Cold Resistance of Euonymus japonicus Beihaidao Leaves and Its Chloroplast Genome Structure and Comparison with Celastraceae Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Observation of the External Morphology of the Blade
2.2. Physiological Index Determination
2.2.1. Determination of Color Differences on the Leaf Surface
2.2.2. Determination of Photosynthetic Pigment Content
- (1)
- Chlorophyll levels were measured using the absolute ethanol extraction method. A sample of 0.1 g fresh and clean plant leaf was cut into filaments and then placed in a test tube. After adding 10 mL absolute ethanol, the samples were placed in the dark for chlorophyll extraction at room temperature for about 24 h until the leaves were colorless or white (the soaking time can be appropriately shortened or extended according to the color state of the leaves).
- (2)
- The specific type of chlorophyll was determined using the colorimetry method. The test solution was poured into a cuvette, while a blank control cuvette contained anhydrous ethanol. Using a spectrophotometer, absorbances were measured at wavelengths of 663, 647, and 470 nm. The content of each pigment was calculated using the following formulas:chla = (12.25 D663 − 2.79 D647) ∗ V ∗ 1000 m,chlb = (21.50 D647 − 5. 10 D663) ∗ V ∗ 1000 m,where D647, D663, and D470 are the absorbances of the sample at 647, 663, and 470 nm, respectively. V is the volume of liquid measured, and m is the blade mass.Carotenoids = (1000 D470 − 1.82 chla − 85.02 chlb) ∗ 198 V ∗ 1000 m,
2.2.3. Determination of Chlorophyll Fluorescence Parameters
2.2.4. Determination of Net Photosynthetic Rate
2.3. Sequencing and Analysis of Chloroplast Genome
2.3.1. Plant Material and Genome Sequencing
2.3.2. Chloroplast Genome Assembly and Annotated
2.3.3. Repeat Sequence Analysis
2.3.4. Analysis of Codon Usage
2.3.5. Comparative Analysis of the Chloroplast Genome Sequences of the Celastraceae
2.3.6. Adaptive Evolutionary Analysis
2.3.7. Phylogenetic Analysis
3. Results
3.1. Performance of Freezing Injury of E. japonicus Beihaidao during Overwintering
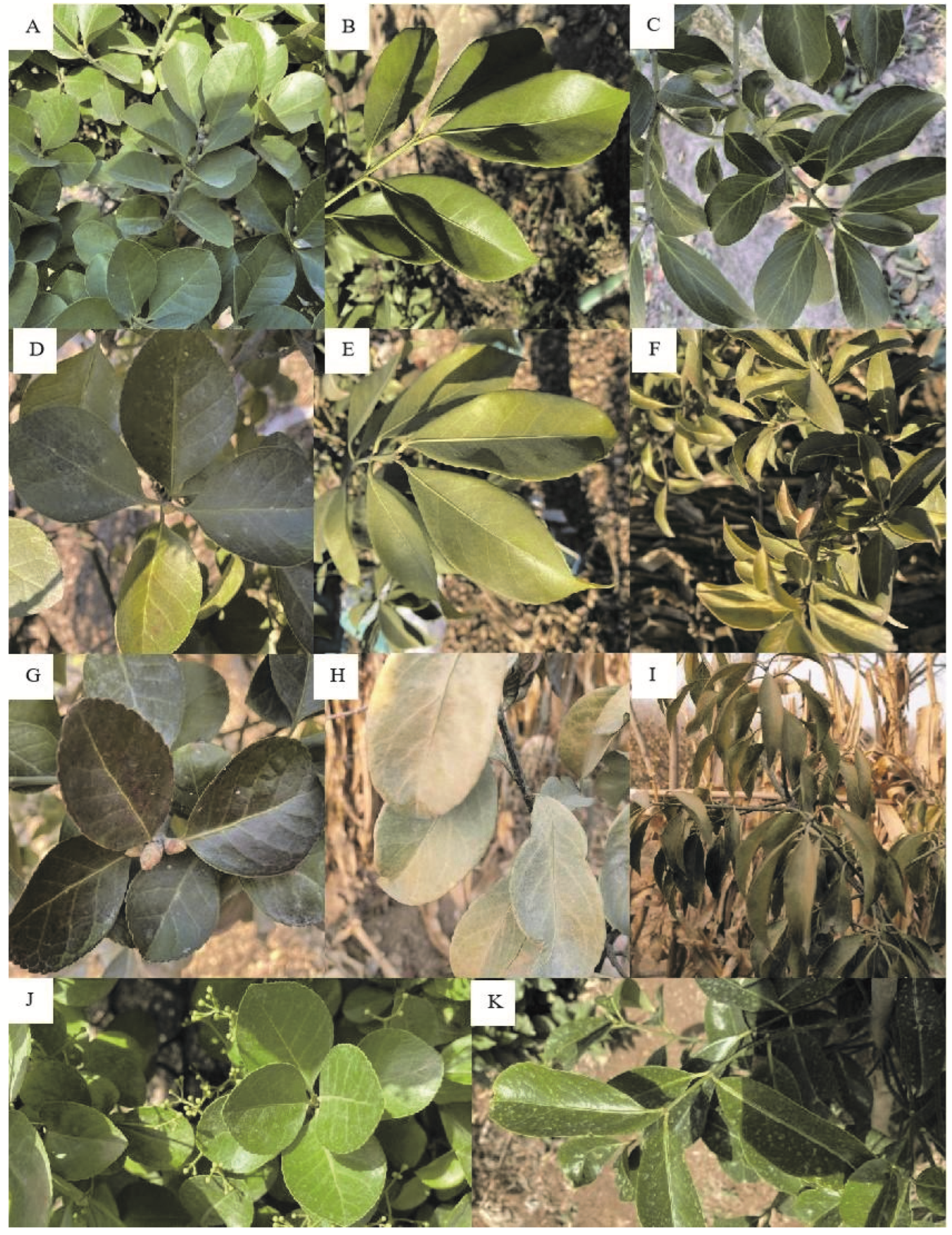
3.1.1. Comparison of Leaf Freezing Damage Characteristics
3.1.2. Comparison of Anatomical Structures of Leaves
3.2. Changes in Physiological Indexes of E. japonicus Beihaidao during Overwintering
3.2.1. Changes in Color Difference of the Leaf Surface
3.2.2. Changes in Photosynthetic Pigment Content
3.2.3. Changes in Chlorophyll Fluorescence Parameters
3.2.4. Changes in Net Photosynthetic Rate
3.3. Analysis of E. japonicus Beihaidao Chloroplast Genome
3.3.1. Basic Characteristics of the E. japonicus Beihaidao Chloroplast Genome
3.3.2. IR Boundary Analysis
3.3.3. Comparative Sequence Analysis of Repetitive Sequences of E. japonicus Beihaidaos and Genus euonymus
3.3.4. Changes in Net Photosynthetic Rate
3.3.5. Analysis of Differential Loci
3.3.6. Adaptive Evolutionary Analysis
Screening of Positive Selection Genes
Positive Selection Genes Annotated and Related Important Metabolic Pathways Analysis
Expression Analysis of Positive Selection Genes in Different Periods
4. Discussion
4.1. Responses of Leaf Morphological Structure and Physiological Indexes of E. japonicus Beihaidao to Wintering
4.2. IR Contraction and Expansion
4.3. Genome Sequence Variation
4.4. Phylogenetic Evolution
4.5. Genetic Adaptive Evolution
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, R.; Peng, J.; Guo, J.; Wan, M.; Huang, H.; Li, L. Modern research on pharmacological action of Euonymus alatus (Thunb.) Sieb. Glob. Trad. Chin. Med. 2015, 8, 245–249. [Google Scholar]
- Qin, X.; Yan, S.; Wei, F. Geographical distribution of plants of Celastraceae in China. J. Northeast For. Univ. 2011, 39, 120–123. [Google Scholar]
- Zhang, T.T.; Sun, J.; Xu, L.; Yang, Y.Y.; Zhan, Z.L.; Xing, Y.P.; Zhao, R.; Li, S.N.; Zhang, D.C.; Kang, T.G. The complete mitochondrial genome of Euonymus alatus (celastraceae, Euonymus L.). Mitochondrial DNA Part B-Resour. 2021, 6, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Lijing, L.; Zhiwei, Z.; Yun, X.; Jiahang, Z.; Liebao, H.; Lixin, X. Effects of low temperature stress on chlorophyll metabolism of Zoysia japonica. J. Beijing For. Univ. 2022, 44, 91–99. [Google Scholar]
- Chen, Z.F.; Wang, Z.; Yang, Y.G.; Li, M.; Xu, B.C. Abscisic acid and brassinolide combined application synergistically enhances drought tolerance and photosynthesis of tall fescue under water stress. Sci. Hortic. 2018, 228, 1–9. [Google Scholar] [CrossRef]
- Steponkus, P.L.; Garber, M.P.; Myers, S.P.; Lineberger, R.D. Effects of cold acclimation and freezing on structure and function of chloroplast thylakoids. Cryobiology 1977, 14, 303–321. [Google Scholar] [CrossRef]
- Jensen, M.; Oettmeier, W. Effects of freezing on the structure of chloroplast membranes. Cryobiology 1984, 21, 465–473. [Google Scholar] [CrossRef]
- Venzhik, J.V.; Titov, D.F.; Talanova, V.V.; Miroslavov, E.D.; Koteeva, N.K. Structural and functional reorganization of photosynthetic apparatus in cold adaptation of wheat plants. Tsitologiia 2012, 54, 916–924. [Google Scholar] [CrossRef]
- Li, J. Effects of Low Temperature Stress on Chill-resistance Physiological Index of Rice Seedlings. Anhui Agric. Sci. Bull. 2014, 20, 21–22+68. [Google Scholar]
- Wang, Y.; Yuan, M.; An, R. Effects of low temperature on the cold resistance physiology index of camphor tree. J. For. Environ. 2020, 40, 512–518. [Google Scholar]
- Chen, J.; Jin, X.; Ning, Y.; Wu, J.; Zeng, W.; Li, R.; Cao, J. Identification and comprehensive evaluation of cold resistance indexes of three Michelia plants. J. Henan Agric. Sci. 2016, 45, 113–118. [Google Scholar]
- Huang, Q.; Huang, X.; Sun, F.; Sun, B.; Yang, L.; Li, Y. Effects of Chilling Stress on Protein and Related Gene Expression in Chloroplasts of Sugarcane. Sci. Agric. Sin. 2012, 45, 4978–4987. [Google Scholar]
- Xu, Y. The Physiological and Molecular Mechanisms of Winter Turnip Rape (Brassica rapa L.) Responses to Cold Stress. Ph.D. Thesis, Gansu Agriculture University, Lanzhou, China, 2020. [Google Scholar]
- Lin, M. Mechanism of Cold Resistance on Kiwifruit Based on Genome Re-Sequencing and BSR-Seq. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2020. [Google Scholar]
- Xing, S. Progress in chloroplast genome analysis. Prog. Biochem. Biophys. 2006, 35, 21–28. [Google Scholar]
- Zhang, X.; Zhou, T.; Kanwal, N.; Zhao, Y.; Bai, G.; Zhao, G. Completion of Eight Gynostemma BL. (Cucurbitaceae) Chloroplast Genomes: Characterization, Comparative Analysis, and Phylogenetic Relationships. Front. Plant Sci. 2017, 8, 1583. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.X.; Daniell, H. The Engineered Chloroplast Genome Just Got Smarter. Trends Plant Sci. 2015, 20, 622–640. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.M. The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Neuhaus, H.E.; Emes, M.J. Nonphotosynthetic metabolism in plastids. Annu. Rev. Plant Physiol. Plant Molec. Biol. 2000, 51, 111–140. [Google Scholar] [CrossRef]
- Gao, L.Z.; Liu, Y.L.; Zhang, D.; Li, W.; Gao, J.; Liu, Y.; Li, K.; Shi, C.; Zhao, Y.; Zhao, Y.J.; et al. Evolution of Oryza chloroplast genomes promoted adaptation to diverse ecological habitats. Commun. Biol. 2019, 2, 13. [Google Scholar] [CrossRef]
- Wang, R.J.; Cheng, C.L.; Chang, C.C.; Wu, C.L.; Su, T.M.; Chaw, S.M. Dynamics and evolution of the inverted repeat-large single copy junctions in the chloroplast genomes of monocots. BMC Evol. Biol. 2008, 8, 14. [Google Scholar] [CrossRef]
- Daniell, H.; Lin, C.S.; Yu, M.; Chang, W.J. Chloroplast genomes: Diversity, evolution, and applications in genetic engineering. Genome Biol. 2016, 17, 29. [Google Scholar] [CrossRef]
- Oldenburg, D.J.; Bendich, A.J. The linear plastid chromosomes of maize: Terminal sequences, structures, and implications for DNA replication. Curr. Genet. 2016, 62, 431–442. [Google Scholar] [CrossRef]
- Bendich, A.J. Circular chloroplast chromosomes: The grand illusion. Plant Cell 2004, 16, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Menezes, A.P.A.; Resende-Moreira, L.C.; Buzatti, R.S.O.; Nazareno, A.G.; Carlsen, M.; Lobo, F.P.; Kalapothakis, E.; Lovato, M.B. Chloroplast genomes of Byrsonima species (Malpighiaceae): Comparative analysis and screening of high divergence sequences. Sci. Rep. 2018, 8, 2210. [Google Scholar] [CrossRef] [PubMed]
- Iram, S.; Hayat, M.Q.; Tahir, M.; Gul, A.; Abdullah; Ahmed, I. Chloroplast Genome Sequence of Artemisia scoparia: Comparative Analyses and Screening of Mutational Hotspots. Plants 2019, 8, 476. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.Y.; Li, J.R.; Li, D.; Shi, L. Complete chloroplast genome sequence of the medical fern Drynaria roosii and its phylogenetic analysis. Mitochondrial DNA Part B-Resour. 2017, 2, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Henriquez, C.L.; Abdullah; Ahmed, I.; Carlsen, M.M.; Zuluaga, A.; Croat, T.B.; McKain, M.R. Evolutionary dynamics of chloroplast genomes in subfamily Aroideae (Araceae). Genomics 2020, 112, 2349–2360. [Google Scholar] [CrossRef] [PubMed]
- Abdullah; Henriquez, C.L.; Mehmood, F.; Carlsen, M.M.; Islam, M.; Waheed, M.T.; Poczai, P.; Croat, T.B.; Ahmed, I. Complete Chloroplast Genomes ofAnthurium huixtlenseandPothos scandens(Pothoideae, Araceae): Unique Inverted Repeat Expansion and Contraction Affect Rate of Evolution. J. Mol. Evol. 2020, 88, 562–574. [Google Scholar] [CrossRef]
- Yi, X.; Gao, L.; Wang, B.; Su, Y.J.; Wang, T. The Complete Chloroplast Genome Sequence of Cephalotaxus oliveri (Cephalotaxaceae): Evolutionary Comparison of Cephalotaxus Chloroplast DNAs and Insights into the Loss of Inverted Repeat Copies in Gymnosperms. Genome Biol. Evol. 2013, 5, 688–698. [Google Scholar] [CrossRef]
- Li, X.W.; Gao, H.H.; Wang, Y.T.; Song, J.Y.; Henry, R.; Wu, H.Z.; Hu, Z.G.; Yao, H.; Luo, H.M.; Luo, K.; et al. Complete chloroplast genome sequence of Magnolia grandiflora and comparative analysis with related species. Sci. China-Life Sci. 2013, 56, 189–198. [Google Scholar] [CrossRef]
- Shinozaki, K.; Ohme, M.; Tanaka, M.; Wakasugi, T.; Hayashida, N.; Matsubayashi, T.; Zaita, N.; Chunwongse, J.; Obokata, J.; Yamaguchi-Shinozaki, K.; et al. The complete nucleotide sequence of the tobacco chloroplast genome: Its gene organization and expression. EMBO J. 1986, 5, 2043–2049. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Yang, K.; Li, Z. A comparison study on extraction methods for genomic DNA from tissues of Camellia plants with modified CTAB method. Acta Agric. Univ. Jiangxiensis 2017, 39, 785–791. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Dierckxsens, N.; Mardulyn, P.; Smits, G. NOVOPlasty: De novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 2017, 45, 9. [Google Scholar] [CrossRef]
- Kent, W.J. BLAT—The BLAST-like alignment tool. Genome Res. 2002, 12, 656–664. [Google Scholar] [CrossRef]
- Zheng, S.Y.; Poczai, P.; Hyvonen, J.; Tang, J.; Amiryousefi, A. Chloroplot: An Online Program for the Versatile Plotting of Organelle Genomes. Front. Genet. 2020, 11, 576124. [Google Scholar] [CrossRef]
- Thiel, T.; Michalek, W.; Varshney, R.K.; Graner, A. Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L.). Theor. Appl. Genet. 2003, 106, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Choudhuri, J.V.; Ohlebusch, E.; Schleiermacher, C.; Stoye, J.; Giegerich, R. REPuter: The manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 2001, 29, 4633–4642. [Google Scholar] [CrossRef]
- Rice, P.; Longden, I.; Bleasby, A. EMBOSS: The European molecular biology open software suite. Trends Genet. 2000, 16, 276–277. [Google Scholar] [CrossRef]
- Frazer, K.A.; Pachter, L.; Poliakov, A.; Rubin, E.M.; Dubchak, I. VISTA: Computational tools for comparative genomics. Nucleic Acids Res. 2004, 32, W273–W279. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef]
- Amiryousefi, A.; Hyvonen, J.; Poczai, P. IRscope: An online program to visualize the junction sites of chloroplast genomes. Bioinformatics 2018, 34, 3030–3031. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Suyama, M.; Torrents, D.; Bork, P. PAL2NAL: Robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res. 2006, 34, W609–W612. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Y.; Zhang, Z.; Zhu, J.; Yu, J. KaKs_Calculator 2.0: A toolkit incorporating gamma-series methods and sliding window strategies. Genom. Proteom. Bioinform. 2010, 8, 77–80. [Google Scholar] [CrossRef]
- Hurst, L.D. The Ka/Ks ratio: Diagnosing the form of sequence evolution. Trends Genet. 2002, 18, 486–487. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Zeng, G.; Zhou, L.; Li, X. Changes in physiological and biochemical indexes and anatomical structure of leaf of Camellia sinensis during natural overwintering period. J. Plant Resour. Environ. 2017, 26, 63–68. [Google Scholar]
- Gao, C.; Zhao, L.; Wang, Q.; Ren, Z.; Zhao, J.; Yin, H. Analysis of Cold Resistance of Seven PARIS Species Based on Leaf Anatomical Structure during the Natural Growth and the Overwintering Periods. Chin. J. Appl. Enivronmental Biol. 2020, 28, 117–127. [Google Scholar]
- Fan, S.; Wang, Y. Effects of Low Temperature on Light Absorption, Conversion and Usage for Rape Leaves. J. Anhui Agric. Sci. 2021, 49, 53–54+59. [Google Scholar]
- Wang, X.; Yi, T.; Zhu, J.; Song, Y. Effect of Low Temperature Stress on Leaf Submicroscopic Structure and Chlorophyll Fluorescence Parameters of Cassava ‘F200’Seedlings. Chin. J. Trop. Crop. 2021, 42, 1925. [Google Scholar]
- Choi, K.S.; Park, S. The complete chloroplast genome sequence of Euonymus japonicus (Celastraceae). Mitochondrial DNA Part A 2016, 27, 3577–3578. [Google Scholar] [CrossRef] [PubMed]
- Hirao, T.; Watanabe, A.; Kurita, M.; Kondo, T.; Takata, K. Complete nucleotide sequence of the Cryptomeria japonicia D. Don. chloroplast genome and comparative chloroplast genomics: Diversified genomic structure of coniferous species. BMC Plant Biol. 2008, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Hoot, S.B.; Palmer, J.D. Structural rearrangements, including parallel inversions, within the chloroplast genome of Anemone and related genera. J. Mol. Evol. 1994, 38, 274–281. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, H.J.; He, J.; Ding, C.H.; Lyu, R.D.; Pei, L.Y.; Cheng, J.; Xie, L. Comparative Analysis of Complete Chloroplast Genomes of Anemoclema, Anemone, Pulsatilla, and Hepatica Revealing Structural Variations Among Genera in Tribe Anemoneae (Ranunculaceae). Front. Plant Sci. 2018, 9, 1097. [Google Scholar] [CrossRef] [PubMed]
- Tangphatsornruang, S.; Uthaipaisanwong, P.; Sangsrakru, D.; Chanprasert, J.; Yoocha, T.; Jomchai, N.; Tragoonrung, S. Characterization of the complete chloroplast genome of Hevea brasiliensis reveals genome rearrangement, RNA editing sites and phylogenetic relationships. Gene 2011, 475, 104–112. [Google Scholar] [CrossRef]
- Zhu, T.; Zhang, L.; Chen, W.; Yin, J.; Li, Q. Analysis of chloroplast genomes in 1342 plants. Genom. Appl. Biol. 2017, 36, 4323–4333. [Google Scholar]
- Wei, F.; Tang, D.F.; Wei, K.H.; Qin, F.; Li, L.X.; Lin, Y.; Zhu, Y.X.; Khan, A.; Kashif, M.H.; Miao, J.H. The complete chloroplast genome sequence of the medicinal plant Sophora tonkinensis. Sci. Rep. 2020, 10, 12473. [Google Scholar] [CrossRef]
- Kuang, D.Y.; Wu, H.; Wang, Y.L.; Gao, L.M.; Zhang, S.Z.; Lu, L. Complete chloroplast genome sequence of Magnolia kwangsiensis (Magnoliaceae): Implication for DNA barcoding and population genetics. Genome 2011, 54, 663–673. [Google Scholar] [CrossRef]
- Martin, G.; Baurens, F.C.; Cardi, C.; Aury, J.M.; D’Hont, A. The Complete Chloroplast Genome of Banana (Musa acuminata, Zingiberales): Insight into Plastid Monocotyledon Evolution. PLoS ONE 2013, 8, e67350. [Google Scholar] [CrossRef]
- Yang, Y.C.; Zhou, T.; Duan, D.; Yang, J.; Feng, L.; Zhao, G.F. Comparative Analysis of the Complete Chloroplast Genomes of Five Quercus Species. Front. Plant Sci. 2016, 7, 959. [Google Scholar] [CrossRef]
- Hu, Y.H.; Woeste, K.E.; Zhao, P. Completion of the Chloroplast Genomes of Five Chinese Juglans and Their Contribution to Chloroplast Phylogeny. Front. Plant Sci. 2017, 7, 1955. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Yang, Y.C.; Zhou, T.; Liu, Z.L. Comparative Plastid Genomes of Primula Species: Sequence Divergence and Phylogenetic Relationships. Int. J. Mol. Sci. 2018, 19, 1050. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, P.M.; Forrest, L.L.; Spouge, J.L.; Hajibabaei, M.; Ratnasingham, S.; van der Bank, M.; Chase, M.W.; Cowan, R.S.; Erickson, D.L.; Fazekas, A.J.; et al. A DNA barcode for land plants. Proc. Natl. Acad. Sci. USA 2009, 106, 12794–12797. [Google Scholar] [CrossRef]
- Doorduin, L.; Gravendeel, B.; Lammers, Y.; Ariyurek, Y.; Chin-A-Woeng, T.; Vrieling, K. The Complete Chloroplast Genome of 17 individuals of Pest Species Jacobaea vulgaris: SNPs, Microsatellites and Barcoding Markers for Population and Phylogenetic Studies. DNA Res. 2011, 18, 93–105. [Google Scholar] [CrossRef]
- Huang, W. DNA Barcoding Identificatin of Fagopyrum and Its Wild Relatives. Master’s Thesis, Minzu University of China, Beijing, China, 2014. [Google Scholar]
- Park, S.; An, B.; Park, S. Reconfiguration of the plastid genome in Lamprocapnos spectabilis: IR boundary shifting, inversion, and intraspecific variation. Sci. Rep. 2018, 8, 14. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, H.Z.; Qu, C.Q.; Meng, X.X.; Meng, F.; Yao, X.Y.; Wu, J.; Guo, X.H.; Han, B.X.; Xing, S.H. Comprehensive analysis of chloroplast genome of Albizia julibrissin Durazz. (Leguminosae sp.). Planta 2022, 255, 16. [Google Scholar] [CrossRef]
- Anderson, W.R. An Integrated System of Classification of Flowering Plants. Brittonia 1982, 34, 268–270. [Google Scholar] [CrossRef]
- Hong, D.Y. Flora Reipublicae Popularis Sinicae; Science Press: Beijing, China, 1983. [Google Scholar]
- Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot. J. Linn. Soc. 2009, 161, 105–121. [Google Scholar]
- Li, J.F.; Yang, Q.; Xu, B.; Liu, Z.L. The complete chloroplast genome of Parnassia wightiana (Celastraceae). Mitochondrial DNA Part B-Resour. 2019, 4, 3987–3988. [Google Scholar] [CrossRef]
- Yu, H.Y.; Guo, F.Y.; Liu, D.; Zhang, Z.X. Complete chloroplast genome sequence of Parnassia palustris (Celastraceae). Mitochondrial DNA Part B-Resour. 2019, 4, 1503–1504. [Google Scholar] [CrossRef]
- Wang, B.; Gao, L.; Su, Y.; Wang, T. Adaptive Evolutionary Analysis of Chloroplast Genes in Euphyllophytes Based on Complete Chloroplast Genome Sequences. Acta Sci. Nat. Univ. Sunyatseni 2012, 51, 108–113+146. [Google Scholar]
- Yin, K.Q.; Zhang, Y.; Li, Y.J.; Du, F.K. Different Natural Selection Pressures on the atpF Gene in Evergreen Sclerophyllous and Deciduous Oak Species: Evidence from Comparative Analysis of the Complete Chloroplast Genome of Quercus aquifolioides with Other Oak Species. Int. J. Mol. Sci. 2018, 19, 1042. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.L.; Wang, R.N.; Zhang, N.Y.; Fan, W.B.; Fang, M.F.; Li, Z.H. Molecular Evolution of Chloroplast Genomes of Orchid Species: Insights into Phylogenetic Relationship and Adaptive Evolution. Int. J. Mol. Sci. 2018, 19, 716. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.J.; Jiang, S.F.; Xie, D.F.; Yu, L.Y.; Huang, Y.; Zhang, Z.J.; Liu, Y.Y. Analysis of complete chloroplast genomes of Curcuma and the contribution to phylogeny and adaptive evolution. Gene 2020, 732, 10. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Singh, A.K. Reference gene validation for qRT-PCR based gene expression studies in different developmental stages and under biotic stress in apple. Sci. Hortic. 2015, 197, 597–606. [Google Scholar] [CrossRef]
- Salazar-Salas, N.Y.; Chairez-Vega, D.A.; Vega-Alvarez, M.; Gonzalez-Nunez, D.G.; Pineda-Hidalgo, K.V.; Chavez-Ontiveros, J.; Delgado-Vargas, F.; Lopez-Valenzuela, J.A. Proteomic changes in mango fruit peel associated with chilling injury tolerance induced by quarantine hot water treatment. Postharvest Biol. Technol. 2022, 186, 15. [Google Scholar] [CrossRef]















| Serial Number | Species | Registry Number Accession | Serial Number | Species | Registry Number Accession |
|---|---|---|---|---|---|
| 1 | Euonymus maackii | NC_057059 | 20 | Drosera regia | NC_035415 |
| 2 | Euonymus fortunei | NC_057058 | 21 | Drosera rotundifolia | NC_029770 |
| 3 | Euonymus phellomanus | NC_057060 | 22 | Saxifraga stolonifera | NC_037882 |
| 4 | Euonymus szechuanensis | NC_047463 | 23 | Mitella diphylla | NC_042925 |
| 5 | Euonymus hamiltonianus | NC_037518 | 24 | Mitella formosana | NC_042926 |
| 6 | Euonymus schensianus | NC_036019 | 25 | Mukdenia rossii | NC_037495 |
| 7 | Euonymus japonicus | NC_028067 | 26 | Bergenia scopulosa | NC_036061 |
| 8 | Maytenus guangxiensis | NC_047301 | 27 | Tiarella cordifolia | NC_042927 |
| 9 | Salacia amplifolia | NC_047214 | 28 | Oresitrophe rupifraga | NC_037514 |
| 10 | Parnassia palustris | NC_045280 | 29 | Tiarella polyphylla | NC_042928 |
| 11 | Parnassia trinervis | NC_043951 | 30 | Tiarella trifoliata | NC_042929 |
| 12 | Catha edulis | KT861471 | 31 | Chrysosplenium aureobracteatum | NC_039740 |
| 13 | Ilex paraguariensis | NC_031207 | 32 | Hydrangea luteovenosa | NC_035662 |
| 14 | Ilex cornuta | NC_044416 | 33 | Hydrangea petiolaris | NC_034935 |
| 15 | Ilex integra | NC_044417 | 34 | Hydrangea serrata | NC_034934 |
| 16 | Pentaphylax euryoides | NC_035710 | 35 | Schizophragma hydrangeoides | NC_034936 |
| 17 | Tapiscia sinensis | NC_036960 | 36 | Itea chinensis | NC_037884 |
| 18 | Aldrovanda vesiculosa | NC_035416 | 37 | Penthorum chinense | NC_023086 |
| 19 | Drosera erythrorhiza | NC_035241 |
| Species | Frost Damage Level | |
|---|---|---|
| December 2020 | January 2021 | |
| E. japonicus Beihaidao | Grade 1 | Grade 2 |
| E. oblongifolius | Grade 1 | Grade 3 |
| E. microcarpus | Grade 3 | Grade 3 |
| Sample | Species | Time | Thickness of Palisade Tissue | Thickness of Spongy Tissue | CTR | SR |
|---|---|---|---|---|---|---|
| 1 | E. japonicus Beihaidao | 2020.09 | 116.42 ± 9.17 a | 153.05 ± 24.96 a | 36.89 ± 6.20 a | 48.02 ± 7.50 a |
| 2 | E. japonicus Beihaidao | 2020.12 | 90.00 ± 10.00 b | 105.00 ± 5.00 b | 38.52 ± 1.48 a | 45.37 ± 4.63 a |
| 3 | E. japonicus Beihaidao | 2021.05 | 103.66 ± 19.24 a | 139.04 ± 17.76 a | 34.28 ± 4.94 a | 46.36 ± 6.91 a |
| 4 | E. oblongifolius | 2020.09 | 106.66 ± 5.00 a | 130.00 ± 15.00 a | 40.95 ± 3.56 a | 49.82 ± 0.81 a |
| 5 | E. oblongifolius | 2020.12 | 146.33 ± 31.14 a | 166.25 ± 20.75 a | 41.46 ± 5.44 a | 47.43 ± 3.13 a |
| 6 | E. oblongifolius | 2021.05 | 115.71 ± 52.61 a | 141.43 ± 29.49 a | 36.55 ± 8.81 a | 48.42 ± 7.18 a |
| 7 | E. microcarpus | 2020.09 | 107.17 ± 14.68 a | 142.08 ± 8.40 a | 36.60 ± 9.04 a | 47.67 ± 5.70 a |
| 8 | E. microcarpus | 2020.12 | 105.00 ± 17.08 ab | 108.33 ± 16.75 b | 37.69 ± 5. 10 a | 38.86 ± 5.00 a |
| E. japonicus Beihaidao | E. hamiltonianus | E. phellomanus | E. schensianus | E. szechuanensis | |
|---|---|---|---|---|---|
| Total length Total length (bp) | 157,661 | 157,360 | 157,543 | 157,702 | 157,465 |
| Total GC content Total GC (%) | 37.26 | 37.25 | 37.30 | 37.19 | 37.18 |
| Length of LSC zone LSC Length (bp) | 85,932 | 86,399 | 86,299 | 86,026 | 86,257 |
| GC content in LSC area GC content in LSC region (%) | 35.09 | 35.10 | 35.18 | 35.02 | 35.00 |
| SSC zone length SSC Length (bp) | 18,363 | 18,317 | 18,536 | 18,528 | 18,472 |
| GC content in SSC area GC content in SSC region (%) | 31.77 | 31.70 | 31.78 | 31.70 | 31.63 |
| IR zone length IR Length (bp) | 26,683 | 26,322 | 26,354 | 26,574 | 26,368 |
| GC content in the IR region GC content in IR region (%) | 42.65 | 42.72 | 42.71 | 42.62 | 42.70 |
| Length of coding area Coding region length (bp) | 79,512 | 79,968 | 79,005 | 78,069 | 80,871 |
| GC content of the coding region GC content in coding region (%) | 38.11 | 38.03 | 38.15 | 38.19 | 38.02 |
| Length of non-coding area Noncoding region length (bp) | 78,149 | 77,392 | 78,538 | 79,633 | 76,594 |
| GC content of non-coding regions GC content in non-coding region (%) | 36.40 | 36.44 | 36.44 | 36.21 | 36.29 |
| Number of protein-coding genes Protein-coding gene number | 87 | 86 | 85 | 86 | 89 |
| GC content of protein-coding region GC content in protein-coding region (%) | 38.11 | 38.03 | 38.15 | 38.19 | 38.02 |
| rRNA GC content rRNA GC content (%) | 55.41 | 55.36 | 55.40 | 55.38 | 55.38 |
| tRNA GC content tRNA GC content (%) | 53.39 | 53.13 | 52.99 | 53.10 | 53.32 |
| Number of tRNAs Total tRNA | 37 | 37 | 37 | 37 | 37 |
| rRNA number Total rRNA | 8 | 8 | 8 | 8 | 8 |
| Number of genes Total gene number | 132 | 131 | 130 | 131 | 134 |
| High Variable Marker | Length | Nucleotide Diversity |
|---|---|---|
| trnH_psbA | 479 | 0.0827796 |
| trnS_trnG | 990 | 0.0598214 |
| trnG_trnR | 646 | 0.0419463 |
| petN_psbM | 581 | 0.0375138 |
| psbZ_trnG | 896 | 0.0727320 |
| trnM_atpE; trnV_trnM | 683 | 0.0477591 |
| rbcL_accD | 1400 | 0.0531015 |
| rpl22_rps19 | 397 | 0.0391588 |
| ycf1_ndhF | 1236 | 0.0712147 |
| ndhF_rpl32 | 857 | 0.0549155 |
| rpl2_rps19 | 491 | 0.0446429 |
| High Variable Marker | Length | Nucleotide Diversity |
|---|---|---|
| trnH_psbA | 581 | 0.1703297 |
| trnS_trnG | 758 | 0.2466063 |
| trnG_trnR | 102 | 0.1406121 |
| psbZ_trnG | 329 | 0.1709402 |
| trnP_ndhJ | 418 | 0.1515152 |
| psaI_ycf4 | 244 | 0.1283296 |
| trnP_psaJ | 237 | 0.1576169 |
| ycf1_ndhF | 692 | 0.1348788 |
| ycf1 | 420 | 0.1278873 |
| Positive Selection Genes | Group | Length | Ka/Ks | p-Value |
|---|---|---|---|---|
| atpE | E. hamiltonianus vs. E. japonicus Beihaidao | 399 | 50 | 0 |
| E. szechuanensis vs. E. japonicus Beihaidao | 399 | 50 | 0 | |
| atpI | E. schensianus vs. E. japonicus Beihaidao | 741 | 50 | 0 |
| E. szechuanensis vs. E. japonicus Beihaidao | 741 | 50 | 0 | |
| ndhB | S. amplifolia vs. E. japonicus Beihaidao | 1530 | 50 | 0 |
| ndhC | E. phellomanus vs. E. japonicus Beihaidao | 360 | 50 | 0 |
| E. schensianus vs. E. japonicus Beihaidao | 360 | 50 | 0 | |
| E. szechuanensis vs. E. japonicus Beihaidao | 360 | 50 | 0 | |
| petL | C. edulis vs. E. japonicus Beihaidao | 93 | 50 | 0 |
| E. hamiltonianus vs. E. japonicus Beihaidao | 93 | 50 | 0 | |
| E. phellomanus vs. E. japonicus Beihaidao | 93 | 50 | 0 | |
| psaI | E. phellomanus vs. E. japonicus Beihaidao | 111 | 50 | 0 |
| psbH | E. szechuanensis vs. E. japonicus Beihaidao | 219 | 50 | 0 |
| rpl20 | E. szechuanensis vs. E. japonicus Beihaidao | 351 | 50 | 0 |
| E. schensianus vs. E. japonicus Beihaidao | 351 | 50 | 0 | |
| rpl2 | E. schensianus vs. E. japonicus Beihaidao | 822 | 50 | 0 |
| E. szechuanensis vs. E. japonicus Beihaidao | 822 | 50 | 0 | |
| rps12 | C. edulis vs. E. japonicus Beihaidao | 369 | 50 | 0 |
| E. fortunei vs. E. japonicus Beihaidao | 369 | 50 | 0 | |
| rps8 | E. schensianus vs. E. japonicus Beihaidao | 393 | 50 | 0 |
| E. szechuanensis vs. E. japonicus Beihaidao | 393 | 50 | 0 |
| Qeury Name | Length | NR Gene ID | Swiss ID | COG/KOG | Function | KO/Gene_ID | KEGG Gene Name |
|---|---|---|---|---|---|---|---|
| atpE | 133 | AKF33728.1 | sp|Q09X11 |ATPE_MORIN | COG0355 |KOG1758 | C | K02114 | ATPF1E, atpC |
| atpI | 247 | AKF33710.1 | sp|P69372 |ATPI_TOBAC | COG0356 |KOG4665 | C | K02108 | ATPF0A, atpB |
| ndhB | 510 | AKF33783.1 | sp|P0CC41 |NU2C2_CARPA | COG1007 |KOG4668 | C | K05573 | ndhB |
| ndhC | 120 | AKF33727.1 | sp|A7Y3E3 |NU3C_IPOPU | COG0838 |KOG4662 | C | K05574 | ndhC |
| rpl2 | 274 | AVY52428.1 | sp|Q4VZK5 |RK2_CUCSA | COG0090 |KOG0438 | J | K02886 | RP-L2, MRPL2, rplB |
| rpl20 | 117 | AKF33744.1 | sp|P06386 |RK20_TOBAC | COG0292 |KOG4707 | J | K02887 | RP-L20, MRPL20, rplT |
| rps8 | 131 | AKF33757.1 | sp|Q49KW3 |RR8_EUCGG | COG0096 |KOG1754 | J | K02994 | RP-S8, rpsH |
| rps12 | 123 | ATD85454.1 | sp|Q0ZIZ5 |RR12_VITVI | COG0048 |KOG1750 | J | K02950 | RP-S12, MRPS12, rpsL |
| petL | 31 | _ | sp|A4QJU9 |PETL_OLIPU | _ | _ | _ | _ |
| psaI | 37 | AVY52374.1 | sp|P56768 |PSAI_ARATH | _ | _ | K02696 | psaI |
| psbH | 73 | AKF33751.1 | sp|P69553 |PSBH_OENEH | _ | _ | K02709 | psbH |
| Path Way | Layer 1 | Layer 2 | Pathway Definition | Kos List |
|---|---|---|---|---|
| ko00190 | 1. Metabolism | 1.2 Energy metabolism | Oxidative phosphorylation | atpE: K02114; atpI: K02108; ndhB: K05573; ndhC: K05574 |
| ko00195 | 1. Metabolism | 1.2 Energy metabolism | Photosynthesis | atpE: K02114; atpI: K02108; psaI: K02696; psbH: K02709 |
| ko01100 | 1. Metabolism | 1.0 Global and overview maps | Metabolic pathways | atpE: K02114; atpI: K02108; ndhB: K05573; ndhC: K05574 |
| ko03010 | 2. Genetic Information Processing | 2.2 Translation | Ribosome | rpl20: K02887; rpl2: K02886; rps12: K02950; rps8: K02994 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, H.; Gu, X.; Li, Y.; Ren, Y.; Yan, S.; Yang, M. Cold Resistance of Euonymus japonicus Beihaidao Leaves and Its Chloroplast Genome Structure and Comparison with Celastraceae Species. Plants 2022, 11, 2449. https://doi.org/10.3390/plants11192449
Cai H, Gu X, Li Y, Ren Y, Yan S, Yang M. Cold Resistance of Euonymus japonicus Beihaidao Leaves and Its Chloroplast Genome Structure and Comparison with Celastraceae Species. Plants. 2022; 11(19):2449. https://doi.org/10.3390/plants11192449
Chicago/Turabian StyleCai, Hongyu, Xiaozheng Gu, Yongtan Li, Yachao Ren, Shufang Yan, and Minsheng Yang. 2022. "Cold Resistance of Euonymus japonicus Beihaidao Leaves and Its Chloroplast Genome Structure and Comparison with Celastraceae Species" Plants 11, no. 19: 2449. https://doi.org/10.3390/plants11192449
APA StyleCai, H., Gu, X., Li, Y., Ren, Y., Yan, S., & Yang, M. (2022). Cold Resistance of Euonymus japonicus Beihaidao Leaves and Its Chloroplast Genome Structure and Comparison with Celastraceae Species. Plants, 11(19), 2449. https://doi.org/10.3390/plants11192449





