Impact of Geraniol and Geraniol Nanoemulsions on Botrytis cinerea and Effect of Geraniol on Cucumber Plants’ Metabolic Profile Analyzed by LC-QTOF-MS
Abstract
:1. Introduction
2. Results
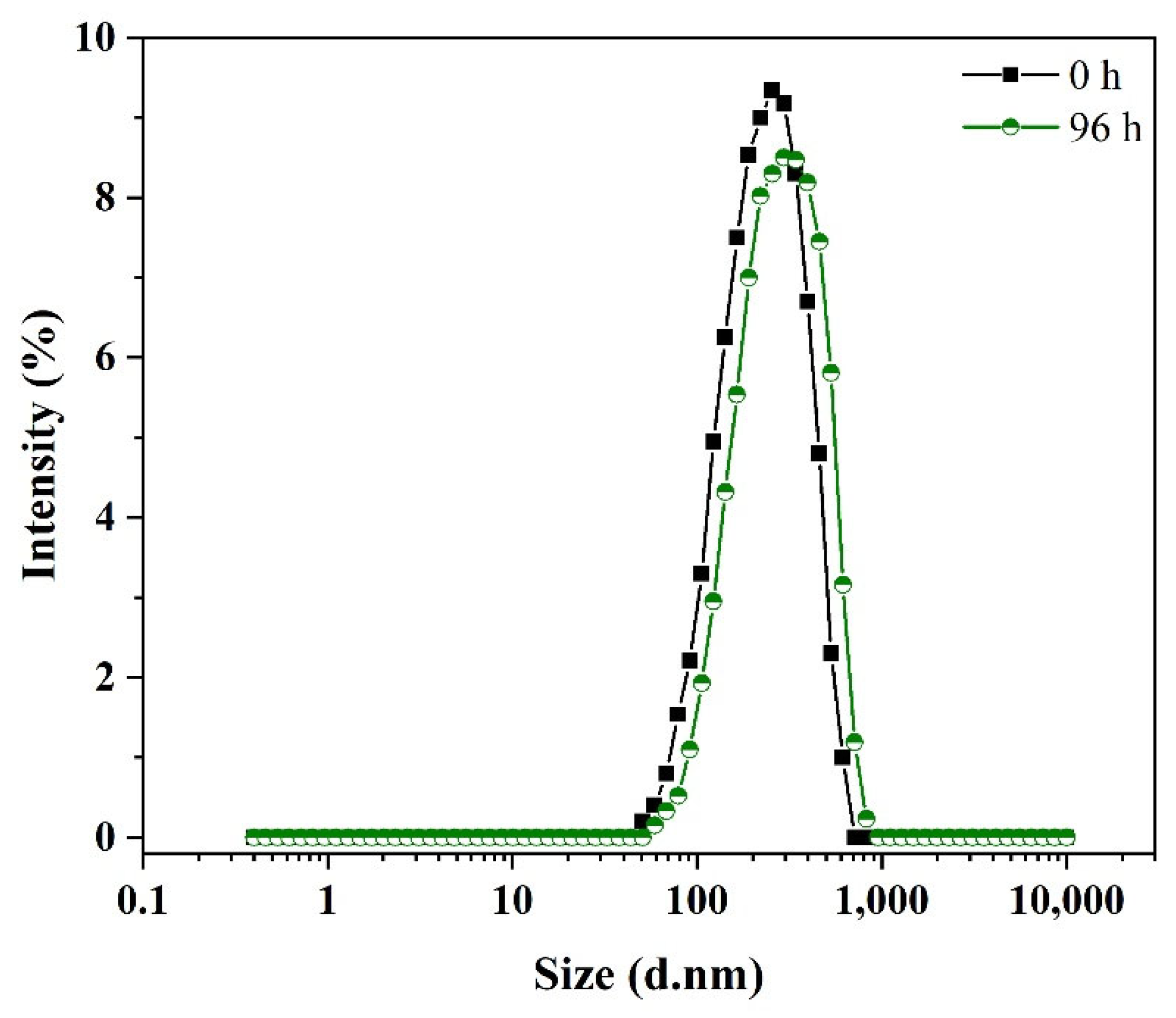
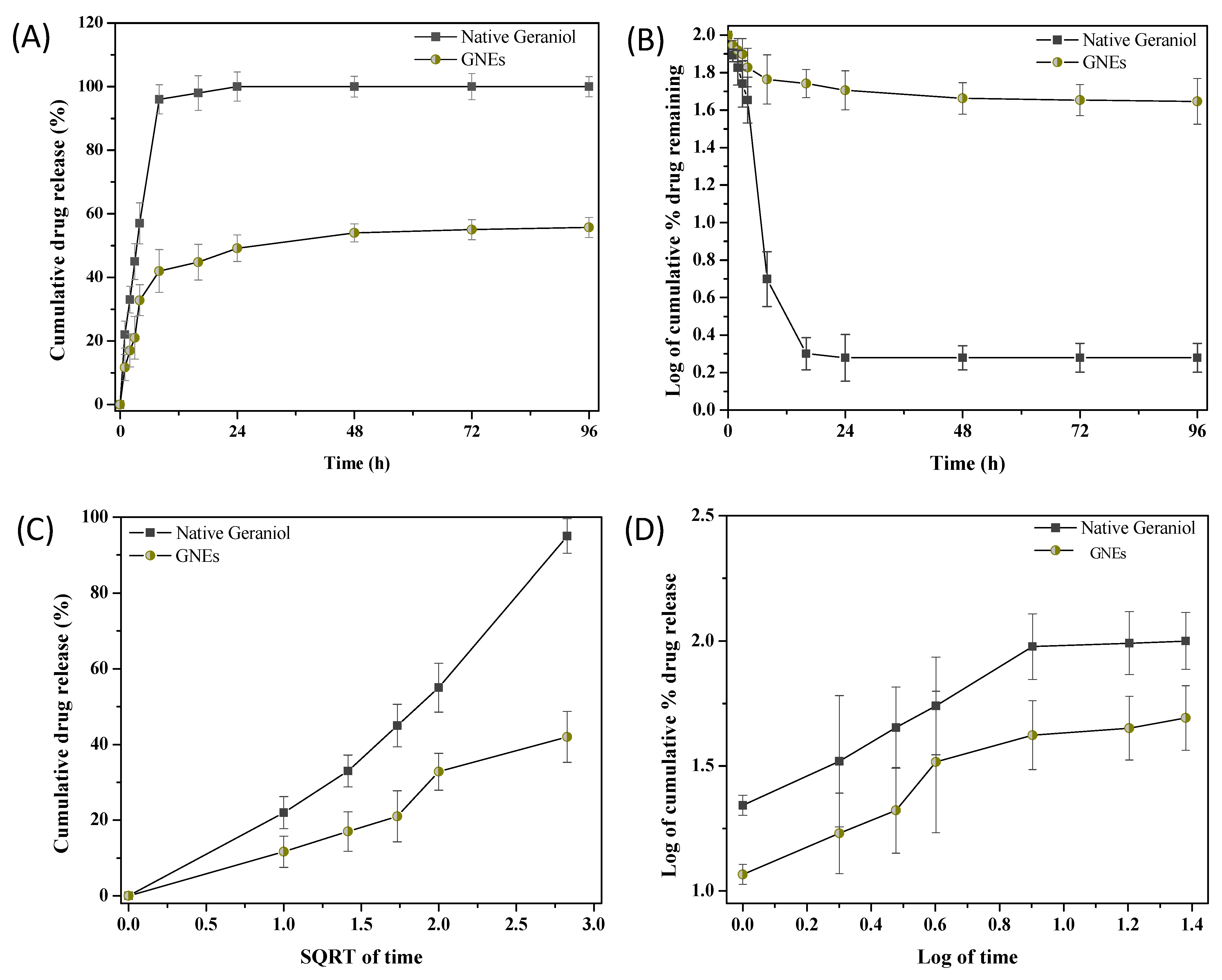
2.1. GNEs: Characterization and Pharmacokinetic Study
2.2. In vitro Antifungal Activity
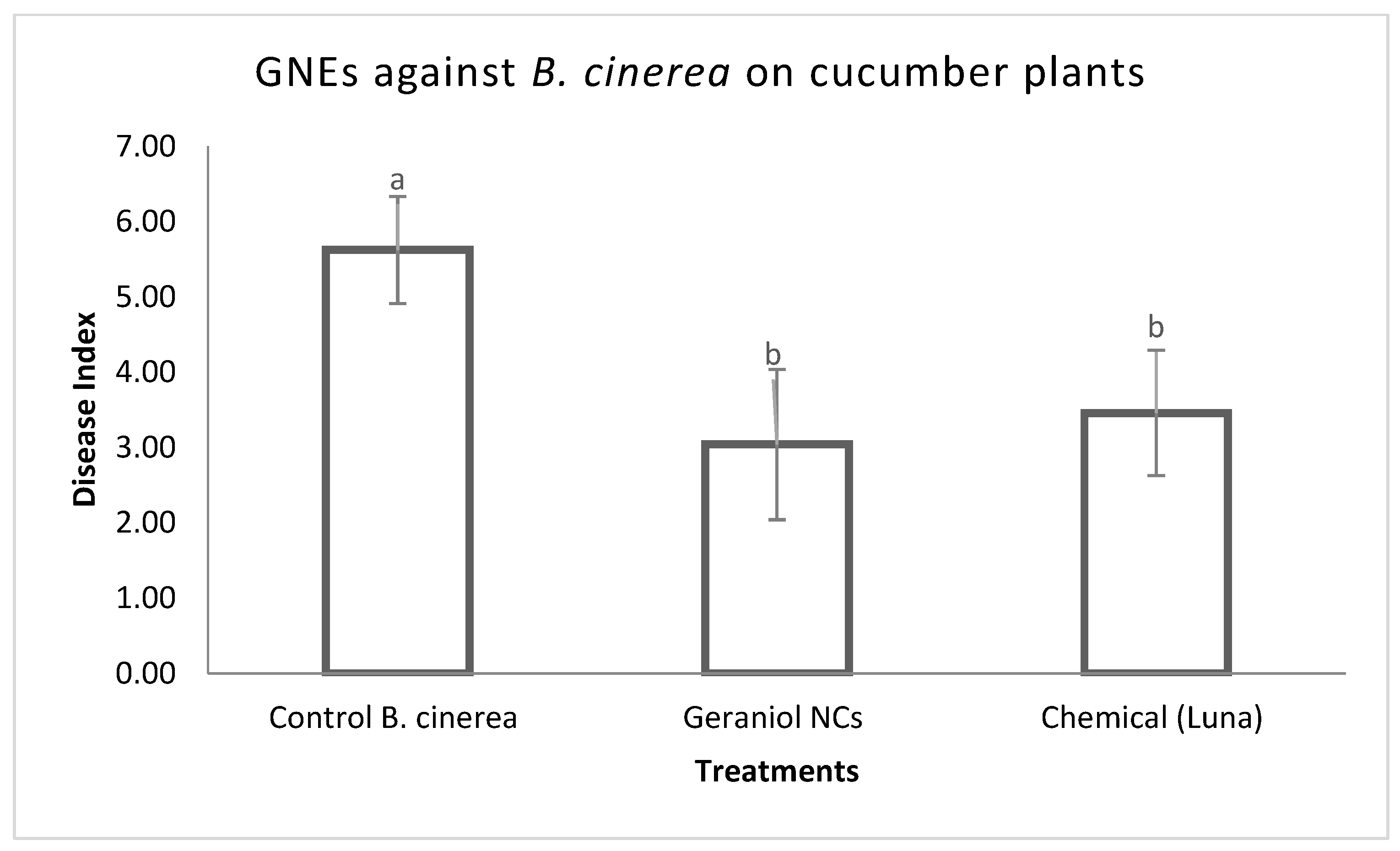
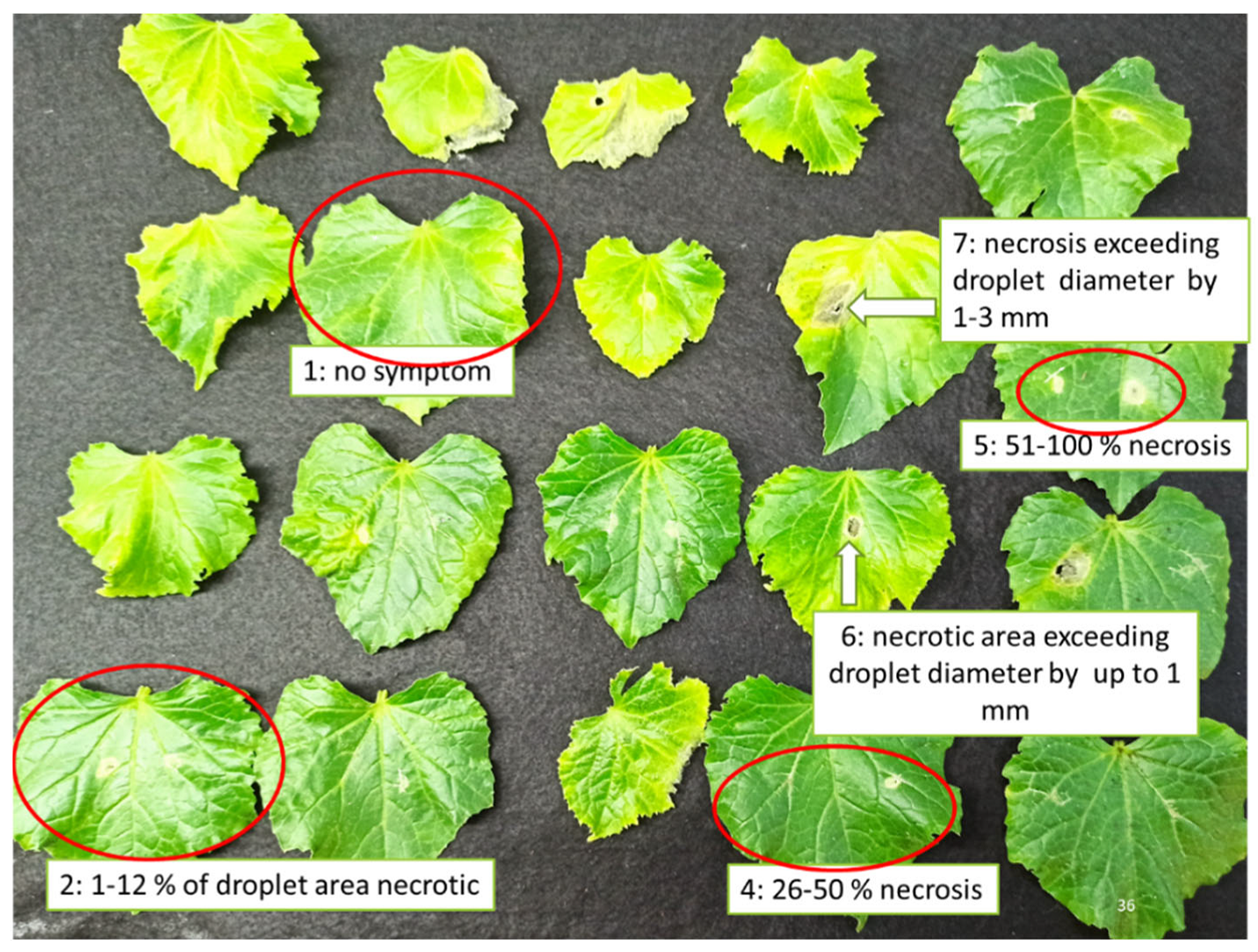
2.3. In Planta Antifungal Activity
2.4. Biochemical Assays
2.5. LC-QTOF/MS Analysis
3. Discussion
4. Materials and Methods
4.1. Preparation of GNEs
4.1.1. Physicochemical Characterization of Geraniol Nanoemulsions
4.1.2. Geraniol Release
4.2. In Vitro Antifungal Activity
4.3. In Planta Antifungal Activity
4.4. Malondialdehyde (MDA) Content
4.5. LC-QTOF-MS Analysis
4.5.1. Standards and Reagents
4.5.2. Sample Preparation
4.5.3. Chromatographic Analysis
4.5.4. Target and Suspect Screening Strategies
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. FAOSTAT Statistical Database. Available online: https://www.fao.org/faostat/en/#home (accessed on 12 May 2022).
- Keinath, A.P.; Wintermantel, W.M.; Zitter, T.A. (Eds.) Compendium of Cucurbit Diseases and Pests, 2nd ed.; APS Press: St. Paul, MN, USA, 2017. [Google Scholar]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology: Top 10 fungal pathogens. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Moser, R.; Pertot, I.; Elad, Y.; Raffaelli, R. Farmers’ attitudes toward the use of biocontrol agents in IPM strawberry production in three countries. Biol. Control 2008, 47, 125–132. [Google Scholar] [CrossRef]
- Hahn, M. The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J. Chem. Biol. 2014, 7, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Zhao, Y.; Ma, Z. Advances in Understanding Fungicide Resistance in Botrytis cinerea in China. Phytopathology 2021, 111, 455–463. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential Oils in Combination and Their Antimicrobial Properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Bajguz, A.; Hayat, S. The role of quercetin in plants. Plant Physiol. Biochem. 2021, 166, 10–19. [Google Scholar] [CrossRef]
- Ntalli, N.; Parlapani, A.B.; Tzani, K.; Samara, M.; Boutsis, G.; Dimou, M.; Menkissoglu-Spiroudi, U.; Monokrousos, N. Thymus Citriodorus (Schreb) Botanical Products as Ecofriendly Nematicides with Bio-Fertilizing Properties. Plants 2020, 9, 202. [Google Scholar] [CrossRef]
- Chen, W.; Viljoen, A.M. Geraniol—A review of a commercially important fragrance material. S. Afr. J. Bot. 2010, 76, 643–651. [Google Scholar] [CrossRef]
- Yegin, Y.; Perez-Lewis, K.L.; Zhang, M.; Akbulut, M.; Taylor, T.M. Development and characterization of geraniol-loaded polymeric nanoparticles with antimicrobial activity against foodborne bacterial pathogens. J. Food Eng. 2016, 170, 64–71. [Google Scholar] [CrossRef]
- Syed, I.; Sarkar, P. Ultrasonication-assisted formation and characterization of geraniol and carvacrol-loaded emulsions for enhanced antimicrobial activity against food-borne pathogens. Chem. Pap. 2018, 72, 2659–2672. [Google Scholar] [CrossRef]
- Gateva, S.; Jovtchev, G.; Stankov, A.; Georgieva, A.; Dobreva, A.; Mileva, M. The potential of geraniol to reduce cytotoxic and genotoxic effects of MNNG in plant and human lymphocyte test-systems. S. Afr. J. Bot. 2019, 123, 170–179. [Google Scholar] [CrossRef]
- Jovtchev, G.; Stankov, A.; Georgieva, A.; Dobreva, A.; Bakalova, R.; Aoki, I.; Mileva, M. Cytotoxic and genotoxic potential of Bulgarian Rosa alba L. essential oil—In vitro model study. Biotechnol. Biotechnol. Equip. 2018, 32, 513–519. [Google Scholar] [CrossRef]
- Ložienė, K.; Vaičiulytė, V. Geraniol and Carvacrol in Essential Oil Bearing Thymus pulegioides: Distribution in Natural Habitats and Phytotoxic Effect. Molecules 2022, 27, 986. [Google Scholar] [CrossRef]
- Petit, A.-N.; Fontaine, F.; Vatsa, P.; Clément, C.; Vaillant-Gaveau, N. Fungicide impacts on photosynthesis in crop plants. Photosynth. Res. 2012, 111, 315–326. [Google Scholar] [CrossRef]
- Lubbe, A.; Verpoorte, R.; Choi, Y.H. Effects of fungicides on galanthamine and metabolite profiles in Narcissus bulbs. Plant Physiol. Biochem. 2012, 58, 116–123. [Google Scholar] [CrossRef]
- Bernillon, S.; Biais, B.; Deborde, C.; Maucourt, M.; Cabasson, C.; Gibon, Y.; Hansen, T.H.; Husted, S.; De Vos, R.C.H.; Mumm, R.; et al. Metabolomic and elemental profiling of melon fruit quality as affected by genotype and environment. Metabolomics 2012, 9, 57–77. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Profiling of phenolic and other polar constituents from hydro-methanolic extract of watermelon (Citrullus lanatus) by means of accurate-mass spectrometry (HPLC–ESI–QTOF–MS). Int. Food Res. J. 2013, 51, 354–362. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Quirantes-Piné, R.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Comparative characterization of phenolic and other polar compounds in Spanish melon cultivars by using high-performance liquid chromatography coupled to electrospray ionization quadrupole-time of flight mass spectrometry. Int. Food Res. J. 2013, 54, 1519–1527. [Google Scholar] [CrossRef]
- Iswaldi, I.; Gómez-Caravaca, A.M.; Lozano-Sánchez, J.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Profiling of phenolic and other polar compounds in zucchini (Cucurbita pepo L.) by reverse-phase high-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Int. Food Res. J. 2013, 50, 77–84. [Google Scholar] [CrossRef]
- Haq, F.U.; Ali, A.; Khan, M.N.; Shah, S.M.Z.; Kandel, R.C.; Aziz, N.; Adhikari, A.; Choudhary, M.I.; Ur-Rahman, A.; El-Seedi, H.R.; et al. Metabolite Profiling and Quantitation of Cucurbitacins in Cucurbitaceae Plants by Liquid Chromatography coupled to Tandem Mass Spectrometry. Sci. Rep. 2019, 9, 15992. [Google Scholar] [CrossRef] [Green Version]
- Kalogiouri, N.P.; Palaiologou, E.; Papadakis, E.N.; Makris, D.P.; Biliaderis, C.G.; Mourtzinos, I. Insights on the impact of deep eutectic solvents on the composition of the extracts from lemon (Citrus limon L.) peels analyzed by a novel RP-LC–QTOF-MS/MS method. Eur. Food Res. Technol 2022, 1–15. [Google Scholar] [CrossRef]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A public repository for sharing mass spectral data for life sciences. J. Mass Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef] [PubMed]
- FooDB, The Food Components Database. 2016. Available online: http://foodb.ca/ (accessed on 10 June 2022).
- Kalogiouri, N.P.; Kritikou, E.; Martakos, I.C.; Lazarou, C.; Pentogennis, M.; Thomaidis, N.S. Characterization of the Phenolic Fingerprint of Kolovi Extra Virgin Olive Oils from Lesvos with Regard to Altitude and Farming System Analyzed by UHPLC-QTOF-MS. Molecules 2021, 26, 5634. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Schmeda-Hirschmann, G. Direct identification of phenolic constituents in Boldo Folium (Peumus boldus Mol.) infusions by high-performance liquid chromatography with diode array detection and electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 443–449. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, X.; Liu, J.; Kang, L.; Chen, S.; Ma, B.; Guo, B. Quantitative and Qualitative Analysis of Flavonoids and Phenolic Acids in Snow Chrysanthemum (Coreopsis tinctoria Nutt.) by HPLC-DAD and UPLC-ESI-QTOF-MS. Molecules 2016, 21, 1307. [Google Scholar] [CrossRef]
- Vijayan, K.P.R.; Raghu, A.V. Tentative characterization of phenolic compounds in three species of the genus Embelia by liquid chromatography coupled with mass spectrometry analysis. Spectrosc. Lett. 2019, 52, 653–670. [Google Scholar] [CrossRef]
- Nguyen, N.A.; Cao, N.T.; Nguyen, T.H.H.; Ji, J.-H.; Cha, G.S.; Kang, H.-S.; Yun, C.-H. Enzymatic Production of 3-OH Phlorizin, a Possible Bioactive Polyphenol from Apples, by Bacillus megaterium CYP102A1 via Regioselective Hydroxylation. Antioxidants 2021, 10, 1327. [Google Scholar] [CrossRef]
- De Oliveira Filho, J.G.; da Cruz Silva, G.; de Aguiar, A.C.; Cipriano, L.; de Azeredo, H.M.C.; Junior, S.B.; Ferreira, M.D. Chemical composition and antifungal activity of essential oils and their combinations against Botrytis cinerea in strawberries. Food Meas. 2021, 15, 1815–1825. [Google Scholar] [CrossRef]
- Aguilar-González, A.E.; Palou, E.; López-Malo, A. Antifungal activity of essential oils of clove (Syzygium aromaticum) and/or mustard (Brassica nigra) in vapor phase against gray mold (Botrytis cinerea) in strawberries. Innov. Food Sci. Emerg. Technol. 2015, 32, 181–185. [Google Scholar] [CrossRef]
- Pedrotti, C.; Ribeiro, R.T.D.S.; Schwambach, J. Control of postharvest fungal rots in grapes through the use of Baccharis trimera and Baccharis dracunculifolia essential oils. Crop Prot. 2019, 125, 104912. [Google Scholar] [CrossRef]
- Ziedan, E.-S.H.E.; Saad, M.M.; El-Kafrawy, A.A.; Sahab, A.F.; Mossa, A.-T.H. Evaluation of essential oils nanoemulsions formulations on Botrytis cinerea growth, pathology and grey mould incidence on cucumber fruits. Bull. Natl. Res. Cent. 2022, 46, 88. [Google Scholar] [CrossRef]
- Martinezromero, D.; Guillen, F.; Valverde, J.; Bailen, G.; Zapata, P.J.; Serrano, M.; Castillo, S.; Valero, D. Influence of carvacrol on survival of Botrytis cinerea inoculated in table grapes. Int. J. Food Microbiol. 2007, 115, 144–148. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Chen, H.; Fan, Y.; Shi, Z. Antifungal activity of eugenol against Botrytis cinerea. Trop. Plant Pathol. 2010, 35, 137–143. [Google Scholar] [CrossRef]
- Olea, A.F.; Bravo, A.; Martínez, R.; Thomas, M.; Sedan, C.; Espinoza, L.; Zambrano, E.; Carvajal, D.; Silva-Moreno, E.; Carrasco, H. Antifungal Activity of Eugenol Derivatives against Botrytis cinerea. Molecules 2019, 24, 1239. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, S.; Du, S.; Chen, S.; Sun, H. Antifungal activity of thymol and carvacrol against postharvest pathogens Botrytis cinerea. J. Food Sci. Technol. 2019, 56, 2611–2620. [Google Scholar] [CrossRef]
- Khan, A.; Ahmad, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.A.; Manzoor, N. Ocimum sanctum essential oil and its active principles exert their antifungal activity by disrupting ergosterol biosynthesis and membrane integrity. Res. Microbiol. 2010, 161, 816–823. [Google Scholar] [CrossRef]
- Tang, X.; Shao, Y.-L.; Tang, Y.-J.; Zhou, W.-W. Antifungal Activity of Essential Oil Compounds (Geraniol and Citral) and Inhibitory Mechanisms on Grain Pathogens (Aspergillus flavus and Aspergillus ochraceus). Molecules 2018, 23, 2108. [Google Scholar] [CrossRef]
- Vamvakidis, K.; Mourdikoudis, S.; Makridis, A.; Paulidou, E.; Angelakeris, M.; Dendrinou-Samara, C. Magnetic hyperthermia efficiency and MRI contrast sensitivity of colloidal soft/hard ferrite nanoclusters. J. Colloid Interface Sci. 2018, 511, 101–109. [Google Scholar] [CrossRef]
- Gago, C.M.L.; Artiga-Artigas, M.; Antunes, M.D.C.; Faleiro, M.L.; Miguel, M.G.; Martín-Belloso, O. Effectiveness of nanoemulsions of clove and lemongrass essential oils and their major components against Escherichia coli and Botrytis cinerea. J. Food Sci. Technol. 2019, 56, 2721–2736. [Google Scholar] [CrossRef]
- Qian, C.; McClements, D.J. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: Factors affecting particle size. Food Hydrocoll. 2010, 25, 1000–1008. [Google Scholar] [CrossRef]
- Niu, F.; Pan, W.; Su, Y.; Yang, Y. Physical and antimicrobial properties of thyme oil emulsions stabilized by ovalbumin and gum rabic. Food Chem. 2016, 212, 138–145. [Google Scholar] [CrossRef]
- Hussein, J.; El-Bana, M.; Refaat, E.; El-Naggar, M.E. Synthesis of carvacrol-based nanoemulsion for treating neurodegenerative disorders in experimental diabetes. J. Funct. Foods 2017, 37, 441–448. [Google Scholar] [CrossRef]
- Dokić, L.; Krstonošić, V.; Nikolić, I. Physicochemical characteristics and stability of oil-in-water emulsions stabilized by OSA starch. Food Hydrocoll. 2012, 29, 185–192. [Google Scholar] [CrossRef]
- Kutawa, A.B.; Ahmad, K.; Ali, A.; Hussein, M.Z.; Wahab, M.A.A.; Adamu, A.; Ismaila, A.A.; Gunasena, M.T.; Rahman, M.Z.; Hossain, M.I. Trends in Nanotechnology and Its Potentialities to Control Plant Pathogenic Fungi: A Review. Biology 2021, 10, 881. [Google Scholar] [CrossRef]
- Dixon, R. Genistein. Phytochemistry 2002, 60, 205–211. [Google Scholar] [CrossRef]
- Balmer, D.; de Papajewski, D.V.; Planchamp, C.; Glauser, G.; Mauch-Mani, B. Induced resistance in maize is based on organ-specific defence responses. Plant J. 2013, 74, 213–225. [Google Scholar] [CrossRef]
- Niggeweg, R.; Michael, A.J.; Martin, C. Engineering plants with increased levels of the antioxidant chlorogenic acid. Nat. Biotechnol. 2004, 22, 746–754. [Google Scholar] [CrossRef]
- Martínez, G.; Regente, M.; Jacobi, S.; Del Rio, M.; Pinedo, M.; de la Canal, L. Chlorogenic acid is a fungicide active against phytopathogenic fungi. Pestic. Biochem. Physiol. 2017, 140, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Bollina, V.; Kushalappa, A.C.; Choo, T.M.; Dion, Y.; Rioux, S. Identification of metabolites related to mechanisms of resistance in barley against Fusarium graminearum, based on mass spectrometry. Plant Mol. Biol. 2011, 77, 355–370. [Google Scholar] [CrossRef]
- Chen, X.; Fang, X.; Zhang, Y.; Wang, X.; Zhang, C.; Yan, X.; Zhao, Y.; Wu, J.; Xu, P.; Zhang, S. Overexpression of a soybean 4-coumaric acid: Coenzyme a ligase (GmPI4L) enhances resistance to Phytophthora sojae in soybean. Funct. Plant Biol. 2019, 46, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphreys, J.M.; Hemm, M.R.; Chapple, C. New routes for lignin biosynthesis defined by biochemical characterization of recombinant ferulate 5-hydroxylase, a multifunctional cytochrome P450-dependent monooxygenase. Proc. Natl. Acad. Sci. USA 1999, 96, 10045–10050. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Dixon, R.A. Altering the Cell Wall and Its Impact on Plant Disease: From Forage to Bioenergy. Annu. Rev. Phytopathol. 2014, 52, 69–91. [Google Scholar] [CrossRef]
- Miedes, E.M.; Evanholme, R.; Boerjan, W.; Emolina, A. The role of the secondary cell wall in plant resistance to pathogens. Front. Plant Sci. 2014, 5, 358. [Google Scholar] [CrossRef] [PubMed]
- Mellidou, I.; Ainalidou, A.; Papadopoulou, A.; Leontidou, K.; Genitsaris, S.; Karagiannis, E.; Van de Poel, B.; Karamanoli, K. Comparative Transcriptomics and Metabolomics Reveal an Intricate Priming Mechanism Involved in PGPR-Mediated Salt Tolerance in Tomato. Front. Plant Sci. 2021, 12, 713984. [Google Scholar] [CrossRef]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and Artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef]
- Schmid-Siegert, E.; Loscos, J.; Farmer, E.E. Inducible Malondialdehyde Pools in Zones of Cell Proliferation and Developing Tissues in Arabidopsis. J. Biol. Chem. 2012, 287, 8954–8962. [Google Scholar] [CrossRef]
- Muñoz, P.; Briones, M.; Munné-Bosch, S. Photoinhibition and photoprotection during flower opening in lilies. Plant Sci. 2018, 272, 220–229. [Google Scholar] [CrossRef]
- Aly, A.A.; Mohamed, H.I.; Mansour, M.T.M.; Omar, M.R. Suppression of Powdery Mildew on Flax by Foliar Application of Essential Oils. J. Phytopathol. 2013, 161, 376–381. [Google Scholar] [CrossRef]
- Chen, C.; Cai, N.; Chen, J.; Wan, C. Clove Essential Oil as an Alternative Approach to Control Postharvest Blue Mold Caused by Penicillium italicum in Citrus Fruit. Biomolecules 2019, 9, 197. [Google Scholar] [CrossRef]
- Xue, Y.; Wu, M.; Liu, Z.; Song, J.; Luo, S.; Li, H.; Li, Y.; Jin, L.; Guan, B.; Lin, M.; et al. In vitro and in vivo evaluation of chitosan scaffolds combined with simvastatin-loaded nanoparticles for guided bone regeneration. J. Mater. Sci. Mater. Med. 2019, 30, 47. [Google Scholar] [CrossRef]
- Kapustová, M.; Granata, G.; Napoli, E.; Puškárová, A.; Bučková, M.; Pangallo, D.; Geraci, C. Nanoencapsulated Essential Oils with Enhanced Antifungal Activity for Potential Application on Agri-Food, Material and Environmental Fields. Antibiotics 2021, 10, 31. [Google Scholar] [CrossRef]
- Dash, V. Release Kinetic Studies of Aspirin Microcapsules from Ethyl Cellulose, Cellulose Acetate Phthalate and their Mixtures by Emulsion Solvent Evaporation Method. Sci. Pharm. 2010, 78, 93–101. [Google Scholar] [CrossRef]
- Lokhandwala, H.; Deshpande, A.; Deshpande, S.H.I.R.I.S.H. Kinetic modeling and dissolution profiles comparison: An overview. Int. J. Pharm. Bio. Sci. 2013, 4, 728–773. [Google Scholar]
- Pandey, D.K.; Tripathi, N.N.; Tripathi, R.D.; Dixit, S.N. Fungitoxic and phytotoxic properties of the essential oil of Hyptis suaveolens/Fungitoxische und phytotoxische Eigenschaften des ätherischen Öis von Hyptis suaveolens. Z. Pflanzenkrankh. Pflanzenschutz J. Plant Dis. Prot. 1982, 89, 344–349. [Google Scholar]
- Elad, Y.; Kohl, J.; Fokkema, N.J. Control of Infection and Sporulation of Botrytis cinerea on Bean and Tomato by Saprophytic Yeasts. Phytopathology 1994, 84, 1193–1200. [Google Scholar] [CrossRef]
- Papadopoulou, A.; Matsi, T.; Kamou, N.; Avdouli, D.; Mellidou, I.; Karamanoli, K. Decoding the potential of a new Pseudomonas putida strain for inducing drought tolerance of tomato (Solanum lycopersicum) plants through seed biopriming. J. Plant Physiol. 2022, 271, 153658. [Google Scholar] [CrossRef]
- Kritikou, E.; Kalogiouri, N.P.; Kolyvira, L.; Thomaidis, N.S. Target and Suspect HRMS Metabolomics for the Determination of Functional Ingredients in 13 Varieties of Olive Leaves and Drupes from Greece. Molecules 2020, 25, 4889. [Google Scholar] [CrossRef]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef] [Green Version]
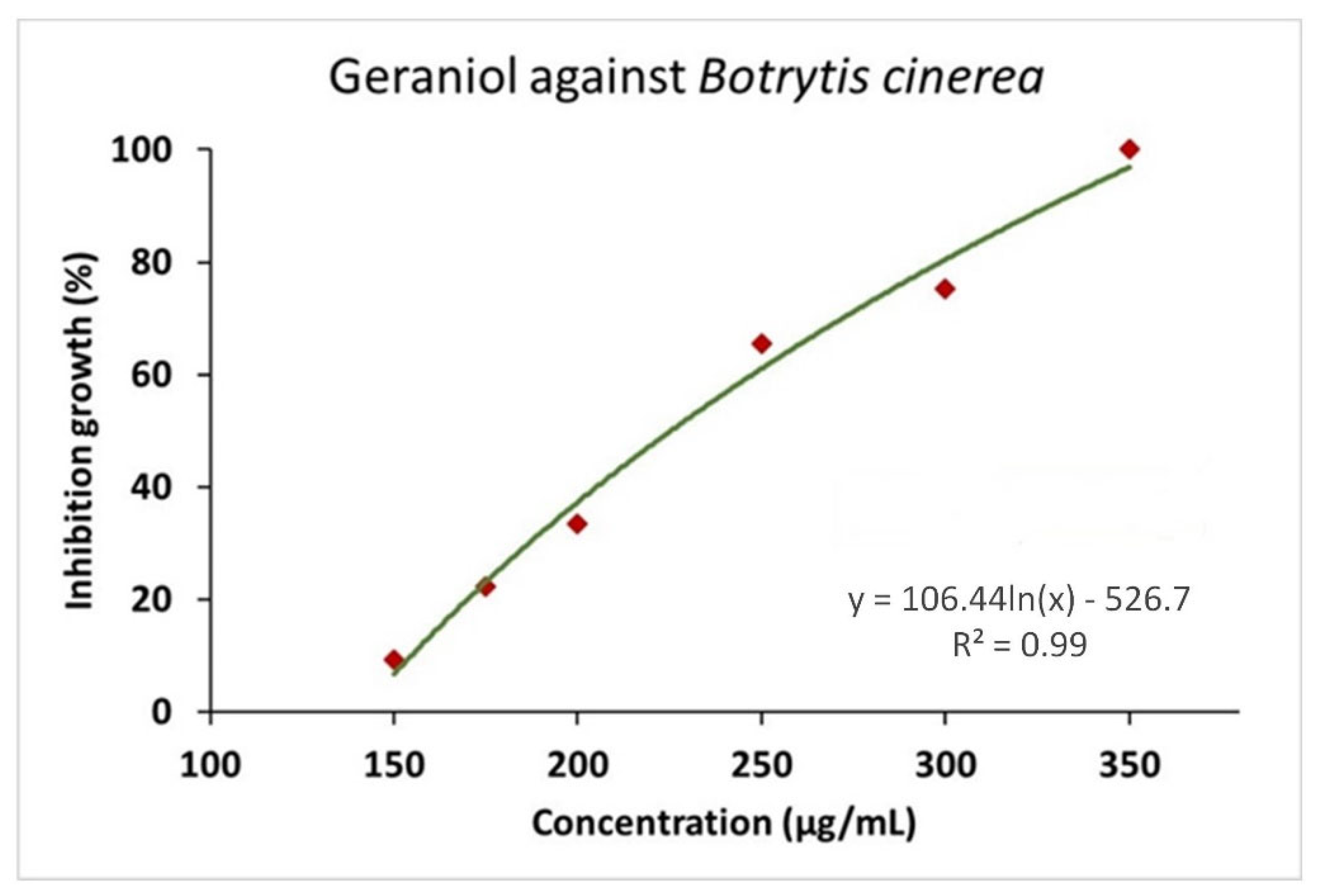
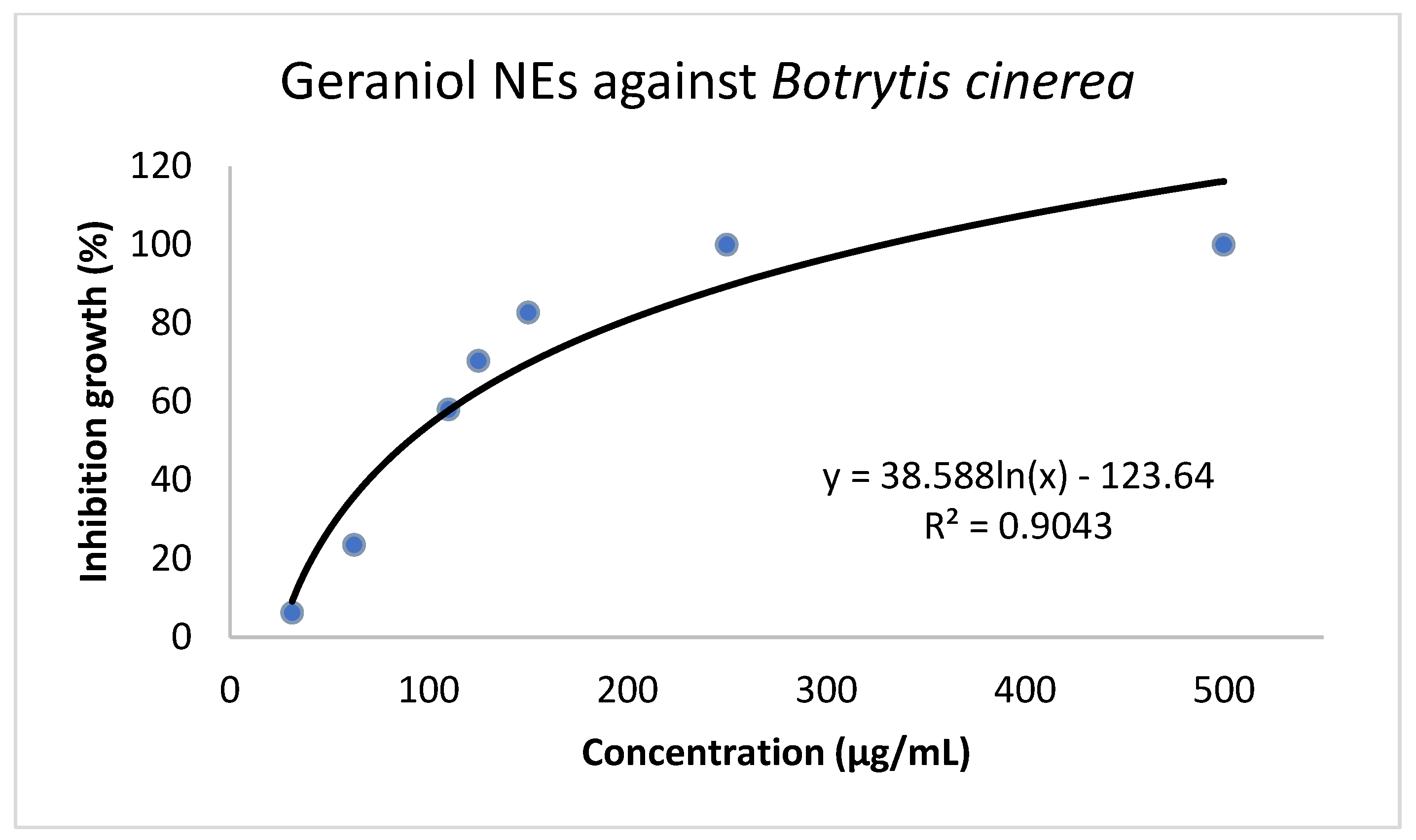


| Treatments | EC50 Values (μg/mL) |
|---|---|
| Geraniol | 235 |
| GNEs | 105 |
| Compound | Molecular Formula | [M-H]- | *RT (min) |
|---|---|---|---|
| Caffeic acid | C9H8O4 | 139.075 | 4.96 |
| p-coumaric acid | C9H8O3 | 163.040 | 5.85 |
| Ferulic acid | C10H10O4 | 193.051 | 6.13 |
| Sinapic acid | C11H12O5 | 223.061 | 6.15 |
| Myricitrin | C21H20O12 | 463.088 | 6.59 |
| Myricetin | C15H10O8 | 317.030 | 7.03 |
| Luteolin | C15H10O6 | 285.040 | 8.25 |
| Apigenin | C15H10O5 | 269.046 | 8.96 |
| Compound | Molecular Formula | [M-H]- | *RT (min) | MS/MS Spectra Comparison |
|---|---|---|---|---|
| Coumaric acid glucoside | C15H18O8 | 325.093 | 4.83 | FOODB record: FDB019119 |
| Quercetin rhamnosylrutinoside | C39H50O25 | 755.204 | 5.01 | [27] |
| Astragalin | C21H20O11 | 447.093 | 5.93 | MassBank record: MSBNK-RIKEN_ReSpect-PS042211 |
| Coreopsin | C21H22O10 | 433.114 | 6.04 | [28] |
| Salicylic acid | C7H6O3 | 137.024 | 6.15 | MassBank record: MSBNK-Keio_Univ-KO000602 |
| Kaempferol rhamnosyl glucoside | C33H40O20 | 593.151 | 6.26 | [27] |
| Tiliroside | C30H26O13 | 593.130 | 6.32 | MassBank record: MSBNK-RIKEN-PR100968 |
| Narcissin | C28H32O16 | 623.162 | 6.36 | [29] |
| Kaempferol rhamnoside | C21H20O10 | 431.098 | 6.44 | [27] |
| Phloridizin | C21H24O10 | 435.130 | 6.58 | [30] |
| Hyperoside/ Isoquercitrin | C21H20O12 | 463.088 | 6.62 | MassBank record: MSBNK-Fiocruz-FIO00168 |
| Naringenin glucoside | C21H22O10 | 433.114 | 6.73 | MassBank record: MSBNK-RIKEN-PR306421 |
| Isorhamnetin glucoside | C22H22O12 | 477.104 | 7.25 | MassBank record: [MSBNK-RIKEN-PR040095] |
| Chlorogenic acid | C16H18O9 | 353.088 | 7.85 | [28] |
| Coniferaldehyde | C10H10O3 | 177.056 | 7.99 | MassBank record: MSBNK-RIKEN_ReSpect-PT200060 |
| Sinapaldehyde | C11H12O4 | 207.066 | 8.09 | MassBank record: MSBNK-RIKEN-PR309000 |
| Naringenin | C15H12O5 | 271.161 | 8.22 | MassBank record: MSBNK-IPB_Halle-PN000004 |
| Genistein | C15H10O5 | 269.046 | 8.98 | MassBank record: MSBNK-MSSJ-MSJ00976 |
| Diosmetin | C16H12O6 | 299.056 | 9.06 | MassBank record: MSBNK-BS-BS003183 |
| Compounds | Cucumber Control | Geraniol Control | B. cinerea control | B. cinerea + geraniol |
|---|---|---|---|---|
| Sinapic acid | * 0.5876 a ± 0.08 | 0.6288 ab ± 0.76 | 0.9812 b ± 0.09 | 0.9584 b ± 0.18 |
| Myricetin | 0.2156 a ± 0.001 | 0.2178 b ± 0.001 | 0.2155 a ± 0.001 | 0.2167 ab ± 0.001 |
| Chlorogenic acid | 100.59 a ± 2.74 | 152.54 c ± 6.55 | 120.31 ab ± 2.22 | 147.96 bc ± 18.47 |
| Genistein | 0.7252 a ± 0.001 | 0.7199 a ± 0.002 | 0.7332 b ± 0.002 | 0.7359 b ± 0.003 |
| Kaempferol rhamnoside | 0.9080 a ± 0.002 | 1.1860 b ± 0.02 | 0.9480 a ± 0.004 | 1.7620 c ± 0.14 |
| Compound | Type | Calibration Equation | R2 |
|---|---|---|---|
| Caffeic acid | Linear | y = 4E + 06x + 49264 | 0.998 |
| p-coumaric acid | Linear | y = 2E + 06x + 187478 | 0.995 |
| Ferulic acid | Linear | y = 1E + 06x + 45615 | 0.996 |
| Sinapic acid | Linear | y = 151632x − 142.29 | 0.999 |
| Myricitrin | Linear | y = 30610x − 1114.8 | 0.999 |
| Myricetin | Linear | y = 8E + 06x − 344334 | 0.997 |
| Luteolin | Linear | y = 1E + 07x + 721454 | 0.996 |
| Apigenin | Linear | y = 7E + 07x + 3E + 06 | 0.991 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamou, N.N.; Kalogiouri, N.P.; Tryfon, P.; Papadopoulou, A.; Karamanoli, K.; Dendrinou-Samara, C.; Menkissoglu-Spiroudi, U. Impact of Geraniol and Geraniol Nanoemulsions on Botrytis cinerea and Effect of Geraniol on Cucumber Plants’ Metabolic Profile Analyzed by LC-QTOF-MS. Plants 2022, 11, 2513. https://doi.org/10.3390/plants11192513
Kamou NN, Kalogiouri NP, Tryfon P, Papadopoulou A, Karamanoli K, Dendrinou-Samara C, Menkissoglu-Spiroudi U. Impact of Geraniol and Geraniol Nanoemulsions on Botrytis cinerea and Effect of Geraniol on Cucumber Plants’ Metabolic Profile Analyzed by LC-QTOF-MS. Plants. 2022; 11(19):2513. https://doi.org/10.3390/plants11192513
Chicago/Turabian StyleKamou, Nathalie N., Natasa P. Kalogiouri, Panagiota Tryfon, Anastasia Papadopoulou, Katerina Karamanoli, Catherine Dendrinou-Samara, and Urania Menkissoglu-Spiroudi. 2022. "Impact of Geraniol and Geraniol Nanoemulsions on Botrytis cinerea and Effect of Geraniol on Cucumber Plants’ Metabolic Profile Analyzed by LC-QTOF-MS" Plants 11, no. 19: 2513. https://doi.org/10.3390/plants11192513
APA StyleKamou, N. N., Kalogiouri, N. P., Tryfon, P., Papadopoulou, A., Karamanoli, K., Dendrinou-Samara, C., & Menkissoglu-Spiroudi, U. (2022). Impact of Geraniol and Geraniol Nanoemulsions on Botrytis cinerea and Effect of Geraniol on Cucumber Plants’ Metabolic Profile Analyzed by LC-QTOF-MS. Plants, 11(19), 2513. https://doi.org/10.3390/plants11192513









