06094 | APETALA2 (AP2) | AT4G36920.1 | 5.76 × 10−128 | OeAP2_2_RTfor | 5′-GAAGAATTTGTGCATGTACTTCG-3′ |
| OE6A068128T1 | Oe6_s00162 | 5.42 × 10−125 | |||||
| OE6A099997T2 | Oe6_s07897 | 4.88 × 10−123 | OeAP2_2_RTrev | 5′-TATCATAAGCCCTGGCAGC-3′ | |||
| OE6A079258T1 | Oe6_s07718 | 1.35 × 10−63 | |||||
| OeAP2_2.1 | OE6A099997T2 | Oe6_s07897 | APETALA2 (AP2) | AT4G36920.1 | 4.88 × 10−123 | OeAP2_2.1_RTfor | 5′-TGCCGAAGCACCAAGTGA-3′ |
| OeAP2_2.1_RTrev | 5′-TTGGCTTAGGAGCTGCGTG-3′ | ||||||
| OeAP2_2.3 | OE6A079258T1 | Oe6_s07718 | APETALA2 (AP2) | AT4G36920.1 | 1.35 × 10−63 | OeAP2_2.3_RTfor | 5′-AGCTTAATTTCCAGAATGGACTTG-3′ |
| OeAP2_2.3_RTrev | 5′-CCTACCGGTGAAGAGAACATT-3′ | ||||||
| OeAP2_2.4 | OE6A061030T2 | Oe6_s06094 | APETALA2 (AP2) | AT4G36920.1 | 5.76 × 10−128 | OeAP2_2.4_RTfor | 5′-GAAGTCTGCAGCGGCTGAG-3′ |
| OeAP2_2.4_RTrev | 5′-GCTGTATCAAATCCACCCAA-3′ | ||||||
| Parameter | Irrigated | Souri | ||
|---|---|---|---|---|
| Barnea (a) | Souri (b) | Rainfed (c) | Irrigated (d) | |
| Number of buds per branch (n) | 19.17 | 25.31 *** | 15.58 | 25.31 *** |
| Number of inflorescences per branch (k) | 6.91 | 9.95 *** | 7.22 | 9.95 ** |
| Percent buds forming inflorescences (i) | 32.70 | 38.70 * | 45.00 | 38.70 |
| Percent inflorescences that kept fruit (f) | 50.61 *** | 30.44 | 16.54 | 30.44 *** |
| Number of fruits on inflorescences that kept fruit (m) | 1.55 *** | 1.18 | 1.11 | 1.18 * |
| Number of fruits per branch (x) | 5.47 *** | 3.47 | 1.82 | 3.47 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wechsler, T.; Bakhshian, O.; Engelen, C.; Dag, A.; Ben-Ari, G.; Samach, A. Determining Reproductive Parameters, which Contribute to Variation in Yield of Olive Trees from Different Cultivars, Irrigation Regimes, Age and Location. Plants 2022, 11, 2414. https://doi.org/10.3390/plants11182414
Wechsler T, Bakhshian O, Engelen C, Dag A, Ben-Ari G, Samach A. Determining Reproductive Parameters, which Contribute to Variation in Yield of Olive Trees from Different Cultivars, Irrigation Regimes, Age and Location. Plants. 2022; 11(18):2414. https://doi.org/10.3390/plants11182414
Chicago/Turabian StyleWechsler, Tahel, Ortal Bakhshian, Chaim Engelen, Arnon Dag, Giora Ben-Ari, and Alon Samach. 2022. "Determining Reproductive Parameters, which Contribute to Variation in Yield of Olive Trees from Different Cultivars, Irrigation Regimes, Age and Location" Plants 11, no. 18: 2414. https://doi.org/10.3390/plants11182414
APA StyleWechsler, T., Bakhshian, O., Engelen, C., Dag, A., Ben-Ari, G., & Samach, A. (2022). Determining Reproductive Parameters, which Contribute to Variation in Yield of Olive Trees from Different Cultivars, Irrigation Regimes, Age and Location. Plants, 11(18), 2414. https://doi.org/10.3390/plants11182414









