Distribution of Biodiversity of Wild Beet Species (Genus Beta L.) in Armenia under Ongoing Climate Change Conditions
Abstract
1. Introduction
- Distribution of the biodiversity of the wild beet species in the context of the impact of climate change over the last decade.
- Identification of Beta x intermedium Aloyan, the new natural hybrid between B. corolliflora and B. macrorhiza, and its phylogenic relation with the wild beet species recorded in the region.
- Conservation measures for the preservation and recovery of endangered or have been lost and may have become extinct genetic resources.
2. Results
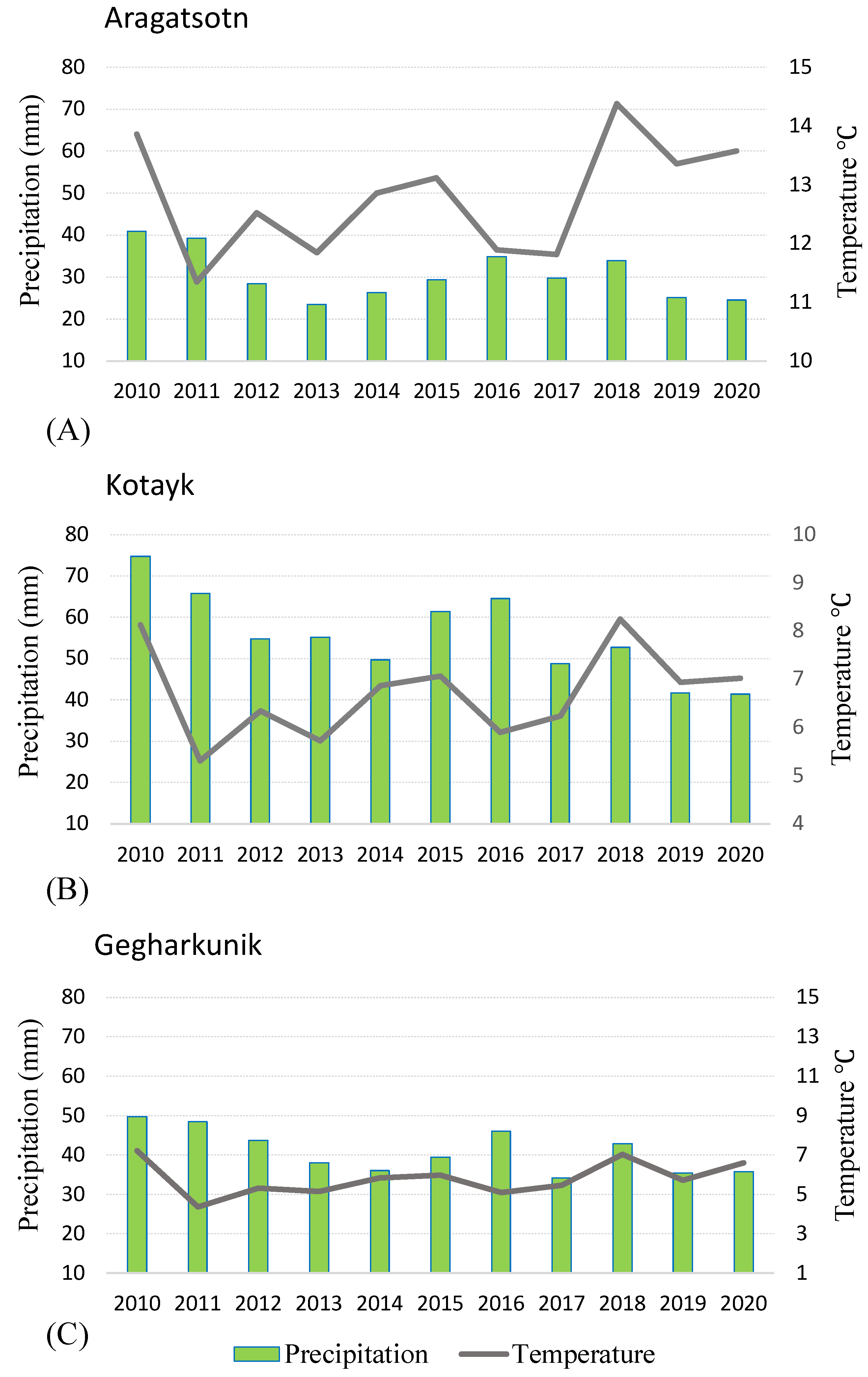
2.1. Distribution and Conservation
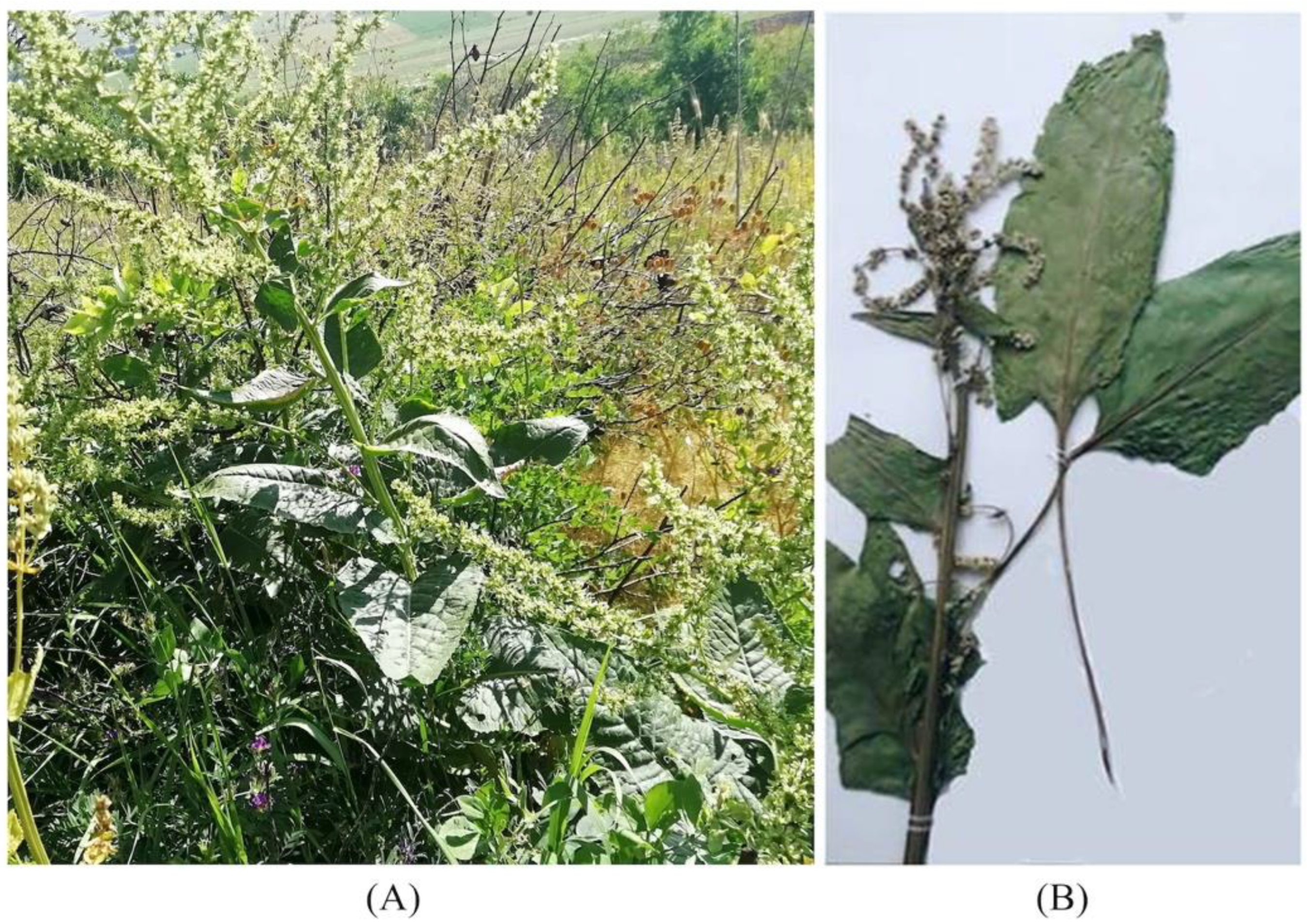
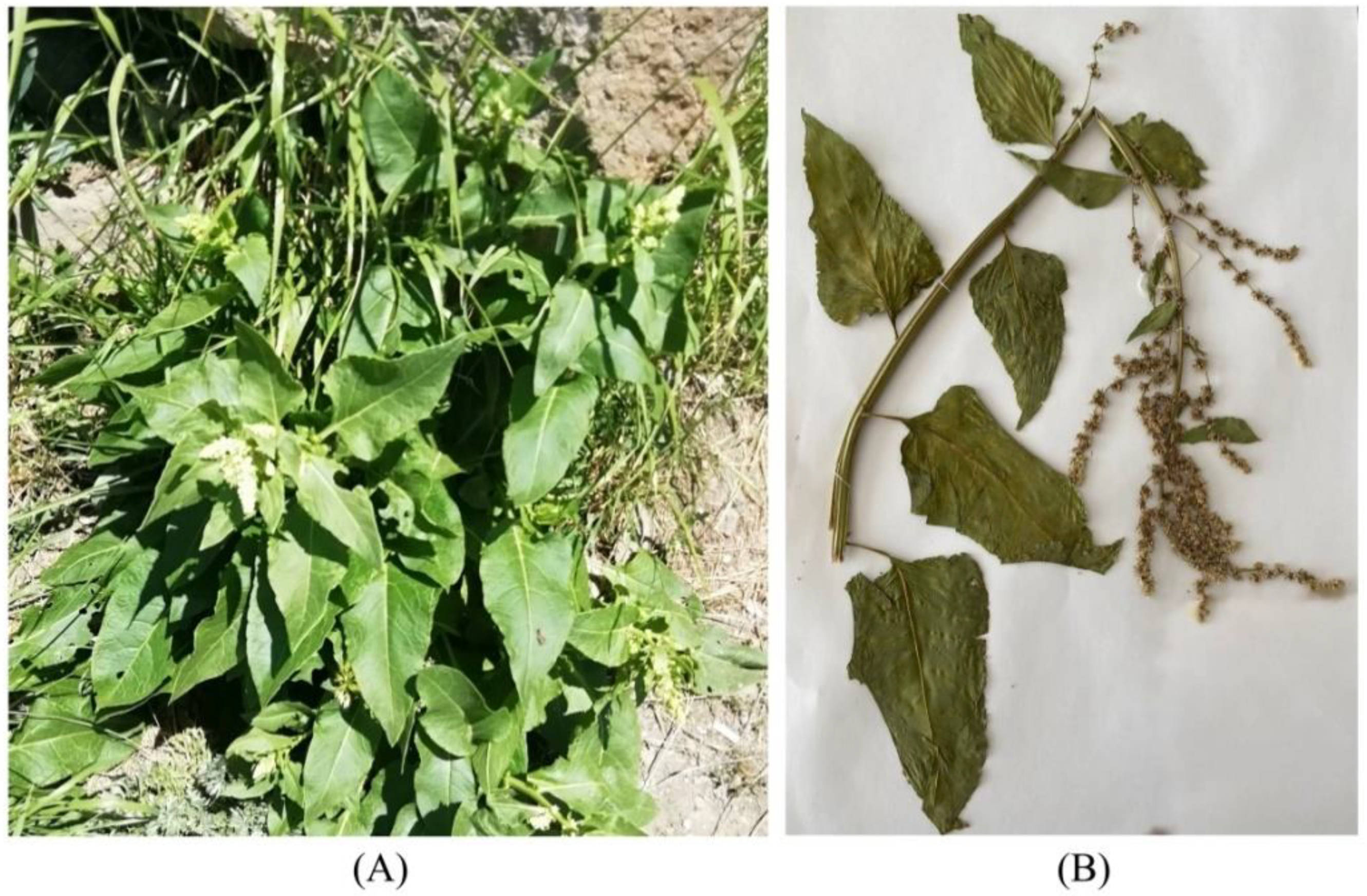
2.2. Morphology
2.3. Identification of Unknown Beta Sample
3. Discussion
4. Materials and Methods
4.1. Study Design and Sample Collection
4.2. Morphological Study
4.3. DNA Extraction, Amplification and Sequencing
4.4. Sequence Alignment and Phylogenetic Analysis
5. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fifth National Report of the Republic of Armenia to the Convention on Biological Diversity; Yerevan, Armenia, 2014; 107 p, Available online: https://www.cbd.int/doc/world/am/am-nr-05-en.pdf (accessed on 12 December 2021).
- Sixth National Report to the Convention on Biological Diversity of the Republic of Armenia; Yerevan, Armenia, 2019; 165 p, Available online: https://www.cbd.int/doc/nr/nr-06/am-nr-06-en.pdf. (accessed on 15 December 2021).
- Dostatny, D.F.; Żurek, G.; Kapler, A.; Podyma, W. The Ex Situ Conservation and Potential Usage of Crop Wild Relatives in Poland on the Example of Grasses. Agronomy 2021, 11, 94. [Google Scholar] [CrossRef]
- Hübner, S.; Kantar, M.B. Tapping Diversity From the Wild: From Sampling to Implementation. Front. Plant Sci. 2021, 12, 626565. [Google Scholar] [CrossRef] [PubMed]
- Burenin, V.I. Genetic Resources Genus Beta L. (Beet); VIR Publishing House: St. Petersburg, Russia, 2007; 274 p. (In Russian) [Google Scholar]
- Panella, L.; Lewellen, R.T. Broadening the Genetic Base of Sugar Beet: Introgression from Wild Relatives. Euphytica 2007, 154, 383–400. [Google Scholar] [CrossRef]
- Zhukovsky, P.M. Cultivated Plants and Their Wild Relatives; Kolos: Moscow, Russia, 1971; pp. 280–291. (In Russian) [Google Scholar]
- WBG Climate Change Knowledge Portal (CCKP, 2020). Climate Data: Historical. Available online: https://climateknowledgeportal.worldbank.org/country/armenia/climate-data-historical (accessed on 20 August 2022).
- Gevorgyan, A.; Melkonyan, H.; Aleksanyan, T.; Iritsyan, A.; Khalatyan, Y. An assessment of observed and projected temperature changes in Armenia. Arab. J. Geosci. 2016, 9, 27. [Google Scholar] [CrossRef]
- Gevorgyan, A. Verification of daily precipitation amount forecasts in Armenia by ERA-Interim model. Int. J. Climatol. 2013, 33, 2706–2712. [Google Scholar] [CrossRef]
- Gevorgyan, A. Surface and tropospheric temperature trends in Armenia. Int. J. Climatol. 2014, 34, 3559–3573. [Google Scholar] [CrossRef]
- Lydolph, P.E. Climates of the Soviet Union; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands; New York, NY, USA, 1977; pp. 1–443. [Google Scholar]
- Ministry of Environment. Republic of Armenia—Fourth National Communication on Climate Change under the UNFCCC; Yerevan, Armenia, 2020; 213 p, Available online: https://unfccc.int/sites/default/files/resource/NC4_Armenia_.pdf (accessed on 2 December 2021).
- Suonan, J.; Classen, A.T.; Sanders, N.J.; He, J. Plant phenological sensitivity to climate change on the Tibetan Plateau and relative to other areas of the world. Ecosphere 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Malcolm, J.R.; Liu, C.; Neilson, R.P.; Hansen, L.; Hannah, L. Global Warming and Extinctions of Endemic Species from Biodiversity Hotspots. Conserv. Biol. 2006, 20, 538–548. [Google Scholar] [CrossRef]
- Climate Risk Country Profile: Armenia: The World Bank Group and the Asian Development Bank; Yerevan, Armenia, 2021; 22 p, Available online: https://www.adb.org/publications/climate-risk-country-profile-armenia (accessed on 5 January 2022).
- Ceclu, L.; Oana-Viorela, N. Red Beetroot: Composition and Health Effects—A Review. J. Nutr. Med. Diet Care 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Babarykin, D.; Smirnova, G.; Pundinsh, I.; Vasiljeva, S.; Krumina, G.; Agejchenko, V. Red Beet (Beta vulgaris) Impact on Human Health. J. Biosci. Med. 2019, 07, 61–79. [Google Scholar] [CrossRef]
- Heratsi, M. Relief of Fevers; Institute of Language after H. Acharian: Yerevan, Armenia, 2019; 1231 p. (In Armenian) [Google Scholar]
- Hamedi, S.; Honarvar, M. Beta vulgaris—A Mini Review of Traditional Uses in Iran, Phytochemistry and Pharmacology. Curr. Drug Discov. Technol. 2019, 16, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Biancardi, E.; Panella, L.W.; Lewellen, R.T. Beta Maritima. The Origin of Beets; Springer: New York, NY, USA, 2012; ISBN 978-1-4614-0841-3. [Google Scholar]
- Pin, P.A.; Zhang, W.; Vogt, S.H.; Dally, N.; Büttner, B.; Schulze-Buxloh, G.; Jelly, N.S.; Chia, T.Y.P.; Mutasa-Göttgens, E.S.; Dohm, J.C.; et al. The Role of a Pseudo-Response Regulator Gene in Life Cycle Adaptation and Domestication of Beet. Curr. Biol. 2012, 22, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Alishan, G. Haybusak; San Lazzaro: Venice, Italy, 1895; pp. 395–396. (In Armenian) [Google Scholar]
- Vincent, H.; Wiersema, J.; Kell, S.; Fielder, H.; Dobbie, S.; Castañeda-Álvarez, N.P.; Guarino, L.; Eastwood, R.; Leόn, B.; Maxted, N. A Prioritized Crop Wild Relative Inventory to Help Underpin Global Food Security. Biol. Conserv. 2013, 167, 265–275. [Google Scholar] [CrossRef]
- Kadereit, G.; Hohmann, S.; Kadereit, J.W. A Synopsis of Chenopodiaceae Subfam. Betoideae and Notes on the Taxonomy of Beta. Willdenowia 2006, 36, 9–19. [Google Scholar] [CrossRef]
- Takhtajian, A.L. Flora of Armenia, V.2; Academy of Sciences of the Armenian SSR: Yerevan, Armenia, 1956; pp. 280–291. (In Russian) [Google Scholar]
- Melikyan, A.S. Biological Peculiarities and Variety of Usage of Several Wild Vegetable Plants Spread Throughout Armenia; Melikyan, A.S., Ed.; Gayison: Yerevan, Armenia, 2001; pp. 4–26. (In Armenian) [Google Scholar]
- Gabrielian, E.; Zohary, D. Wild relatives of food crops native to Armenia and Nakhichevan. Flora Mediterr. 2004, 14, 80. [Google Scholar]
- Herbarium collection of the Institute of Botany after A. Takhtajyan NAS RA. Available online: https://www.sci.am/orgsview.php?id=12&langid=2 (accessed on 10 July 2021).
- GBIF.org. Available online: https://doi.org/10.15468/dl.t4pkfb (accessed on 28 December 2021).
- Aleksidze, G.; Akparov, Z.; Melikyan, A.; Nasser Arjmand, M. Biodiversity of Beta species in the Caucasus Region (Armenia, Azerbaijan, Georgia, Iran). In Report of a Working Group on Beta and the World Beta Network; Bioversity International, Frese, L., Maggioni, L., Lipman, E., Eds.; 2009; pp. 38–40. ISBN 978-92-9043-815-1. Available online: https://www.ecpgr.cgiar.org/fileadmin/bioversity/publications/pdfs/1353_Report_Working_Group_Beta_World_Beta_Network.pdf (accessed on 20 December 2021).
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Published on the Internet. 2022. Available online: http://www.plantsoftheworldonline.org/ (accessed on 25 August 2022).
- The Red Book of Plants of the Republic of Armenia, 2nd ed.; Zangak-97: Yerevan, Armenia, 2012; pp. 210–211. (In Armenian)
- Melikyan, A.S. The Features of ex situ and in situ Conservation of Beta in Armenia. ECPGR Work. Gr. Beta WBN Jt. Meet. Capelle-en-Pévèle, Abstr. Broch. 2012, 23. [Google Scholar]
- Petkeviciene, B. The effects of climate factors on sugar beet early sowing timing. Agron. Res. 2009, 7, 436–443. [Google Scholar]
- Compagnoni, A.; Levin, S.; Childs, D.Z.; Harpole, S.; Paniw, M.; Römer, G.; Burns, J.H.; Che-Castaldo, J.; Rüger, N.; Kunstler, G.; et al. Herbaceous perennial plants with short generation time have stronger responses to climate anomalies than those with longer generation time. Nat. Commun. 2021, 12, 1824. [Google Scholar] [CrossRef]
- Hu, A.; Zhang, J.; Chen, X.; Chang, S.; Hou, F. Winter Grazing and Rainfall Synergistically Affect Soil Seed Bank in Semiarid Area. Rangel. Ecol. Manag. 2019, 72, 160–167. [Google Scholar] [CrossRef]
- van Langevelde, F.; Tessema, Z.K.; de Boer, W.F.; Prins, H.H.T. Soil seed bank dynamics under the influence of grazing as alternative explanation for herbaceous vegetation transitions in semi-arid rangelands. Ecol. Modell. 2016, 337, 253–261. [Google Scholar] [CrossRef]
- Akopian, J. On the flowering biology of Beta corolliflora and Hablitzia tamnoides (Betoideae, Chenopodiaceae). Takhtajania 2020, 6, 42–46. [Google Scholar]
- Martins, A.A.; Opedal, Ø.H.; Armbruster, W.S.; Pélabon, C. Rainfall seasonality predicts the germination behavior of a tropical dry-forest vine. Ecol. Evol. 2019, 9, 5196–5205. [Google Scholar] [CrossRef] [PubMed]
- Gutterman, Y.; Gozlan, S. Amounts of winter or summer rain triggering germination and “the point of no return” of seedling desiccation tolerance, of some Hordeum spontaneum local ecotypes in Israel. Plant Soil 1998, 204, 223–234. [Google Scholar] [CrossRef]
- Travis, J.M.J. Climate change and habitat destruction: A deadly anthropogenic cocktail. Proc. R. Soc. London. Ser. B Biol. Sci. 2003, 270, 467–473. [Google Scholar] [CrossRef]
- Jantz, S.M.; Barker, B.; Brooks, T.M.; Chini, L.P.; Huang, Q.; Moore, R.M.; Noel, J.; Hurtt, G.C. Future habitat loss and extinctions driven by land-use change in biodiversity hotspots under four scenarios of climate-change mitigation. Conserv. Biol. 2015, 29, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Schierenbeck, K.A. Population-level genetic variation and climate change in a biodiversity hotspot. Ann. Bot. 2017, 119, 215–228. [Google Scholar] [CrossRef]
- Bonin, A.; Ehrich, D.; Manel, S. Statistical analysis of amplified fragment length polymorphism data: A toolbox for molecular ecologists and evolutionists. Mol. Ecol. 2007, 16, 3737–3758. [Google Scholar] [CrossRef]
- Gray, S.B.; Brady, S.M. Plant developmental responses to climate change. Dev. Biol. 2016, 419, 64–77. [Google Scholar] [CrossRef]
- Guerin, G.R.; Wen, H.; Lowe, A.J. Leaf morphology shift linked to climate change. Biol. Lett. 2012, 8, 882–886. [Google Scholar] [CrossRef]
- Kalisz, S.; Kramer, E.M. Variation and constraint in plant evolution and development. Heredity 2008, 100, 171–177. [Google Scholar] [CrossRef]
- Gaut, B.; Yang, L.; Takuno, S.; Eguiarte, L.E. The Patterns and Causes of Variation in Plant Nucleotide Substitution Rates. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 245–266. [Google Scholar] [CrossRef]
- Ministry of Nature Protection, Republic of Armenia. Vulnerability of Water Resources in the Republic of Armenia under Climate Change. Prepared under the United Nations Development Programme/Global Environment Facility (UNDP/GEF) Project Implemented by UNDP Armenia and Executed by Ministry of Nature Protection of the Republic of Armenia. Yerevan, Armenia. 2009; 27p. Available online: http://www.nature-ic.am/Content/announcements/7319/WATER-Resources-Vulnerability_Eng_2009_1.pdf (accessed on 21 August 2022).
- Margaryan, L.A. Assessment of the Climate Change Impact on the Quality and Quantity of Drinking Water Sources in Armenia. Russ. J. Gen. Chem. 2017, 87, 3160–3165. [Google Scholar] [CrossRef]
- Ministry of Nature Protection. Republic of Armenia—Second National Communication on Climate Change; Yerevan, Armenia, 2010; 101 p, Available online: https://unfccc.int/resource/docs/natc/armnc2e.pdf (accessed on 15 December 2021).
- Huang, J.; Yu, H.; Guan, X.; Wang, G.; Guo, R. Accelerated dryland expansion under climate change. Nat. Clim. Chang. 2016, 6, 166–171. [Google Scholar] [CrossRef]
- McFARI.ANE, J.S. Naturally Occurring Hybrids Between Sugarbeet and Beta macrocarpa in the Imperial Valley of California. J. Am. Soc. Sugar Beet Technol. 1975, 18, 245–251. [Google Scholar] [CrossRef]
- Abe, J.; Tsuda, C. Distorted Segregation in the Backcrossed Progeny between Beta vulgaris L. and B. macrocarpa Guss. Ikushugaku Zasshi 1988, 38, 309–318. [Google Scholar] [CrossRef]
- Coons, G.H. The wild species of Beta. Proc. Am. Soc. Sugar Beet Technol. 1954, 8, 142–147. [Google Scholar]
- Pivovarov, V.F.; Burenin, V.I. Beet; Leytan, V.G., Ed.; VIR: Saint Petersburg, Russia, 1998; 214 p. (In Russian) [Google Scholar]
- Van Geyt, J.P.C.; Lange, W.; Oleo, M.; De Bock, T.S.M. Natural variation within the genus Beta and its possible use for breeding sugar beet: A review. Euphytica 1990, 49, 57–76. [Google Scholar] [CrossRef]
- Chunco, A.J. Hybridization in a warmer world. Ecol. Evol. 2014, 4, 2019–2031. [Google Scholar] [CrossRef]
- Klonner, G.; Dullinger, I.; Wessely, J.; Bossdorf, O.; Carboni, M.; Dawson, W.; Essl, F.; Gattringer, A.; Haeuser, E.; van Kleunen, M.; et al. Will climate change increase hybridization risk between potential plant invaders and their congeners in Europe? Divers. Distrib. 2017, 23, 934–943. [Google Scholar] [CrossRef]
- Counterman, B.A. Hybrid Speciation. In Encyclopedia of Evolutionary Biology; Kliman, R.M., Ed.; Elsevier: Cambridge, MA, USA, 2016; pp. 242–248. [Google Scholar]
- Leal, B.S.S.; Brandão, M.M.; Palma-Silva, C.; Pinheiro, F. Differential gene expression reveals mechanisms related to habitat divergence between hybridizing orchids from the Neotropical coastal plains. BMC Plant Biol. 2020, 20, 554. [Google Scholar] [CrossRef]
- Goudarzi, F.; Hemami, M.-R.; Rancilhac, L.; Malekian, M.; Fakheran, S.; Elmer, K.R.; Steinfartz, S. Geographic separation and genetic differentiation of populations are not coupled with niche differentiation in threatened Kaiser’s spotted newt (Neurergus kaiseri). Sci. Rep. 2019, 9, 6239. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y. Sewall Wright and Gustave Malécot on Isolation by Distance. Philos. Sci. 2009, 76, 784–796. [Google Scholar] [CrossRef]
- Sexton, J.P.; Hangartner, S.B.; Hoffmann, A.A. Genetic Isolation by Environment or Distance: Which Pattern of Gene Flow is Most Common? Evolution 2014, 68, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.M.; Chen, G.F.; Watt, L.R.; Schemske, D.W. The Biology of Speciation. Evolution 2010, 64, 295–315. [Google Scholar] [CrossRef]
- Wright, S. Isolation by Distance. Genetics 1943, 28, 114–138. [Google Scholar] [CrossRef]
- Chichorro, F.; Juslén, A.; Cardoso, P. A review of the relation between species traits and extinction risk. Biol. Conserv. 2019, 237, 220–229. [Google Scholar] [CrossRef]
- Ascarini, F.; Ragonezi, C. Assessing the Diversity of Sea Beet (Beta vulgaris L. ssp. maritima) Populations. J. Agric. Sci. Technol. 2021, 23, 685–698. [Google Scholar]
- Touzet, P.; Villain, S.; Buret, L.; Martin, H.; Holl, A.-C.; Poux, C.; Cuguen, J. Chloroplastic and nuclear diversity of wild beets at a large geographical scale: Insights into the evolutionary history of the Beta section. Ecol. Evol. 2018, 8, 2890–2900. [Google Scholar] [CrossRef]
- Veloso, M.M.; Simões-Costa, M.C.; Guimarães, J.B.; Ribeiro, C.M.; Evaristo, I.; Espírito-Santo, D.; Pinto-Ricardo, C.; Paulo, O.S.; Duarte, M.C. Genetic Diversity and Population Structure of Wild Beets (Beta spp.) from the Western Iberian Peninsula and the Azores and Madeira Islands. Diversity 2021, 13, 593. [Google Scholar] [CrossRef]
- Ribeiro, I.C.; Pinheiro, C.; Ribeiro, C.M.; Veloso, M.M.; Simoes-Costa, M.C.; Evaristo, I.; Paulo, O.S.; Ricardo, C.P. Genetic Diversity and Physiological Performance of Portuguese Wild Beet (Beta vulgaris spp. maritima) from Three Contrasting Habitats. Front. Plant Sci. 2016, 7, 1293. [Google Scholar] [CrossRef]
- Andrello, M.; Henry, K.; Devaux, P.; Desprez, B.; Manel, S. Taxonomic, Spatial and Adaptive Genetic Variation of Beta Section Beta. Theor. Appl. Genet. 2016, 129, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Albano, A.; Alessandrini, A.; Ardenghi, N.M.G.; Astuti, G.; Bacchetta, G.; Ballelli, S.; Banfi, E.; et al. An Updated Checklist of the Vascular Flora Native to Italy. Plant Biosyst. 2018, 152, 179–303. [Google Scholar] [CrossRef]
- Buttler, K.P. Revision von Beta Section Corollinae (Chenopodiaceae) I. Selbststerile Basisarten. Mitt. Bot. Staatssamml Munch 1977, 13, 255–336. [Google Scholar]
- Shen, Y.; Ford-Lloyd, B.V.; Newbury, H.J. Genetic relationships within the genus Beta determined using both PCR-based marker and DNA sequencing techniques. Heredity 1998, 80, 624–632. [Google Scholar] [CrossRef]
- Physical Geography of the Armenian SSR; Publishing House of the Academy of Sciences of the Armenian SSR: Yerevan, Armenia, 1971; 471 p. (In Armenian)
- Taberlet, P.; Gielly, L.; Pautou, G.; Bouvet, J. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol. Biol. 1991, 17, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- McWilliam, H.; Li, W.; Uludag, M.; Squizzato, S.; Park, Y.M.; Buso, N.; Cowley, A.P.; Lopez, R. Analysis Tool Web Services from the EMBL-EBI. Nucleic Acids Res. 2013, 41, W597–W600. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Hohmann, S.; Kadereit, J.W.; Kadereit, G. Understanding Mediterranean-Californian disjunctions: Molecular evidence from Chenopodiaceae-Betoideae. Taxon 2006, 55, 67–78. [Google Scholar] [CrossRef]
- Harutyunyan, M.; Avagyan, A.; Melyan, G.; Hovhannisyan, M. The Conservation of Agro-Biodiversity of Armenia in the National Seed Collections. In Proceedings of the International Conference on "Protection of Agrobiodiversity and Sustainable Develop-ment of Agriculture", Tbilisi, Georgia; 2010; pp. 165–167. (In Russian). [Google Scholar]
- Avagyan, A. Crop wild relatives in Armenia: Diversity, legislation and conservation issues. In Crop Wild Relative Conservation and Use; CABI: Wallingford, UK, 2007; pp. 58–66. [Google Scholar]
- Skedsmo, P.W.; Andersen, R. Governing crop genetics in post-Soviet countries: Lessons from the biodiversity hotspot Armenia. Euphytica 2021, 217, 94. [Google Scholar] [CrossRef]
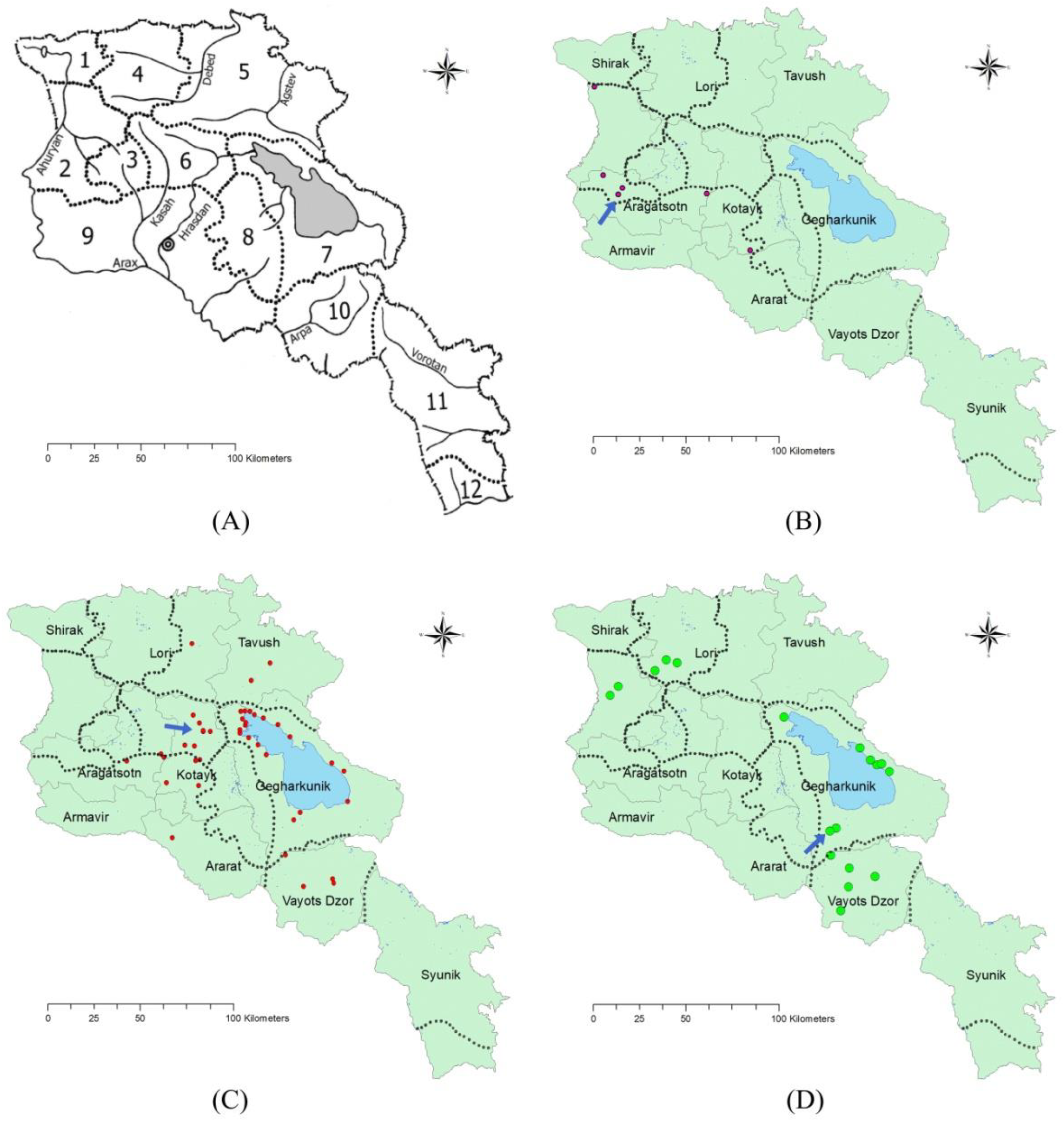
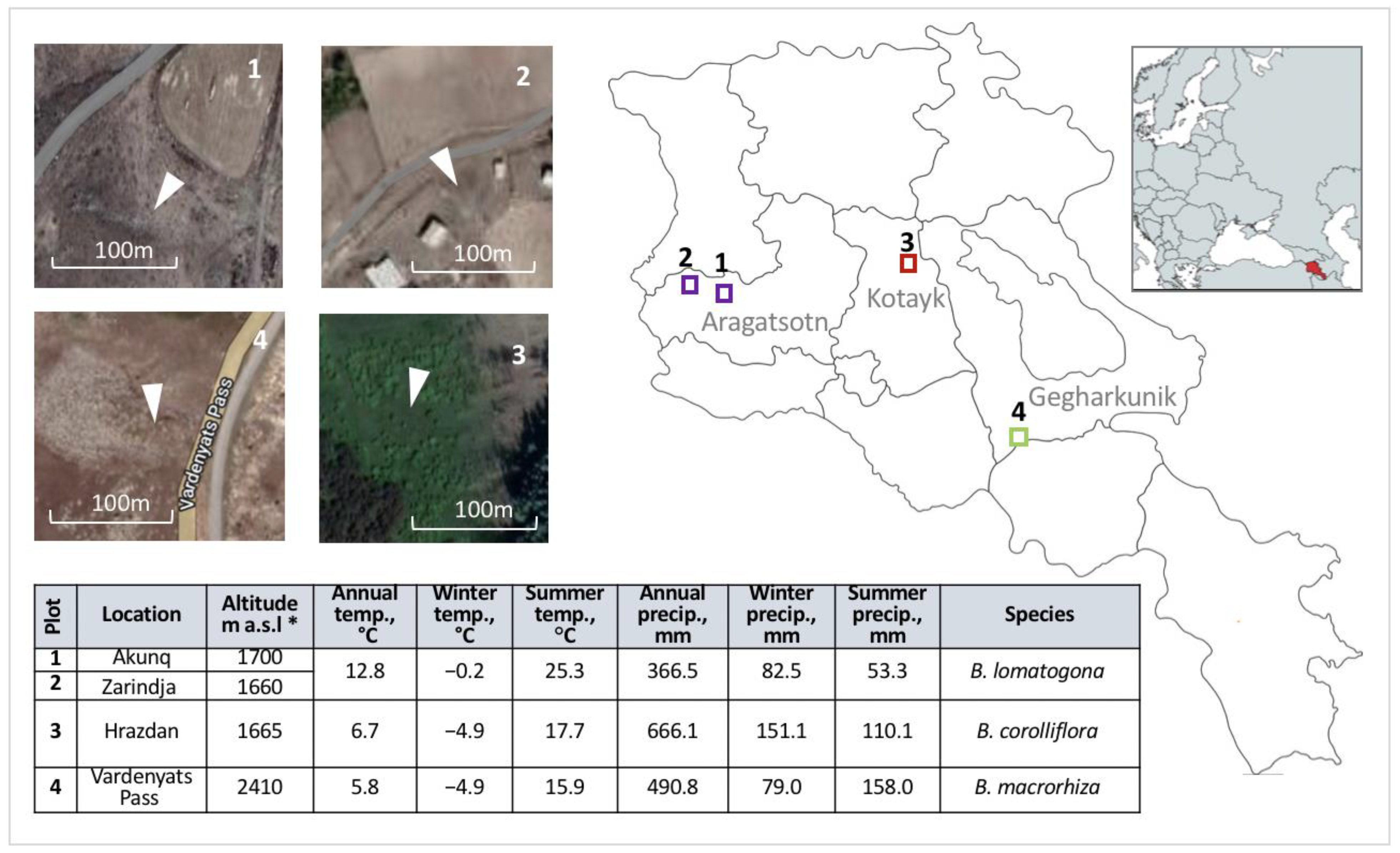




| Species | Floristic Region | Province (Marz) | Location | Collection Year | Altitude, m a.s.l. | Source and/or Reference |
|---|---|---|---|---|---|---|
| B. lomatogona | Shirak | Aragatsotn | Zarindja | 1988 | 1660 | EURISCO catalog, Acc. № 8L03, [27] |
| Akunq | 1987 | 1700 | EURISCO catalog, Acc. № 8L01, [27] | |||
| 2006 | 1700 | EURISCO catalog, Acc. № 8L04, This study | ||||
| 2021 | 1789 | EURISCO catalog, Acc. № 8L05, This study | ||||
| Shirak | Dzithankov | 1987 | 1740 | EURISCO catalog, Acc. № 8L02, [27] | ||
| Gtashen | - | 1880 | [28] | |||
| Yerevan | Yerevan Kotayk | North from Yerevan | 1933 | 1500 | [29] | |
| 1956 | 1390 | [26,28] | ||||
| Aparan | Kotayk | Mount Ara | 2002 | 1990 | [30] | |
| B. corolliflora | Ijevan | Tavush | Haghartsin, Hovq | - | 1300 | [28] |
| Lori | Lori | Dsegh | 1987 | 1350 | EURISCO catalog, Acc. № 8C06; [27] | |
| Darelegis | Vayots Dzor | Gladzor | 1987 | 2000 | EURISCO catalog, Acc. № 8C07 | |
| Karmrashen | 1980 | 2065 | EURISCO catalog, Acc. № 8C01 | |||
| 1999 | - | [29] | ||||
| Between Karmrashen and Herher | 2003 | 1820 | [30] | |||
| Aparan | Aragatsotn | Aparan reservoir | 1988 | 1800 | EURISCO catalog, Acc. № 8C11; [27,28,31] | |
| Amberd castle | 1988 | 2300 | EURISCO catalog, Acc. № 8C12; [27] | |||
| 2001 | [29] | |||||
| Mount Ara | 1980 | 2240 | [30] | |||
| 2021 | 2360 | This study | ||||
| Kotayk | Teghenik | 1987 | 1600 | EURISCO catalog, Acc. № 8C02; [27] | ||
| Akunq | 1988 | 1400 | EURISCO catalog, Acc. № 8C13 | |||
| Fantan | 1987 | 1800 | EURISCO catalog, Acc. № 8C04; [27] | |||
| Hatis | 1987 | - | EURISCO catalog, Acc. № 8C05; [27] | |||
| Hrazdan | 1956 | - | [29] | |||
| - | 1550 | [30] | ||||
| 2016- 2019 | 1675 | EURISCO catalog, Acc. № 8C18; This study | ||||
| 2021 | EURISCO catalog, Acc. № 8C19; This study | |||||
| Aghavnadzor | 2017 | 1750 | EURISCO catalog, Acc. № 8C16; This study | |||
| Meghradzor | 1987 | 1960 | EURISCO catalog, Acc. № 8C03; [27] | |||
| Tsakhkadzor | 1984 | 1980 | [30] | |||
| 2017 | 1825 | EURISCO catalog, Acc. № 8C17; This study | ||||
| Arzakan | 2002 | 1670 | [30] | |||
| Yerevan | Abovyan | 2002 | 1630 | [28,29] | ||
| Sevan | Gegharkunk | Sevan Lake district | 1989 | 1900 | EURISCO catalog, Acc. № 8C14; [27] | |
| 2007 | - | [29] | ||||
| Pambak | 1987 | 1985 | EURISCO catalog, Acc. № 8C09; [27] | |||
| 2000 | 1985 | EURISCO catalog, Acc. № 8C15; [27] | ||||
| Areguni mountain range | 1987 | 2220 | EURISCO catalog, Acc. № 8C08; [27] | |||
| Vardenyats (Selim) Mountain Pass | 1987 | 2100 | [30] | |||
| Tsovagyugh | 1988 | 2023 | EURISCO catalog, Acc. № 8C10; [27] | |||
| Areguni | - | 2000 | [28] | |||
| Vaghashen | - | 1956 | [28] | |||
| Geghhovit | - | 2080 | [28] | |||
| B. macrorhiza | Sevan | Gegharkunk | Sevan (Areguni mountain range) | 1956 | 2014 | [26] |
| Tsapatax | 2001 | 1950 | [30] | |||
| Pambak | 2000 | 1920 | [30] | |||
| 2009 | 2180 | [30] | ||||
| Vardenyats (Selim) Mountain Pass | 1987 | 2200 | EURISCO catalog, Acc. № 8M03; [27,31] | |||
| - | 2110 | [28] | ||||
| 2016- 2021 | 2200 | This study | ||||
| 2019 | 2410 | EURISCO catalog, Acc. № 8M04; This study | ||||
| 2021 | 2410 | EURISCO catalog, Acc. № 8M05; This study | ||||
| Darelegis | Vayots Dzor | Gnishik | 1956 | 2020 | [26,33] | |
| Yehegis river gorge | 1956 | - | [26,33] | |||
| Gladzor | 1987 | 1350 | EURISCO catalog, Acc. № 8M01; [27] | |||
| road Goghtanik—Herher | 2005 | 2070 | [30] | |||
| Shirak | Shirak | Gyumri-Spitak highway | 1987 | - | EURISCO catalog, Acc. № 8M02; [27] | |
| Lori | Lori | |||||
| B. trigyna | Aparan | Kotayk | Mount Ara | 1928 | 2480 | [30] |
| Tsakhkadzor | 1929 | 2265 | [30] | |||
| Aragatsotn | Amberd castle | - | 2140 | [30] | ||
| Gegham | Ararat | Khosrov Forest State Reserve | - | 2750 | [30] | |
| Sevan | Gegharkunk | Sevan | - | 2280 | [30] | |
| Vayots Dzor | Vardenyats (Selim) Mountain Pass | - | 2165 | [30] | ||
| B. vulgarissubsp maritima | Ijevan | - | - | 800–1800 | [26,28,31] | |
| Yerevan | - | - | ||||
| Darelegis | - | - |
| Species | Location | 2016 | 2017 | 2018 | 2019 | 2020 * | 2021 |
|---|---|---|---|---|---|---|---|
| B. lomatogona | Akunq/Zarindja | 0 | 0 | 0 | 0 | - | 8 |
| B. corolliflora | Hrazdan region | 18 | 22 | 24 | 30 | - | 27 |
| B. macrorhiza | Vardenyats Mountain Pass | 10 | 15 | 17 | 20 | - | 16 |
| B. lomatogona | B. corolliflora | B. macrorhiza | ||
|---|---|---|---|---|
| Stems | Shape/ Cross-section | Erect stem, round with small ridges | Strong thick stem, angular | Erect or procumbent stem, angular |
| Height (cm) | 40–75 | 50–150 | 70–95 | |
| Diameter (cm) | 0.3–0.7 | 0.8–2.0 | 3.0–4.0 | |
| Rosette | Shape | With semi-erect leaves | With erect leaves | With erect or semi-erect leaves |
| Leaves length (cm) | 12–15 | 15–19 | 14–16 | |
| Leaves width (cm) | 2–5 | 7–9 | 5–7 | |
| Petiole length (cm) | 10–12 | 15–17 | 5–7 | |
| Leaves | Shape | Oblong, triangular, lanceolate, ovate; smooth and shiny surface | Oval, lanceolate (other types possible) with a cordate leaf base; weak fluffy surface | Spear-shaped, large, wide, with a blunt base; smooth surface |
| Roots | Shape | Narrowed, fusiform, woody | Cylindrical, woody | Cylindrical, conical, soft |
| Length (cm) | 75–100 | 100 (or more) | 120–150 | |
| Diameter (cm) | 6–8 | up to 15 | 8–15 | |
| Color | Red brownish | Whitish | Whitish | |
| Flowers/Seeds | Tepals are greenish, wide, white membranous, unequally dented at apex. Single flowers are on a long spiciform inflorescence and are arranged in tight axillary glomeruli. Weight of 1000 glomeruli 12–14 g. | Tepals are corolla-like whitish or yellowish, broadly open during flowering, slightly curved inward. Bracts are linear, not exceeding flowers. Inflorescences are compound, pyramidal, dichasial, with sympodial branching, and with branched leaf-shaped bracts. Flowers (2)3 coalescing into glomeruli. Weight of 1000 glomeruli 43–56 g. | Tepals are greenish, yellowish-greenish, flat, wide open. Bracts broadly ovate, exceeding the flowers. Inflorescences are elongated spikes. Flowers 4–6(8) coalescing into glomeruli. Weight of 1000 glomeruli 55–56 g. | |
| Species | Stem Height (cm) | Rosette Shape | Leaves | Root | |||||
|---|---|---|---|---|---|---|---|---|---|
| Shape | Surface | Length * (cm) | Width * (cm) | Petiole Length * (cm) | Diameter (cm) | Color | |||
| B. macrorhiza | 90 | Erect | Spear-shaped | Smooth | 15 ± 1.1 a | 6 ± 0.7 a | 6 ± 0.7 a | 12 | White |
| Unknown Beta | 40 | Semi-erect | Ovate | Smooth and shiny | 14 ± 1.0 a | 4 ± 1.2 b | 9 ± 1.0 b | 8 | Red |
| Gene/ Fragment | Accession Number | Definition | Marked in the Phylogenetic Tree as | Description/Reference |
|---|---|---|---|---|
| adh | KP747951.1 | B. lomatogona alcohol dehydrogenase (adh) gene, partial cds | Beta lomatogona/GenBank | [70] |
| adh | KP747950.1 | B. macrorhiza alcohol dehydrogenase (adh) gene, partial cds | Beta macrorhiza/GenBank | [70] |
| adh | KP747982.1 | B. vulgaris subsp. maritima isolate 33HAT alcohol dehydrogenase (adh) gene, partial cds | Beta vulgaris subsp. maritima/GenBank | [70] |
| adh | OM857605 | B. lomatogona alcohol dehydrogenase (adh) gene, partial cds | Beta lomatogona | Location: Akunq, Aragatsotn This study, collection of 2006 |
| adh | OM857606 | B. corolliflora alcohol dehydrogenase (adh) gene, partial cds | Beta corolliflora | Location: Hrazdan, Kotayk This study |
| adh | OM857607 | B. macrorhiza alcohol dehydrogenase (adh) gene, partial cds | Beta macrorhiza | Location: Vardenyats mountain Pass, Gegharkunik This study |
| adh | OM857608 | Beta x intermedium Aloyan alcohol dehydrogenase (adh) gene, partial cds | Unknown Beta | Location: Vardenyats mountain Pass, Gegharkunik This study |
| LF | KP747769.1 | B. lomatogona trnL-trnF intergenic spacer, partial sequence; chloroplast | Beta lomatogona/GenBank | [70] |
| LF | KP747770.1 | B.macrorhiza trnL-trnF intergenic spacer, partial sequence; chloroplast | Beta macrorhiza/GenBank | [70] |
| LF | AY858608.1 | B.corolliflora tRNA-Leu (trnL) gene, partial sequence; trnL-trnF intergenic spacer, complete sequence; and tRNA-Phe (trnF) gene, paritial sequence; chloroplast | Beta corolliflora/GenBank | [81] |
| LF | AY858605.1 | B. trigyna tRNA-Leu (trnL) gene, partial sequence; trnL-trnF intergenic spacer, complete sequence; and tRNA-Phe (trnF) gene, paritial sequence; chloroplast | Beta trigyna/GenBank | [81] |
| LF | KP747794.1 | B. vulgaris subsp. maritima isolate 33HAT trnL-trnF intergenic spacer, partial sequence; chloroplast | Beta vulgaris subsp. maritima/GenBank | [70] |
| LF | OM857609 | B. lomatogona trnL-trnF intergenic spacer, partial sequence; chloroplast | Beta lomatogona | Location: Akunq, Aragatsotn This study, collection of 2006 |
| LF | OM857610 | B. corolliflora trnL-trnF intergenic spacer, partial sequence; chloroplast | Beta macrorhiza | Location: Hrazdan, Kotayk This study |
| LF | OM857611 | B. macrorhiza trnL-trnF intergenic spacer, partial sequence; chloroplast | Beta corolliflora | Location: Vardenyats mountain Pass, Gegharkunik This study |
| LF | OM857612 | Beta x intermedium Aloyan trnL-trnF intergenic spacer, partial sequence; chloroplast | Unknown Beta | Location: Vardenyats mountain Pass, Gegharkunik This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avetisyan, A.; Aloyan, T.; Iskandaryan, A.; Harutyunyan, M.; Jaakola, L.; Melikyan, A. Distribution of Biodiversity of Wild Beet Species (Genus Beta L.) in Armenia under Ongoing Climate Change Conditions. Plants 2022, 11, 2502. https://doi.org/10.3390/plants11192502
Avetisyan A, Aloyan T, Iskandaryan A, Harutyunyan M, Jaakola L, Melikyan A. Distribution of Biodiversity of Wild Beet Species (Genus Beta L.) in Armenia under Ongoing Climate Change Conditions. Plants. 2022; 11(19):2502. https://doi.org/10.3390/plants11192502
Chicago/Turabian StyleAvetisyan, Anna, Tatevik Aloyan, Amalya Iskandaryan, Margarita Harutyunyan, Laura Jaakola, and Andreas Melikyan. 2022. "Distribution of Biodiversity of Wild Beet Species (Genus Beta L.) in Armenia under Ongoing Climate Change Conditions" Plants 11, no. 19: 2502. https://doi.org/10.3390/plants11192502
APA StyleAvetisyan, A., Aloyan, T., Iskandaryan, A., Harutyunyan, M., Jaakola, L., & Melikyan, A. (2022). Distribution of Biodiversity of Wild Beet Species (Genus Beta L.) in Armenia under Ongoing Climate Change Conditions. Plants, 11(19), 2502. https://doi.org/10.3390/plants11192502








