Identification of a BAHD Acyltransferase Gene Involved in Plant Growth and Secondary Metabolism in Tea Plants
Abstract
:1. Introduction
2. Results
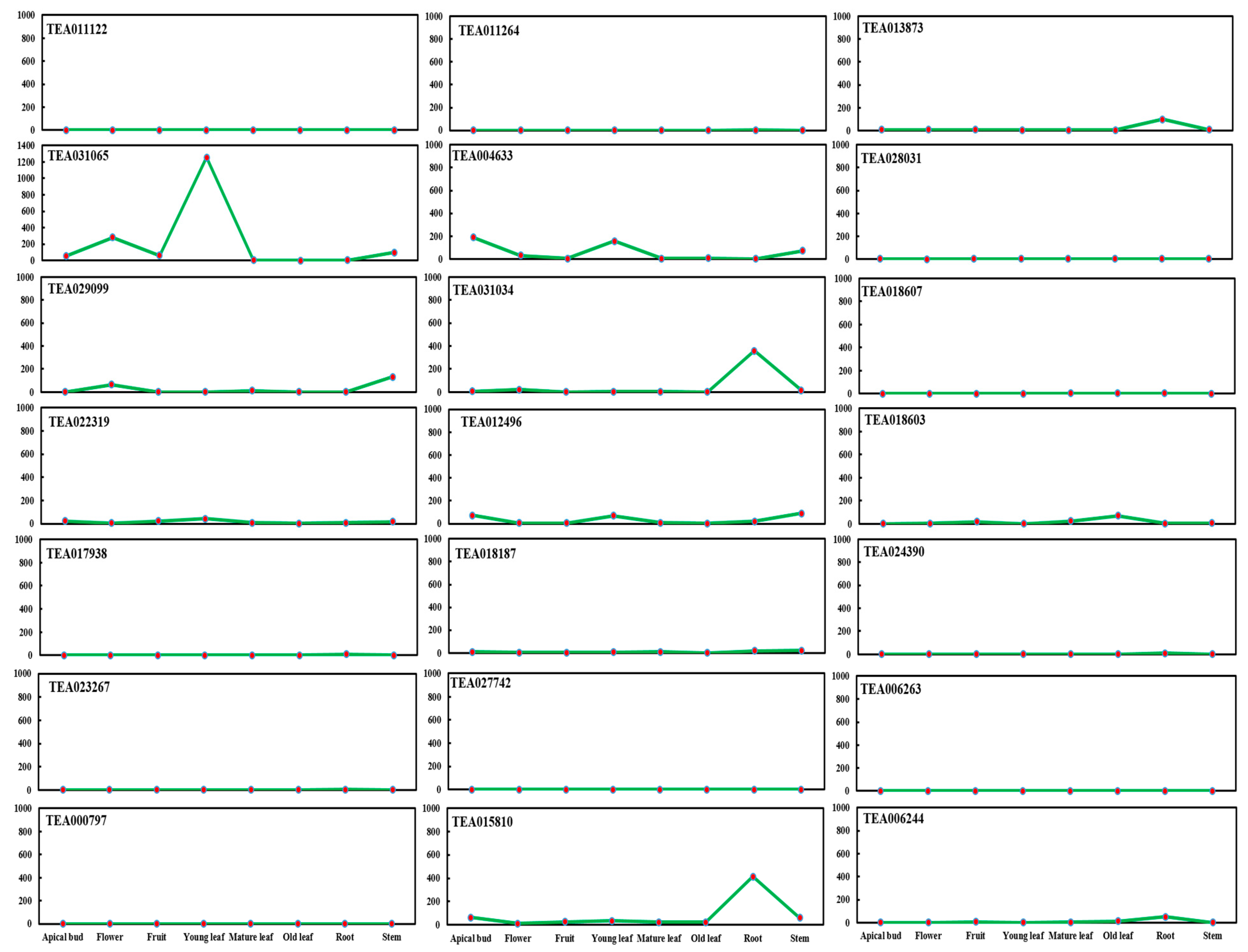
2.1. BAHD Acyltransferase Genes’ Expression in Tea Plant (C. sinensis)
2.2. BAHD Acyltransferase-DCR(TEA031065) Gene Expression in Three Different Tissues in Tea Plant (C. sinensis)
2.3. Phenotypical Expression and Statistical Analysis of Arabidopsis Root (A. thaliana) among Transgenic Plant and WT
2.4. Phenotypical Expression and Statistical Analysis of Arabidopsis Young Shoots (A. thaliana) among Transgenic Plant and WT
2.5. Anthocyanin Contents in Transgenic Leaves in A. thaliana under ½ MS Media
2.6. Transcriptional Changes in DEGs and the Reference Genome in Transgenic Plants (A. thaliana)
2.7. Analysis of DEGs (Differentially Expressed Genes) Involved in Plant Growth and Development under ½ MS Media
2.8. Analysis of Candidate Genes with Anthocyanin Production in A. thaliana
2.9. Analysis of DEGs (Differentially Expressed Genes) Involved in Flavonoid Biosynthesis under ½ MS Media
3. Discussion
3.1. TEA031065 Expression Pattern of BAHD Acyltransferase Genes Family in Various Tissues of Tea (C. sinensis) Plants
3.2. Phenotyping Expression of TEA031065 in A. thaliana
3.3. TEA031065 Might Play a Role as a Regulator for Plant Growth and Development Gene in Tea (C. sinensis) Plant
3.4. The Importance of TEA031065 in Tea (C. sinensis) Secondary Metabolites
4. Materials and Methods
4.1. Generation of Transgenic Arabidopsis Seedlings Overexpressing BAHD Acyltransferase (TEA031065)
4.2. Plant Growth Conditions and Over-Expression Lines
4.3. Anthocyanin Analysis
4.4. Analysis of RNA Sequencing
4.5. Identification of DEGs
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DCR | Defective in Cuticular Ridges |
| OX | Over expression |
| TF | Transcription factor |
| DEGs | Differentially Expressed Genes |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| ALDH7B4 | Aldehyde dehydrogenase |
| CAT | Catalase-related immune-responsive |
| UGT | UDP-glucoronosyl and UDP-glucosyl transferase |
| WRKY | WRKY DNA-binding domain |
| SAG | Protein phosphatase 2C |
| 4CL | 4-coumarate CoA ligase |
| CHI | Chalcone-flavanone isomerase |
| LODX | Leucoanthocyanidin dioxygenase |
| FPKM | Fragments per kb per million |
| ERF | Apetala2/ethylene response factor |
| NDK | Nucleoside diphosphate kinase |
| NCBI | Non-redundant protein database |
| SPSS | Statistical Package for the Social Sciences |
| FW | Fresh Weight |
| AHP | Histidine-containing phosphor-transmitters |
| GH3.5 | GH3 auxin-responsive promoter |
| PYL | Abscisic acid receptor PYL6 |
| SPCH | Basic helix-loop-helix (bHLH) DNA-binding superfamily protein |
| NIT2 | Carbon-nitrogen hydrolase |
| MS | MS media (Murashige–Skoog) |
| WT | Wild type |
| ETR | Response regulator receiver domain |
| 1AA | Auxin |
| GA | Gibberellin |
| ARR | Arabidopsis Response regulator receiver |
| PER | Peroxidase |
| PAL | Phenylalanine ammonia-lyase |
| CCR | Regulator of chromosome condensation |
| PCL | Primary congenital lymphedema |
| bHLH | Basic helix-loop-helix DNA-binding domain |
| CGA | Chlorogenic Acid |
| CHS | Chalcone and stilbene synthases |
| NDK | Nucleoside diphosphate kinase |
| PCA | Principal component analysis |
| ERS | His Kinase A (phospho-acceptor) domain |
| GSTF12 | Glutathione S-transferase F12 |
| 3AT | anthocyanin acyltransferase |
| GO | Gene Ontology |
| CCOAMT | O-methyltransferase |
| SAUR | Small auxin upregulated RNAs |
| CYP | Cytochrome P450 |
| BGLU | Glycosyl hydrolase |
| CAD | Cinnamyl alcohol dehydrogenase |
| MYB | A regulatory factor in anthocyanin production |
References
- Yu, S.W.; Li, P.H.; Zhao, X.C.; Tan, M.M.; Ahmad, M.Z.; Xu, Y.J.; Tadege, M.; Zhao, J. CsTCPs regulate shoot tip development and catechin biosynthesis in tea plant (Camellia sinensis). Hortic. Res. 2021, 8, 104. [Google Scholar]
- Zhao, M.T.; Li, J.; Zhou, S.S.; Rao, G.W.; Xu, D.M. Effects of tetracycline on the secondary metabolites and nutritional value of oilseed rape (Brassica napus L.). Environ. Sci. Pollut. Res. 2022, 1156, 1–12. [Google Scholar]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Ruocco, M.; Woo, S.; Lorito, M. Trichoderma Secondary Metabolites that Affect Plant Metabolism. Nat. Prod. Commun. 2012, 7, 1545–1550. [Google Scholar]
- Luo, J.; Nishiyama, Y.; Fuell, C.; Taguchi, G.; Elliott, K.; Hill, L.; Tanaka, Y.; Kitayama, M.; Yamazaki, M.; Bailey, P.; et al. Convergent evolution in the BAHD family of acyl transferases: Identification and characterization of anthocyanin acyl transferases from Arabidopsis thaliana. Plant J. 2007, 50, 678–695. [Google Scholar]
- Bontpart, T.; Cheynier, V.; Ageorges, A.; Terrier, N. BAHD or SCPL acyltransferase? What a dilemma for acylation in the world of plant phenolic compounds. New Phytol. 2015, 208, 695–707. [Google Scholar]
- Dudareva, N.; D’Auria, J.C.; Nam, K.H.; Raguso, R.A.; Pichersky, E. Acetyl-CoA:benzylalcohol acetyltransferase—An enzyme involved in floral scent production in Clarkia breweri. Plant J. 1998, 14, 297–304. [Google Scholar]
- Fujiwara, H.; Tanaka, Y.; Yonekura-Sakakibara, K.; Fukuchi-Mizutani, M.; Nakao, M.; Fukui, Y.; Yamaguchi, M.; Ashikari, T.; Kusumi, T. cDNA cloning, gene expression and subcellular localization of anthocyanin 5-aromatic acyltransferase from Gentiana triflora. Plant J. 1998, 16, 421–431. [Google Scholar]
- Yang, Q.; Trinh, H.X.; Imai, S.; Ishihara, A.; Zhang, L.; Nakayashiki, H.; Tosa, Y.; Mayama, S. Analysis of the involvement of hydroxyanthranilate hydroxycinnamoyltransferase and caffeoyl-CoA 3-O-methyltransferase in phytoalexin biosynthesis in oat. Mol. Plant Microbe Interact. 2004, 17, 81–89. [Google Scholar]
- St-Pierre, B.; Luca, V.D. Chapter Nine Evolution of acyltransferase genes: Origin and diversification fo the BAHD superfamily of acyltransferases involved in secondary metabolism. Plant Cell Rep. 2000, 34, 285–315. [Google Scholar]
- Hoffmann, L.; Besseau, S.; Geoffroy, P.; Ritzenthaler, C.; Meyer, D.; Lapierre, C.; Pollet, B.; Legrand, M. Acyltransferase-catalysed p- coumarate ester formation is a committed step of lignin biosynthesis. Plant Biosyst. 2005, 139, 50–53. [Google Scholar]
- Suzuki, H.; Nakayama, T.; Yonekura-Sakakibara, K.; Fukui, Y.; Nakamura, N.; Nakao, M.; Tanaka, Y.; Yamaguchi, M.A.; Kusumi, T.; Nishino, T. Malonyl-CoA:anthocyanin 5-O-glucoside-6‴-O-malonyltransferase from scarlet sage (Salvia splendens) flowers. Enzyme purification, gene cloning, expression, and characterization. J. Biol. Chem. 2001, 276, 49013–49019. [Google Scholar]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar]
- Panikashvili, D.; Shi, J.X.; Schreiber, L.; Aharoni, A. The Arabidopsis DCR encoding a soluble BAHD acyltransferase is required for cutin polyester formation and seed hydration properties. Plant Physiol. 2009, 151, 1773–1789. [Google Scholar]
- Lolle, S.J.; Hsu, W.; Pruitt, R.E. Genetic analysis of organ fusion in Arabidopsis thaliana. Genetics 1998, 149, 607–619. [Google Scholar]
- Matzke, C.M.; Shore, J.S. The Turnera Style S-Locus Gene TsBAHD Possesses Brassinosteroid-Inactivating Activity When Expressed in Arabidopsis thaliana. Plants 2020, 9, 1566. [Google Scholar]
- Sonnante, G.; D’Amore, R.; Emanuela, B.; Luo, J.; Pignone, D.; Martin, C. Isolation and Characterization of Genes for the Synthesis of Chlorogenic Acid in Artichoke. Plant Physiol. 2009, 942, 377–384. [Google Scholar]
- Barriere, Y.; Guillaumie, S.; Denoue, D.; Pichon, M.; Goffner, D.; Martinant, J.P. Investigating the unusually high cell wall digestibility of the old INRA early flint F4 maize inbred line. Maydica 2017, 62, 1–12. [Google Scholar] [CrossRef]
- Wilson, A.E.; Matel, H.D.; Tian, L. Glucose ester enabled acylation in plant specialized metabolism. Phytochem. Rev. 2016, 15, 1057–1074. [Google Scholar]
- Nakayama, T.; Suzuki, H.; Nishino, T. Anthocyanin acyltransferases: Specificities, mechanism, phylogenetics, and applications. J Mol. Catal. B-Enzym. 2003, 23, 117–132. [Google Scholar]
- Bartley, L.E.; Peck, M.L.; Kim, S.R.; Ebert, B.; Manisseri, C.; Chiniquy, D.M.; Sykes, R.; Gao, L.; Rautengarten, C.; Vega-Sánchez, M.E.; et al. Overexpression of a BAHD acyltransferase, OsAt10, alters rice cell wall hydroxycinnamic acid content and saccharification. Plant Physiol. 2013, 161, 1615–1633. [Google Scholar]
- Koyama, K.; Numata, M.; Nakajima, I.; Goto-Yamamoto, N.; Matsumura, H.; Tanaka, N. Functional characterization of a new grapevine MYB transcription factor and regulation of proanthocyanidin biosynthesis in grapes. J. Exp. Bot. 2014, 65, 4433–4449. [Google Scholar]
- Rinaldo, A.R.; Cavallini, E.; Jia, Y.; Moss, S.M.A.; McDavid, D.A.J.; Hooper, L.C.; Robinson, S.P.; Tornielli, G.B.; Zenoni, S.; Ford, C.M. A Grapevine Anthocyanin Acyltransferase, Transcriptionally Regulated by VvMYBA, Can Produce Most Acylated Anthocyanins Present in Grape Skins. Plant Physiol. 2015, 169, 1897–1916. [Google Scholar]
- Wang, M.J.; Liu, X.Y.; Wang, R.; Li, W.C.; Rodermel, S.; Yu, F. Overexpression of a putative Arabidopsis BAHD acyltransferase causes dwarfism that can be rescued by brassinosteroid. J. Exp. Bot. 2012, 63, 5787–5801. [Google Scholar]
- Sato-Izawa, K.; Ito, M.; Nuoendagula; Kajita, S.; Nakamura, S.; Matsumoto, T.; Ezura, H. Distinct deposition of ester-linked ferulic and p-coumaric acids to the cell wall of developing sorghum internodes. Plant Biotechnol. 2020, 37, 15–23. [Google Scholar]
- Li, Y.; Beisson, F.; Koo, A.J.; Molina, I.; Pollard, M.; Ohlrogge, J. Identification of acyltransferases required for cutin biosynthesis and production of cutin with suberin-like monomers. Proc. Natl. Acad. Sci. USA 2007, 104, 18339–18344. [Google Scholar]
- Panikashvili, D.; Shi, J.X.; Bocobza, S.; Franke, R.B.; Schreiber, L.; Aharoni, A. The Arabidopsis DSO/ABCG11 transporter affects cutin metabolism in reproductive organs and suberin in roots. Mol. Plant 2010, 3, 563–575. [Google Scholar]
- Wu, L.; Zhou, Z.Y.; Zhang, C.G.; Chai, J.; Zhou, Q.; Wang, L.; Hirnerova, E.; Mrvkova, M.; Novak, O.; Guo, G.Q. Functional Roles of Three Cutin Biosynthetic Acyltransferases in Cytokinin Responses and Skotomorphogenesis. PLoS ONE 2015, 10, e0121943. [Google Scholar]
- Feng, Z.M.; Wu, C.Y.; Wang, C.M.; Roh, J.; Zhang, L.; Chen, J.; Zhang, S.Z.; Zhang, H.; Yang, C.Y.; Hu, J.L.; et al. SLG controls grain size and leaf angle by modulating brassinosteroid homeostasis in rice. J. Exp. Bot. 2016, 67, 4241–4253. [Google Scholar]
- Zhao, J.; Li, P.; Xia, T.; Wan, X. Exploring plant metabolic genomics: Chemical diversity, metabolic complexity in the biosynthesis and transport of specialized metabolites with the tea plant as a model. Crit. Rev. Biotechnol. 2020, 40, 667–688. [Google Scholar]
- Gilbert, N. The science of tea’s mood-altering magic. Nature 2019, 566, S8–S9. [Google Scholar]
- Li, P.H.; Xia, E.H.; Fu, J.M.; Xu, Y.J.; Zhao, X.C.; Tong, W.; Tang, Q.; Tadege, M.; Fernie, A.R.; Zhao, J. Diverse roles of MYB transcription factors in regulating secondary metabolite biosynthesis, shoot development, and stress responses in tea plants (Camellia sinensis). Plant J. 2022, 110, 1144–1165. [Google Scholar]
- Wu, L.Y.; Fang, Z.T.; Lin, J.K.; Sun, Y.; Du, Z.Z.; Guo, Y.L.; Liu, J.H.; Liang, Y.R.; Ye, J.H. Complementary iTRAQ Proteomic and Transcriptomic Analyses of Leaves in Tea Plant (Camellia sinensis L.) with Different Maturity and Regulatory Network of Flavonoid Biosynthesis. J. Proteome Res. 2019, 18, 252–264. [Google Scholar]
- Ye, Q.; Liu, Y.; Chen, J.; Gai, X.; Ling, Y.; Dong, Y.; Tian, C. Research progress on chemical constituents and pharmacological activities of green tea. J. Drug Eval. 2021, 44, 2711–2719. [Google Scholar]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar]
- Grienenberger, E.; Besseau, S.; Geoffroy, P.; Debayle, D.; Heintz, D.; Catherine, L.; Pollet, B.; Heitz, T.; Legrand, M. A BAHD acyltransferase is expressed in the tapetum of Arabidopsis anthers and is involved in the synthesis of hydroxycinnamoyl spermidines. Plant J. 2009, 58, 246–259. [Google Scholar]
- Liao, Y.; Zhou, X.; Zeng, L. How does tea (Camellia sinensis) produce specialized metabolites which determine its unique quality and function: A review. Crit. Rev. Food Sci. 2021, 62, 3751–3767. [Google Scholar]
- Zhang, Y.; Wei, K.; Li, H.; Wang, L.; Ruan, L.; Pang, D.; Cheng, H. Identification of key genes involved in catechin metabolism in tea seedlings based on transcriptomic and HPLC analysis. Plant Physiol. Bioch. 2018, 133, 107–115. [Google Scholar]
- Wei, K.; Wang, L.; Zhang, Y.; Ruan, L.; Li, H.; Wu, L.; Xu, L.; Zhang, C.; Zhou, X.; Cheng, H.; et al. A coupled role for CsMYB75 and CsGSTF1 in anthocyanin hyperaccumulation in purple tea. Plant J. 2019, 97, 825–840. [Google Scholar]
- Aktar, S.; Zhang, Y.; Mao, P.; Lei, Y.; Bai, P.; Wang, Y.; Ruan, L.; Xun, H.; Wu, L.; Cheng, H.; et al. Responses of secondary metabolites and transcriptomes in the tea cultivar ‘Zhong Ming 6’ (Camellia sinensis) to blue light and red light. Plant Growth Regul. 2022, 1–16. [Google Scholar] [CrossRef]
- D’Auria, J.C. Acyltransferases in plants: A good time to be BAHD. Curr. Opin. Plant Biol. 2006, 9, 331–340. [Google Scholar]
- Bunsupa, S.; Okada, T.; Saito, K.; Yamazaki, M. An acyltransferase-like gene obtained by differential gene expression profiles of quinolizidine alkaloid-producing and nonproducing cultivars of Lupinus angustifolius. Plant Biotechnol. 2011, 28, 89–94. [Google Scholar]
- D’Auria, J.C.; Pichersky, E.; Schaub, A.; Hansel, A.; Gershenzon, J. Characterization of a BAHD acyltransferase responsible for producing the green leaf volatile (Z)-3-hexen-1-yl acetate in Arabidopsis thaliana. Plant J. 2007, 49, 194–207. [Google Scholar]
- Zhang, Y.; Gao, W.; Cui, C.; Zhang, Z.; He, L.; Zheng, J.; Hou, R. Development of a method to evaluate the tenderness of fresh tea leaves based on rapid, in-situ Raman spectroscopy scanning for carotenoids. Food Chem. 2020, 308, 125648. [Google Scholar]
- MacGregor, D.R.; Deak, K.I.; Ingram, P.A.; Malamy, J.E. Root System Architecture in Arabidopsis Grown in Culture Is Regulated by Sucrose Uptake in the Aerial Tissues. Plant Cell 2008, 20, 2643–2660. [Google Scholar]
- Franke, R.; Höfer, R.; Briesen, I.; Remus-Emsermann, M.; Efremova, N.; Yephremov, A.; Schreiber, L. The DAISY gene from Arabidopsis encodes a fatty acid elongase condensing enzyme involved in the biosynthesis of aliphatic suberin in roots and the chalaza-micropyle region of seeds. Plant J. 2008, 57, 80–95. [Google Scholar]
- Jetter, R. Examination of the Processes Involved in the Emission of Scent Volatiles from Flowers. Plant Sci. 2006, 1201, 125–144. [Google Scholar]
- Braynen, J.; Yang, Y.; Yuan, J.; Xie, Z.; Cao, G.; Wei, X.; Shi, G.; Zhang, X.; Wei, F.; Tian, B. Comparative transcriptome analysis revealed differential gene expression in multiple signaling pathways at flowering in polyploid Brassica rapa. Cell Biosci. 2021, 11, 17. [Google Scholar]
- Li, A.; Zhou, M.; Wei, D.; Chen, H.; You, C.; Lin, J. Transcriptome Profiling Reveals the Negative Regulation of Multiple Plant Hormone Signaling Pathways Elicited by Overexpression of C-Repeat Binding Factors. Front. Plant Sci. 2017, 8, 1647. [Google Scholar]
- Kurdyukov, S.; Faust, A.; Nawrath, C.; Bär, S.; Voisin, D.; Efremova, N.; Franke, R.; Schreiber, L.; Saedler, H.; Metraux, J.-P.; et al. The epidermis-specific extracellular BODYGUARD controls cuticle development and morphogenesis in Arabidopsis. Plant Cell 2006, 18, 321–339. [Google Scholar]
- Panikashvili, D.; Savaldi-Goldstein, S.; Mandel, T.; Yifhar, T.; Franke, R.; Höfer, R.; Schreiber, L.; Chory, J.; Aharoni, A. The arabidopsis DESPERADO/AtWBC11 transporter is required for cutin and wax secretion. Plant Physiol. 2008, 145, 1345–1360. [Google Scholar]
- Moglia, A.; Acquadro, A.; Eljounaidi, K.; Milani, A.M.; Cagliero, C.; Rubiolo, P.; Genre, A.; Cankar, K.; Beekwilder, J.; Comino, C. Genome-Wide Identification of BAHD Acyltransferases and In vivo Characterization of HQT-like Enzymes Involved in Caffeoylquinic Acid Synthesis in Globe Artichoke. Front. Plant Sci. 2016, 7, 1424. [Google Scholar]
- Tohge, T.; Nishiyama, Y.; Hirai, M.Y.; Yano, M.; Nakajima, J.; Awazuhara, M.; Inoue, E.; Takahashi, H.; Goodenowe, D.B.; Kitayama, M.; et al. Functional genomics by integrated analysis of metabolome and transcriptome of Arabidopsis plants over-expressing an MYB transcription factor. Plant J. 2005, 42, 218–235. [Google Scholar]
- Honda, C.; Kotoda, N.; Wada, M.; Kondo, S.; Kobayashi, S.; Soejima, J.; Zhang, Z.; Tsuda, T.; Moriguchi, T. Anthocyanin biosynthetic genes are coordinately expressed during red coloration in apple skin. Plant Physiol. Bioch. 2002, 40, 955–962. [Google Scholar]
- Ban, Y.; Kondo, S.; Ubi, B.E.; Honda, C.; Bessho, H.; Moriguchi, T. UDP-sugar biosynthetic pathway: Contribution to cyanidin 3-galactoside biosynthesis in apple skin. Planta 2009, 230, 871–881. [Google Scholar]
- Kumar, A.; Sharma, D.P.; Kumar, P.; Sharma, G.; Suprun, I.I. Comprehensive insights on Apple (Malus × domestica Borkh.) bud sport mutations and epigenetic regulations. Sci. Hortic. 2022, 297, 110979. [Google Scholar]
- Curaba, J.; Bostan, H.; Cavagnaro, P.F.; Senalik, D.; Mengist, M.F.; Zhao, Y.; Simon, P.W.; Iorizzo, M. Identification of an SCPL Gene Controlling Anthocyanin Acylation in Carrot (Daucus carota L.) Root. Front. Plant Sci. 2019, 10, 1770. [Google Scholar]
- Bent, A. Arabidopsis thaliana floral dip transformation method. Agrobact. Prot. 2006, 343, 87–104. [Google Scholar]
- Zhang, X.; Henriques, R.; Lin, S.S.; Niu, Q.W.; Chua, N.H. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 2006, 1, 641–646. [Google Scholar]
- Zheng, D.; Han, X.; An, Y.I.; Guo, H.; Xia, X.; Yin, W. The nitrate transporter NRT2.1 functions in the ethylene response to nitrate deficiency in Arabidopsis. Plant Cell Environ. 2013, 36, 1328–1337. [Google Scholar]
- Wei, K.; Zhang, Y.; Wu, L.; Li, H.; Ruan, L.; Bai, P.; Zhang, C.; Zhang, F.; Xu, L.; Wang, L.; et al. Gene expression analysis of bud and leaf color in tea. Plant Physiol. Bioch. 2016, 107, 310–318. [Google Scholar]
- Wei, K.; Wang, L.Y.; Zhang, C.C.; Wu, L.Y.; Li, H.L.; Zhang, F.; Cheng, H. Transcriptome Analysis Reveals Key Flavonoid 3′-Hydroxylase and Flavonoid 3′,5′-Hydroxylase Genes in Affecting the Ratio of Dihydroxylated to Trihydroxylated Catechins in Camellia sinensis. PLoS ONE 2015, 10, e0137925. [Google Scholar]
- Naeem, M.; Shahzad, K.; Saqib, S.; Shahzad, A.; Nasrullah; Younas, M.; Afridi, M.I. The Solanum melongena COP1LIKE manipulates fruit ripening and flowering time in tomato (Solanum lycopersicum). Plant Growth Regul. 2022, 96, 369–382. [Google Scholar]
- Wei, C.; Yang, H.; Wang, S.; Zhao, J.; Liu, C.; Gao, L.; Xia, E.; Lu, Y.; Tai, Y.; She, G.; et al. Draft genome sequence of Camellia sinensis var. sinensis provides insights into the evolution of the tea genome and tea quality. Proc. Natl. Acad. Sci. USA 2018, 115, E4151–E4158. [Google Scholar]
- Trapnell, C.; Hendrickson, D.G.; Sauvageau, M.; Goff, L.; Rinn, J.L.; Pachter, L. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 2013, 31, 46–53. [Google Scholar]
- Hu, M.; Fang, J.; Wang, H.; Zhou, S. Proteome and Phosphoproteome Analyses Reveal the Kinase Regulatory Network Involved in Glycogen Synthesis Kinase 3β. Front Genet. 2021, 12, 657140. [Google Scholar]







| Sample | Number of Reads (Million) | Total Base (Gb) | Q20% | Q30 (%) | Total Mapped (Million) | Mapping Rate (%) |
|---|---|---|---|---|---|---|
| WT_1 | 40.33 | 6.28 | 97.24 | 92.12 | 39.39 | 97.67 |
| WT_2 | 43.43 | 6.74 | 97.07 | 91.76 | 42.37 | 97.56 |
| WT_3 | 43.41 | 6.88 | 97.24 | 92.11 | 42.34 | 97.53 |
| OX1_1 | 43.83 | 6.86 | 97.3 | 92.26 | 42.82 | 97.68 |
| OX1_2 | 44.78 | 7.11 | 97.33 | 92.33 | 43.67 | 97.50 |
| OX1_3 | 40.83 | 6.52 | 97.2 | 92.02 | 39.76 | 97.38 |
| OX2_1 | 43.21 | 6.87 | 97.25 | 92.14 | 41.97 | 97.13 |
| OX2_2 | 42.52 | 6.75 | 97.32 | 92.29 | 41.42 | 97.41 |
| OX2_3 | 42.23 | 6.88 | 97.42 | 92.6 | 40.95 | 96.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aktar, S.; Bai, P.; Wang, L.; Xun, H.; Zhang, R.; Wu, L.; He, M.; Cheng, H.; Wang, L.; Wei, K. Identification of a BAHD Acyltransferase Gene Involved in Plant Growth and Secondary Metabolism in Tea Plants. Plants 2022, 11, 2483. https://doi.org/10.3390/plants11192483
Aktar S, Bai P, Wang L, Xun H, Zhang R, Wu L, He M, Cheng H, Wang L, Wei K. Identification of a BAHD Acyltransferase Gene Involved in Plant Growth and Secondary Metabolism in Tea Plants. Plants. 2022; 11(19):2483. https://doi.org/10.3390/plants11192483
Chicago/Turabian StyleAktar, Shirin, Peixian Bai, Liubin Wang, Hanshuo Xun, Rui Zhang, Liyun Wu, Mengdi He, Hao Cheng, Liyuan Wang, and Kang Wei. 2022. "Identification of a BAHD Acyltransferase Gene Involved in Plant Growth and Secondary Metabolism in Tea Plants" Plants 11, no. 19: 2483. https://doi.org/10.3390/plants11192483
APA StyleAktar, S., Bai, P., Wang, L., Xun, H., Zhang, R., Wu, L., He, M., Cheng, H., Wang, L., & Wei, K. (2022). Identification of a BAHD Acyltransferase Gene Involved in Plant Growth and Secondary Metabolism in Tea Plants. Plants, 11(19), 2483. https://doi.org/10.3390/plants11192483






