Micropropagation of Citronella mucronata D. Don, a Vulnerable Chilean Endemic Tree Species
Abstract
:1. Introduction
2. Results
2.1. In Vitro Establishment
2.2. In Vitro Shoot Multiplication
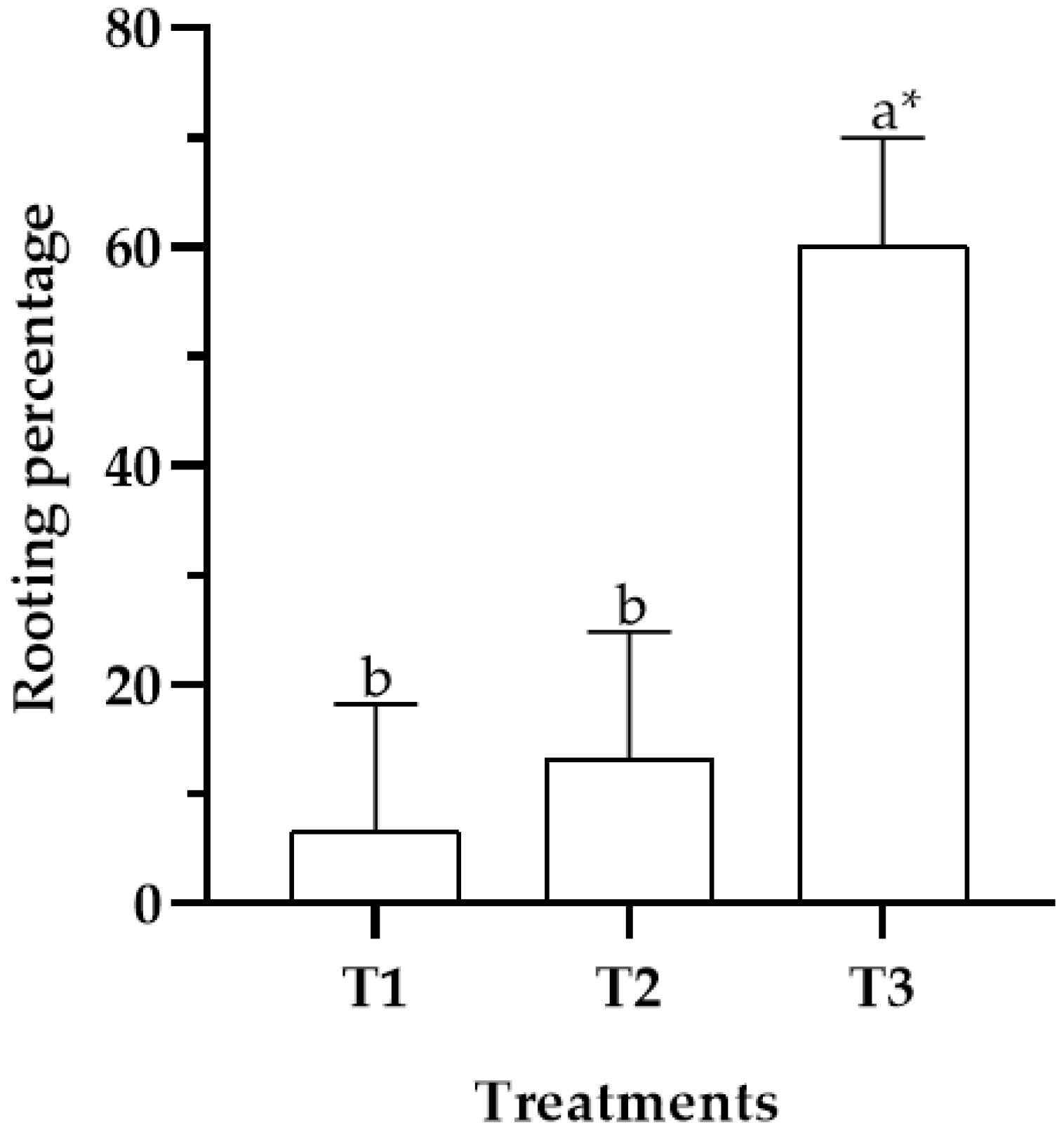
2.3. In Vitro Rooting
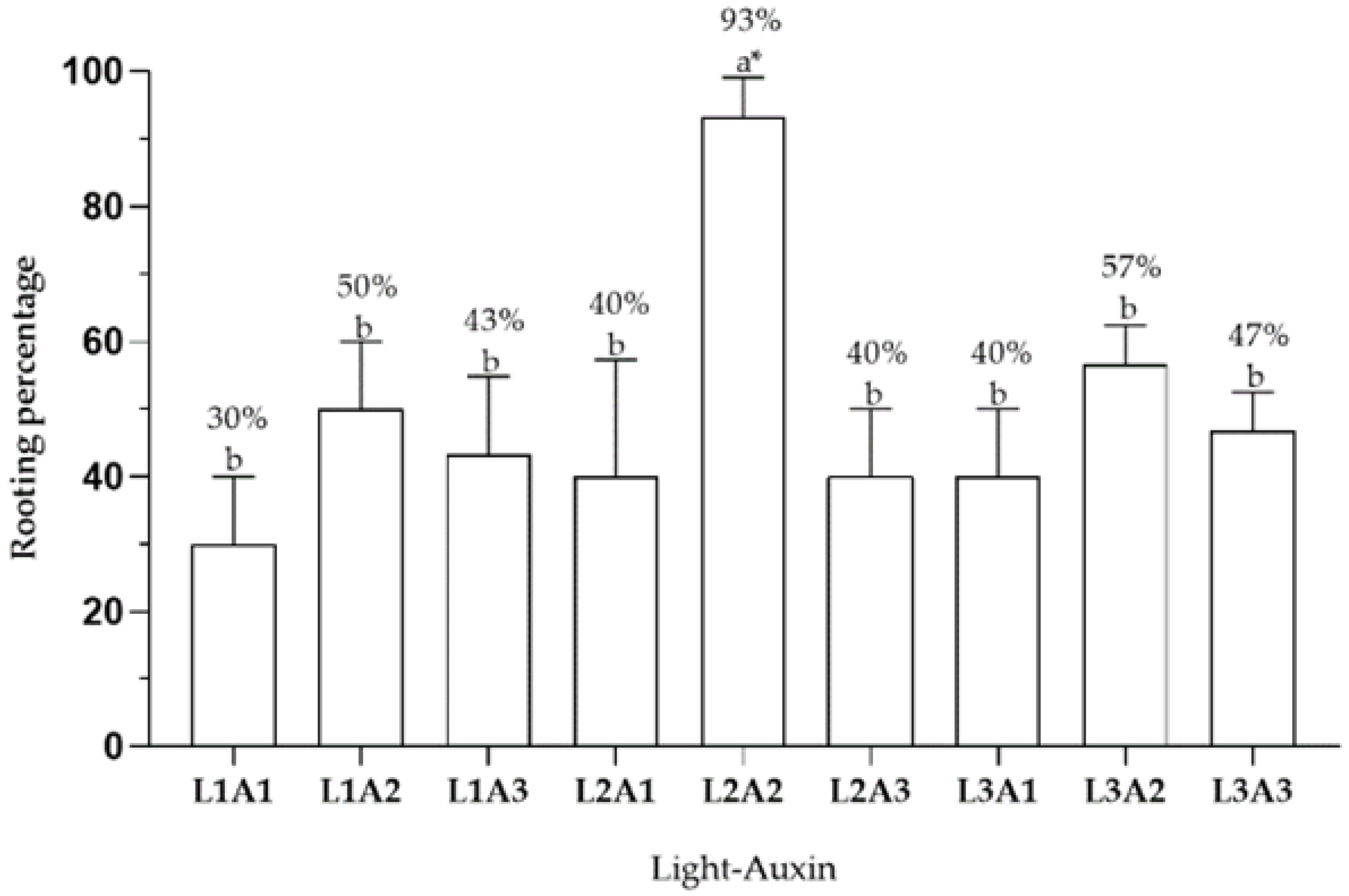
2.4. Effect of Light Type on Rooting
3. Discussion
4. Materials and Methods
4.1. In Vitro Establishment
4.2. In Vitro Shoot Multiplication
4.3. In Vitro Rooting
4.4. Effect of Light Type on Rooting
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garrido, N.; Echeverría, C. Clasificación de Especie. Available online: https://clasificacionespecies.mma.gob.cl/wpcontent/uploads/2019/10/Citronella_mucronata_12RCE_FIN.pdf (accessed on 17 March 2020).
- Ministerio del Medio Ambiente (MMA). Inventario Nacional de Especies de Chile. Available online: http://especies.mma.gob.cl/CNMWeb/Web/WebCiudadana/ficha_indepen.aspx?EspecieId=395&Version=1 (accessed on 20 March 2020).
- Donoso, C. Ecología Forestal. El Bosque y su Medioambiente; Editorial Universitaria: Santiago, Chile, 1981; p. 369. [Google Scholar]
- Gajardo, R. La Vegetación Natural de Chile. Clasificación y Distribución Geográfica; Editorial Universitaria: Santiago, Chile, 1994; p. 165. [Google Scholar]
- Hechenleitner, P.; Gardner, M.; Thomas, P.; Echeverría, C.; Escobar, B.; Brownless, P.; Martínez., C. Plantas Amenazadas del Centro-Sur de Chile. Distribución, Conservación y Propagación; Universidad Austral de Chile y Real Jardín Botánico de Edimburgo: Valdivia, Chile, 2005; p. 188. [Google Scholar]
- Figueroa, J.A.; Jaksic, F.M. Latencia y banco de semillas en plantas de la región mediterránea de Chile central. Rev. Chil. Hist. Nat. 2004, 77, 201–215. [Google Scholar] [CrossRef]
- Filho, A.R.; Vesco, L.D.; Nodari, R.; Lischka, R.; Müller, C.; Guerra, M. Tissue culture for the conservation and mass propagation of Vriesea reitzii Leme and Costa, a bromeliad threatened of extinction from the Brazilian Atlantic Forest. Biodivers. Conserv. 2005, 14, 1799–1808. [Google Scholar] [CrossRef]
- Tyagi, R.K.; Agrawal, A.; Mahalakshmi, C.; Hussain, Z.; Tyagi, H. Low-cost media for in vitro conservation of turmeric (Curcuma longa L.) and genetic stability assessment using RAPD markers. In vitro Cell. Dev. Biol. Plant 2007, 43, 51–58. [Google Scholar]
- Hussain, A.; Qarshi, I.A.; Nazir, H.; Ullah, I. Plant tissue culture: Current status and opportunities. Recent Adv. Plant Vitr. Cult. 2012, 6, 1–28. [Google Scholar]
- Chen, C. Fluorescent Lighting Distribution for Plant Micropropagation. Biosyst. Eng. 2005, 90, 295–306. [Google Scholar] [CrossRef]
- Aitken-Christie, J.; Kozai, T.; Smith, M.A.L. Automation and Environmental Control in Plant Tissue Culture; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; p. 586. [Google Scholar]
- Miler, N.; Kulus, D.; Woźny, A.; Rymarz, D.; Hajzer, M.; Wierzbowski, K.; Nelke, R.; Szeffs, L. Application of wide-spectrum light-emitting diodes in micropropagation of popular ornamental plant species: A study on plant quality and cost reduction. In Vitr. Cell. Dev. Biol. Plant 2019, 55, 99–108. [Google Scholar] [CrossRef]
- Wangchuk, K.; Manochai, B.; Chulaka, P.; Wongchaochant, S.; Chintakovid, W.; Pumprasert, J. Monitoring of active constituents of turmeric (Curcuma longa L.) rhizome stored under supplemented white LED-light with different light intensities. Acta Hortic. 2019, 1245, 131–138. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially-feasible micro-propagation of Mountain laurel, Kalmia latifolia, by use of shoot tip culture. Comb. Proc. Int. Plant Prop. Soc. 1980, 30, 421–427. [Google Scholar]
- Su, Y.H.; Liu, Y.B.; Zhang, X.S. Auxin–Cytokinin Interaction Regulates Meristem Development. Mol.Plant 2011, 4, 616–625. [Google Scholar] [CrossRef]
- Silva, J.; Winarto, B.; Dobránszki, J.; Zeng, S. Disinfection procedures for in vitro propagation of Anthurium. Folia Hortic. 2015, 27, 3–14. [Google Scholar]
- Sivanesan, I.; Jana, S.; Jeong, R.R. Regeneración de brotes in vitro y desarrollo de microcormos en Crocus vernus (L.) Hill. Pak. J. Bot. 2014, 46, 693–697. [Google Scholar]
- Cui, Y.; Deng, Y.; Zheng, K.; Hu, X.; Zhu, M.; Deng, X.; Xi, R. An efficient micropropagation protocol for an endangered ornamental tree species (Magnolia sirindhorniae Noot. & Chalermglin) and assessment of genetic uniformity through DNA markers. Sci. Rep. 2019, 9, 1–10. [Google Scholar]
- Khatri, P.; Rana, J.S.; Sindhu, A.; Jamdagni, P. Effect of additives on enhanced in-vitro shoot multiplication and their functional group identification of Chlorophytum borivilianum Sant. Et Fernand. SN Appl. Sci. 2019, 1, 1105. [Google Scholar] [CrossRef]
- Bello, A.; Aliabad, K.K.; Saravi, A.; Zade, H.S. Determination of the Best Culture Medium and Plant Growth Regulators for Micropropagation of Neem Tree (Azadirachta indica A.Juss). Int. J. Hortic. Sci. Technol. 2022, 9, 237–245. [Google Scholar]
- Nowakowska, K.; Pacholczak, A.; Tepper, W. The effect of selected growth regulators and culture media on regeneration of Daphne mezereum L. ‘Alba’. Rend. Lincei. Sci. Fis. Nat. 2019, 30, 197–205. [Google Scholar] [CrossRef]
- Wen, S.; Chen, L.; Tian, R.N. Micropropagation of tree peony (Paeonia sect. Moutan): A review. Plant Cell Tissue Organ Cult. 2019, 141, 1–14. [Google Scholar] [CrossRef]
- Klerk, G.J. Rooting of microcuttings: Theory and practice. In Vitr. Cell. Dev. Biol. Plant 2002, 38, 415–422. [Google Scholar] [CrossRef]
- Amiri, S.; Panahi, B.; Mohammadi, R.; Fattahi, F. Effect of Plant Growth Regulator Combination on Direct In Vitro Regeneration of Persian Lilac (Melia azedarach L.). Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 90, 261–265. [Google Scholar] [CrossRef]
- Li, Q.; Gu, M.; Deng, M. In Vitro Propagation of Oriental White Oak Quercus aliena Blume. Forests 2019, 10, 463. [Google Scholar] [CrossRef]
- Ho, W.-J.; Huang, Y.-K.; Huang, W.-W.; Chung, J.-P. Effective in vitro culture using dormant bud of nodal sections from a mature Acacia tree. In Vitr. Cell. Dev. Biol. Plant 2022, 58, 437–446. [Google Scholar] [CrossRef]
- Song, G.; Chen, Q.; Callow, P.; Mandujano, M.; Han, X.; Cuenca, B.; Bonito, G.; Medina-Mora, C.; Fulbright, D.; Guyer, D. Efficient Micropropagation of Chestnut Hybrids (Castanea spp.) Using Modified Woody Plant Medium and Zeatin Riboside. Hortic. Plant J. 2021, 7, 174–180. [Google Scholar] [CrossRef]
- Mao, A.A.; Vijayan, D.; Singha, R.K.N.; Pradhan, S. In vitro propagation of Rhododendron wattii Cowan—A critically endangered and endemic plant from India. In Vitr. Cell. Dev. Biol. Plant 2018, 54, 45–53. [Google Scholar] [CrossRef]
- Guo, Y.-X.; Zhao, Y.-Y.; Zhang, M.; Zhang, L.-Y. Development of a novel in vitro rooting culture system for the micropropagation of highbush blueberry (Vaccinium corymbosum) seedlings. Plant Cell Tissue Organ Cult. 2019, 139, 615–620. [Google Scholar] [CrossRef]
- Massa, G.D.; Kim, H.-H.; Wheeler, R.M.; Mitchell, C.A. Plant Productivity in Response to LED Lighting. HortScience 2008, 43, 1951–1956. [Google Scholar] [CrossRef]
- Hung, C.D.; Hong, C.-H.; Jung, H.-B.; Kim, S.-K.; Van Ket, N.; Nam, M.-W.; Choi, D.-H.; Lee, H.-I. Growth and morphogenesis of encapsulated strawberry shoot tips under mixed LEDs. Sci. Hortic. 2015, 194, 194–200. [Google Scholar] [CrossRef]
- Gupta, S.D.; Jatothu, B. Fundamentals and applications of light-emitting diodes (LEDs) in in vitro plant growth and morphogenesis. Plant Biotechnol. Rep. 2013, 7, 211–220. [Google Scholar] [CrossRef]
- Makarov, S.; Rodin, S.; Kuznetsova, I.; Chudetsky, A.; Tsaregradskaya, S. Effect of Light on Rhizogenesis of Forest Berry Plants during Clonal Micropropagation. Food Process. Tech. Technol. 2021, 51, 520–528. [Google Scholar] [CrossRef]
- Ramírez-Mosqueda, M.; Iglesias-Andreu, L.; Luna-Sánchez, I. Light quality affects growth and development of in vitro plantlet of Vanilla planifolia Jacks. S. Afr. J. Bot. 2017, 109, 288–293. [Google Scholar] [CrossRef]
- Correll, M.J.; Kiss, J.Z. The Roles of Phytochromes in Elongation and Gravitropism of Roots. Plant Cell Physiol. 2005, 46, 317–323. [Google Scholar] [CrossRef]
- Araujo, A.; Pasqual, L.; Miyata, Y.; Castro, E.; Rocha, H. Qualidade de luz na biometria e anatomia foliar de plântulas de Cattleya loddigessi L. (Orchidaceae) micropropagadas. Cienc. Rural 2009, 39, 2506–2511. [Google Scholar] [CrossRef]
- Lin, Y.; Li, J.; Li, B.; He, T.; Chun, Z. Effects of light quality on growth and development of protocorm-like bodies of Dendrobium officinale in vitro. Plant Cell Tissue Organ Cult. 2010, 105, 329–335. [Google Scholar] [CrossRef]
- Wu, H.-C.; Lin, C.-C. Red Light-emitting Diode Light Irradiation Improves Root and Leaf Formation in Difficult-to-propagate Protea cynaroides L. Plantlets In Vitro. HortScience 2012, 47, 1490–1494. [Google Scholar] [CrossRef]
- Kurilčik, A.; Miklušytė-Čanova, R.; Dapkūnienė, S.; Žilinskaitė, S.; Kurilčik, G.; Tamulaitis, G.; Duchovskis, P.; Žukauskas, A. In vitro culture of Chrysanthemum plantlets using light-emitting diodes. Cent. Eur. J. Biol 2008, 3, 161–167. [Google Scholar] [CrossRef]
- Batista, D.; Felipe, S.H.S.; Silva, T.D.; de Castro, K.M.; Mamedes-Rodrigues, T.C.; Miranda, N.A.; Ríos-Ríos, A.M.; Faria, D.V.; Fortini, E.A.; Chagas, K.; et al. Light quality in plant tissue culture: Does it matter? In Vitr. Cell. Dev. Biol. Plant 2018, 54, 195–215. [Google Scholar] [CrossRef]
- Jung, W.-S.; Chung, I.-M.; Hwang, M.; Kim, S.-H.; Yu, C.; Ghimire, B. Application of Light-Emitting Diodes for Improving the Nutritional Quality and Bioactive Compound Levels of Some Crops and Medicinal Plants. Molecules 2021, 26, 1477. [Google Scholar] [CrossRef]
- Hashim, M.; Ahmed, B.; Drouet, S.; Hano, C.; Abbasi, B.H.; Anjum, S. Comparative Effects of Different Light Sources on the Production of Key Secondary Metabolites in Plants In Vitro Cultures. Plants 2021, 10, 1521. [Google Scholar] [CrossRef]
- Santibáñez, F. Atlas Agroclimático de Chile. Estado Actual y Tendencias del Clima. Tomo III: Regiones de Valparaíso, Metropolitana, O’Higgins y Maule; Fundación para la Innovación Agraria: Santiago, Chile, 2017; p. 37. [Google Scholar]


| Treatment | Survival (%) | Oxidation (%) | Fungal Contamination (%) | Bacterial Contamination (%) |
|---|---|---|---|---|
| T1: MS | 79 ± 2.3 a* | 5 ± 1.3 b | 6 ± 1.7 b | 11 ± 1.1 a |
| T2: WPM | 50 ± 2.8 b | 17 ± 1.7 b | 17 ± 2.7 a | 17 ± 2.2 a |
| T3: MSm | 35 ± 2.5 b | 44 ± 1.7 a | 11 ± 2.4 b | 11 ± 2.0 a |
| Treatment | Concentration BAP (μM) | Shoot Length (cm) | Lateral Shoot Length (cm) | Number of Lateral Shoots |
|---|---|---|---|---|
| T1 | 0 | 3.0 ± 0.6 bc* | 2.0 ± 0.5 cd | 2.0 ± 1.2 b |
| T2 | 1.78 | 3.3 ± 0.5 bc | 2.4 ± 0.4 bc | 2.2 ± 1.3 b |
| T3 | 3.55 | 3.4 ± 0.2 b | 2.6 ± 0.2 b | 3.6 ± 1.4 a |
| T4 | 4.44 | 4.0 ± 0.3 a | 3.2 ± 0.4 a | 3.9 ± 1.6 a |
| T5 | 11.10 | 3.0 ± 0.6 c | 1.9 ± 0.6 d | 2.1 ± 1.2 b |
| Light Type | Red | Blue | Red:Blue Ratio | DLI (mol m−2) |
|---|---|---|---|---|
| (YPFD, μmol m−2s−1) | ||||
| L1 | 2 | 2 | 1:1 | 0.8 |
| L2 | 7 | 14 | 1:2 | 3.8 |
| L3 | 6 | 6 | 1:1 | 2.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra, F.; Badilla, L.; Cautin, R.; Castro, M. Micropropagation of Citronella mucronata D. Don, a Vulnerable Chilean Endemic Tree Species. Plants 2022, 11, 2425. https://doi.org/10.3390/plants11182425
Guerra F, Badilla L, Cautin R, Castro M. Micropropagation of Citronella mucronata D. Don, a Vulnerable Chilean Endemic Tree Species. Plants. 2022; 11(18):2425. https://doi.org/10.3390/plants11182425
Chicago/Turabian StyleGuerra, Francesca, Loreto Badilla, Ricardo Cautin, and Mónica Castro. 2022. "Micropropagation of Citronella mucronata D. Don, a Vulnerable Chilean Endemic Tree Species" Plants 11, no. 18: 2425. https://doi.org/10.3390/plants11182425
APA StyleGuerra, F., Badilla, L., Cautin, R., & Castro, M. (2022). Micropropagation of Citronella mucronata D. Don, a Vulnerable Chilean Endemic Tree Species. Plants, 11(18), 2425. https://doi.org/10.3390/plants11182425







